Abstract
Salinomycin (Sal) is a potent inhibitor with effective anti-breast cancer properties in clinical therapy. The occurrence of various side effect of Sal greatly limits its application. The epidermal growth factor receptor (EGFR) family is a family of receptors highly expressed in most breast cancer cells. GE11 is a dodecapeptide which shows excellent EGFR affinity. A series of nanoparticles derivatives with GE11 peptide conjugated PLGA/TPGS were synthesized. Nanoprecipitation method was used to prepare the Sal loaded nanoparticles at the optimized concentration. The characterization, targeting efficacy, and antitumor activity were detected both in vitro and in vivo. Encapsulation of Sal in GE11 modified PLGA/TPGS nanoparticles shows an improved therapy efficacy and lower systemic side effect. This represents the delivery system a promising strategy to enhance the therapeutic effect against EGFR highly expressed breast cancer.
Keywords: breast cancer, GE11, salinomycin, nanoparticles, targeting delivery
Introduction
Breast cancer is the second most common cancer among female globally. 1 Effective treatments for breast cancer with early-stage include surgical resection and concurrent chemoradiotherapy, which can lead to a 5-year survival of ≤ 75%. About 50% of breast cancer patients eventually cause tumor recurrence and/or metastasis, which hampers the long-lasting and effective therapy. 2
The chemotherapeutic drug of Salinomycin (Sal), which isolated from Streptomyces albus, has been reported to eliminate various tumors, such as liver cancer, breast cancer, and lung cancer. 3,4 Significantly, more and more evidence established that the Sal inhibits the breast cancer stem cells via blocking the Wnt/β-catenin pathway both in vitro and in vivo. 5 Therefore, the Sal is regarding as a potentially effective agent for treatment of breast cancer. However, Sal possesses poor aqueous solubility and un-substantial cancer cells cytotoxicity. 6 These disadvantages induce the limitation of the Sal application on breast cancer therapy. Direct delivery of Sal to the breast cancer cells would be a valuable breakthrough for its clinical application.
Targeted nanoparticles have developed to be excellent drug delivery systems due to their promising performance and unique characteristics, such as improving the inapplicable properties of drugs or pharmacokinetic properties. 7,8 In addition, the chemotherapeutic drugs could accumulate at the solid tumor site owing to the enhanced permeability and retention (EPR) effect, leading to the clinical benefit with decreased side effects. 9,10 The biodegradable polymers and biocompatible liposomes are the widely used categories in nanomedicine. The liposomes are the spherical lipid carriers with single or multiple bilayers and favorable in feasible surface modification and long circulation time in vivo. However, the prepared liposomes are restricted by uncontrollable drug release, instability, and insufficient drug loading. While the combination of polymer-lipid hybrid nanoparticles could dissolve the limitations, some researchers developed these nanoparticles as powerful drug delivery systems on the treatments of cancers. PLGA, poly lactic-co-glycolic acid, is characterized by long-lasting drugs release from many days to many weeks and easy medicated with many approaches such as surface embellish, novel synthesized, and polyporous. In addition, PLGA shows advanced biocompatibility in vivo with a rigid structure. 11 -14 TPGS, D-α-Tocopheryl polyethylene glycol 1000 succinate, is a water-soluble derivative of natural vitamin E which can be used as solvent, stabilizer, and emulsifier for PLGA modification. 15 These modifications can cause the size of PLGA nanoparticles be smaller and the drug encapsulation efficiency be higher. 16 Moreover, both the PLGA and TPGS are approved by US food and drug administration (FDA) as safe pharmaceutical excipients. 17 Some researchers have used PLGA/TPGS particles to deliver drugs for improving chemotherapy about multi-drug resistant breast cancer. 18
Epidermal growth factor receptor (EGFR) is greatly overexpressed on the surface of various human malignancies and regarding as a valuable target for cancer therapy include breast cancer. 19 -21 A screened small peptide GE11 by the phage display was demonstrated to have high affinity against EGFR significantly upregulated cancer cells. 22 The affinity between GE11 and EGFR is significantly high (kd = 22 nM). 23 GE11 has only 12 amino acids (YHWYGYTPQNVI) and is much smaller than the EFGR’s ligand EGF. 24 This small peptide targets only to one region of EGFR. These characters make it a prospecting targeting candidate for EGFR-targeted therapy of breast cancer. 25,26
In present study, we designed and synthesized Sal-loaded nanoparticles (NPs) functionalized with GE11 targeting peptide to achieve the efficient delivery of Sal to breast cancer cells. The chemotherapeutic drugs Sal were successfully encapsulated in NPs via the 1-step nanoprecipitation process. 27 NPs-Sal-GE11 was further prepared by binding anti-EGFR peptide with NP-Sal by a maleimide-thiol reaction. The physicochemical characterization, targeting efficacy, and antitumor activity of the Sal-NPs-GE11 against breast cancer cells were evaluated in this work.
Materials and Methods
Materials
Salinomycin sodium, poly(d, l-lactide-co-glycolide) (PLGA, 50:50, Mw 40,000-75,000 Da), was purchased from Sigma-Aldrich (St Louis, MO, USA). Soybean lecithin was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy(polyethylene glycol)-2000) (DSPE-PEG2000), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(maleimide(polyethylene glycol)-2000) (DSPE-PEG2000-Mal), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-carboxyfluorescein (CFPE) were obtained from Avanti Polar Lipids (Alabaster, USA). The EGFR-targeted peptide GE11, CGGGSYHWYGYTPQNVI, was synthesized by Apeptide Ltd. (Shanghai, China), with the “GGGS” sequence as a spacer and the N-terminal amino cysteine of the peptide reacting with the maleimide group of Sal-NPs. Cell Counting Kit-8 (CCK-8) was obtained from Dojindo (Kumamoto, Japan). All other chemicals were of analytical grade.
Cell Culture
Human breast carcinoma cell lines MCF-7 cells, which EGFR higher expression, were obtained from ATCC (USA). MCF-7 cells were cultured in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum and 1% penicillin–streptomycin at 37°C in 5% CO2 atmosphere.
Synthesis of Polymer–Lipid Hybrid NPs
The polymer–lipid hybrid particles were prepared by a 1-step process of nanoprecipitation. 3,27 Briefly, 1 mg of Sal was dissolved with 1 mg/mL PLGA acetonitrile solution. 0.25 mg soybean lecithin and 0.075 mg DSPE-PEG2000 were dissolved in a 4% ethanol aqueous solution. Then, the mixture was heated to 65°C. The above PLGA solution was added into the preheated lipid aqueous solution dropwise with the speed of 1 mL/min. The mixture was stirred gently for 3 h at 25°C. Then, the free agent and the excess organic solvent were removed by PBS using a dialysis tube (Spectra/Por 6 membrane, MWCO 1,000; Spectrum Labs, Cincinnati, OH, USA). The NPs were mixed with 3% w/w sucrose as a cryoprotectant and freeze-dried to obtain a fine powder. The GE11 peptide was mixed with 2 mL of the nanoparticles prepared above. The mixture was then incubated at 16°C overnight to form polymer–lipid hybrid anti-EGFR nanoparticles. The unconjugated GE11 peptide was removed using Ultra-4 centrifugal filter devices (Merck Millipore, Billerica, MA, USA) with 1200 rpm at 4°C for half an hour. The Sal-loaded polymer–lipid hybrid nanoparticles functionalized with GE11 targeting peptide (NP-Sal-TP) was obtained at last. The control nanoparticles Sal-loaded polymer–lipid nanoparticles (NP-Sal), polymer–lipid-GE11 nanoparticles (NPs-GE11), and FAM(Carboxyfluorescein-5-succimidyl ester)-labeled Sal-loaded polymer–lipid-GE11 nanoparticles (FAM-Sal-NPs-GE11) were designated.
Characterization of Nanoparticles
The size and zeta potential of the NPs were determined by a dynamic light-scattering detector (Zetasizer, Nano-ZS; Malvern Instruments, Malvern, UK). Nanoparticle morphology was measured by a transmission electron microscopy (TEM) (H-600; Hitachi, Tokyo, Japan).
Sal Encapsulation Efficacy of Nanoparticles
The drug encapsulation and loading efficacy of Sal in nanoparticles were determined by high-performance liquid chromatography (HPLC, L-2000; Hitachi). HPLC was performed by a reverse-phase C-18 column (Diamonsil, 250 × 4.6 mm, 5 μm; Dikma Technologies, Inc, Lake Forest, CA, USA). The mobile phase was acetonitrile/deionized water/tetrahydrofuran/phosphoric acid (85:10:5:0.01, v/v) with the flow rate 1.0 mL/min. Drug-loading efficiency was calculated as DE/DN × 100% (DE: the mass of encapsulated Sal; DN: the mass of NPs) and the drug encapsulation efficiency was calculated as DE/DT × 100% (DT: the mass of total NPs). The FAM concentration in NPs was calculated according to FAM calibration curves constructed.
Cellular Uptake In Vitro
Intracellular drug release of NP-Sal-TP was determined by confocal laser scanning microscopy. MCF-7 cells were treated with FAM-NP-Sal-TP at the concentration of 10 μg/mL for 2 hours of incubation at 37°C. Cells were washed 3 times with ice-cold PBS and fixed by 4% paraformaldehyde for 10 mins. The nuclei of cells were strained with DAPI (10 μg/mL) for 5 minutes and washed thoroughly with ice-cold PBS before fluorescent imaging.
Cells Migration Assay
The breast cancer cells MCF-7 cells (2 × 105 cells/well) were seeded overnight in a 24-well tissue culture plate and then NP-Sal-TP, NP-Sal, GE11, Sal, and PBS were added into cells for 24 h. Then, 5 × 103 the cells were trypsinized and seeded into the upper 8.0-µm pore size membrane per insert (polycarbonate, from Corning, USA) in 200 µL of FBS free medium. To the low chambers were added 600 µL of medium with 10% FBS. Cells that attached to the upper side of the membrane were wiped off, and cells that had migrated and attached to the lower side of the membrane were fixed by methanol and stained with crystal violet. And then cells were counted under a microscope with a 20 × objective.
Cellular Cytotoxicity of NPs In Vitro
The cytotoxic effects of NPs against breast cancer cells MCF-7 were determined by the Cell Counting Kit 8 (CCK-8) assay. Briefly, the cells were seeded at a density of 2 × 103 cells/well in 96-well plates for 12 h. The medium was replaced with fresh medium containing varying concentrations of NP-Sal-TP, NP-Sal, GE11, Sal, or PBS incubated for 48 h. Then, 10 µL CCK-8 solution and 90 µL medium was added to each well for 4 h at 37°C in 5% CO2. After that, the absorbance of each well was measured at 450 nm with the microplate reader.
Antitumor Assays of NPs in Mice In Vivo
All animal experiments were carried out in agreement with the National Institutes of Health (NIH) requirements for the care and use of laboratory animals and approved by the Shanghai University Animal Study Committee(SHU-20-247x). The mice were maintained on standard SPF laboratory. The mice were divided into 5 groups, 5 mice each group, randomly. Mice were treated with PBS, GE11, free Sal, NP-Sal, and NP-Sal-TP. The therapeutic effect of the Sal-NPs-GE11 was measured in mice bearing subcutaneous breast cancer in vivo. Briefly, 1 × 107 MCF-7 cells in 100 µL PBS were injected s.c. into 4-6 weeks BALB/c nude mice. When the tumors volume reached about 100 mm3, mice were treated with single iv. injections of NP-Sal-TP, NP-Sal, GE11, or free Sal (2 mg/kg) via the tail vein. Treatment was carried out on days 0, 7, 14, 21, and 35. Tumors were evaluated by using a caliper every 5 days. The tumor volume was calculated by using the formula: (a2 × b)/2 (a: width, b: length). The body weight of the mice was monitored every 5 days.
Statistical Analysis
Data in this study was conducted by using the software GraphPad Prism 5.01. Statistical analysis was performed by a Student’s t-test for direct comparisons and 1-way analysis of variance for multiple groups. A P value of < 0.05 was considered statistically significant.
Results and Discussions
Preparation and Characteristics of NPs
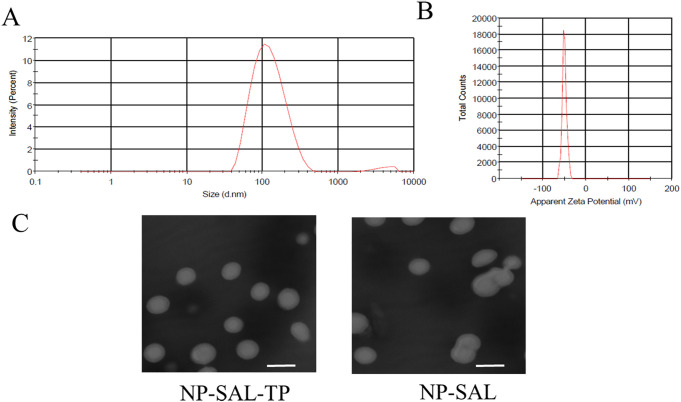
Sal-NP was prepared by a 1-step nanoprecipitation process and NP-Sal-TP was further prepared by modifying GE11 with Sal-NP by a maleimide-thiol reaction. As shown in Figure 1A and 1B, the size of the NP-Sal-TP nanoparticles was 132.6 nm and the zeta potential was -51.2 mV. The TEM analysis demonstrated that both the 2 types of particles showed a spherical shape, and the dim ring around the core indicated the well-defined core–shell structure of the particles (Figure 1C).
Figure 1.
Characterization of nanoparticles (PLGA/TPGS). Size distribution (A) and zeta potential of nanoparticles (B), as detected by dynamic light scattering. The TEM image of nanoparticles (C). Scale bar:100 nm. One representative image is shown. Data are presented as means ± standard deviations (n = 3).
In Vitro Cellular Uptake Study
As shown in Figure 2A, the cellular uptake efficiency of Sal-NP and Sal-NP-TP were determined by flow cytometry. The mean fluorescence intensity (MFI) of MCF-7 cells treated with Sal-NP-TP was about 3-fold then those treated with Sal-NP after 2 hours incubation, respectively (Figure 2B). This result indicated that the targeting group shows a stronger nanoparticles internalization capability of MCF-7 cells compared to the Sal-NP treated cells.
Figure 2.
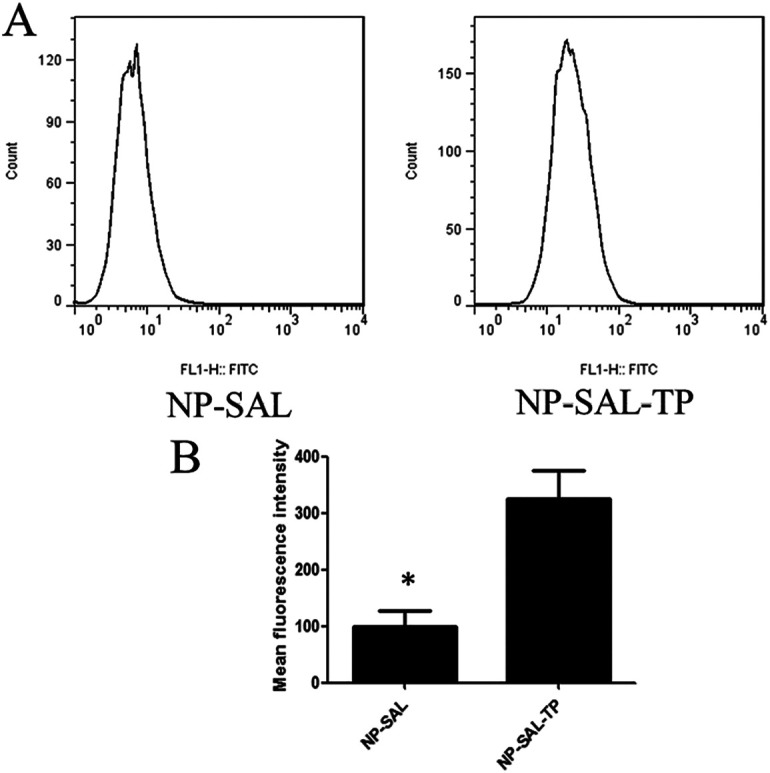
GE11 modified nanoparticle targeting to breast cancer cells MCF-7. (A) In vitro cellular uptake analysis in MCF-7 cells were treated with the NP-SAL or NP-SAL-TP nanoparticles for 24 h. After then, the cells were trypsinized, washed and the mean fluorescence intensity was determined by flow cytometry. (B) Quantification of the mean fluorescence intensity with different groups. Data represented as mean ± SD (n = 3). *P < 0.01.
Migration Inhibition of Sal-NP-TP Toward Breast Cancer Cells
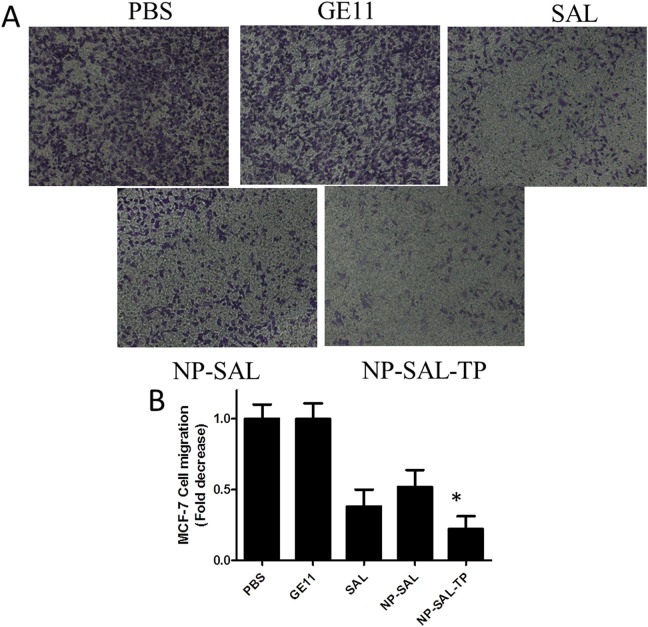
Cell migration was considerate as a vital role in numerous biological phenomena including tumor metastasis and morphogenesis. 28 Because of the ability of tumor cells migration is very related to human tumor metastasis and morphogenesis, we then determined whether targeting group Sal-NP-TP could lead to the inhibition of breast cancer cells migration in vitro. The capability of breast cancer cells migration was examined by the counting of the cells number displayed at the lower side of the membrane of the Transwell system. After 18 h incubation, the results showed that free Sal, NP-Sal, and NP-Sal-TP inhibited cellular migration compared with the PBS or GE11 groups (Figure 3 A and B). Significantly, compared with the none targeting treated cells, NP-Sal-TP group showed dramatic suppression of cell migration. This suggests that NP-Sal-TP can specifically suppress cell migration in EGFR-overexpressing breast cancer cells, whereas NP-Sal had no such effect.
Figure 3.
Suppression of the migration of breast cancer cell MCF-7. (A) The amount of migrating MCF-7 cells markedly suppressed with the targeting nanoparticles. Cells at the lower of the members are stained and images shown here. (B) Quantification of the migrating MCF-7 cells with different groups. *P < 0.05.
Cytotoxicity of Sal-NP-TP Toward Breast Cancer Cells
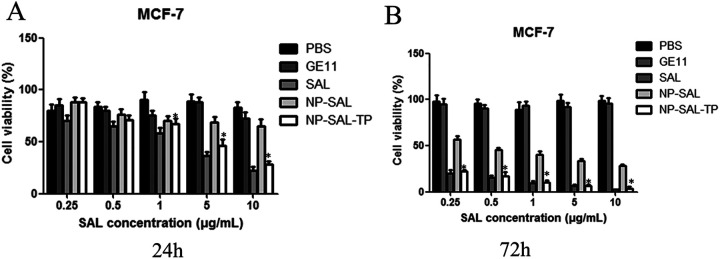
The antiproliferative effect of different nanoparticles against MCF-7 cells was evaluated using CCK-8 assays. As shown in Figure 4, preliminary of evaluation of PBS and GE11 showed negligible anti-tumor activity, whereas free Sal, NP-Sal, and NP-Sal-TP inhibited cellular proliferation in a dose-dependent manner (0.25-10 μg/mL) both at 24 h and 72 h. Among the 5 groups, free Sal showed the dramatic efficacy of inhibition of breast cancer cell proliferation after 72 h. As expected, the targeting group NP-Sal-TP exhibited stronger cytotoxicity compared with none targeting group NP-Sal. The modification of NP-Sal with TP enhanced the cytotoxicity against breast cancer cells, which is consistent with the cellular uptake in flow cytometry.
Figure 4.
The concentration-dependent cytotoxicity induced by particles in MCF-7 cells after 24 h (A) or 72 h (B). The cells were incubated with varying concentrations of different groups, and the cell viability was determined by CCK-8 assays. Data are presented as means ± standard deviations (n = 3). *P < 0.05.
In Vivo Antitumor Assay
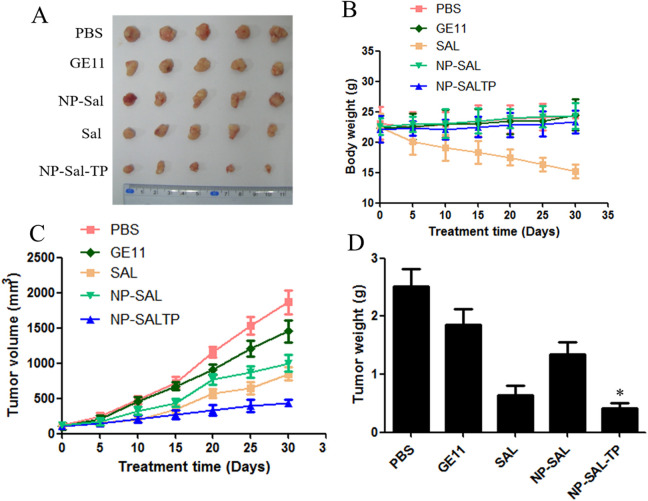
To validate the ability of NP-Sal-TP for the antitumor potential in vivo, the antitumor efficacy was evaluated using BALB/C nude mice bearing MCF-7 breast cancer xenografts. The tumor volume was measured every 5 days for up to 30 days. As shown in Figure 5A, free Sal, NP-Sal, and NP-Sal-TP groups demonstrated distinct tumor growth inhibition efficiency, whereas the tumor size grew swiftly in the control groups using PBS or GE11 (Figure 5B and 5C). In particularly, the targeting group NP-Sal-TP displayed a marked reduction in tumor volume during the total test process up to 30 days compared with other groups. At the end of the experiment, the final tumor weight of isolated tumors for different treated groups further confirmed the superior efficiency of NP-Sal-TP.
Figure 5.
Antitumor assay in mice bearing subcutaneous MCF-7 tumors in vivo. (A) Pictures of excised tumors of each group at the end of experiment. (B) Variation in body weight. (C) Tumor growth volume. (D) The excised tumors were weighed at the end point. The 2 groups were compared by 1-way ANOVA with the Newman–Keuls method. Data are expressed as mean ± SD (n = 5). *P < 0.05.
Conclusions
In conclusion, we present a PLGA/TPGS nanoparticle NP-Sal-TP based on GE11 conjugate specially target the breast cancer not only by EPR effect, but also via active targeting endocytosis mechanism. The results demonstrated that the targeting NP-Sal-TP performed the predominant EGFR targeting effect in enhancing antitumor efficacy both in vitro and in vivo. Thus, we believe that nanoparticle-Sal-TP is considerable as a promising and desirable vehicle for future application in cancer therapy and drug delivery like Sal.
Footnotes
Authors’ Note: All animal experiments were carried out in agreement with the National Institutes of Health (NIH) requirements for the care and use of laboratory animals and approved by the Shanghai University Animal Study Committee(SHU-20-247x).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was funded by special project of clinical research of health industry of Shanghai Municipal Health Commission (No.201940178), by the scientific research project of Hongkou District Health Committee of Shanghai (No.2002-17), and by the research project of Shanghai Fourth People’s Hospital (No.0443&0446).
ORCID iD: Jie Gao  https://orcid.org/0000-0003-1317-2445
https://orcid.org/0000-0003-1317-2445
References
- 1. Yu Y, Nie Y, Feng Q, et al. Targeted covalent inhibition of GRB2–SOS1 interaction through proximity-induced conjugation in breast cancer cells. Mol Pharm. 2017;14(5):1548–1557. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3. Gao J, Liu J, Xie F, Lu Y, Yin C, Shen X. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomedicine. 2019;14:9199–9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta P, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 2011;108(32):13253–13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyazaki Y, Shibuya M, Sugawara H, Kawaguchi O, Hirose C. Salinomycin, a new polyether antibiotic. J Antibiot (Tokyo). 1974;27(11):814–821. [DOI] [PubMed] [Google Scholar]
- 7. Gao J, Yu Y, Zhang Y, et al. EGFR-specific pegylated immunoliposomes for active siRNA delivery in hepatocellular carcinoma. Biomaterials. 2012;33(1):270–282. [DOI] [PubMed] [Google Scholar]
- 8. Liu J, Qu J, Zhou W, et al. Syntenin-targeted peptide blocker inhibits progression of cancer cells. Eur J Med Chem. 2018;154:354–366. [DOI] [PubMed] [Google Scholar]
- 9. Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–135. [DOI] [PubMed] [Google Scholar]
- 10. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1-2):271–284. [DOI] [PubMed] [Google Scholar]
- 11. Mir M, Ahmed N, Rehman AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159:217–231. [DOI] [PubMed] [Google Scholar]
- 12. Shakerizadeh A, Shiran MB, Khoee S, Sharifi A, Ghaznavi H, Khoei S. A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumor, and pro-apoptotic effects on ct26 cells allograft model. J Biomater Appl. 2014;29(4):548–556. [DOI] [PubMed] [Google Scholar]
- 13. Tang X, Liang Y, Feng X, Zhang R, Jin X, Sun L. Co-delivery of docetaxel and poloxamer 235 by PLGA–TPGS nanoparticles for breast cancer treatment. Mater Sci Eng C Mater Biol Appl. 2015;49:348–355. [DOI] [PubMed] [Google Scholar]
- 14. Danhier F, Ansorena E, Silva J, Coco R, Breton AL, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Z, Tan S, Feng S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. [DOI] [PubMed] [Google Scholar]
- 16. Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of vitamin E TPGS in drug delivery. Eur J Pharm Sci. 2013;49(2):175–186. [DOI] [PubMed] [Google Scholar]
- 17. Tan S, Zou C, Zhang W, Yin M, Gao X, Tang Q. Recent developments in d-α-tocopheryl polyethylene glycol-succinate-based nanomedicine for cancer therapy. Drug Deliv. 2017;24(1):1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu H, Chen H, Zeng X, et al. Co-delivery of chemotherapeutic drugs with vitamin E TPGS by porous PLGA nanoparticles for enhanced chemotherapy against multi-drug resistance. Biomaterials. 2014;35(7):2391–2400. [DOI] [PubMed] [Google Scholar]
- 19. Brand TM, Iida M, Luthar N, Starr MM, Huppert EJ, Wheeler DL. Nuclear EGFR as a molecular target in cancer. Radiother Oncol. 2013;108(3):370–377. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 20. Yarden Y. The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(4):3–8. [DOI] [PubMed] [Google Scholar]
- 21. Rochalima C, Soares HP, Raez LE, Singal R. EGFR targeting of solid tumors. Cancer Control. 2007;14(3):295–304. [DOI] [PubMed] [Google Scholar]
- 22. Genta I, Chiesa E, Colzani B, Modena T, Conti B, Dorati R. Ge11 peptide as an active targeting agent in antitumor therapy: a minireview. Pharmaceutics. 2017;10(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012;24(28):3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z, Zhao R, Wu X, et al. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J. 2005;19(14):1978–1985. [DOI] [PubMed] [Google Scholar]
- 25. Mickler FM, Möckl L, Ruthardt N, Ogris M, Wagner E, Bräuchle C. Tuning nanoparticle uptake: live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012;12(7):3417–3423. [DOI] [PubMed] [Google Scholar]
- 26. Song S, Liu D, Peng J, et al. Novel peptide ligand directs liposomes toward EGFR high-expressing cancer cells in vitro and in vivo. FASEB J. 2009;23(5):1396–1404. [DOI] [PubMed] [Google Scholar]
- 27. Chen D, Pan X, Xie F, et al. Codelivery of doxorubicin and elacridar to target both liver cancer cells and stem cells by polylactide-co-glycolide/d-alpha-tocopherol polyethylene glycol 1000 succinate nanoparticles. Int J Nanomed. 2018;13:6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu Y, Liu M, Ng TT, et al. PDZ-reactive peptide activates Ephrin-B reverse signaling and inhibits neuronal chemotaxis. ACS Chem Biol. 2016;11(1):149–158. [DOI] [PubMed] [Google Scholar]