Abstract
Free fatty acids (FFA) observed as independent risk factors of cardiovascular diseases (CVD). In this study we investigated FFA levels in patients with CVD, and its risk factors. In this case-control study, 214 patients experienced coronary angiography and 222 healthy subjects were enrolled. Participants were categorized into two groups: who had >50% and <30% stenosis were assigned to the angiogram positive (N=90) and negative (N=124) group, respectively. Several risk factors were assessed and the levels of FFAs were determined using gas chromatography. Serum FFA concentrations were compared between healthy and patients with positive and negative angiograms. The association of serum FFA levels with four major risk factors (hypertension, FBG level, high BMI and WHR) were also assessed. Our data showed that median of FFAs was higher in patients than healthy subjects (p<0.0001), such as SFA and n6-FFAs (in patients; 1.59 (1.27) and 1.22 (1.06) and in healthy subjects 0.33 (0.38) and 0.36 (0.35), respectively). According to anthropometric and biochemical data, there were not statistical differences between the groups, except FBG, SBP and hs-CRP that showed significantly higher levels in patients than controls (p<0.0001, p=0.001). Also, lower median levels of total cholesterol, LDL-C, HDL-C and DBP were observed in patients which can due to lipid-lowering medication use like Statins. High serum levels of FFAs are considered as an independent risk factor for CVDs, while various types of FFAs can have different influences on CVD risk factors. Therefore, longitudinal studies are needed to clarify the association between FFAs and CVD risk factors. (www.actabiomedica.it)
Keywords: Free fatty acids, cardiovascular diseases, Risk factors
Abbreviations
- High sensitivity C reactive protein:
Hs-CRP
- High-density lipoprotein:
HDL
- Low-density ipoprotein:
LDL
- Triglycerides:
TG
- Waist circumference:
WC
- Diastolic/systolic blood pressure:
DBP/SBP
- Fasting blood glucose:
FBG
- Mashhad University of Medical Science:
MUMS
- Body mass index:
BMI
- Standard deviation:
SD
- Interquartile range:
IQR
- Cardiovascular disease:
CAD
- Free fatty acid:
FFA
- Waist to hip ratio:
WHR
- Reaction species oxygen:
ROS
- Nuclear factor-kB:
NF-kB
- National Institutes for Medical Research Development:
NIMAD
- Acute coronary syndrome:
ACS
- Enzyme-linked immunosorbent assay:
ELISA
- Protein kinas C:
PKC
- Monounsaturated fatty acids:
MUFAs
- Polyunsaturated fatty acids:
PUFAs
- Saturated fatty acids:
SFAs
Introduction
Cardiovascular disease (CVD) is reported as a major cause of mortality in the world and is considered responsible for one-third of all deaths in over 35 year-individuals in west countries (1, 2). Some risk factors including high blood pressure, increased level of lipid profiles (total cholesterol, TG, LDL-C, HDL-C) and also diabetes have considerable attention because of their important role in the occurrence and development of CVD (3-5). In addition, some previous studies have suggested that a high level of fatty acids can be related to the incidence of cardiovascular disease (6-8) plasma free fatty acids (FFAs) are mainly generated during lipolysis in adipose tissues and provide a source of energy to the heart cells (9, 10). Oxidation of FFAs can lead to the accumulation of toxic intermediates, inflammatory responses and results in abnormalities known as heart diseases (11-13). The increased concentration of FFAs in plasma leads to enhance the binding activity of intranuclear NF-kB that acts to increase ROS generation and is an important step in the induction of inflammation (7, 14, 15).
Plasma FFA can negatively affect insulin activity and partly results in insulin resistance in type 2 diabetic subjects (16). Induced oxidative stress by FFA oxidation leads to endothelial apoptosis and increases insulin resistance (9, 17). Also, in diabetic individuals, lipolysis regulation of circulating FFAs is disrupted which leads to increased FFA oxidation and decreasing glycogenesis in turn (18). It was reported that oxidative modification of LDL-C leads to biological conditions, from foam cells to plaques formation, which results in heart diseases (19-21). In addition, high serum FFA levels may be an independent risk factor for hypertension (22). According to these results, in patients with hypertension and insulin resistance simultaneously, the level of fatty acids significantly increased (22).
A study on Chinese individuals with stable coronary artery disease suggested that increased plasma level of FFAs is an independent risk factor of CVD (23). Also, it was reported that higher levels of FFAs persist and may reflect the severity of ischemia and necrosis during the subacute phase of ACS (acute coronary syndrome) attack (24). According to findings, increased FFA level can be valuable as well as high TG levels and WC measurements for the prediction of CVD and even can be applied as a clinical guide for the early treatment of heart diseases (25). Although elevated plasma FFA concentrations are considered related to CVD, a single fasting measurement of plasma FFA levels does not appear to be an absolute risk factor when the information of other risk factors are considered (8). In addition, one study has investigated the association of FFAs as a risk factor with several cardiovascular diseases in subjects who underwent coronary angiography (26). According to these results, FFA levels are not related to the presence of angiographic CVD (26). In the present study, we aimed to assess the FFA levels in patients with CVD, and its risk factors.
Figure 1.
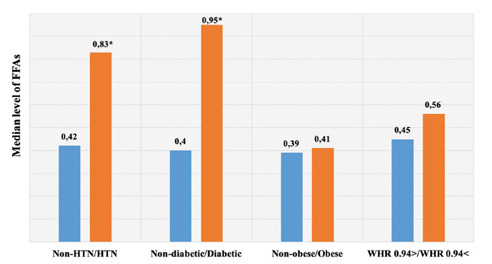
Levels of FFAs in subjects with and without the main risk fators of CVD. * significant difference
Materials and methods
Study population
A total of 437 subjects mean aged 59-61 years old were recruited. All subjects were classified into one of three categories: 1) control group were those with no evidence of coronary artery disease, 2) who had >50% stenosis were assigned to the angiogram positive group 3) those with <30% stenosis were assigned to the angiogram negative group. In this study, patients were under treatments with lipid-lowering drugs such as Statin. In addition, subjects were divided into two groups based on the presence of four major CVD risk factors, including hypertension (SBP >140 mm HG and DBP >90 mmHg), fasting serum glucose (>120 mg/d L) and obesity as mean WHR (>0.94) and BMI (>30 Kg/m2). Exclusion criteria include: pregnancy, and metabolic diseases for CVD group and subjects with confirmed data about taking medicinal drugs, or definite diagnosis of diabetes mellitus, hypertension, osteoporosis, autoimmune diseases, cancers, hematologic disorders, cardiovascular diseases, and hepatitis were excluded from control group. Informed consent was obtained from all participants using protocols approved by the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Anthropometric and blood pressure measurements
Height was measured to the nearest 0.1 cm, and weight was measured to the nearest 0.1 Kg without shoes. BMI (body mass index) was calculated as weight (Kg)/height (m2). Blood pressure (mmHg.) was measured three times using a DONG BANG ACUPRIME device as each subject was requested to sit for approximately 30 minutes before every measurement. Three systolic and diastolic blood pressures (SBP and DBP respectively) were recorded and the average used for the analysis.
Biochemical analysis
All subjects were asked to fast for 12 to 14 hours before the blood sampling. The serum of blood was used to determine the levels triglycerides (TG), total cholesterol (TC), fasting blood glucose (FBG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) as lipid profiles using commercial kits and an Alycon auto-analyzer (ABBOTT, Chicago, IL, USA). Also, serum hs-CRP levels were estimated using the enzyme-linked immunosorbent assay (ELISA) kits (27).
Measurement of fatty acids levels
The first step included preparation and lipid extraction from plasma. Briefly, 500μl of the sample, 2ml chloroform, and 1ml ethanol were mixed vigorously. Then 600μl NaCl (0.9%) was added to the mixture and the reaction mixture was, vortexed uniformly and centrifuged at 2000 rpm for 3 minutes. After that, the lower phase was then collected and the solvent (chloroform) was removed by the rotary device evaporator (474 mbar, 40 °C, laborota 4003, heidolph rotary evaporator, Germany). For transesterification, the dried lipid extract was dissolved in 500 μl heptane and 100 μl of methanolic KoH (2 mol/L). Then the mixture was shaken vigorously and maintained at 78 °C for 15 minutes. Finally, the samples were centrifuged at 4000 rpm for 5 minutes and the upper phase was collected into a microtube and stored at -70 °C prior to GC injection. An amount of 1 μl was used for injection to the Gas Chromatography (GC) device (variant 450, USA) to detect the fatty acids. The device was equipped with a CP-Sill 88 coated with silicon-based polymers (polysiloxanes), polyethylene glycols and solid adsorbent (EU) fused silica capillary column (100 × 0.25 mm ID × 0.2 mm film thickness). The conditions were as followed: initial temperature 120 °C (holding for 5 minutes), temperature ramp 3 °C per minutes, 240 °C (holding for 10 minutes), and injector temperature at 260 °C. The detector was FID model, carrier gas was nitrogen, and split ratio was 1:20. The fatty acids were detected by comparing of retention time of samples with a synthetic standard (37 component FAME mix, SUPELCO, USA) with known FA composition, Then, a standard curve was prepared using the 3-point linear plot of the different dilutions of the standard, and the concentrations of fatty acids of each sample was reported as mg/ml.
Statistical analysis
Statistical analysis was performed using version 18 of SPSS. Continuous and categorical variables are reported as median with IQR and frequency percentage, respectively. A Student’s T-test was used for variables with a normal distribution. The Mann–Whitney U and Kruskal–Wallis tests were used not normally distributed data. For categorical variables a chi-square test was applied. P-value less than 0.05 was considered significant.
Results
All participants were 437 including control group (n=222) and patients (n=215). We compared baseline characteristics between healthy individuals and patients with CVD (angiography and non-angiography) in Table 1. The mean age of including subjects in both groups was in a similar range from 59 to 63 years old. Data showed the patients had a higher FBG level (106.66±52.63 mg/d Lit) than the control group (90.81±29.88 mg/d Lit) (p<0.0001). Although the ratio of waist to hip (WHR) and BMI revealed no statistical difference between the two groups, the blood pressure in patients differed significantly from the healthy group (p<0.0001). The mean of systolic blood pressure in patients was higher than controls (130.01±26.42 mm HG and 122.34±17.90 mm Hg, respectively), while it has not been shown for diastolic blood pressure. Interestingly, the level of lipid profiles had inconsistent results including higher levels of total cholesterol, HDL-C and LDL-C in control groups (190.83±41.28, 42.29±9.53 and 121.50±36.66 mg/d Lit respectively) and had no significant difference in TG level between controls and patients (p=0.21) did not show. Also, the higher level of hs-CRP was observed in patients (median 1.62 (2.83) mg/d Lit) in compared with a healthy group (median 1.33 (1.97) mg/d Lit) (p<0.0001).
Table 1.
Baseline characteristics of the study population.
| Control n=222 | Total n=215 | P-value | |
| Age (year) | 63.49±95.84 | 59.75±69.80 | 0.51 |
| FBG (mg/d Lit) | 90.81±29.88 | 106.66±52.63 | >0.0001 |
| Total cholesterol (mg/d Lit) | 190.83±41.28 | 183.53±48.22 | >0.0001 |
| HDL (mg/d Lit) | 42.29±9.53 | 42.00±10.94 | 0.02 |
| LDL (mg/d Lit) | 121.50±36.66 | 114.46±39.42 | >0.0001 |
| WHR | 0.93±0.05 | 0.94±0.07 | 0.29 |
| SBP (mm Hg) | 122.34±17.90 | 130.01±26.42 | >0.0001 |
| DBP (mm Hg) | 80.31±10.46 | 77.27±12.68 | >0.0001 |
| BMI (Kg/m2) | 27.90±4.72 | 27.63±5.31 | 0.20 |
| Control Median (IQR) | Total Median (IQR) | P-value | |
| TG (mg/d Lit) | 125.00 (95.00) | 127 (100) | 0.21 |
| Hs-CRP (mg/d Lit) | 1.33 (1,97) | 1.62 (2.83) | 0.001 |
| N (%) | N (%) | ||
| Hypertension(> 140/90) | 40 (20.1) | 97 (52.4) | < 0.001 |
| WHR(> 0.94) | 104 (51.7) | 93 (50.8) | 0.85 |
| Obesity(BMI > 30) | 62 (30.8) | 31 (25.2) | 0.27 |
| FBG(>120 mg/d L) | 15 (7.4) | 57 (35) | < 0.001 |
Abbreviations: FBG = fast blood sugar, HDL = high density lipoprotein, LDL = low density lipoprotein, WHR = waist/hip ratio, SBP = systolic blood pressure, DBP = diastolic blood pressure, BMI = Body Mass Index, TG = triglyceride, Hs-CRP = high sensitivity C-reactive protein. *The data reported as median. *t-test, Mann–Whitney test was used.
The plasma levels of FFAs in the control group and patients were shown in Table 2. We also compared the level of various types of FFAs into separate groups of patients including angiography (Angio +) and non-angiography (Angio -) (n= 91 and n=124 respectively). Data indicated the higher FFAs levels in patients as mean significant differences in C17:0 (p<0.01), C18:3n3 (p=0.013), C14:0, C18:0, C20:3n6 (p<0.0001). Particularly, a dramatic increase has detected in C16:0, C16:1, C18:2n6c, and C18:1 oleic level (p<0.0001) in patients. Also, lower levels of SFA, MUFA, and PUFA were observed in the control group (p<0.0001) and according to data, the median level of SFA in patients (1.59 (1.27)) had much more difference in comparison to median of MUFA and PUFA levels (1.59 (1.27) and 0.69 (0.43)) respectively). The median levels of n6 and n3 fatty acids in patients (1.22 (1.06) and 0.48 (0.33) respectively) were higher than healthy group (0.36 (0.35) and 0.00 (.08) respectively) (p<0.0001). The level of N6-FFAs was nearly three times less in controls and almost no n3-FFAs were observed in the healthy group. Notably, in patients, the median level of n6-FFA was dramatically higher in comparison to the n3-FFA level. In addition, Levels of FFAs in subjects with and without the main risk factors of CVD were investigated (Graph 1). According to this date, the level of FFAs had no significant difference in BMI and WHR categorized (p=0.51 and p=0.14), while showed revealed a significant difference in blood pressure and FBG categorized (p<0.0001). Interestingly, the mode of CVD subjects with hypertension is 52.4% and with high FBG is 35% (shown in Table 1). Also, the level of FFAs of individuals with hypertension was nearly double than of normal subjects (0.83 (1.20) and 0.42 (1.00) respectively) (p=0.007). Moreover, patients with high FBG showed a high level of FFAs circa two times more than the normal group (0.95 (1.24) and 0.4 (0.89) respectively).
Table 2.
Fatty acid levels in CVD and control groups
| Fatty acid Type | Sample Size | Control Median (IQR) | Sample Size | Angio - Median (IQR) | Sample Size | Angio + Median (IQR) | P value | Sample Size | All patients Median (IQR) |
| SFA | 222 | 0.33 (0.38) | 124 | 1.36 (1.39) | 90 | 1.76 (1.39) | <0.0001 | 214 | 1.59 (1.27)* |
| C12:0 | 17 | 0.05 (0.00) | 35 | 0.57 (0.00) | 8 | 0.05 (0.00) | 0.56 | 43 | 0.058 (0.00) |
| C14:0 | 81 | 0.10 (0.01) | 96 | 0.12 (0.02) | 47 | 0.11 (0.04) | <0.0001 | 143 | 0.124 (0.034)* |
| C15:0 | 21 | 0.07 (0.00) | 67 | 0.072 (0.00) | 17 | 0.077 (0.01) | 0.05 | 84 | 0.073 (0.00) |
| C16:0 | 221 | 0.14 (0.10) | 124 | 0.45 (0.33) | 90 | 0.51 (0.38) | <0.0001 | 214 | 0.48 (0.36)* |
| C17:0 | 32 | 0.08 (0.022) | 79 | 0.092 (0.03) | 35 | 0.096 (0.04) | 0.018 | 114 | 0.094 (0.038)* |
| C18:0 | 217 | 0.11 (0.06) | 124 | 0.26 (0.13) | 90 | 0.27 (0.14) | <0.0001 | 214 | 0.26 (0.148)* |
| C20:0 | 2 | 0.070 (0.00) | 4 | 0.065 (0.00) | 0 | 0.00 | 0.35 | 4 | 0.065 (0.00) |
| C24:0 | 4 | 0.065 (0.01) | 20 | 0.063 (0.006) | 5 | 0.066 (0.008) | 0.21 | 25 | 0.64 (0.00) |
| MUFA | 222 | 0.33 (0.38) | 124 | 1.36 (1.390 | 90 | 1.76 (1.39) | <0.0001 | 214 | 1.59 (1.27)* |
| C14:1 | 1 | 0.00 | 6 | 0.07 (0.00) | 5 | 0.07 (0.00) | 0.36 | 11 | 0.071 (0.00) |
| C16:1 | 120 | 0.08 (0.04) | 98 | 0.16 (0.12) | 68 | 0.21 (0.25) | <0.0001 | 166 | 0.183 (0.16)* |
| C17:1 | 10 | 0.069 (0.00) | 47 | 0.069 (0.00) | 46 | 0.072 (0.01) | 0.066 | 93 | 0.070 (0.01) |
| C18:1 elaidic | 16 | 0.11 (0.05) | 84 | 0.102 (0.03) | 76 | 0.108 (0.04) | 0.53 | 160 | 0.106 (0.03) |
| C18:1 oleic | 215 | 0.14 (0.14) | 124 | 0.564 (0.54) | 90 | 0.761 (0.58) | <0.0001 | 214 | 0.63 (0.50)* |
| C20:1 | 9 | 0.081 (0.012) | 17 | 0.084 (0.01) | 16 | 0.088 (0.021) | 0.22 | 33 | 0.084 (0.014) |
| PUFA | 222 | 0.082 (0.18) | 124 | 0.72 (0.47) | 90 | 0.67 (0.41) | <0.0001 | 214 | 0.69 (0.43)* |
| C18:2n6c | 209 | 0.30 (0.31) | 122 | 1.03 (1.06) | 90 | 1.14 (0.82) | <0.0001 | 212 | 1.06 (0.953)* |
| C18:3n6 | 18 | 0.082 (0.01) | 57 | 0.083 (0.013) | 39 | 0.084 (0.025) | 0.51 | 96 | 0.084 (0.016) |
| C18:3n3 | 46 | 0.078 (0.011) | 64 | 0.078 (0.015) | 46 | 0.083 (0.017) | 0.013 | 110 | 0.081 (0.016)* |
| C20:2 | 17 | 0.081 (0.01) | 58 | 0.082 (0.007) | 41 | 0.082 (00.00) | 0.42 | 99 | 0.082 (0.007) |
| C20:3n3 | 32 | 0.4 (0.32) | 120 | 0.38 (0.26) | 90 | 0.40 (0.20) | <0.0001 | 210 | 0.39 (0.23) |
| C20:3n6 | 134 | 0.095 (0.04) | 122 | 0.139 (0.54) | 90 | 0.15 (0.059) | 0.46 | 212 | 0.143 (0.05) |
| C22:2 | 19 | 0.079 (0.020) | 8 | 0.076 (0.00) | 31 | 0.078 (0.012) | 0.48 | 39 | 0.076 (0.011) |
| C22:6n3 | 33 | 0.090 (0.02) | 81 | 0.090 (0.028) | 17 | 0.087 (0.033) | 0.99 | 98 | 0.089 (0.02) |
| N3 | 222 | 0.00 (.08) | 124 | 0.51 (0.36) | 90 | 0.45 (0.27) | <0.0001 | 214 | 0.48 (0.33)* |
| N6 | 222 | 0.36 (0.35) | 124 | 1.20 (1.19) | 90 | 1.2 (0.97) | <0.0001 | 214 | 1.22 (1.06)* |
Values are expressed as median with range (IQR). The concentration of fatty acids was in mg/ml
Discussion
The major findings of our study revealed that the plasma level of fatty acids in patients with CVD is higher in comparison to healthy individuals which may be associated with some risk factors of heart diseases observed in patients (both angiography and non-angiography groups). Although some studies suggested that elevated plasma concentration of FFA has no relation with increasing the risk of ischemic heart disease and CVD mortality (8, 28), some other results reported that FFAs are independently related to cardiac mortality in patients with angiographic cardio artery diseases (26) and even is reported as a risk factor for future sudden death in such patients after nearly 7 years (29). Also, it was found high levels of FFAs play a role in arrhythmia and myocardial dysfunction (30-32).
According to evidence, high FFAs concentration is considered as toxic because of inducing oxidative stress (9, 13), subsequently leading to vascular endothelial dysfunction and inflammation (33). Both in-vivo and in-vitro studies reported that inflammatory cytokines such as IL-6 and TNF-α can be added to adipocytes which results in enhancing lipolysis and FFA levels (34). Elevating in circulating FFAs can active PKC-mediated inflammatory pathways and also increases the generation of oxidants (35, 36). All these results in endothelial dysfunction and inflammation (34-37). C-reactive protein is a highly sensitive biomarker of inflammation and tissue damage which is suggested for predicting major cardiovascular events (38, 39). In our study, we observed a statistical association between high levels of FFA and increased hs-CRP in patients who were in agreement with the known inflammatory action of elevated level FFA. Based on some studies (40, 41) n-3 fatty acid intakes have developed effects on TG concentration, inflammatory factors, and arrhythmia, while n-6 PUFA is the main precursors of eicosanoids (C20) and has been considered as pro-inflammatory molecules (42). Our findings showed no significant difference in n-3 fatty acids between healthy and patient groups, however, the level of C20:3n6 (n-6 PUFA) was double in patients which can confirm the previous results.
The results of observational studies that investigated the association of FFA levels in the blood or adipose tissue to blood pressure are contradictory (43-47). It was reported no relation between serum fatty acid composition and blood pressure (46), while another found an inverse association between special FFA levels and blood pressure (43). Cholesterol ester stearic acid (18:0) has shown an inverse correlation with both systolic and diastolic pressure and particularly, the higher levels of stearic acid were related to lower levels of diastolic pressure (47, 48). Although Stearic acid is a saturated fatty acid (SFA) which is expected to raise blood pressure, it is less atherogenic than other SFAs and has an inverse association with blood pressure (49, 50). Also, dihomogammalinolenic acid (20:3) has an inverse relation with diastolic pressure, which results from lowering endothelial prostacyclin levels (48, 51). It was suggested that differences in the level of such prostaglandin may mediate the inverse correlation of DBP with dihomogammalinolenic (48). In agreement with such results, our findings showed that the level of stearic and dihomogammalinolenic acid in plasma of patients was more double in comparison with the control group. Likewise, diastolic blood pressure (DBP) was lower in patients. Some studies also reported that higher serum and adipose tissue levels of palmitoleic acid (16:1) are associated with heart diseases which refer to its independent correlation with a higher level of systolic blood pressure (48, 52). Palmitoleic acid, a monounsaturated fatty acid, is a metabolite of palmitic acid (16:0) and It’s relation to systolic blood pressure may deal with synthesis and metabolism of palmitic acid (48). Our data revealed high levels of plasma palmitic and palmitoleic acid in patients nearly three times more than a healthy group which may be the reason for the high level of systolic pressure in the patient group.
Elevated plasma FFAs play an important role to cause insulin resistance in non-diabetic people (53). FFAs are considered to exert atherogenic effects because of inducing oxidative stress which leads to apoptosis of endothelial and insulin resistance (9, 54). In the patient with heart diseases, a disruption of cardiac energy metabolism has often seen (25). B-oxidation blocks due to reducing transport of FFAs into the mitochondria (55). Some intermediates as fatty acyl-CoA and acylcarnitine accumulate in the cytosol are considered harmful (1, 55). They also increase mitochondrial uncoupling proteins which ultimately leads to less synthesis of efficient myocardial ATP (56). It also results in inhibition of Na+ K+ ATPase pump which reduces transport of glucose-4 into cells and the efficient myocardial glucose metabolism (57). According to such mechanism, high levels of FFAs can be effective on high levels of glucose and subsequently cardiovascular diseases (26). It is in agreement with our finding that higher levels of both FFAs and FBG observed in patients.
Our finding revealed the level of HDL-C, total cholesterol and LDL-C were lower in patients. A lower level of LDL-C can result from Statin treatment in patient groups. As reported, the main effect of statins is reducing plasma LDL-C concentrations (58). In addition, high levels of FFA can stimulate exchange the HDL and LDL which ultimately leads to low levels of HDL and presence of LDL, independent risk factors of CVD (54, 58). Some studies also have reported that MUFAs can elicit an effect by which lower the level of total cholesterol and PUFAs lower total cholesterol half as much as SFA raise it (59-62). Regarding high levels of FFA in patients, this may explain the lower levels of total cholesterol and HDL-C in our patient groups. Although statin therapy can reduce triglycerides, there was no difference in TG levels between both patient groups and controls. On the other hand, the effects of Statin on FFA levels have varied. One study reported that high-potency of Statins decreased serum docosahexaenoic acid (DHA) (c22:6n3) level significantly (63, 64), whereas one other result showed no significant effects on DHA level (65) which was in agreement with our findings. It was also reported that Statins can mainly reduce n-3 PUFAs (63) which was in contrast with presented results that simvastatin can increase the formation of long-chain PUFAs (66) which was similar to our data of PUFAs levels. Nevertheless, statin type, treatment duration and dose can be effective on changes in lipid profiles and FFAs levels (67), then it is suggested to investigate more about such fields.
According to some results, high level of TG combined with increased WHR were considered as indicators for CVD (68) and the FFA level is relevant to them (69), whereas, in our study, the levels of TG, WHR and BMI in both groups showed no significant difference (26). Nevertheless, we investigated the correlation between FFAs levels and four main risk factors (shown in Table 1) in all subjects (healthy and patients). Our findings showed that hypertension and high level of FBG correlated significantly with higher FFAs levels which were in agreement with other studies (22, 23, 48). In spite of some previous reports (25, 26, 70, 71), no relation was observed in WHR and BMI with the level of FFAs. Although all these findings may be influenced by diet, medicine treatments, environmental and genetic factors and change in lifestyle in subjects.
Conclusion
The high level of fatty acids is considered as a risk factor for CVDs, while the impacts of various FFAs are different and each one can have specific influences on other known risk factors. The level of FBG and hypertension have a correlation with high levels of FFAs which can be considered an independent risk factor for CVDs. Regarding contradictory findings obtained from previous studies, more investigations are required to assess the association between Types of FFAs with CVD risk factors.
Acknowledgement
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [958759] from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Conflicts of interest:
The authors have no conflict to interest to declare.
Funding:
This study was support by grant from Mashhad University of Medical Sciences (MUMS) and the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
References
- Pilz S, März W. Free fatty acids as a cardiovascular risk factor. C Clin Chem Lab Med. 2008;46(4):429–34. doi: 10.1515/CCLM.2008.118. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4(13) doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Kannel WB. Multiple risk functions for predicting coronary heart disease: the concept, accuracy, and application. Am Heart J. 1982;103(6):1031–9. doi: 10.1016/0002-8703(82)90567-1. [DOI] [PubMed] [Google Scholar]
- Kannel W, McGee D. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes care. 1979;2(2):120–6. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Dawber TR. Diabetes, Blood Lipids, and the Role of Obesity in Coronary Heart Disease Risk for Women. Ann Intern Med. 1977;87:393–7. doi: 10.7326/0003-4819-87-4-393. [DOI] [PubMed] [Google Scholar]
- Roy VK, Kumar A, Joshi P, Arora J, Ahanger AM. Plasma free Fatty Acid concentrations as a marker for acute myocardial infarction. J Clin Diagn Res. 2013;7(11):2432. doi: 10.7860/JCDR/2013/7682.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–7. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- Pirro M, Mauriège P, Tchernof A, Cantin B, Dagenais GR, Després J-P, et al. Plasma free fatty acid levels and the risk of ischemic heart disease in men: prospective results from the Quebec Cardiovascular Study. Atherosclerosis. 2002;160(2):377–84. doi: 10.1016/s0021-9150(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. The Journal of clinical investigation. 1997;100(5):1230–9. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89(2):463–78. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- Hendrickson SC, Louis JDS, Lowe JE, Abdel-aleem S. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol Cell Biochem. 1997;166(1-2):85–94. doi: 10.1023/a:1006886601825. [DOI] [PubMed] [Google Scholar]
- Oliver EF, Opie L. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. The. Lancet. 1994;343(8890):155–8. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol. 2010;9(1):9. doi: 10.1186/1475-2840-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishehbor MH, Brennan M-L, Aviles RJ, Fu X, Penn MS, Sprecher DL, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108(4):426–31. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- Shishehbor MH, Aviles RJ, Brennan M-L, Fu X, Goormastic M, Pearce GL, et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA Netw Open. 2003;289(13):1675–80. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93(6):2438–46. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HG, Liu L, Zhang Y, Huang YY, Tao YH, Zhang S, et al. Glutathione Prevents Free Fatty Acids-Induced Oxidative Stress and Apoptosis in Human Brain Vascular Endothelial Cells Through A kt Pathway. CNS Neurosci Ther. 2013;19(4):252–61. doi: 10.1111/cns.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfitano C, de Souza Junior AL, Carbonaro M, Bolsoni-Lopes A, Figueroa D, de Souza LE, et al. Glucose and fatty acid metabolism in infarcted heart from streptozotocin-induced diabetic rats after 2 weeks of tissue remodeling. Cardiovasc Diabetol. 2015;14(1):149. doi: 10.1186/s12933-015-0308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SL, Zhang R. Combined F2-isoprostane and myeloperoxidase detection, a risk indicator for cardiovascular disease. Google Patents. 2015 [Google Scholar]
- Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105(17):2107–11. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28(12):1815–26. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- Guo S-X, Yan Y-Z, Mu L-T, Niu Q, He J, Liu J-M, et al. Association of serum free fatty acids with hypertension and insulin resistance among rural uyghur adults in far Western China. Int J Environ Res Public Health. 2015;12(6):6582–90. doi: 10.3390/ijerph120606582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-W, Zhao X, Guo Y-L, Zhu C-G, Wu N-Q, Sun J, et al. Free fatty acids and cardiovascular outcome: a Chinese cohort study on stable coronary artery disease. Nutr Metab. 2017;14(1):41. doi: 10.1186/s12986-017-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P, Han L, Lv Z, Chen W, Hu H, Tu J, et al. In-hospital free fatty acids levels predict the severity of myocardial ischemia of acute coronary syndrome. BMC Cardiovasc Disord. 2016;16(1):29. doi: 10.1186/s12872-016-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R-F, Zhang H, Wang Z, Liu X-Y, Lin Z. A study on the relationship between waist phenotype, hypertriglyceridemia, coronary artery lesions and serum free fatty acids in adult and elderly patients with coronary diseases. Immun Ageing. 2018;15(1):14. doi: 10.1186/s12979-018-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wellnitz B, Boehm BO, et al. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab. 2006;91(7):2542–7. doi: 10.1210/jc.2006-0195. [DOI] [PubMed] [Google Scholar]
- Tajfard M, Tavakoly Sany SB, Avan A, Latiff LA, Rahimi HR, Moohebati M, et al. Relationship between serum high sensitivity Creactive protein with angiographic severity of coronary artery disease and traditional cardiovascular risk factors. J Cell Physiol. 2019;234(7):10289–99. doi: 10.1002/jcp.27945. [DOI] [PubMed] [Google Scholar]
- Aline Charles M, Fontbonne A, Thibult N, Claude J-R, Warnet J-M, Rosselin G, et al. High plasma nonesterified fatty acids are predictive of cancer mortality but not of coronary heart disease mortality: results from the Paris Prospective Study. Am J Epidemiol. 2001;153(3):292–8. doi: 10.1093/aje/153.3.292. [DOI] [PubMed] [Google Scholar]
- Pilz S, Scharnagl H, Tiran B, Wellnitz B, Seelhorst U, Boehm BO, et al. Elevated plasma free fatty acids predict sudden cardiac death: a 6.85-year follow-up of 3315 patients after coronary angiography. Eur Heart J. 2007;28(22):2763–9. doi: 10.1093/eurheartj/ehm343. [DOI] [PubMed] [Google Scholar]
- Khawaja O, Bartz TM, Ix JH, Heckbert SR, Kizer JR, Zieman SJ, et al. Plasma free fatty acids and risk of atrial fibrillation (from the Cardiovascular Health Study) Am J Cardiol. 2012;110(2):212–6. doi: 10.1016/j.amjcard.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havmoeller R, Reinier K, Teodorescu C, Ahmadi N, Kwok D, Uy-Evanado A, et al. Elevated plasma free fatty acids are associated with sudden death: a prospective community-based evaluation at the time of cardiac arrest. Heart Rhythm. 2014;11(4):691–6. doi: 10.1016/j.hrthm.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp H, Zarain-Herzberg A, Maisch B. The use of partial fatty acid oxidation inhibitors for metabolic therapy of angina pectoris and heart failure. Herz. 2002;27(7):621–36. doi: 10.1007/s00059-002-2428-x. [DOI] [PubMed] [Google Scholar]
- Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233(1):104–12. doi: 10.1016/j.atherosclerosis.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Pond CM. Interactions of noradrenalin and tumour necrosis factor α, interleukin 4 and interleukin 6 in the control of lipolysis from adipocytes around lymph nodes. Cytokine. 1999;11(5):334–46. doi: 10.1006/cyto.1998.0442. [DOI] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88(7):3005–10. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- Li H, Li H, Bao Y, Zhang X, Yu Y. Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-κB pathway in rat aorta. Int J Cardiol. 2011;152(2):218–24. doi: 10.1016/j.ijcard.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Tang Y, Li G. Chronic exposure to high fatty acids impedes receptor agonist-induced nitric oxide production and increments of cytosolic Ca2+ levels in endothelial cells. J Mol Endocrinol. 2011;47(3):315. doi: 10.1530/JME-11-0082. [DOI] [PubMed] [Google Scholar]
- Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol. 2013;168(6):5126–34. doi: 10.1016/j.ijcard.2013.07.113. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Inflammation, C-reactive protein, and cardiovascular disease: moving past the marker versus mediator debate. Circ Res. 2014;114:594–595. doi: 10.1161/CIRCRESAHA.114.303215. [DOI] [PubMed] [Google Scholar]
- Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65(5):1645S–54S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- Geelen A, Brouwer IA, Zock PL, Katan MB. Antiarrhythmic effects of n-3 fatty acids: evidence from human studies. Curr Opin Lipidol. 2004;15(1):25–30. doi: 10.1097/00041433-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36(9):1007–24. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- Oster P, Arab L, Schellenberg B, Heuck CC, Mordasini R, Schlierf G. Blood pressure and adipose tissue linoleic acid. Research in Experimental Medicine. 1979;175(3):287–91. doi: 10.1007/BF01851285. [DOI] [PubMed] [Google Scholar]
- Miettinen T, Naukkarinen V, Huttunen J, Mattila S, Kumlin T. Fatty-acid composition of serum lipids predicts myocardial infarction. Br Med J. 1982;285(6347):993–6. doi: 10.1136/bmj.285.6347.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry EM, Hirsch J. Does dietary linolenic acid influence blood pressure? Am J Clin Nutr. 1986;44(3):336–40. doi: 10.1093/ajcn/44.3.336. [DOI] [PubMed] [Google Scholar]
- Ciocca S, Arca M, Montali A, Fazio S, Bucci A, Angelico F. Lack of association between arterial blood pressure and erythrocyte fatty acid composition in an Italian population sample. Scand J Clin Lab Invest. 1987;47(2):105–10. [PubMed] [Google Scholar]
- Leng G, Smith F, Fowkes F, Horrobin D, Ells K, Morse-Fisher N, et al. Relationship between plasma essential fatty acids and smoking, serum lipids, blood pressure and haemostatic and rheological factors. Prostaglandins Leukot Essent Fatty Acids. 1994;51(2):101–8. doi: 10.1016/0952-3278(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Simon JA, Fong J, Bernert Jr JT. Serum fatty acids and blood pressure. Hypertension. 1996;27(2):303–7. doi: 10.1161/01.hyp.27.2.303. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D. Stearic acid metabolism and atherogenesis: history. Am J Clin Nutr. 1994;60(6):997S–1001S. doi: 10.1093/ajcn/60.6.997S. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am J Clin Nutr. 1994;60(6):986S–90S. doi: 10.1093/ajcn/60.6.986S. [DOI] [PubMed] [Google Scholar]
- Goodnight Jr SH, Harris WS, Connor WE, Illingworth D. Polyunsaturated fatty acids, hyperlipidemia, and thrombosis. Arteriosclerosis: J Am Heart Assoc. 1982;2(2):87–113. doi: 10.1161/01.atv.2.2.87. [DOI] [PubMed] [Google Scholar]
- Cambien F, Warnet J-M, Vernier V, Ducimetiére P, Jacqueson A, Flament C, et al. An epidemiologic appraisal of the associations between the fatty acids esterifying serum cholesterol and some cardiovascular risk factors in middle-aged men. Am J Epidemiol. 1988;127(1):75–86. doi: 10.1093/oxfordjournals.aje.a114793. [DOI] [PubMed] [Google Scholar]
- Jin J-L, Guo Y-L, Li J-J. Plasma free fatty acids in relation with the severity of coronary artery disease in non-diabetics: a Gensini score assessment. IJC Metab Endocr. 2017;14:48–52. [Google Scholar]
- Semenkovich CF. Fatty acid metabolism and vascular disease. Trends Cardiovasc Med. 2004;14(2):72–6. doi: 10.1016/j.tcm.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Arsenault BJ, Lemieux I, Després J-P, Wareham NJ, Kastelein JJ, Khaw K-T, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. Cmaj. 2010;182(13):1427–32. doi: 10.1503/cmaj.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallhaus TR, Taylor M, DeGrado TR, Russell DC, Stanko P, Nickles RJ, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103(20):2441–6. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. The Lancet. 2004;364(9447):1786–8. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN. Insulin resistance and cardiovascular disease. Trends Cardiovasc Med. 2000;106(4):453–8. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism. 1965;14(7):776–87. doi: 10.1016/0026-0495(65)90004-1. [DOI] [PubMed] [Google Scholar]
- Hegsted D, McGandy R, Myers M, Stare F. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr. 1965;17(5):281–95. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12(8):911–9. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- Yu S, Derr J, Etherton TD, Kris-Etherton PM. Plasma cholesterol-predictive equations demonstrate that stearic acid is neutral and monounsaturated fatty acids are hypocholesterolemic. Am J Clin Nutr. 1995;61(5):1129–39. doi: 10.1093/ajcn/61.4.1129. [DOI] [PubMed] [Google Scholar]
- Kurisu S, Ishibashi K, Kato Y, Mitsuba N, Dohi Y, Nishioka K, et al. Effects of lipid-lowering therapy with strong statin on serum polyunsaturated fatty acid levels in patients with coronary artery disease. Heart Vessels Suppl. 2013;28(1):34–8. doi: 10.1007/s00380-011-0213-6. [DOI] [PubMed] [Google Scholar]
- Nozue T, Michishita I. Statin treatment alters serum n-3 to n-6 polyunsaturated fatty acids ratio in patients with dyslipidemia. Lipids Health Dis. 2015;14(1):67. doi: 10.1186/s12944-015-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Hamazaki T, Jokaji H, Minami S, Kobayashi M. Effect of HMG-CoA reductase inhibitors on plasma polyunsaturated fatty acid concentrations in patients with hyperlipidemia. Int J Clin Lab Res. 1998;28(3):192. doi: 10.1007/s005990050043. [DOI] [PubMed] [Google Scholar]
- Jula A, Marniemi J, Rönnemaa T, Virtanen A, Huupponen R. Effects of diet and simvastatin on fatty acid composition in hypercholesterolemic men: a randomized controlled trial. Arterioscler Thromb. 2005;25(9):1952–9. doi: 10.1161/01.ATV.0000177812.84927.fa. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Simental-Mendía LE, Pedone C, Ferretti G, Nachtigal P, Bo S, et al. Statin therapy and plasma free fatty acids: a systematic review and meta-analysis of controlled clinical trials. Br J Clin Pharmacol. 2016;81(5):807–18. doi: 10.1111/bcp.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanko LB, Bagger YZ, Qin G, Alexandersen P, Larsen PJ, Christiansen C. Enlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal women. Circulation. 2005;111(15):1883–90. doi: 10.1161/01.CIR.0000161801.65408.8D. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Davinelli S, Kaneko T, Koverech G, Koverech A, Calabrese EJ, et al. Dose response biology of resveratrol in obesity. J Cell Commun Signal. 2014;8(4):385–91. doi: 10.1007/s12079-014-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P, Rydén M. Fatty acids, obesity and insulin resistance. Obes Facts. 2015;8(2):147–55. doi: 10.1159/000381224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatz S, Conrad Z, Johnson L, Picklo M, Jahns L. Relationship of the reported intakes of fat and fatty acids to body weight in US adults. Nutrients. 2017;9(5):438. doi: 10.3390/nu9050438. [DOI] [PMC free article] [PubMed] [Google Scholar]


