Abstract
Background and Purpose:
In a comprehensive stroke center, we analyzed the stroke code activations (SCA), assessed the impact of Covid-19, and the measures taken by the local government to lessen the spread of the disease.
Methods:
We retrospectively reviewed SCA and classified them into 2 groups: pre-pandemic activations (February 15 to March 10) and Covid-19 pandemic activations (March 11 to April 30). The primary outcome was the ratio of true positive diagnoses of stroke relative to the total number of SCA in the 2 time periods.
Results:
Among the 212 SCA, 83 (39.2%) were from the pre-pandemic period, whereas 129 (60.8%) were from the pandemic period, 147 (69.3%) in the Emergency Department (ED) versus 65 (30.7%) in the inpatient service. In the ED cohort, a rapid decrease in the number of SCA at the beginning of the pandemic was followed by a gradual increase to pre-pandemic levels and a significant increase in the number of true positive strokes over time (44.2% vs 61.1%, p = 0.037). An increase in door-to-CT time (p = 0.001) and an increase in the rate of diagnostic error in patients admitted from the ED (p = 0.016) were also seen. The in-hospital cohort had a sustained decrease in the number of SCA following the pandemic declaration, with no difference in the rate of true positive stroke.
Conclusions:
We observed a rapid decline and slow recovery in ED SCA with a shift toward increased true positive cases following the Covid-19 pandemic. Also, delays in obtaining CT and diagnostic error was increased, however, no difference in early clinical outcomes were seen between groups.
Keywords: Covid-19, acute stroke, pandemic, stroke code, stroke mimics
Introduction
The Covid-19 pandemic is a global health crisis that has impacted medical care at all levels worldwide and the medical care for acute stroke patients is no exception. 1 -3 Though the first cases occurred on January 24, 2020 in Illinois, community transmission was not suspected until March 8. 4 On March 9, a disaster proclamation was issued by the Governor of Illinois, followed by pandemic declaration by the World Health Organization (WHO) on March 11, school closures statewide on March 17, and a state-at-home order on March 19. 5,6 At the time of the submission of this manuscript, Illinois had 382,985 diagnosed Covid-19 cases and 9,568 deaths secondary to Covid-19, with more than half in Chicago. 7
The rising number of Covid-19 cases has increased the burden on emergency services and raised the risk of viral contagion among health care personnel, patients, and families. With the need to protect health care workers and limit exposure to Covid-19 patients, health systems have rapidly implemented modified acute stroke protocols. Moreover, several investigators have reported a decrease in the number of stroke presentations to emergency departments (ED), 8 with delays in the time to presentation and a decrement in patients undergoing mechanical thrombectomy. 1,3 However, there have been few data on the effects of stroke code modifications on acute stroke process metrics and none on diagnostic error.
We sought to understand the impact of Covid-19 pandemic and public measures to mitigate the spread of the disease (e.g., stay-at-home orders) on acute stroke care process and time metrics including diagnostic error at single large comprehensive stroke center in Chicago, an area severely affected by Covid-19.
Methods
Study Design
We conducted an analysis of all stroke code activations from February 15, 2020 through April 30, 2020 at the University of Chicago Medical Center (UCMC), a comprehensive stroke center on the South Side of Chicago. All data supporting the findings of this analysis are available upon request to the corresponding author.
The patients included in the study were classified into 2 groups depending on the time of the stroke code activation as follows: 1) pre-pandemic from February 15 to March 10 and 2) Covid-19 pandemic from March 11 to April 30. March 11, 2020 was selected based on the date the WHO declared Covid-19 a pandemic. The study was reviewed and approved by The University of Chicago Institutional Review Board (IRB19-1339) and written consent was waived.
Studied Population
Consecutive patients, evaluated during the study period, were included either if activation occurred in the emergency department (ED) or the in-hospital (IH) setting. Patients with a final diagnosis of stroke who did not present as stroke code activations were excluded from the study. Institutional protocol for stroke code activation recommends single pager stroke team activation for any patient with stroke like symptoms with last known normal within 24 hours.
Data Collection
Patient charts were reviewed by 4 independent researchers (F.S.V, R.A.D, J.C.P and Z.B). We collected the following variables for each stroke code activation: baseline demographics, vascular risk factors, antithrombotic, time from symptom onset (last known well) to stroke code activation, baseline functional status according to the modified Rankin Scale (mRS), stroke severity according to the National Institutes of Health Stroke Scale (NIHSS), acute reperfusion interventions including intravenous thrombolysis (t-PA) and mechanical thrombectomy, process times, final diagnosis, and clinical outcomes (discharge mRS, discharge disposition). Final diagnosis was confirmed as stroke versus stroke mimic by the attending vascular neurologist and reviewed independently for accuracy by the authors (FSV, RAD, JCP, ZB, SP). True positive stroke was any acute stroke activation confirmed as stroke. Stroke was defined as the sudden occurrence of a focal deficit with a demonstrated lesion via neuroimaging in a definite vascular territory and included ischemic and hemorrhagic events. 9,10 Any other event not considered to be a vascular in nature was considered as a stroke mimic. Process times of interest were: 1) time from hospital arrival to head CT (door-to-CT); 2) time from hospital arrival to intravenous thrombolysis (door-to-needle); 3) time of hospital arrival to initiation of mechanical thrombectomy (door-to-puncture). For IH stroke code activations, we substituted stroke code activation time for hospital arrival to calculate activation to CT, needle, and puncture times.
We calculated the rate of diagnostic error. False positive stroke was defined as stroke code activation or neurology admission with diagnosis of stroke with an alternative non-stroke final diagnosis (stroke mimic). False negative stroke was defined as stroke code activation not initiated in the ED, but rather after admission due to the same presenting symptoms and in which a stroke was later confirmed.
Study Outcomes
We compared (1) demographics, presenting clinical findings, last known well to stroke code activation, and stroke severity, (2) time from door (or activation for IH stroke codes) to initial head computed tomography (CTH) and to acute interventions, (3) length of stay, (4) discharge clinical findings and disability using the mRS, and (5) discharge disposition between pre-pandemic and pandemic patient groups. A non-blinded analysis of the data was performed. The primary outcome was the difference in the proportion of true positive strokes among total stroke code activations in the 2 time periods. Secondary outcomes included the proportion of patients with diagnostic error (false positive + false negative) in the 2 periods.
Statistical Analysis
Baseline characteristics were reported by descriptive statistics (e.g., mean and standard deviation [SD] for continuous normally distributed data, median and interquartile range [IQR] for continuous and not-normally distributed data, and frequency tabulations for categorical variables) for each group and compared using one-way analysis of variance (ANOVA) or Kruskal-Wallis test and Fisher’s exact test (for continuous and categorical, respectively). To describe the pattern of stroke code activations during the study period, we created time series plots of stroke activations overall and by confirmed stroke versus stroke mimics. We performed an exponential smoothing (St = αyt-1 + (1 − α) St-1) using a weighted moving average with exponentially decreasing weights (damping factor 0.9) to optimize visualization of trends.
Results
During the study period, a total of 212 stroke code activations were included in the analysis. Overall, the mean age was 64.5 (SD15.2) and 54.2% were female. Eighty-three (39.2%) cases were in the pre-pandemic period, whereas 129 (60.8%) cases were during the Covid-19 pandemic. We analyzed the ED stroke code activations (n = 147, 69.3%) and IH stroke code activation (n = 65, 30.7%) cohorts separately.
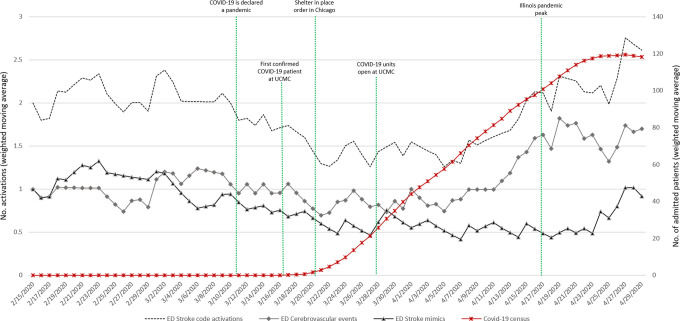
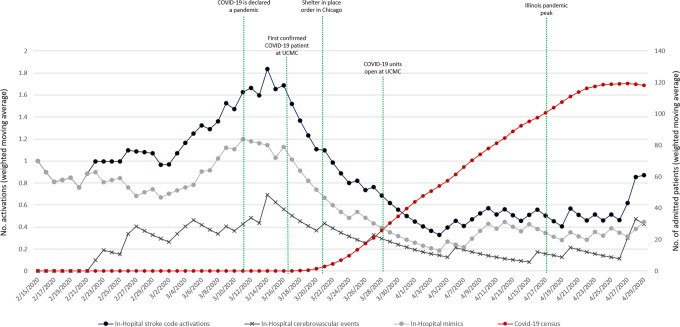
Comparing pre-pandemic to pandemic periods in the ED group, the median daily number of stroke code activations was not significantly different (pre-pandemic 2 IQR [1-3] vs. pandemic 1 IQR [1-2]); p = 0.052). However, there was a trend toward a decrease in the daily stroke codes activations during the first 2 weeks following the stay-at-home order with a gradual return to pre-pandemic levels afterward (Figure 1). In the IH group, daily stroke code activations steadily decreased during the pandemic period compared to pre-pandemic phase (pre-pandemic 1 IQR [1-2] vs. pandemic 0 IQR [0-1]; p < 0.001) (Figure 2).
Figure 1.
Emergency department stroke code activations time series, with daily cerebrovascular event and mimic counts along with hospital Covid-19 census.
Figure 2.
In-hospital stroke code activations time series, with daily cerebrovascular event and mimic counts along with hospital Covid-19 census.
ED Cohort
A total of 52 activations occurred in the pre-pandemic period and 95 activations during the pandemic in the ED. There were no significant differences in baseline characteristics, vascular risk factors, antithrombotic medications on presentation between the pre-pandemic and pandemic groups (Table 1). Overall, we found an increase in the proportion of confirmed strokes (true positives) relative to stroke mimics during the pandemic compared to the pre-pandemic period (67.4% vs. 32.5%; p = 0.039). Additionally, we observed an increase in misdiagnosis of stroke (false negatives + false positive strokes) during the Covid-19 pandemic when compared to before the pandemic (16.2% vs. 2.2%; p=0.016).
Table 1.
Baseline Characteristics of ED Stroke Code Activations.
| Emergency department stroke code activations | |||||
|---|---|---|---|---|---|
| Pre-Pandemic (n = 52) | Covid-19 Pandemic (n = 95) | P-value | |||
| Age, mean (SD) | 64.36 | 14.9 | 65.4 | 15.0 | 0.681 |
| Woman, n% | 26 | 50.0% | 54 | 56.9% | 0.426 |
| Vascular risk factors | |||||
| Hypertension, No. (%) | 39 | 75.0% | 73 | 76.8% | 0.802 |
| Hyperlipidemia, No. (%) | 11 | 21.2% | 31 | 32.6% | 0.141 |
| Diabetes mellitus, No. (%) | 13 | 25.0% | 30 | 31.6% | 0.402 |
| Smoker, No. (%) | 14 | 26.9% | 18 | 19.0% | 0.104 |
| Atrial Fibrillation, No. (%) | 5 | 9.6% | 11 | 11.6% | 0.789 |
| Active cancer, No. (%) | 1 | 1.9% | 7 | 7.4% | 0.261 |
| LVAD, No. (%) | 2 | 3.8% | 0 | 0% | 0.124 |
| Antithrombotic at presentation | 0.724 | ||||
| Aspirin, No. (%) | 11 | 21.2% | 30 | 31.6% | |
| Clopidogrel, No. (%) | 2 | 3.8% | 2 | 2.1% | |
| DAPT, No. (%) | 2 | 3.8% | 2 | 2.1% | |
| Warfarin, No. (%) | 1 | 1.9% | 2 | 2.1% | |
| NOAC, No. (%) | 2 | 3.8% | 3 | 3.2% | |
| Combination, No. (%) | 2 | 3.8% | 7 | 7.4% | |
| None, No. (%) | 32 | 61.5% | 49 | 51.6% | |
| Last known well to activation, Median (IQR) | 200 | (77-780) | 158 | (60-601) | 0.473 |
| Baseline mRS, median (IQR) | 1 | (0-2) | 0 | (0-2) | 0.723 |
| Baseline NIHSS, median (IQR) | 6 | (2-15.5) | 6 | (2-14) | 0.682 |
LVAD: left ventricular assist device; DAPT: dual antiplatelet therapy; NOAC: non-vitamin K oral anticoagulant; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale.
The frequency of t-PA administration, mechanical thrombectomies performed, and post-reperfusion complications were not different between groups. The median last known well to stroke activation time (p = 0.473) and door-to-needle time (p = 0.112) were not different between periods. However, a significant increase in the median door-to-CT times (pandemic 14.5 minutes IQR [8-23] vs. pre-pandemic 8 minutes, IQR [2-15]; p = 0.001) and door-to-puncture times (pandemic 207 minutes IQR [149-286] vs. pre-pandemic 125 minutes IQR [111-137]; p = 0.031) was observed during the pandemic compared to pre-pandemic period. No differences were observed in discharge disposition, NIHSS score at discharge, mRS at discharge, or length of stay (Table 2).
Table 2.
ED and in-Hospital Stroke Code Activation Diagnostic Evaluation and Treatments.
| Emergency department stroke code activations | In-house stroke code activations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Pandemic (n = 52) | Covid-19 Pandemic (n = 95) | P-value | Pre-Pandemic (n = 31) | Covid-19 Pandemic (n = 34) | P-value | |||||
| Final event diagnosis | 0.039 | 0.306 | ||||||||
| Vascular event, No. (%) | 26 | 50.0% | 64 | 67.4% | 9 | 29.0% | 14 | 41.2% | ||
| Mimic, No. (%) | 26 | 50.0% | 31 | 32.5% | 22 | 71.0% | 20 | 58.8% | ||
| Diagnostic imaging obtained | 0.212 | 0.009 | ||||||||
| None, No. (%) | 20 | 38.5% | 27 | 28.4% | 25 | 80.7% | 17 | 50.0% | ||
| Advanced vascular Imaging (CTA/P, MRA/P), No. (%) | 32 | 61.5% | 68 | 71.6% | 6 | 19.4% | 17 | 50.0% | ||
| Vascular Diagnosis | 0.884 | 0.498 | ||||||||
| Ischemic stroke, No. (%) | 14 | 26.9% | 34 | 35.8% | 6 | 19.4% | 11 | 32.4% | ||
| Intracerebral hemorrhage, No. (%) | 9 | 17.3% | 24 | 25.3% | 1 | 3.2% | 3 | 8.9% | ||
| TIA, No. (%) | 3 | 5.8% | 6 | 6.3% | 2 | 6.5% | 0 | 0% | ||
| t-PA administration, No. (%) | 4 | 7.7% | 7 | 7.4% | 1 | 0 | 0% | 0 | 0% | NA |
| Door to needle, median (IQR) | 36 | (36-40) | 47 | (40-82) | 0.112 | – | – | – | – | NA |
| Mechanical Thrombectomy, No. (%) | 3 | 5.8% | 7 | 7.4% | 1 | 0 | 0% | 2 | 5.9% | 0.493 |
| Door to puncture, median (IQR) | 125 | (111-137) | 207 | (149-286) | 0.031 | – | – | 123.5 | (120-127) | NA |
CTA: computed tomography angiogram; CTP: computed tomography perfusion; MRA: magnetic resonance angiography; MRP: magnetic resonance perfusion; TIA: transient ischemic attack; t-PA: tissue plasminogen activator.
In-hospital Cohort
During the pre-pandemic period, there were 31 IH stroke code activations compared to 34 during the pandemic period; the daily count of activations declined significantly (pre-pandemic 1 IQR [1-2] vs. pandemic 0 IQR [0-1]; p < 0.001). The proportions of true positive strokes and stroke mimics were not different between periods (Table 2).
There were no differences in baseline characteristics, vascular risk factors, and clinical presentation between pre-pandemic and pandemic groups (Table 3). We noted that the baseline mRS was higher pre-pandemic (3 IQR [1-4]) compared to during the pandemic (1 IQR [0-3]; p = 0.003). None of the patients included in the IH cohort received t-PA; however, 2 patients underwent mechanical thrombectomy during the pandemic compared to none pre-pandemic. Additionally, we observed a significant increase in the proportion of patients who obtained advanced vascular imaging during the pandemic period (50.0% vs. 19.5%; p = 0.009). Time to CT increased from pre-pandemic (14 minutes, IQR [6-31]) to pandemic periods (30.5 minutes, IQR [14-43]; p = 0.024). We observed no differences in NIHSS score on discharge and discharge disposition between periods.
Table 3.
Outcomes at Hospital Discharge in ED and in-Hospital Groups, Before and After Covid-19 Pandemic.
| Emergency department stroke code activations | In-hospital stroke code activations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Pandemic (n = 52) | Covid-19 Pandemic (n = 95) | P-value | Pre-Pandemic (n = 31) | Covid-19 Pandemic (n = 34) | P-value | |||||
| Discharge disposition | 0.732 | 0.581 | ||||||||
| Home, No. (%) | 29 | 55.8% | 54 | 56.9% | 12 | 38.7% | 13 | 38.2% | ||
| Acute Rehabilitation, No. (%) | 14 | 26.9% | 25 | 26.3% | 6 | 19.4% | 9 | 26.4% | ||
| Subacute rehabilitation, No. (%) | 1 | 1.9% | 5 | 5.3% | 2 | 6.5% | 0 | 0% | ||
| Long-term facility, No. (%) | 1 | 1.9% | 0 | 0% | 1 | 3.2% | 1 | 2.9% | ||
| Hospice, No. (%) | 2 | 3.8% | 3 | 3.2% | 1 | 3.2% | 2 | 5.9% | ||
| Death, No. (%) | 4 | 7.7% | 7 | 7.4% | 9 | 29.0% | 6 | 17.7% | ||
| Inpatient psychiatric unit, No. (%) | 1 | 1.9% | 0 | 0% | 0 | 0% | 1 | 2.9% | ||
| Length of stay, median (IQR) | 5 | (2-9.5) | 4 | (2-9) | 0.825 | – | – | – | – | – |
| NIHSS at discharge, median (IQR) | 2 | (0-11) | 1 | (0-6) | 0.723 | 0 | (0-4) | 2 | (0-6.5) | 0.506 |
| mRS at discharge, median (IQR) | 2 | (1-4) | 2 | (0-4) | 0.633 | 4 | (3-6) | 2.5 | (1-5) | 0.023 |
| Baseline vs. Discharge mRS difference | 1.35 | 1.97 | 1.11 | 1.81 | 0.627 | 1.21 | 1.75 | 1.20 | 1.93 | 0.846 |
| Baseline vs. Discharge NIHSS difference | -3.43 | 5.83 | -3.52 | 6.60 | 0.542 | -5 | 11.11 | -5.08 | 7.17 | 0.812 |
NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale.
Discussion
In this single center retrospective study, we observed a reduction in stroke code activations both in ED and hospitalized patients in the early period of the Covid-19 pandemic in Chicago. While this reduction persisted within the in-hospital setting, the number of stroke code activations in the ED gradually increased and returned to pre-pandemic levels by early April 2020. This is contrary to a recent study from a tertiary hospital in southern New Jersey, where they observed a sustained decrease in the number of acute stroke events which was attributed to a marked decrease in transfers, telestroke consultations, and emergency department admissions. 1,11 Notably, we also observed an increment in the proportion of true positive cases during the Covid-19 pandemic when compared to the pre-pandemic period. Moreover, the increase we observed later in pandemic with a shift toward greater true positive cases and fewer false positive cases suggests that while some patients with mimics may have stayed home, true cerebrovascular events may have actually increased in the South Side of Chicago during the pandemic. A similar observation from Asia supports this conclusion. 2 The initial reduction in the ED cases we observed was likely secondary to a reluctance to seek medical care following the issuance of stay-at-home orders by state and city officials. Another, plausible explanation would be that during Covid-19 pandemic stroke patients could have been shifted away from larger comprehensive stroke center (CSC) to smaller institutions. However, it is unclear if other CSC in the area had increased numbers as there is no data available in the matter.
Importantly, a recent study published by Uchino et.al reported a marked decrease in stroke code presentations to multiple EDs across the area of North Ohio. 12 An additional hypothesis for the variation in number of stroke code activations could be month-to-month variability. Nonetheless, prior studies published by our group from data collected in our center have shown that the numbers of mimic vs. vascular events remain constant at our institution with predominance for mimics rather than vascular events in a period of 4 years studies including 1965 Stroke code activations. Therefore, suggesting that the changes observed in this analysis are rather secondary to the effects of Covid-19 pandemic instead of month to month variability. 13,14
These data suggest that while there may have been a decrease in ED visits for cerebrovascular conditions in the first few weeks after the pandemic in Chicago, this was short-lived. This finding might correlate with a recent report in which an initial decrease was seen in advanced neuroimaging acquired at many centers throughout the United States during the early pandemic period with a subsequent increase in neuroimaging. 8
We observed increased door-to-CT time that may be attributable to the precautions to ensure appropriate personal protective equipment taken by the neurology stroke team evaluating the patients as well as potential delays to evaluate patients for Covid-19 symptoms rather than neurological symptoms, the latter particularly in the ED cohort. We also observed increased door-to-puncture time that may be attributable to the need for a negative pressure room for intubation prior to angiography along with the adequate personal protective equipment that must be worn and the time this entails. 15,16 All of this not taking in consideration additional delays that were secondary to the screening procedures instituted at our institution that included temperature checks, pre-screen for upper respiratory symptoms, among others.
We also analyzed the impact of Covid-19 on misdiagnosis of acute stroke in the ED, finding an increase in false-negative and false-positive strokes. The increase in false-negative strokes suggests that some patients who presented through the ED with stroke symptoms were initially missed, only to be detected in the hospital. We speculate that a plausible reason for an increment in the false negative rates was a higher thresholds to activate stroke codes in the ED, in part to limit exposure of the acute stroke team to Covid-19 patients, shifted diagnostic focus on Covid-19 symptoms, and/or reduced staffing and neurologic evaluations may have contributed to this increase in missed strokes in the ED. 17
We also found an increase in false-positive stroke admissions from the ED, also likely secondary to an incomplete neurological examination or an examination done by a non-neurologist as many were directly admitted to Neurology without in-person consultation to minimize exposure risk during the early stages of the pandemic. Despite the above findings, we did not observe a significant difference in clinical outcomes.
Notwithstanding the fact that there were delays in some of stroke metrics, there was no difference in early clinical outcomes when the pandemic cohort is compared to the pre-pandemic group. Recent literature showed similar results regarding early clinical outcome in stroke patients during the Covid-19 pandemic. 18 The small sample size in our series precludes any robust conclusion. The impact of Covid-19 in stroke outcomes deserves further larger studies.
Study limitations are related to a single-center retrospective design and the relatively small sample size of our cohort. Even though our pre-pandemic data availability was limited resulting in a 2:1 sample size between study periods, we reviewed historical data previously published by our group and showed that our sample was consistent, therefore, an accurate representative of the pre-pandemic data from our institution. Although disparities in sample size may influence our findings, historical records from our institution showed stroke code activations had similar features to those found in the pre-pandemic sample. 13 External validation of our results may be limited by incomplete catchment of the South Side Chicago population and by disparities in the impact of Covid-19 between states. Our institution had the advantage of learning ahead of time from other centers such as the ones in New York State. 19
In summary, we observed a decline and then gradual recovery in ED acute stroke activations with a shift toward increased true cerebrovascular diagnoses following the Covid-19 pandemic. Times to CT and groin puncture were prolonged and an increase in stroke misdiagnosis was noted in the ED during the pandemic, however early clinical outcomes were not impacted. Larger, multicenter studies are necessary to assess specific changes and effects of these metric changes observed and its impact on patient outcomes.
Footnotes
Authors’ Note: Faddi G. Saleh Velez and Ronald Alvarado-Dyer are equally contributing first authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosures: Dr. Faddi G. Saleh Velez has nothing to disclose. Dr. Ronald Alvarado-Dyer has nothing to disclose. Dr. Victor J. Del Brutto has nothing to disclose. Dr. Julián Carrión-Penagos has nothing to disclose. Dr. Zachary Bulwa has nothing to disclose. Dr. Shyam Prabhakaran has nothing to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Faddi G. Saleh Velez  https://orcid.org/0000-0002-2626-6259
https://orcid.org/0000-0002-2626-6259
Zachary Bulwa  https://orcid.org/0000-0002-3857-8188
https://orcid.org/0000-0002-3857-8188
References
- 1. Siegler JE, Heslin ME, Thau L, Smith A, Jovin TG. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center: cover title: falling stroke rates during COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8):104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teo KC, Leung WCY, Wong YK, et al. Delays in stroke onset to hospital arrival time during COVID-19. Stroke. 2020;51(7):2228–2231: STROKEAHA120030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kerleroux B, Fabacher T, Bricout N, et al. Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 outbreak: decreased activity, and increased care delays. Stroke. 2020;51(7):2012–2017: STROKEAHA120030373. [DOI] [PubMed] [Google Scholar]
- 4. Ghinai I, McPherson TD, Hunter JC, et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395(10230):1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Public Health Officials Announce First Illinois Coronavirus Disease Death. Illinois department of public health. Published 2020. Accessed October 28, 2020. http://www.dph.illinois.gov/news/public-health-officials-announce-first-illinois-coronavirus-disease-death
- 6. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. World Health Organization. Published 2020. Accessed October 28, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 7. Coronavirus (COVID-19) Global Data Tracker. Tableau Software, LLC. Published 2020. Accessed Octobery 27, 2020. https://www.tableau.com/covid-19-coronavirus-data-resources/global-tracker
- 8. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ropper AH, Samuels MA, Klein JP, Prasad S. Stroke and cerebrovascular diseases. In: Adams and Victor’s Principles of Neurology, 11e. McGraw-Hill Education; 2019. [Google Scholar]
- 10. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McNamara D. COVID-19: are acute stroke patients avoiding emergency care? 2020. Published April 8, 2020. Accessed October 28, 2020. https://www.mdedge.com/neurology/article/220491/stroke/covid-19-are-acute-stroke-patients-avoiding-emergency-care
- 12. Uchino K, Kolikonda MK, Brown D, et al. Decline in stroke presentations during COVID-19 surge. Stroke. 2020;51(8):2544–2547. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Brutto VJ, Ardelt A, Loggini A, et al. Clinical characteristics and emergent therapeutic interventions in patients evaluated through the in-hospital stroke alert protocol. J Stroke Cerebrovasc Dis. 2019;28(5):1362–1370. [DOI] [PubMed] [Google Scholar]
- 14. Bulwa Z, Del Brutto VJ, Loggini A, et al. Mechanical thrombectomy for patients with in-hospital ischemic stroke: a case-control study. J Stroke Cerebrovasc Dis. 2020;29(5):104692. [DOI] [PubMed] [Google Scholar]
- 15. Harrington RA, Elkind MSV, Benjamin IJ. Protecting medical trainees on the COVID-19 frontlines saves us all. Circulation. 2020;141(18):e775–e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020;51(6):1891–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leadership AASC. Temporary emergency guidance to US stroke centers during the coronavirus disease 2019 (COVID-19) pandemic: on behalf of the American Heart Association/American Stroke Association stroke council leadership. Stroke. 2020;51(6):1910–1912. [DOI] [PubMed] [Google Scholar]
- 18. Jasne AS, Chojecka P, Maran I, et al. Stroke code presentations, interventions, and outcomes before and during the COVID-19 pandemic. Stroke. 2020;51(9):2664–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waldman G, Mayeux R, Claassen J, et al. Preparing a neurology department for SARS-CoV-2 (COVID-19): early experiences at Columbia University Irving Medical Center and the New York Presbyterian Hospital in New York City. Neurology. 2020;94(20):886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]