Abstract
Background:
Growth-differentiation factor-15 (GDF-15) is a distant member of the transforming growth factor-beta cytokine superfamily expressed in human atherosclerotic plaque macrophages. In this study, we sought to compare GDF-15 between patients with coronary artery disease and control group.
Methods:
In this cross-sectional study, 176 subjects were enrolled, consisted of 88 coronary artery disease patients (CAD group) and 88 non-CAD participants (control group. Clinical and demographic data, comprising of family history of CAD, history, and lifestyle factors, hypertension, diabetes, and some blood parameters (e.g. glucose, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C)), triglyceride, high-sensitivity C-reactive protein (hs-CRP)).
Results:
Mean age of the patients was 55.5±11.1 years (age range: 28–80 years). Of all the participants, 91 (51.7%) were male and 85 (48.3%) female. Hs-CRP, LDL-C, and GDF-15 levels were significantly higher in the CAD patients (P=0.091, P=0.008, and P<0.001, respectively). Total cholesterol, hematocrit, and hemoglobin were significantly higher in the controls (P=0.002, P=0.011, and P=0.055, respectively). The area under the receiver operating characteristic curve yielded the satisfactory result of 0.9 (95% CI, 0.8-0.9; P<0.001). The optimum cut-off value of GDF-15 was 1233 ng/L with 71% specificity and 71% sensitivity for CAD diagnosis.
Conclusion:
These data suggest that serum GDF-15 might be useful in prediction of CAD. (www.actabiomedica)
Keywords: Growth differentiation factor-15, Coronary artery disease, Angiography
Introduction
Coronary artery disease (CAD) is a worldwide health concern with high morbidity and mortality rates. It is a chronic inflammatory atherosclerosis process lead to CAD and acute coronary syndromes (1). Cytokine or growth factors produced in the inflamed intima induce monocytes to enter the plaque to differentiate into macrophages leading to the development of atherosclerosis (2).
Effector molecules from immune cells that dominate the early stage of atherosclerotic lesions accelerate lesion progression and further elicit acute coronary syndromes. Inflammatory factors such as C-reactive protein or monocyte chemoattractant protein-1 are attractive biomarkers in order to the prediction of risk for developing coronary artery disease (3, 4). Although these biomarkers are promising, they only have a moderate predictive value and are not widely used in clinical practice (5).
Therefore, there is a growing interest in using blood circulating biomarkers to obtain pathophysiological insight and improve the management of CAD patients.
Microarray analyses have been used to identify downstream targets of nitric oxide (NO) when growth differentiation factor-15 (GDF-15) first came to attention (6). GDF-15 is a distant member of the transforming growth factor beta (TGF-β) cytokine superfamily that was initially cloned from an activated macrophage cell line (7).
Like other TGF-β-related cytokines, GDF-15 is synthesized as a precursor protein that undergoes disulfide-linked dimerization (8). under physiological circumstances, GDF-15 is weakly produced in most tissues (9), its expression level may increase in response to pathological stress associated with tissue injury or inflammation. Along that line, GDF-15 was detected in macrophages in human carotid atherectomy samples (10).
Cardiomyocytes express and secrete GDF-15 following ischemia or any reperfusion injury, stimulation with reactive oxygen species or pro-inflammatory cytokines, and exposure to mechanical stretch (7). GDF-15 expression in the myocardium has been shown to increase in mouse models of myocardial infarction, aortic stenosis, and dilated cardiomyopathy (11, 12).
GDF-15 is also expressed in the myocardium of patients with acute myocardial infarction (11).
However, GDF-15 is not a cardiac-specific cytokine, oxidized low-density lipoproteins and pro-inflammatory cytokines induce GDF-15 are expressed in cultured macrophages (10, 13). Consistent with these in-vitro observations, GDF-15 is expressed in human atherosclerotic plaque macrophages (14). GDF-15 is also upregulated in the endothelial cells exposed to antiangiogenic stress (15, 16) and in vascular smooth muscle cells upon stimulation with triglyceride-rich lipoproteins (17). Adipocytes synthesize and secrete GDF-15 upon exposure to oxidative stress and may release paracrine factors that promote GDF-15 expression in the neighboring cells (17). Hence, GDF-15 is expressed by multiple cardiovascular cell types under stressful circumstances. With this background in mind, in the present study, we aimed to compare GDF-15 between CAD patients and healthy cases.
Materials and Methods
In this cross-sectional study, 176 subjects were enrolled, which consisted of 88 CAD patients (CAD group) and 88 non-CAD participants (control group) recruited from cardiac catheterization lab of Ghaem Hospital, Mashhad, Iran, during January-December 2015.
The diagnosis of CAD was confirmed by coronary angiography performed with Judkins technique using quantitative coronary angiographic system. CAD diagnosis was defined by angiography with at least one main coronary vessel > 50% luminal narrowing. The control group was defined as angiography with no or < 50% luminal narrowing. All the CAD patients were new case.
Inclusion and exclusion criteria
The inclusion criteria were being candidate for angiography and aged 20-80 years old; the exclusion criteria comprised of history of myocardial infarction and history of using statin drugs.
Data collection
Demographic information, family history of CAD, history, and lifestyle factors were obtained through interview and recorded in a questionnaire. Hypertension was diagnosed in individuals with systolic blood pressure at or above 140 mmHg, diastolic blood pressure at or above 90 mmHg, and/or consumption of anti-hypertension medication.
Diabetes mellitus was defined as fasting blood sugar (FBS) of ≤126 mg/dl or consumption of oral hypoglycemic agents or insulin. Blood samples (10 cc) were collected in the morning of the angiography day after an overnight fast from the femoral artery of the angiographic catheter insertion site. Serum and plasma were separated by centrifugation at 1500 g for 15 min and stored at -80°c until testing. The GDF-15 assay was constructed using Human GDF-15 Duoset Kit (Biotechne, R&D, Minneapolis, United states) by ELISA method.
Statistical analysis
Statistical analyses were performed in SPSS, version 16. The normal distribution assumptions were assessed by Kolmogorov-Smirnov test. Mean±standard deviation was applied for quantitative normally distributed variables, median (plus range) for quantitative non-parametric data, and number (plus percentage) for qualitative variables. Quantitative normally distributed variables were compared using the independent samples t-test, and quantitative non-parametric variables were compared by performing the Mann–Whitney test. P-value less than 0.05 was considered statistically significant. Serum GDF-15 concentrations were used to draw receiver operating characteristic (ROC) curve, and the specificity, sensitivity, and area under the ROC curve (AUC) were determined.
Ethical consideration
Written informed consent was obtained from each participant. The confidentiality of the subjects the study was approved by the Ethics Committee of Mashhad University of Medical Sciences (ethical code: IR.MUMS.REC.1391.23). Peripheral blood sample was obtained from each participant.
Results
Mean age of the patients was 55.52±11.10 years (range: 28–80 years). We also compared women with the age-matched men. The mean age of the male patients was 61.66±7.94 years, while it was 61.92±9.67 years in females (P=0.846). Other demographic data are presented in Table 1.
Table 1.
Baseline of clinical and laboratory data according to gender
| Characteristics | Male (n=91) | Female (n=85) | P-value |
| Age (years) | 61.66±7.94 | 61.92±9.67 | 0.846 |
| Height | 163.23±11.56 | 155.21±6.89 | <0.001 |
| Weight | 71.47±11.85 | 67.02±13.55 | 0.033 |
| BMI (kg/m2) | 25.70±3.64 | 25.16±3.31 | 0.306 |
| Hypertension n (%) | 32(40.0%) | 35 (44.9%) | 0.536 |
| Diabetes n (%) | 23(28.8%) | 19(23.8%) | 0.472 |
| hs-CRP (mg/dl) | 3.14(1.50-8.81) | 2.85(1.19-6.66) | 0.324 |
| TC (mg/dl) | 182.66±63.27 | 187.48±66.77 | 0.623 |
| TG (mg/dl) | 190.23±64.82 | 201.86±63.44 | 0.231 |
| LDL-C (mg/dl) | 126.38±38.36 | 127.62±3369 | 0.820 |
| HDL-C (mg/dl) | 47.89±15.85 | 44.41±13.26 | 0.129 |
| FBS (mg/dl) | 114.43±56.03 | 107.48±41.37 | 0.594 |
| HCT | 39.53±5.27 | 39.49±4.90 | 0.960 |
| Height/Weight | 0.43±0.08 | 0.42±0.08 | 0.356 |
| Hb | 13.10±1.90 | 13.06±1.68 | 0.883 |
| GDF-15 (ng/l) | 923.49±665.09 | 873.79±538.39 | 0.920 |
All values are mean±standard deviation for quantitative normal distribution variables and median (range) for quantitative non-parametric data; also number (percentage) for Qualitative variables. BMI (body mass index), hs-CRP (high sensitivity-C reactive protein), TC (total cholesterol), TG (total triglyceride), LDL-C (low-density lipoprotein cholesterol), HDL-C (high-density lipoprotein cholesterol), FBS (fasting blood sugar), HCT (hematocrit), Height/Weight, Hb (hemoglobin) and GDF-15 (Growth Differentiation Factor-15)
As presented in table 1 there were significant differences in height and weight between males and females (P<0.001 and P=0.033, respectively). Other variables including age, body mass index (BMI), hypertension, diabetes, high sensitivity-C reactive protein (hs-CRP), total cholesterol (TC), total triglyceride (TG), low density lipoprotein-C (LDL-C), high density lipoprotein-C (HDL-C), fasting blood sugar (FBS), hematocrit (HCT), height/weight, hemoglobin (Hb), and GDF-15 were not significantly different between the two genders.
The mean heights of the patients were 163.23±11.56 and 155.21±6.89 cm in male and female patients, respectively. The mean weights of the patients were 71.47±11.85 and 67.02±13.55 kg in male and female patients, respectively. Also, the mean BMI of the patients were 25.70±3.64 and 25.16±3.31 kg/m2 in male and female patients, respectively. Further, 32 (40%) and 35 (44.9%) of the male and female patients were hypertensive, respectively, and 23 (28.8%) and 19 (23.8%) of the male and female patients were diabetic, respectively. The mean TC levels of the patients were 182.66±63.27 and 187.48±66.77 mg/dl in male and female patients, respectively.
The mean ranges of TG in the male and female patients were 190.23±64.82 and 201.86±63.44 mg/dl, respectively. In addition, the mean ranges of LDL-C in male and female patients were 126.38±38.36 and 127.62±33.69 mg/dl, respectively. The mean ranges of HDL-C in male and female patients were 47.89±15.85 and 44.41±13.26 mg/dl, respectively. The mean FBS levels were 114.43±56.03 and 107.48±41.37 mg/dl in male and female patients, respectively. The mean ranges of HCT in the male and female patients were 39.53±5.27 and 39.49±4.90 mg/dl, respectively. The mean ranges of Hb in male and female patients were 13.10±1.90 and 13.06±1.68 mg/dl, respectively. Finally, the mean ranges of GDF-15 in male and female patients were 923.49±665.09 and 873.79±538.39 mg/dl, respectively.
According to Table 2, hs-CRP, LDL-C, and GDF-15 levels were significantly higher in the CAD patients compared to the non-CAD group (P=0.091, P=0.008, and P<0.001, respectively); besides, TC, HCT, and Hb levels were significantly higher in the control group (P=0.002, P=0.011, and P=0.055, respectively).
Table 2.
Baseline clinical and laboratory data according to coronary artery disease
| Characteristics | Case (n=88) | Control (n=88) | P-value |
| Age (years) | 61.07±8.42 | 62.50±9.15 | 0.281 |
| Height | 160.52±10.44 | 157.45±9.91 | 0.065 |
| Weight | 69.89±12.26 | 68.37±13.95 | 0.472 |
| Female n (%) | 44 (50.0%) | 41 (46.6%) | 0.651 |
| Male n (%) | 44(50.0%) | 47 (53.4%) | |
| BMI (kg/m2) | 25.33±3.47 | 25.55±3.51 | 0.680 |
| Hypertension n (%) | 42(45.7%) | 25(37.9%) | 0.330 |
| Diabetes n (%) | 28(30.4%) | 14(20.6%) | 0.162 |
| hs-CRP (mg/dl) | 3.16(1.54-8.48) | 2.54(0.99-6.63) | 0.091 |
| TC (mg/dl) | 169.69±64.14 | 200.28±62.22 | 0.002 |
| TG (mg/dl) | 201.90±61.20 | 189.80±66.94 | 0.212 |
| LDL-C (mg/dl) | 134.10±37.02 | 119.86±33.85 | 0.008 |
| HDL-C (mg/dl) | 46.10±15.46 | 46.32±14.03 | 0.832 |
| FBS (mg/dl) | 115.49±55.35 | 106.66±42.67 | 0.279 |
| HCT | 38.64±4.50 | 40.37±5.50 | 0.011 |
| Height/Weight | 0.42±0.08 | 0.42±0.08 | 0.992 |
| Hb | 12.82±1.69 | 13.34±1.86 | 0.055 |
| GDF-15(ng/l) | 1069.58±677.46 | 729.40±470.36 | <0.001 |
All values are mean ± standard deviation for quantitative normal distribution variables and median (range) for quantitative non-parametric data; also number (percentage) for Qualitative variables. BMI (body mass index), hs-CRP (high-sensitivity C reactive protein), TC (total cholesterol), TG (total triglyceride), LDL-C (low-density lipoprotein cholesterol), HDL-C (high-density lipoprotein cholesterol), FBS (fasting blood sugar), HCT (hematocrit), Height/Weight, Hb (hemoglobin) and GDF-15 (Growth Differentiation Factor-15)
The other parameters including age (years), height, weight, BMI, hypertension, diabetes, TG, HDL-C, FBS, and height/weight were not significantly different between the CAD and non-CAD groups. The mean heights of the patients were 160.52±10.44 and 157.45±9.91 cm in the case and control groups, respectively. The mean weights were 69.89±12.26 and 68.37±13.95 kg in the case and control groups, respectively. Moreover, 44 (50%) and 47 (53.4%) of the patients in the case and control groups were male, respectively.
Also, the mean BMIs were 25.33±3.47 and 25.55±3.51 kg/m2 in the case and control groups, respectively. Additionally, 42 (45.7%) and 25 (37.9%) of the case and control patients were hypertensive, respectively, 28 (30.4%) and 14 (20.6%) of the case and control patients were diabetic, respectively. The medians and quartile ranges of Hs-CRP in the case and control groups were 3.16 (1.54-8.48) and 2.54 (0.99-6.63), respectively. The mean TCs were 169.69±64.14 and 200.28±62.22 mg/dl in the case and control groups, respectively.
The mean ranges of TG in the case and control groups were 201.90±61.20 and 189.80±66.94 mg/dl, respectively. Also, the mean levels of LDL-C in the case and control groups were 134.10±37.02 and 119.86±33.85 mg/dl, respectively. The mean ranges of HDL-C in the case and control groups were 46.10±15.46 and 46.32±14.03 mg/dl, respectively. The mean FBS levels were 115.49±55.35 and 106.66±42.67 mg/dl in the case and control patients, respectively. The mean ranges of HCT in the case and control patients were 38.64±4.50 and 40.37±5.50 mg/dl, respectively. The mean ranges of Hb in the case and control patients were 12.82±1.69 and 13.34±1.86 mg/dl, respectively. Finally, the mean ranges of GDF-15 in the case and control patients were 1069.58±677.46 and 729.40±470.36 mg/dl, respectively.
To determine the diagnostic predictive value of GDF-15 for CAD, ROC curve analysis was performed. As shown in Figure 1, the area under the ROC curve yielded a satisfactory result of 0.912 (95% CI, 0.866-0.959; P<0.001). The optimum cut-off value of GDF-15 was 1233 ng/L with 71% specificity and 71% sensitivity for CAD diagnosis.
Figure 1.
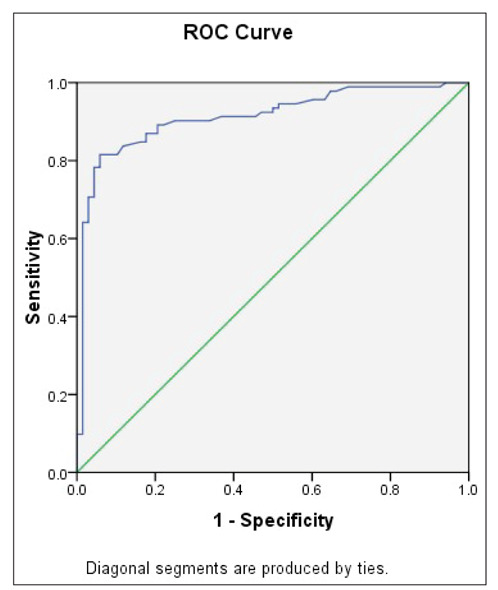
Receiver operating curve (ROC) for evaluating the diagnostic role of growth differentiation factor-15 (GDF-15) on coronary artery disease (CAD); serum GDF-15 concentrations in CAD and control groups were used to draw ROC, and the specificity, sensitivity, and areas under the curve were determined ([AUC] 0.912, CI 0.866–0.959; P<0.0001, optimum cut-off value of GDF-15 was 1233 ng/L with 71% specificity and 71% sensitivity).
Discussion
According to our results, LDL-C and GDF-15 were significantly higher in the CAD patients compared to the controls. TC and HCT levels were significantly higher in the control group. The optimum cut-off value of GDF-15 was 1233 ng/L with 71% specificity and 71% sensitivity for CAD diagnosis. These data suggest that serum GDF-15 can predict CAD.
In a study conducted by Khan et al. in 2009 in the UK, GDF-15 was found as a prognostic marker of death and heart failure (HF) in patients with acute myocardial infraction. According to the ROC analysis, NT-pro BNP and GDF-15 were of similar accuracy in the prediction of death or HF (18). According to previous studies, GDF-15 rapidly increases in response to cardiovascular injury, hypertension, HF, ischemia, and atherosclerosis (7, 19, 20). Also, GDF-15 is strongly correlated with age in both healthy and unhealthy adults (21, 22). Ho et al. showed that GDF-15 is significantly higher in healthy elderlies than younger adults (23). Additionally, GDF-15 is positively associated with diabetes, CAD (21, 22, 24, 25), and chronic kidney disease (26, 27).
Recent studies also exhibited that GDF-15 is correlated with inflammatory biomarkers such as C-reactive protein and N-terminal pro b-type natriuretic peptide, suggesting a link between GDF-15 and inflammation (19, 28, 29).
Prospective investigation of the vasculature in Uppsala Seniors study reported that GDF-15 is related to the male gender, current smoking, and diabetes. In this study observed an independent relationship between GDF-15 and TG level, low LDL, and low HDL, supporting a link between GDF-15 and lipid metabolism (30).
Notably, GDF15 could predict cardiovascular events, even after correction for the Framingham risk score, endothelium-dependent vasodilation, and intima-media thickness (IMT), emphasizing the strong and independent relation of this biomarker with cardiovascular diseases (30).
Conclusion
GDF-15 was significantly higher in CAD patients relative to the controls. The optimum cut-off value of GDF-15 was 1233 ng/L with 71% specificity and 71% sensitivity for CAD diagnosis. These data suggest that serum GDF-15 has a good capability in predicting CAD.
Limitations
This study has some limitations. First, this was a cross-sectional study and had no follow up; thus, we could not clearly establish a causal relationship between GDF-15 and development of CAD. Second, this study only included Iranian subjects; therefore, our results may not fully apply to the general population. Third, the sample size was limited, and further larger studies are required in this regard.
Acknowledgments:
The results presented in this study have been derived from student’s thesis (ID number: 900923). Mashhad University of Medical Sciences funded this study. We are particularly grateful to the patients and their family members who volunteered to participate in this study.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Tong DC, Wilson AM, Layland J. Novel risk factors for acute coronary syndromes and emerging therapies. International journal of cardiology. 2016;220:815–24. doi: 10.1016/j.ijcard.2016.06.148. [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, Van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–7. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SA, Register TC, Riley RF, D’Agostino Jr RB, Stopyra JP, Miller CD. Monocyte chemoattractant protein-1 as a predictor of coronary atherosclerosis in patients receiving coronary angiography. Critical pathways in cardiology. 2018;17(2):105. doi: 10.1097/HPC.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilely K, Fumagalli S, Rosbjerg A, Genster N, Skjoedt M-O, Perego C, et al. C-reactive protein binds to cholesterol crystals and co-localizes with the terminal complement complex in human atherosclerotic plaques. Frontiers in immunology. 2017;8:1040. doi: 10.3389/fimmu.2017.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diercks DB, Kirk JD, Naser S, Turnipseed S, Amsterdam EA. Value of high-sensitivity C-reactive protein in low risk chest pain observation unit patients. International journal of emergency medicine. 2011;4(1):37. doi: 10.1186/1865-1380-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C Tongers, Jr, et al. The transforming growth factor-β superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circulation research. 2006;98(3):351–60. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- Milks MW, Nambi V. Elsevier: Biomarkers in Cardiovascular Disease; 2019. Cardiac Injury, Maladaptation, and Heart Failure Incidence; pp. 81–96. [Google Scholar]
- Horsch A, Zdunek D, Hess G. GDF-15 and/or Troponin T for Predicting Kidney Failure in Heart Surgery Patients. Google Patents. 2016 [Google Scholar]
- George M, Jena A, Srivatsan V, Muthukumar R, Dhandapani V. GDF 15-a novel biomarker in the offing for heart failure. Current cardiology reviews. 2016;12(1):37–46. doi: 10.2174/1573403X12666160111125304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell and tissue research. 2004;318(2):325–33. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- Piek A, Du W, de Boer RA, Sillje HH. Novel heart failure biomarkers: why do we fail to exploit their potential? Critical reviews in clinical laboratory sciences. 2018;55(4):246–63. doi: 10.1080/10408363.2018.1460576. [DOI] [PubMed] [Google Scholar]
- Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circulation research. 2006;98(3):342–50. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- deFilippi C, Christenson R, Joyce J, Park EA, Wu A, Fitch KV, et al. Statin effects on myocardial fibrosis markers in people living with HIV. Journal of acquired immune deficiency syndromes (1999) 2018;78(1):105. doi: 10.1097/QAI.0000000000001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf T, Wollert KC. Growth differentiation factor-15: a new biomarker in cardiovascular disease. Herz. 2009;34(8):594–9. doi: 10.1007/s00059-009-3317-3. [DOI] [PubMed] [Google Scholar]
- Secchiero P, Corallini F, Gonelli A, Dell’Eva R, Vitale M, Capitani S, et al. Antiangiogenic activity of the MDM2 antagonist nutlin-3. Circulation research. 2007;100(1):61–9. doi: 10.1161/01.RES.0000253975.76198.ff. [DOI] [PubMed] [Google Scholar]
- Ferrari N, Pfeffer U, Dell’Eva R, Ambrosini C, Noonan DM, Albini A. The transforming growth factor-β family members bone morphogenetic protein-2 and macrophage inhibitory cytokine-1 as mediators of the antiangiogenic activity of N-(4-hydroxyphenyl) retinamide. Clinical Cancer Research. 2005;11(12):4610–9. doi: 10.1158/1078-0432.CCR-04-2210. [DOI] [PubMed] [Google Scholar]
- Kim Y, Noren Hooten N, Evans MK. CRP Stimulates GDF15 expression in endothelial cells through p53. Mediators of inflammation. 2018;2018 doi: 10.1155/2018/8278039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, et al. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. European heart journal. 2009;30(9):1057–65. doi: 10.1093/eurheartj/ehn600. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Zeller T. Standard and Novel Biomarkers. Chronic Coronary Artery Disease: A Companion to Braunwald’s Heart Disease E-Book. 2017;103:98. [Google Scholar]
- Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clinical chemistry. 2017;63(1):140–51. doi: 10.1373/clinchem.2016.255174. [DOI] [PubMed] [Google Scholar]
- Doerstling S, Hedberg P, Öhrvik J, Leppert J, Henriksen E. Growth differentiation factor 15 in a community-based sample: age-dependent reference limits and prognostic impact. Upsala journal of medical sciences. 2018;123(2):86–93. doi: 10.1080/03009734.2018.1460427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen LL, Zhang Q. Increased Serum Level of Growth Differentiation Factor 15 (GDF-15) is Associated with Coronary Artery Disease. Cardiovascular therapeutics. 2016;34(3):138–43. doi: 10.1111/1755-5922.12184. [DOI] [PubMed] [Google Scholar]
- Ho JE, Mahajan A, Chen M-H, Larson MG, McCabe EL, Ghorbani A, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clinical chemistry. 2012;58(11):1582–91. doi: 10.1373/clinchem.2012.190322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf T, Guba-Quint A, Torgerson J, Magnone MC, Haefliger C, Bobadilla M, et al. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. European Journal of Endocrinology. 2012;167(5):671. doi: 10.1530/EJE-12-0466. [DOI] [PubMed] [Google Scholar]
- Tzikas S, Palapies L, Bakogiannis C, Zeller T, Sinning C, Baldus S, et al. GDF-15 predicts cardiovascular events in acute chest pain patients. PloS one. 2017;12(8) doi: 10.1371/journal.pone.0182314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszyk E, Lukaszyk M, Koc-Zorawska E, Bodzenta-Lukaszyk A, Malyszko J. GDF-15, iron, and inflammation in early chronic kidney disease among elderly patients. International urology and nephrology. 2016;48(6):839–44. doi: 10.1007/s11255-016-1278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adela R, Banerjee SK. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. Journal of diabetes research. 2015;2015 doi: 10.1155/2015/490842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, James SK, Gabrysch K, Storey RF, Himmelmann A, Cannon CP, et al. Association of multiple biomarkers with risk of all-cause and cause-specific mortality after acute coronary syndromes: a secondary analysis of the PLATO biomarker study. JAMA cardiology. 2018;3(12):1160–6. doi: 10.1001/jamacardio.2018.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggen VJ, van den Bosch AE, Eindhoven JA, Schut A-RW, Cuypers JA, Witsenburg M, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide, troponin-T, and growth-differentiation factor 15 in adult congenital heart disease. Circulation. 2017;135(3):264–79. doi: 10.1161/CIRCULATIONAHA.116.023255. [DOI] [PubMed] [Google Scholar]
- Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. European heart journal. 2009;30(19):2346–53. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]


