Abstract
Corneal endothelial dysfunction is a principal cause of visual deficiency. Corneal transplantation is the most effective treatment for corneal endothelial dysfunction. However, a severe shortage of available donor corneas or human corneal endothelial cells (HCECs) remains a global challenge. Previously, we acquired corneal endothelial cell-like cells (CEC-like cells) derived from human skin-derived precursors (SKPs). CEC-like cells were injected into rabbit and monkey corneal endothelial dysfunction models and exerted excellent therapeutic effect. In this study, we prolonged the clinical observation in the monkey experiment for 2 years. Polymerase chain reaction (PCR) and DNA sequencing were carried out to confirm the existence of CEC-like cells. Histological examinations were carried out to show the corneal morphology. Further transcriptome sequencing was also carried out on HCEC, CEC-like cells before transplantation and after transplantation. We found that the monkeys cornea remained transparent and normal thickness. The total endothelial cell density decreased gradually, but tended to be stable and remained in a normal range during 2-year observation. The CEC-like cells persist during observation and could adapt to the microenvironment after transplantation. The gene expression pattern of CEC-like cells was similar to HCEC and changed slightly after transplantation. In conclusion, this study presented a brand-new insight into CEC-like cells and further provided a promising prospect of cell-based therapy for corneal endothelial dysfunction. The renewable cell source, novel derivation method and simple treatment strategy may be clinically applied in regenerative medicine in the future.
Keywords: corneal endothelial dysfunction, skin-derived precursors, corneal endothelial cell-like cells, transcriptome sequencing, cell-based therapy
Background
Corneal blindness, the 4th cause of blindness globally (5.1%), is one of the principal causes of visual deficiency 1,2 . Corneal endothelial dysfunction which is characterized by excessive endothelial loss is a major reason for corneal blindness. Severe endothelial cell loss may occur as a result of previous ocular surgeries, infections, trauma, and some corneal disease such as Fuchs’ corneal endothelial dystrophy (FCED), posterior polymorphous corneal dystrophy (PPCD), and iridocorneal endothelial syndrome (ICE) 3 . Corneal transplantation, such as penetrating keratoplasty (PKP) and corneal endothelial keratoplasty, is an effective way for treating corneal blindness 4,5 . Corneal endothelial dysfunction is the leading indication for endothelial keratoplasty which account for about 39% of all corneal transplantation 6,7 . However, there is a worldwide shortage of cornea donors which is an obstacle for their application 8 .
A new source of functional CECs combine with cell-based therapy is an extremely good choice to overcome the shortage of cornea donors. Human corneal endothelial cells (HCECs) have limited proliferative capacity in vivo and cannot be sub-cultured for more than a few passages in vitro 9,10 . However, we previously invented a new method that could acquire corneal endothelial cells (CEC-like cells). We co-cultured skin-derived precursors (SKPs) with B4G12 cells using Transwell inserts in human endothelial serum free medium (SFM). B4G12 cells is the immortalized cell population of HCECs. They are characterized by polygonal monolayers and express junctional proteins and Na+/K+ ATPase. The acquired CEC-like cells had similar morphology and characteristic with HCECs. After they were injected into the anterior chamber of rabbit and monkey corneal endothelial dysfunction models, they had exerted excellent therapeutic effect 11 . The invention provided a new treatment strategy for corneal endothelial dysfunction and also provided efficient source of seed cells to construct tissue engineered corneas 12 .
In this study, the observation after CEC-like cell transplantation into the monkey endothelial dysfunction model was prolonged for 2 years. In addition, further transcriptome sequencing was carried out on HCEC, CEC-like cells before transplantation and after transplantation. Results showed that CEC-like cells could adapt to the microenvironment and had a good therapeutic effect after being transplanted into the monkey endothelial dysfunction model in long-term observation. The gene expression pattern of CEC-like cells was similar to HCEC and changed slightly after transplantation. Our studies have provided a promising prospect of cell-mbased therapy with CEC-like cells for corneal endothelial dysfunction in future clinical applications.
Materials and Methods
Cell Culture
Human SKPs were isolated and cultured as previously described 11,13,14 . Human skin was taken from 10 patients (age: 32.9± 15.82 years, mean ± S.D.) that underwent a double-eyelid operation. Samples were collected following written informed consents, and the study was performed in adherence to the Declaration of Helsinki. All experiments were approved by the Medical Ethics Committee of Qilu Hospital of Shandong University and were conducted following the institutional guidelines (approval number 12028). Briefly, SKPs were cultured to a cell density of 25,000 or more cells/ml. The growth medium was DMEM/F12 Nutrient Mixture (3:1; Thermo Fisher) supplemented with 1% penicillin/streptomycin (Solarbio), 50μg/mL fungizone, 2% B27 supplement (Thermo Fisher), 40 ng/mL human recombinant bFGF, and 20 ng/mL epidermal growth factor (both from Peprotech). SKPs spheres were passaged every 1–2 weeks. SKPs between passages 2 and 4 were used for further experiments.
The immortalized HCEC population B4G12 cells were generously provided by Monika Valtink and were cultured according to the previous protocol 15,16 . Briefly, B4G12 cells were cultured in human endothelial SFM (Thermo Fisher) supplemented with 10 ng/ml human recombinant bFGF in T25 culture flasks.
CEC-like cells were derived from human SKPs as our previously protocol 11 . Briefly, human SKPs spheres were separated into single cells by 0.05% trypsin-0.02% EDTA, and then they were plated into 6-well plates coated with 10 mg/ml chondroitin sulphate and 10μg/ml laminin (both from Sigma). The B4G12 cells were cultured in six-well Transwell inserts (Corning) and the Transwell inserts were added to the 6-well plates and co-cultured with the human SKPs in B4G12 culture medium for 8 days to acquire CEC-like cells. CEC-like cells were passaged and cultured in CEC-like cells culture medium consisting of human endothelial SFM supplemented with 10 ng/ml bFGF. CEC-like cells between passages 2 and 3 were used for further experiments.
Animals
Five rhesus monkeys weighting 2.5–3.5 kg (Hongli Medical Animal Experimental Research Center, Jinan, Shandong Province, China) were used for corneal endothelial transplantation. All animals were treated in accordance with the Institutional Animal Care guideline of Hongli Medical Animal Experimental Research Center, Shandong Province, China and approved by their Association for Laboratory Animal Care (approval number 2016-0803). The schematic diagram of study design was shown in Supplemental Fig. S1.
CEC-Like Cells Transplantation Into the Monkey Corneal Endothelial Dysfunction Models
CEC-like cells were transplanted according to our previous method 11 . Briefly, the corneal endothelium was mechanically scraped with a lacrimal passage irrigator (Shandong Weigao) from the Descemet’s membrane to create monkey corneal endothelial dysfunction models. 50 µl aqueous humor was first extracted from the models’ anterior chamber. Then, 4.0 × 105 CEC-like cells suspended in 50 µl culture medium supplemented with 1.6 µg of Y-27632 (Selleck) were injected into the anterior chamber of four monkeys. And 50 µl culture medium supplemented with 1.6 µg of Y-27632 was injected into the anterior chamber of one monkey. Four monkeys that had cells injected were the experimental group and the other one monkey that had no cells injected was the control group. Peribulbar and subconjunctival injection of triamcinolone and dexamethasone were given after surgery. The eyes of all monkeys were kept in a face-down position for 6 hours under general anesthesia. 0.3% Tobramycin and 0.1% Dexamethasone were given topically 3–4 times a day. The corneas were examined by a slit-lamp microscope (Topcon), Visante OCT (Carl Zeiss), non-contact specular microscopy (Topcon), tenonometer (Suzhou liuliu), gonioscope (Volk), B-ultrasonography (Suoer), and fundus camera (Carl Zeiss) at certain times. The contralateral normal eyes of the monkeys were observed as the normal group.
Groups and Corneal Endothelium Acquisition After Euthanasia
Monkeys were euthanized by an intravenous overdose of pentobarbital sodium. The postoperative and normal eyes were removed. The two monkeys in the experimental group were euthanized at the end of first year and labeled as the EXP1 group. The other two monkeys in the experimental group were euthanized at the end of second year and labeled as the EXP2 group. The monkey in the control group was euthanized at the end of second year. The normal monkey eyes in normal groups were labeled as the MON group. Human eyes were obtained from discarded eyes after penetrating keratoplasty and from the Eye Tissue Bank of Shandong Province, China. The normal human eyes group was labeled as the HCEC group. The Descemet membranes with corneal endothelial cells were torn off monkey and human eyes of different groups with microscopic tweezers.
Polymerase Chain Reaction and DNA-Sequencing
DNA was extracted from CEC-like cells and corneal endothelial cells of EXP1, EXP2, MON, and HCEC groups using a DNA extraction kit (Tiangen) according to the manufacturer’s protocol. Human and monkey specific Cytb gene fragments were amplified by polymerase chain reaction (PCR) with the Ultra HiFidelity PCR Kit (Tiangen) according to the manufacturer’s protocol. Primers designed by BioSune Biotech (Shanghai, China) are listed in Table 1. Agarose gel electrophoresis was carried out with the PCR product according to the standard method. Amplified DNA was purified using the TIANgel Midi Purification Kit (Tiangen). The sequencing of DNA was performed in BioSune Biotech (Shanghai, China) with the ABI3730XL sequenator. Data was analyzed with Chramas.
Table 1.
Primers Used for PCR,
| Forward | Reverse | |
|---|---|---|
| Homo-Cytb | 5’-GGCATTATCCTC CTGCTTGC-3’ | 5’-GATGTGGGGA GGGGTGTTTA-3’ |
| Macaca-Cytb | 5’-ACCCAGACA ACTACATCCCG-3 | 5’-AAGAGTGCTAG TACGCCTCC-3’ |
Histological Examination
Part of the corneas was frozen and embedded in OCT compound. Part of the corneas were fixed in 4% formaldehyde and embedded in paraffin. Sections were cut at 5 µm slices, and subjected to immunofluorescence, immunohistochemistry and HE staining according to standard methods. For immunofluorescence and immunohistochemistry, the primary antibody was anti-Na+/K+ ATPase (1:100, Millipore), anti-Pitx2 (1:100, Novus) and anti-Col8a2 (1:50, Novus).
RNA-Sequencing and Data Analysis
The total RNA of CEC-like cells and corneal endothelial cells of EXP1 and EXP2 groups were isolated using TRIzol reagent (Life Technologies) according to the manufacturer’s protocol. RNA-seq transcriptome library was prepared following the TruSeqTM RNA sample preparation Kit from Illumina (San Diego, CA) using 1µg of total RNA. Sequencing was performed in Majorbio Biotech (Shanghai, China) using the Illumina HiSeq 4000 150 bp Paired-End Platform. The raw paired end reads were trimmed and quality controlled by SeqPrepand Sickle with default parameters. Then clean reads were separately aligned to human reference genome with orientation mode using HIASAT software. The mapped reads of each sample were assembled by StringTie. The sequencing data was deposited to NCBI SRA and the BioProject No. is PRJNA722271. The HCEC RNA-sequencing raw paired end reads data was downloaded from GEO datasets (BioProject No. is PRJNA395734).
To identify DEGs (differential expression genes) between two different samples, the expression level was calculated according to the TPM method. RSEM was used to quantify gene abundances. R statistical package software EdgeR was utilized for differential expression analysis. Genes with FDR < 0.05 and |log2FC| ≥ 1 were considered as significant. Venn analysis (TPM>1) was used to demonstrate common and uniquely expressed genes transcripts between samples. The correlation analysis provides basic reference for the analysis of differential genes. GO functional enrichment analysis were performed by Goatools to identify which DEGs were significantly enriched at Bonferroni-corrected P-value ≤ 0.05 compared with the whole-transcriptome background.
Statistical Analyses
All data are presented as means. The Student’s t test was used to examine differences between the two groups. All data analyses were calculated by GraphPad Prism version 6 (GraphPad Software, Inc.).
Results
Cell Culture
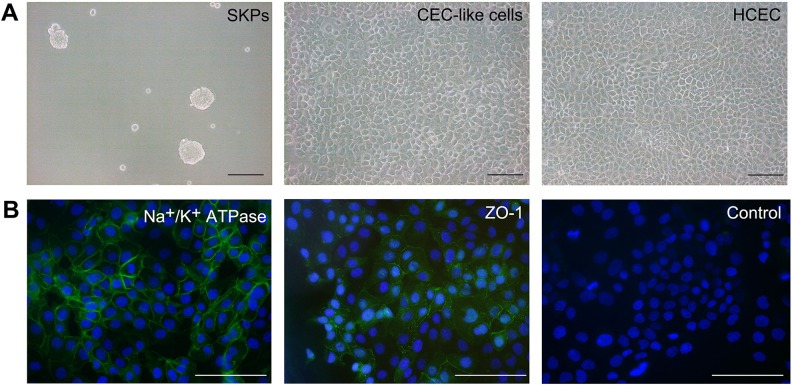
Human SKPs were successfully isolated and presented as small floating spheres. CEC-like cells were well achieved using our previous differentiation method. The CEC-like cells displayed hexagonal or polygonal shapes and formed a mosaic monolayer, which were similar to HCEC. These cells strongly expressed HCEC major markers, Na+/K+ ATPase and ZO-1(Fig. 1A, B).
Figure 1.
Cell culture. (A) Human SKPs were small floating spheres. CEC-like cells displayed hexagonal or polygonal shapes and formed a mosaic monolayer, which were similar to HCEC. (B) CEC-like cells expressed Na+/K+ ATPase and ZO-1. Scar bars 100 μm.
Long-Term Observation of CEC-Like Cells Transplantation Into the Monkey Corneal Endothelial Dysfunction model
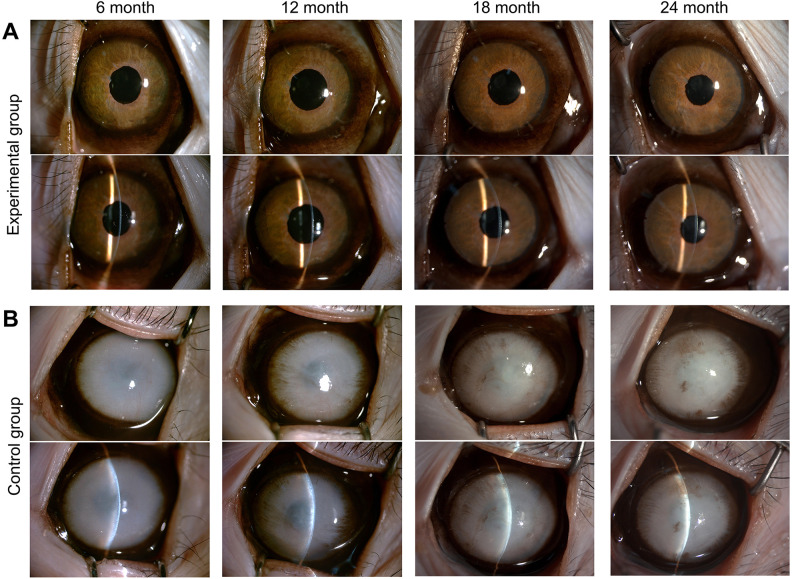
In the experimental group, the cornea remained transparent, and almost no keratic precipitates or corneal neovascularization appeared (Fig. 2A). In addition, the pupil was round with a positive light reflex, the iris texture was clear, the anterior chamber depth was normal, the aqueous fluid was clear, and the lens was transparent. In the control group, the cornea remained opaque with edema and as a consequence the iris could not be seen (Fig. 2B). Clinical observations of the other three monkeys are shown in Supplemental Fig. 2.
Figure 2.
Clinical observation of the experimental group and control group during 2 years. (A) In the experimental group, the corneas remained transparent, and almost no keratic precipitates or corneal neovascularization appeared. (B) In the control group, the corneas remained opaque with edema and as a consequence the iris could not be seen. Monkey 1 and 2 are shown here. The other three monkeys are shown in Supplemental figure 2.
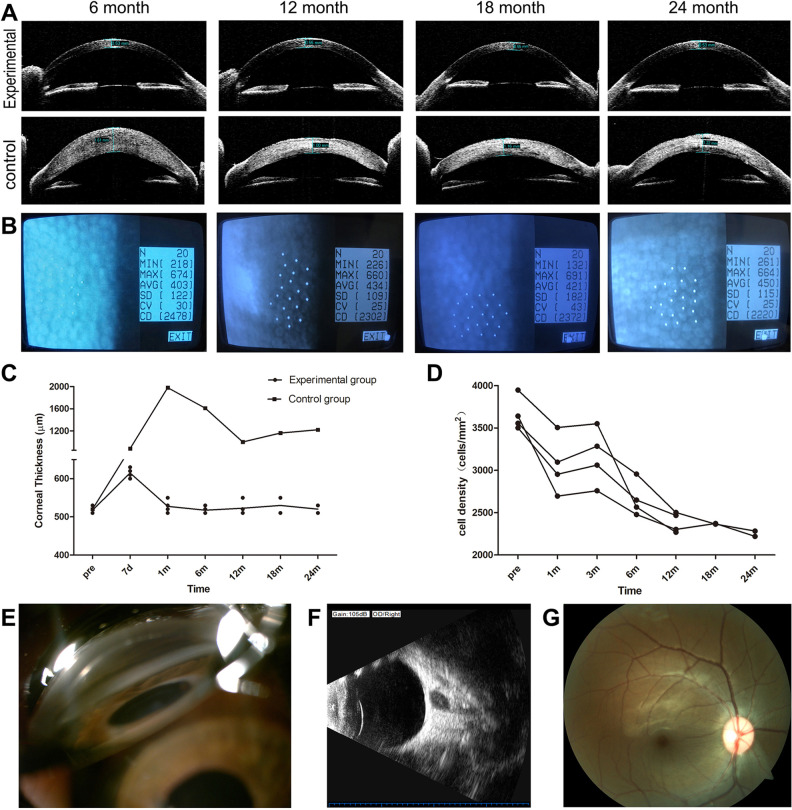
The corneal thickness in the experimental group remained steady within the range of 0.5 mm–0.55 mm from 1 month to 2 years after transplantation. The corneal thickness in the control group continued to be above 1.0 mm 2 years after transplantation, and neovascularization appeared (Fig. 3A,C). The cells after transplantation were in the form of a polygonal monolayer (Fig. 3B). The total endothelial cell density continued to increase in the first 3 months, peaking at an average of 3042 cells/mm2, 84% of pre-operation. But after 3 months it gradually fell. The average cell density rapidly decreased in the first year and slowly in the second year. It decreased by about 20% during the first year and by 6% during the second year compared to the third month. However, the cell density was still more than 2000 cells/mm2 within 2 years, 63% of pre-operation (Fig. 3D). However, because of corneal opacity, this could not be detected in the control group. Other ophthalmic examinations including intraocular pressure, chamber angle, B-ultrasonography, and fundus imaging showed no apparent pathological abnormity (Fig. 3E–G).
Figure 3.
Ophthalmic examinations of the experimental group and control group during 2-year observation. (A) Visante OCT showed significant corneal thickness differences in the experimental group and the control group (Monkey 1 and 2). (B) Non-contact specular microscopy showed that cells after transplantation were in the form of a polygonal monolayer (Monkey 1). (C) The changes of corneal thickness in the experimental group and control group. (D) The changes of cell density in the experimental group. (E) Chamber angle examination. (F) B-ultrasonography. (G) Fundus imaging. (E–G: Monkey 1 at 2-year time is shown)
Polymerase Chain Reaction and DNA-Sequencing after Transplantation
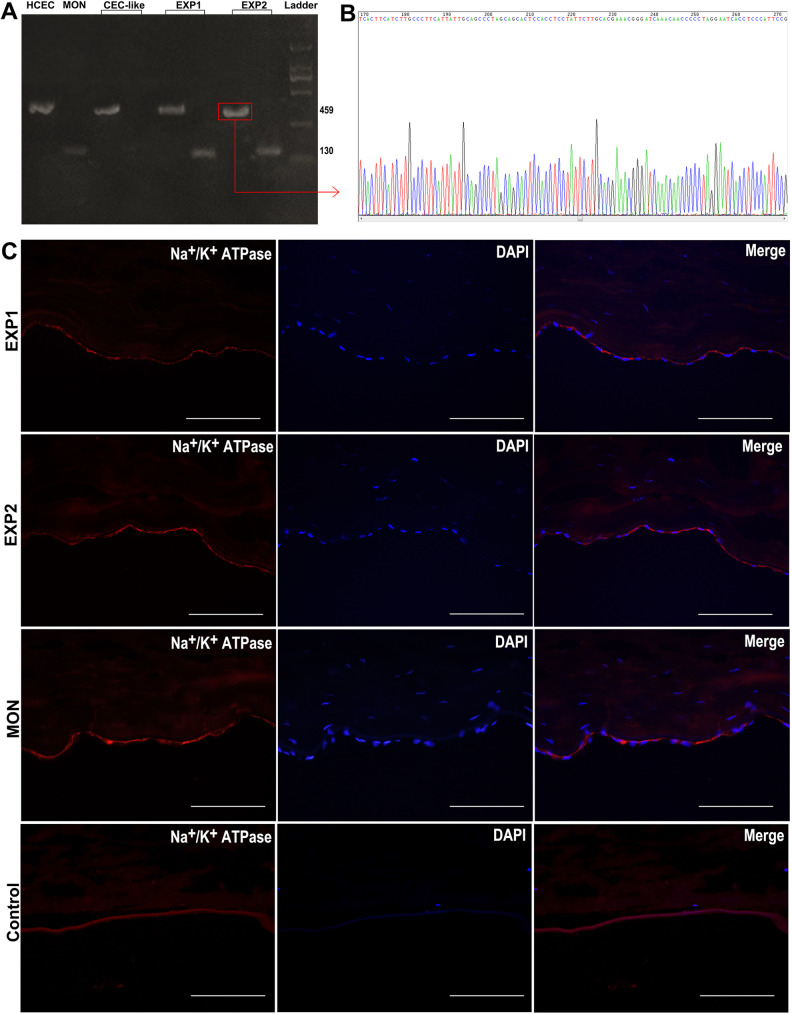
PCR was carried out to show the existence of human or monkey DNA in the samples. We designed primers for human and monkey specific Cytb gene fragments to identify human or monkey DNA. With the human Cytb gene fragments primer, a 459 bp gene fragment in HCEC group was obtained (Fig. 4A HCEC). With the monkey Cytb gene fragments primer, a 130 bp gene fragment in MON group was obtained (Fig. 4A MON). But, with the two primers, only a 459 bp but not a 130 bp gene fragment in CEC-like cells was obtained (Fig. 4A CEC-like). However, with the two primers, both a 459 bp and a 130 bp gene fragment in the EXP1 group were obtained (Fig. 4A EXP1), the same as the EXP2 group (Fig. 4A EXP2).
Figure 4.
DNA-sequencing and immunofluorescent staining after transplantation. (A) Polymerase chain reaction of HCEC, MON cells, CEC-like cells, EXP1 cells, EXP2 cells. (B) DNA-sequencing of EXP2 456 bp gene fragment products. (C) Immunofluorescent staining of the EXP1, EXP2, MON and control group. Scar bars 100 μm.
DNA-sequencing of the 459 bp gene fragment products in EXP2 group was carried out. It showed that the base pairs sequences were highly corresponded to that of the designed human Cytb DNA fragment target sequences (Fig. 4B and Table 2). This indicated that the amplified DNA products acquired in the experimental group had a high homology with human DNA, which proved the long-term existence of injected CEC-like cells in the 1-year and 2-year experimental groups (EXP1 and EXP2).
Table 2.
DNA Sequences.
| Target sequences |
|---|
| GGCATTATCCTCCTGCTTGCAACTATAGCAACAGCCTTCATAGGCTATGTCCTCCCGTGAGGCCAAATATCATTCTGAGGG GCCACAGTAATTACAAACTTACTATCCGCCATCCCATACATTGGGACAGACCTAGTTCAATGAATCTGAGGAGGCTACTC AGTAGACAGTCCCACCCTCACACGATTCTTTACCTTTCACTTCATCTTGCCCTTCATTATTGCAGCCCTAGCAACACTCCA CCTCCTATTCTTGCACGAAACGGGATCAAACAACCCCCTAGGAATCACCTCCCATTCCGATAAAATCACCTTCCACCCTT ACTACACAATCAAAGACGCCCTCGGCTTACTTCTCTTCCTTCTCTCCTTAATGACATTAACACTATTCTCACCAGACCTCC TAGGCGACCCAGACAATTATACCCTAGCCAACCCCTTAAACACCCCTCCCCACATC |
| Sequencing results |
| GGCATTATCCTCCTGCTTGCAACTATAGCAACAGCCTTCATAGGCTATGTCCTCCCGTGAGGCCAAATATCATTCTGAGGG GCCACAGTAATTACAAACTTACTATCCGCCATCCCATACATTGGGACAGACCTAGTTCAATGAATCTGAGGAGGCTACTC AGTAGACAGTCCCACCCTCACACGATTCTTTACCTTTCACTTCATCTTGCCCTTCATTATTGCAGCCCTAGCAGCACTCC ACCTCCTATTCTTGCACGAAACGGGATCAAACAACCCCCTAGGAATCACCTCCCATTCCGATAAAATCACCTTCCACCCT TACTACACAATCAAAGACGCCCTCGGCTTACTTCTCTTCCTTCTCTCCTTAATGACATTAACACTATTCTCACCAGACCTC CTAGGCGACCCAGACAATTATACCCTAGCCAACCCCTTAAACACCCCTCCCCACATCA |
The base pairs sequences were highly corresponded to that of the designed human Cytb DNA fragment target sequences.
Histological Examination After Transplantation
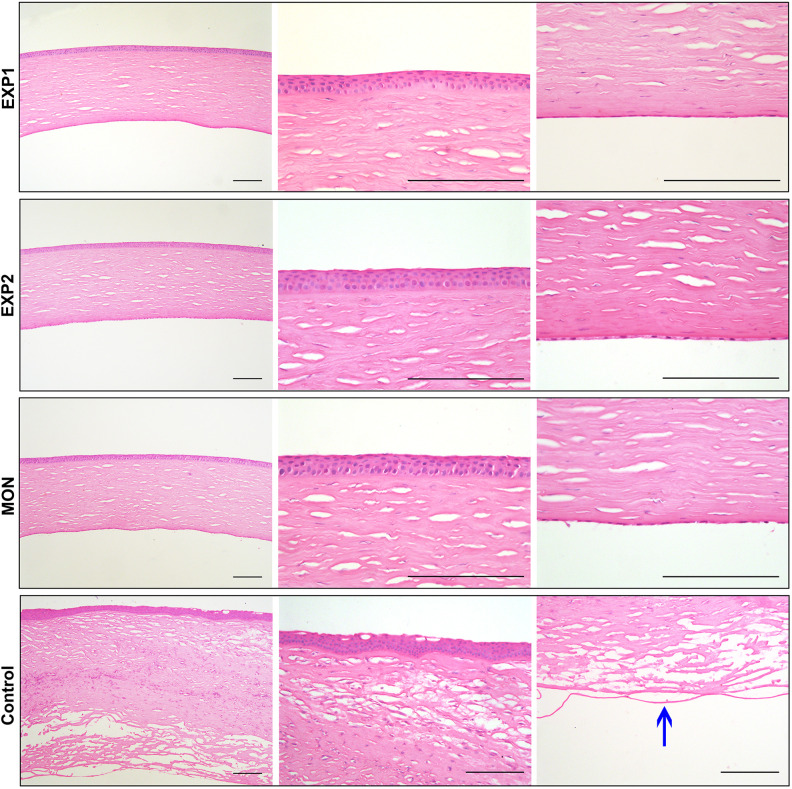
Immunofluorescent staining of frozen section showed Na+/K+ ATPase expression in EXP1 and EXP2 group, indicating the persistent pump function similar to the normal group. In the control group, the Descemet’s membranes were bare and almost no cells were detected (Fig. 4C). CEC specific markers Col8a2 and Pitx2 were also expressed and shown in Supplemental Fig. S3. Through HE staining it could be seen that the corneal thickness in the EXP1 and EXP2 group was similar to the normal group. Under magnified observation, the CEC-like cells tightly adhered to the posterior surface of the cornea in a monolayer. The morphology of the corneal epithelium, stromal cells, and endothelium in the EXP1 and EXP2 group were all similar to the normal group. However, in the control group, the cornea was very thick and edematous. Under magnified observation, the Descemet’s membranes were bare and almost no cells were detected. The corneal stromal layer had severe edema and a lot of inflammatory cells. Collagen fibers were irregularly arranged, layered, and broken. Stromal cells had an abnormal morphology. The corneal epithelial layer was also vacuolate (Fig. 5).
Figure 5.
HE staining of the EXP1, EXP2, MON and control group. The morphology of EXP1 and EXP2 group were all similar to the normal group. However, in the control group, the cornea was very thick and edematous. The arrow points to bare Descemet’s membrane in control group. Scar bars 100 μm.
Functional Pattern of Gene Expression in HCEC, CEC-Like Cells, EXP1 Cells, and EXP2 Cells
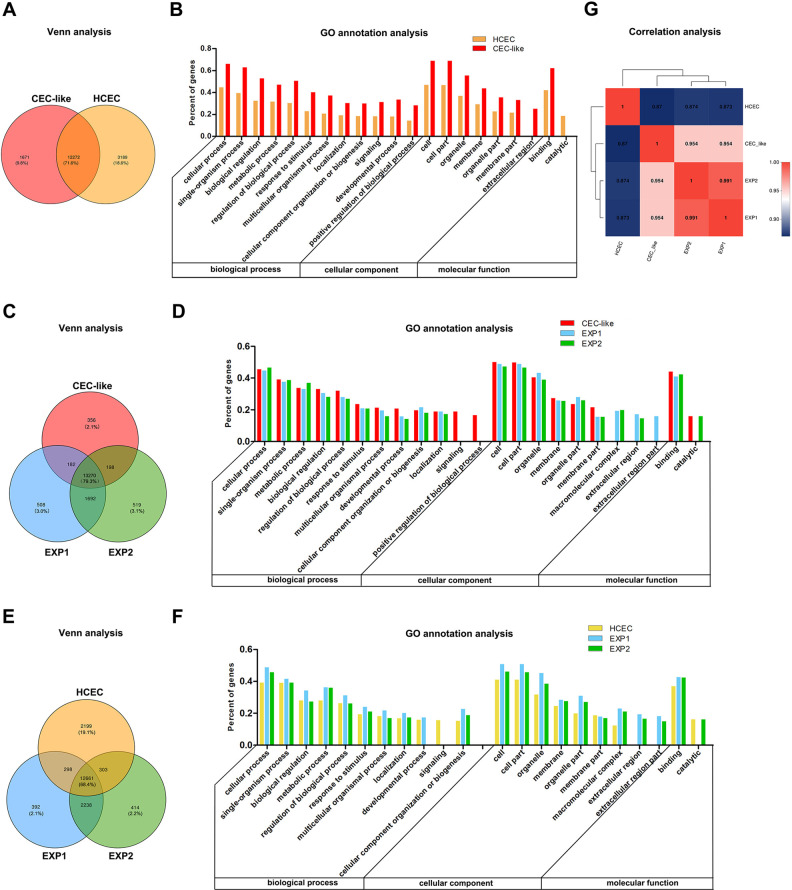
Venn analysis was carried out to show co-expressed and specially expressed genes between samples or groups. The GO annotation analysis showed the top 20 GO sets of abundance in specially expressed genes, including classification of the biological process, cellular component, and molecular function.
The Venn analysis of HCEC and CEC-like cells showed that 71.6% of all expressed genes were co-expressed. The number of specific genes of CEC-like cells and HCEC was 1671 and 3186, accounting for 9.8% and 18.6% (Fig. 6A). The GO annotation analysis of specific genes in the two groups showed that the abundance ranking in the two groups was almost the same with only few differences. The percent of the signaling and developmental process ranked higher in CEC-like cells than that of HCEC. The percent of the extracellular region ranked higher than the catalytic activity in CEC-like cells, whereas in HCEC they were the opposite (Fig. 6B).
Figure 6.
Functional pattern of gene expression in HCEC, CEC-like cells, EXP1 cells, and EXP2 cells. (A) Venn analysis of CEC-like cells and HCEC; (B) GO annotation analysis of CEC-like cells and HCEC. (C) Venn analysis of CEC-like cells, EXP1 cells, EXP2 cells. (D) GO annotation analysis of CEC-like cells, EXP1, EXP2. (E) Venn analysis of HCEC, EXP1 cells, EXP2 cells. (F) GO annotation analysis of HCEC, EXP1 cells, EXP2 cells. (G) The correlation analysis of CEC-like cells, HCEC, EXP1 cells, EXP2 cells.
The Venn analysis of CEC-like/EXP1/EXP2 cells showed that 79.3% of all expressed genes were co-expressed. The number of specific genes of CEC-like/EXP1/EXP2 cells was 356/508/519, accounting for 2.1%/3.0%/3.1%, respectively (Fig. 6C). The GO annotation analysis of specific genes in the three groups showed that the abundance ranking was similar with only a few differences. The percent of signaling and positive regulation among biological process ranked higher in CEC-like cells. However, the macromolecular complex and extracellular region among cellular component ranked higher in EXP1 and EXP2 (Fig. 6D).
The Venn analysis of HCEC/EXP1/EXP2 cells showed that 68.4% of all expressed genes were co-expressed. The number of specific genes of HCEC/EXP1/EXP2 cells was 2119/392/414, accounting for 19.1%/2.1%/2.2%, respectively (Fig. 6E). Through GO annotation analysis it was found that the abundance ranking was similar to that in CEC-like/EXP1/EXP2 cells GO annotation analysis (Fig. 6F).
The correlation analysis was carried out to detect the relativity between samples or groups. The r value between each biological repeat sample of HCEC and CEC-like cells was more than 0.98 (data not shown), demonstrating good biological repeatability. The mean r value between CEC-like cells and EXP1 or EXP2 cells was both about 0.954. The r value between EXP1 and EXP2 cells was 0.991. The mean r value between CEC-like cells and HCEC was about 0.87, but after 1–2 years transplantation the r value between EXP1 or EXP2 cells and HCEC was 0.873 and 0.874, respectively (Fig. 6G).
Differential Expression Gene Analysis of CEC-Like Cells, EXP1 Cells, and EXP2 Cells
Expression variance analysis was used to detect differential expression genes (DEGs). Genes with FDR < 0.05 and |log2FC| ≥ 1 were considered as significant. GO enrichment analysis was carried out to obtain the GO Term of significant DEGs enrichment.
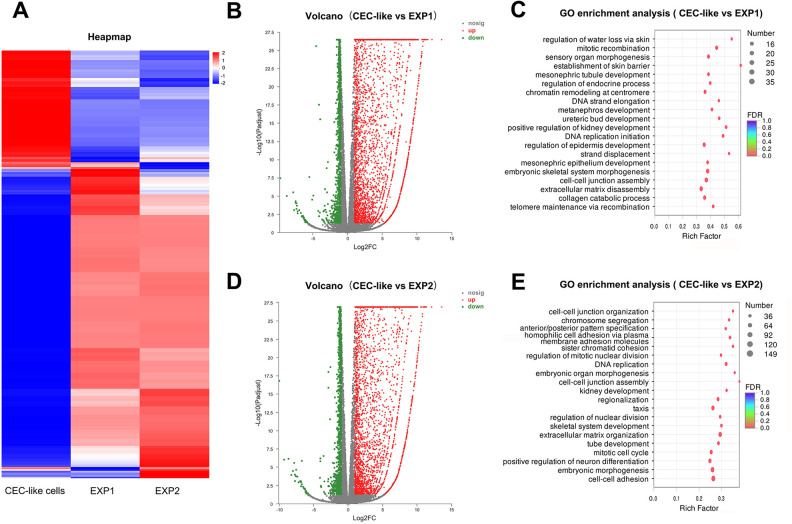
4566 significant DEGs were detected between CEC-like cells and EXP1 cells, among which 3385 (74.1%) genes were up-regulated and 1181 (25.9%) were down-regulated. The GO enrichment analysis showed that the significant DEGs in EXP1 cells versus CEC-like cells mainly took part in regulation of water loss, mitotic recombination, sensory organ morphogenesis, and kidney development (Fig. 7B,C).
Figure 7.
Differential expression gene analysis of CEC-like cells, EXP1 cells, and EXP2 cells. (A) Heapmap of CEC-like cells versus EXP1 cells versus EXP2 cells. (B) Volcano of CEC-like cells versus EXP1 cells. (C) GO enrichment analysis of CEC-like cells versus EXP1 cells. (D) Volcano of CEC-like cells versus EXP2 cells. (E) GO enrichment analysis of CEC-like cells versus EXP2 cells.
4524 significant DEGs were detected between CEC-like cells and EXP2 cells, among which 3360 (74.3%) genes were up-regulated and 1164 (25.7%) were down-regulated. Significant DEGs in EXP2 cells versus CEC-like cells mainly took part in cell-cell junction organization/assembly, cell adhesion, chromosome segregation, pattern specification, and DNA replication (Fig. 7D,E).
Nineteen significant DEGs were detected between EXP1 cells and EXP2 cells, among which 12 genes were up-regulated and 7 were down-regulated. The genes could not be GO enriched.
Discussion
In our previous study, we invented a creative method to acquire CEC-like cells. We co-cultured skin-derived precursors (SKPs) with B4G12 cells using Transwell inserts in human endothelial SFM. B4G12 cells were for the first time applied in cell induction and the serum free medium were for the first time used for corneal endothelial cells culture. The CEC-like cells we acquired had similar morphology and characteristics to normal human CECs. CEC-like cells could be passaged in vitro. CEC-like cells could survive after being injected into anterior chamber of the monkey corneal endothelial dysfunction model and had an excellent therapeutic effect within 3 months 11 .
In this study, we prolonged the observation to 2 years and discovered that the CEC-like cells still worked. The cornea remained transparent and its thickness was constant. The count of total corneal endothelial cells gradually increased within 3 months, indicating that the injected CEC-like cells survived well in the microenvironment and still proliferated early after transplantation. During 3 months to 1 year, the cell density significantly decreased, possibly because CEC-like cells stopped proliferating and some CEC-like cells that could not adapt to the microenvironment gradually died. After 1 year, the rest of the CEC-like cells had adapted and the cell density tended to be stable remaining above 2000 cells/mm2 within 2 years. A few cells died possibly due to the physiological aging process 17 . Cell density of corneal endothelial cells in normal adults is above 2000 cells/mm2. Therefore, the cell density in our experiment conforms to the normal physiological state.
To verify that the origin of endothelial cells in the long-term experimental group, we extracted the endothelial cell DNA for PCR and sequencing. Nucleotide sequences of human and animal mitochondrial cytochrome b genes are known to be species-specific and have been used for species identification in forensic investigations 18,19 . In order to distinguish human and monkey species, we designed specific Cytb primers. The results showed that the corneal endothelial cells in the experimental group contained both CEC-like cells and monkey cells. This suggests that CEC-like cells can survive and persist in the monkey corneal dysfunction model for a long time, despite a few residual monkey cells. Because of the lack of tracking technology, we were unable to determine the percentage of CEC-like cells and monkey cells. But the proportion of monkey cells was very low for the following reasons. First, the RNA-sequencing data we used for analysis was the mapped data which mean the clean reads obtained after comparing with the human genome. In general, if there is no or little contamination associated with experiment, the mapping rate of the sequence is usually higher than 65%. In our experiment, the mapping rate of the CEC-like cells was 96.78%. The mapping rate of the EXP1 and EXP2 was 94.48% and 94.58% separately. In addition, we also carried out the RNA-seq of monkey CECs and the mapping rate was 37.34%. So, we can see that the EXP1 and EXP2 cells were almost human CEC-like cells. On the other hand, in the control group, the cornea was very thick and edematous all the time and there were almost no CECs on the Descemet’s membrane, indicating that our corneal dysfunction model was very successful. Therefore, the relatively stable cell density after 2 years is a result of the peaceful coexistence of CEC-like cells and very few monkey cells.
RNA-sequencing was also used in this study to analyze the gene expression pattern of HCEC, CEC-like cells, and cells after transplantation (EXP1, 2). The three Venn analyses showed a high proportion of co-expressed genes in these cells. This indicates that the gene expression pattern of the SKP-derived CEC-like cells are similar to HCEC and after transplantation CEC-like cells still retain their characteristics. The three GO annotation analyses of specially expressed genes showed the top 20 GO sets of abundance, including classification of biological process, cellular component, and molecular function. The abundance ranks in the three analyses were almost same with only a few differences. This also indicates the similarities between these four kinds of cells. The gene transcriptional sequencing analysis reveals the reason why the CEC-like cells can effectively work on the primate models and work so long.
Correlation analysis provides basic reference for the analysis of differential genes. The correlation analysis showed a high correlation between HCEC, CEC-like cells, and cells after transplantation (EXP1, 2), suggesting a high similarity of their gene expression pattern. The r value between EXP1 and EXP2 cells reached 0.991, indicating that cells tended to be stable after 1 year of transplantation. The r value between EXP cells and HCEC was higher than that between CEC-like cells and HCEC, suggesting that long after transplantation these CEC-like cells become more and more similar to human corneal endothelial cells. This might be due to the influence of the monkey anterior chamber microenvironment in vivo 20 . CEC-like cells retained their characteristics after transplantation and only slightly changed. Thus, we carried out gene differential analysis of CEC-like cells and EXP cells. Differential analysis showed that most (EXP1:74.1%, EXP2:74.3%) genes were up-regulated and a few were down-regulated. The up-regulated genes function focused on mitotic recombination, DNA replication, organ development and regulation, cell junction, and adhesion. Therefore, CEC-like cells still proliferated and cell attachment was enhanced after transplantation.
Although Descemet’s stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasties (DMEK) are now the most commonly used surgical method of endothelial keratoplasty 21 –23 , the anterior chamber injection method may be a very promising method to be clinically used. Recently, Okumura reported serial studies showing that injection of cultivated corneal endothelial cells with Rock inhibitor (Y-27632) into rabbit or monkey anterior chambers could exert good therapeutic effect for corneal endothelial dysfunction 24 –27 . The Y-27632 could significantly promote cell adhesion and proliferation, and inhibit apoptosis of corneal endothelial cells 28 . This method does not cause adverse effects, such as rejection, secondary glaucoma, or aberrant ectopic cell transplantation. Their clinical trials also succeeded in 11 corneal endothelial dysfunction patients 29 . The anterior chamber injection method is simple and easy to do. In our study, we not only applied the anterior chamber injection method with Rock inhibitor but also used our novel CEC-like cells. CEC-like cells could be produced abundantly and avoid using cultivated human endothelial cells. However, in clinical practice, corneal endothelial dysfunction is usually accompanied with Descemet’s membrane defects. Actually, there are some differences between mechanical abrasion in animal models and diseased surfaces. But in the process of mechanically scraping there were also some damages and fractures on the Descemet’s membranes, which resembles diseased Descemet’s membranes. CEC-like cells could express collagen type VIII (Col8a2) which are components of the Descemet’s membranes. CEC-like cells could attach and survive well on the Descemet’s membranes during 2 years observation. So, CEC-like cells could adapt to the microenvironment in anterior chamber and will have a good therapeutic effect for endothelial dysfunction in clinical trials in the future.
In our experiment, there was no apparent immune rejection during observation period. Few inflammatory keratic or anterior chamber precipitates appeared. This demonstrates the low immunogenicity of CEC-like cells. The anterior-chamber-associated immune deviation (ACAID) mechanism, the less irritating surgical procedure, and the serum free medium culture of CEC-like cells were the possible factors that could reduce cell immunogenicity 30 –32 . This’s also why CEC-like cells were stable and could adapt to the microenvironment after transplantation. In addition, it is reported that SKPs express HLA-ABC molecules, but not HLA-DR, rendering them poorly immunogenic. SKP do not express the complete pattern of molecules required to fully activate T-lymphocytes. SKPs are able to suppress the allogeneic activation of T-lymphocytes, resulting in an improved health status of animals suffering from a graft-versus-host reaction 33 . We speculate that SKPs’ ability has passed to CEC-like cells. If our cells are applicated in the clinical trials in the future, the immune rejection is also expected to be low. What is more, the monkey is the species most similar to humans 34 . Though the number of experimental monkeys was limited because of several constraints. But we have provided long-term observation results in higher animals. There were very few articles that could provide this. Thus, our research is preclinical research that is significantly meaningful for cell-based therapy of corneal endothelial dysfunction. We are ready do clinical trials in the near future.
Conclusions
CEC-like cells could adapt to the microenvironment and had a good therapeutic effect after being transplanted into the monkey endothelial dysfunction model during long-term observation. The cornea remained transparent and cell density remained in a normal range. The gene expression pattern of CEC-like cells was similar to HCEC and slightly changed after transplantation. We have acquired a deep understanding of CEC-like cells in terms of gene, protein, and animal experiment. Our researches provided a promising prospect of cell-based therapy with CEC-like cells for corneal endothelial dysfunction. The renewable cell source, novel derivation method and simple treatment strategy may be clinically applied in cellular replacement therapy or regenerative medicine in the future.
Supplemental Material
Supplemental Material, sj-docx-1-cll-10.1177_09636897211017830 for Long-Term Observation and Sequencing Analysis of SKPs-Derived Corneal Endothelial Cell-Like Cells for Treating Corneal Endothelial Dysfunction by Lin Shen, Peng Sun, Liqun Du, Jing Zhu, Chengqun Ju, Hui Guo and Xinyi Wu in Cell Transplantation
Acknowledgments
Thanks to Dr. Edward C. Mignot, Shandong University, for linguistic advice.
Footnotes
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval: Ethical approval to this study was obtained from the Medical Ethics Committee of Qilu Hospital of Shandong University, China (approval number 12028) and the Association for Laboratory Animal Care of Hongli Medical Animal Experimental Research Center, Shandong Province, China (approval number 2016-0803).
Statement of Human and Animal Rights: All procedures involving human in this study were conducted in accordance with protocols approved by the Medical Ethics Committee of Qilu Hospital of Shandong University, China (approval number 12028). All animals were treated in accordance with the Institutional Animal Care guideline of Hongli Medical Animal Experimental Research Center, Shandong Province, China and approved by their Association for Laboratory Animal Care (approval number 2016-0803).
Statement of Informed Consent: Human samples were collected following written informed consents.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Natural Science Foundation of Shandong Province (grant number ZR2020QH145 and ZR2017MH022), the National Natural Science Foundation of China (grant number 81770893 and 82070925), China Postdoctoral Science Foundation (grant number 2018M642665).
ORCID iD: Lin Shen  https://orcid.org/0000-0002-4741-9584
https://orcid.org/0000-0002-4741-9584
Supplemental Material: Supplemental material for this article is available online.
Reference
- 1. Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease S. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 2. Lee JG, Ko MK, Kay EP. Endothelial mesenchymal transformation mediated by IL-1beta-induced FGF-2 in corneal endothelial cells. Exp Eye Res. 2012;95(1):35–39. [DOI] [PubMed] [Google Scholar]
- 3. Rolev K, Coussons P, King L, Rajan M. Experimental models of corneal endothelial cell therapy and translational challenges to clinical practice. Exp Eye Res. 2019;188:107794. [DOI] [PubMed] [Google Scholar]
- 4. Ono T, Ishiyama S, Hayashidera T, Mori Y, Nejima R, Miyata K, Amano S. Twelve-year follow-up of penetrating keratoplasty. Jpn J Ophthalmol. 2017;61(2):131–136. [DOI] [PubMed] [Google Scholar]
- 5. Fuest M, Ang M, Htoon HM, Tan D, Mehta JS. Long-term visual outcomes comparing descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2017;182:62–71. [DOI] [PubMed] [Google Scholar]
- 6. Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167–173. [DOI] [PubMed] [Google Scholar]
- 7. Okumura N, Koizumi N. Regeneration of the corneal endothelium. Curr Eye Res. 2020;45(3):303–312. [DOI] [PubMed] [Google Scholar]
- 8. Hanson C, Arnarsson A, Hardarson T, Lindgard A, Daneshvarnaeini M, Ellerstrom C, Bruun A, Stenevi U. Transplanting embryonic stem cells onto damaged human corneal endothelium. World J Stem Cells. 2017;9(8):127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wahlig S, Peh GSL, Adnan K, Ang HP, Lwin CN, Morales-Wong F, Ong HS, Lovatt M, Mehta JS. Optimisation of storage and transportation conditions of cultured corneal endothelial cells for cell replacement therapy. Sci Rep. 2020;10(1):1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meir YJ, Chen HC, Chen CC, Ma HD. Revisiting existing evidence of corneal endothelial progenitors and their potential therapeutic applications in corneal endothelial dysfunction. Adv Ther. 2020;37(3):1034–1048. [DOI] [PubMed] [Google Scholar]
- 11. Shen L, Sun P, Zhang C, Yang L, Du L, Wu X. Therapy of corneal endothelial dysfunction with corneal endothelial cell-like cells derived from skin-derived precursors. Sci Rep. 2017;7(1):13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang C, Du L, Sun P, Shen L, Zhu J, Pang K, Wu X. Construction of tissue-engineered full-thickness cornea substitute using limbal epithelial cell-like and corneal endothelial cell-like cells derived from human embryonic stem cells. Biomaterials. 2017;124:180–194. [DOI] [PubMed] [Google Scholar]
- 13. Biernaskie JA, McKenzie IA, Toma JG, Miller FD. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1(6):2803–2812. [DOI] [PubMed] [Google Scholar]
- 14. De Kock J, Najar M, Bolleyn J, Al Battah F, Rodrigues RM, Buyl K, Raicevic G, Govaere O, Branson S, Meganathan K, Gaspar JA, et al. Mesoderm-derived stem cells: the link between the transcriptome and their differentiation potential. Stem Cells Dev. 2012;21(18):3309–3323. [DOI] [PubMed] [Google Scholar]
- 15. Valtink M, Gruschwitz R, Funk RH, Engelmann K. Two clonal cell lines of immortalized human corneal endothelial cells show either differentiated or precursor cell characteristics. Cells Tissues Organs. 2008;187(4):286–294. [DOI] [PubMed] [Google Scholar]
- 16. Ju C, Gao L, Wu X, Pang K. A human corneal endothelium equivalent constructed with acellular porcine corneal matrix. Indian J Med Res. 2012;135(6):887–894. [PMC free article] [PubMed] [Google Scholar]
- 17. Van den Bogerd B, Dhubhghaill SN, Koppen C, Tassignon MJ, Zakaria N. A review of the evidence for in vivo corneal endothelial regeneration. Surv Ophthalmol. 2018;63(2):149–165. [DOI] [PubMed] [Google Scholar]
- 18. Matsuda H, Seo Y, Kakizaki E, Kozawa S, Muraoka E, Yukawa N. Identification of DNA of human origin based on amplification of human-specific mitochondrial cytochrome b region. Forensic Sci Int. 2005;152(2-3):109–114. [DOI] [PubMed] [Google Scholar]
- 19. Lopez-Oceja A, Gamarra D, Borragan S, Jimenez-Moreno S, de Pancorbo MM. New cyt b gene universal primer set for forensic analysis. Forensic Sci Int Genet. 2016;23:159–165. [DOI] [PubMed] [Google Scholar]
- 20. Koizumi N, Sakamoto Y, Okumura N, Okahara N, Tsuchiya H, Torii R, Cooper LJ, Ban Y, Tanioka H, Kinoshita S. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007;48(10):4519–4526. [DOI] [PubMed] [Google Scholar]
- 21. Zhu L, Zha Y, Cai J, Zhang Y. Descemet stripping automated endothelial keratoplasty versus descemet membrane endothelial keratoplasty: a meta-analysis. Int Ophthalmol. 2018;38(2):897–905. Epub 2017 Apr 17. [DOI] [PubMed] [Google Scholar]
- 22. Maier AK, Gundlach E, Gonnermann J, Klamann MK, Bertelmann E, Rieck PW, Joussen AM, Torun N. Retrospective contralateral study comparing descemet membrane endothelial keratoplasty with descemet stripping automated endothelial keratoplasty. Eye (Lond). 2015;29(3):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arbelaez JG, Feng MT, Pena TJ, Price MO, Price FW, Jr. A year of cornea in review: 2013. Asia Pac J Ophthalmol (Phila). 2015;4(1):40–50. [DOI] [PubMed] [Google Scholar]
- 24. Okumura N, Matsumoto D, Fukui Y, Teramoto M, Imai H, Kurosawa T, Shimada T, Kruse F, Schlotzer-Schrehardt U, Kinoshita S, Koizumi N. Feasibility of cell-based therapy combined with descemetorhexis for treating Fuchs endothelial corneal dystrophy in rabbit model. PLoS One. 2018;13(1):e0191306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okumura N, Sakamoto Y, Fujii K, Kitano J, Nakano S, Tsujimoto Y, Nakamura S, Ueno M, Hagiya M, Hamuro J, Matsuyama A, et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci Rep. 2016;6:26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okumura N, Koizumi N, Ueno M, Sakamoto Y, Takahashi H, Tsuchiya H, Hamuro J, Kinoshita S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181(1):268–277. [DOI] [PubMed] [Google Scholar]
- 27. Okumura N, Kinoshita S, Koizumi N. Application of rho kinase inhibitors for the treatment of corneal endothelial diseases. J Ophthalmol. 2017;2017:2646904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peh GS, Adnan K, George BL, Ang HP, Seah XY, Tan DT, Mehta JS. The effects of Rho-associated kinase inhibitor Y-27632 on primary human corneal endothelial cells propagated using a dual media approach. Sci Rep. 2015;5:9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kinoshita S, Koizumi N, Ueno M, Okumura N, Imai K, Tanaka H, Yamamoto Y, Nakamura T, Inatomi T, Bush J, Toda M, et al. Injection of cultured cells with a rock inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995–1003. [DOI] [PubMed] [Google Scholar]
- 30. Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J Immunol. 2016;196(10):3983–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamada J, Ueno M, Toda M, Shinomiya K, Sotozono C, Kinoshita S, Hamuro J. Allogeneic sensitization and tolerance induction after corneal endothelial cell transplantation in mice. Invest Ophthalmol Vis Sci. 2016;57(11):4572–4580. [DOI] [PubMed] [Google Scholar]
- 32. Hos D, Tuac O, Schaub F, Stanzel TP, Schrittenlocher S, Hellmich M, Bachmann BO, Cursiefen C. Incidence and clinical course of immune reactions after descemet membrane endothelial keratoplasty: retrospective analysis of 1000 consecutive eyes. Ophthalmology. 2017;124(4):512–518. [DOI] [PubMed] [Google Scholar]
- 33. De Kock J, Meuleman P, Raicevic G, Rodrigues RM, Branson S, Meganathan K, De Boe V, Sachinidis A, Leroux-Roels G, Vanhaecke T, Lagneaux L, et al. Human skin-derived precursor cells are poorly immunogenic and modulate the allogeneic immune response. Stem Cells. 2014;32(8):2215–2228. [DOI] [PubMed] [Google Scholar]
- 34. Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL, 3rd. Normal ocular development in young rhesus monkeys (macaca mulatta). Vision Res. 2007;47(11):1424–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-cll-10.1177_09636897211017830 for Long-Term Observation and Sequencing Analysis of SKPs-Derived Corneal Endothelial Cell-Like Cells for Treating Corneal Endothelial Dysfunction by Lin Shen, Peng Sun, Liqun Du, Jing Zhu, Chengqun Ju, Hui Guo and Xinyi Wu in Cell Transplantation