Abstract
Avian metapneumovirus (aMPV) is a viral pathogen that causes respiratory signs and drops in egg production most notably in turkeys but also affects chickens, ducks and other species. Four subgroups (aMPV-A, -B, -C and -D) have been characterized to date, which differ genetically, antigenically and in terms of geographic distribution and host range. Despite the rising awareness towards aMPV, many aspects of its epidemiology are still poorly understood.
In this pilot study, a serological survey was carried out to assess the possible circulation of subgroups A, B, and C in intensively raised ducks in Northern Italy. A total of 220 sera from 10 Pekin duck flocks and one mallard flock were collected at slaughter and tested by subgroup-specific aMPV-A, B, and C indirect ELISA assays. None of the tested birds had been vaccinated against aMPV and no symptoms had been reported. No aMPV-A, B or C antibodies were detected in any bird of the Pekin duck flocks, whereas the entire mallard flock tested positive for aMPV-C antibodies. This is the first report of aMPV-C antibodies in ducks in Italy, where only aMPV-B has been reported to circulate in recent years. Further research efforts will be targeted towards the screening of a larger panel of samples, in the hope of obtaining positive samples from which full length genome sequences and infectious viruses can be isolated for virus characterization. Finally, the demonstration of aMPV-C specific antibodies in the serum of all sampled mallards indirectly suggests that this species is readily infected by aMPV-C and supports a possible role of wild anatids as a transmission vector of the virus.
Key words: Avian metapneumovirus, ELISA, Italy, duck, mallard
INTRODUCTION
Avian metapneumovirus (aMPV) is an enveloped, single-stranded negative-sense RNA virus that belongs to the genus Metapneumovirus of the family Pneumoviridae. This pathogen is associated with respiratory signs which are referred to with different names, such as turkey rhinotracheitis and avian rhinotracheitis, and also to infections of the reproductive tract resulting in drops in egg production. The disease burden is particularly notable for turkey farming, in which routine vaccination is often adopted to achieve proper protection, but also poses a significant threat to the chicken and duck industries. Concurrent bacterial or viral infections may also be involved in the pathogenesis, possibly exacerbating the severity of clinical manifestations. These concurrent bacterial infections in chickens often result in a swelling of the head, a condition known as swollen head syndrome (Rautenschlein 2020).
To date, 4 different subgroups (aMPV-A, -B, -C and -D) have been well-characterized based on genetic and antigenic differences. Two genetic lineages of aMPV-C, one in the North America (US) and the other Eurasian have also been described and very recently, 2 studies from North America suggested the existence of other subgroups (Canuti et al., 2019; Retallack et al., 2019). Subgroups A, B, and D are genetically closer than subgroup C, which is more closely related to human metapneumoviruses, possibly suggesting a common origin (Rautenschlein 2020).
Interestingly, differences exist between the different subgroups in terms of host range: aMPV-A, B, C (US lineage) and D are considered as viruses of galliforms, in particular turkeys, and aMPV-C (Eurasian lineage) as viruses of ducks. However, experimental evidence has shown that some level of infection can occur in the different species by the different subgroups (Brown et al., 2019).
Regarding other species, aMPVs have also been detected in geese, guinea fowls, sparrows, gulls, parakeets, and various species of waterfowl, and is known to elicit seroconversion in several others including pheasants, pigeons, and crows (Rautenschlein 2020; Canuti et al., 2019; Retallack et al., 2019). However, the degree to which minor and wild species are susceptible to aMPV is not entirely clear.
Since the vast majority of epidemiological studies have been conducted on turkeys and chickens, little is known about aMPV prevalence in ducks. It is no coincidence that reports of aMPV infections in commercially raised ducks are mainly limited to China and France, 2 of the largest producers of duck meat (http://www.fao.org/faostat). Nonetheless, the possible aMPV circulation in ducks deserves to be also investigated in countries where the interest towards this sector is less significant. For this reason, a pilot study was conducted in Italy to screen intensively raised ducks by serological means.
MATERIALS AND METHODS
Blood samples were taken from ten Pekin duck (Anas platyrhynchos domesticus) flocks (flocks A-J) and one mallard (Anas platyrhynchos) flock (flock K) in a duck abattoir located in Northern Italy during the summer of 2019. Pekin ducks had been farmed in Veneto and mallards in Lombardy, 2 regions of Northern Italy. Twenty samples were taken from each flock for a total of 220 sera.
Pekin duck flocks were unique sex flocks composed of either males or females. Seven female flocks were slaughtered at an age comprised between 58 and 66 d, and 3 male flocks were slaughtered at an age comprised between 87 and 89 d. The mallard flock was composed of both males and females and these were slaughtered at 130 d of age. None of the animals had been vaccinated against aMPV.
The sera were obtained by drawing 20 mL of blood from each bird at jugulation. Blood samples were allowed to clot and then centrifuged for 20 min at 1,600 RPM. The sera were kept at −20°C until processing. Sera were centrifuged for 10 min at 2,500 RPM and diluted 1:5 in PBS. A 25% kaolin solution (weight/volume) was added, then the mixtures were agitated, incubated at room temperature for 20 min and centrifuged for 3 min at 2,000 RPM. Lastly, the supernatants were diluted 1:10 in a solution of PBS/Tween 20 (0,002%)/ non-fat dry milk (5%) and 10% foetal calf serum.
Samples were analyzed by a panel of indirect ELISAs for the detection of anti-aMPV IgGs specific to subgroup A, B, and C. ELISA procedures and validation methods followed those previously described by Giraud et al. (1987).
In-house negative SPF duck serum and three subgroup-specific (A, B, or C) positive duck sera were used as references and the results were interpreted as positive when the corrected optical density (OD) value was higher than 0.200. The OD value of a sample serum tested on the negative antigen had to be lower than 0.200 for the result to be considered interpretable.
The corrected OD (cOD) value was calculated as follows:
Corrected OD=(X-Z)-(A-B)/(C-D)-(A-B)
X: OD of the sample serum tested on the positive antigen
Z: OD of the sample serum tested on the negative antigen
A: OD of the negative reference serum tested on the positive antigen
B: OD of the negative reference serum tested on the negative antigen
C: OD of the positive reference serum tested on the positive antigen
D: OD of the positive reference serum tested on the negative antigen
RESULTS AND DISCUSSION
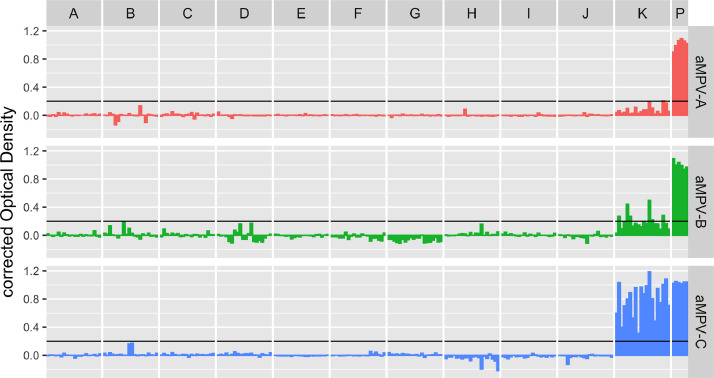
All Pekin duck groups (A-J) proved negative for each of the 3 subgroups, whereas all mallards (group K) were positive for aMPV subgroup C (cOD range = 0.314–1.186, mean = 0.779, SD = 0.244). Six mallards were positive to aMPV-B (cOD range = 0.221–0.497, mean = 0.330, SD = 0.111) and one was also borderline positive to aMPV-A with a cOD value equal to 0.206 (Figure 1). Across 6 measurements, the OD values detected in the positive controls were in the range between 0.898 and 1.089 for subgroup A (mean = 1.020, SD = 0.069), 0.940 to 1.089 for subgroup B (mean = 1.004, SD = 0.053) and 1.018 to 1.047 for subgroup C (mean = 1.033, SD = 0.011).
Figure 1.
Individual subgroup-specific antibody values found in the different flocks. Results were considered positive when the corrected OD value was higher than 0.200 (black threshold line). Group A-J were composed of Pekin ducks, and group K of mallards. Group P shows the OD values measured by testing the positive reference serum in the different runs. Abbreviation: OD, optical density.
Anamnestic data about the mallard flock were retrospectively obtained. The flock was raised indoors for the entire productive cycle, during which no signs were reported. The mallards did not show any clinical sign at slaughter.
This is the first evidence of aMPV-C presence in Italy, where only aMPV-B has been accounted to circulate in recent years. Subgroup B has been reported to be by far the dominant subgroup in Europe (Franzo et al., 2020). However, this prevalence only relates to aMPV in turkeys and chickens as almost all studies have been conducted uniquely on these 2 species. Very little studies about the type of aMPV infection in ducks have been undertaken, and thus the circulation of the different subgroups of aMPV can only be considered in respect to the species tested.
To date, aMPV-C has been detected in North America, Asia, and Europe (primarily France). This subgroup may be further divided into 2 distinct lineages: the North American strains belong to a turkey-adapted lineage, whereas the Eurasian ones are found in ducks and other anatids (Brown et al., 2019). Based on both location and host species, it may be hypothesized that the herein detected aMPV-C antibodies were elicited by strains belonging to the duck lineage. For confirmation however, molecular diagnosis and characterization of genomic RNA would be needed.
Only the investigated mallard group was found serologically positive to aMPV. The animals did not show any clinical sign at slaughter, and no symptoms were reported throughout their life. Mallards, from which the majority of domestic duck breeds (including Pekin ducks) originated, have already been reported to be susceptible to aMPV-C in a study in which this subgroup was detected in a wild mallard in the Netherlands (van Boheemen et al., 2012).
Mallards are one of the most abundant wild avian species in Italy, especially in the North-Eastern regions where the study was conducted and poultry farming activities are markedly concentrated. Even if the positive flock was intensively raised, this discovery may corroborate the hypothesis that wild waterfowl may act as carrier and reservoir for aMPV-C (Turpin et al., 2008).
Six mallards tested borderline positive to the subgroup B-specific assay, and one also to the subgroup A assay. The occurrence of nonspecific reactions is considered inherent to ELISA methods especially when chicken or duck sera are used (World Organisation for Animal Health, 2020), and the detection of antibodies raised against subgroup C with ELISAs based on A or B has already been described (Cook and Cavanagh, 2002). These borderline positive results were thus considered nonsignificant.
Based on the results of this pilot study, aMPV-C seem to circulate in intensively raised mallard ducks in Northern Italy. However, further studies aimed at detecting and characterizing aMPV presence from a molecular standpoint, possibly on a larger scale, are required to shed light on this subject. The molecular characterization of Italian aMPV-C strains would allow to determine whether they cluster within the duck C lineage or if they possess country- or species-specific features. Additionally, the possible isolation of these strains would enable in vivo pathogenicity studies in other duck species, and would be especially important if the strains found in mallards are revealed to be divergent from the currently available subgroup C sequences. Considering the peculiarities of the Italian poultry sector, with different species bred in close proximity to each other, expanding the survey to other susceptible species could also prove interesting.
ACKNOWLEDGMENTS
The authors deeply thank dr. Piraska Sabbion for the support in the sampling activities and dr. Stefania Laverta for providing the anamnestic information about the mallard flock.
Disclosures
The authors declare no conflicts of interest.
REFERENCES
- World Organisation for Animal Health . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE; Paris, France: 2020. Chapter 3.3.15. Turkey rhinotracheitis (avian metapneumovirus)https://www.oie.int/standard-setting/terrestrial-manual/access-online Accessed Aug. 2020. [Google Scholar]
- Brown P.A., Allée C., Courtillon C., Szerman N., Lemaitre E., Toquin D., Mangart J.M., Amelot M., Eterradossi N. Host specificity of avian metapneumoviruses. Avian Pathol. 2019;48:311–318. doi: 10.1080/03079457.2019.1584390. [DOI] [PubMed] [Google Scholar]
- Canuti M., Kroyer A., Ojkic D., Whitney H.G., Robertson G.J., Lang A.S. Discovery and characterization of novel RNA viruses in aquatic North American Wild Birds. Viruses. 2019;11:768. doi: 10.3390/v11090768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K.A., Cavanagh D. Detection and differentiation of avian pneumoviruses (metapneumoviruses) Avian Pathol. 2002;31:117–132. doi: 10.1080/03079450120118603. [DOI] [PubMed] [Google Scholar]
- Franzo G., Legnardi M., Mescolini G., Tucciarone C.M., Lupini C., Quaglia G., Catelli E., Cecchinato M. Avian Metapneumovirus subgroup B around Europe: a phylodynamic reconstruction. Vet Res. 2020;51:88. doi: 10.1186/s13567-020-00817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud P., Toquin D., Picault J.P., Guittet M., Bennejean G. Utilisation de la méthode ELISA pour le siérodiagnostic de I'infection par le virus de la rhinotrachéite infectieuse chez la dinde, la poule et la pintade. Bull. Lab. Vét. 1987;27-28:65–70. [Google Scholar]
- 7.Rautenschlein, S. 2020. Avian metapneumovirus. Pages 135–143 in Diseases of Poultry. D. E. Swayne, ed. 14th ed. Wiley Blackwell, Hoboken, NJ.
- Retallack H., Clubb S., DeRisi J.L. Genome sequence of a divergent Avian Metapneumovirus from a Monk Parakeet (Myiopsitta monachus) Microbiol. Resour. Announc. 2019 doi: 10.1128/MRA.00284-19. 8:e00284–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin E.A., Stallknecht D.E., Slemons R.D., Zsak L., Swayne D.E. Evidence of avian metapneumovirus subtype C infection of wild birds in Georgia, South Carolina, Arkansas and Ohio, USA. Avian Pathol. 2008;37:343–351. doi: 10.1080/03079450802068566. [DOI] [PubMed] [Google Scholar]
- van Boheemen S., Bestebroer T.M., Verhagen J.H., Osterhaus A.D.M.E., Pas S.D., Fouchier R.A.M. A family-wide RT-PCR assay for detection of paramyxoviruses and application to a large-scale surveillance study. PLOS ONE. 2012;7:e34961. doi: 10.1371/journal.pone.0034961. [DOI] [PMC free article] [PubMed] [Google Scholar]