Abstract
Poultry house dust is composed of fine particles which likely originate from a diverse range of materials such as feed, litter, excreta, and feathers. Little is known about the contribution of these sources to broiler house airborne dust so the present study was designed to identify the relative contributions of these sources. Samples of feed, excreta, feather, and bedding, known mixtures of these and settled dust from 28 broiler chicken flocks were tested for the concentration of 18 chemical elements. A chemometrics approach (the application of multivariate statistical techniques to chemical analysis data) was used to identify the primary source material in broiler chicken house dust samples. Scanning electron microscopy (SEM) was also used to analyze dust sample particulates based on examination of source materials. Excreta was found to be the main component of broiler chicken house dust, both by SEM and chemometric analysis. SEM of experimental flock dust between 7 and 35 days of age (d) revealed that the contribution of excreta to dust increased with age from 60% at 7 d to 95% at 28 d (P < 0.001). The proportion of bedding and feed in dust declined with age while the contribution of feather material remained low throughout. This study demonstrates that excreta provides the bulk of the material in poultry dust samples with bedding material, feed and feather material providing lower proportions. The relative contributions of these materials to dust varies with age of birds at dust collection. Additional research is required to determine the health and diagnostic implications of this variation.
Key words: poultry dust, source, chemometric, chemistry, scanning electron microscopy
INTRODUCTION
Dust found in poultry housing is a complex substance originating from nonorganic and organic material inputs and the biological shedding of birds including floor bedding, feed, excreta, feather, exfoliated epithelium (dander), and microorganisms (Feddes et al., 1992; Aarnink et al., 1999; Just et al., 2009). Natural forces such as desiccation and mechanical processes including human and bird movement result in the breakdown and drying of source material which is then dispersed as airborne dust (WHO, 2020). The dust particles circulating inside a poultry house vary in size and shape (Shen et al., 2018; Li et al., 2021), are easily disturbed, and can be suspended in the air or settle on building surfaces depending on gravitational, hydrophobicity and electrostatic forces. Certain particles (<1 µm) may stay airborne (WHO, 2020). Poultry dust is likely to differ from atmospheric dust or dust from other livestock due to its source materials (Cambra-López et al., 2011). Approximately 85% of broiler house settled dust mass has been found to consist of organic material (Hartung and Saleh, 2007) while the inorganic portion of poultry dust has been reported to consist of mainly calcium, sodium, iron, zinc, magnesium, and aluminum (Nakaue et al., 1981). Deposited dust of poultry origin has also been reported to be comprised of 92% dry matter, of which 60% was crude protein, 9% crude fat, and 4% cellulose (Koon, 1963).
Poultry house dust is a matter of occupational and environmental concern and an important topic of interdisciplinary research. Exposure to poultry dust and microorganisms in it may impair the health of both poultry and farmworkers (Cambra-López et al., 2010; Viegas et al., 2013; Wei et al., 2018). The health effects of dust and its emission to the environment are closely related to morphology and source of dust particles (Cambra-López et al., 2010). Poultry dust is also important as a reservoir and transmission mechanism for poultry diseases such as Marek's disease (Carrozza et al., 1973). Furthermore, several studies have demonstrated the potential of poultry dust as a population level sample material for tracking pathogen incidence and assessing vaccination efficacy (Walkden-Brown et al., 2013; Ahaduzzaman et al., 2019; Nguyen et al., 2019). Given these aspects, it is important to understand the composition of poultry dust and how it changes during the production cycle. While a study characterizing airborne dust particles in turkey barns found that excreta is the main constituent (Feddes et al., 1992), there has been little research undertaken on characterization of dust components in broiler chicken housing. This study was therefore designed to determine the ratio of different originating materials in dust samples using chemical and physical analysis.
Chemical analysis such as inductively coupled plasma optical emission spectrometry (ICP-OES) and combustion analysis can be used to estimate the elemental composition of organic compounds (Hunt and Ohno, 2007; Odenigbo et al., 2014) and offer a viable option to investigate and identify the components of a complex organic entity like dust. Both ICP-OES and combustion analyses are economical, less labor intensive and safer than conventional acid digestion methods and can provide accurate estimation of elemental composition (Wright and Bailey, 2001). In contrast to ICP-OES and combustion analysis, the non-destructive X-ray diffraction method (XRD) allows direct identification of crystalline chemical compounds in environmental samples and has been utilized for analysis of residential household and atmospheric dust samples (Queralt et al., 2001; Kumar and Rajkumar, 2014; Li et al., 2019). Scanning electron microscopy (SEM) imagery has also proven to be useful in enabling a compositional description of morphological microstructure of dust and agricultural aerosols, including chemical microanalysis or Energy Dispersive X-Ray Spectroscopy (EDS) (Feddes et al., 1992; Hiranumaa et al., 2008). These analytical methods combined with chemometric classification approaches based on similarities between test samples have been used to analyze rabbit house dust (Cambra-López and Torres, 2008), street dust (Gunawardana et al., 2012; Azid et al., 2014; Azimi et al., 2018), soil (Minasny and McBratney, 2008; Sila et al., 2016) and household dust (Meglen, 1991). In the present study, we applied these approaches to determine the source components of poultry dust and test the following propositions: 1) that the individual dust components (feed, excreta, feather, and bedding) will exhibit distinct chemical profiles, 2) this chemical profile will be useful for predicting their contribution to the composition of poultry dust samples, 3) SEM analysis of dust samples will support the chemical profile prediction, and 4) the composition of poultry dust will change during the production cycle resulting in an increased excreta content over time.
MATERIALS AND METHODS
The approach taken in this study was to analyze samples of the likely components of poultry house dust (feed, excreta, feather material, and various bedding materials referred to as individual component samples) and mixtures of known proportions of these samples (referred as mixture samples) and to use these individual component samples or mixture samples as training sets to predict the relative contribution of source components in commercial broiler poultry dust samples of unknown composition using chemometrics and SEM image analysis. Details are provided below.
Sampling and Sample Processing
Three groups of samples were used as described below.
Individual Component Samples
Four types of individual component samples assumed to be the key source materials for poultry house dust (feed, excreta, feather and bedding) were collected. Six broiler pellet feed samples (starter feed n = 2, grower feed n = 2, and finisher feed n = 2) from two feed companies were included. Two pooled types of excreta samples were used, either collected from the terminal large intestine of 14-day-old euthanized broiler birds or freshly voided by 35-day-old broiler birds. Two pooled feather samples were collected from euthanized broiler birds at 14 and 32 days of age. Bedding material samples comprised 2 samples of fresh pine wood shavings and 2 samples of fresh rice hulls both of which are common bedding materials in Australia where new bedding provided for every batch of chickens is the norm. Feather and excreta samples were collected from trials with approval from the Animal Ethics Committee of the University of New England (AEC19-051 and AEC19-102). All feed, excreta, feather and bedding samples were dried at 70°C for 48 h using a fan-forced hot air oven (C 7995, Labmaster, Australia) and ground to pass a 0.5-mm sieve at 18,000 RPM using an ultra-centrifugal grinder (ZM 200, Retsch, Germany). Samples were then stored in airtight PVC containers until use.
Mixture Samples
Mixtures were made in the laboratory by mixing known proportions (by weight) of dried and ground feed, excreta, feathers, and rice hull bedding. These samples were constructed to test if the components for these samples could be predicted by chemical analysis. Eight known different mixtures were made with differing proportions of bedding (rice hulls): feed (finisher): excreta (35-day-old birds): feather (late-stage) as follows 50: 30: 15: 05 (mix 1), 50: 15: 30: 05 (mix 2), 30: 15: 50: 05 (mix 3), 30: 50: 15: 05 (mix 4), 50: 15: 25: 10 (mix 5), 50: 30: 19: 01 (mix 6), 60: 10: 29: 01 (mix 7), and 10: 60: 29: 01 (mix 8).
Dust Samples From Broiler Chicken Flocks
Two sets of dust samples were collected: 1) Commercial flock dust: Settled dust samples from 28 broiler flocks (1 sample/flock) from all 6 Australian states were collected using the settle plate method at the end of batch (35−49 d) in the study of Groves et al. (2008). The plates were placed 1.5 meters above the floor for 7 d in order to collect settled dust. Flocks comprised commercial meat chickens of the Ross or Cobb breed in enclosed poultry houses containing between 16,000 and 40,000 chickens with natural or fan forced ventilation systems and a range of bedding types. Of the 28 samples, n = 7 dust samples were taken from flocks with wood shavings bedding material, n = 7 dust samples from flocks with sawdust bedding material, n = 7 dust samples from flocks with rice hull bedding material, and n = 7 dust samples from flocks with chopped straw bedding material. All samples were dried at 70°C for 48 h using a fan-forced hot air oven and stored in airtight PVC containers. This sample set was used to test if the key components of dust could be predicted using both chemometrics and SEM image analysis. 2) Experimental flock dust: Weekly dust samples (3 samples/wk) from an experimental broiler flock (Ahaduzzaman et al., 2020) were collected for a duration of 5 weeks using the settle plate method. This sample set was used to predict the changes in components of dust over time using SEM image analysis. Chemical analysis with this sample set was not possible due to deposition of insufficient amount of dust (~0.1 g/settle plate/sampling day).
Chemical Analysis
Individual component, mixture and commercial broiler chicken flock samples were analyzed using combustion, ICP-OES, XRD and SEM-EDS techniques. Briefly, a sample of 0.15 g was used for combustion analysis (Dumas method) using a carbon and nitrogen analyzer (TruSpec Series, LECO Corporation, USA) to measure the total concentration of carbon (C) and nitrogen (N) in the sample. For ICP-OES, 0.5 g of sample (0.2 g in case of feather) was pre-digested in 4 mL of nitric acid (70%) and 1 mL of deionized water in a Teflon tube for 1 h. The sample was then digested using an automated four step ultra-microwave method (Milestone UltraWAVE, Italy) for 40 min (10 min/step). The minimum operating temperature for all four steps was 70°C, while the maximum operating temperature was 110°C and 180°C for steps 1 and 2,respectively, and 240°C for both steps 3 and 4. The pressure was 110 bars for all four steps with a power output range of 800 to 1,500 W. The quantitative elemental analysis was performed using an ICP-OES system (Agilent, Australia) measuring the total concentration of the following 16 chemical elements: aluminum (Al), boron (B), calcium (Ca), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), molybdenum (Mo), sodium (Na), phosphorus (P), sulphur (S), selenium (Se) and zinc (Zn). The XRD analysis was conducted using an 600 watt X-ray diffractometer (Aeris-Malvern Panalytical, UK) with Cu Kα radiation operated at a voltage of 40 kV and a current of 15 mA and following setup: slit size 1˚, beam knife low, start angle 5˚ (2θ), end angle 60˚, step size 0.0027, time per step 80 s, scan speed 0.009. A total 20246 number of steps were counted. Processing of the spectra was done with XRD data analysis software HighScore V.4.9 utilizing PDF4+ and the PAN-ICSD databases. In addition to above mentioned methods, chemical analysis and imagery was also performed using the SEM-EDS function integrated with the SEM (JEOL JSM-6010LA, USA) to enable qualitative and quantitative measurement, elemental distribution mapping, and chemical state analysis. EDS was performed in conjunction with the analysis of the SEM image.
Image Analysis
Prior to analysis, approximately 5 mg of ground (except dust) sample material were mounted on aluminum stubs and coated with gold using a gold coater (JEOL Smart Coater, USA). High-resolution images of the individual sample then were captured at 100X, 200X and 400X magnifications and in different fields of view to allow characterization of the morphology of the particles present in the sample. Morphology of individual components was determined based on observed variation in the individual component samples and reference to published images from turkey farm dust (Feddes et al., 1992). Quantification of the prevalence of similar particles in the image was performed manually after uploading the image to ImageJ software (NIH, USA) (refer to Supplementary Figure 1). The findings are presented as proportion particles in number (%) in the sample classified as feed particles (%), excreta particles (%) feather particles (%) and bedding particles (%).
Statistical Analysis
JMP-14 software (SAS Institute, Cary, NC) was used for statistical analyses apart from the Linear Discriminant Analysis (LDA) which was performed using R software (R Core Team, 2019). The following 18 chemical elements derived from combustion and ICP-OES analyses were included in univariate and multivariate statistical analyses: C, N, Al, B, Ca, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, P, S, Se and Zn. All data were tested for normal distribution prior to statistical analysis.
In the univariate analysis, one-way analysis of variance was used to test differences in mean concentrations of each of the chemical elements by sample type (feed, excreta, feather, wood shaving, and rice hull bedding) for the component samples and by bedding material type (wood shaving, sawdust, rice hulls and straw) for the dust samples from commercial broiler flocks (Yij = μ+xi+ϵij, where Yij represents the j-th observation (j = 1,2,…,ni) on the i-th sample type (i = 1,2,..,5 level of x). The significance of differences between sample type means was determined by using Tukey's honestly significant difference test and results are presented as least squares means ± standard error of means (LSM±SEM). Differences between statistical parameters were considered to be significant if P-values were <0.05.
For the multivariate analyses, Principal component analysis (PCA) was used to evaluate the degree of association between the different sample types, based on the full suite of 18 elements measured. Prior to PCA analysis, the PCA scree plot was constructed to determine the contributions of each principal component, quantified by Eigenvalue with the most significant contributions coming from the first two components. The LDA method was used to predict the major component of dust samples (feed, excreta, feather, wood shavings or rice hull bedding) in the commercial dust samples. An initial LDA algorithm, based on linear combinations of predictor variables (18 separate chemical elements), was determined for 2 different training sets of substrate type: 1) individual sample components and 2) mixed samples of known component proportions. The probability of belonging to the different groups within those 2 substrate types was then computed for each individual feed or dust sample. The first training set comprised of chemical data of the individual component samples (e.g., excreta, feed, feather, bedding, n = 13). One of the starter feeder samples with zero Se content was removed from the training set to improve prediction accuracy. In an additional analysis, a second training set comprising the 8 mixed samples of known content of the initial components were used as a training set to determine which known mixtures the dust samples from commercial flocks most closely resembled.
RESULTS
Chemical Profile of Individual Component Samples
The means (±SE) of chemical element variables for the individual component samples are shown in Table 1. Individual components (feed, excreta etc.) differed statistically for 15 out of the 18 chemical elements, these being C, N, Al, Ca, Co, Cr, Fe, K, Mg, Mn, Mo, Na, P, S, and Zn (P < 0.05). No statistical difference was evident between the different types of samples for B, Cu and Se. The feed samples had a reasonable level of almost all the elements measured and shared high values for Na, Mo, Cu and Zn with excreta. Excreta samples had high concentrations of most of the elements (Al, Ca, Co, Fe, K, Mg, Mn, Mo, Na, P, and Zn) with clearest differentiation from other samples by higher levels of Co, Al, Ca, P, and Mg. Feather samples were distinguished by very high levels of N and S reflecting the amino acid composition and disulphide bonds of beta keratin, the main constituent of feathers. Wood shavings were distinguished by high C and low content of N and most minerals. Rice hulls shared the low N content of wood shavings but had a much lower content of C and higher mineral content, being distinguished from all other sample materials by having the highest levels of Cr and Fe and sharing the highest Mn content with excreta. There was wide variation between the sample types for most minerals with CV >100% for 13 of the minerals. The most extreme variation was for Co (CV 283%) due the low level in all samples other than excreta, while the least variation was in C content (CV 10%) reflecting the organic nature of all the source materials (Table 1).
Table 1.
Chemical profile (LSM±SEM) of individual component samples representing substrates contributing to poultry dust as determined by combustion and ICP-OES analyses.
| Chemical analysis (LSM±SEM) of samples of different potential components of dust |
|||||||
|---|---|---|---|---|---|---|---|
| Element | Feed (n = 6) | Excreta (n = 2) | Feather (n = 2) | Wood shavings (n = 2) | Rice hulls (n = 2) | P-value | Coefficient of Variation (CV %) |
| C (%) | 43.3 ± 0.5b | 36.2 ± 0.8cd | 39.9 ± 0.8c | 47.0 ± 0.8a | 34.9 ± 0.8d | <0.001 | 10 |
| N (%) | 3.7 ± 0.1b | 4.0 ± 0.2b | 13.3 ± 0.2a | 0.1 ± 0.2c | 0.4 ± 0.2c | <0.001 | 102 |
| Al (µg/g) | 85 ± 76b | 1024 ± 131a | 57 ± 131b | 41 ± 131b | 125 ± 131b | 0.002 | 176 |
| B (µg/g) | 15 ± 5a | 33 ± 9a | 2 ± 9a | 3 ± 9a | 15.3 ± 9a | 0.22 | 104 |
| Ca (%) | 1.4 ± 0.3b | 4.9 ± 0.6a | 0.1 ± 0.6b | 0.1 ± 0.0b | 0.1 ± 0.0b | <0.001 | 132 |
| Co (µg/g) | 0.0 ± 0.1b | 0.3 ± 0.1a | 0.0 ± 0.1b | 0.0 ± 0.1b | 0.0 ± 0.1b | 0.002 | 283 |
| Cr (µg/g) | 2 ± 4b | 10 ± 6b | 24 ± 6b | 7 ± 6b | 193 ± 6a | <0.001 | 198 |
| Cu (µg/g) | 67 ± 18a | 71 ± 31a | 8 ± 31a | 3 ± 31a | 4 ± 31a | 0.22 | 118 |
| Fe (µg/g) | 172 ± 45b | 780 ± 77a | 188 ± 77b | 47 ± 77b | 1105 ± 77a | <0.001 | 105 |
| K (%) | 1.0 ± 0.2ab | 2.1 ± 0.4a | 0.2 ± 0.4b | 0.1 ± 0.4b | 0.9 ± 0.4ab | 0.02 | 87 |
| Mg (%) | 0.2 ± 0.1b | 0.6 ± 0.1a | 0.1 ± 0.1b | 0.1 ± 0.1b | 0.1 ± 0.1b | <0.001 | 104 |
| Mn (µg/g) | 130 ± 32b | 364 ± 55a | 11 ± 55b | 45 ± 55b | 366 ± 55a | 0.003 | 87 |
| Mo (µg/g) | 2.9 ± 0.8b | 5.1 ± 1.0a | 0.0 ± 1.0b | 0.1 ± 1.0b | 0.0 ± 1.0b | 0.04 | 124 |
| Na (µg/g) | 2338 ± 831b | 8046 ± 1440a | 1580 ± 1440ab | 49 ± 1440b | 112 ± 1440b | 0.02 | 129 |
| P (%) | 0.8 ± 0.1b | 2.0 ± 0.3a | 0.1 ± 0.1c | 0.0 ± 0.0b | 0.1 ± 0.0a | <0.001 | 110 |
| S (%) | 0.3 ± 0.0b | 0.4 ± 0.0b | 2.3 ± 0.0a | 0.0 ± 0.0c | 0.0 ± 0.0c | <0.001 | 144 |
| Se (µg/g) | 0.8 ± 0.2a | 1.0 ± 0.4a | 0.7 ± 0.4a | 0.2 ± 0.4a | 0.1 ± 0.4a | 0.31 | 93 |
| Zn (µg/g) | 127 ± 25b | 331 ± 43a | 178 ± 43ab | 3 ± 43b | 10 ± 43b | 0.003 | 91 |
Means within rows that do not share a common letter in the superscript differ significantly (P < 0.05).
P-values shown in bold font indicate a significant difference between high (bold) and low (underlined) group means.
Results of the PCA of individual component samples indicated that the first 2 principal components accounted for 65% of the total variation between samples (Figure 1). Each material clustered separately with the feed, feather and wood shavings clusters being fairly closely spaced and the rice hulls and excreta clusters being more distant.
Figure 1.
The PCA score plot (left) of the first two principal components of the data set comprising the individual component samples. The first component accounts for 45.3% of the variation, and the second component 19.7%. The PCA loading plot (right) of the same data set indicates partial contribution of variables in PCA analysis. Abbreviations: PCA, Principal component analysis.
Predicting Mixtures of Base Components Using Chemometrics
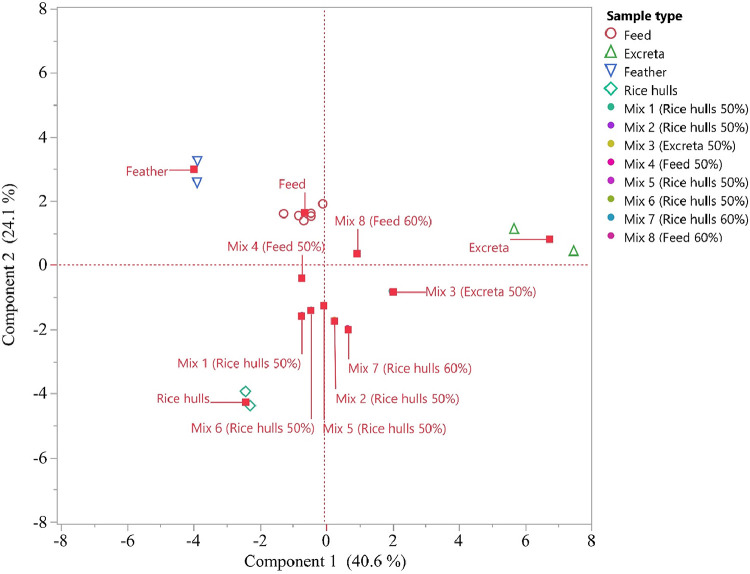
The PCA score plot of individual components and mixtures showed that mixture samples, not surprisingly, tended to be located at the middle of the score plot suggesting that they have average properties, while the individual component samples were more distant from each other (Figure 2). However, as expected, mixtures tended towards the source material making up the majority of the mixture.
Figure 2.
The PCA score plot of the first two principal components of a data set of individual component samples (feed, excreta, feather, and rice hulls) and mixture samples (Mix 1−8). The first component explains 40.6% of the variation, and the second component 24.1%. Proportions of rice hull: feed: excreta: feather for the mixtures are: Mix 1 (50:30:15:05); Mix 2 (50:15:30:05; Mix 3 (30:15:50:05); Mix 4 (30:50:15:05; Mix 5 (50:15:25:10); Mix 6 (50:30:19:01); Mix 7 (60:10:29:01) and Mix 8 (10:60:29:01).
Using chemical analyses of the 13 individual component samples as the LDA training set for the known mixture samples, four discriminant functions (representing linear combinations of quantitation variables) explained 55.5%, 34.9%, 9.3%, and 3.1% of the total variance respectively. The classification results of the 8 mixture samples based on the major individual components (Table 2) were very satisfactory, allowing 100% of the samples to be correctly predicted.
Table 2.
Linear discriminant analysis (LDA) prediction of majority sample component in mixture samples based on individual component sample training sets.
| Type of sample | Proportion of individual components in mixture (rice hulls: feed: excreta: feather) | Component with the highest proportion1 | LDA prediction of majority component2 |
|---|---|---|---|
| Mix 1 | 50: 30: 15: 05 | Rice hulls | Rice hulls |
| Mix 2 | 50: 15: 30: 05 | Rice hulls | Rice hulls |
| Mix 3 | 30: 15: 50: 05 | Excreta | Excreta |
| Mix 4 | 30: 50: 15: 05 | Feed | Feed |
| Mix 5 | 50: 15: 25: 10 | Rice hulls | Rice hulls |
| Mix 6 | 50: 30: 19: 01 | Rice hulls | Rice hulls |
| Mix 7 | 60: 10: 29: 01 | Rice hulls | Rice hulls |
| Mix 8 | 10: 60: 29: 01 | Feed | Feed |
Predominant component for classification (≥50%).
Based on prior probability of groups: (excreta = 15.4%, feather = 15.4%, feed = 38.5%, rice hulls = 15.4%, wood litter = 15.4%).
Chemical Profile of Broiler Flock Dust
Means (±SE) of the elemental composition of dust from broiler chicken flocks housed on different bedding material are given in Table 3. There were significant differences between dust types for 11 of the 18 elements, most notably for N, Cu, K, Mo and S (P < 0.001). The chemical profile of dust originating from flocks on wood shavings and sawdust tended to be similar and distinct from those on rice hulls and straw bedding which also tended to be similar. The former dusts were characterized by higher C and lower N than the latter suggestive of greater contributions of sawdust and shavings bedding material to the dust and reduced contribution of excreta, feed and feather. This is supported by the higher content of most minerals in the samples originating from flocks on rice hulls and straw, particularly S and N (very high in feather material), and Na and K (high in excreta and feed). Overall, the variation between the elemental compositions of dust samples from broilers on different bedding types varied less than the composition of the source material samples in Table 3 with CV % exceeding 100% for only 4 elements (Zn, B, Cr and Mo) and being less than 40% for 9 elements (Table 3).
Table 3.
Chemical profiles (LSM±SEM) of dust from commercial broiler flocks housed on various bedding materials. Dust samples were collected using settle plate method at end of batch (35−49 days of age).
| Chemical analysis (LSM±SEM) of dust samples from commercial broiler flocks raised on different bedding materials |
||||||
|---|---|---|---|---|---|---|
| Element | Wood shavings bedding (n = 7) | Sawdust bedding (n = 7) | Rice hulls bedding (n = 7) | Straw bedding (n = 7) | P value | Coefficient of variation (CV %) |
| C (%) | 41.8 ± 0.6ab | 42.1 ± 0.6a | 39.2 ± 0.6c | 39.6 ± 0.6bc | 0.004 | 5 |
| N (%) | 9.1 ± 0.6b | 10.5 ± 0.6b | 13.7 ± 0.6a | 14.1 ± 0.6a | <0.001 | 22 |
| Al (µg/g) | 2543 ± 535a | 729 ± 535a | 2146 ± 535a | 2096 ± 535a | 0.12 | 80 |
| B (µg/g) | 204 ± 44a | 15 ± 44b | 18 ± 44b | 16 ± 44b | 0.01 | 216 |
| Ca (%) | 1.2 ± 0.1b | 1.6 ± 0.1a | 1.7 ± 0.1a | 1.4 ± 0.1ab | 0.004 | 20 |
| Co (µg/g) | 1.0 ± 0.2a | 0.3 ± 0.2b | 0.9 ± 0.2a | 0.3 ± 0.2b | 0.01 | 92 |
| Cr (µg/g) | 11 ± 4a | 11 ± 4a | 1 ± 4a | 2 ± 4a | 0.25 | 189 |
| Cu (µg/g) | 209 ± 28a | 163 ± 28ab | 26 ± 28c | 68 ± 28bc | <0.001 | 88 |
| Fe (µg/g) | 1580 ± 484a | 2041 ± 484a | 1168 ± 484a | 1476 ± 484a | 0.65 | 80 |
| K (%) | 1.9 ± 0.1b | 1.7 ± 0.1b | 2.7 ± 0.1a | 2.4 ± 0.1a | <0.001 | 22 |
| Mg (%) | 0.6 ± 0.1b | 0.6 ± 0.1b | 0.7 ± 0.1ab | 0.8 ± 0.1a | 0.02 | 25 |
| Mn (µg/g) | 306 ± 39a | 297 ± 39a | 340 ± 39a | 361 ± 39a | 0.63 | 31 |
| Mo (µg/g) | 0.3 ± 0.4b | 0.2 ± 0.4b | 3.0 ± 0.4a | 0.4 ± 0.4b | <0.001 | 154 |
| Na (µg/g) | 3808 ± 268b | 3662 ± 268b | 4336 ± 268ab | 4979 ± 268a | 0.008 | 20 |
| P (%) | 0.8 ± 0.1a | 1.1 ± 0.1a | 1.2 ± 0.1a | 1.0 ± 0.1a | 0.16 | 29 |
| S (%) | 0.5 ± 0.1c | 0.6 ± 0.1bc | 0.8 ± 0.1ab | 0.8 ± 0.1a | <0.001 | 25 |
| Se (µg/g) | 1.1 ± 0.4a | 1.2 ± 0.4a | 1.6 ± 0.4a | 1.4 ± 0.4a | 0.72 | 71 |
| Zn (µg/g) | 278 ± 1318a | 790 ± 1318a | 312 ± 1318a | 3837 ± 1318a | 0.20 | 277 |
Means within rows that do not share a common letter in the superscript differ significantly (P < 0.05).
P-values shown in bold font indicate a significant difference between high (bold) and low (underlined) group means.
Predicting Components in Dust Using Chemometrics
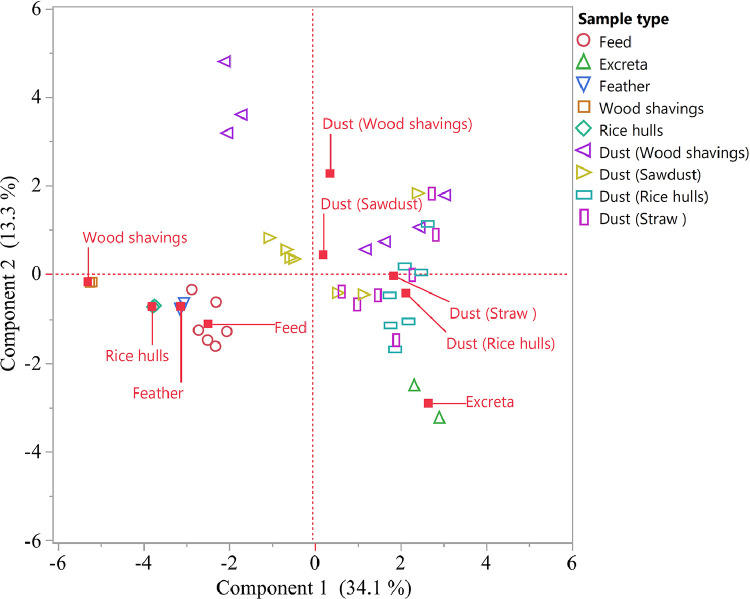
The PCA score plot including source materials and dust samples suggests that dust samples originating from commercial broiler flocks housed on different bedding materials are heterogeneous. The majority of dust samples were distant from bedding materials, feed and feather, with some showing closer proximity to excreta with proximity influenced by bedding type (Figure 3). As noted above from the chemical analysis, dusts from flocks on straw and rice hulls tended to group together, closer to excreta than those from flocks on wood shavings and sawdust.
Figure 3.
The PCA score plot of the first two principal components of a data set of individual component samples and dust from commercial broiler flocks housed on various bedding materials (n = 7 wood shavings, n = 7 sawdust, n = 7 rice hulls, n = 7 straw). The first component explains 34.1% of the variation, and the second component 13.3%. Components close to each other have similar profile.
Linear discriminant analysis using both the 13 individual component samples and the 8 mixture samples (Mix 1−8) as different training sets was then applied to predict the major component in the 28 dust samples from commercial broiler flocks (Table 4). Based on the individual component training set, results indicated that excreta was the major component in 26 out of 28 dust samples (92.9%). Based on the mixture sample training set the results indicated that 24 out of the 28 dust samples (85.7%) were most similar to the excreta mix (Mix 3) – the other 4 samples were predicted by LDA to be most similar to the rice hull dominant mixtures (Mix 1, 2, 5, 6 and 7) which all had an excreta content of ≤30%. The mixture training set was based on 2 discriminant functions (representing linear combinations of quantitation variables) explaining 83.4%, and 16.1% of the total variance respectively.
Table 4.
Linear discriminant analysis (LDA) prediction of majority sample component in 28 dust samples comparing both the individual component samples or mixture samples as training sets. Prior probabilities for LDA prediction on mixture samples was excreta mix (12.5%), feed mix (25%), and rice mix (62.5%). Prior probabilities for LDA prediction on individual component samples was excreta (5.4%), feather (15.4%), feed (38.5%), rice hulls (15.4%), and wood litter (15.4%).
| Training set | Dust sampleN | Bedding material of flock of origin of the dust sample | LDA prediction of majority component from the training set |
|---|---|---|---|
| 13 individual component samples (Feed, wood shavings, sawdust, excreta) | 7 | Wood shavings | Excreta (7/7, 100%) |
| 7 | Sawdust | Excreta (7/7, 100%) | |
| 7 | Rice hulls | Excreta (6/7, 85.7%) Feed (1/7, 14.3%) |
|
| 7 | Straw | Excreta (6/7, 85.7%) Wood shavings (1/7, 14.3%) |
|
| 8 mixture samples (See Table 2 for mixture details- Mix 3 ≥50% excreta) | 7 | Wood shavings | Excreta mix (4/7, 57.1%) Rice hull mix (3/7, 42.9%) |
| 7 | Sawdust | Excreta mix (7/7, 100%) | |
| 7 | Rice hulls | Excreta mix (7/7, 100%) | |
| 7 | Straw | Excreta mix (6/7, 85.7%) Rice hull mix (1/7, 14.3%) |
Characterization of Dust Components Using XRD and SEM/EDS
The XRD analysis showed non-specific peaks which did not enable quantification of crystalline minerals in dust. However, the wave pattern suggests that dust could have feed, excreta, feather, and bedding materials as components (Supplementary Figure 2). The SEM/EDS data showed wide variation in the chemical data among particles within the same type of sample, possibly due to use of ground materials. This method therefore failed to provide a definitive means of testing the study hypotheses.
Characterization of Dust Components Using SEM Image Analysis
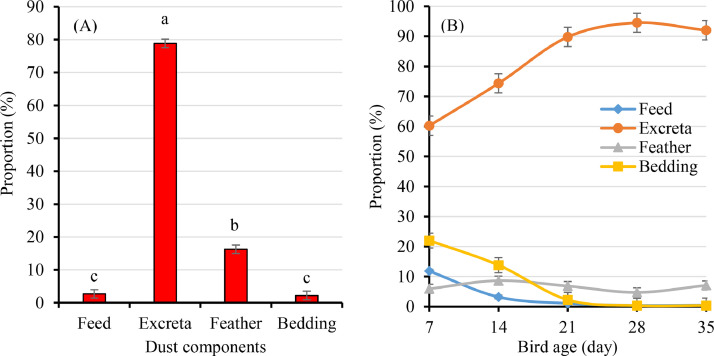
Detailed morphology of possible source materials in poultry dust was investigated using SEM (Figure 4). On the basis of similarity with particle morphology classified by SEM of individual component and mixture samples, the primary particles identified in settled plate dust from broiler sheds appeared to be excreta particles (~80% in most dust samples) (Figure 5). The proportion of feed, excreta, feather and bedding materials in settle plate dust obtained at the end of batch could not easily be determined as the majority of bedding material particles appeared to be degraded or clumped together (particularly excreta particles for example) possibly due to storage at −20°C for a prolonged period (several years). Quantification of proportions of differing particles was possible in 11 out of 28 dust samples and revealed a preponderance of excreta particles (79%) followed by feather (16%), feed (3%) and bedding (2%) (Figure 5A). However, weekly settle plate dust samples from the experimental broiler flock which were stored at −20˚C for a short period (<1 yr) appeared unaffected and it was possible to quantify the proportions of differing particles in all samples and percentage change over time (Figure 5B). The proportion of excreta particles in settle dust increased over time (P < 0.001), and the proportion of feed (P < 0.001) and bedding materials (P < 0.001) declined over time. The proportion of feather particles remained unchanged (P = 0.48) throughout the observation period in this study.
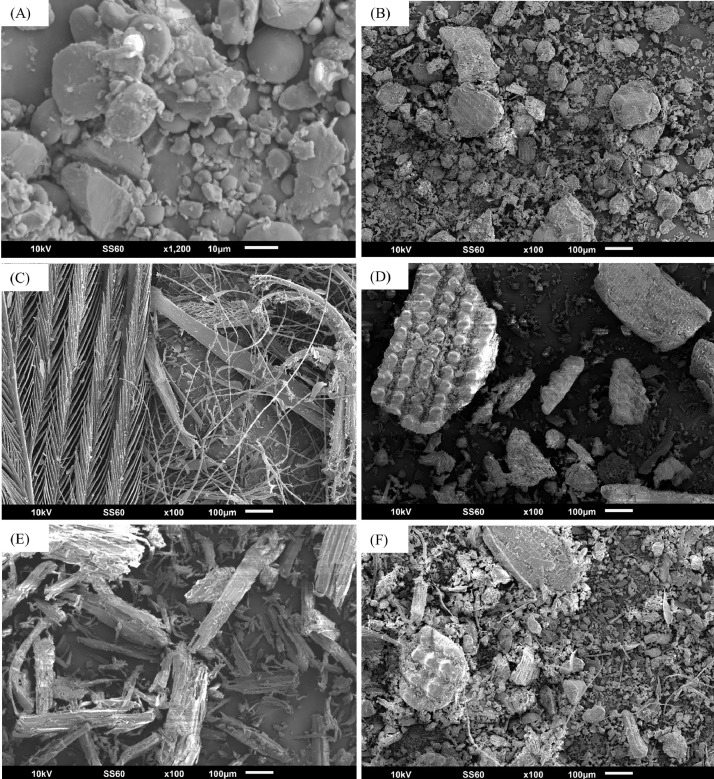
Figure 4.
SEM images of individual component samples representing key source materials for poultry house dust: (A) Feed particles: spherical or angular appearance with smooth surface. (B) Excreta particles: irregular appearance with rough surface. (C) Feather: flat or filamentous with barbules and calamus. (D) Rice hulls: plate shape, vascular, thick and flat. (E) Wood shavings: woodchip appearance. (F) A known mixture (mix 2) of rice hulls: feed: excreta: feather. All samples were ground to pass a 0.5-mm sieve and coated with gold before SEM. Abbreviations: Scanning electron microscopy.
Figure 5.
(A) Proportion of feed, excreta, feather and bedding particles (LSM±SE) identified in end of batch (35−49 d) broiler house dust (n = 11). (B) Mean proportion of feed, excreta, feather and bedding particles in dust by bird age (7−35d) in an experimental flock on wood shavings with dust samples collected weekly by settle plate. Characterization of particles was done visually using SEM and ImageJ analyses.
DISCUSSION
This study corroborates previous findings that excreta material is a primary constituent of airborne poultry dust (Feddes et al., 1992), and demonstrates that chemometric analysis is a useful tool to predict the source components of dust samples. SEM image analysis proved useful for the detailed study of dust sample morphology and relative particle composition, which enabled validation of the chemometric analysis results.
The results reported here clearly support the first proposition that the individual dust components (feed, excreta, feather, and bedding) will exhibit distinct chemical profiles. Significant differences in the chemical profile of individual component samples were observed for the majority of chemical elements studied. Feed and excreta tended to have high mineral content relative to bedding materials with wood shavings being highest in carbon but generally low in minerals, particularly N. Rice hulls had a lower carbon and higher mineral content than wood shavings, being particularly characterized by high Cr values. Feather material was distinguished by very high levels of N and S reflecting the amino acid composition and disulphide bonds of beta keratin, the main constituent of feathers. Despite the shared high mineral content, feed, and excreta were most clearly differentiated by the high levels of Co, Al, Ca, P, and Mg in excreta relative to feed. The PCA score plot in Figure 1 showed that feed samples were located near the center of the plot, consistent with having a reasonable level of almost all the elements measured. A higher level of Al, Ca, Co, Fe, K, Mg, Mn, Mo, Na, P, and Zn was recorded in the excreta samples compared to feed samples and that signature differentiated them in PCA analysis where they sat well to the right of feed on the component 1 plot axis. The high mineral content of bird excreta samples observed in this study was similar to that reported in excreta samples sourced from broiler chickens reared in battery cages (van Ryssen et al., 1977). In chickens, excreta contains the waste products of both the digestive and urinary systems so the higher levels of minerals in excreta may be derived not only from unabsorbed feed but also from excretion or loss of endogenous minerals (Bao and Choct, 2009). In this study, feather and wood shavings samples located in the upper left quadrant of PCA score plot consistent with the main contributors to PC2 (C, N, and S) with that feather being rich in N and S, and wood shavings is rich in C. The main constituent of wood is carbon, ranging between 45 and 50% (Chandrasekaran et al., 2012), which is similar to the carbon result for wood shavings (47%) found in this study. A high level of Cr, Fe, P, and Mn and a moderate level of Al and B in rice hulls distinguished them from the other 4 individual component samples (feed, excreta feather, and wood shavings) in the PCA.
The second proposition, that chemical profile of source materials will be useful for predicting their contribution to the composition of poultry dust samples, was supported by these results. In the present study the major component of known mixtures was predicted successfully based on the chemical profile of individual components. The alignment of both LDA and SEM analysis indicated that the main component of poultry house dust is excreta as predicted in 93% of samples by the application of chemometrics and 100% of samples analyzed using SEM. The use of mixtures as a training set in LDA further identified the closest fit of dust samples was to mixture 3 (24/28, 85.7% of predictions). Mixture 3 was excreta dominant having a composition of 50% excreta, 30% rice hulls, 15% feed, and 5% feather. These results, based on LDA are broadly consistent with those of Aarnink et al. (1999), which reported that the relative proportion of feed, excreta (crystalline and non-crystalline) and feather particles determined in settled dust samples by light microscopy is likely to be 3%, >10% and >10%, respectively in broiler dust during the fattening period (>21 d). In that study, fresh bedding materials was provided at age 21 d and settle dust was collected at age 42 d. In support of LDA used in this study, the univariate analysis of chemical data also showed that there is a relatively high concentration of Al, Fe, and Na in settle dust samples, which is similar to the chemical signature of excreta. The PCA score plot of source materials and dust samples also showed closer proximity of dust samples to excreta with proximity influenced by bedding type. On the contrary, LDA analysis of dust in this study based on the mixture training set also suggests that a lower number of dust samples (4/28, 14.3%) collected at the end of batch (35−49 d) had a higher proportion of bedding materials (50−60%) and moderate proportion of excreta (15−30%) and a lower proportion of feed (10−30%) and feather (1−5%). This variation in LDA outcome depending on training set may be due to true heterogeneity or due to the limitations of the LDA analysis. Chemometrics using LDA to predict the major components of dust is attractive as the chemistry involved is automated and comparatively inexpensive. However, a significant amount of dust (0.2−0.5 g) is required.
The SEM results of this study supported our third proposition that SEM analysis of dust samples will support the chemical profile prediction. In SEM analysis, excreta was identified as the primary component (>80% of particles) of poultry house dust which is consistent with the chemometrics analysis. Other major component particles of dust such as feed, feather and bedding materials were also identified using SEM were similar to the findings of chemometrics. Overall, excreta is the primary component found in settled dust in this study, in agreement with the findings of Feddes et al., 1992 that airborne dust in turkey barns consists mainly of excreta as determined by SEM. Despite the dominance of excreta in dust samples, there were statistically significant differences in the chemical signature of dusts from chickens on different bedding types. In broad terms, dusts from chickens on straw or rice hulls bedding, were somewhat different from those on wood shavings and sawdust bedding, being characterized by lower C and higher N values and a mineral signature closer to those of excreta (high Na and K) and feathers (high S and N). This is suggestive of a lower contribution of high carbon bedding material to dust on straw and rice hulls bedding with a corresponding increase in the proportion from excreta, feed, and feathers. This in turn suggests a slower breakdown rate of rice hulls and straw bedding materials to fine dust particles.
SEM imagery of weekly dust samples collected from an experimental flock between 7 and 35 days of age in this study supported the fourth proposition that the composition of poultry dust will change during the course of production cycle with an increasing excreta content with time. This is to be expected as excreta forms an increasing component of the overall bedding material as chicken age. Also given passage through the digestive tract, particle size in excreta has already been reduced by digestion enabling ready aerosol formation once dried and subjected to physical breakdown. In the present study chickens were placed on new bedding material as is the general practice in Australia but if litter is reused multiple times (as is the standard practice in many major poultry producing countries) it can be expected that the excreta concentration will be even higher than in the current examples. SEM proved useful for determining the relative proportion of particle types in small dust samples, something not provided by LDA analysis which predicted the major component. However, SEM is labor and capital equipment intensive and so a comparatively expensive methodology without standardized methods for partitioning dust particles into different components based on origin.
The high propensity of excreta particles in dust may pose direct or indirect risks to human and poultry health (Viegas et al., 2013). The excreta particles observed on SEM were the finest particles detected by SEM, therefore, could carry a wide range of vegetative organisms (Chinivasagam et al., 2009; Chinivasagam et al., 2010), spores or toxins deep into the respiratory system. The high excreta content is dust is also suggestive that dust could be a potential population level sample for the monitoring of pathogens excreted in excreta and indeed this has been reported for Eimeria and Clostridium spp. (Ahaduzzaman et al., 2020). In addition, dust has also been shown to be useful for the monitoring of pathogens that are primarily transmitted by feather dander such as Marek's disease virus (Walkden-Brown et al., 2013). Respiratory pathogens such as infectious laryngotracheitis virus (Ahaduzzaman et al., 2019), Newcastle disease virus and infectious bronchitis virus (Tran et al., 2020) are detectable in dust samples using PCR although it is not clear whether this represents infective virus from the respiratory tract or inactivated viral nucleic acids present in excreta following passage through the gut (Bindari et al., 2020; Yegoraw et al., 2020). It can be expected that pathogens originating in litter material (e.g., litter derived Aspergillus fumigatus) will also be well represented in dust, however, it should be noted that the proportion of bedding in dust declined to low levels over time as observed in this study.
In conclusion, use of chemometrics and SEM to determine the source materials of dust from broiler chicken houses revealed excreta to be the major component in broiler chicken house dust. SEM analysis of dust between 7 and 35 days of age revealed that the contribution of excreta to dust was high (>60%) and increased with age, bedding and feed in dust declined with age while the contribution of feather material remained low throughout. Comparison of both chemometrics and SEM analyses indicated that the major components of dust could be suitability and accurately predicted by both methods, however, SEM is relatively more expensive and time consuming than chemical analysis, but requires less sample material. These findings improve our understanding of this material which has significant importance for the health of chickens and poultry workers and considerable diagnostic potential.
Acknowledgments
ACKNOWLEDGMENTS
The project was supported by UNE-IPRA research fund. Thanks to Md Momenuzzaman Bhuiyan and Shahram Barzegar for providing the feed and excreta samples, respectively. We are thankful to Leanne Lisle, Malcolm Lambert, Michael Raue, Craig Johnson, John Pesor, Calista McLachlan and Justin Bailey for technical support.
DISCLOSURES
The authors declare that they have no competing interests.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2021.101188.
Appendix. Supplementary materials
Supplementary Figure 1. Identification of feed (green), excreta (yellow), feather (blue) and bedding (red) particles in SEM image of broiler chicken dust. Identification was conducted manually using ImageJ. The settle plate dust sample was collected at bird age 7d from a broiler chicken flock with wood shavings bedding material
Supplementary Figure 2. XRD pattern of individual component samples (feed, excreta, feather and bedding material) and commercial broiler flock dust from sheds with different bedding material (wood shavings, sawdust, straw and rice hulls). Peaks/bumps at identical points (2 degrees theta) are suggestive of similarity
REFERENCES
- Aarnink A., Roelofs P., Ellen H., Gunnink H. Dust sources in animal houses. Proc. Intl. Symp. Dust Ctrl. Anim. Prod. Facs. 1999:34–40. (Abstr.) [Google Scholar]
- Ahaduzzaman M., Groves P.J., Sharpe S.M., Williamson S.L., Gao Y.K., Nguyen T.V., Gerber P.F., Walkden-Brown S.W. A practical method for assessing infectious laryngotracheitis vaccine take in broilers following mass administration in water: spatial and temporal variation in viral genome content of poultry dust after vaccination. Vet. Microbiol. 2020 doi: 10.1016/j.vetmic.2019.108545. [DOI] [PubMed] [Google Scholar]
- Ahaduzzaman M., Keerqin C., Kumar A., Musigwa S., Morgan N., Kheravii S., Sharpe S., Williamson S., Wu S., Walkden-Brown S., Gerber P. Detection and quantification of Clostridium perfringens and netB toxin gene from poultry dust using real-time PCR. Avian Dis. 2020;65:75–85. doi: 10.1637/aviandiseases-D-20-00084. [DOI] [PubMed] [Google Scholar]
- Azid A., Juahir H., Toriman M.E., Kamarudin M.K.A., Saudi A.S.M., Hasnam C.N.C., Aziz N.A.A., Azaman F., Latif M.T., Zainuddin S.F.M. Prediction of the level of air pollution using principal component analysis and artificial neural network techniques: a case study in Malaysia. Water Air Soil Pollut. 2014;225:2063. [Google Scholar]
- Azimi A., Bakhtiari A.R., Tauler R. Chemometrics analysis of petroleum hydrocarbons sources in the street dust, runoff and sediment of urban rivers in Anzali port-South of Caspian Sea. Environ. Pollut. 2018;243:374–382. doi: 10.1016/j.envpol.2018.08.073. [DOI] [PubMed] [Google Scholar]
- Bao Y., Choct M. Trace mineral nutrition for broiler chickens and prospects of application of organically complexed trace minerals: a review. Anim. Prod. Sci. 2009;49:269–282. [Google Scholar]
- Bindari Y.R., Walkden-Brown S.W., Gerber P.F. Methods to prevent PCR amplification of DNA from non-viable virus were not successful for infectious laryngotracheitis virus. Plos One. 2020;15 doi: 10.1371/journal.pone.0232571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambra-López M., Aarnink A.J., Zhao Y., Calvet S., Torres A.G. Airborne particulate matter from livestock production systems: a review of an air pollution problem. Environ. Pollut. 2010;158:1–17. doi: 10.1016/j.envpol.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Cambra-López M., Torres A. Vol. 26. Proc. Intl. Conf. Agric. Eng.; 2008. An approach to source apportionment of dust in animal houses: the case of rabbit rearing facilities. (Abstr.) [Google Scholar]
- Cambra-López M., Torres A., Aarnink A.J.A., Ogink N.W. Source analysis of fine and coarse particulate matter from livestock houses. Atmos. Environ. 2011;45:694–707. [Google Scholar]
- Carrozza J.H., Fredrickson T.N., Prince R.P., Luginbuhl R.E. Role of desquamated epithelial cells in transmission of Marek's disease. Avian Dis. 1973;17:767–781. [PubMed] [Google Scholar]
- Chandrasekaran S.R., Hopke P.K., Rector L., Allen G., Lin L. Chemical composition of wood chips and wood pellets. Energ. Fuel. 2012;26:4932–4937. [Google Scholar]
- Chinivasagam H., Tran T., Maddock L., Gale A., Blackall P. Mechanically ventilated broiler sheds: a possible source of aerosolized Salmonella, Campylobacter, and Escherichia coli. Appl. Environ. Microb. 2009;75:7417–7425. doi: 10.1128/AEM.01380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinivasagam H., Tran T., Maddock L., Gale A., Blackall P. The aerobiology of the environment around mechanically ventilated broiler sheds. J. Appl. Microbiol. 2010;108:1657–1667. doi: 10.1111/j.1365-2672.2009.04571.x. [DOI] [PubMed] [Google Scholar]
- Feddes J.J., Zuidhof M.J., Cook H. Characterization of airborne dust particles in turkey housing. Can. Agric. Eng. 1992;34:273–280. [Google Scholar]
- Groves P., Walkden-Brown S., Islam A., Reynolds P., King M., Sharpe S. An epidemiological survey of MDV in Australian broiler flocks. Proc. 8th Intl. Marek's Dis. Symp. 2008;24 (Abstr.) [Google Scholar]
- Gunawardana C., Goonetilleke A., Egodawatta P., Dawes L., Kokot S. Source characterisation of road dust based on chemical and mineralogical composition. Chemosphere. 2012;87:163–170. doi: 10.1016/j.chemosphere.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Hartung J., Saleh M. Composition of dust and effects on animals. Landbau. 2007;308:111–116. [Google Scholar]
- Hiranumaa N., Brooksa Sarah D., Auvermannb Brent W., Littleton R. Using environmental scanning electron microscopy to determine the hygroscopic properties of agricultural aerosols. Atmos. Environ. 2008;42:1983–1994. [Google Scholar]
- Hunt J.F., Ohno T. Characterization of fresh and decomposed dissolved organic matter using excitation− emission matrix fluorescence spectroscopy and multiway analysis. J. Agric. Food Chem. 2007;55:2121–2128. doi: 10.1021/jf063336m. [DOI] [PubMed] [Google Scholar]
- Just N., Duchaine C., Singh B. An aerobiological perspective of dust in cage-housed and floor-housed poultry operations. J. Occup. Med. Toxicol. 2009;4:13. doi: 10.1186/1745-6673-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon J. Poultry dust: origin and composition. J. Agric. Eng. 1963;44:608–609. [Google Scholar]
- Kumar R.S., Rajkumar P. Characterization of minerals in air dust particles in the state of Tamilnadu, India through FTIR, XRD and SEM analyses. Infrared. Phys. Technol. 2014;67:30–41. [Google Scholar]
- Li L., Qiu Y., Gustafsson Å., Krais A.M., Weiss J.M., Lundh T., Bergman Å. Characterization of residential household dust from Shanghai by particle size and analysis of organophosphorus flame retardants and metals. Environ. Sci. Eur. 2019;31:1–12. [Google Scholar]
- Li Z., Zheng W., Wang Y., Li B., Wang Y. Spatiotemporal variations in the association between particulate matter and airborne bacteria based on the size-resolved respiratory tract deposition in concentrated layer feeding operations. Environ. Int. 2021;150 doi: 10.1016/j.envint.2021.106413. [DOI] [PubMed] [Google Scholar]
- Meglen R.R. Examining large databases: a chemometric approach using principal component analysis. J. Chemom. 1991;5:163–179. [Google Scholar]
- Minasny B., McBratney A.B. Regression rules as a tool for predicting soil properties from infrared reflectance spectroscopy. Chemom. Intell. Lab. Syst. 2008;94:72–79. [Google Scholar]
- Nakaue H., Koelliker J., Buhler D., Arscott G. Distribution of inorganic elements in poultry house dust. Poultry Sci. 1981;60:1386–1391. doi: 10.3382/ps.0601386. [DOI] [PubMed] [Google Scholar]
- Nguyen T.V., Ahaduzzaman M., Campbell D.L., Groves P.J., Walkden-Brown S.W., Gerber P.F. Spatial and temporal variation of Marek's disease virus and infectious laryngotracheitis virus genome in dust samples following live vaccination of layer flocks. Vet. Microbiol. 2019 doi: 10.1016/j.vetmic.2019.108393. [DOI] [PubMed] [Google Scholar]
- Odenigbo C., Makonnen Y., Asfaw A., Anastassiades T., Beauchemin D. Towards the use of ICP-OES for the elemental analysis of organic compounds such as glucosamine. J. Anal. At. Spectrom. 2014;29:454–457. [Google Scholar]
- Queralt I., Sanfeliu T., Gomez E., Alvarez C. X-ray diffraction analysis of atmospheric dust using low-background supports. J. Aerosol Sci. 2001;32:453–459. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Shen D., Wu S., Dai P., Li Y., Li C. Distribution of particulate matter and ammonia and physicochemical properties of fine particulate matter in a layer house. Poultry. Sci. 2018;97:4137–4149. doi: 10.3382/ps/pey285. [DOI] [PubMed] [Google Scholar]
- Sila A.M., Shepherd K.D., Pokhariyal G.P. Evaluating the utility of mid-infrared spectral subspaces for predicting soil properties. Chemom. Intell. Lab. Syst. 2016;153:92–105. doi: 10.1016/j.chemolab.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T., Yegoraw A., Assen A., Walkden-Brown S., Gerber P. Genomic stability for PCR detection of infectious laryngotracheitis virus and infectious bronchitis virus in poultry dust samples stored under different conditions. Avian. Dis. 2020;64:565–570. doi: 10.1637/0005-2086-64.4.565. [DOI] [PubMed] [Google Scholar]
- van Ryssen J., Channon P., Stielau W. Minerals and nitrogen in poultry manure. S. Afr. J. Anim. Sci. 1977;7:195–199. [Google Scholar]
- Viegas S., Faísca V.M., Dias H., Clérigo A., Carolino E., Viegas C. Occupational exposure to poultry dust and effects on the respiratory system in workers. J. Toxicol. Environ. Health Sci. Part A. 2013;76:230–239. doi: 10.1080/15287394.2013.757199. [DOI] [PubMed] [Google Scholar]
- Walkden-Brown S.W., Islam A., Groves P.J., Rubite A., Sharpe S.M., Burgess S.K. Development, application and results of routine monitoring of Marek's disease virus in broiler house dust using real-time quantitative PCR. Avian. Dis. 2013;57:544–554. doi: 10.1637/10380-92112-REG.1. [DOI] [PubMed] [Google Scholar]
- Wei J., Zhou J., Cheng K., Wu J., Zhong Z., Song Y., Ke C., Yen H.-L., Li Y. Assessing the risk of downwind spread of avian influenza virus via airborne particles from an urban wholesale poultry market. Build. Environ. 2018;127:120–126. doi: 10.1016/j.buildenv.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Dust: Definitions and Concepts. https://www.who.int/occupational_health/publications/en/oehairbornedust3.pdf. Accessed July 2020. [Google Scholar]
- Wright A.F., Bailey J.S. Organic carbon, total carbon, and total nitrogen determinations in soils of variable calcium carbonate contents using a Leco CN-2000 dry combustion analyzer. Comm. Soil Sci. Plant Anal. 2001;32:3243–3258. [Google Scholar]
- Yegoraw A.A., Nazir S., Gerber P.F., Walkden-Brown S.W. Airborne transmission of vaccinal and wild type infectious laryngotracheitis virus and non-infectivity of extracts of excreta from infected chickens. Avian. Dis. 2020;65:30–39. doi: 10.1637/aviandiseases-D-20-00073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Identification of feed (green), excreta (yellow), feather (blue) and bedding (red) particles in SEM image of broiler chicken dust. Identification was conducted manually using ImageJ. The settle plate dust sample was collected at bird age 7d from a broiler chicken flock with wood shavings bedding material
Supplementary Figure 2. XRD pattern of individual component samples (feed, excreta, feather and bedding material) and commercial broiler flock dust from sheds with different bedding material (wood shavings, sawdust, straw and rice hulls). Peaks/bumps at identical points (2 degrees theta) are suggestive of similarity