Abstract
Silicon (Si) application enhanced the tolerance of plants against different environmental stresses. Therefore, objective of the study revealed that foliar applied Si alleviates the adverse effect of Cd by enhancing the growth, metabolite accumulation, strengthening the antioxidant defense system, reducing oxidative injury, improving plant nutrient status, and decreasing the Cd uptake in wheat. The surface sterilized seeds of Sahar-2006 (tolerant) and Inqalab-91 (sensitive) having the differential metal tolerance capacity were sown in plastic pots containing normal and Cd spiked sandy loamy soil. The design of experiments was completely randomized with 3 replicates per treatment. Two weeks after germination, plants were sprayed with different concentrations of Si (1.5 and 3 mM) with 0.1% surfactant in the form of Tween-20. The plants were harvested after 2 weeks of Si application to determine various attributes. High concentration of Cd (25 mg kg-1) decreased growth-related-attributes, essential nutrient uptake and increase the levels of oxidative stress indicators. The application of Si increased the growth-related attributes, photosynthetic pigments, essential nutrient uptake and also enhanced the activities of various antioxidant compounds (superoxide dismutase (SOD), peroxidase (POD, ascorbate peroxidase (APX) and catalase (CAT) by decreasing the contents of oxidative stress indicators and Cd uptake in root and shoot of both wheat cultivars. Sahar-2006 cultivar showed more tolerance to Cd regimes than that of Inqalab-91 as clear from greater plant dry masses. Thus, our results showed that the applied Si level (3 mM) is an efficient strategy for field use in the areas, where slightly Cd polluted soils limit the agriculture production.
Keywords: silicon, oxidative stress, antioxidant compounds, cadmium, wheat, nutrient uptake
Introduction
Metals stress is the major ecological issue, now a days for agricultural soil and arises from the geological sources such as vast industrialization and some agricultural practices. 1 Moreover, anthropogenic activities such as mine and smelters, 2 rapid industrialization, 3 untreated wastewater irrigation, 4 atmospheric deposition, 5 disposal of wastes, gasoline and paints, fertilizers, 6 bio solids, 7 and pesticides 8 are major causes to increase the concentrations of various toxic pollutants, especially Cd in the soil. 9,10 The Cd is a hazardous trace element because of its higher solubility, mobility and toxicity in the nature. 11 And is widely recognized as carcinogenic, mutagenic and toxic metal. 12 Plant roots simply absorbed Cd from the soil and then uptake it to vegetative tissues, enters into the food chain and then transferred to the end consumers leading to human health problems. 13,14 Excessive Cd influences the many physiological processes in plants such as changes the membrane permeability, 15 inhibits physiologically active enzymes, 16 inactivates photosystems, 17 and disturbing mineral metabolism. 18,19 Cadmium toxicity induces oxidative stress, disrupts the pigments function and changes in protein activity. 19 Reacting to Cd stress, hyper generation of reactive oxygen species (ROS) in plants that cause damage to cell structures in plants such as oxidation of proteins and lipids, 20,21 nucleic acid damages, 22 enzyme inhibition and ultimately cell death. 23,24 However, to protect cells and tissues from injury and dysfunction, plants have evolved a broad range antioxidative defense systems containing peroxidase (POD), ascorbate peroxidase (APX), superoxide dismutase (SOD), and catalase (CAT) contents 12 and non-enzymatic antioxidants such as anthocyanin, proline, 25 cysteine, 26 ascorbic acid, 27 and glutathione 24 to scavenge the ROS 28 and protect the cellular structures from oxidative damage. 29,30 Hence, higher accumulation of Cd may produce significant change in morpho-physiological and biochemical attributes that ultimately contribute to low yield and productivity. 19
Silicon (Si) is known as quasi-essential element because of its multiple beneficial role. 31 Silicon is second most abundant metalloid in the soil 32 and found in a form of mono-silicic acid. 33 Application of Si gives freshness to the plants and its foliar spray enhance stress tolerance by changing the various biological and physical processes. 34 -36 Therefore, when Si promotes nutrient absorption by the stressed plant, as found in other species such as banana. 37 Silicon is taken up from the soil by plants through roots via aquaporin channels 38 and accumulated in plants, mostly depends on environmental factors such as water contents, Si availability and temperature. 39 Silicon is a universal player to mitigate the metal toxicity by enhancing the metabolic activities in polluted environments. 32,36 Silicon through polymerization which act as barriers to symplastic transport of Cd 34,40 and reduce transport from root to xylem. 41 Silicon nutrients play a vital structural and enzymatic role in physiological processes and in the antioxidant defense system, based on the production of compounds, such as carotenoids. 42,43 Moreover, it accumulates in cell walls, 44 increases photosynthetic activity 45,46 and mineral uptakes 47 and also activates the antioxidant systems 48 to mitigate the negative effects of reactive oxygen species (ROS) in plant. 49,50
Wheat is used as staple food in various regions of the globe and is extremely sensitive to Cd stress. We used 2 wheat cultivars to determine whether the impact of foliar applied Si differs with cultivars. It was hypothesized that Si application may confer the growth improvement in Cd stressed wheat plants by maintaining optimum levels of Cd. Therefore, this study was conducted to assess (i) the effects of different Si levels on plant growth and biomass of wheat under Cd stress by strengthens the antioxidant capacity, (ii) the silicon application may improve essential mineral uptakes by reducing oxidative injury under Cd stress, (iii) the role of Si application in regulating the main metabolic processes in wheat exposed to Cd stress.
Materials and Methods
Plant Materials and Growing Conditions
The seeds of 2 wheat cultivars having the differential metal tolerance capacity 51 namely, Sahar-2006 and Inqalab-91 were collected from the Ayub Agricultural Research Institute (AARI) Faisalabad, Pakistan. In order to contaminate the dry, sandy loamy soil, the calculated amount of Cd (10 and 25 mg kg-1) was applied to soil collected from Agriculture Field Faisalabad for spiking process to make contaminated soil artificially for 3 months. Cadmium chloride (CdCl2) was used as source for Cd stress. Prior to sowing, seeds were washed with Cd free water and then surface sterilized with 10% H2O2 to minimize the microbial contamination and rinsed with distilled water thoroughly. The plastic pots containing 25-cm diameter and 15-cm depth were filled with normal and Cd spiked sandy loamy soil (5 kg). The physico-chemical properties of dry sandy loamy soil are given in Table 1. In each pot, 10 seeds were sown in each pot and placed in an open greenhouse. All pots were arranged in completely randomized design with 3 replicate per treatment. An average of 19 ± 3/10 ± 2°C was the day/ night temperatures, an average of 62.0% to 65.1% was the relative humidity, and an average of 10 to 11 was the day length. Upon full emergence, thinning was done and only 5 seedlings were kept in each pot. Then half strength Hoagland’s nutrient solution made in Cd-free water 52 was supplied to all plants at every 5 days-interval. Soil pH was adjusted back to control, pH of 6.0 to 6.2 with diluted H2SO4. A control group without Cd application was maintained for comparison. Two weeks after germination, plants were sprayed with different concentration of Si (1.5 and 3 mM Si) using 30 mL of Na2SiO3 (Merck, pH 11.76) solution to each wheat seedling. An equivalent amount of Na+ (as NaCl) was added to the controlled plants to compensate the differences resulting from Na2SiO3 application. The controlled plants were sprayed with distilled water in the same amount. The plants were fertilized with a Hoagland nutrient solution to keep the moisture contents. Plants were harvested after 2 weeks of Si application and fresh leaves were kept in a freezer at −20°C for recording of different growth and biochemical attributes.
Table 1.
Mean Values of Physico-Chemical Properties of Dry Loamy Soil.
| Soil characteristics | Values | Soil characteristics | Values |
|---|---|---|---|
| Soil texture | Clay loam | Carbonate (meq L-1) | Nil |
| Saturation percentage (%) | 34.1 | Bicarbonate (meq L-1) | 4.0 |
| ECe (dS m-1) | 6.7 | Total nitrates (mg kg-1) | 10.5 |
| pH | 7.7 | Total nitrogen (mg kg-1) | 14.5 |
| Organic matter (%) | 0.4-0.6 | Phosphates | 8.1-10.7 |
| Calcium (meq L-1) | 3.53 | Potassium (mg kg-1) | 108-204 |
| Magnesium (meq L-1) | 5.67 |
Growth Attributes
The uprooting of the plants from the pot soil were carried in such a way that there will be no damage to whole intact plant. The uprooted intact plants were washed with the water to remove all the dust and soil particles before being subjected to phenotypic analysis. The root and shoot tissues are completely segregated and the metric scale was used to estimate the root and shoot lengths. Their fresh weight measured immediately after rooted-up and packed some plant samples in a paper bag (which were labelled) and oven dried for 70°C for 72 h to measure plant dry weight.
Photosynthetic Pigments
Uppermost leaves that are fully expanded were harvested at 9 to 10 am and used for the estimation of chlorophyll (a, b), total chlorophyll and carotenoid contents using the method described by Lichtenthaler (1987). 53 The supernatant was used to find out the absorbance at 645, 663, and 480 nm by using UV-VIS (Hitachi U–2910) spectrophotometer. Carotenoids contents were measured by the method of Davies (1976). 54 The chlorophyll contents and total carotenoid contents were calculated by using the following formulas.
Hydrogen Peroxide (H2O2) Contents
For determination of H2O2, 0.15 g fresh leaves were homogenized in 0.1% TCA and then centrifuged for 15 min at 12000 × g, the supernatant mixed with 10 mM potassium phosphate buffer (pH 7.0) and 1 mL potassium iodide (1 M). H2O2 utilized to establish a standard curve. Absorbance at 390 nm were used to estimate the H2O2 55 by using the UV-VIS spectrophotometer (Hitachi U–2910).
Malondialdehyde (MDA) Contents
The content of the MDA was assessed by using the TBA method as described by Hodge et al (1999). 56 For analysis, fresh leaves (0.15 g) of wheat were grinded with 5.0 mL of 5% (w/v) TCA in mortar placed on ice bath, centrifuged, and the MDA content was measured at 532 and 600 nm by using UV-VIS (Hitachi U–2910) spectrophotometrically.
Electrolyte Leakage
Electrolyte leakage (EL) was assayed according to the method of Dionisio-Sese and Tobita (1998). 57 Fresh leaves of wheat seedlings (0.5 g) were cut into uniform sized leaf discs, and put in a test tube with 10 ml deionized water. Leaf discs are incubated in distilled water, and the electrical conductivity (EC1) was measured before and (EC2) after boiling for taking the respective electrical conductivities. The electrolyte leakage was estimated by using the following formula
Total Soluble Protein Contents
Total soluble protein content in fresh leaf materials (0.1 g) of wheat was estimated by using the 2 mL of phosphate buffer saline (pH 7.2) as described by Bradford (1976) 58 and then homogenized material was centrifuged for 10 minutes at 12000 g. The sample was incubated at room temperature for 30 minutes and measured the absorbance at 595 nm using UV-VIS spectrophotometer (Hitachi U-2910, Tokyo, Japan) and bovine serum albumin (BSA) was used as standard.
Anthocyanin Contents
Total anthocyanin content was measured using the procedure of Hodges and Nozzolillo (1996). 59 Fresh leaves (0.15 g) of wheat were grinded in in 2 mL of acidified methanol (methanol+1% HCI) by using the mortar and pestle. The grinded material was moved to the test tube and boiled at 100°C in the water bath for 30 min and then homogenate was centrifuged at 12000×g for 15 min and absorbance was read at 540 and 600 nm on a spectrophotometer.
Antioxidant Enzymes Assay
The fresh leaves of wheat were grinded in phosphate buffer (50 mM; pH 7.0) and 1 mM dithiothreitol. Then centrifuged the material at 15,000 rpm for 15 min and was stored at 4°C until being utilized to assay superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX) activities. 60 Superoxide dismutase (SOD) activity of fresh leaves of wheat was estimated with NBT (Nitro blue tetrazolium) method to assay the activity of SOD. 61 Briefly, 20 mL of enzyme extract was mixed with a reaction mixture containing 50 µM phosphate buffer (pH 7.8), 50 µM NBT, 1.3 µM riboflavin, 75 µM EDTA and methionine. A light source was used to expose the reaction mixture in the test tube and then absorbance was measured at 560 nm using a spectrophotometer (Hitachi U–1800). Accordingly, one Unit SOD activity was equal to the amount of enzyme required to inhibit 50% reduction of NBT as observed at 560 nm. Chance & Maehly method (1995) 62 was followed for the estimation of CAT and POD activities. Accordingly, the CAT solution mixture (3 mL) was composed of enzyme extract (0.1 mL), phosphate buffer (50 mM; pH 7.0), and H2O2 (5.9 mM). The CAT reaction mixture was irradiate and decrease in absorbance read at 240 nm as an outcome of H2O2 consumption after a 20 s interval indicates the activity of the enzyme. Likewise, POD solution mixture (3 mL) contained 50 mM phosphate buffer (pH 5.0), 0.1 mL enzyme extract, 10 mM H2O2 and 20 mM guaiacol. Peroxidase solution mixture was irradiated and absorbance after a 20 s interval was measured at 470 nm using a spectrophotometer (Hitachi U–1800). Per-minute change in absorbance of 0.01 Units was equal to one Unit of each CAT and POD activities. The guaiacol oxidation rate in H2O2 presence read at 470 nm indicates the enzyme activity. The method of Nakano and Asada (1981) 63 was used for the assay of APX activity. The reaction mixture of APX contained 1 mL of 50 mM phosphate buffer (pH 7.5), 0.5 mM ascorbic acid, 0.1 mM H2O2 and 200 µL enzyme extract. The absorbance of the reaction mixture of APX was measured spectrophotometerically (Hitachi U–1800) at 290 nm after every 20 s for 2 min and EU mg-1 protein expresses the APX activity. One unit of APX is the quantity of protein used to break down 1.0 μmol of substrate per min at 25°C.
Mineral Uptake and Cd Content Accumulation in Root and Shoot
Plant root and shoot dry sample (0.1 g) was added to a test tube having HNO3 and H2SO4 (3:1) and placed it on a hot plate for 2 to 3 hours. The temperature of hot plate was progressively increased to 200°C. The mixture was turned black in test tubes. HClO4 (0.5 mL) was added to the test tubes, and again heated until the solution become discoloured. Then removed the test tubes from the hot plate for cooling. Then diluted the solution with distilled water up to 50 mL. Nutrients with the procedure of Wolf (1982) 64 were measured from the solution. Magnesium (Mg2+), zinc (Zn2+), iron (Fe2+) and Cd2+ contents in the root and shoot of wheat were determined by using atomic absorption spectrophotometer (Hitachi, Z-2000, Tokyo, Japan).
Silicon Content in Root and Shoot
The plant samples (shoot and root) were dried in an oven at 70ºC for 2 to 3 h and then powdered by grinding prior to analysis. The dry sample (0.1 g) was digested microwave digestion system containing a mixture of 2 mL of 30% H2O2, 1 mL of 40% hydrofluoric acid and 7 mL HNO3 (70 percent) for 10 minutes. 65 The digested samples were diluted with 4% boric acid up to 50 mL. The Si contents were measured in the digested solution as described below: Digested aliquot (0.5 mL) was shifted to a plastic centrifuge tube, and then added 0.5 mL ammonium molybdate (10%), 3.75 mL of HCl (0.2 N), and amino naphtholsulphonic acid (0.5 mL) and 0.5 mL of tartaric acid (20%) was added and then made the volume with distilled water up to 12.5 ml. Optical density was recorded at 390 nm with a UV visible spectrophotometer after1 h, Standards of Si (0, 0.2, 0.4, 0.8 and 1.2 ppm) were prepared by using Merck Certipur® Si standard solution (1000 mgL-1) and then measured according to method of Ma et al (2002). 66
Statistical Analysis
The experiment was conducted in completely randomized design (CRD) with 3 replicates per treatment. All variables studied in this experiment was analyzed by means of 3-way ANOVA using STATISTIX software (version 8.1). Least significant difference (LSD) test was used for the comparison of the different mean values (control and treatment values), and the significance was estimated at the P ≤ 0.05.
Results
Growth Attributes
Cadmium stress caused a reduction (P ≤ 0.001) in root length, shoot length, root fresh, shoot fresh, and their dry weights (Table 2). Both Cd treatments (10 and 25 mg kg-1) imposed a remarkable reduction in these growth attributes. Root length (19%, 49% and 13%, 46%), shoot length (23%, 45% and 32%, 61%), shoot fresh weight (12%, 39% and 23%, 49%), shoot dry weight (13%, 40% and 25%, 57%), root fresh weight (15%, 38% and 24%, 59%) and root dry weight (25%, 51% and 29%, 59%) was reduced under Cd stress (10 and 25 mg kg-1) in both Sahar-2006 and Inqalab-91 cultivars, respectively. However, less reduction was observed in Sahar-2006 cultivar as compared to Inqalab-91 cultivar. At the highest level of Cd (25 mg/kg), there was a consistent decline of all the growth attributes in Inqalab-91 cultivar. After Si application (1.5 and 3 mM) without Cd stress, the root (6% and 25%) and shoot lengths (6%), shoot fresh (12% and 7%) and dry weights (11%), and root fresh (8% and 4%) and dry weights (13% and 26%) increased in Sahar-2006 and Inqalab-91, respectively; Si addition to Cd showed a remarkable impact on these variables. For example, higher shoot (25%, 45% and 31%, 57%) and root lengths (18%, 39% and 22%, 51%), shoot fresh (14%, 42% and 22%, 51%) and dry weights (13%, 40% and 24%, 58%) was seen in plants with Si application (1.5 mM) in both Sahar-2006 and Inqalab-91 cultivars, respectively under Cd stress (10 and 25 mg kg-1), whereas values of these parameters greatly enhanced at higher level of Si-application (3 mM) in both cultivars under Cd stress. Sahar-2006 cultivar shows better performance in all growth attributes as compared to Inqalab-91, which has been less performed and recorded as sensitive one (Table 3).
Table 2.
Mean Sum of Squares From ANOVA of Data for Growth and Physio-Chemical Attributes of Wheat Cultivars Treated With Si Under Cadmium Stress.
| Source of Variance | Cultivars (CV), df = 1 | Cadmium (Cd), df = 2 | Silicon (Si), df = 2 | CV × Cd, df = 2 | CV × Si, df = 2 | Cd × Si, df = 4 | CV × Cd × Si, df = 4 | Error, df = 36 |
|---|---|---|---|---|---|---|---|---|
| Root length | 62.875*** | 36.622*** | 3.922*** | 0.430*** | 0.281*** | 0.081** | 0.159*** | 0.0299 |
| Shoot length | 49.832*** | 110.262*** | 2.399*** | 0.223* | 0.120ns | 0.032ns | 0.042ns | 0.068 |
| Shoot fresh weight | 18.963*** | 31.062*** | 2.657*** | 0.054ns | 0.146ns | 0.113ns | 0.038ns | 0.0622 |
| Shoot dry weight | 1.356*** | 1.439*** | 0.128*** | 0.005* | 0.009** | 0.004* | 0.0007ns | 0.00165 |
| Root fresh weight | 3.190*** | 4.461*** | 0.481*** | 0.040** | 0.004ns | 0.018ns | 0.003ns | 0.00895 |
| Root dry weight | 0.199*** | 0.355*** | 0.038*** | 0.0001ns | 0.007** | 0.0002ns | 0.00004ns | 0.00140 |
| Chlorophyll a | 0.6350*** | 1.0485*** | 0.0288*** | 0.0091** | 0.00008ns | 0.00068ns | 0.00042ns | 0.00113 |
| Chlorophyll b | 0.9180*** | 0.6608*** | 0.0233*** | 0.01701*** | 0.00057ns | 0.00064ns | 0.00019ns | 0.00113 |
| Total chlorophyll | 3.0816*** | 3.3665*** | 0.1036*** | 0.0472*** | 0.00103ns | 0.00224ns | 0.00030ns | 0.00127 |
| Total carotenoid | 0.0126*** | 0.0089*** | 2.72 × 10-4*** | 1.73 × 10-5ns | 4.31 × 10-6ns | 3.78 × 10-6ns | 3.37 × 10-6ns | 8.56 × 10-6 |
| Hydrogen peroxide | 245.08*** | 95.595*** | 10.050*** | 5.845*** | 1.635*** | 0.398** | 0.111ns | 0.128 |
| Malondialdehyde | 41.724*** | 21.929*** | 0.849*** | 1.273*** | 0.1599*** | 0.042** | 0.0325ns | 0.0237 |
| Electrolyte leakage | 3855.18** | 2681.42*** | 197.67*** | 145.23*** | 9.62* | 1.16ns | 3.12ns | 3.28 |
| TSP | 6.738*** | 3.362*** | 0.357*** | 0.085*** | 0.0103ns | 0.0032ns | 0.00146ns | 0.00490 |
| Anthocyanin | 1.689*** | 2.325*** | 0.268*** | 0.014** | 0.013** | 0.006* | 0.004ns | 0.00277 |
| SOD | 484.69*** | 322.47*** | 119.47*** | 2.135ns | 0.294ns | 3.999* | 0.389ns | 1.513 |
| POD | 5162.5*** | 5271.11*** | 1498.09*** | 25.66ns | 15.16ns | 8.39ns | 10.45ns | 33.25 |
| CAT | 121793*** | 37923*** | 9378*** | 892*** | 37ns | 81ns | 19ns | 79 |
| APX | 43896*** | 25669.7*** | 6910.3*** | 404.8*** | 54.5ns | 41.3ns | 89.8* | 39.1 |
| Root Fe2+ | 42629*** | 31543.5*** | 6487.1*** | 478.9*** | 1875.6*** | 34.6ns | 20.8ns | 56.9 |
| Shoot Fe2+ | 20193*** | 11275.2*** | 2066.2*** | 67.2ns | 46.8ns | 41.6ns | 5.8ns | 33.7 |
| Root Zn2+ | 12138** | 2681*** | 333.1*** | 232.2*** | 9.6ns | 2.5ns | 14ns | 12.6 |
| Shoot Zn2+ | 1262.4*** | 497.31*** | 34.30*** | 14.64*** | 0.20ns | 0.37ns | 0.17ns | 0.88 |
| Root Mg2+ | 116361*** | 25978*** | 5828*** | 770*** | 10ns | 154** | 125** | 39 |
| Shoot Mg2+ | 51746*** | 25126.2*** | 3317.7*** | 109.4** | 131.6** | 49.7ns | 10.0ns | 27.6 |
| Root Cd2+ | 12.033*** | 152.368*** | 11.655*** | 2.317*** | 0.034ns | 2.150*** | 0.034ns | 0.048 |
| Shoot Cd2+ | 2.8921*** | 17.764*** | 1.1299*** | 0.591*** | 0.036*** | 0.1734*** | 0.0218*** | 0.0050 |
| Root Si | 0.6828*** | 0.9447*** | 0.7219*** | 0.0788*** | 0.0155*** | 0.0417*** | 0.0047** | 0.00146 |
| Shoot Si | 0.2790*** | 0.2440*** | 0.0221*** | 0.0085*** | 0.0032*** | 0.0004ns | 0.0005ns | 0.00051 |
Abbreviations: TSP, total soluble protein; SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase; Fe2+, iron ion; Zn2+, zinc ion; Mg2+, magnesium ion; Cd2+, cadmium; Si, silicon.
***, **, * = significant at 0.001, 0.01 and 0.05 levels, respectively.
Table 3.
Effect of Si Spray on Growth Attributes of 2 Wheat Cultivars Under Cd Regimes.
| Cultivars | Cd Stress (mg/kg) | Silicon (Si) (mM) | Root length (cm) | Shoot length (cm) | Root fresh weight (g) | Root dry weight (g) | Shoot fresh weight (g) | Shoot dry weight (g) |
|---|---|---|---|---|---|---|---|---|
| Sahar-2006 | 0 | 0 | 7.2c ± 0.057 | 10.00c ± 0.100 | 2.03cd ± 0.09 | 0.54c ± 0.031 | 5.66cd ± 0.24 | 1.171cd ± 0.004 |
| 1.5 | 7.63b ± 0.066 | 10.60b ± 0.06 | 2.20b ± 0.057 | 0.61b ± 0.032 | 6.33b ± 0.20 | 1.30b ± 0.028 | ||
| 3.0 | 8.23a ± 0.033 | 11.23a ± 0.145 | 2.50a ± 0.115 | 0.68a ± 0.016 | 7.00a ± 0.252 | 1.45a ± 0.029 | ||
| 10 | 0 | 5.84f ± 0.024 | 7.66h ± 0.088 | 1.73fg ± 0.03 | 0.40ef ± 0.04 | 5.00ef ± 0.12 | 1.02g ± 0.044 | |
| 1.5 | 6.27e ± 0.126 | 7.97gh ± 0.120 | 1.93de ± 0.03 | 0.47d ± 0.006 | 5.47cd ± 0.12 | 1.13de ± 0.044 | ||
| 3.0 | 6.75d ± 0.083 | 8.33fg ± 0.328 | 2.12bc ± 0.07 | 0.54c ± 0.022 | 5.85c ± 0.08 | 1.23bc ± 0.033 | ||
| 25 | 0 | 3.69k ± 0.078 | 5.53k ± 0.152 | 1.26j ± 0.025 | 0.26hi ± 0.04 | 3.44k ± 0.25 | 0.70kl ± 0.015 | |
| 1.5 | 4.63h ± 0.328 | 5.84jk ± 0.217 | 1.31ij ± 0.02 | 0.33g ± 0.005 | 3.70jk ± 0.199 | 0.77jk ± 0.026 | ||
| 3.0 | 5.30g ± 0.058 | 6.28i ± 0.217 | 1.47hi ± 0.02 | 0.38fg ± 0.005 | 4.08ij ± 0.06 | 0.84i ± 0.034 | ||
| Inqalab-91 | 0 | 0 | 4.57h ± 0.053 | 8.58ef ± 0.251 | 1.69fg ± 0.06 | 0.46de ± 0.03 | 4.83fg ± 0.04 | 0.94h ± 0.012 |
| 1.5 | 5.34g ± 0.062 | 8.93de ± 0.123 | 1.83ef ± 0.06 | 0.49cd ± 0.004 | 5.33de ± 0.03 | 1.05fg ± 0.005 | ||
| 3.0 | 5.67f ± 0.070 | 9.13d ± 0.017 | 2.06bcd ± 0.05 | 0.51cd ± 0.031 | 5.50cd ± 0.06 | 1.09ef ± 0.005 | ||
| 10 | 0 | 3.98jk ± 0.044 | 5.82jk ± 0.197 | 1.29j ± 0.035 | 0.32gh ± 0.017 | 3.71jk ± 0.056 | 0.71kl ± 0.012 | |
| 1.5 | 4.179ij ± 0.05 | 6.13ij ± 0.007 | 1.37ij ± 0.07 | 0.35fg ± 0.007 | 4.16hi ± 0.05 | 0.80ij ± 0.005 | ||
| 3.0 | 4.41hi ± 0.036 | 6.39i ± 0.034 | 1.59gh ± 0.03 | 0.38fg ± 0.009 | 4.50gh ± 0.05 | 0.85ij ± 0.004 | ||
| 25 | 0 | 2.43m ± 0.048 | 3.37m ± 0.070 | 0.69l ± 0.043 | 0.19j ± 0.005 | 2.46l ± 0.231 | 0.40n ± 0.022 | |
| 1.5 | 2.59m ± 0.084 | 3.80lm ± 0.026 | 0.77kl ± 0.020 | 0.21ij ± 0.010 | 2.59l ± 0.036 | 0.44mn ± 0.015 | ||
| 3.0 | 2.95l ± 0.055 | 3.99l ± 0.026 | 0.89k ± 0.016 | 0.23ij ± 0.009 | 2.77l ± 0.063 | 0.48m ± 0.007 |
Means are followed by standard errors. Three-way ANOVA was performed and different mean values (control and treatment values) were tested by least significant difference (LSD) at P ≤ 0.05 and different letters following means within the same column indicate the significant differences between the variables in the table.
Photosynthetic Pigments
Cadmium toxicity significantly (P ≤ 0.001) reduced the chlorophyll molecules and non-significant (P ≥ 0.05) carotenoids in both wheat cultivars (Table 2). The decline in photosynthetic pigments was significantly in wheat plants exposed to Cd stress (10 and 25 mg kg-1). Cadmium (10 mg kg-1) reduced the chlorophyll (a, b), total chlorophyll and carotenoid contents in both Sahar-2006 (22, 14, 19 and 19%) and Inqalab-91 (32%, 34%, 33% and 23%) cultivars, respectively, but higher reduction was observed at 25 mg kg-1 Cd in both cultivars. The decline in photosynthetic pigment was greater in Inqalab-91 than that in the Sahar-2006 cultivar. Exogenous application of Si (1.5 and 3 mM) in control plants had a significant rise in chlorophyll a (5%, 8%, and 4%, 10%), chlorophyll b (6%, 12%, and 4%, 12%), total chlorophyll contents (5%, 10%, and 4%, 11%) and total carotenoid contents (2%, 7%, and 4%, 11%) in both Sahar-2006 and Inqalab-91 cultivars, respectively. A Si application under different Cd regimes, significantly increased the chlorophyll (a, b), total chlorophyll and total carotenoid content in the range of 16% to 56% in both wheat cultivars. Generally, the Si (3 mM) induced increases were better in Sahar-2006 than Inqalab-91 cultivars, particularly under stress conditions. However, cultivars difference was also evident; Sahar-2006 cultivar accumulated more photosynthetic pigments as compared Inqalab-91 (Table 4).
Table 4.
Effect of Si Spray on Photosynthetic Pigments and Biochemical Attributes of 2 Wheat Cultivars Under Cd Regimes.
| Cultivars | Cd stress (mg/kg) | Silicon (Si) (mM) |
Chl. a
(mg g 1 FW) |
Chl. b
(mg g 1 FW) |
Total Chl. (mg g-1 FW) |
Total Car. (mg g-1 FW) |
TSP (mg g-1 FW) | Anthocyanin (Unit g-1 FW) |
|---|---|---|---|---|---|---|---|---|
| Sahar-2006 | 0 | 0 | 1.128b ± 0.040 | 0.916b ± 0.027 | 2.04c ± 0.039 | 0.120b ± 0.003 | 1.93b ± 0.042 | 1.50c ± 0.026 |
| 1.5 | 1.185a ± 0.007 | 0.969b ± 0.019 | 2.15b ± 0.017 | 0.123b ± 0.001 | 2.18a ± 0.006 | 1.71b ± 0.029 | ||
| 3.0 | 1.219a ± 0.005 | 1.026a ± 0.005 | 2.24a ± 0.004 | 0.129a ± 0.001 | 2.29a ± 0.039 | 1.88a ± 0.069 | ||
| 10 | 0 | 0.879f ± 0.019 | 0.784def ± 0.005 | 1.66h ± 0.017 | 0.097c ± 0.002 | 1.62d ± 0.043 | 1.15f ± 0.019 | |
| 1.5 | 0.918ef ± 0.012 | 0.813cde ± 0.003 | 1.73fg ± 0.011 | 0.100bc ± 0.001 | 1.78c ± 0.033 | 1.28de ± 0.023 | ||
| 3.0 | 0.972de ± 0.005 | 0.846c ± 0.005 | 1.82e ± 0.005 | 0.106b ± 0.002 | 1.96b ± 0.045 | 1.46c ± 0.071 | ||
| 25 | 0 | 0.687hi ± 0.039 | 0.608h ± 0.020 | 1.29jk ± 0.019 | 0.074fgh ± 0.001 | 1.02j ± 0.062 | 0.85k ± 0.008 | |
| 1.5 | 0.717gh ± 0.006 | 0.632gh ± 0.006 | 1.35j ± 0.099 | 0.077fg ± 0.001 | 1.16hi ± 0.048 | 0.94ij ± 0.014 | ||
| 3.0 | 0.756g ± 0.010 | 0.684g ± 0.027 | 1.44i ± 0.031 | 0.082ef ± 0.001 | 1.30fg ± 0.030 | 1.06gh ± 0.02 | ||
| Inqalab-91 | 0 | 0 | 0.947e ± 0.013 | 0.746f ± 0.042 | 1.69gh ± 0.031 | 0.087de ± 0.004 | 1.21gh ± 0.027 | 1.20ef ± 0.006 |
| 1.5 | 1.011cd ± 0.003 | 0.769ef ± 0.023 | 1.78ef ± 0.020 | 0.091cd ± 0.002 | 1.37ef ± 0.023 | 1.30d ± 0.023 | ||
| 3.0 | 1.055c ± 0.010 | 0.826cd ± 0.013 | 1.88d ± 0.006 | 0.097c ± 0.002 | 1.48e ± 0.076 | 1.36d ± 0.033 | ||
| 10 | 0 | 0.640i ± 0.022 | 0.490j ± 0.033 | 1.13l ± 0.011 | 0.067h ± 0.002 | 0.85k ± 0.063 | 0.86jk ± 0.013 | |
| 1.5 | 0.650i ± 0.022 | 0.514ij ± 0.022 | 1.16l ± 0.018 | 0.069gh ± 0.001 | 1.02j ± 0.005 | 0.99hi ± 0.019 | ||
| 3.0 | 0.688hi ± 0.007 | 0.550i ± 0.014 | 1.24k ± 0.010 | 0.073gh ± 0.001 | 1.09ij ± 0.007 | 1.14fg ± 0.027 | ||
| 25 | 0 | 0.469k ± 0.029 | 0.325j ± 0.004 | 0.79n ± 0.025 | 0.047i ± 0.0005 | 0.51m ± 0.041 | 0.53m ± 0.014 | |
| 1.5 | 0.510jk ± 0.016 | 0.342k ± 0.010 | 0.85mn ± 0.027 | 0.049i ± 0.001 | 0.63l ± 0.019 | 0.60lm ± 0.008 | ||
| 3.0 | 0.540j ± 0.021 | 0.366k ± 0.026 | 0.91m ± 0.023 | 0.052i ± 0.001 | 0.69l ± 0.029 | 0.67l ± 0.018 |
Abbreviations: Chl., chlorophyll; Car., carotenoids; TSP, total soluble protein.
Means are followed by standard errors. Three-way ANOVA was performed and different mean values (control and treatment values) were tested by least significant difference (LSD) at P ≤ 0.05 and different letters following means within the same column indicate the significant differences between the variables in the table.
Total Soluble Protein (TSP) and Anthocyanin Contents
Compared with the control group, Cd treatments (10 and 25 mg kg-1) significantly (P ≤ 0.001) decreased the TSP and anthocyanin contents in both Sahar-2006 (16%-47% and 23%-43%) and Inqalab-91 (29%-58% and 28%-55%) cultivars. Besides, the enhancement in TSP (14%-47% and 25%-54%) and anthocyanin contents (23%-45% and 17%-54%) were triggered in leaves of Sahar-2006 and Inqalab-91 cultivars, respectively, with Si (1.5 and 3 mM) plus Cd stress (10 and 25 mg kg-1) as compared to the stress treatments alone. Similarly, higher significant increase in TSP and anthocyanin was identified only in wheat plants with Si (3 mM) in both Sahar-2006 (18% and 26%) and Inqalab-91 (27% and 24%) cultivars (Table 4).
Oxidative Stress Attributes
Hydrogen peroxide, MDA and EL are key markers in assessing the degree of oxidative damage in the plants. We have documented, highly significant (P ≤ 0.001) increase in cellular levels of H2O2, MDA and EL in plants exposed to Cd toxicity (Table 2). MDA contents were increased by 31% and 74% in Sahar-2006 and 46% and 77% in Inqalab-91cultivar under Cd stress (10 and 25 mg kg-1), respectively, as compared to their corresponding controls. After Si application (1.5 and 3 mM) without Cd stress, MDA contents (3% and 6%) in Sahar-2006 and Inqalab-91 (1% and 6%), respectively. In different Cd regimes, Si (1.5 and 3 mM) considerably reduced the MDA contents in both cultivars. Sahar-2006 (22%-77%) cultivar was more responsive to foliar applied Si (3 mM) than Inqalab-91 (43%-73%) in terms of MDA in response to Cd stress (Figure 1). Si application (1.5 and 3 mM) in controlled environment, reduced the H2O2 contents in Sahar-2006 (6% and 9%) cultivar as well as Inqalab-91 (2% and 3%), respectively. Cadmium stress caused an increased in H2O2 and EL in both cultivars. Thus, Inqalab-91 cultivar (41% and 53%) showed significantly more H2O2 contents than Sahar-2006 (31% and 48%) under Cd stress (10 and 25 mg kg-1), respectively. Similarly, EL significantly increased (27%-80%) under both levels of Cd-stress in both cultivars, but more permeability was observed in both cultivars under Cd stress (25 mg kg-1). Silicon significantly decreased the H2O2 and EL in plants of both Sahar-2006 and Inqalab-91cultivars. The response of both cultivars to interactive effect of Si and Cd stress was almost same for H2O2 and EL and the effect of Si (1.5 and 3 mM) in Sahar-2006 (20%-59% and 28%-94%) cultivar was greater than that of the Inqalab-91 (37%-56% and 50%-93%) cultivar. Of the 2 wheat cultivars, elevated levels of H2O2 and EL were found in Inqalab-91 being a sensitive cultivar under Cd (25 mg kg-1) stress (Figure 1).
Figure 1.
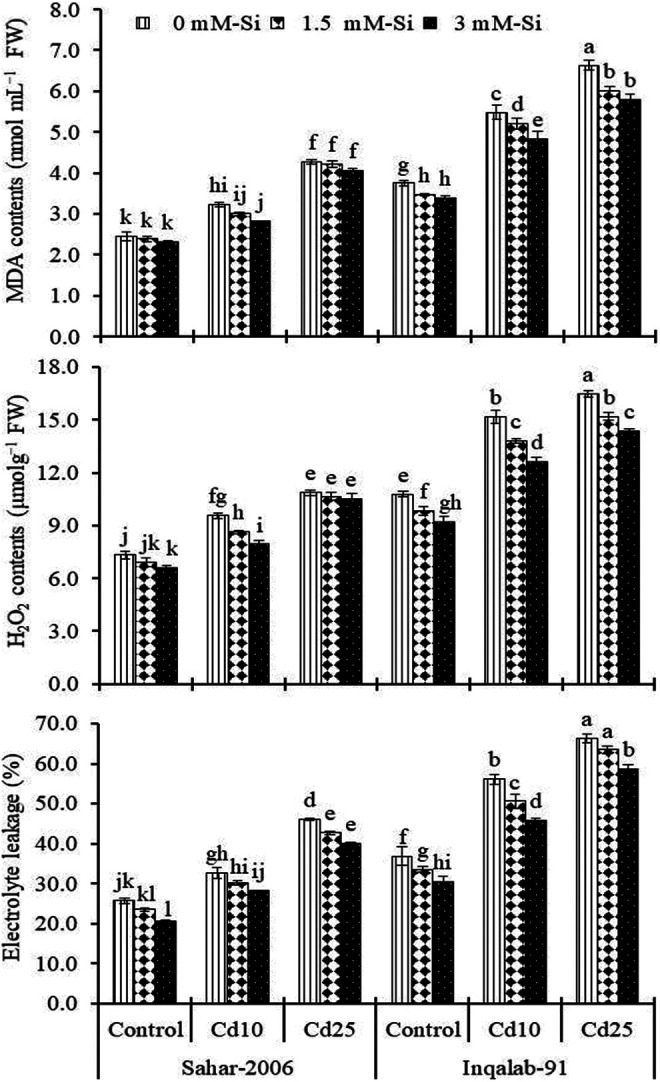
Effect of Si spray on MDA contents, H2O2 contents and EL in the shoot of 2 wheat cultivars seedlings grown under different levels of Cd stress (n = 3 ± SE). Three-way ANOVA was performed and mean values (control and treatment values) were tested by least significant difference (LSD) at P ≤ 0.05 and different lowercase letters indicate significant difference between the treatments.
Antioxidant Enzyme Activity
A marked increase in the activities of SOD, POD, CAT and APX was measured in 2 wheat cultivars subjected to Cd stress (Table 2). Cadmium toxicity (10 and 25 mg kg-1) increased the SOD, POD, CAT and APX contents in both Sahar-2006 (19%-60%, 20%-59%, 31%-56%, and 14%-40%) and Inqalab-91 (28%-81%, 30%-86%, 33%-72%, and 46%-84%) cultivars, respectively as compared to their corresponding controls. However, Si-application at both levels (1.5 and 3 mM) further increased the antioxidant activities to a significant level in both cultivars under control and stressed wheat seedlings. Silicon enhanced the SOD content under Cd stress (17%-48% and 30%-51%) and non-stress conditions (13%-35% and 4%-14%) in both Sahar-2006 and Inqalab-91 cultivars, respectively. Similarly, Si (1.5-3 mM) significantly increased in POD (11%-44% and 18%-74%), CAT (21%-44% and 16%-51%), and APX (16%-38% and 29%-81%) contents in both cultivars under Cd stress, while values of these attributes greatly enhanced at higher level of Si-application (3 mM) in both cultivars under Cd stress. The cultivar difference was also pertinent, Inqalab-91 (which was sensitive against Cd stress), while in Sahar-2006 cultivar, higher antioxidant activity was recorded and declared as tolerant against cadmium (25 mg kg-1) stress (Figure 2).
Figure 2.
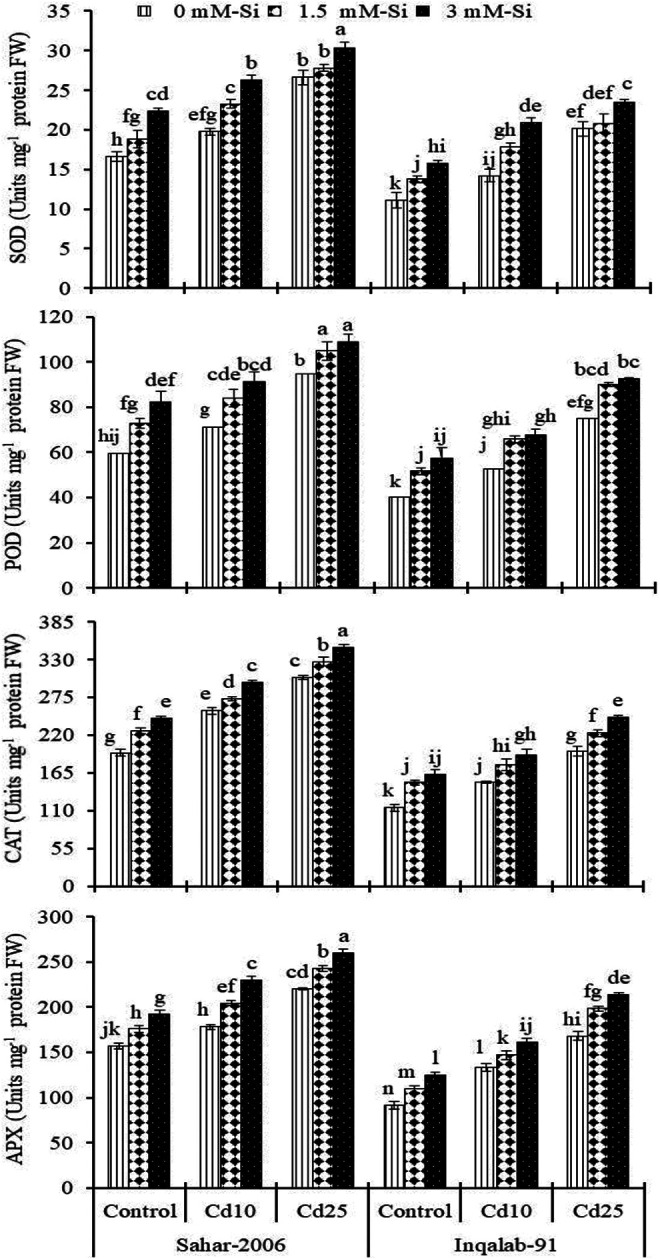
Effect of Si spray on SOD, POD, CAT and APX contents in the shoot of 2 wheat cultivars seedlings grown under different levels of Cd stress (n = 3 ± SE). Three-way ANOVA was performed and mean values (control and treatment values) were tested by least significant difference (LSD) at P ≤ 0.05 and different lowercase letters indicate the significant difference between the treatments.
Mineral Nutrient Contents
Cadmium stress resulted in a significant (P ≤ 0.001) decrease in iron (Fe2+) in root and shoot of Sahar-2006 (25%-35% and 18%-28%) and Inqalab-91 (34%-49% and 23%-44%) cultivars, respectively. However, exogenous Si (1.5 and 3 mM) improved the Fe2+ contents in the root (17%-31% and 32%-50%) and shoot (13%-29% and 17%-42%) of both Sahar-2006 and Inqalab-91 cultivars under Cd stress compared with the control plants. However, there was a conspicuous difference between 2cultivars for root and shoot Fe contents, where the Sahar-2006 cultivar had greater values for Fe contents. (Table 5). Similarly, Zn contents considerably reduced (P ≤ 0.001) in the root and shoot of Sahar-2006 (16%-20% and 22%-31%) and Inqalab-91 (18%-48% and 24%-55%) cultivars under Cd stress, while Mg contents are also reduced in different plant parts such as root and shoot of both Sahar-2006 (13%-22% and 17%-31%) and Inqalab-91 (14%-39% and 25%-51%) cultivars, when subjected to Cd toxicity, respectively. Silicon with Cd addition, enhanced the Zn contents in root and shoot of Sahar-2006 (15%-24% and 19%-30%) cultivar, respectively. Whereas Si (1.5-3 mM) mitigated the effect of Cd stress and as a result, Mg content was greater in both root and shoot tissues of Sahar-2006 (9%-22% and 15%-34%) and Inqalab-91 (21%-43% and 23%-49%) cultivars, respectively. Over all, greater increases in mineral nutrients (Fe2+ Zn2+ and Mg2+) was identified only in both root and shoot tissues with Si (3 mM) in both wheat cultivars (Table 5).
Table 5.
Effect of Si Spray on Mineral Nutrient in 2 Wheat Cultivars Under Cd Stress.
| Cultivars | Cd stress (mg/kg) | Silicon (Si) (mM) | Root Fe2+ (µg g-1 DW) | Shoot Fe2+ (µg g-1 DW) | Root Zn2+ (µg g-1 DW) | Shoot Zn2+ (µg g-1 DW) | Root Mg2+ (µg g-1 DW) | Shoot Mg2+ (µg g-1 DW) |
|---|---|---|---|---|---|---|---|---|
| Sahar-2006 | 0 | 0 | 201.49d ± 1.70 | 154.20b ±2.72 | 87.76b ±1.81 | 31.06b ± 0.61 | 278.51cd ±4.19 | 199.62b ±1.91 |
| 1.5 | 234.82b ± 5.71 | 164.20a ±3.29 | 93.37b ±5.04 | 32.01b ± 0.93 | 295.90b ±4.37 | 226.79a ±3.75 | ||
| 3.0 | 259.11a ± 1.92 | 172.46a ± 4.76 | 99.26a ±2.54 | 33.78a ± 0.62 | 315.05a ±4.08 | 234.54a ±2.86 | ||
| 10 | 0 | 151.50f ± 7.88 | 127.13e ±3.71 | 73.64de ±1.37 | 24.15de ± 0.67 | 242.42e ±4.13 | 164.84de ±2.81 | |
| 1.5 | 183.85e ± 4.87 | 143.40cd ± 3.51 | 79.17cd ±2.37 | 25.27d ± 0.40 | 268.73d ±3.01 | 182.74c ±2.05 | ||
| 3.0 | 214.97c ± 3.84 | 150.26bc ± 1.91 | 79.96c ±1.51 | 27.41c ± 0.64 | 283.28c ±3.06 | 199.30b ±1.67 | ||
| 25 | 0 | 131.70g ± 7.13 | 111.20gh ± 4.48 | 70.30ef ±1.53 | 21.54g ± 0.55 | 217.13f ±6.15 | 136.79g ±5.88 | |
| 1.5 | 162.36f ± 6.12 | 116.40fg ±4.89 | 71.96ef ±2.21 | 22.39fg ± 0.48 | 238.39e ±3.58 | 150.19f ±2.25 | ||
| 3.0 | 185.75e ± 3.79 | 124.20ef ±3.25 | 75.42cde ± 0.93 | 23.60ef ± 0.42 | 245.00e ±1.76 | 164.35e ±1.11 | ||
| Inqalab-91 | 0 | 0 | 175.63e ± 4.42 | 114.00g ±2.14 | 60.19h ± 2.75 | 21.31g ± 0.77 | 185.99g ±2.62 | 148.79f ±2.10 |
| 1.5 | 186.01e ± 3.60 | 129.93e ±4.75 | 62.79gh ±1.04 | 22.33fg ± 0.38 | 219.70f ±3.12 | 165.76de ±2.50 | ||
| 3.0 | 199.2d ± 4.28 | 140.00d ±3.47 | 67.11fg ±1.71 | 23.54ef ± 0.61 | 241.48e ±1.79 | 173.19d ±1.43 | ||
| 10 | 0 | 115.29h ± 3.09 | 88.24i ±2.51 | 49.60i ±1.71 | 16.24i ± 0.45 | 160.02i ±3.87 | 112.01h ±1.71 | |
| 1.5 | 126.23gh ± 4.66 | 103.72h ± 2.99 | 53.49i ±1.92 | 17.76i ± 0.23 | 171.72h ±1.62 | 120.21h ±1.14 | ||
| 3.0 | 133.77g ± 2.72 | 116.79fg ±2.50 | 61.45gh ± 0.70 | 19.55h ± 0.40 | 189.61g ±5.90 | 132.73g ± 4.13 | ||
| 25 | 0 | 89.71i ± 1.40 | 64.37k ±2.02 | 31.11k ±1.11 | 9.65k ± 0.19 | 113.60k ± 4.11 | 72.52j ± 5.75 | |
| 1.5 | 93.83i ± 2.30 | 74.81j ±3.27 | 34.36k ± 0.93 | 11.26j ± 0.36 | 128.55j ±1.50 | 84.19i ±1.97 | ||
| 3.0 | 100.48i ± 1.67 | 83.54ij ±1.43 | 40.88j ± 1.34 | 12.56j ± 0.49 | 138.16j ± 0.79 | 92.56i ± 2.99 |
Abbreviations: Fe2+= iron ion; Zn2+= Zinc; Mg2+= magnesium ion.
Means are followed by standard errors. Three-way ANOVA was performed and different mean values (control and treatment values) were tested by least significant difference (LSD) at P ≤ 0.05 and different letters following means within the same column indicate the significant differences between the variables in the table.
Cadmium and Si Accumulation in Root and Shoot Tissues
The cadmium concentrations were significantly (P ≤ 0.001) increased in root and shoot tissue of both Sahar-2006 (950%-1456% and 816%-1076%) and Inqalab-91 (980%-1125% and 696%-913%) cultivars under stress condition compared to controls. Exogenous applied Si enhanced the impairment and lessen the Cd uptake and accumulation of Cd in the root and shoot of Sahar-2006 (1076%-2313% and 1062%-1487%) and Inqalab-91 (885%-1390% and 830%-1274%) cultivars, respectively (Figure 3). Under Cd stress, Si concentration was significantly decreased in root and shoot tissues of Sahar-2006 (21%-52% and 17%-52%) and Inqalab-91 (10%-27% and 25%-56%) cultivars, respectively. In normal seedlings, Si (3 mM) resulted in 69% to 76% and 28% to 33% higher root and shoot Si content in both Sahar-2006 and Inqalab-91 cultivars with respect to Si (1.5 mM), respectively. More Si accumulated in the plant roots than leaves. In addition, compared to Si (1.5-3 mM) treated seedlings exhibited more Si content in root (17%-50% and 16%-50%) and shoot (22%-50% and 22%-49%) in both cultivars, respectively under Cd stress. Moreover, Sahar-2006 was more responsive to Si application (3 mM) than Inqalab-91cultivar (Figure 4).
Figure 3.
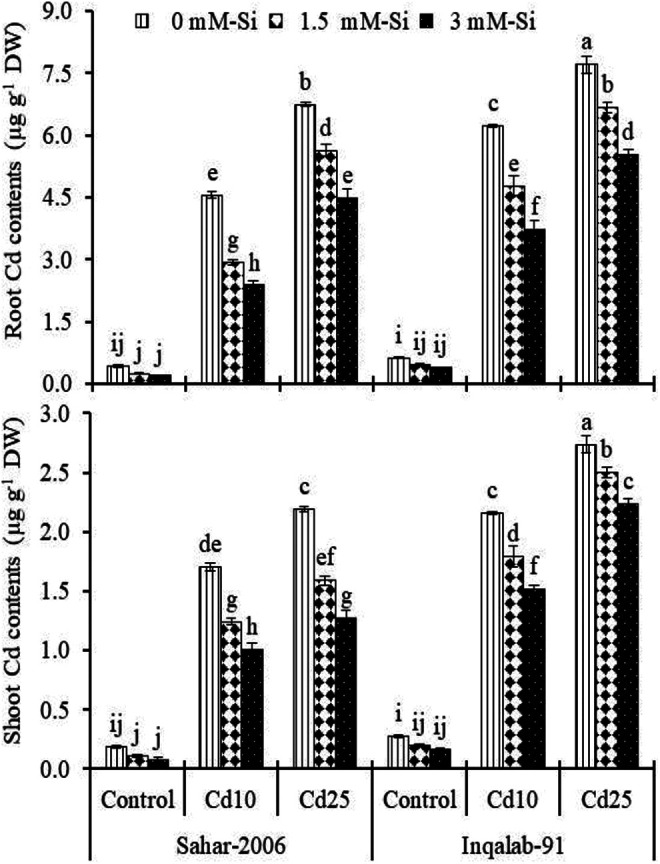
Effect of Si spray on Si contents in the root and shoot of 2 wheat cultivars seedlings grown under different levels of Cd stress (n = 3 ± SE). Three-way ANOVA was performed and mean values (control and treatment values) were tested by least significant difference (LSD) at P ≤ 0.05 and different lowercase letters indicate the significant difference between the treatments.
Figure 4.

Effect of Si spray on Cd contents in the root and shoot of 2 wheat cultivars seedlings grown under different levels of Cd stress (n = 3 ± SE). Three-way ANOVA was performed and mean values (control and treatment values) were tested by least significant difference (LSD) at P ≤ 0.05 and different lowercase letters indicate the significant difference between the treatments.
Discussion
Metal contamination in agricultural soils has become a main environmental issue nowadays due to its toxic effects on soil-plant system, food chain and human beings. 67 Soil contamination is generally caused by more release of metals from anthropogenic sources such as mining, smelting, irregular agricultural inputs (pesticides and fertilizers) and inadequate battery recycling. 68 The most abundant metals like the Cd normally co-exist in the environment, and disturb the metabolism of living organisms at the cellular level and affected the plant growth and yield. 69 Furthermore, Cd interferes with several metabolic processes, resulting inhibited plant growth, stunted development and plant death in mustard. 20 This inhibition by Cd stress might be related to the damaged photosynthesis system. 70,71 The present study examines the role of foliar applied Si in mitigation of Cd stress in 2 wheat cultivars (sensitive and tolerant), and investigates the primary physio-chemical mechanism. In this study, Cd stress harshly inhibited the fresh and dry masses of both cultivars, and this decline was more conspicuous in Inqalab-91 (sensitive) cultivar might also be the result of oxidative stress (Table 3). Although, a number of reports are available in the literature, where Cd-induced reduction in wheat growth attributes, and photosynthesis, might be due to reduce cell division and cell expansion owing to oxidative damage and decline in activities of important antioxidant enzyme activities. 70,72 Cadmium induced decline in chlorophyll and carotenoid contents has been reported in many plants, e.g. strawberry, 73 faba bean, 74 cotton, 44 and Artemisia annua. 75 Furthermore, Cd toxicity decreased plant growth and affected photosynthetic efficiency in various crops and also affects the nutrient uptake and disrupt the membrane structure and properties. 71,76 The similar trend was found in wheat plant grown under Cd stress in our study.
Silicon application improves the plant growth and biomass might be due to photosynthesis improvement, 34 enhanced nutrient uptake 77 and immobilized the Cd. 45 Furthermore, Si is known to enhance the internodal elongation of shoot by increasing the cell division and cell expansion, 78,79 which could be a reason for better growth performance under stress condition. However, exogenous application of Si was effective in improving the harmful effects of Cd stress on wheat growth and photosynthetic pigments, as shown by Si-induced increase in shoot and root growth (Table 3), chlorophyll pigments (Table 4). Moreover, Si application enhances the chlorophyll pigments might have also contributed in enhancing the growth performance of wheat under stress and non-stress conditions. Previous studies considered the Si, as a growth regulator-like compound that enhanced the cell division and extension in different crops, including maize, 72 mustard, 80 alfalfa, 78 barley 79 and rice 81 has a defensive role against many biotic and abiotic stresses. 82 Silicon improved the photosynthetic pigments and protect them from oxidative injury by increasing the synthesis and accumulation of chlorophyll and carotenoid contents. 83,84 In the present study, Si application lessens the oxidative injury in wheat caused by Cd, which might have provoked the chlorophyll and carotenoid contents accumulation in wheat seedling (Table 4).
The equilibrium between the generation of ROS and its removal from the plant cell or tissue disturb and causes oxidative damage to membrane-bound organelles when a plant subjected to abiotic stress. 24,85 Cadmium toxicity inhibits the activities of antioxidant enzymes in plants due to more accumulation of MDA and H2O2 in plants, which reflected the damaging of cell membrane and thus, increases the EL in plants. 86 In present study, greater production of ROS (e.g., H2O2) considerably increased the MDA content and EL in wheat cultivars under Cd (25 mg kg-1) stress (Figure 1). Earlier, Cd toxicity induced generation of H2O2 and MDA contents has been described in different plant species. For instance, rice, 87 rapeseed, 24 tomato, 88 and maize. 89 While the plant has a strong defense mechanism which scavenged ROS production by increasing the activities of various antioxidants and plants with a strong defense system are known as more resistant plant species against oxidative stress. 77,79,90
The results of this study have shown that Si application reduced ROS generation and accumulation that afterward limited the lipid peroxidation with the help of increased activities of antioxidant enzymes (Figure 1). Furthermore, Si has a defensive role to inhibit the generation of ROS, maintain the membrane stability, and alleviate the lipid peroxidation indirectly under stress conditions. 91 We also recorded a Cd-induced reduction in the accumulation of non-enzymatic antioxidants (low molecular weight) such as phenolics, flavonoids, and anthocyanins. The Sahar-2006 cultivar was superior to Inqalab-91cultivar for non-enzymatic antioxidant compounds. Non-enzymatic antioxidants also protect the plants against pests and diseases, play a role in plant development and regarded as a powerful antioxidant compounds 92 and protect the plants from ROS-induced oxidative stress. 93 Our results have shown that rise in the endogenous levels of anthocyanin in plants with Si application because anthocyanin possess potent antioxidant activity that enables them to play pivotal role in plant abiotic stress tolerance (Table 4). Plants tend to accumulate more anthocyanin contents under abiotic stress. 94 Metal stress induced the production of anthocyanin contents, which has been reported in many plants, e.g. barley, 79 and Echium amoenum. 95 Anthocyanin contents have strong potential to scavenge the free radicals and prevent the plants from oxidative damage. 96
In the present study, Cd toxicity significantly reduced the soluble protein contents in wheat (Table 4); similar results have been reported for Tall fescue seedlings. 86 Osmotic adjustment is also an important way to resist adverse effect of stress on plants. The osmotic adjustment substances such as proline and soluble protein plays vital roles in maintaining the osmotic equilibrium and integrity of membranes. 97 The reduction in soluble protein contents might be due to inhibition of the uptake of nitrate ions in root by metals, and may partly reflect the changes in various antioxidant enzymes to cope with metal induced oxidative stress. 98 Application of Si (3 mM) more increased the soluble protein content in Cd treated wheat plants. This increase in soluble protein may possibly be due to increase of nitrate reductase biosynthesis with the inhibition of their degradation, 80 thus enhanced the accumulation of soluble protein in leaves of wheat plants.
Cadmium can promote the production of reactive oxygen species (ROS) thus causing oxidative stress in plants. 29 Increased activities of antioxidant enzymes also contribute significantly to the antioxidant potential of plants. 99 Cadmium is generally known to inhibit the activity of various enzymatic antioxidants such as SOD, POD, APX and CAT. 100,101 For example, Cd stress decreased the SOD and APX activities in cotton 102 and reduced CAT activity in wheat. 45 Silicon (3 mM) application further increased the activities of SOD, POD, APX and CAT under Cd stress (Figure 2), the increase antioxidant in wheat activated the defense mechanism in plants, thus enhanced the Cd resistance in wheat seedling. These antioxidants coordinately played a key role in alleviating the oxidative damage in Si treatments seedlings. Similar results were reported in rapeseed, 24 cucumber, 103 Brassica chinensis, 104 and soybean 105 under environmental stresses.
The shortage of essential nutrients in plants is directly linked to the growth and yield of plants. Abiotic stresses hinder the uptake of nutrients by down regulating the expression of membrane transporters. 106 Cadmium, being a bivalent cation, usually competes with Mg, Ca, Zn, and Fe during membrane transport. 107,108 Cation transporters can uptake toxic elements (Cd) potentially. 109 Our results suggested that Cd stress considerably decreased the Mg2+, Zn2+, and Fe 2+ accumulation in root and shoot of both cultivars (Figure 3). However, Si treatment reduced the Cd uptake and considerably improved the macro- and micronutrient concentration in leaf, stem and root tissues. Increased uptake of mineral elements like Mg, Zn, Fe and Si in Si treated plants may have directly affected the metabolism by regulating the mechanisms such as chlorophyll synthesis, protein synthesis, and enzyme activity. 42 In the present study, Si alleviated the Cd effect on the mechanisms related to uptake of nutrients, thereby contributing to the maintenance of plant growth and development under Cd stress (Figure 3).
The toxicity of Cd relies on Cd species, which influences its uptake, accumulation, and translocation of Cd. In the current study, significant increase Cd contents in the root and leaf by the addition of Cd, which was then taken up by the wheat plants. In addition, more Cd contents were accumulated in root of both cultivars than those in the shoot (Figure 4). Thind et al (2020) 84 described that Si inhibited the uptake of Cd and decreased its transport in different plant tissues by chelating mechanism. Si being a non-essential nutrient for crop plants can affect plant growth and development in stressed 110 or normal conditions. 38 In this study, application of Si (3 mM) decreased the Cd content in root and shoot of both cultivars under Cd stress (Figure 4). Moreover, Si prevent the Cd transport in plants via symplastic pathway. Silicon inhibited the Cd accumulation and increased the yield because of more photosynthetic pigment synthesis. 49 These findings showed that our easy and practical approach is appropriate for agricultural practices to combat metal stress environment.
Conclusions
Cadmium stress a severely hampered the growth attributes, photosynthetic pigments, and nutrient uptake in wheat cultivars, while enhancing the levels of oxidative stress indicators (MDA and H2O2) contents. However, Si application significantly increased the plant growth under Cd stress. Cadmium regime (25 mg kg-1) revealed a substantial increase in the activities of antioxidant enzymes. Higher activities of these antioxidants associated with the lesser oxidative damage in terms of minimal cellular H2O2 and MDA contents. The response of both wheat cultivars was significantly variable with respect to Cd tolerance. Inqalab-91 cultivar was found to be sensitive having higher MDA and H2O2 contents and poor antioxidative defense system, and more degradation of chlorophyll under Cd stress. The application of Si increased growth-related-attributes, improved photosynthetic machinery, enhanced essential nutrient uptake and increased the activities of various antioxidant compounds by decreasing the MDA and H2O2 contents and Cd uptake in various tissues of plants. In addition, the use of Si might provide interesting information to future researchers about chelating mechanism, which involved in Si induced metal tolerance of plants under stress conditions. Overall, foliar applied Si relieves Cd stress by regulating growth, physiological, and biochemical, and antioxidant attributes in wheat.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The findings of this study are a part PhD dissertation of MS. Sumaira Thind and financially supported by GCUF-RSP (4325-4359) and Project Code: 48-BOT-7.
ORCID iD: Iqbal Hussain  https://orcid.org/0000-0002-8449-0494
https://orcid.org/0000-0002-8449-0494
References
- 1. Riaz M, Kamran M, Fang Y, et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: a critical review. J Hazard Mater. 2021;402(155):123919. [DOI] [PubMed] [Google Scholar]
- 2. Zeng L, Ning D, Xu L, Mao X, Chen X. Sedimentary evidence of environmental degradation in Sanliqi Lake, Daye City (a typical mining city, central China). Bull Environ Contam Toxicol. 2015;95(3):317–324. [DOI] [PubMed] [Google Scholar]
- 3. Proshad R, Kormoker T, Mursheed N, et al. Heavy metal toxicity in agricultural soil due to rapid industrialization in Bangladesh: a review. Int J Adv Geosci. 2018;6(1):83–88. [Google Scholar]
- 4. Qishlaqi A, Moore F, Forghani G. Impact of untreated wastewater irrigation on soils and crops in Shiraz suburban area, SW Iran. Environ Monit Assess. 2008;141(1-3):257–273. [DOI] [PubMed] [Google Scholar]
- 5. Zhang S, Chen M, Li T, Xu X, Deng L. A newly found cadmium accumulator-Malva sinensis Cavan. J Hazard Mater. 2010;173(1-3):705–709. [DOI] [PubMed] [Google Scholar]
- 6. Munir M, Khan ZI, Ahmad K, et al. Transfer of heavy metals from different sources of fertilizers in wheat variety (Galaxy-13). Asian J Biol Sci. 2019;12(4):832–841. [Google Scholar]
- 7. Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA. Plant growth promotion by phosphate solubilizing fungi–current perspective. Arch Agron Soil Sci. 2010;56(1):73–98. [Google Scholar]
- 8. Jones LHP, Jarvis SC. The Fate of Heavy Metals. The Chemistry of Soil Process; 1981:593–620. [Google Scholar]
- 9. Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut. 2008;152(3):686–692. [DOI] [PubMed] [Google Scholar]
- 10. Kumar V, Chopra AK. Toxicity of chromium in agricultural crops with respect to its chemical speciation-a review. World Appl Sci J. 2015;33(6):944–969. [Google Scholar]
- 11. Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C. Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics. 2019;11(2):255–277. [DOI] [PubMed] [Google Scholar]
- 12. Xuebin Q, Yatao X, Ahmad MI, Shehzad M, Zain M. Silicon and its application methods improve physiological traits and antioxidants in Triticum aestivum (L.) under cadmium stress. J Soil Sci Plant Nutr. 2020;20(3):1110–1121. [Google Scholar]
- 13. Sarwar N, Imran M, Shaheen MR, et al. Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere. 2017;171:710–721. [DOI] [PubMed] [Google Scholar]
- 14. Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Inter. 2019;125:365–385. [DOI] [PubMed] [Google Scholar]
- 15. Mahmood T, Gupta KJ, Kaiser WM. Cadmium stress stimulates nitric oxide production by wheat roots. Pak J Bot. 2009;41(3):6. [Google Scholar]
- 16. Khan MJ, Jan MT, Khan K. Effect of organic and inorganic amendments on the heavy metal content of soil and wheat crop irrigated with wastewater. Sarhad J Agric. 2013;29(1):49–57. [Google Scholar]
- 17. Pizzeghello D, Francioso O, Ertani A, Muscolo A, Nardi S. Isopentenyladenosine and cytokinin-like activity of different humic substances. J Geochem Explor. 2013;129:70–75. [Google Scholar]
- 18. Gadd GM. Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bio weathering and bioremediation. Mycol Res. 2007;111(1):3–49. [DOI] [PubMed] [Google Scholar]
- 19. Hussain S, Khaliq A, Noor MA, et al. Metal toxicity and nitrogen metabolism in plants: an overview. In: Datta R, Meena R, Pathan S, Ceccherini M, eds. Carbon and Nitrogen Cycling in Soil. Springer; 2020;221–248. [Google Scholar]
- 20. Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LS. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss) by calcium application involves various physiological and biochemical strategies. PLoS One. 2015;10(1):e0114571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anjum NA, Sofo A, Scopa A, Roy Choudhury A, Gill SS, Ahmad I. Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res. 2015;22(6):4099–4121. [DOI] [PubMed] [Google Scholar]
- 22. Gratao PL, Monteiro CC, Tezotto T, et al. Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals. 2015;28(5):803–816. [DOI] [PubMed] [Google Scholar]
- 23. Adrees M, Ali S, Rizwan M, et al. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf. 2015;119:186–197. [DOI] [PubMed] [Google Scholar]
- 24. Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BHN, Fujita M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci. 2017;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zarattini M, Forlani G. Toward unveiling the mechanisms for transcriptional regulation of proline biosynthesis in the plant cell response to biotic and abiotic stress conditions. Front Plant Sci. 2017;8:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujishima K, Wang KM, Palmer JA, et al. Reconstruction of cysteine biosynthesis using engineered cysteine-free enzymes. Sci Rep. 2018;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akram NA, Shafiq F, Ashraf M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci. 2017;8:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malecka A, Piechalak A, Tomaszewska B. Reactive oxygen species production and antioxidative defense system in pea root tissues treated with lead ions: the whole roots level. Acta Physiol Plant. 2009;31(5):1053–1063. [Google Scholar]
- 29. Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. [DOI] [PubMed] [Google Scholar]
- 30. Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biolog Sci. 2015;11(8):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meharg C, Meharg AA. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ Exp Bot.2015;120:8–17. [Google Scholar]
- 32. Luyckx M, Hausman JF, Lutts S, Guerriero G. Silicon and plants: current knowledge and technological perspectives. Front Plant Sci. 2017;8:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Currie HA, Perry CC. Silica in plants: biological, biochemical and chemical studies. Ann Bot. 2007;100(7):1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang M, Gao L, Dong S, Sun Y, Shen Q, Guo S. Role of silicon on plant–pathogen interactions. Front Plant Sci. 2017;8:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alhousari F, Greger M. Silicon and mechanisms of plant resistance to insect pests. Plants. 2018;7(2):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viciedo DO, de Mello Prado R, et al. Silicon supplementation alleviates ammonium toxicity in sugar beet (Beta vulgaris L.). J Soil Sci Plant Nutr. 2019;19(2):413–419. [Google Scholar]
- 37. Long M, Guo L, Li J, et al. Effects of water and exogenous Si on element concentrations and ecological stoichiometry of plantain (Plantago lanceolata L.). J Plant Nutr. 2018;41(10):1263–1275. [Google Scholar]
- 38. Guntzer F, Keller C, Meunier JD. Benefits of plant silicon for crops: a review. Agron Sustain Dev. 2012;32(1):201–213. [Google Scholar]
- 39. Meunier JD, Barboni D, Anwar-ul-Haq M, et al. Effect of phytoliths for mitigating water stress in durum wheat. New Phytol. 2017;215(1):229–239. [DOI] [PubMed] [Google Scholar]
- 40. Ma J, Cai H, He C, Zhang W, Wang L. A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol. 2015;206(3):1063–1074. [DOI] [PubMed] [Google Scholar]
- 41. Naeem A, Ghafoor A, Farooq M. Suppression of cadmium concentration in wheat grains by silicon is related to its application rate and cadmium accumulating abilities of cultivars. J Sci Food Agric. 2015;95(12):2467–2472. [DOI] [PubMed] [Google Scholar]
- 42. Marschner H. Marschner’s Mineral Nutrition of Higher Plants. 3rd ed Academic; 2012. [Google Scholar]
- 43. Emamverdian A, Ding Y, Xie Y, Sangari S. Silicon mechanisms to ameliorate heavy metal stress in plants. Biomed Res Int. 2018;2018;8492898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu X, Li L, Bian R, et al. Effect of biochar amendment on soil-silicon availability and rice uptake. J Plant Nutr Soil Sci. 2014;177(1):91–96. [Google Scholar]
- 45. Hussain I, Ashraf MA, Rasheed R, Asghar A, Sajid MA, Iqbal M. Exogenous application of silicon at the boot stage decreases accumulation of cadmium in wheat (Triticum aestivum L.) grains. Braz J Bot. 2015;38(2):223–234. [Google Scholar]
- 46. Maghsoudi K, Emam Y, Pessarakli M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J Plant Nutr. 2016;39(7):1001–1015. [Google Scholar]
- 47. Keller C, Rizwan M, Davidian JC, et al. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta. 2015;241(4):847–860. [DOI] [PubMed] [Google Scholar]
- 48. Bharwana SA, Ali S, Farooq MA, Iqbal N, Abbas F, Ahmad MS. Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J Bioremediat Biodegrad. 2013;4(4):187. [DOI] [PubMed] [Google Scholar]
- 49. Liang Y, Sun W, Zhu YG, Christie P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut. 2007;147(2):422–428. [DOI] [PubMed] [Google Scholar]
- 50. Gunes A, Pilbeam DJ, Inal A, Coban S. Influence of silicon on sunflower cultivars under drought stress, I: growth, antioxidant mechanisms, and lipid peroxidation. Commun Soil Sci Plant. 2008;39(13-14):1885–1903. [Google Scholar]
- 51. Ahmad I, Akhtar MJ, Zahir ZA, Jamil A. Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak J Bot. 2012;44(5):1569–1574. [Google Scholar]
- 52. Hoagland DR, Arnon DI. The Water Culture Method for Growing Plant Without Soil. Circular 347. California Agricultural Experiment Station. The College of Agriculture, University of California, Berkeley. 1950;328:1–60. [Google Scholar]
- 53. Lichtenthaler HK. Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. In: Paker L, Douce R, eds. Methods in Enzymology. CRC Press; 1987:350–382. [Google Scholar]
- 54. Davies BH. Carotenoids. In: Goodwin TW, ed. Chemistry and Biochemistry of Plant Pigments. Academic Press; 1976:199–217. [Google Scholar]
- 55. Sergiev L, Alexieva E, Karanov E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and markers in plants. Comp Ren Del Academie Buldes Sci. 1997;51(3):121–124. [Google Scholar]
- 56. Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207(4):604–611. [DOI] [PubMed] [Google Scholar]
- 57. Dionisio-Sese ML, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135(1):1–9. [Google Scholar]
- 58. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. [DOI] [PubMed] [Google Scholar]
- 59. Hodges DM, Nozzolillo C. Anthocyanin and anthocyanoplast content of cruciferous seedlings subjected to mineral nutrient deficiencies. J Plant Physiol. 1996;47(6):749–754. [Google Scholar]
- 60. Dixit V, Pandey V, Shyam R, Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot. 2001;52(358):1101–1109. [DOI] [PubMed] [Google Scholar]
- 61. Giannopolitis CN, Ries SK. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol. 1977;59(2):309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chance B, Maehly AC. The assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- 63. Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–880. [Google Scholar]
- 64. Wolf B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal. 1982;13(12):1035–1059. [Google Scholar]
- 65. Chen W, Yao X, Cai K, Chen J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol Trace Elem Res. 2011;142(1):67–76. [DOI] [PubMed] [Google Scholar]
- 66. Ma J F, Tamai K, Ichii M, Wu K. A rice mutant defective inactive Si uptake. Plant Physiol. 2002;130(4):2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett. 2010;8(3):199–216. [Google Scholar]
- 68. Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011;2011:402647. [Google Scholar]
- 69. Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Saidi I, Ayouni M, Dhieb A, Chtourou Y, Chaïbi W, Djebali W. Oxidative damages induced by short-term exposure to cadmium in bean plants: protective role of salicylic acid. S Afr J Bot. 2013;85:32-38. [Google Scholar]
- 71. Paunov M, Koleva L, Vassilev A, Vangronsveld J, Goltsev V. Effects of different metals on photosynthesis: cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int J Mol Sci. 2018;19(3):787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dresler S, Wójcik M, Bednarek W, Hanaka A, Tukiendorf A. The effect of silicon on maize growth under cadmiums tress. Russ J Plant Physiol. 2015;62(1):86–92. [Google Scholar]
- 73. Muradoglu F, Gundogdu M, Ercisli S, et al. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol Res. 2015;48(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Piršelová B, Kuna R, Lukáč P, Havrlentová M. Effect of cadmium on growth, photosynthetic pigments, iron and cadmium accumulation of Faba Bean (Vicia faba cv. Aštar). Agriculture. 2016;62(2):72–79. [Google Scholar]
- 75. Li X, Zhao M, Guo L, Huang L. Effect of cadmium on photosynthetic pigments, lipid peroxidation, antioxidants, and artemisinin in hydroponically grown Artemisia annua. J Environ Sci. 2012;24(8):1511–1518. [DOI] [PubMed] [Google Scholar]
- 76. Hussain HA, Men S, Hussain S, Zhang Q, Ashraf U, Anjum SA, Ali I, Wang L. Maize tolerance against drought and chilling stresses varied with root morphology and antioxidative defense system. Plants. 2020. b;9:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shahid M, Shamshad S, Rafiq M, et al. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere. 2017;178:513–533. [DOI] [PubMed] [Google Scholar]
- 78. Kabir AH, Hossain MM, Khatun MA, Mandal A, Haider SA. Role of silicon counteracting cadmium toxicity in alfalfa (Medicago sativa L.). Front Plant Sci. 2016;7:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hussain I, Parveen A, Rasheed R, Ashraf MA, Ibrahim M, Riaz S, Afzaal Z, Iqbal M. Exogenous silicon modulates growth, physio-chemicals and antioxidants in barley (Hordeum vulgare L.) exposed to different temperature regimes. Silicon. 2019;11(6):2753–2762. [Google Scholar]
- 80. Ashfaque F, Inam A, Iqbal S, Sahay S. Response of silicon on metal accumulation, photosynthetic inhibition and oxidative stress in chromium-induced mustard (Brassica juncea L.). S Afr J Bot. 2017;111:153–160. [Google Scholar]
- 81. Bari MA, Prity SA, Das U, et al. Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol. 2020;22(3):472–479. [DOI] [PubMed] [Google Scholar]
- 82. Tripathi P, Tripathi RD, Singh RP, et al. Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol Eng. 2013;52:96–103. [Google Scholar]
- 83. Wahid A, Arshad M, Farooq M. Cadmium phytotoxicity: responses, mechanisms and mitigation strategies. In: Lichtfouse E, ed. Advances in Sustainable. Springer; 2009:371–340. [Google Scholar]
- 84. Thind S, Hussain I, Ali S, et al. Physiological and biochemical bases of foliar silicon-induced alleviation of cadmium toxicity in wheat. J Soil Sci Plant Nutr. 2020;20(4):2714–2730. [Google Scholar]
- 85. Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BHN, Fujita M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci. 2017;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li H, Liu X, Wassie M, Chen L. Selenium supplementation alleviates cadmium-induced damages in tall fescue through modulating antioxidant system, photosynthesis efficiency, and gene expression. Environ Sci Pollut Res. 2020;9:1–3. [DOI] [PubMed] [Google Scholar]
- 87. Srivastava M, Ma LQ, Rathinasabapathi B, Srivastava P. Effects of selenium on arsenic uptake in arsenic hyperaccumulator Pteris vittata L. Bioresour Technol. 2009;100(3):1115–1121. [DOI] [PubMed] [Google Scholar]
- 88. Nasraoui-Hajaji A, Gouia H, Carrayol E, Haouari-Chaffei C. Ammonium alleviates redox state in solanum seedlings under cadmium stress conditions. J Environ Anal Toxicol. 2012;2:116–120. [Google Scholar]
- 89. Anjum SA, Tanveer M, Hussain S, Ullah E, Wang L, Khan I, Samad RA, Tung SA, Anam M, Shahzad B. Morpho‐physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. CLEAN–Soil Air Water. 2016;44:29–36. [Google Scholar]
- 90. Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019;8(4):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim Y-H, Khan A, Waqas M, Shahzad R, Lee I-J. Silicon mediated mitigation of wounding stress acts by up-regulating the rice antioxidant system. Cereal Res Commun. 2016;44(1):111–121. [Google Scholar]
- 92. Shabbir Z, Sardar A, Shabbir A, et al. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere. 2020;259:127436. [DOI] [PubMed] [Google Scholar]
- 93. Luo J, Zhu T, Wang X, et al. Toxicological mechanism of excessive copper supplementation: Effects on coloration, copper bioaccumulation and oxidation resistance in mud crab Scylla paramamosain. J Hazard Mater. 2020;395(55):122600. [DOI] [PubMed] [Google Scholar]
- 94. Orororo OC, Asagba SO, Tonukari NJ, Okandeji OJ, Mbanugo JJ., Hibiscus Sabdarrifa L. Anthocyanins-induced changes in reproductive hormones of cadmium-exposed rats. Int J Sci Res. 2018;8(4):308–311. [Google Scholar]
- 95. Amiri J, Entesari S, Delavar K, Saadatmand M, Rafie NA. The effect of silicon on cadmium stress in Echium amoenum. World Acad Sci Eng Technol. 2012;62:242–245. [Google Scholar]
- 96. Kovinich N, Kayanja G, Chanoca A, Riedl K, Otegui MS, Grotewold E. Not all anthocyanins are born equal: distinct patterns induced by stress in Arabidopsis. Planta. 2014;240(5):931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wu M, Wang PY, Sun LG, et al. Alleviation of cadmium toxicity by cerium in rice seedlings is related to improved photosynthesis, elevated antioxidant enzymes and decreased oxidative stress. Plant Growth Regul. 2014;74(3):251–260. [Google Scholar]
- 98. Adhikari S, Ghosh S, Azahar I, et al. Sulfate improves cadmium tolerance by limiting cadmium accumulation, modulation of sulfur metabolism and antioxidant defense system in maize. Environ Exp Bot. 2018;153:143–162. [Google Scholar]
- 99. Mahmud JA, Hasanuzzaman M, Nahar K, Bhuyan MHMB, Fujita M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol Environ Saf. 2018;147:990–1001. [DOI] [PubMed] [Google Scholar]
- 100. Hossain MA, Piyatida P, da Silva JAT, Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot. 2012;2012:872875. [Google Scholar]
- 101. Rasheed R, Ashraf MA, Kamran S, Iqbal M, Hussain I. Menadione sodium bisulphite mediated growth, secondary metabolism, nutrient uptake and oxidative defense in okra (Abelmoschus esculentus Moench) under cadmium stress. J Hazard Mater. 2018;360:604–614. [DOI] [PubMed] [Google Scholar]
- 102. Farooq MA, Ali S, Hameed A, et al. Cadmium stress in cotton seedlings: physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S Afr J Bot. 2016;104:61–68. [Google Scholar]
- 103. Liu JJ, Lin SH, Xu PL, Wang XJ, Bai JG. Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed cucumber leaves. Agr Sci China. 2009;8(9):1075–1086. [Google Scholar]
- 104. Song A, Li Z, Zhang J, Xue G, Fan F, Liang Y. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J Hazard Mater. 2009;172(1):74–83. [DOI] [PubMed] [Google Scholar]
- 105. Shen X, Zhou Y, Duan L, Li Z, Eneji AE, Li J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J Plant Physiol. 2010;167(15):1248–1252. [DOI] [PubMed] [Google Scholar]
- 106. Bista DR, Heckathorn SA, Jayawardena DM, Mishra S, Boldt JK. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and-tolerant grasses. Plants. 2018;7(2):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pourrut B, Lopareva-Pohu A, Pruvot C, et al. Assessment of fly ash-aided phytostabilisation of highly contaminated soils after an 8-year field trial: Part 2. Influence on plants. Sci Total Environ. 2011;409(21):4504–4510. [DOI] [PubMed] [Google Scholar]
- 108. Pereira TS, Pereira TS, de Carvalho Souza CL, Lima EJ, Batista BL, da Silva Lobato AK. Silicon deposition in roots minimizes the cadmium accumulation and oxidative stress in leaves of cowpea plants. Physiol Mol Biol Plant. 2018;24(1):99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. De Maria S, Rivelli AR, Kuffner M, et al. Interactions between accumulation of trace elements and macronutrients in Salix caprea after inoculation with rhizosphere microorganisms. Chemosphere. 2011;84(9):1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rodrigues FA, McNally DJ, Datnoff LE, et al. Silicon enhances the accumulation of diterpenoid phytoalexins in rice: a potential mechanism for blast resistance. Phytopathol. 2004;94(2):177–183. [DOI] [PubMed] [Google Scholar]


