Abstract
Purpose
Colleges and universities across the United States are developing and implementing data-driven prevention and containment measures against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Identifying risk factors for SARS-CoV-2 seropositivity could help to direct these efforts. This study aimed to estimate the associations between demographic factors and social behaviors and SARS-CoV-2 seropositivity and self-reported positive SARS-CoV-2 diagnostic test.
Methods
In September 2020, we randomly sampled Indiana University Bloomington undergraduate students. Participants completed a cross-sectional online survey about demographics, SARS-CoV-2 testing history, relationship status, and risk behaviors. Additionally, during a subsequent appointment, participants were tested for SARS-CoV-2 antibodies using a fingerstick procedure and SARS-CoV-2 IgM/IgG rapid assay kit. We used unadjusted modified Poisson regression models to evaluate the associations between predictors of both SARS-CoV-2 seropositivity and self-reported positive SARS-CoV-2 infection history.
Results
Overall, 1,076 students were included in the serological testing analysis, and 1,239 students were included in the SARS-CoV-2 infection history analysis. Current seroprevalence of SARS-CoV-2 was 4.6% (95% confidence interval: 3.3%, 5.8%). Prevalence of self-reported SARS-CoV-2 infection history was 10.3% (95% confidence interval: 8.6%, 12.0%). Greek membership, having multiple romantic partners, knowing someone in one's immediate environment with SARS-CoV-2 infection, drinking alcohol more than 1 day a week, and hanging out with more than five people when drinking alcohol increased both the likelihood of seropositivity and SARS-CoV-2 infection history.
Conclusion
Our findings have implications for American colleges and universities and could be used to inform SARS-CoV-2 prevention and control strategies on such campuses.
Keywords: College students, COVID-19, SARS-CoV-2, Antibody test, Risk factors, Alcohol drinking in college, College fraternities and sororities
Implications and Contribution.
Among a random sample of college students, this study found that Greek membership, relationship status, knowing someone with SARS-CoV-2 infection, alcohol drinking, and number of people hanging out with when drinking alcohol are predictors for SARS-CoV-2 seropositivity. These findings are helpful when developing future strategies for combating the pandemic at colleges.
The ongoing coronavirus disease 2019 (COVID-19) pandemic has caused major challenges for both colleges and students including school closures, shifts to remote and hybrid educational formats, and negative financial impacts on institutions and students [1]. More importantly, the disease burden on college campuses has been substantial with at least 700,000 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases and at least 100 deaths reported at more than 1,900 colleges as of May 26, 2021 [2]. Furthermore, because of the collegiate semester schedules, there are mounting concerns that infected asymptomatic students might spread the virus to their family members when traveling back home [3]. Identifying predictors of SARS-CoV-2 test positivity can help to plan and coordinate mitigation testing programs, containment efforts, and vaccination strategies.
Previous studies among the general U.S. adult population have established that race, gender, and age are associated with SARS-CoV-2 positivity [4,5]. However, these characteristics have not been thoroughly studied among college students. Moreover, there are demographic factors specific to college student populations, such as participation in Greek life [6], collegiate dating, and year in school, which might be significant predictors of SARS-CoV-2 positivity in this particular population.
Finally, because of the drinking culture, social context of drinking among college students [7], and alcohol disinhibition effects [8], alcohol use patterns may be another potential predictor of SARS-CoV-2 positivity in this population. Young adults with a hazardous drinking problem have reported complying less with the stay-at-home order on days that they were drinking compared with days that they did not drink [9]. The effects of alcohol are compounded in the social setting of college drinking: when the number of friends present in an alcohol drinking event increases, the number of consumed alcohol drinks increases (at an individual level) [10]. Crowded social events also, by definition, limit the ability to maintain physical distance. Because alcohol consumption is prevalent among college students [11], assessing the relationship between this behavior and SARS-CoV-2 positivity is imperative to better understand the dynamics of SARS-CoV-2 transmission among college students. Therefore, in the present study, we examined the relationship between drinking behaviors and SARS-CoV-2 positivity.
Objective
The primary aim of the present study was to estimate the associations between different demographic characteristics and social behaviors and SARS-CoV-2 seropositivity and self-reported positive test history outcomes among college students. We also estimated the seroprevalence of SARS-CoV-2 antibody (in September 2020) and the prevalence of self-reported SARS-CoV-2 positive test history among Indiana University (IU) Bloomington undergraduate students.
Methods
We used the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [12] to report our findings of the baseline characteristics of IU COVID-19 Serosurvey Study participants and predictors of SARS-CoV-2 positivity.
Study design
The parent study design was a randomized controlled trial (RCT) to test whether receiving SARS-CoV-2 antibody test results alters students' protective behaviors against infection [13]. The RCT data collection was longitudinal and lasted for two months. We collected data at baseline and every two weeks after baseline. In the present study, we used data from the baseline survey and baseline antibody test results in a cross-sectional study design. Participants were compensated with a maximum of $30 for completing all the steps in the RCT. The IU Human Subjects and Institutional Review boards provided ethical approval for this study protocol (Protocol #2008293852).
Setting
We conducted the study at IU Bloomington, a public university with more than 33 thousand undergraduate students in Bloomington, Indiana. Several requirements, restrictions, and university policies were in place at the time of this study (i.e., September 2020), including mitigation testing, physical distancing, mask wearing, class spacing, contact tracing, and quarantine and self-isolation mandates.
Participants and study procedures
We selected a random sample of IU Bloomington undergraduate students (n = 7,499) from the sampling frame of all IU Bloomington undergraduate students (n~33,084). Selected students were eligible to participate in this study if they were (1) of age 18 years or older, (2) a current IU Bloomington undergraduate student, and (3) currently residing in Monroe County, Indiana.
Study invitation emails were sent to a random sample of 7,499 IU Bloomington undergraduate students. The emails included information about the study and a student-specific link to an online survey. The online survey consisted of an eligibility criteria instrument, an online consent form, a laboratory test appointment scheduler for the SARS-CoV-2 antibody test, and a baseline survey. The baseline survey measured participant demographics, SARS-CoV-2 testing history, and risk behaviors. Eligible students who consented to participate were able to schedule a laboratory test appointment and complete the online baseline survey. Study invitation and reminder emails were sent on September 8–20, 2020. Students scheduled their baseline appointments and responded to the baseline survey between September 8 and September 30, 2020.
The SARS-CoV-2 antibody laboratory tests were conducted in-person outdoors on the IU Bloomington campus, between September 14 and 30. During laboratory tests, recommended protocols to reduce the likelihood of SARS-CoV-2 transmission at the study site were used, including physical distancing, mask wearing, glove wearing, and disinfection of laboratory equipment. Students were advised not to attend their appointment if they were experiencing COVID-19 symptoms, had tested positive for SARS-CoV-2 in the last two weeks before their appointment, or had been directed to isolate or quarantine. Participants checked in with their unique study ID, which they had created in the online survey. Using a fingerstick procedure, trained nursing staff took a small blood sample from each participant and placed the blood sample on the antibody testing kit. Trained field staff read the antibody test results from the test kit, took a high-quality picture of the kit and uploaded it to a secured cloud drive, and entered the test results into the REDCap (Research Electronic Data Capture) data management system. To increase the accuracy of the antibody test readings, a trained research assistant independently assessed the results using the pictures that field staff had taken from the test kits. Discordant results were adjudicated by five research team members.
Variables and data sources/measurement
Outcomes
Objective outcome: The main outcome was the participants' SARS-CoV-2 antibody laboratory test result. The virus can cause an immune response in both symptomatic and asymptomatic individuals [14,15]. The antibody test kits we used were SARS-CoV-2 IgM/IgG rapid assay kit (Colloidal Gold method). These kits can detect IgM and IgG antibodies against SARS-CoV-2 in the blood and provide accurate and rapid results at the testing site. If the antibody test result was negative for both IgM and IgG antibodies, the antibody test result was coded as negative. Otherwise, if the test kit results for any of the two types of antibodies were positive, the outcome was coded positive.
Self-reported outcome: The second outcome of interest was self-reported SARS-CoV-2 testing history. This was measured by the following questions in the baseline survey.
-
1)
“Have you ever been tested for SARS-CoV-2 (COVID-19) before? Note: By this, we mean testing for active infections, usually done with a nasal swab or saliva test” (Responses: “Yes,” “No,” “Don't Know”).
-
2)
[Displayed if one equals Yes] “Have you ever tested positive for a SARS-CoV-2 (COVID-19) infection?" (Responses: “Yes,” “No,” “Don't Know”)
Participants who responded “Yes” to both questions were categorized as ever tested positive for SARS-CoV-2.
Demographic and behavioral predictors
We collected data on the following baseline characteristics and potential risk factors for a positive SARS-CoV-2 test result: age (≥22 years vs. <22 years), sex at birth (female vs. male), race (Asian, black, multiracial, other, White), Hispanic or Latinx ethnicity (yes vs. no), year in school (first through fifth), residence (on-campus vs. off-campus), Greek membership (yes vs. no), relationship status (multiple partners, single partner, no partner), and know others who were infected (yes vs. no). Moreover, among those who self-reported a positive SARS-CoV-2 testing history, we collected data about their symptoms, symptomatic (yes vs. no). Age was collected as a continuous variable but was dichotomized for analysis. Undergraduate students are mainly 18–22 years of age. We recoded this continuous variable as categorical with 22 years of age as the cutoff point.
Moreover, we collected alcohol use data using the following two variables: (1) Number of days per week drinking alcohol: This variable was collected continuously and could range from 0 to 7 days a week. In our inferential analyses, we used the median of one as the cutoff point for this variable (>1 day in a week vs. ≤1 day in a week). (2) Number of people hanging out with while drinking: This continuous variable measured the number of people students hung out with while drinking. It was only measured among those who reported drinking alcohol (i.e., drinking more than 0 days a week) and could range from 0 to 1,000. In our inferential analyses, we used the median of five as the cutoff point for this variable (>5 people vs. ≤5 people). All the aforementioned predictors were self-reported in the online baseline survey. Appendix A includes the survey questions used. Figures in Appendix B show the distribution for continuous variables.
Bias
We took several measures to reduce different sources of bias, such as nonresponse and selection biases. We used a random sample to decrease selection bias. Besides the initial study invitation email, we sent two reminders to participants to increase the response rate. We also identified different types of partial responses and sent reminder emails to participants who had only completed part of the baseline study. Moreover, to maximize the number of participants showing up for their SARS-CoV-2 antibody testing appointment, we sent appointment reminders to participants 6–12 hours before their appointments.
Study size
The sample size calculation for the RCT was calculated before conducting that study. However, the power analysis was specific to the RCT aims, and therefore, no sample size calculation was conducted for the current cross-sectional analysis of the baseline data.
Statistical methods
We used the normal approximation interval (Wald interval) to calculate the point prevalence/seroprevalence estimates and 95% confidence intervals (CIs) for positive SARS-CoV-2 antibody test and self-reported history of positive SARS-CoV-2 test. We used Poisson regression with a robust error variance to calculate the unadjusted/crude prevalence ratios (i.e., the measure of choice in cross-sectional studies [16,17]) between different baseline variables and the self-reported SARS-CoV-2 testing history and SARS-CoV-2 antibody laboratory test outcome variables. We report the unadjusted prevalence ratios and 95% CIs for these associations. Values of “Don't Know” were recoded as missing in the analysis. We used complete case analysis. Finally, in a sensitivity analysis, to remove any biases that age outliers were potentially introducing to our findings, we restricted our sample to students younger than 30 years of age and reran the models.
Results
Participants
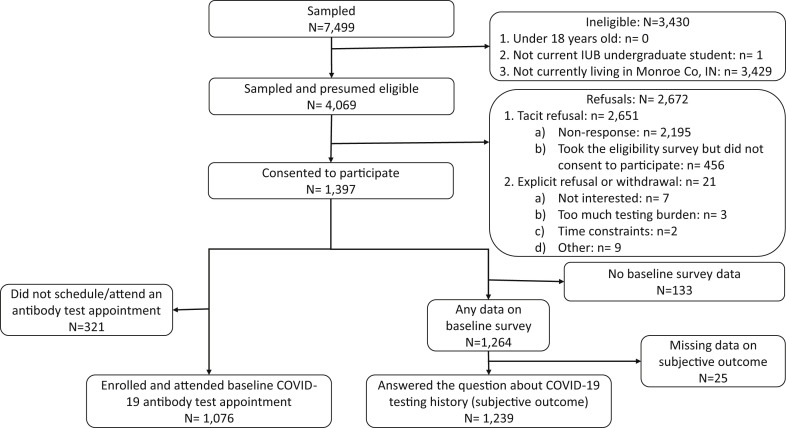
We sampled 7,499 IU Bloomington undergraduate students; of them, 4,069 students were likely eligible based on county of residence, 1,397 confirmed eligibility and consented to participate in the study, and 1,076 attended a laboratory test visit and provided SARS-CoV-2 antibody test data. Overall, 21 students explicitly refused to participate in the study, while 2,651 tacitly refused via nonresponse or by not signing the consent form. Moreover, 321 students did not schedule or attend a baseline antibody test appointment.
For the self-reported SARS-CoV-2 testing history outcome, of the 1,397 students who consented to participate in the study, 133 did not answer any of the questions in the baseline survey and 25 students had missing values for the self-reported outcome. Overall, 1,239 answered the survey questions about SARS-CoV-2 testing history (Figure 1 ). We calculated the response rate to be 28.7% for the antibody testing outcome and 31.7% for the self-reported SARS-CoV-2 testing history outcome.
Figure 1.
Flow diagram of the study sample.
Descriptive data
For the SARS-CoV-2 antibody laboratory test outcome, participants were on average 20 years of age (median, standard deviation: 20, 2.4), female (64%), white (79%), non-Hispanic (93%), senior student (28%), off-campus residents (69%), and non-Greek affiliated (76%). Participants reported different relationship statuses; most students (40%) were single and not dating, 33% were in a relationship but not living together, 13% were single and dating/hooking up with one specific person, and 9% were single and dating/hooking up with multiple people. Moreover, 51% of participants knew others with SARS-CoV-2 positive history, 46% reported drinking alcohol >1 day a week, and 34% reported hanging out with >5 people while drinking (Table 1 ). Similar trends in demographic and behavioral variables were found for the self-reported SARS-CoV-2 testing history outcome.
Table 1.
Characteristics of the study participants, Indiana University Bloomington undergraduate students, September 2020
| Antibody test |
Self-reported positive SARS-CoV-2 test |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall 1076 |
Negative 1027 |
Positive 49 |
Overall 1239 |
No 1111 |
Yes 128 |
|||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Age | ||||||||||||
| 18 years | 208 | (20.6) | 198 | (20.5) | 10 | (21.7) | 247 | (21.2) | 217 | (20.9) | 30 | (24.0) |
| 19 years | 224 | (22.2) | 215 | (22.3) | 9 | (19.6) | 253 | (21.7) | 223 | (21.5) | 30 | (24.0) |
| 20 years | 228 | (22.6) | 219 | (22.7) | 9 | (19.6) | 254 | (21.8) | 231 | (22.2) | 23 | (18.4) |
| 21 years | 255 | (25.3) | 241 | (25.0) | 14 | (30.4) | 297 | (25.5) | 263 | (25.3) | 34 | (27.2) |
| 22 + years | 95 | (9.4) | 91 | (9.4) | 4 | (8.7) | 113 | (9.7) | 105 | (10.1) | 8 | (6.4) |
| Missing | 66 | 63 | 3 | 75 | 72 | 3 | ||||||
| Sex at birth | ||||||||||||
| Female | 689 | (64.3) | 655 | (64.0) | 34 | (70.8) | 786 | (63.5) | 709 | (63.8) | 77 | (60.6) |
| Male | 382 | (35.7) | 368 | (36.0) | 14 | (29.2) | 452 | (36.5) | 402 | (36.2) | 50 | (39.4) |
| Missing | 5 | 4 | 1 | 1 | 0 | 1 | ||||||
| Race | ||||||||||||
| Asian | 80 | (7.5) | 77 | (7.5) | 3 | (6.1) | 95 | (7.7) | 90 | (8.1) | 5 | (3.9) |
| Black | 13 | (1.2) | 13 | (1.3) | 0 | (.0) | 24 | (1.9) | 24 | (2.2) | 0 | (.0) |
| Multiracial | 85 | (7.9) | 80 | (7.8) | 5 | (10.2) | 101 | (8.2) | 84 | (7.6) | 17 | (13.3) |
| Other | 46 | (4.3) | 43 | (4.2) | 3 | (6.1) | 59 | (4.8) | 58 | (5.2) | 1 | (.8) |
| White | 847 | (79.1) | 809 | (79.2) | 38 | (77.6) | 959 | (77.5) | 854 | (76.9) | 105 | (82.0) |
| Missing | 5 | 5 | 0 | 1 | 1 | 0 | ||||||
| Hispanic or Latinx ethnicity | ||||||||||||
| No | 998 | (92.8) | 955 | (93.0) | 43 | (87.8) | 1146 | (92.5) | 1028 | (92.5) | 118 | (92.2) |
| Yes | 78 | (7.3) | 72 | (7.0) | 6 | (12.2) | 93 | (7.5) | 83 | (7.5) | 10 | (7.8) |
| Year in school | ||||||||||||
| 1st | 236 | (22.1) | 224 | (21.9) | 12 | (24.5) | 280 | (22.7) | 249 | (22.5) | 31 | (24.4) |
| 2nd | 246 | (23.0) | 235 | (23.0) | 11 | (22.4) | 277 | (22.4) | 245 | (22.1) | 32 | (25.2) |
| 3rd | 264 | (24.7) | 255 | (25.0) | 9 | (18.4) | 302 | (24.4) | 276 | (24.9) | 26 | (20.5) |
| 4th | 297 | (27.8) | 281 | (27.5) | 16 | (32.7) | 343 | (27.8) | 306 | (27.6) | 37 | (29.1) |
| 5th | 27 | (2.5) | 26 | (2.5) | 1 | (2.0) | 34 | (2.8) | 33 | (3.0) | 1 | (.8) |
| Missing | 6 | 6 | 0 | 0 | 3 | 2 | 1 | |||||
| Residence | ||||||||||||
| Off campus | 738 | (68.9) | 705 | (69.0) | 33 | (67.3) | 844 | (68.2) | 764 | (68.8) | 80 | (62.5) |
| On campus | 333 | (31.1) | 317 | (31.0) | 16 | (32.7) | 394 | (31.8) | 346 | (31.2) | 48 | (37.5) |
| Missing | 5 | 5 | 0 | 1 | 1 | 0 | ||||||
| Greek membership | ||||||||||||
| No | 812 | (75.9) | 788 | (77.2) | 24 | (49.0) | 943 | (76.3) | 870 | (78.5) | 73 | (57.0) |
| Yes | 258 | (24.1) | 233 | (22.8) | 25 | (51.0) | 293 | (23.7) | 238 | (21.5) | 55 | (43.0) |
| Missing | 6 | 6 | 0 | 3 | 3 | 0 | ||||||
| Relationship status | ||||||||||||
| Single and not dating/hooking up with anyone | 432 | (40.3) | 415 | (40.6) | 17 | (34.7) | 499 | (40.3) | 448 | (40.4) | 51 | (39.8) |
| Single and dating/hooking up with multiple people | 101 | (9.4) | 91 | (8.9) | 10 | (20.4) | 121 | (9.8) | 94 | (8.5) | 27 | (21.1) |
| Single and dating/hooking up with one specific person | 138 | (12.9) | 133 | (13.0) | 5 | (10.2) | 165 | (13.3) | 151 | (13.6) | 14 | (10.9) |
| In a relationship but not living together | 358 | (33.4) | 342 | (33.4) | 16 | (32.7) | 404 | (32.6) | 368 | (33.2) | 36 | (28.1) |
| Living together but not married | 40 | (3.7) | 40 | (3.9) | 0 | (.0) | 46 | (3.7) | 46 | (4.1) | 0 | (.0) |
| Married and living together | 3 | (.3) | 2 | (.2) | 1 | (2.0) | 3 | (.2) | 3 | (.3) | 0 | (.0) |
| Missing | 4 | 4 | 0 | 1 | 1 | 0 | ||||||
| Self-report positive test | ||||||||||||
| No | 961 | (90.8) | 944 | (93.2) | 17 | (37.0) | -- | -- | -- | -- | -- | -- |
| Yes | 98 | (9.3) | 69 | (6.8) | 29 | (63.0) | -- | -- | -- | -- | -- | -- |
| Missing | 17 | 14 | 3 | -- | -- | -- | -- | -- | -- | |||
| Symptomatica | ||||||||||||
| No | 23 | (24.0) | 18 | (26.9) | 5 | (17.2) | 31 | (24.6) | 0 | (.0) | 31 | (24.6) |
| Yes | 73 | (76.0) | 49 | (73.1) | 24 | (82.8) | 95 | (75.4) | 0 | (.0) | 95 | (75.4) |
| Missing | 980 | 960 | 20 | 1113 | 1111 | 2 | ||||||
| Know others who were infected | ||||||||||||
| No | 510 | (49.4) | 501 | (50.9) | 9 | (18.8) | 581 | (48.6) | 551 | (51.5) | 30 | (23.8) |
| Yes | 523 | (50.6) | 484 | (49.1) | 39 | (81.3) | 614 | (51.4) | 518 | (48.5) | 96 | (76.2) |
| Missing | 43 | 42 | 1 | 44 | 42 | 2 | ||||||
| Number of days in a week drinking alcohol | ||||||||||||
| 1 day or less | 577 | (54.0) | 558 | (54.7) | 19 | (39.6) | 652 | (53.3) | 600 | (54.6) | 52 | (41.6) |
| More than 1 day | 491 | (46.0) | 462 | (45.3) | 29 | (60.4) | 572 | (46.7) | 499 | (45.4) | 73 | (58.4) |
| Missing | 8 | 7 | 1 | 15 | 12 | 3 | ||||||
| Number of people hanging out with while drinkingb | ||||||||||||
| 5 people or less | 476 | (66.5) | 457 | (67.6) | 19 | (47.5) | 542 | (65.5) | 496 | (67.7) | 46 | (48.9) |
| More than 5 people | 240 | (33.5) | 219 | (32.4) | 21 | (52.5) | 285 | (34.5) | 237 | (32.3) | 48 | (51.1) |
| Missing | 360 | 351 | 9 | 412 | 378 | 34 | ||||||
SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Among participants who self-reported a positive test.
Among participants who self-reported alcohol drinking.
Outcome data
Overall, 49 students (of 1076) tested positive for SARS-CoV-2 antibodies (prevalence [95% CI]: 4.6% [3.3%, 5.8%]) and 128 students (of 1239) self-reported ever having tested positive for SARS-CoV-2 infection (prevalence [95% CI]: 10.3% [8.6%, 12.0%]) (Table 1).
Main results
Objective outcome: SARS-CoV-2 antibody laboratory test
Students affiliated with Greek fraternities or sororities were 3.28 (95% CI: 1.91, 5.64) times more likely to have a positive SARS-CoV-2 antibody test result compared with non-Greek students. Those with multiple partners were 2.52 (95% CI: 1.19, 5.33) times more likely to have a positive antibody test compared with students with no partner. However, those with a single partner relationship status had a similar distribution of positive antibody tests compared with those with no partner (PR [95% CI]: 1.04 [0.56, 1.93]). Students who knew others in their immediate environment with SARS-CoV-2 positive history were 4.23 (95% CI: 2.07, 8.63) times more likely to test positive for SARS-CoV-2 antibodies compared with those who did not know anyone with SARS-CoV-2 infection. Students who self-reported drinking alcohol more than one day a week were 1.79 (95% CI: 1.02, 3.16) times more likely to have a positive antibody test compared with those who self-reported drinking alcohol equal to or less than one day a week. Similarly, students who socialized with more than five people when drinking alcohol were 2.19 (95% CI: 1.20, 4.00) times more likely to have a positive antibody test result compared with those who socialized with five or less people while drinking alcohol (Table 2 ). Because there were few to zero observations in many of the cells of race and outcome variables cross-tabulations, we could not fit the unadjusted models with race as an independent variable (models with race variable did not converge).
Table 2.
Bivariate prevalence ratios for the associations between risk factors and positive SARS-CoV-2 antibody test and self-reported history of positive SARS-CoV-2 test
| Predictor | Outcomes |
|
|---|---|---|
| Positive SARS-CoV-2 antibody test (objective outcome) |
Self-reported history of positive SARS-CoV-2 test (secondary outcome) |
|
| PR (95% CI) | PR (95% CI) | |
| Age | n = 1010 | n = 1164 |
| ≥22 years | .92 (.34, 2.50) | .64 (.32, 1.27) |
| <22 years | Ref. | Ref. |
| Sex at birth | n = 1071 | n = 1238 |
| Female | 1.35 (.73, 2.48) | .89 (.63, 1.24) |
| Male | Ref. | Ref. |
| Hispanic or Latinx ethnicity | n = 1076 | n = 1239 |
| Yes | 1.79 (.78, 4.06) | 1.04 (.57, 1.92) |
| No | Ref. | Ref. |
| Year in school | n = 1070 | n = 1236 |
| 1st | .97 (.47, 1.99) | 1.10 (.70, 1.72) |
| 2nd | .85 (.41, 1.79) | 1.15 (.74, 1.79) |
| 3rd | .65 (.29, 1.43) | .85 (.53, 1.37) |
| 4th or 5th | Ref. | Ref. |
| Residence | n = 1071 | n = 1238 |
| On -campus | 1.07 (.60, 1.92) | 1.29 (.92, 1.80) |
| Off-campus | Ref. | Ref. |
| Greek membership | n = 1070 | n = 1236 |
| Yes | 3.28 (1.91, 5.64) | 2.42 (1.75, 3.35) |
| No | Ref. | Ref. |
| Relationship status | n = 1072 | n = 1238 |
| Multiple partners | 2.52 (1.19, 5.33) | 2.18 (1.43, 3.33) |
| Single partner | 1.04 (.56, 1.93) | .79 (.55, 1.15) |
| No partner | Ref. | Ref. |
| Self-reported history of positive SARS-CoV-2 test | n = 1059 | |
| Yes | 16.73 (9.54, 29.33) | -- |
| No | Ref. | -- |
| Symptomatica | n = 96 | |
| Yes | 1.51 (.65, 3.51) | -- |
| No | Ref. | -- |
| Know others who were infected | n = 1033 | n = 1195 |
| Yes | 4.23 (2.07, 8.63) | 3.03 (2.04, 4.49) |
| No | Ref. | Ref. |
| Number of days per week drinking alcohol | n = 1068 | n = 1224 |
| More than 1 day | 1.79 (1.02, 3.16) | 1.60 (1.14, 2.24) |
| 1 day or less | Ref. | Ref. |
| Number of people hanging out with while drinkingb | n = 716 | n = 827 |
| More than 5 people | 2.19 (1.20, 4.00) | 1.98 (1.36, 2.90) |
| 5 people or less | Ref. | Ref. |
Boldface indicates p < .05.
CI = confidence interval; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Among participants who self-reported positive SARS-CoV-2 test.
Among participants who self-reported alcohol drinking.
Self-reported SARS-CoV-2 testing history (secondary outcome)
Similar results were observed for the associations between the aforementioned factors and the self-reported SARS-CoV-2 testing history outcome (Table 2). However, the point estimates for this outcome were calculated more precisely with tighter confidence intervals, likely because of the larger sample size. For all associations, the magnitude of the prevalence ratios attenuated, yet they remained significantly and substantially greater than null. The largest attenuation in the magnitude occurred for knowing others who were infected variable, from 4.23 to 3.03 (95% CI: 2.04, 4.49).
Other analyses: the relationship between self-reported and objective outcomes
We also evaluated the association between self-reported SARS-CoV-2 testing history and SARS-CoV-2 antibody laboratory test outcomes (Table 2). Of the 46 students who tested positive for SARS-CoV-2 antibodies and had complete self-reported testing data, 29 self-reported they had previously tested positive for an active SARS-CoV-2 infection and 17 self-reported they had never tested positive for an active SARS-CoV-2 infection. The magnitude of the association was large (PR [95% CI]: 16.73 [9.54, 29.33]). Moreover, similar results were found in our sensitivity analysis of restricting the sample size to students younger than 30 years of age (Appendix C). Finally, we calculated the number of days between SARS-CoV-2 self-reported positive test date and antibody positive test date; both dates were available for n = 29 participants (median: 28 days, min: 16 days, max: 83 days).
Discussion
Key results
In September 2020, near the beginning of the fall semester, the seroprevalence of SARS-CoV-2 in our random study sample of IU Bloomington undergraduate students was 4.6%, while the prevalence of students who self-reported SARS-CoV-2 infection history was 10.3%. We found that students who had Greek membership, had multiple romantic partners, knew others in their immediate environment with SARS-CoV-2 infection, drank alcohol more than one day a week, and hanged out with more than five people when drinking were more likely to be tested positive for SARS-CoV-2 antibody test and self-report positive SARS-CoV-2 test history.
Interpretation
The SARS-CoV-2 seroprevalence among IU Bloomington undergraduate students was lower than the nationwide seroprevalence estimate in July 2020 (9.3%) [18] and higher than the Indiana statewide estimate in April 2020 (1.1%) [19]. However, our findings are comparable with that of other large universities in the United States [2,20]. Selection bias might have influenced our seroprevalence estimate because we asked students not to attend their laboratory test appointment if they were tested positive for SARS-CoV-2 in the last two weeks, had been directed to isolate or quarantine, or were experiencing COVID-19 symptoms. Some of the participants were able to reschedule their antibody test for our later testing days. This selection bias could have altered our seroprevalence estimate in either direction. However, because people with COVID-19 symptoms are more likely to have SARS-CoV-2 infection, the bias likely caused an underestimation of the true seroprevalence. Selection bias did not affect our estimate for the prevalence of students who self-reported ever having a SARS-CoV-2 positive test because this information was collected on the baseline online survey.
We also found that the prevalence of a self-reported SARS-CoV-2 positive test was higher than the seroprevalence collected via the laboratory test visit. At least some of this difference could be explained by the time lag between SARS-CoV-2 infection and antibody development and the fact that some infected individuals might never develop antibodies against the virus [21]. However, it is less likely that the difference is because of immune memory loss in previously infected students. A recent study (yet to be peer-reviewed) found that antibodies might last for years in recovered individuals [22,23]. This difference could also be owing to the selection bias explained in the previous paragraph. Finally, antibody test performance is not always perfect [24], and some of the observed discrepancies might be owing to the measurement error inherent to antibody testing. We have collected data on self-reported SARS-CoV-2 positivity date and other relevant variables; our team plans future analyses to further evaluate the reasons for the observed difference in the outcomes' prevalence estimates.
Living in one of IU's fraternities and sororities was a strong risk factor for seropositivity. Similarly, on other campuses, clusters of COVID-19 cases have been linked to Greek houses [25]. Congregate living settings and the unofficial activities and gatherings, such as rush events, could possibly explain this strong association [25]. We further found that students who were dating/hooking up with multiple people were more likely to self-report a positive SARS-CoV-2 test or have a positive SARS-CoV-2 antibody test result. To our knowledge, this is the first study that quantitatively evaluated this association. SARS-CoV-2 is primarily transmitted through direct contact with infected individuals or contaminated surfaces (i.e., fomite transmission) and/or exposure to large and small droplets that contain the virus [26], all of which are possible when students are dating/hooking up with multiple partners. Likewise, students who knew others with SARS-CoV-2 infection in their immediate environment were more likely to self-report a positive SARS-CoV-2 test or have a positive SARS-CoV-2 antibody test result. These students could also have been exposed to SARS-CoV-2 because of being in prolonged contact with the infected individuals.
Drinking alcohol more than once a week and drinking in groups of larger than five increased the likelihood of SARS-CoV-2 seropositivity. Young adults might adhere less strictly to COVID-19 prevention measures when drinking alcohol [9], probably because of cognitive distortion that follows drinking [27]. In a social drinking event, students are likely to drink more when more friends are present [10] because of peer pressure, which can exacerbate the cognitive distortion and correspondingly cause further noncompliance with COVID-19 prevention measures. More importantly, presence of more friends in a drinking event brings in more possible sources of SARS-CoV-2 infections. Holding social events via online video-conferencing technologies, such as Zoom, or in settings where social distancing is possible, avoiding excessive drinking, and drinking only with people who live in one's household could help to reduce transmission of SARS-CoV-2 among college students. Moreover, we suggest future studies with robust study designs and detailed alcohol use data collection tool kits further explore the associations between alcohol use and SARS-CoV-2 infection among college students.
Limitations and generalizability
In this study, because we used cross-sectional baseline data, we cannot assess temporal ordering between different study variables and outcomes. Our response rates (28.7% for the objective outcome and 31.7% for the self-reported outcome) may seem smaller than optimum levels; however, they are considered greater than average and comparable with other studies among college students [[28], [29], [30]]. Although confounding is usually a limitation in observational studies, adjusting for confounding was not necessary in the present study because our research questions were descriptive/predictive and they were not about causal inference [31]. Finally, all data, except SARS-CoV-2 antibody laboratory test results, were collected through self-reported surveys. Different sources of bias, for example, measurement and recall biases, could affect the quality of self-reported data, including the predictors and the self-reported outcome. However, we found a very strong association between a positive SARS-CoV-2 antibody laboratory test result and a positive self-reported SARS-CoV-2 testing history, suggesting measurement bias may not be a significant concern for the self-reported data. Finally, we did not provide a definition for hooking up in the survey and it might have been interpreted in different ways by different participants.
Despite the limitations, our study provides insight into the dynamics of SARS-CoV-2 seropositivity among college students. Although educational administrators and policy makers cannot necessarily enforce change in some of the identified predictors (e.g., dating or alcohol drinking behaviors), they can use our findings when developing future strategies for combating the pandemic at college campuses. Particularly, as we used random sampling methods in this study to increase the external validity of our results, our findings may be applicable to other large universities in the United States.
Funding Sources
This study was supported by private contributions to the Indiana University Foundation. The United Arab Emirates provided the testing kits. The funding sources did not have any role in study design; the collection, analysis, and interpretation of data; or the writing of the report and the decision to submit the manuscript for publication.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Parent study (RCT) registry site and number: ClinicalTrials.gov, NCT04620798.
Ethics approval: The Indiana University Office of Research Compliance approved the study protocol (protocol #2008293852).
Data statement: Data are available upon reasonable request.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jadohealth.2021.05.003.
Supplementary data
References
- 1.Smalley A. Higher education responses to coronavirus (COVID-19) 2020. https://www.ncsl.org/research/education/higher-education-responses-to-coronavirus-covid-19.aspx Available at:
- 2.The New York Times Tracking coronavirus cases at U.S. Colleges and universities. The New York Times. 2021 online. [Google Scholar]
- 3.Parker-Pope T., Halpert J. How can my college student come home safely for thanksgiving? The New York Times. 2020 [Google Scholar]
- 4.Kabarriti R., Brodin N.P., Maron M.I. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw open. 2020;3:e2019795. doi: 10.1001/jamanetworkopen.2020.19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price-Haywood E.G., Burton J., Fort D. Hospitalization and mortality among black patients and white patients with Covid-19. New Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmon A., Robles F., Blinder A., Fuller T. ‘Frats Are Being Frats’: Greek life is stoking the virus on some campuses. The New York Times. 2020 [Google Scholar]
- 7.Wechsler H., Dowdall G.W., Davenport A. Correlates of college student binge drinking. Am J Public Health. 1995;85:921–926. doi: 10.2105/ajph.85.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field M., Wiers R.W., Christiansen P. Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcohol Clin Exp Res. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suffoletto B., Ram N., Chung T. In-person contacts and their relationship with alcohol consumption among young adults with hazardous drinking during a pandemic. J Adolesc Health. 2020;67:671–676. doi: 10.1016/j.jadohealth.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thrul J., Kuntsche E. The impact of friends on young adults’ drinking over the course of the evening—an event-level analysis. Addiction. 2015;110:619–626. doi: 10.1111/add.12862. [DOI] [PubMed] [Google Scholar]
- 11.O'Malley P.M., Johnston L.D. Epidemiology of alcohol and other drug use among American college students. J Stud alcohol Suppl. 2002;63:23–40. doi: 10.15288/jsas.2002.s14.23. [DOI] [PubMed] [Google Scholar]
- 12.Von Elm E., Altman D.G., Egger M. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg M. ClinicalTrials.gov; Bethesda, Maryland: 2020. Longitudinal COVID-19 antibody testing in Indiana university undergraduate students.https://ClinicalTrials.gov/show/NCT04620798 [Google Scholar]
- 14.Long Q.-X., Liu B.-Z., Deng H.-J. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:1–4. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 15.Hung I.F.-N., Cheng V.C.C., Li X. SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: A case series. The Lancet Infect Dis. 2020;20:1051–1060. doi: 10.1016/S1473-3099(20)30364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barros A.J., Hirakata V.N. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 18.Anand S., Montez-Rath M., Han J. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: A cross-sectional study. The Lancet. 2020;396:1335–1344. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menachemi N., Yiannoutsos C.T., Dixon B.E. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample—Indiana, April 25–29, 2020. Morbidity Mortality Weekly Rep. 2020;69:960. doi: 10.15585/mmwr.mm6929e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilley K., Ayvazyan V., Martinez L. A cross-sectional study examining the seroprevalence of severe acute respiratory syndrome coronavirus 2 antibodies in a university student population. J Adolesc Health. 2020;67:763–768. doi: 10.1016/j.jadohealth.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Test for Past infection. 2020. https://www.cdc.gov/coronavirus/2019-ncov/testing/serology-overview.html Available at:
- 22.Dan J.M., Mateus J., Kato Y. Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. bioRxiv. 2020 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandavilli A. Immunity to the Coronavirus May last years, new data hint. The New York Times. 2020 [Google Scholar]
- 24.Administration USFaD EUA Authorized Serology test performance. 2021. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance Available at:
- 25.Wilson E., Donovan C.V., Campbell M. Multiple COVID-19 clusters on a university campus—North Carolina. Morbidity Mortality Weekly Rep 2020. 2020;69:1416. doi: 10.15585/mmwr.mm6939e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Scientific Brief: SARS-CoV-2 and potential Airborne transmission. 2020. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html Available at: [PubMed]
- 27.Weissenborn R., Duka T. Acute alcohol effects on cognitive function in social drinkers: Their relationship to drinking habits. Psychopharmacology. 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- 28.Eastman-Mueller H., Fu T-c, Dodge B.M. The relationship between college students’ campus sexual health resource utilization and self-reported STI testing: Findings from an undergraduate probability survey. J Am Coll Health. 2020:1–9. doi: 10.1080/07448481.2020.1775607. [DOI] [PubMed] [Google Scholar]
- 29.Engagement NSoS NSSE response rates: Frequently asked questions. https://nsse.indiana.edu/nsse/psychometric-portfolio/responserate-faq.html Available at:
- 30.Cantor D., Fisher B., Chibnall S. The Association of American Universities; Westat, Rockville, Maryland: 2019. Report on the AAU campus climate survey on sexual assault and misconduct. [Google Scholar]
- 31.Conroy S., Murray E.J. Let the question determine the methods: Descriptive epidemiology done right. Br J Cancer. 2020;123:1351–1352. doi: 10.1038/s41416-020-1019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.