Abstract
Background
The burden of HIV is especially concerning for Eastern and Southern Africa (ESA), as despite expansion of test-and-treat programmes, this region continues to experience significant challenges resulting from high rates of morbidity, mortality and new infections. Hard-won lessons from programmes on the ground in ESA should be shared.
Objectives
This report summarises relevant evidence and regional experts’ recommendations regarding challenges specific to ESA.
Method
This commentary includes an in-depth review of relevant literature, progress against global goals and consensus opinion from experts.
Results
Recommendations include priorities for essential research (surveillance data collection, key and vulnerable population education and testing, in-country testing trials and evidence-based support services to improve retention in care) as well as research that can accelerate progress towards the prevention of new infections and achieving ambitious global goals in ESA.
Conclusion
The elimination of HIV in ESA will require continued investment, commitment to evidence-based programmes and persistence. Local research is critical to ensuring that responses in ESA are targeted, efficient and evaluated.
Keywords: HIV epidemiology, public health, risk factors, vulnerable populations, prevention and control, early diagnosis
Introduction
In the decades since HIV-1 first emerged, the response has been marked by strong global commitments, extensive education campaigns and the development of improved tests and life-saving antiretroviral treatments (ART) that are reaching more and more individuals.1 With evidence-based prevention and treatment strategies available, nations have united to set goals, with the end of the HIV epidemic potentially attainable by 2030.2
One hallmark concept in the fight against HIV has been the ‘know your epidemic, know your response’ approach to deliver programmes in different settings.3 More than 70% of persons living with HIV (PLWH) reside in sub-Saharan Africa (SSA), where resources for healthcare are disproportionately limited. Eastern and Southern Africa (ESA), in particular, continues to record the highest rates of HIV-1 prevalence and incidence worldwide.4 In this region, knowing where and among whom the infection is spreading has been challenging, and key populations are only recently being highlighted.
A second hallmark of the fight against HIV has been the Joint United Nations Programme on HIV/AIDS (UNAIDS) ‘Fast-Track’ targets, whereby 90% of PLWH should know their status, 90% of those diagnosed should receive ART and 90% of those on ART should achieve viral suppression by 2020 (‘90-90-90’).5 Despite remarkable progress towards these targets in ESA, the sheer scale of the epidemic in this region leaves much to be done.6 In the next decade, efforts must be redoubled for raised targets of 95-95-95 by 2030.7 Programmes on the ground have identified region-specific challenges to be overcome and lessons that should be broadly shared with ESA and potentially with many communities globally.
An important barrier preventing progress in ESA is the timely detection and treatment of acute HIV infections.The earliest stage of HIV infection is characterised by high viral loads and a high potential for onward transmission, but it is typically missed using existing testing algorithms.8 As ART coverage improves, the proportion of transmissions attributable to undiagnosed acute HIV infection increases.9 Furthermore, new HIV infections disproportionately affect key populations.10 Affordable testing solutions for acute HIV detection in high-prevalence, resource-limited settings are needed.9
As the 2020 deadline passed, trends indicated that the 90-90-90 targets were not reached across most of ESA.2
Renewed efforts looking ahead to the 2030 UNAIDS targets of 95-95-95 will be required. To this end, a group of regional experts was invited to collate their expertise, with a view to addressing the local challenges that prevent the achievement of global goals.
State of the global epidemic
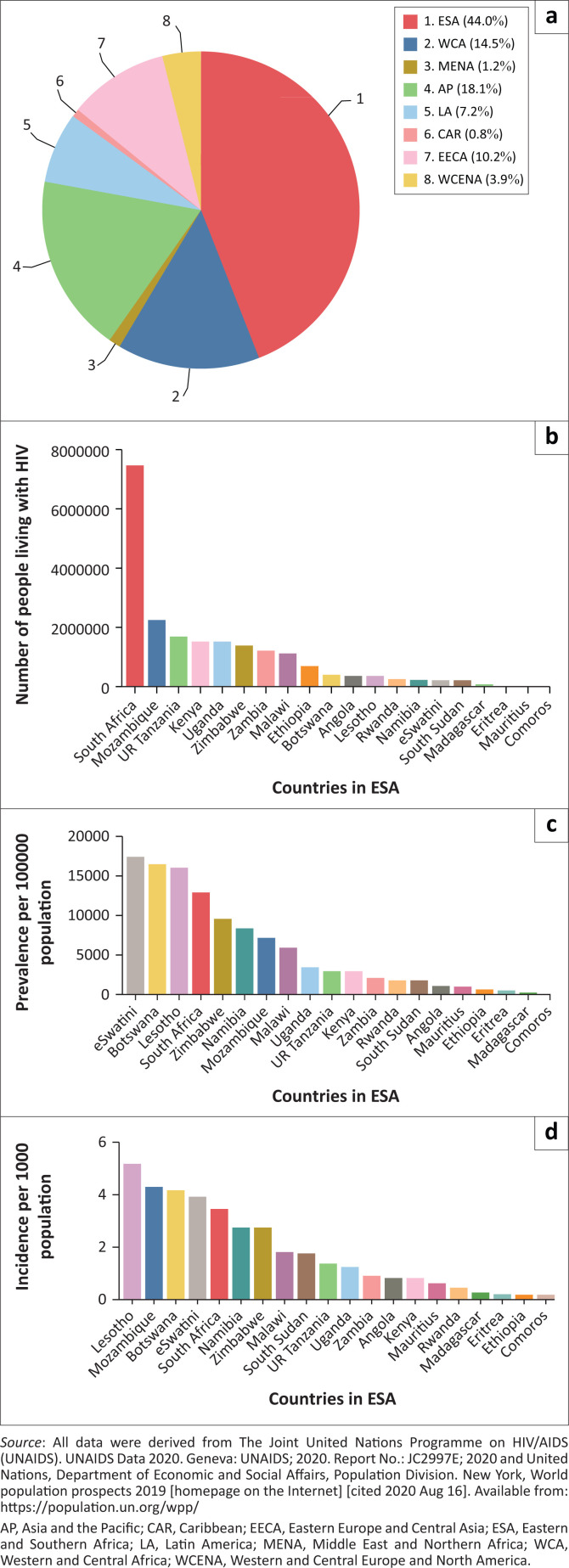
According to UNAIDS, there were an estimated 38 million PLWH worldwide at the end of 2019, with 1.7 million new infections and 690 000 AIDS-related deaths that year (Figure 1).10 A successful vaccine and functional cure for HIV are yet to be developed, and lifelong ART remains the cornerstone of management.
FIGURE 1.
(a) Worldwide distribution of new HIV infections identified by UNAIDS in 2019. (b) Distribution of people living with HIV infection in ESA in 2019. (c) Prevalence of HIV infection per 100 000 population among countries in ESA. (d) Incidence of HIV infection per 1000 population among countries in ESA.
The 2020 UNAIDS report highlights a ‘prevention crisis’.10 Programmes aiming to prevent new HIV infections (such as education, barrier contraception, voluntary male medical circumcision and pre-exposure prophylaxis [PreP]) must be a priority alongside test-and-treat programmes and must appropriately target key populations and their partners, who comprise 62% of new HIV infections globally.10
HIV epidemiology in ESA
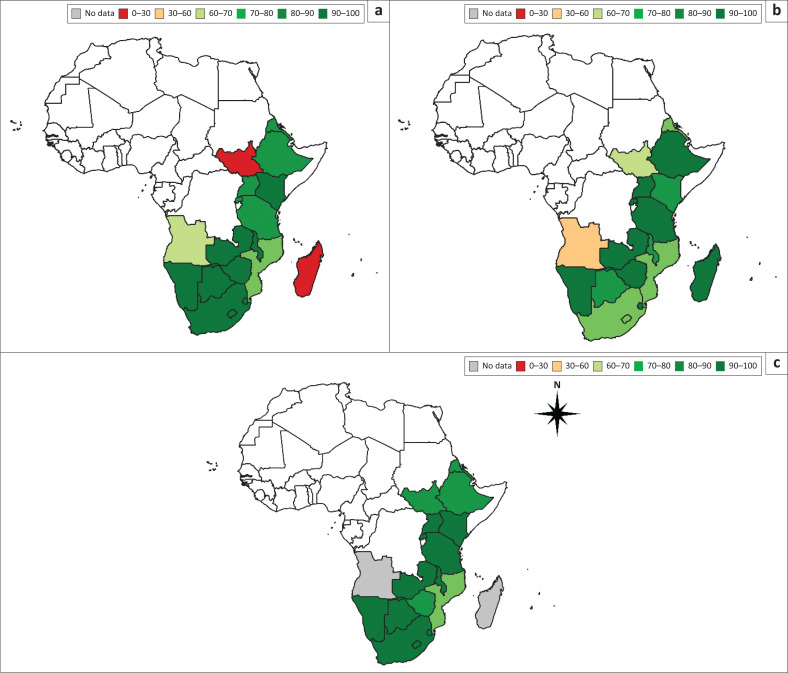
Regional HIV epidemics look markedly different across the world and require tailored responses. In ESA, there are 20.7 million adults and children living with HIV (54% of global HIV prevalence),10 and in 2019 44% of all new infections occurred here.10 Key populations make up an estimated 28% of new infections in ESA.10 In some areas, reasonable progress has been made towards the Fast-Track targets (e.g. eSwatini, Namibia and Zambia); in other areas, progress is more limited (e.g. Mauritius and South Sudan). An estimated 87% of PLWH in ESA are aware of their status (the ‘first 90’; Figure 2); however, this figure ranges from 15% to 98% between countries.10
FIGURE 2.
Data on the ESA 90-90-90 goals in all ages by country from the UNAIDS data 2020 report.10 (a) First 90; (b) second 90; (c) third 90.
Of those diagnosed with HIV in ESA, approximately 83% have commenced ART (the ‘second 90’).10 This figure ranges from 37% to 98% between countries,10 highlighting deep inequities within and across countries. Of those on treatment, 90% have achieved viral suppression (the ‘third 90’); this figure ranges from 68% to 97% between countries, with 2 of 21 countries unable to provide estimates. Combatting the epidemic in ESA is a multifaceted challenge, and progress must occur within a broader context of socio-economic development. Despite some successes, the 2020 milestones were not achieved in many countries across ESA, and the greatest challenges persist as the focus shifts to achieving the new 95-95-95 targets.
Challenges for achieving 95-95-95 in ESA
The first 95
In high HIV prevalence settings, obtaining accurate measures of the first 95 is challenging. In ESA, HIV care commonly takes place in rural settings, utilising paper-based records.11 To estimate the first 95, the denominator is typically the number of people testing positive for HIV during randomised household or community-based serosurveys and/or at antenatal clinics; the numerator is those among them known to have previous positive results (either disclosed to surveyors or identified in medical records).12
Rates of non-disclosure can be high.13 When people are retested in southern Mozambique, non-disclosure of previous results occurs in over one-third of people, but the rate is higher for tests performed in a community setting (38.9%) or initiated by the provider (29.4%) than in those presenting for voluntary testing (13%).13 Similar findings have been described in Tanzania and Malawi.14,15 Cross-checking survey responses against medical records is impossible in many countries where HIV testing is performed anonymously.11 The high percentage of HIV-positive people who do not disclose, and are thus repeatedly deemed recently infected, leads to an overestimation of new HIV cases and an underestimation of progress towards the first 95. In Mozambique, non-disclosure resulted in underestimation of the first 95 by around 8.5%.13
Improving testing coverage to achieve the first 95 is feasible but must be accompanied by interventions that support the whole care cascade. In the Treatment as Prevention (TasP) trial of universal test-and-treat in KwaZulu-Natal, South Africa, repeated rounds of home-based testing increased the proportion of people knowing their HIV status to 91.5%.16 However, only 58.0% of these individuals commenced ART; many did not link to care.16 This suggests that to reach 95-95-95, all three targets must be understood and addressed in parallel.17
The second 95
The second 95 is more readily measured, as countries (or their health facilities and non-governmental organisations) generally have stronger records on the number of people receiving ART. According to World Health Organization recommendations, early ART commencement has reduced HIV/AIDS-related mortality, with some models showing an estimated 75% fewer deaths per annum.18 Test-and-treat strategies recommending commencement of ART within 14 days of a positive diagnosis (independent of the CD4+ T-cell count) are relatively recent in most of SSA,19 and uptake has been commendable.20 Broader implementation is limited not only by the political will but also by the resources required to upskill staff and provide a sustainable treatment supply. Given the scale of the epidemic in ESA, the rollout of any advances in treatment regimens to the front lines can present a formidable challenge. The system’s fragility has been highlighted by COVID-19 over the past year, with reports of delays in the delivery of treatment stock from international suppliers, depleted national stockpiles and periods of lockdown limiting individuals’ access to HIV medications.21
Based on country guidelines, in 2014–2015, of those eligible for ART in Manhiça, Mozambique, 83.7% started ART within 3 months.22 In July 2016, Mozambique phased in the implementation of test-and-treat and undertook qualitative research into the patterns of ART initiation or refusal.23,24 The acceptance of treatment depends on the availability and accessibility of services, as well as appropriate and considered explanations following diagnosis.25
Linkage to care is improved by the desire to live, family support and subjective illness.23,24 Barriers to linkage to care include the fear of dissemination of one’s HIV status, feeling subjectively healthy, migration, health system issues and fears of discrimination.23,24,26
The third 95
Achieving viral suppression requires retention in care, maintenance of ART and regular testing of the HIV viral load. Retention in care is improved by feeling better after ART initiation, confidence in the health system and support from family and providers.27 Communication about continuing treatment despite feeling better also helps.24 Barriers to retention include provider authoritarianism, which limits patient autonomy and engagement in their healthcare, and the adverse effects of ART.27 Men across the region are a hard-to-reach group; they test less, and more abandon ART after initiation.22 There are additional complexities related to paediatric care, such as the health literacy of parents and their confidence in managing HIV. For children, retention is highest when both mother and child register concurrently.28 Innovative strategies to improve testing uptake and support early ART initiation and nutritional supplementation can improve retention.29,30
Before the introduction of the universal test-and-treat programme, there were concerns that commencing ART among people with high CD4+ T-cell counts would overburden the health system and that those feeling healthy would not adhere to treatment.31 However, of the PLWH in KwaZulu-Natal with CD4+ T-cell counts of > 500 cells/µL, 78% accepted ART,32 86% were adherent33 and 96% achieved viral suppression.34 Furthermore, retention in care and viral suppression were similar among people who initiated ART with CD4+ T-cell counts of > 500 cells/µL compared to those with lower CD4+ T-cell counts.35
Measuring the third 95 requires country-wide laboratory systems capable of processing large volumes of viral load requests and returning results; thus, many ESA countries score poorly.10 For example, in Mozambique, only 45% of PLWH achieve documented viral suppression10; however, viral load testing is available to few, particularly in rural settings.36,37 Under-developed laboratory systems also delay diagnoses of virological failure, leading to increased transmission, illness progression and treatment resistance.38 Point-of-care (POC) viral load testing improved viral suppression, retention in care and the communication of results to patients in KwaZulu-Natal,39 and it proved feasible and cost-effective in Botswana and Zambia.40,41 Further development of centralised, high-throughput laboratory-based testing alongside decentralised POC testing will be crucial to ensure adequate monitoring of viral suppression throughout ESA.
The impending challenge of acute HIV infections
Acute HIV infection is commonly defined as the period prior to seroconversion, between 3 and 12 weeks in duration.42,43 Gene expression is vastly upregulated in the initial months, driving inflammation, immune responses and cell turnover.44 This correlates with a substantial peak in viral load, meaning the risk of onward transmission during acute HIV is 8–25 times higher than during chronic infection.45,46,47 The estimated prevalence of acute HIV infection in ESA is 1% – 3%.48,49,50,51 Undiagnosed acute HIV is particularly concerning for the following: pregnant and breastfeeding women who have poorer health outcomes as well as increased perinatal transmission risk52,53,54,55; people who received blood transfusions screened for HIV serology but not viral load56,57; and those who started PreP when already infected, as this may confer an increased risk of drug resistance mutations.58
The earliest time period that an acute HIV infection can be detected is 5–14 days by nucleic acid amplification.59 This is not feasible in low-resource settings, so other options include viral load POC testing (Gene Xpert60 and AlereQ61), p24 antigen testing (if developed into rapid tests),62 non-viral immune response biomarkers (e.g. IP-10)63 or a symptom/risk score.8,64 Rapid testing and ART for all HIV-seropositive individuals remains the priority; however, a focus on this alone will miss seronegative HIV-infected individuals. As ART coverage increases, the proportion of HIV transmission attributable to acute HIV will increase. Affordable rapid tests for p24 or non-viral immune markers combined with a risk score may be the best way to identify acutely infected individuals in high-HIV-burden, low-resource settings.
Disproportionate impact of new HIV infections on key and vulnerable populations
Of the 1.7 million new HIV infections in 2019, 62% occurred in key populations and their sexual partners.6 Key populations include men who have sex with men,65 people who inject drugs,66 female sex workers67 and transgender people.6 Vulnerable populations at increased HIV risk in ESA include prisoners,6,68 long-haul truck drivers,69 mobile mining workers,70 migrants71 and serodiscordant couples.6 Also at disproportionately high risk of HIV infection are young women,72 who are 2–3 times73,74 more likely to be newly infected than their 15–24-year-old male counterparts.
Pregnant and breastfeeding women and their infants are an often-overlooked vulnerable population.52 Infants of mothers who acquired HIV during pregnancy or postpartum are at increased risk of HIV transmission compared to infants of chronically HIV-infected mothers.52
Approximately 45% of new global infections in 2019 were in ESA.6,10 No country in ESA has sufficient data to describe the size of their key populations,10,75 although several have commenced population-specific mapping (Table 1).76 Control of HIV in these populations will contribute to the deceleration of the HIV epidemic in the general population. National surveys of key populations biennially are recommended, as knowing the epidemic is the first key to design the response.
TABLE 1.
Prevalence of HIV among certain key and vulnerable populations in ESA.
| Country | HIV prevalence among |
Reference | |||
|---|---|---|---|---|---|
| MSM (%) | Sex workers (%) | PWID (%) | Prisoners (%) | ||
| Angola | 2.0 [2017] | 8.0 [2017] | - | 15.9 [2017] | 10 |
| Botswana | 14.8 [2018] | 42.2 [2018] | - | - | 75 |
| Comoros | 0.0 [2018] | 0.3 [2017] | 1.8 [2017] | - | 10 |
| Eritrea | - | 10.4 [2014] | - | 1.4 [2019] | 10, 75 |
| eSwatini | 12.6 [2015] | 60.5 [2015] | - | 34.9 [2015] | 75 |
| Ethiopia | - | 24.3 [2014] | 6 [2018] | 4.2 [2016] | 75, 77, 78 |
| Kenya | 18.2 [2011] | 29.3 [2011] | 18.3 [2011] | 5.7 [2016] | 78, 79 |
| Lesotho | 32.9 [2014] | 71.9 [2014] | - | 31.4 [2017] | 10, 75 |
| Madagascar | 14.9 [2014] | 5.5 [2016] | 8.5 [2016] | 0.3 [2018] | 75 |
| Malawi | 6.8 [2019] | 55.0 [2018] | - | 19.0 [2019] | 10 |
| Mauritius | 17.2 [2015] | 15.0 [2015] | 32.3 [2017] | 17.3 [2017] | 75 |
| Mozambique | 3.1–9.1 [2015] | 17.8–31.2 [2016] | 19.9–50.1 [2019] | 24.0 [2019] | 75, 80, 81, 82 |
| Namibia | 12.4 [2009] | 40.7 [2016] | - | - | 75, 83 |
| Rwanda | 4.0 [2016] | 45.8 [2016] | - | - | 75 |
| Seychelles | 13.2 [2013] | 4.6 [2015] | 23.0 [2019] | 9.9 [2019] | 10, 79 |
| South Africa | 18.1 [2018] | 57.7 [2015] | 21.8 [2018] | 11.1 [2019] | 75, 79 |
| South Sudan | - | 11.4 [2019] | - | 5.3 [2016] | 10, 84 |
| Uganda | 13.2 [2013] | 31.3 [2017] | 17.0 [2017] | 4.0 [2019] | 10, 79 |
| UR Tanzania | 8.4 [2018] | 15.4 [2018] | 15.5 [2013] | 6.7 [2015] | 10, 79 |
| Zambia | - | 48.8 [2017] | - | 27.4 [2015] | 10 |
| Zimbabwe | 21.1 [2019] | 42.2 [2019] | - | 28.0 [2015] | 10 |
Note: Data on the HIV prevalence among transgender people are not presented in this table because of a lack of data on this key population in ESA; of the vulnerable populations, only prisoners and incarcerated people have sufficient data in ESA to be presented in this table.
MSM, men who have sex with men; PWID, people who inject drugs; [year], year of publication.
Recommendations for research priorities
Promote and expand local prevention research, including programme and policy evaluations.
Investigate and implement methods to improve the accessibility of HIV education and testing, including routine surveillance, particularly for key populations.
Support in-country trials of viral load and CD4 T-cell count POC testing and the surrounding services required to improve ART adherence, clinical management and retention in care.
Conclusion
The road to HIV elimination in ESA requires continued strong and sustained national and international investment, commitment to evidence-based programmes and persistence. The region contains over half of the world’s population of PLWH and continues to have major challenges to achieving 90-90-90, let alone the looming target of 95-95-95. The priority must remain diagnosing, treating and virally suppressing all existing HIV infections. However, in high-prevalence settings, the prevention of new infections and early diagnosis of acute infections remain important goals. Research must ensure that responses in the region are targeted, efficient and evaluated. In particular, ESA will benefit from strengthened surveillance and key and vulnerable population research, in-country development and validation of HIV tests, and supported rapid transition to new ART regimens to ensure sustainable progress towards important global goals.
Acknowledgements
The authors thank Eva Kiwango, the UNAIDS country director for Mozambique, for the contribution to this article.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors’ contributions
E.P. and M.J. are co-first authors and have contributed equally to the article. E.P., M.A.J., E.M., T.N., D.N. and P.N.L.S. planned and organised the collaboration; all authors contributed region-specific knowledge and expertise. E.P., M.A.J., D.N. and P.N.L.S. wrote the manuscript, with review and revision by all authors.
Ethical considerations
This article followed all ethical standards for research without direct contact with human or animal subjects.
Funding information
This study was supported by the Manhiça Foundation, Mozambique, and a Research Impact Grant (RA/1/2799/40) from the University of Western Australia.
Data availability
Publicly available data sets were accessed from https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf (associated with Figures 1 and 2) and https://population.un.org/wpp/ (associated with Figure 1).
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
Footnotes
How to cite this article: Parker EL, Judge MA, Macete E, et al. HIV infection in Eastern and Southern Africa: Highest burden, largest challenges, greatest potential. S Afr J HIV Med. 2021;22(1), a1237. https://doi.org/10.4102/sajhivmed.v22i1.1237
References
- 1.Ford N, Ball A, Baggaley R, et al. The WHO public health approach to HIV treatment and care: Looking back and looking ahead. Lancet Infect Dis. 2018;18(3):e76–e86. 10.1016/S1473-3099(17)30482-6 [DOI] [PubMed] [Google Scholar]
- 2.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . Seizing the moment. Geneva: UNAIDS; 2020. Report No.: JC2991. [Google Scholar]
- 3.Wilson D, Halperin DT. “Know your epidemic, know your response”: A useful approach, if we get it right. Lancet. 2008;372(9637):423–426. 10.1016/S0140-6736(08)60883-1 [DOI] [PubMed] [Google Scholar]
- 4.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . Global AIDS Update 2019 – Communities at the Centre. Geneva: UNAIDS; 2019. Report No: JC2956. [Google Scholar]
- 5.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. Report No.: JC2684. [Google Scholar]
- 6.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . Miles to go: Closing gaps, breaking barriers, righting injustices. Geneva: UNAIDS; 2018. Report No.: JC2924. [Google Scholar]
- 7.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . Fast track – Ending the AIDS epidemic by 2030. Geneva: UNAIDS; 2014. Report No.: JC2686. [Google Scholar]
- 8.Sanders EJ, Wahome E, Powers KA, et al. Targeted screening of at-risk adults for acute HIV-1 infection in sub-Saharan Africa. AIDS. 2015;29(Suppl 3):S221–S230. 10.1097/QAD.0000000000000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutstein SE, Ananworanich J, Fidler S, et al. Clinical and public health implications of acute and early HIV detection and treatment: A scoping review. J Int AIDS Soc. 2017;20(1):21579. 10.7448/IAS.20.1.21579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . UNAIDS Data 2020. Geneva: UNAIDS; 2020. Report No.: JC2997E; 2020. [PubMed] [Google Scholar]
- 11.Bernardo EL, Fuente-Soro L, Lopez-Varela E, Naniche D. Anonymity in HIV testing: Implications for public health. Lancet. 2017;390(10112):2546. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS Reference Group on Estimates, Modelling and Projections . Annex on methods. Geneva: UNAIDS; 2018. [Google Scholar]
- 13.Fuente-Soro L, Lopez-Varela E, Augusto O, et al. Monitoring progress towards the first UNAIDS target: Understanding the impact of people living with HIV who re-test during HIV-testing campaigns in rural Mozambique. J Int AIDS Soc. 2018;21(4):e25095. 10.1002/jia2.25095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rentsch CT, Reniers G, Machemba R, et al. Non-disclosure of HIV testing history in population-based surveys: Implications for estimating a UNAIDS 90-90-90 target. Glob Health Action. 2018;11(1):1553470. 10.1080/16549716.2018.1553470 [DOI] [Google Scholar]
- 15.Choko AT, MacPherson P, Webb EL, et al. Uptake, accuracy, safety, and linkage into care over two years of promoting annual self-testing for HIV in Blantyre, Malawi: A community-based prospective study. PLoS Med. 2015;12(9):e1001873. 10.1371/journal.pmed.1001873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Universal test and treat and the HIV epidemic in rural South Africa: A phase 4, open-label, community cluster randomised trial. Lancet HIV. 2018;5(3):e116–e125. [DOI] [PubMed] [Google Scholar]
- 17.Fox MP. Are we shifting attrition downstream in the HIV cascade? Lancet HIV. 2016;3(12):e554–e555. 10.1016/S2352-3018(16)30149-7 [DOI] [PubMed] [Google Scholar]
- 18.Johnson LF, May MT, Dorrington RE, et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study. PLoS Med. 2017;14(12):e1002468. 10.1371/journal.pmed.1002468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tymejczyk O, Brazier E, Yiannoutsos CT, et al. Changes in rapid HIV treatment initiation after national ‘treat all’ policy adoption in 6 sub-Saharan African countries: Regression discontinuity analysis. PLoS Med. 2019;16(6):e1002822. 10.1371/journal.pmed.1002822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford N, Vitoria M, Doherty M. Providing antiretroviral therapy to all who are HIV positive: The clinical, public health and programmatic benefits of Treat All. J Int AIDS Soc. 2018;21(2):e25078. 10.1002/jia2.25078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: Results from multiple mathematical models. Lancet HIV. 2020;7(9):e629–e640. 10.1016/S2352-3018(20)30211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Varela E, Fuente-Soro L, Augusto OJ, et al. Continuum of HIV care in rural Mozambique: The implications of HIV testing modality on linkage and retention. J Acquir Immune Defic Syndr. 2018;78(5):527–535. 10.1097/QAI.0000000000001720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magaço A, Dovel K, Cataldo F, et al. ‘Good health’ as a barrier and facilitator to ART initiation: A qualitative study in the era of test-and-treat in Mozambique. Cult Health Sex. 2019;21(9):1059–1073. 10.1080/13691058.2018.1535091 [DOI] [PubMed] [Google Scholar]
- 24.Nhassengo P, Cataldo F, Magaco A, et al. Barriers and facilitators to the uptake of test and treat in Mozambique: A qualitative study on patient and provider perceptions. PLoS One. 2018;13(12):e0205919. 10.1371/journal.pone.0205919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: A systematic review. AIDS. 2012;26(16):2059–2067. 10.1097/QAD.0b013e3283578b9b [DOI] [PubMed] [Google Scholar]
- 26.Fuente-Soro L, Iniesta C, López-Varela E, et al. Tipping the balance towards long-term retention in the HIV care cascade: A mixed methods study in southern Mozambique. PLoS One. 2019;14(9):e0222028. 10.1371/journal.pone.0222028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maixenchs M, Boene H, Anselmo R, et al. Post-ART symptoms were not the problem: A qualitative study on adherence to ART in HIV-infected patients in a Mozambican rural hospital. PLoS One. 2015;10(9):e0137336. 10.1371/journal.pone.0137336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nhampossa T, Fernandez S, Augusto O, et al. Discordant retention of HIV-infected mothers and children: Evidence for a family-based approach from Southern Mozambique. Medicine (Baltimore). 2020;99(32):e21410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: The RapIT randomized controlled trial. PLoS Med. 2016;13(5):e1002015. 10.1371/journal.pmed.1002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saghayam S, Wanke C. The impact of nutritional status and nutrition supplementation on outcomes along the HIV treatment cascade in the resource-limited setting. Curr Opin HIV AIDS. 2015;10(6):472–476. 10.1097/COH.0000000000000202 [DOI] [PubMed] [Google Scholar]
- 31.Geffen N, Low M. When to start antiretroviral treatment? A history and analysis of a scientific controversy. S Afr J HIV Med. 2017;18(1):a734. 10.4102/sajhivmed.v18i1.734 [DOI] [Google Scholar]
- 32.Garrett N, Norman E, Leask K, et al. Acceptability of early antiretroviral therapy among South African women. AIDS Behav. 2018;22(3):1018–1024. 10.1007/s10461-017-1729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwuji C, McGrath N, Calmy A, et al. Universal test and treat is not associated with sub-optimal antiretroviral therapy adherence in rural South Africa: The ANRS 12249 TasP trial. J Int AIDS Soc. 2018;21(6):e25112. 10.1002/jia2.25112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorward J, Drain PK, Osman F, et al. Early antiretroviral therapy is associated with better viral suppression and less HIV drug resistance after implementation of universal treatment in South Africa. AIDS Res Hum Retroviruses. 2020;36(4):297–299. 10.1089/AID.2019.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorward J, Sookrajh Y, Gate K, et al. HIV treatment outcomes among people with initiation CD4 counts >500 cells/µL after implementation of Treat All in South African public clinics: a retrospective cohort study. Journal of the International AIDS Society. 2020;23(4):e25479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mozambique Ministry of Health (MISAU) . 2017 annual report of activities related to HIV/AIDS. Maputo, Mozambique: MISAU; April 2018. [Google Scholar]
- 37.Swannet S, Decroo T, De Castro SMTL, et al. Journey towards universal viral load monitoring in Maputo, Mozambique: Many gaps, but encouraging signs. Int Health. 2017;9(4):206–214. 10.1093/inthealth/ihx021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen ML, Tran L, Geng EH, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS. 2014;28(14):2097–2107. 10.1097/QAD.0000000000000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drain PK, Dorward J, Violette LR, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): Findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV. 2020;7(4):e229–e237. 10.1016/S2352-3018(19)30402-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moyo S, Mohammed T, Wirth KE, et al. Point-of-care Cepheid Xpert HIV-1 viral load test in rural African communities is feasible and reliable. J Clin Microbiol. 2016;54(12):3050–3055. 10.1128/JCM.01594-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girdwood SJ, Nichols BE, Moyo C, Crompton T, Chimhamhiwa D, Rosen S. Optimizing viral load testing access for the last mile: Geospatial cost model for point of care instrument placement. PLoS One. 2019;14(8):e0221586. 10.1371/journal.pone.0221586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis. 2010;202(Suppl 2):S270–S277. 10.1086/655651 [DOI] [PubMed] [Google Scholar]
- 43.Hecht FM, Wellman R, Busch MP, et al. Identifying the early post-HIV antibody seroconversion period. J Infect Dis. 2011;204(4):526–533. 10.1093/infdis/jir304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker E, Judge M, Pastor L, et al. Signature of the storm: RNA-seq analysis of the extensive host transcriptome dysregulation caused by acute HIV-1 infection in a Mozambican cohort. Abstracts of AIDS 2018; 2018 Jul 23–27; Amsterdam; Abstract WEPEA018. Geneva: International AIDS Society; 2018. [Google Scholar]
- 45.Pilcher CD, Tien HC, Eron JJ, Jr., et al. Brief but efficient: Acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–1792. 10.1086/386333 [DOI] [PubMed] [Google Scholar]
- 46.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 Transmission per Coital Act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409. 10.1086/429411 [DOI] [PubMed] [Google Scholar]
- 47.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: A modelling study. Lancet. 2011;378(9787):256–268. 10.1016/S0140-6736(11)60842-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilcher CD, Price MA, Hoffman IF, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004;18(3):517–524. 10.1097/00002030-200402200-00019 [DOI] [PubMed] [Google Scholar]
- 49.Fiscus SA, Pilcher CD, Miller WC, et al. Rapid, real-time detection of acute HIV infection in patients in Africa. J Infect Dis. 2007;195(3):416–424. 10.1086/510755 [DOI] [PubMed] [Google Scholar]
- 50.Serna-Bolea C, Munoz J, Almeida JM, et al. High prevalence of symptomatic acute HIV infection in an outpatient ward in southern Mozambique: Identification and follow-up. AIDS. 2010;24(4):603–608. 10.1097/QAD.0b013e328335cda3 [DOI] [PubMed] [Google Scholar]
- 51.Bebell LM, Pilcher CD, Dorsey G, et al. Acute HIV-1 infection is highly prevalent in Ugandan adults with suspected malaria. AIDS. 2010;24(12):1945–1952. 10.1097/QAD.0b013e32833bb732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: A systematic review and meta-analysis. PLoS Med. 2014;11(2):e1001608. 10.1371/journal.pmed.1001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Schacht C, Hoffman HJ, Mabunda N, et al. High rates of HIV seroconversion in pregnant women and low reported levels of HIV testing among male partners in Southern Mozambique: Results from a mixed methods study. PLoS One. 2014;9(12):e115014. 10.1371/journal.pone.0115014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Schacht C, Mabunda N, Ferreira OC, et al. High HIV incidence in the postpartum period sustains vertical transmission in settings with generalized epidemics: A cohort study in Southern Mozambique. J Int AIDS Soc. 2014;17(1):18808. 10.7448/IAS.17.1.18808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González R, Rupérez M, Sevene E, et al. Effects of HIV infection on maternal and neonatal health in southern Mozambique: A prospective cohort study after a decade of antiretroviral drugs roll out. PLoS One. 2017;12(6):e0178134. 10.1371/journal.pone.0178134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: A systematic review. AIDS. 2014;28(10):1509–1519. 10.1097/QAD.0000000000000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansasuta P, Rowland-Jones SL. HIV-1 transmission and acute HIV-1 infection. Br Med Bull. 2001;58(1):109–127. 10.1093/bmb/58.1.109 [DOI] [PubMed] [Google Scholar]
- 58.Misra KHJ, Daskalakis D, Udeagu C. editor. Impact of PrEP on drug resistance and acute HIV infection, New York City, 2015–2017. Conference on Retroviruses and Opportunistic Infections; 2019 Mar 04–07. Seattle, WA; 2019. [Google Scholar]
- 59.Ananworanich J, Phanuphak N, De Souza M, et al. Incidence and characterization of acute HIV-1 infection in a high-risk Thai population. J Acquir Immune Defic Syndr. 2008;49(2):151–155. [DOI] [PubMed] [Google Scholar]
- 60.Nash M, Huddart S, Badar S, Baliga S, Saravu K, Pai M. Performance of the Xpert HIV-1 viral load assay: A systematic review and meta-analysis. J Clin Microbiol. 2018;56(4):e01673-17. 10.1128/JCM.01673-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jani IV, Meggi B, Vubil A, et al. Evaluation of the whole-blood Alere Q NAT point-of-care RNA assay for HIV-1 viral load monitoring in a primary health care setting in Mozambique. J Clin Microbiol. 2016;54(8):2104. 10.1128/JCM.00362-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pilcher CD, Christopoulos KA, Golden M. Public health rationale for rapid nucleic acid or p24 antigen tests for HIV. J Infect Dis. 2010;201(Suppl 1):S7–S15. 10.1086/650393 [DOI] [PubMed] [Google Scholar]
- 63.Pastor L, Casellas A, Carrillo J, et al. IP-10 Levels as an accurate screening tool to detect acute HIV infection in resource-limited settings. Sci Rep. 2017;7(1):8104. 10.1038/s41598-017-08218-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Powers KA, Miller WC, Pilcher CD, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: Development of a risk score algorithm. AIDS. 2007;21(16):2237–2242. 10.1097/QAD.0b013e3282f08b4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Institute of Health (INS), United States Centers for Disease Control (CDC), University of California San Francisco (UCSF), Pathfinder International Population Services International (PSI), Mozambican Association for the Defence of Sexual Minorities (LAMBDA), International Training and Education Center for Health (I-TECH) . Final Report: Integrated, biological and behavioral survey among men who have sex with men, Mozambique. San Francisco, CA: UCSF; 2011. Contract No.:U2GPS001468. [Google Scholar]
- 66.Mozambique Ministry of Health (MISAU), National Institute of Health (INS), United States Centers for Disease Control (CDC), et al. Final Report: Integrated biological and behavioral survey among people who inject drugs in Mozambique. Maputo: UCSF; 2014. [Google Scholar]
- 67.National Institute of Health (INS), United States Centers for Disease Control (CDC), University of California San Francisco (UCSF), Pathfinder International Population Services International (PSI), International Training and Education Center for Health (I-TECH) . Final Report: Integrated biological and behavioral survey among female sex workers, Mozambique. San Francisco, CA: UCSF; 2011–2012. Contract No.:U2GPS001468. [Google Scholar]
- 68.United Nations Office on Drugs and Crime, The Joint United Nations Programme on HIV/AIDS (UNAIDS), The World Bank Group . HIV and prisons in sub-Saharan Africa. Geneva: UNAIDS; 2007. [Google Scholar]
- 69.Mozambique Ministry of Health (MISAU), National Institute of Health (INS), United States Centers for Disease Control (CDC), et al. Final Report: Integrated survey biological and behavioral analysis among Long Haul truck drivers. Maputo: MISAU; 2012. Contract No.:U2GPS001468. [Google Scholar]
- 70.Mozambique Ministry of Health (MISAU), National Institute of Health (INS), Ministry of Labour (MITRAB), United States Centers for Disease Control (CDC), University of California San Francisco (UCSF), International Training and Education Center for Health (I-TECH) . Final Report: Integrated biological and behavioral survey among Mozambican workers in the mines of the Republic of South Africa. Maputo: UCSF; 2012. Contract No.:U2GPS001468. [Google Scholar]
- 71.International Organization for Migration (IOM) . Briefing note on HIV and labour migration in Mozambique. Pretoria: IOM; 2007. [Google Scholar]
- 72.De Oliveira T, Kharsany AB, Graf T, et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: A community-wide phylogenetic study. Lancet HIV. 2017;4(1):e41–e50. 10.1016/S2352-3018(16)30186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . Country overview – Mozambique Geneva [homepage on the Internet] [cited 2020 Jan 4]. Available from: https://www.unaids.org/en/regionscountries/countries/mozambique
- 74.Republic of Mozambique National Council for the Fight against HIV/AIDS . National strategic plan for the response to HIV and AIDS 2015–2019. Maputo: CNCS; 2015. [Google Scholar]
- 75.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . UNAIDS Data 2019 – Eastern and Southern Africa. Geneva: UNAIDS; 2019. Report No.:JC2959E. [Google Scholar]
- 76.Abdul-Quader AS, Gouws-Williams E, Tlou S, Wright-De Agüero L, Needle R. Key populations in sub-Saharan Africa: Population size estimates and high risk behaviors. AIDS Behav. 2015;19 (Suppl 1):S1–S2. 10.1007/s10461-014-0963-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demissie M, Johnston LG, Muleta M, et al. Prevalence of HIV and other infections and injection behaviours among people who inject drugs in Addis Ababa, Ethiopia. Afr J AIDS Res. 2018;17(3):259–264. 10.2989/16085906.2018.1511604 [DOI] [PubMed] [Google Scholar]
- 78.Telisinghe L, Charalambous S, Topp SM, et al. HIV and tuberculosis in prisons in sub-Saharan Africa. Lancet (London, England). 2016;388(10050):1215–1227. 10.1016/S0140-6736(16)30578-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.The Joint United Nations Programme on HIV/AIDS (UNAIDS) . UNAIDS key population atlas Geneva [homepage on the Internet] [cited 2021 Mar 31]. Available from: https://kpatlas.unaids.org/dashboard
- 80.Nalá R, Cummings B, Horth R, et al. Men who have sex with men in Mozambique: Identifying a hidden population at high-risk for HIV. AIDS Behav. 2015;19(2):393–404. 10.1007/s10461-014-0895-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Augusto Âdo R, Young PW, Horth RZ, et al. High burden of HIV infection and risk behaviors among female sex workers in three main urban areas of Mozambique. AIDS Behav. 2016;20(4):799–810. 10.1007/s10461-015-1140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Semá Baltazar C, Horth R, Boothe M, et al. High prevalence of HIV, HBsAg and anti-HCV positivity among people who injected drugs: Results of the first bio-behavioral survey using respondent-driven sampling in two urban areas in Mozambique. BMC Infect Dis. 2019;19(1):1022. 10.1186/s12879-019-4655-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baral S, Trapence G, Motimedi F, et al. HIV prevalence, risks for HIV infection, and human rights among men who have sex with men (MSM) in Malawi, Namibia, and Botswana. PLoS One. 2009;4(3):e4997. 10.1371/journal.pone.0004997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heijnen M, Mumtaz GR, Abu-Raddad LJ. Status of HIV and hepatitis C virus infections among prisoners in the Middle East and North Africa: Review and synthesis. J Int AIDS Soc. 2016;19(1):20873. 10.7448/IAS.19.1.20873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.United Nations, Department of Economic and Social Affairs, Population Division . New York, World population prospects 2019 [homepage on the Internet] [cited 2020 Aug 16]. Available from: https://population.un.org/wpp/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available data sets were accessed from https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf (associated with Figures 1 and 2) and https://population.un.org/wpp/ (associated with Figure 1).