Abstract
Epithelioid hemangioendothelioma (EHE) is an ultra-rare, translocated, vascular sarcoma. EHE clinical behavior is variable, ranging from that of a low-grade malignancy to that of a high-grade sarcoma and it is marked by a high propensity for systemic involvement. No active systemic agents are currently approved specifically for EHE, which is typically refractory to the antitumor drugs used in sarcomas. The degree of uncertainty in selecting the most appropriate therapy for EHE patients and the lack of guidelines on the clinical management of the disease make the adoption of new treatments inconsistent across the world, resulting in suboptimal outcomes for many EHE patients. To address the shortcoming, a global consensus meeting was organized in December 2020 under the umbrella of the European Society for Medical Oncology (ESMO) involving >80 experts from several disciplines from Europe, North America and Asia, together with a patient representative from the EHE Group, a global, disease-specific patient advocacy group, and Sarcoma Patient EuroNet (SPAEN). The meeting was aimed at defining, by consensus, evidence-based best practices for the optimal approach to primary and metastatic EHE. The consensus achieved during that meeting is the subject of the present publication.
Key words: sarcoma, epithelioid hemangioendothelioma, diagnosis, treatment, management, guidelines
Highlights
-
•
This consensus paper provides key recommendations on the management of epithelioid hemangioendothelioma (EHE).
-
•
Recommendations followed a consensus meeting between experts and a representative of the EHE advocacy group and SPAEN.
-
•
Authorship includes a multidisciplinary group of experts from different institutions from Europe, North America and Asia.
Introduction
Epithelioid hemangioendothelioma (EHE) is an ultra-rare vascular sarcoma, usually behaving as a low-grade malignancy despite a high propensity for systemic involvement. The degree of uncertainty in selecting the most appropriate treatment of EHE patients is high, treatment options vary and the adoption of new treatments is inconsistent across the world, resulting in suboptimal outcomes for many patients. Also, no active systemic agents are currently approved specifically for EHE, which is typically refractory to the antitumor drugs used in soft tissue sarcomas (STS).
In December 2020, the European Society for Medical Oncology (ESMO) convened a virtual consensus meeting to update its clinical practice guidelines on sarcomas. Recognizing the special need for a global consensus on the management of patients with EHE, ESMO hosted a parallel meeting that included EHE experts from several disciplines from Europe, North America and Asia, with the contribution of the EHE Group (composed by the EHE Rare Cancer Charity, UK and the EHE Foundation), a global, disease-specific patient advocacy group and a patient representative from Sarcoma Patient EuroNet (SPAEN).
Quality of existing evidence
The quality of evidence available for common cancers is considerably stronger than for EHE. No phase II or III randomized studies have ever been conducted and results from only two phase II trials are available. At present, there are only two prospective trials ongoing specifically dedicated to EHE on eribulin and trametinib (ClinicalTrials.gov Identifier: NCT03331250 and NCT03148275). Current clinical practice is therefore based mostly on retrospective case series or case reports (i.e. level IV-V evidence), in which the pathological diagnosis was often not confirmed by an experienced STS pathologist and molecular testing was not available. Many of these reports were published before the identification of characteristic WW domain-containing transcription regulator 1 (WWTR1)-calmodulin binding transcription activator 1 (CAMTA1) or Yes-associated protein 1 (YAP1)-transcription factor E3 (TFE3) gene fusions in EHE.
A degree of uncertainty needs to be accepted when facing regulatory matters and clinical decision making in ultra-rare cancers. In this consensus, however, we grade levels of evidence from I to V and use grades of recommendation from A to D adapted from the system used by the Infectious Diseases Society of America-US Public Health Service Grading System 2 (Supplementary Table S1, Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2021.100170).1
Epidemiology/Clinical presentation
EHE has an incidence of 0.038/100 000/year and a prevalence of <1/1 000 000,2, 3, 4 showing a slight predominance in women. The incidence peaks in the fourth to fifth decade.2,4, 5, 6, 7 EHE is exceedingly rare in children.
EHE can arise anywhere in the body and have various presentations, including unifocal lesion (i.e. localized disease characterized by a single tumor lesion), locoregional metastases (i.e. a disease with multifocal single-organ involvement or multiple lesions to a single anatomic compartment), or systemic metastases (i.e. a disease with multi-organ involvement).2,7, 8, 9 The relative incidence of such presentations is currently unknown. When the onset occurs as a single lesion, this is usually a solitary mass in the soft tissues.5,10,11 However, >50% of patients present with metastatic disease, mostly involving lung, liver and bone.5, 6, 7, 8, 9, 10, 11 An origin from a blood vessel can be demonstrated in ∼50% cases.2,5 The monoclonal origin of multifocal EHE, at least in liver, has also been established using WWTR1-CAMTA1 breakpoint analysis, indicating that multiple lesions arise from spread of a single primary malignant clone.12
The clinical presentation at the time of diagnosis is quite variable. The initial diagnosis can be incidental in completely asymptomatic patients. In symptomatic cases, the most common symptoms are pain (40%), a palpable mass (6%-24%) and weight loss (9%).2,6,9 Angiocentric EHE may present with signs and symptoms of venous obstruction.
Prognostic factors
Data available on prognostic factors in localized and advanced EHE are limited and only retrospective. In most cases, the interpretation of these series is further hampered by the unavailability of standard diagnostic criteria.
There are no clearly validated pathological/molecular prognostic factors for EHE. Grading systems based on a combination of mitotic activity and tumor size,13 or of histologic atypia and tumor size have been proposed, but their prognostic value needs to be confirmed [level of evidence IV, C].14 Conflicting conclusions had been reported on the prognostic value of the gene rearrangement subtype,5,15,16 while recently, the negative prognostic role of synaptophysin expression has been proposed.14
The only prognostic factors are the extent of disease at presentation (tumor size or the evidence of metastatic disease) and the presence of systemic signs and symptoms.2,5,14,16, 17, 18, 19, 20, 21 In patients with serosal involvement with/without effusion, the expected survival ranges from 1 to 11 months. The prognostic impact of locoregional metastases versus systemic metastases is still controversial.2,20
The clinical behavior of metastatic EHE can be extremely variable, ranging from that of a low-grade malignancy to that of a high-grade sarcoma. Reliable molecular and biological features to stratify prognosis for advanced EHE are lacking.
EHE can present with tumor-related systemic symptoms such as fever, weight loss and fatigue. Their presence seems to correlate with a worse quality of life (QoL) and survival.9,17,21 The same symptoms can also occur during surveillance.21 The presence of serosal involvement/effusion, weight loss, fever, fatigue and tumor-related pain should be carefully investigated and recorded. A recent report indicated no impact of pregnancy and lactation on the course of a multifocal EHE.22
As for hematological or biochemical markers, anemia is the only reported negative prognostic factor.17 The prognostic relevance of inflammatory and hormonal circulating biomarkers is currently under investigation. The identification of reliable clinical and/or biological correlates of outcome is indeed crucial to optimize the treatment strategy and design future clinical trials.
At present, many centers use sequential imaging to assess the growth dynamic as a measure of aggressiveness and potential hint for the further course of disease, although the disease burden may be underestimated with conventional imaging techniques.
General principles on EHE management
Due to the ultra-rare nature of EHE, patients should be managed within sarcoma reference centers or reference networks, by a dedicated sarcoma multidisciplinary team including a pathologist, radiologist, surgical oncologist, orthopedic surgeon, radiation oncologist, medical oncologist and palliative care specialist who are familiar with the nuances of this disease23 [III, A]. Other specialists, such as liver transplant (LT) experts, should be involved on an individualized basis.
Pathology and molecular biology
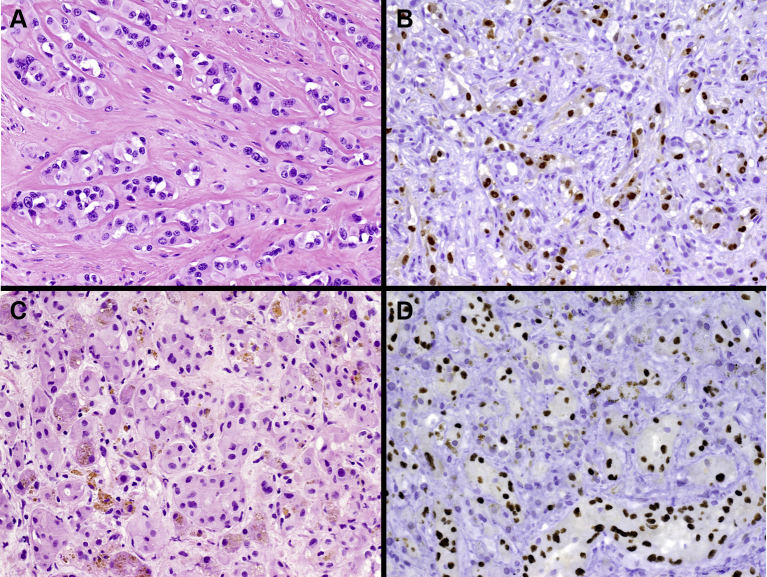
EHE is a malignant vascular neoplasm, featuring an epithelioid endothelial cell population in a distinctive myxohyaline stroma, molecularly characterized by WWTR1-CAMTA1 (90%) or YAP1-TFE3 (10%) gene fusions15,24 (Figure 1). Rare WWTR1 translocations involving gene partners other than CAMTA1 have been reported.25 The immunohistochemical (IHC) or molecular assessment of WWTR1-CAMTA1 and/or YAP1-TFE3 is highly recommended to confirm the diagnosis and rule out other mimics, like angiosarcoma and epithelioid hemangioma [V, A]. In particular, CAMTA1 IHC is a good surrogate to detect translocation, whereas TFE3 IHC is less specific and therefore, when suspected, molecular confirmation is suggested. At present, none of the translocations identified in EHE is druggable. Their identification has currently no prognostic or predictive value, nor can it be used for treatment stratification purposes.
Figure 1.
Pathologic features of epithelioid hemangioendothelioma (EHE).
(Panel A) Hematoxylin–eosin staining in CAMTA1-rearranged EHE: epithelioid cells organized in strands and cords set in a collagenous stroma are typically observed. The neoplastic cells contain intracytoplasmic vacuoles. (Panel B) CAMTA1 immunostaining shows CAMTA1 nuclear expression in tumor cells. (Panel C) Hematoxylin–eosin staining in TFE3-rearranged EHE: tumor cells tend to show larger eosinophilic cytoplasm. (Panel D) TFE3 immunostaining shows the nuclear expression of TFE3.
CAMTA1, calmodulin binding transcription activator 1; TFE3, transcription factor E3.
Pathologic review is strongly recommended if the first diagnosis was made outside a sarcoma reference center [IV, A].
Morphology
Macroscopically, the tumor size can vary from small nodules to large masses. The cut surface is white and firm. Morphologically, EHE is most often composed of epithelioid cells, organized in strands, cords or solid nests, showing glassy eosinophilic cytoplasm often with cytoplasmatic vacuoles, set in a distinctive myxohyaline stroma, occasionally associated with hemorrhagic foci. Rarely, neoplastic cells may organize in a cribriform pattern of growth that may mimic invasive carcinoma. Low mitotic activity and mild nuclear atypia are generally observed. EHE associated with YAP1-TFE3 fusion is composed of epithelioid neoplastic cells with more copious bright eosinophilic cytoplasm, often organized in a solid pattern of growth but featuring at least focally unequivocal vasoformative features, usually not seen in WWTR1-CAMTA1 EHE.24
Approximately 10% of EHE cases feature marked nuclear atypia with prominent nucleoli, focal solid growth pattern, foci of necrosis and higher mitotic activity (>2 mitoses/10 high power field). These features are associated with more aggressive behavior. However, clear morphologic criteria to distinguish EHE variants with a more or less favorable prognosis are missing.
Immunophenotype
Immunohistochemically, EHE consistently expresses endothelial differentiation markers such as CD31, ERG, CD34 and FLI-1.26,27 Focal cytokeratin immunopositivity is observed in <30% of cases.13,28 Only occasionally, the expression of epithelial differentiation markers can be strong and diffuse. As a consequence of both WWTR1-CAMTA1 and YAP1-TFE3 fusions, nuclear expression of CAMTA29 and TFE3,15 respectively, is consistently observed. However, TFE3 expression is less specific than CAMTA1 to predict the presence of the corresponding molecular alteration.7,29
Molecular profile
Up to 90% of EHEs harbor a t(1;3)(p36.3;q25) translocation, leading to the formation of WWTR1-CAMTA1 fusion gene,24,30 whereas a smaller subset shows an alternative genetic aberration represented by a t(X;11)(p11;q22) translocation, leading to a YAP1-TFE3 fusion.15 Patients with YAP1-TFE3 fused EHE tend to be younger than those with a WWTR1-CAMTA1 fusion.15,16 Other rare fusions can be seen.25
The translocation is present irrespective of anatomical site and clinical behavior. The WWTR1 (also call TAZ) protein is one of two end effectors of the Hippo pathway.31 WWTR1 and YAP both exert a pro-oncogenic transcriptional function. Fusion of WWTR1 to CAMTA1 results in dysregulation of the Hippo pathway, such that WWTR1-CAMTA1 resides constitutively in the nucleus, driving oncogenic transformation.32
Differential diagnosis and diagnostic pitfalls
The main differential diagnosis is with primary or metastatic carcinomas. However, consistent expression of endothelial differentiation markers is a helpful diagnostic clue.26 Pancytokeratin, however, may be expressed focally in EHE, further increasing diagnostic uncertainty.16,25 In pleural EHE, another differential diagnosis is malignant mesothelioma. Expression of calretinin and WT1, as well as negativity for endothelial differentiation markers, generally allows accurate diagnosis. Epithelioid sarcoma (ES) may enter the differential diagnosis. In fact, in addition to epithelioid morphology, ES may share with EHE the expression of vascular markers such as CD34 and, less frequently, ERG. However, CD31 is consistently negative, and loss of nuclear INI1 expression is almost invariably observed in ES.33 When EHE exhibits greater cytologic atypia and cellularity, epithelioid angiosarcoma needs to be excluded. The presence of striking cytologic atypia, elevated mitotic count as well as the presence of a solid or sheet-like growth pattern is a valuable diagnostic clue. Importantly, expression of CAMTA1 or TFE3 has never been observed in angiosarcoma.34 Pseudomyogenic hemangioendothelioma (PE) is another differential diagnosis. By difference to EHE, PE shows in most cases spindled cytomorphology, rather than epithelioid, and lacks cytoplasmic vacuoles.35 While sharing expression of endothelial antigens with EHE, in contrast to EHE, pseudomyogenic hemangioendothelioma typically expresses FOSB due to FOSB-related fusion and is negative for CAMTA1 and TFE3.36
Radiology
Baseline staging for EHE consists of whole-body imaging including the brain, by computed tomography (CT) or magnetic resonance imaging (MRI), or the combinations of both modalities (e.g. chest CT and abdominal/whole-body MRI) [V, A]. A whole-body MRI, or whole-body [18F]-fluorodeoxyglucose (FDG)-PET/CT (including limbs) depending on locally available resources, is advisable to detect bone and limb involvement. If whole-body MRI or PET/CT are unavailable, a bone scan is recommended to exclude bone lesions [V, A]. As a general rule, to monitor the status of the disease, any relevant radiological study that is positive at baseline should be repeated while on treatment or surveillance.
Even in the absence of prospective studies on the radiological assessment of EHE, CT of the chest, abdomen and pelvis is the imaging modality of choice [V, B], because of its wide coverage and optimal assessment of pulmonary disease.37 Triple phase imaging of the liver is particularly helpful for hepatic involvement.
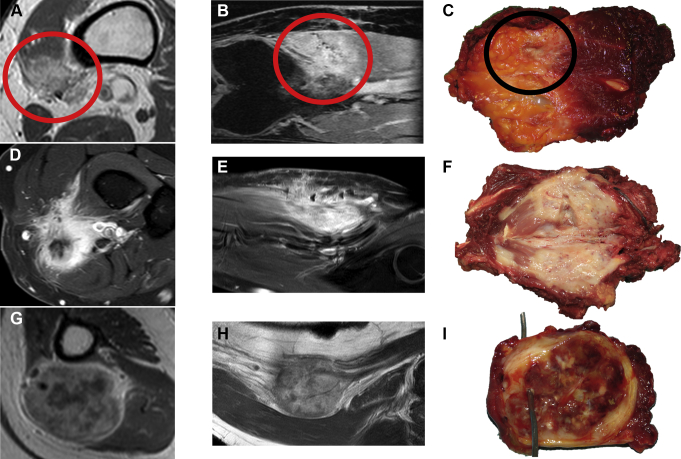
MRI is recommended for the assessment of primary soft tissue disease [IV, C] (Figure 2) and should be considered for the monitoring of liver and bone involvement and response assessments at these sites [IV, B].38, 39, 40 Where available, whole-body MRI should be considered for detection, monitoring and response assessment of bone disease because of increased sensitivity and wide coverage [IV, B].
Figure 2.
Primary, soft tissue epithelioid hemangioendothelioma (EHE).
Contrast-enhanced T1-weighted magnetic resonance imaging (MRI), axial view (A) and contrast-enhanced T1- Spectroscopie Dans le Proche Infrarouge (SPIR)-weighted MRI coronal view (B), showing a classic EHE with an infiltrative growth pattern in the vastus medialis (red circle). Surgical specimen cut along the longitudinal axis showing the macroscopic appearance of the tumor (black circle, C). Contrast-enhanced T1-SPIR-weighted MRI, axial view (D) and coronal view (E), showing a more aggressive EHE with infiltrative growth pattern and necrosis in the anterior aspect of the proximal forearm. Surgical specimen cut along the longitudinal axis showing the macroscopic appearance of the tumor (F). Contrast-enhanced T1-weighted MRI, axial view (G) and coronal view (H) showing a classic EHE with a nodular growth pattern in the medial aspect of the arm arising from the brachial vessels. Surgical specimen cut along the axial axis, showing the macroscopic appearance of the tumor (I, brachial artery is cannulated by the wire).
FDG uptake is usually mild-to-moderate.37,41, 42, 43 However, patients with higher standardized uptake value (SUV) lesions seem to have significantly worse survival and higher progression rates.41,44
Imaging features and differential diagnosis
The presence of multifocal, coalescent hepatic nodules in a peripheral subcapsular location with capsular retraction39,40,45 and a target appearance at portal venous phase contrast-enhanced CT, should raise the possibility of EHE46,47 (Figure 3). The target appearance may also be seen on T2-weighted and dynamic contrast-enhanced MRI.38,39,46 The ‘lollipop’ sign (hepatic or portal vein tapering and termination at the periphery or just within a well-defined hypoenhancing lesion) may also be visible.46,48 However, a biopsy is required for definitive diagnosis, in particular to exclude mass-forming peripheral intrahepatic cholangiocarcinoma.
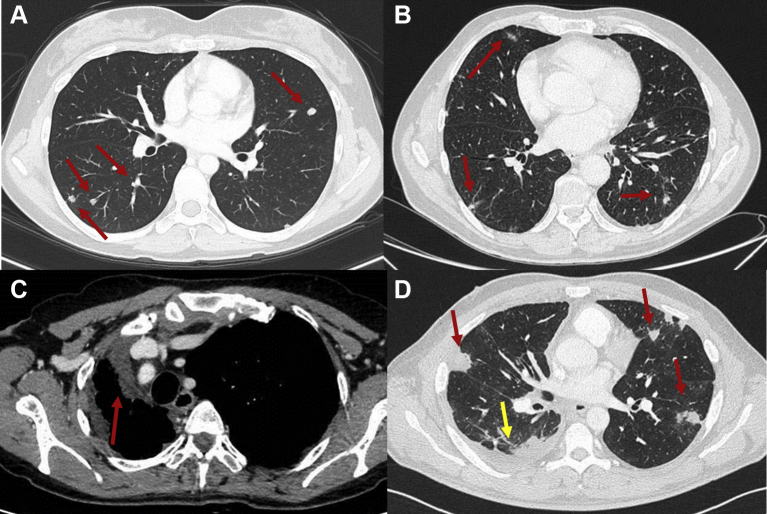
Figure 3.
Thoracic epithelioid hemangioendothelioma (EHE).
(A) Computed tomography (CT) scan of the chest, lung window, axial view, showing solid pulmonary nodules in both lungs (red arrows). (B) CT scan of the chest, lung window, axial view, showing multifocal areas of reticulonodular pattern in both lungs (red arrows). (C) Contrast enhanced CT scan of the chest, venous phase, soft tissue window, axial view, showing diffuse pleural involvement (red arrow) with mediastinal shift. (D) CT scan of the chest, lung window, axial view, showing pleural thickening (yellow arrow) and parenchymal lesions (red arrows).
Thoracic EHE can present with four main patterns: multiple pulmonary nodules,2,19,38 reticulonodular opacities,36,49 diffuse pleural thickening and parenchymal nodule/mass with pleural invasion which simulates malignant pleural mesothelioma50 (Figure 4). Sometimes pulmonary nodules may exhibit a surrounding ground glass halo similar in morphology to pulmonary metastatic angiosarcoma. Patients with diffuse pleural thickening and effusion seem to have a poor prognosis.19,50
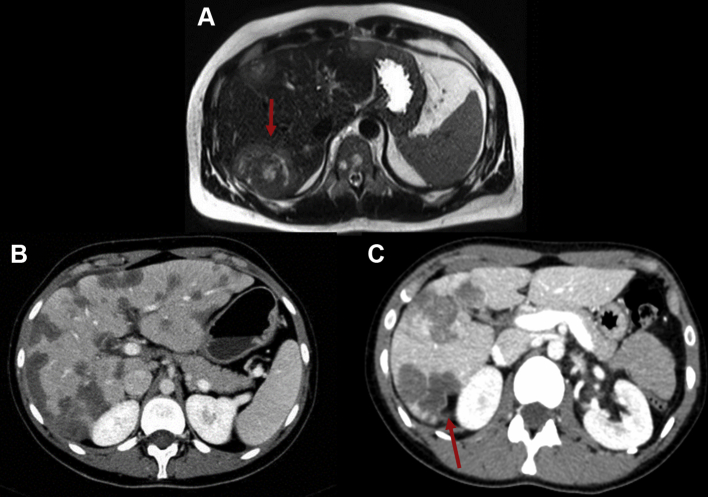
Figure 4.
Hepatic epithelioid hemangioendothelioma (EHE).
(A) T2-weighted magnetic resonance imaging of the abdomen, axial view, showing hepatic lesions with a target sign (red arrow). (B) Contrast enhanced computed tomography (CT) scan of the abdomen, portal phase, axial view, showing multiple peripheral low attenuation hepatic lesions. (C) Contrast enhanced CT scan of the abdomen, portal phase, axial view, showing multiple peripheral low attenuation hepatic lesions and capsular retraction (red arrow).
When EHE involves bones, the axial skeleton and lower limbs are the most common sites.51 Bone lesions are predominantly poorly demarcated and lytic, some with a sclerotic rim at initial presentation.5,7 Expansible mixed density lesions are also recognized. On MRI, lesions are hypointense on T1 and hyperintense on T2/STIR sequences.37,40 Hemangiomas can be distinguished from EHE on CT as they appear as well-defined lucent lesions containing high-density rounded foci which correspond to thickened vertical trabeculae mixed with low attenuation fat. On MRI, the majority of asymptomatic hemangiomas also have characteristic imaging features and exhibit high signal intensity on T1- and T2-weighted images owing to the presence of fat. However, extraspinal hemangiomas are less characteristic and can appear as non-specific, well-defined osteolytic lesions without the web-like coarse trabecular pattern.40 The poorly marginated, lytic radiological appearance of angiosarcoma of bone is also non-specific and it is not possible to differentiate angiosarcoma of bone from EHE on imaging alone.40
Response assessment
The definition of radiological progression and the assessment of treatment response are a challenge in EHE. The occurrence of serosal effusion and of slow-growing variants makes the use of RECIST 1.1 of limited value. Improvement of serosal effusion, reduction in size <30% and symptom control should be valued in the treatment response assessment. Symptomatic deterioration may not be reflected by radiological changes, particularly for thoracic disease, therefore assessment of symptoms and QoL are an essential component of response assessment. Preliminary data suggest that changes in SUV on FDG-PET/CT and signal/apparent diffusion coefficient (ADC) on MRI can be of help in response assessment.
Diagnosis
Percutaneous core-needle biopsy is recommended [V, A]. Biopsy should be carried out using 14-16-gauge needles with preferred coaxial technique. The biopsy tract should be placed in such a way to be safely removed at the time of definitive surgery.52
Treatment
Surgery
The treatment of choice for confirmed unifocal EHE (most often located in soft tissue) is surgery [II, A]. Resection should be carried out in referral centers with experience in sarcoma surgery.53, 54, 55, 56
The primary aim of surgery is the complete resection of EHE with microscopic negative (R0) margins, with an expected cure rate of 70%-80%.5 However, the inherent contribution of surgery to prognosis in this group is difficult to determine, as the tendency to progress in the individual case cannot be (reliably) predicted. Surgery can be complemented with radiation therapy (RT) when R1 excisions occur [V, A]. When severe morbidity is anticipated or a macroscopic complete resection (R0/R1) is not possible, surgery can be substituted by definitive RT, or ablative procedures, or even isolated limb perfusion (ILP), depending on local/institutional expertise/policies [V, A].
Given the possible curative impact of local treatments and the risk of locoregional and/or systemic progression of EHE, at this state of knowledge, a prolonged watchful waiting policy for unifocal EHE is not suggested [V, A]. Active surveillance should be considered only for patients who are not surgical candidates due to the presence of comorbidities or technical challenges.2,5,9,57 [V, A].
On the contrary, active surveillance is the initial recommended option in cases presenting with asymptomatic locoregional or systemic metastases53 [V, A].
Prolonged disease stability and occasional regression have been reported.58 Although active surveillance has never been formally studied, it is common practice in experienced centers to keep asymptomatic patients on active surveillance before engaging in treatment, including surgery.2,5,9,53
EHE of soft tissue and bone
Extent of resection should be carried out according to the principles of sarcoma surgery. For soft tissue EHE, the mass should be resected en bloc. Resection should encompass the biopsy tract, and R0 margins should be obtained by leaving a cuff of normal tissue all around the tumor surface, to minimize the risk of local recurrence (LR). For bony EHE, resection should aim at R0 margins with en bloc resection of the bone of origin and of the involved soft tissues. Surgical curettage of the bone with adjuvant or definitive RT in recurrent or metastasized patients, or both, can be considered if surgical resection is not an option.34,52
Amputation should be discouraged in patients who can be treated with a combination of surgical procedures, locoregional treatments and/or adjuvant RT, provided the limb remains functional after multimodality treatment.
In angiocentric tumors, the vessel of origin should be resected en bloc with the mass. The need for vascular reconstruction depends on the vessel type (artery versus vein), site and presence of collateral pathways. After venous resection, the vein is usually reconstructed if originally patent and in the absence of collateral pathways; otherwise, ligation without reconstruction is acceptable. After arterial resection, the artery is usually reconstructed. Preoperative RT is preferred to avoid irradiation of the vascular graft. Musculocutaneous flaps are frequently necessary to cover the defect and the vascular graft.
The role of surgery in metastatic EHE of bone is unknown, but in the absence of alternative treatments, if feasible, surgery is an option. Such decisions should be individualized and take into consideration the pattern of tumor progression, fracture risk, site and number of lesions, expected morbidity, patient symptoms and availability of alternative local therapies, such as ILP, ablation procedures and/or RT.53,57
EHE of liver
Treatment of hepatic EHE should take into consideration the anatomical location within the liver, tumor size, number of nodules, the presence of vascular invasion and the presence of extrahepatic disease.
After a short period of observation to assess the biological behavior of EHE, surgical resection is considered the treatment of choice for stable/slowly progressing unifocal or limited locoregional disease that is technically resectable [IV, B]. The goal is a complete resection of the tumor with R0 margins. Based on the limited literature available, hepatic resections provide cure in at least 50% of patients.44,59, 60, 61, 62, 63 However, none of the studies contained information of the pace of disease growth before surgery.
In selected patients with unresectable hepatic EHE and no extrahepatic disease, LT provides short- and long-term outcomes comparable to patients with other indications. Specifically, LT has been associated with a 5-year post-LT survival rate in excess of 50%.59,61 Similarly to the evidence available for hepatic resections, none of the studies provided information on the pace of disease before LT. It is well known that multifocal hepatic EHE can remain stable for many years without active treatment. On this basis, after full disclosure about the limitations of the current literature, LT should be proposed as a treatment modality for patients with unresectable EHE [IV, B]. LT is also an option for patients with liver-only EHE who develop liver failure due to the tumor, but who are in generally good condition, with an expected good survival after LT.
The median survival in a study of 149 patients after LT was 7.6 years. It is unclear, however, to which extent this is due to the natural history of stable EHE cases or, in part, to the impact of the complete removal of EHE by the replacement of the liver. Risk factors for post-LT recurrence include tumor rupture, macrovascular invasion and the presence of hilar lymph node metastases.44,59, 60, 61, 62 History of tumor rupture should be considered a major contraindication to LT.
The role of LT for unresectable locoregional EHE requires further studies, as the current evidence is based only on retrospective cohorts with a high risk of selection and reporting biases. Therefore, LT is not universally supported by all transplant centers. Nevertheless, LT should be considered and should be proposed to patients after full disclosure of the potential benefits and the risks associated with it. LT should not be proposed to patients with liver EHE and extrahepatic disease [V, A].
Future studies with a long follow-up should provide data about the patient population from which the subgroup of transplanted patients has been drawn.
Stereotactic body RT (SBRT), radiofrequency ablation (RFA) and microwave ablation (MWA) are therapeutic options for patients with unifocal disease who are not surgical candidates and for patients with recurrent liver nodules after liver resection/LT and/or as a bridge to LT (see below) [V, B].
EHE of the thorax
Thoracic EHEs are predominantly found in association with liver and/or bone EHE.19 The presence of pleural involvement is associated with a worse prognosis.2,5,21
Among four different thoracic involvement types, the lung multinodular pattern is associated with a longer survival, whereas the shortest survival is observed in cases with a nodule/mass with pleural involvement. There appears to be no significant difference in survival between thoracic EHE and non-thoracic EHE.19
Surgical resection is the treatment of choice in cases with proven unifocal disease or with reasonably limited locoregional metastases that are technically resectable, after a period of observation. Procedures such as pleural stripping and or pneumonectomy may be considered on an individualized basis, balancing the extent of disease and the expected benefit. Local ablative techniques can also be considered when EHE involvement is isolated to the lungs [V, B].
RT
General principles
There is limited evidence evaluating the role of RT alone in EHE, although EHE is considered relatively radiosensitive. The potential role of radiation should be based upon individualized case considerations. This will primarily depend on the resectability of the tumor and the risk of recurrence and/or the feasibility of further surgery in the event of relapse.
Surgery remains the mainstay of treatment in primary localized, resectable cases. The risk of LR following complete surgical resection is in the range of 10%-15%.64 The role of RT in EHE is based on the principles and management of STS. Adjuvant RT to a total dose of 60 Gy in 30 fractions can be recommended in selected cases where margins are close or positive and there is concern regarding the risk of LR [V, A]. There are no reported cases evaluating the role of preoperative RT for EHE, but following basic STS principles, preoperative RT (50 Gy in 25 fractions) for cases of predicted close or positive surgical margins is reasonable to consider. For inoperable cases, definitive RT to a total dose in the order of 60 Gy in conventional fractionation has been recommended65 [V, A].
Responses to RT have been described at a minimum dose of 30 Gy in conventional fractionation.66 Depending on the clinical presentation of multifocal or metastatic disease, symptoms, disease burden and involved anatomical site, appropriate radiation doses can range from 30 Gy (in conventional fractions) for palliation of symptoms, to a more radical approach in the order of 60 Gy to obtain durable control.
EHE of bone
Post-operative RT with doses ranging from 40 to 60 Gy has been shown to offer excellent local control at 2 years in skeletal EHE.67 Albakr et al.67 summarized a series of 32 publications describing the management of spinal EHE. RT intent for these series ranged from adjuvant RT, primary RT for inoperable disease and palliation. Adjuvant dosing of 60 Gy using conventional fractionation has been reported to provide effective disease control.68,69 Single-fraction high-dose intensity-modulated RT may be indicated in solitary bony lesions.
EHE of liver
Local ablative techniques including SBRT, RFA and MWA, as described below, can be considered in unresectable unifocal disease or oligometastatic disease. The most suitable ablative technique is best determined by a multimodality team and is based on such factors as size and anatomical location of the lesion (e.g. proximity to vessel or bowel).
SBRT delivers very high (ablative) radiation doses over a few fractions with high precision and using small margins. It can be considered in suitable cases at prescribed doses as for other STS when other treatment options are not indicated.70
EHE of the lung
Pulmonary EHE commonly manifests as multifocal disease. In this case, RT is generally considered only for palliation.62,63 SBRT can be considered in the setting of oligometastatic disease delivering doses employed for STS.
Other locoregional treatments
Data on locoregional treatments as an alternative to surgery and/or RT in EHE are limited and only retrospective and do not allow definitive conclusions on the specific role of any of these approaches. They include percutaneous ablation, transarterial chemoembolization (TACE), radioembolization and ILP. No data are available on other local therapies such as high-intensity focused ultrasound in EHE.
Retrospective data suggest that percutaneous ablation (RFA/MWA) can be safe and useful to treat a single and small EHE with curative intent.71,72 Nevertheless, prospective confirmatory data are necessary to make any definitive recommendations.
Prospective data are needed to confirm that TACE is superior to surgical modalities in advanced disease.73 In extrahepatic disease (lymph node, lung, bone) a trend toward better survival and a lower morbidity was seen in four patients who received TACE compared with five patients who underwent resection or LT,73 suggesting that TACE can be considered as a palliative option in most advanced cases. The role of preoperative TACE as an adjunct to LT is not established and warrants further investigation.71, 72, 73, 74, 75, 76
Data on selective internal RT (radioembolization) (SIRT) are lacking. A single case report described significant remission of a multifocal, diffuse, non-resectable EHE 2 months after carrying out SIRT, with a single dose of 1.8 GBq yttrium-90 (48.6 mCi).77
EHE localized to the extremities can be treated by ILP with tumor necrosis factor (TNF) and melphalan. However, while there is a uniform belief of high activity of this treatment in EHE, there are no published data reported to confirm this.57 It remains unclear whether surgery should be carried out to resect residual disease post ILP, or whether patients should continue active surveillance, delaying further treatments to the time of progression.
Systemic treatment
In patients with localized, resectable EHE there is no evidence supporting the use of systemic therapies in the (neo)adjuvant setting [V, D].
For patients with asymptomatic metastatic disease that is not amenable to complete resection with acceptable morbidity, active surveillance is the upfront preferred approach [V, B]. In this scenario, the risk of over-treatment appears to outweigh the damage of a delayed treatment start.
Patients with serosal effusion and/or marked systemic symptoms tend to have a rapidly progressive course, therefore in this case, an early start of systemic therapy should be considered [IV, B].
Patients with metastatic disease and unequivocal evidence of disease progression and/or worsening of symptoms and/or organ dysfunction are candidates for systemic treatment, even though a standard medical approach is currently not established. Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100170 summarizes the data available on systemic agents in EHE, including all reports published from 1999 to 2020 including at least five patients. Conventional chemotherapy available for STS appears to have very limited activity [V, D] and should be limited to more aggressive or rapidly progressive cases, behaving as high-grade STS.78 Participation in clinical trials is encouraged.
Antitumor activity has been seen retrospectively with interferon79,80 [V, C], thalidomide81 [V, C] multi-tyrosine kinase inhibitors [usually with a strong vascular endothelial growth factor receptor (VEGFR) inhibitory property]9,82, 83, 84, 85, 86, 87, 88 [III, B] and mechanistic target of rapamycin (mTOR) inhibitors17,81,89, 90, 91 [IV, B]. Among them, the highest clinical activity has been reported for mTOR inhibitors, with a progression-free survival (PFS) and overall survival in the range of 1 year and 2 years, respectively, and ∼10% of patients having even longer PFS. The panel agrees that these represent the preferred treatment options for patients with advanced and moderately progressive disease. No formal prospective comparison, however, has been carried out and selection of drugs should factor in comorbidities as well as patient preferences.
As with other ultra-rare cancers, as far as reimbursement is concerned, in many countries EHEs are merged with more common sarcomas, even though most trials that led to approval of drugs commonly used in sarcomas have never included patients with EHE.3 The use of off-label drugs may be substantially limited. We strongly advocate treatments for EHE based on the best available clinical evidence, even though the best evidence is limited to small trials and case series.
EHE patients should be considered for clinical studies when available. Based on unpublished preclinical work suggesting the CAMTA1 fusion leads to overexpression of growth factors that may activate the methyl ethyl ketone (MEK) signaling pathway, the MEK-inhibitor trametinib is currently under investigation in the disease. In addition, also eribulin, that is a mitotic inhibitor, is under assessment in patients with EHE within a clinical study currently ongoing in the US. Ongoing efforts are trying to clarify the role of hormonal stimulation and inflammation in EHE pathogenesis, with the aim of possibly identifying new treatment targets.
Palliative care
Palliative care should be an integral part of the care of EHE patients.92 Early palliative care referral can be particularly useful for symptomatic patients and those with serosal effusion.9,93 Identifying clinical and psychosocial support needs for patients and their family caregivers as early as possible across the changing pattern of the disease is crucial and requires an interdisciplinary palliative care approach and research efforts.94 Systematic symptom screening and assessment should be formally present in clinical records and registries.
EHE pain pathophysiology is not well understood. Constitutional symptoms may be related to cytokine release from the tumor, which may also be responsible for increasing local nociceptive mechanisms and justifying relative opioid resistance.9,21 This observation requires specific research efforts.
Some aspects of palliative care for EHE can be challenging, such as predicting the response to analgesia.94 General palliative care principles apply and criteria for referral to specialized palliative care should be personalized92,94 (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100170). Pain management should follow state-of-the-art multimodal approaches, using nonsteroidal anti-inflammatory drugs (NSAIDs), neuropathic pain medications such as gabapentin, when indicated95 and opioids81 with consideration for opioid rotation in case of tolerance or adverse effects to escalating doses [V, B].96 Pain related to EHE can be severe, with spontaneous flares that are difficult to treat with opioids and electively sensitive to NSAIDs.93 Anesthesiologists' and pain specialists' expertise may be needed when interventional procedures are considered. Localized surgical and RT can be necessary for pain management of symptomatic lesions. New pharmacological strategies would be of great benefit.
Follow-up
There are no data to indicate the optimal length and frequency for follow-up of EHE patients treated with complete surgical resection. Currently, routine follow-up schedules differ across institutions. An MRI of the primary tumor site and a whole-body CT scan can be suggested every 6 months for the first 4 to 5 years after diagnosis. Thereafter, if no disease progression is observed, MRI and whole-body CT scans could be done yearly [V, B].
Future prospective
There are several unmet needs that require clinical studies in EHE. Given the rarity of the condition, it is almost inevitable that these trials have to be uncontrolled. The main challenge for uncontrolled studies is the unpredictable behavior of EHE, characterized by stability for many years in a significant proportion of patients. For that reason, patient registries should be encouraged, to generate external controls for uncontrolled studies. Uncontrolled studies, case series analyses and even case reports with all the caveats mentioned above all contribute to the available evidence. Global collaboration will be critical to advance our knowledge, including a better definition of QoL for EHE patients, collection of prospective data and understanding the role of LT in isolated multifocal liver EHE.
Acknowledgements
We thank the European Society for Medical Oncology (ESMO) for their invaluable support to the consensus building process, without which this effort would not have been possible. We are also deeply grateful to the Epithelioid Hemangioendothelioma (EHE) Group for inspiring and helping to spearhead this international consensus-building effort.
Funding
None declared.
Disclosure
SS: Honoraria, consultancy or advisory role: Adaptimmune, Bayer, Daiichi Sankyo, Deciphera, Epizyme, Eli Lilly, Glaxo, Immune Design, Karyopharm, MaxiVAX, Novartis, PharmaMar; Institutional financial interests: Advenchen, Amgen Dompè, Bayer, Epizyme, Eli Lilly, Daiichi Sankyo, Glaxo, Hutchison MediPharma, Karyopharm, Novartis, Pfizer, PharmaMar, SpringWorks. AMF: Institutional financial interests: Advenchen, Amgen Dompè, Bayer, Epizyme, Eli Lilly, Daiichi Sankyo, Glaxo, Hutchison MediPharma, Karyopharm, Novartis, Pfizer, PharmaMar, SpringWorks. AC: Advisory board: Angelini Holding S.p.A, Shionogi B.V., L. Molteni & C., Kyowa Kirin S.r.l.; invited speaker King Faisal Specialist Hospital & Research Center. JB: Uncompensated advisory board: Novartis; research funding (institutional) from Eli Lilly, Novartis, Roche, Samsung Bioepis Co. Ltd, Paxman Coolers Ltd, Sun Pharma. SB: Personal fees from Bayer, Deciphera, Lilly, Daiichi Sankyo, Plexxikon, Exelixis, PharmaMar, Lilly, Roche, GlaxoSmithKline (GSK); grants from Incyte, grants and personal fees from Blueprint Medicines, Novartis; research funding (institutional) Pfizer. SB: Research support from Novartis, Incyte, Blueprint Medicines; honoraria or consultation fees from Novartis, Lilly, Pfizer, PharmaMar, Bayer. JYB: Research support and honoraria from Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Roche, Bayer, Novartis, GSK, Deciphera, OSE Pharma, AstraZeneca. Member of the supervisory board: Innate Pharma. SB: Honoraria from PharmaMar. JVMGB: Research funding from TRACON Pharmaceuticals; Royalties from UptoDate and Wolters Kluwer. KB: Advisory: Bayer; expert testimony: Bayer; invited speaker: Lilly; institutional research funding: Lilly. TB: Advisory board from Bayer; honoraria: Novartis, PharmaMar, Eli Lilly. ME: Consultant for Blueprint Medicines; advisory boards for Clinigen and Bayer. AF; Honoraria: Amgen, Lilly, Roche, MSD; research funding; MSD, Roche, Clovis, AstraZeneca. BMS; Abbvie. VF: Consulting or advisory role: BMS, MSD, Novartis; speakers' bureau: BMS, MSD, Novartis, Pierre Fabre. XGdM: Advisory boards for Ipsen, EUSA Pharma, BMS, Pfizer, Roche, PharmaMar; honoraria from Lilly, Astellas Pharma, Eisai, Pfizer. HG: Reports institutional funding from Novartis, Deciphera. FG: Honoraria from Deciphera, Amgen; stock ownership from Atlanthera; royalties from Zimmer. GG: Grants/research supports: PharmaMar, Novartis, Bayer; honoraria or consultation fees: PharmaMar, Bayer, Merck, Eisai, Novartis, Glaxo. NH: Research grants from PharmaMar, Eisai, Immix BioPharma and Novartis; honoraria and travel grants from PharmaMar; and research funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, Adaptimmune Therapeutics, AROG, Bayer, Eisai, Lixte, Karyopharm, Deciphera, GSK, Novartis, Blueprint, Nektar, Forma, Amgen and Daiichi Sankyo. PH: Honoraria for consultations and speaker's fees from Roche, GSK, AstraZeneca, Pfizer, PharmaMar, Lilly, Nanobiotix, Novartis, EISAI, Nektar, Sirtex and Pekkip Oncology; research funding and educational grants from Novartis, Siemens, Boehringer, Merck, BLUMedicine and Springer; trial support to my institution by Novartis and Siemens. PH: Advisory roles for Sarcoma UK and National Leiomyosarcoma Foundation; research grants from Sarcoma UK, Desmoid Tumor Research Foundation, Children's Cancer and Leukemia Group and Cancer Research UK, funding from EHE Rare Cancer Charity (UK). HJ: Chair of the Orion Pharma Scientific Advisory Board, chair of Neutron Therapeutics Advisory Board; he has been employed by Orion Pharma, and owns stocks of Orion Pharma and Sartar Therapeutics. RJ: Advisory board Adaptimmune, Athenex, Bayer, Boehringer Ingelheim, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immune Design, Lilly, Merck, PharmaMar, SpringWorks, TRACON, UptoDate; honoraria: BMS, MSD, Novartis, Pierre Fabre, Merck, Sanofi. CJ: Travel grants from PharmaMar, Ipsen. BK: Reports advisory board Bayer, Blueprint, Boehringer Ingelheim, GSK, PharmaMar, SpringWorks; research funding (institutional) PharmaMar, SpringWorks. AK: Advisory board Otsuka; invited speaker for Daiichi Sankyo, Eisai, Eli Lilly, Novartis, Taiho. ALC: Advisory boards with Lilly, PharmaMar, Deciphera, Blueprint. AL: Reports institutional financial interest, and educational grant from Alphamed, ImplanTec, Johnson & Johnson, Medacta. JMB: Research grants from PharmaMar, Eisai, Immix BioPharma and Novartis; honoraria from PharmaMar, Lilly, Bayer, Eisai, Daichii. Travel grants from PharmaMar; advisory board: Roche, Lilly, PharmaMar; research funding for clinical studies (institutional) from PharmaMar, Eli Lilly and Company, Adaptimmune Therapeutics, AROG, Bayer, Eisai, Lixte, Karyopharm, Deciphera, GSK, Novartis, Blueprint, Nektar, Forma, Amgen, Celgene, Pfizer, BMS, and Daiichi Sankyo. OMe: Consultation fees from MSD, AstraZeneca, Takeda, Megapharm, Medison, Progenetics; supported lecture: MSD, AstraZeneca, Roche, BMS. OMi: Consultancy for Amgen, AstraZeneca, Bayer, BMS, Eli Lilly, Ipsen, Lundbeck, MSD, Novartis, Pfizer, Roche, Servier, Vifor Pharma; Speakers bureau: Eli Lilly, Roche, Servier; Stock ownership: Amplitude Surgical, Ipsen, Transgene. EP: Advisory boards for Amgen, Daiichi Sankyo, Lilly, Deciphera, EUSA Pharma, and SynOx Therapeutics; research support from BMS, Pfizer, PharmaMar, Daiichi Sankyo, Incyte; travel support from Lilly, PharmaMar and Takeda. MAP: Advisory board Lilly, Pfizer, Roche. SP: Consultant: Deciphera, Daichi Sankyo, Dova, Epizyme, MaxiVAX; Research/Grant Support: Blueprint Medicines, Hutchison MediPharma. SPN: Advisory board for Immunocore. AR: Consulting role with Lilly, Merck, Boehringer Ingelheim; research funding from CASI Pharmaceuticals, Boehringer Ingelheim, Lilly, Novartis, Deciphera, Karyopharm Therapeutics, Pfizer, Roche/Genentech, Boston Biomedical, BMS, MedImmune, Amgen, GSK, Blueprint Medicines, Merck, AbbVie, Adaptimmune. PRe: Advisory roles for Bayer, Clinigen, Roche, MSD, Deciphera, PharmaMar, Mundibiopharma, and speaker's honoraria from Lilly, PharmaMar. BPR: Reports advisory board from Deciphera. PRu: Advisory board MSD, BMS, Merck, Sanofi, Pierre Fabre, Blueprint Medicines; honoraria from BMS, MSD, Novartis, Pierre Fabre, Merck, Sanofi. MS: Travel grant from PharmaMar. SJS: Advisory boards for Lilly and GSK. WDT: Reports fees from Eli Lilly, EMD Serono, Eisai, Mundipharma, C4 Therapeutics, Daiichi Sankyo, Blueprint, GSK, Agios Pharmaceuticals, NanoCarrier, Deciphera; patent Companion Diagnostic for CDK4 inhibitors - 14/854 329 pending to MSKCC/SKI, and patent Enigma and CDH18 as companion Diagnostics for CDK4 inhibition – SKI2016-021-03 pending to MSKCC/SKI and Scientific Advisory Board - Certis Oncology Solutions, Stock Ownership Üo-Founder - Atropos Therapeutics; Stock Ownership/scientific advisory board Innova Therapeutics. WTAWDG: Reports consultancy from SpringWorks to her institute; research grant support from Novartis and Lilly to her institute; advisory for Bayer to her institute. AJW: Consulting: Daiichi Sankyo, Deciphera, Epizyme, Mundipharma; research support to my Institution: Aadi Bioscience, Daiichi Sankyo, Deciphera, Eli Lilly, Karyopharm, Plexxikon. PGC: Honoraria, consultancy or advisory role: Bayer, Deciphera, Eisai, Eli Lilly, Pfizer. Institutional financial interest: Advenchen Laboratories, Amgen Dompé, AROG Pharmaceuticals, Bayer, Blueprint Medicines, Daiichi Sankyo, Deciphera, Eisai, Eli Lilly, Epizyme, Glaxo, Hutchinson MediPharma, Karyopharm, Novartis, Pfizer, PharmaMar. APDT: Honoraria: Roche, Bayer, Pharmamar, MSD. AG: Compensations for Advisory boards from Novartis, Pfizer, Bayer, Lilly, PharmaMar, SpringWorks, Nanobiotix; honoraria from Lilly and PharmaMar; institutional research grants from PharmaMar. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Dykewicz C.A., Centers for Disease Control and Prevention (U.S.), Infectious Diseases Society of America, American Society of Blood and Marrow Transplantation Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33:139–144. doi: 10.1086/321805. (Adapted from: Gross PA, Barrett TL, Dellinger EP, et al. Purpose of quality standards for infectious diseases. Clin Infect Dis.1994;18:421) [DOI] [PubMed] [Google Scholar]
- 2.Lau K., Massad M., Pollak C. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140:1312–1318. doi: 10.1378/chest.11-0039. [DOI] [PubMed] [Google Scholar]
- 3.Stacchiotti S., Frezza A.M., Blay J.Y. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021 doi: 10.1002/cncr.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Pinieux G., Karanian M., Le Loarer F. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS One. 2021;16:e0246958. doi: 10.1371/journal.pone.0246958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbaum E., Jadeja B., Xu B. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol. 2020;33:591–602. doi: 10.1038/s41379-019-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Q., Xue J., Xu L., Shi Z., Zhou B. The clinical features of epithelioid hemangioendothelioma in a Han Chinese population: a retrospective analysis. Medicine (Baltimore) 2017;96(26):e7345. doi: 10.1097/MD.0000000000007345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flucke U., Vogels R.J., de Saint Aubain Somerhausen N. Epithelioid hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. doi: 10.1186/1746-1596-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavhan G.B., Siddiqui I., Ingley K.M., Gupta A.A. Rare malignant liver tumors in children. Pediatr Radiol. 2019;49:1404–1421. doi: 10.1007/s00247-019-04402-8. [DOI] [PubMed] [Google Scholar]
- 9.Shiba S., Imaoka H., Shioji K. Clinical characteristics of Japanese patients with epithelioid hemangioendothelioma: a multicenter retrospective study. BMC Cancer. 2018;18:993. doi: 10.1186/s12885-018-4934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papke D.J., Jr., Hornick J.L. What is new in endothelial neoplasia? Virchows Arch. 2020;476:17–28. doi: 10.1007/s00428-019-02651-4. [DOI] [PubMed] [Google Scholar]
- 11.Wu X., Li B., Zheng C., Hong T., He X. Clinical characteristics of epithelioid hemangioendothelioma: a single-center retrospective study. Eur J Med Res. 2019;24(1):16. doi: 10.1186/s40001-019-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Errani C., Sung Y.S., Zhang L., Healey J.H., Antonescu C.R. Monoclonality of multifocal epithelioid hemangioendothelioma of the liver by analysis of WWTR1-CAMTA1 breakpoints. Cancer Genet. 2012;205:12–17. doi: 10.1016/j.cancergen.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deyrup A.T., Tighiouart M., Montag A.G., Weiss S.W. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol. 2008;32:924–927. doi: 10.1097/pas.0b013e31815bf8e6. [DOI] [PubMed] [Google Scholar]
- 14.Shibayama T., Makise N., Motoi T. Clinicopathologic characterization of epithelioid hemangioendothelioma in a series of 62 cases: a proposal of risk stratification and identification of a synaptophysin-positive aggressive subset. Am J Surg Pathol. 2021;45:616–626. doi: 10.1097/PAS.0000000000001660. [DOI] [PubMed] [Google Scholar]
- 15.Antonescu C.R., Le Loarer F., Mosquera J.M. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer. 2013;52:775–784. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mentzel T., Beham A., Calonje E., Katenkamp D., Fletcher C.D. Epithelioid hemangioendothelioma of skin and soft tissues: clinicopathologic and immunohistochemical study of 30 cases. Am J Surg Pathol. 1997;21:363–374. doi: 10.1097/00000478-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Bagan P., Hassan M., Le Pimpec Barthes F. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg. 2006;82:2010–2013. doi: 10.1016/j.athoracsur.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 18.Woo J.H., Kim T.J., Lee K.S., Kim T.S., Kim B.T. Epithelioid hemangioendothelioma in the thorax: clinicopathologic, CT, PET, and prognostic features. Medicine (Baltimore) 2016;95(30):e4348. doi: 10.1097/MD.0000000000004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stacchiotti S., Simeone N., Lo Vullo S. Activity of sirolimus in patients with progressive epithelioid hemangioendothelioma: a case-series analysis within the Italian rare cancer network. Cancer. 2021;127:569–576. doi: 10.1002/cncr.33247. [DOI] [PubMed] [Google Scholar]
- 20.Amin R.M., Hiroshima K., Kokubo T. Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and a case report. Respirology. 2006;11:818–825. doi: 10.1111/j.1440-1843.2006.00923.x. [DOI] [PubMed] [Google Scholar]
- 21.Frezza A.M., Napolitano A., Miceli R. Clinical prognostic factors in advanced epithelioid haemangioendothelioma: a retrospective series within the Italian Rare Cancers Network. ESMO open. 2021;6:100083. doi: 10.1016/j.esmoop.2021.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yazigi A., Lecointe-Artzner E., Cesne A.L., Ray-Coquard I., Blay J.Y. Pregnancy in women with metastatic sarcomas. Oncologist. 2020;25:e2010–e2012. doi: 10.1002/onco.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blay J.Y., Soibinet P., Penel N. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol. 2017;28:2852–2859. doi: 10.1093/annonc/mdx484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Errani C., Zhang L., Sung Y.S. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644–653. doi: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suurmeijer A.J.H., Dickson B.C., Swanson D. Variant WWTR1 gene fusions in epithelioid hemangioendothelioma-A genetic subset associated with cardiac involvement. Genes Chromosomes Cancer. 2020;59:389–395. doi: 10.1002/gcc.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folpe A.L., Chand E.M., Goldblum J.R., Weiss S.W. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol. 2001;25:1061–1066. doi: 10.1097/00000478-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Rossi S., Orvieto E., Furlanetto A., Laurino L., Ninfo V., Dei Tos A.P. Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody. Mod Pathol. 2004;17:547–552. doi: 10.1038/modpathol.3800065. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen M., Fetsch J.F. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Hum Pathol. 2000;31:1062–1067. doi: 10.1053/hupa.2000.9843. [DOI] [PubMed] [Google Scholar]
- 29.Doyle L.A., Fletcher C.D., Hornick J.L. Nuclear expression of CAMTA1 distinguishes epithelioid hemangioendothelioma from histologic mimics. Am J Surg Pathol. 2016;40:94–102. doi: 10.1097/PAS.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 30.Tanas M.R., Sboner A., Oliveira A.M. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3(98):98ra82. doi: 10.1126/scitranslmed.3002409. [DOI] [PubMed] [Google Scholar]
- 31.Harvey K.F., Zhang X., Thomas D.M. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 32.Tanas M.R., Ma S., Jadaan F.O. Mechanism of action of a WWTR1(TAZ)-CAMTA1 fusion oncoprotein. Oncogene. 2016;35:929–938. doi: 10.1038/onc.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hornick J.L., Dal Cin P., Fletcher C.D. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542–550. doi: 10.1097/PAS.0b013e3181882c54. [DOI] [PubMed] [Google Scholar]
- 34.Righi A., Sbaraglia M., Gambarotti M. Primary vascular tumors of bone: a monoinstitutional morphologic and molecular analysis of 427 cases with emphasis on epithelioid variants. Am J Surg Pathol. 2020;44:1192–1203. doi: 10.1097/PAS.0000000000001487. [DOI] [PubMed] [Google Scholar]
- 35.Hornick J.L., Fletcher C.D.M. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190–201. doi: 10.1097/PAS.0b013e3181ff0901. [DOI] [PubMed] [Google Scholar]
- 36.Hung Y.P., Fletcher C.D.M., Hornick J.L. FOSB is a useful diagnostic marker for pseudomyogenic hemangioendothelioma. Am J Surg Pathol. 2017;41:596–606. doi: 10.1097/PAS.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 37.Epelboym Y., Engelkemier D.R., Thomas-Chausse F. Imaging findings in epithelioid hemangioendothelioma. Clin Imaging. 2019;58:59–65. doi: 10.1016/j.clinimag.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Paolantonio P., Laghi A., Vanzulli A. MRI of hepatic epithelioid hemangioendothelioma (HEH) J Magn Reson Imaging. 2014;40:552–558. doi: 10.1002/jmri.24391. [DOI] [PubMed] [Google Scholar]
- 39.Kim E.H., Rha S.E., Lee Y.J., Yoo IeR., Jung E.S., Byun J.Y. CT and MR imaging findings of hepatic epithelioid hemangioendotheliomas: emphasis on single nodular type. Abdom Imaging. 2015;40:500–509. doi: 10.1007/s00261-014-0229-3. [DOI] [PubMed] [Google Scholar]
- 40.Errani C., Vanel D., Gambarotti M., Alberghini M., Picci P., Faldini C. Vascular bone tumors: a proposal of a classification based on clinicopathological, radiographic and genetic features. Skeletal Radiol. 2012;41:1495–1507. doi: 10.1007/s00256-012-1510-6. [DOI] [PubMed] [Google Scholar]
- 41.Wang W., Liu G., Hu P. Imaging characteristics and prognostic values of hepatic epithelioid hemangioendothelioma on (18)F-FDG PET/CT. Clin Exp Med. 2020;20:557–567. doi: 10.1007/s10238-020-00653-0. [DOI] [PubMed] [Google Scholar]
- 42.Lin E., Agoff N. Recurrent hepatic epithelioid hemangioendothelioma: detection by FDG PET/CT. Clin Nucl Med. 2007;32:949–951. doi: 10.1097/RLU.0b013e31815969bc. [DOI] [PubMed] [Google Scholar]
- 43.Dong A., Dong H., Wang Y., Gong J., Lu J., Zuo C. MRI and FDG PET/CT findings of hepatic epithelioid hemangioendothelioma. Clin Nucl Med. 2013;38:e66–e73. doi: 10.1097/RLU.0b013e318266ceca. [DOI] [PubMed] [Google Scholar]
- 44.Mehrabi A., Kashfi A., Fonouni H. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108–2121. doi: 10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 45.Miller W.J., Dodd G.D., Federle M.P., Baron R.L. Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol. 1992;159:53–57. doi: 10.2214/ajr.159.1.1302463. [DOI] [PubMed] [Google Scholar]
- 46.Ganeshan D., Pickhardt P.J., Morani A.C. Hepatic hemangioendothelioma: CT, MR, and FDG-PET-CT in 67 patients—a bi-institutional comprehensive cancer center review. Eur Radiol. 2020;30:2435–2442. doi: 10.1007/s00330-019-06637-3. [DOI] [PubMed] [Google Scholar]
- 47.Lyburn I.D., Torreggiani W.C., Harris A.C. Hepatic epithelioid hemangioendothelioma: sonographic, CT, and MR imaging appearances. AJR Am J Roentgenol. 2003;180:1359–1364. doi: 10.2214/ajr.180.5.1801359. [DOI] [PubMed] [Google Scholar]
- 48.Alomari A.I. The lollipop sign: a new cross-sectional sign of hepatic epithelioid hemangioendothelioma. Eur J Radiol. 2006;59:460–464. doi: 10.1016/j.ejrad.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Kim E.Y., Kim T.S., Han J., Choi J.Y., Kwon O.J., Kim J. Thoracic epithelioid hemangioendothelioma: imaging and pathologic features. Acta Radiol. 2011;52:161–166. doi: 10.1258/ar.2010.100292. [DOI] [PubMed] [Google Scholar]
- 50.Bahrami A., Allen T.C., Cagle P.T. Pulmonary epithelioid hemangioendothelioma mimicking mesothelioma. Pathol Int. 2008;58:730–734. doi: 10.1111/j.1440-1827.2008.02301.x. [DOI] [PubMed] [Google Scholar]
- 51.Ignacio E.A., Palmer K.M., Mathur S.C., Schwartz A.M., Olan W.J. Epithelioid hemangioendothelioma of the lower extremity. Radiographics. 1999;19:531–537. doi: 10.1148/radiographics.19.2.g99mr11531. [DOI] [PubMed] [Google Scholar]
- 52.Casali P.G., Abecassis N., Aro H.T. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv51–iv67. doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 53.Tong D., Constantinidou A., Engelmann B. The role of local therapy in multi-focal epithelioid haemangioendothelioma. Anticancer Res. 2019;39:4891–4896. doi: 10.21873/anticanres.13675. [DOI] [PubMed] [Google Scholar]
- 54.Lazarides A.L., Kerr D.L., Nussbaum D.P. Soft tissue sarcoma of the extremities: what is the value of treating at high-volume centers? Clin Orthop Relat Res. 2019;477:718–727. doi: 10.1097/01.blo.0000533623.60399.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutierrez J.C., Perez E.A., Moffat F.L., Livingstone A.S., Franceschi D., Koniaris L.G. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg. 2007;245:952–958. doi: 10.1097/01.sla.0000250438.04393.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vos M., Blaauwgeers H.G.T., Ho V.K.Y., Dutch Sarcoma Study Group (DSSG) Increased survival of non low-grade and deep-seated soft tissue sarcoma after surgical management in high-volume hospitals: a nationwide study from the Netherlands. Eur J Cancer. 2019;110:98–106. doi: 10.1016/j.ejca.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Martin-Tellez K.S., van Houdt W.J., van Coevorden F., Colombo C., Fiore M. Isolated limb perfusion for soft tissue sarcoma: current practices and future directions. A survey of experts and a review of literature. Cancer Treat Rev. 2020;88:102058. doi: 10.1016/j.ctrv.2020.102058. [DOI] [PubMed] [Google Scholar]
- 58.Kitaichi M., Nagai S., Nishimura K. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J. 1998;12:89–96. doi: 10.1183/09031936.98.12010089. [DOI] [PubMed] [Google Scholar]
- 59.Brahmbhatt M., Prenner S., Bittermann T. Liver transplantation for hepatic epithelioid hemangioendothelioma is facilitated by exception points with acceptable long-term outcomes. Transplantation. 2020;104:1187–1192. doi: 10.1097/TP.0000000000002982. [DOI] [PubMed] [Google Scholar]
- 60.Lai Q., Feys E., Karam V., European Liver Intestine Transplant Association (ELITA) Hepatic epithelioid hemangioendothelioma and adult liver transplantation: proposal for a prognostic score based on the analysis of the ELTR-ELITA registry. Transplantation. 2017;101:555–564. doi: 10.1097/TP.0000000000001603. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez J.A., Becker N.S., O'Mahony C.A., Goss J.A., Aloia T.A. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg. 2008;12:110–116. doi: 10.1007/s11605-007-0247-3. [DOI] [PubMed] [Google Scholar]
- 62.Lerut J.P., Orlando G., Adam R. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246:949–957. doi: 10.1097/SLA.0b013e31815c2a70. [DOI] [PubMed] [Google Scholar]
- 63.Konstantinidis I.T., Nota C., Jutric Z. Primary liver sarcomas in the modern era: resection or transplantation? J Surg Oncol. 2018;117:886–891. doi: 10.1002/jso.24979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss S.W., Enzinger F.M. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 65.Sardaro A., Bardoscia L., Petruzzelli M.F., Portaluri M. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev. 2014;8:259. doi: 10.4081/oncol.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sybert D.R., Steffee A.D., Keppler L., Biscup R.S., Enker P. Seven-year follow-up of vertebral excision and reconstruction for malignant hemangioendothelioma of bone. Spine (Phila Pa 1976) 1995;20:841–844. doi: 10.1097/00007632-199504000-00020. [DOI] [PubMed] [Google Scholar]
- 67.Albakr A., Schell M., Drew B., Cenic A. Epithelioid hemangioendothelioma of the spine: case report and review of the literature. J Spine Surg. 2017;3:250–259. doi: 10.21037/jss.2017.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yim K.-L., Sumathi V.P., Spooner D. Radiotherapy as an effective primary treatment for epithelioid haemangioendothelioma of the cervical spine. Anticancer Res. 2012;32:4597–4600. [PubMed] [Google Scholar]
- 69.Themistocleous G.S., Papagelopoulos P.J., Petraki K.D., Stilianessi E.V., Partsinevelos A.A., Sapkas G.S. A 23-year-old woman with complete paraplegia and anesthesia below the T8 level. Clin Orthop Relat Res. 2005;430:258–265. doi: 10.1097/01.blo.0000150455.62479.37. [DOI] [PubMed] [Google Scholar]
- 70.Hoyer M., Swaminath A., Bydder S. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82:1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 71.Kamarajah S.K., Robinson D., Littler P., White S.A. Incidental hepatic epithelioid haemangioendothelioma the role of ablative therapy in borderline patients. J Surg Case Rep. 2018;2018(8):rjy223. doi: 10.1093/jscr/rjy223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shiina S., Tateishi R., Arano T. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577. doi: 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cardinal J., de Vera M.E., Marsh J.W. Treatment of hepatic epithelioid hemangioendothelioma. Arch Surg. 2009;144:1035–1039. doi: 10.1001/archsurg.2009.121. [DOI] [PubMed] [Google Scholar]
- 74.Kou K., Chen Y.G., Zhou J.P. Hepatic epithelioid hemangioendothelioma: update on diagnosis and therapy. World J Clin Cases. 2020;8:3978–3987. doi: 10.12998/wjcc.v8.i18.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weitz J., Klimstra D.S., Cymes K. Management of primary liver sarcomas. Cancer. 2007;109:1391–1396. doi: 10.1002/cncr.22530. [DOI] [PubMed] [Google Scholar]
- 76.Thomas R.M., Aloia T.A., Truty M.J. Treatment sequencing strategy for hepatic epithelioid haemangioendothelioma. HPB (Oxford) 2014;16:677–685. doi: 10.1111/hpb.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bostancı E.B., Karaman K., Turhan N. Selective internal radiotherapy for hepatic epithelioid hemangioendothelioma. Turk J Gastroenterol. 2014;25(suppl 1):252–253. doi: 10.5152/tjg.2014.4286. [DOI] [PubMed] [Google Scholar]
- 78.Frezza A.M., Ravi V., Lo Vullo S. Systemic therapies in advanced epithelioid haemangioendothelioma: a retrospective international case series from the World Sarcoma Network and a review of literature. Cancer Med. 2021;10:2645–2659. doi: 10.1002/cam4.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moon S.B., Kwon H.J., Park K.W., Yun W.J., Jung S.E. Clinical experience with infantile hepatic hemangioendothelioma. World J Surg. 2009;33:597–602. doi: 10.1007/s00268-008-9882-4. [DOI] [PubMed] [Google Scholar]
- 80.Radzikowska E., Szczepulska-Wojcik E., Chabowski M., Oniszh K., Langfort R., Roszkowski K. Pulmonary epithelioid haemangioendothelioma, interferon 2-alpha treatment, case report. Pneumonol Alergol Pol. 2008;76:281–285. [PubMed] [Google Scholar]
- 81.Soape M.P., Verma R., Payne J.D., Wachtel M., Hardwicke F., Cobos E. Treatment of hepatic epithelioid hemangioendothelioma: finding uses for thalidomide in a new era of medicine. Case Rep Gastrointest Med. 2015;2015:326795. doi: 10.1155/2015/326795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saada E., Saint Paul M.C., Gugenheim J., Follana P., François E. Metastatic hepatic epithelioid hemangio-endothelioma: long-term response to sunitinib malate. Oncol Res Treat. 2014;37:124–126. doi: 10.1159/000360208. [DOI] [PubMed] [Google Scholar]
- 83.Kollár A., Jones R.L., Stacchiotti S. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017;56:88–92. doi: 10.1080/0284186X.2016.1234068. [DOI] [PubMed] [Google Scholar]
- 84.Chevreau C., Le Cesne A., Ray-Coquard I. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO) Cancer. 2013;119:2639–2644. doi: 10.1002/cncr.28109. [DOI] [PubMed] [Google Scholar]
- 85.Agulnik M., Yarber J.L., Okuno S.H. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257–263. doi: 10.1093/annonc/mds237. [DOI] [PubMed] [Google Scholar]
- 86.Yousaf N., Maruzzo M., Judson I., Judson, Fisher C., Benson C. Systemic treatment options for epithelioid haemangioendothelioma: the Royal Marsden Hospital experience. Anticancer Res. 2015;35:473–480. [PubMed] [Google Scholar]
- 87.Zheng Z., Wang H., Jiang H., Chen E., Zhang J., Xie X. Apatinib for the treatment of pulmonary epithelioid hemangioendothelioma: a case report and literature review. Medicine (Baltimore) 2017;96:e8507. doi: 10.1097/MD.0000000000008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Semenisty V., Naroditsky I., Keidar Z., Bar-Sela G. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma, a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer. 2015;15:402. doi: 10.1186/s12885-015-1395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Engel E.R., Cournoyer E., Adams D.M., Stapleton S. A retrospective review of the use of sirolimus for pediatric patients with epithelioid hemangioendothelioma. J Pediatr Hematol Oncol. 2020;42:e826–e829. doi: 10.1097/MPH.0000000000001643. [DOI] [PubMed] [Google Scholar]
- 90.Riou S., Morelon E., Guibaud L., Chotel F., Chotel F., Marec-Berard P. Efficacy of rapamycin for refractory hemangioendotheliomas in Maffucci's syndrome. J Clin Oncol. 2012;30:e213–e215. doi: 10.1200/JCO.2012.41.7287. [DOI] [PubMed] [Google Scholar]
- 91.Cohen E.E., Wu K., Hartford C. Phase I studies of sirolimus alone or in combination with pharmacokinetic modulators in advanced cancer patients. Clin Cancer Res. 2012;18:4785–4793. doi: 10.1158/1078-0432.CCR-12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferrell B.R., Temel J.S., Temin S. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice guideline update. J Clin Oncol. 2017;35:96–112. doi: 10.1200/JCO.2016.70.1474. [DOI] [PubMed] [Google Scholar]
- 93.Cronin P., Arenberg D. Pulmonary epithelioid hemangioendothelioma: an unusual case and a review of the literature. Chest. 2004;125:789–793. doi: 10.1378/chest.125.2.789. [DOI] [PubMed] [Google Scholar]
- 94.Zimmermann C., Ryan S., Hannon B. Team-based outpatient early palliative care: a complex cancer intervention. BMJ Support Palliat Care. 2019 doi: 10.1136/bmjspcare-2019-001903. [DOI] [PubMed] [Google Scholar]
- 95.Wei S.C., Yang P.M., Chen C.H., Chu J.S., Chen D.S. Case report: successful palliative treatment with intraperitoneal OK-432 injection for epithelioid haemangioendothelioma presenting with intractable ascites. J Gastroenterol Hepatol. 1997;12:39–43. doi: 10.1111/j.1440-1746.1997.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 96.Caraceni A., Hanks G., Kaasa S. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.