Abstract
Administration of substances directly into the cerebrospinal fluid (CSF) that surrounds the brain and spinal cord is one approach that can circumvent the blood-brain barrier to enable drug delivery to the central nervous system (CNS). However, molecules that have been administered by intrathecal injection, which includes intraventricular, intracisternal, or lumbar locations, encounter new barriers within the subarachnoid space. These barriers include relatively high rates of turnover as CSF clears and potentially inadequate delivery to tissue or cellular targets. Nanomedicine could offer a solution. In contrast to the fate of freely administered drugs, nanomedicine systems can navigate the subarachnoid space to sustain delivery of therapeutic molecules, genes, and imaging agents within the CNS. Some evidence suggests that certain nanomedicine agents can reach the parenchyma following intrathecal administration. Here, we will address the preclinical and clinical use of intrathecal nanomedicine, including nanoparticles, microparticles, dendrimers, micelles, liposomes, polyplexes, and other colloidalal materials that function to alter the distribution of molecules in tissue. Our review forms a foundational understanding of drug delivery to the CSF that can be built upon to better engineer nanomedicine for intrathecal treatment of disease.
Keywords: cerebrospinal fluid, subarachnoid space, nanotechnology, polymer, gene delivery, intracisternal, intraventricular, lumbar, intracerebroventricular, central nervous system
1. Introduction
Intrathecal (IT) administration, i.e., the injection of substances directly into the thecal sac that contains cerebrospinal fluid (CSF), is a potentially powerful drug delivery approach. This route of administration can achieve a high concentration of therapeutic agent within the central nervous system (CNS) while minimizing off-target exposure and associated toxicity.[1] However, the distribution of molecules following IT administration when they are provided in free form remains to be optimized.[2, 3] Many hydrophilic agents clear rapidly as CSF turns over, many hydrophobic agents are restricted to delivery near to the injection site, and many macromolecules can experience relatively limited parenchymal penetration.[4] In contrast to the fate of freely administered drugs, colloidal systems (lipids, polymers, metals, other synthetic materials, and biological particles[5]) can effectively deliver genes, imaging agents, and therapeutic molecules via the CSF to reach the surfaces of the brain and spinal cord. In some instances, which we will discuss below, there is evidence to suggest that these carriers or their encapsulated payloads are capable of reaching deep parenchymal structures. The potential significance of IT drug delivery for the treatment of CNS disease is amplified by deepened understanding of dynamic exchange between CSF, interstitial fluid (ISF), and peripheral clearance, which provides an opportunity to alter the distribution of nanomedicine through fluid convection.[6] It is thus not surprising that IT delivery of nanoparticles has found utility in a multitude of preclinical models of CNS disease, including pain, neuroinflammation, infection, metabolism, neurotrauma, neurovascular disease, behavior, and cancer. Several nanomedicine therapeutics have reached clinical trial or are approved for clinical use.
Despite increasing interest in this approach, relatively little field-level consensus has been developed regarding how to best engineer nanomedicine for IT administration. We predict that a better understanding of the relationship between carrier or payload properties and resulting distribution or efficacy will yield insight into how to design colloids for IT administration. Here, we review state-of-the-art strategies for IT drug delivery via nanomedicine. Our review is structured to first discuss the anatomical and physiological characteristics of the subarachnoid space that define CSF production, flow, and clearance. Next, we will explore preclinical and clinical literature describing the use of various forms of nanomedicine in an IT context. Finally, we will summarize current understanding and describe several potential areas of future investigation to guide the development of nanotechnology for drug delivery via the IT route.
2. Anatomy of CSF filled spaces
2.1. Cerebral ventricles:
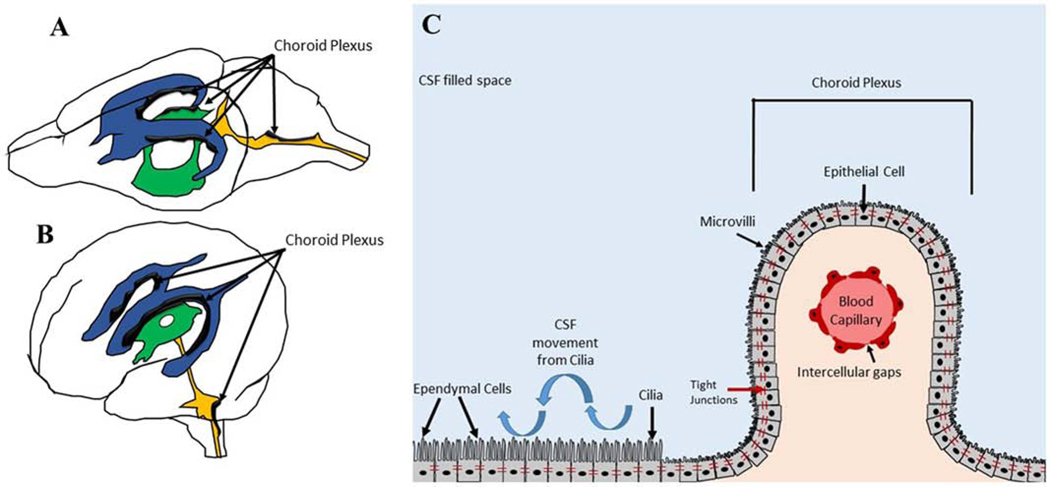
The cerebral ventricles are cavities within the brain that produce and circulate CSF. The lateral ventricles are the two largest ventricles and are positioned directly below the cerebral cortex. The 3rd ventricle is positioned inferiorly to the lateral ventricles, and the 4th ventricle precedes the 3rd ventricle.[7] Ventricular anatomy is similar between humans and rodents, being primarily oriented in the rostral-caudal directions. However, the ventricular system in humans takes a 90º turn compared to the ventricular system in rodents, which is aligned along the ventral-dorsal axis (Figure 1).[8] The cerebral ventricles are lined with ependymal cells that posses beating cilia to promote CSF movement and mixing. The majority of ventricular CSF is produced from branching villi of the choroid plexus, which is composed of capillaries and epithelial cells that are connected by tight junctions to allow exchange of water, nutrients and other molecules from the blood to CSF. The choroid plexus epithelial cells are derived from the surrounding ependymal cells that line the ventricles. Although the choroid plexus is often depicted as being located within the lateral ventricles, it is important to note that choroid plexus can be found through the ventricular system of the brain.
Figure 1. Anatomy of the ventricular system.
Ventricles of a rodent brain (A) and human brain (B). Lateral ventricles (blue), 3rd ventricle (green), and 4th ventricle (orange) with associated choroid plexus in black. Magnified schematic of choroid plexus anatomy in the ventricles (C).
2.2. Subarachnoid space:
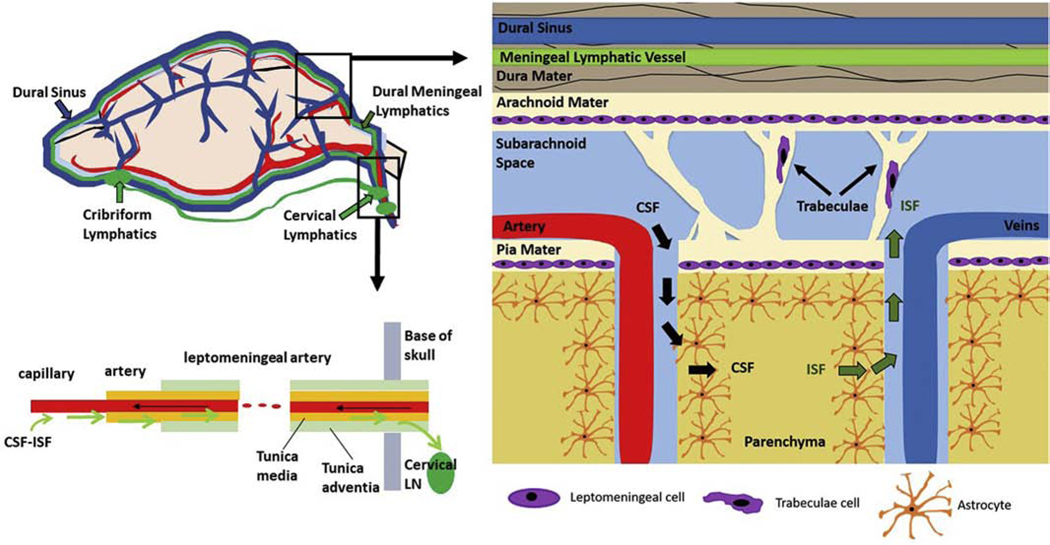
There are three membranes, or meninges, that surround the brain and spinal cord. Beginning from superficial to deep structures, these meninges are called the dura mater, the arachnoid mater, and the pia mater (Figure 2). The dura mater is located closest to the skull and bones of the spinal column, while the pia mater directly contacts surfaces of the brain and spinal cord. The arachnoid mater and pia mater are collectively known as the leptomeninges. CSF flows between the leptomeninges to form the subarachnoid space (SAS). In addition to CSF, the SAS contains fibrous trabeculae, blood vessels, and nerves. The subarachnoid trabeculae (SAT), which are sometimes referred to as leptomeningeal or arachnoid trabeculae, are collagen-rich fibers that either span the distance between the pia mater and arachnoid mater or form web-like coatings to ensheath blood vessels and nerve bundles. The SAT are thought to serve multiple functions, providing structural support to the SAS to stabilize the brain, limiting the movement of nerves and vessels, and providing a conduit through which CSF can flow and mix.[9]
Figure 2. CSF exchange and clearance pathways.
CSF-ISF exchanges and is cleared along arteries to cervical lymph nodes (bottom, left). CSF moves through the perivascular space and associated SAS microanatomy into the brain parenchyma (right).
The pia mater is the innermost meningeal layer, and it is continuous with the gyri, sulci, and fissures of the brain and spinal cord. The pia mater is composed of connective fibers (including collagen, reticular, and elastic) and cells (including fibroblast-like cells and macrophages). These structures extend from the parenchyma to surround and anchor blood vessels that enter and exit the superficial surfaces of the brain and spinal cord. The perivascular spaces formed between the pia mater and blood vessels are referred to as Virchow-Robin spaces and play an important role in CSF absorption and clearance.[10]
Fibroblast-like leptomeningeal cells within the pia mater are connected by desmosomes to form an impermeable barrier between the parenchyma and the subarachnoid space.[11] In humans, the pia mater in the brain is generally only a few cell and fiber layers thick, and it increases in thickness from approximately 8–15 μm in the cervical region of the spinal cord to 130–200 μm in the thoracic region of the spinal cord.[11, 12] Thicker regions of the pia mater within the spinal cord are sometimes referred to as the intermediate layer. In contrast, the arachnoid mater has an average thickness of 35–40 μm that is considered relatively spatially uniform.[13] Similar to the pia mater, the arachnoid mater contains elastin, collagen, and leptomeningeal cells that are connected by desmosomes. The arachnoid mater is partially connected with the dura, and it also forms SAT extensions to the pia mater.[12]
The SAS contains both cells and collagen trabeculae. In early fetal development, the space between the pia and arachnoid membranes is filled by a glycosaminoglycan (GAG) gel, within which mesenchymal stem cells reside. As CSF is produced and begins to flow through the SAS, the gel retracts laterally. This process of retraction yields microstructural cavities between the pia and arachnoid mater that continue developing until the material can no longer retract, leaving behind a stable meshwork of fibers. The mesenchymal cells that remain within this network differentiate into fibroblast-like leptomeningeal cells. Collectively, this retracted network with cells forms the pia and arachnoid barriers and the subarachnoid space within which CSF is contained.[14]
3. CSF circulation
3.1. CSF production and general flow
CSF plays an essential role in CNS homeostasis, providing buoyancy and protection to the brain, preserving solute concentration and intracranial pressure, and facilitating clearance of waste.[15, 16] Ependymal cells and epithelial cells of the choroid plexus form the blood-CSF-brain barrier, which regulates passage of electrolytes, a small amount of protein, and water into the ventricles.[17] Although the majority (approximately 80%) of CSF is produced by the choroid plexus, the parenchyma also serves as an important source of CSF.[18, 19]
The total amount of CSF in the body, the rate of production, and the rate of turnover varies among species (Table 1) and can be affected by disease.[16, 20] CSF is produced by the choroid plexus at an approximate rate of 0.37 mL/min in healthy, young adult humans. Approximately 25% of this fluid is found in the ventricular system,[7] with the remainder circulating throughout the SAS. Importantly, the choroid plexus has evolved to maintain homeostasis within the CSF. Small perturbations in osmolality and pH can stimulate significant changes to CSF secretion that are expected to impact flow.[21]
Table 1.
CSF volume, production, and turnover across species.
| Species | Volume (mL) | Production (mL/min) | Turnover (times/day) | Reference |
|---|---|---|---|---|
| Mouse | 0.035 – 0.040 | 0.00032–0.00035 | 12–14 | [34, 223–225] |
| Rat | 0.150 | 0.0017–0.0028 | 9–12 | [224, 226–228] |
| Rabbit | 2.3 | 0.0078 | n.d. | [229, 230] |
| Cat | n.d. | 0.005–0.02 | n.d. | [231, 232] |
| Dog | 12 | 0.03–0.50 | 5–6 | [225, 231] |
| Sheep | 251 | 0.017–0.063 | 1–6 | [233–236] |
| Monkey | 13–15 | 0.03–0.04 | 4–5 | [224, 225, 237] |
| Human | 100–4002 | 0.35–0.37 | 3–5 | [20, 223–225, 238, 239][240] |
Estimation based on CSF volume of goats
CSF volume in humans increases with advancing age
CSF movement within the SAS is often conceptualized as unidirectional bulk flow, experiencing convection first downstream and then recirculating upstream along the spinal cord and back to the brain.[22] However, a unidirectional pressure gradient does not exist within the CNS, and the mechanisms of CSF flow are both complex and not unidirectional.[23] CSF and interstitial fluid (ISF) experience significant exchange with one another, which is dependent on diurnal cycles. Experimental data demonstrate that respiratory and cardiac pulsations produce a to-and-fro movement of CSF to generate local mixing, which is expected to ehance the distribution of IT administered agents.[22, 24–27]. For example, using the near-infrared dye indocyanine green (ICG), we found that injection into the subarachnoid space of the lumbar spinal cord resulted in dye movement in both rostral and caudal directions, entry into the SAS and perivascular spaces, and eventual cranial drainage into the cervical lymph nodes and lumbar drainage into lumbar lymph nodes.[28] Within the ventricles, ependymal cells also contain extensions called cilia that perform a synchronized beating, generating force to facilitate CSF circulation.[29, 30] CSF from the SAS is absorbed into the parenchyma of the brain and spinal cord via transport through perivascular (Virchow-Robin) spaces. This absorption produces an increase in interstitial pressure that facilitates clearance of ISF components from arterial to venous perivascular spaces.[31] In total, this system enables a dynamic exchange of fluid between ISF and CSF.[18, 32]
3.2. Glymphatic and lymphatic routes for CSF distribution and clearance
The fate of circulating CSF is complex (Figure 2). CSF from the SAS is driven by convection into the arterial perivascular spaces that are bounded by the blood vessel walls and glia limitans, which is a thin barrier of astrocytes processes. CSF must cross this barrier to reach the parenchyma. Narrow gaps (20–30 nm) across the glia limitans allow transport of fluid and solutes out of the perivascular spaces, a process controversially thought to be driven by arterial pulsations [6, 33]. Once outside the arterial perivascular spaces, it is postulated that CSF movement is driven by convective currents created by the water transporter, Aquaporin-4 (AQP4) on the foot processes of astrocytes. Through mechanisms that remain highly controversial and largely unknown, CSF-ISF exchange has been hypothesized to occur as fluid is convected from arterial perivascular spaces toward the venous perivascular channels before fluid flows out of the cranium into the peripheral lymphatics. The CSF-ISF exchange of solutes across the parenchyma via the glial water pathway was discovered by Maiken Nedergaard, who termed the phenomenon “glymphatics” in reference to the role the glial (or astrocyte) cell plays in convecting solute (or cellular waste products) across the brain parenchyma in a fashion similar to peripheral lymphatics but without authentic lymphatic vessels [6]. Other transporters, some which play role in the secretion of CSF in the choroidal plexus, could also mediate water fluxes that provide convective flow necessary for CSF-ISF exchange [34].
An alternate hypothesis by Weller and Carare postulates that exchanged CSF-ISF drains not through the venous perivascular spaces but rather through the basement membranes of cerebral capillaries and subsequently through the basement membranes between smooth muscle cells in the tunica media of cerebral arteriole and arteries.[35] These basement membrane channels were first depicted in microscopy studies by Wagner.[36] Given the presence of initial lymphatics in the adventia, [37] it is certainly possible that CSF-ISF outflow into the cervical lymph nodes occurs via the carotid artery. In this hypothesis, a sheath of CSF-ISF outflow occurs in a direction countercurrent to arterial blood flow.[38, 39] Regardless of whether the direction of CSF-ISF outflow engages co-current venous or countercurrent arterial mechanisms, the anatomical and functional connections between these perivascular spaces and the receiving lymphatic vessels and lymph nodes of the neck are not well understood. What is understood is that CSF transport form the arterial perivascular spaces enables CSF-ISF exchange with deposition in the peripheral lymphatics outside the cranium, which is expected to play a role in the fate of IT administered substances.
3.2.1. Venous outflow
In the brain, arachnoid granulations protrude from the SAS into the arachnoid and dura mater and are postulated to facilitate CSF clearance into venous meningeal blood vessels or arachnoid villi [40]. In the spine, CSF is also re-absorbed through arachnoid granulations along nerve root sheaths and epidural veins.[41] However, while re-absorption into the venous subarachnoid and spinal arachnoid villi has been regarded as the major clearance route of CSF in the past, recent revisions in Starling’s law and the low oncotic pressure in the CSF make significant venous reabsorption less likely [42].
3.2.2. Lymphatic outflow
Recent work shows that the lymphatics represent the major route of CSF outflow [43] [44]. Lymphatic vessels found in the dura of rodents drain CSF via the foramina at the base of the skull [45–47] without exchange with the ISF. Likewise, CSF drains through the cribriform plate and into the lymphatics of the nasal submucosa of rodents and non-human primates [45] [45, 48, 49]. Increasing evidence suggests that intracranial outflow of CSF occurs predominantly through the nasal mucosa (cribriform plate) as well as basal and ventral meningeal lymphatics [46, 50] which ultimately drain through jugular lymphatic channels into the deep cervical lymph nodes. These pathways coincide with perineural pathways along the olfactory nerve and to the nasal mucosa (cribiform plate).[44] It is important to note that the relative significance of each of these clearance pathways varies by species. The nasal mucosa, especially, is an important route of clearance in small animals, whereas higher order species do not experience such high clearance through mucosal routes. Cortical folding – and thus cortical surface area – is also much higher in humans, which is an additional consideration for species scaling of IT drug delivery that has not yet been directly experimentally addressed.[51]
In addition to lymphatic cranial CSF outflow via dural or cribriform lymphatics, spinal CSF outflow has also been observed in preventrebral lymph nodes under conditions of high volume, high pressure intrathecal or intracisternal fluid injections [43, 52]. In rodents with kaolin-induced hydrocephalus, spinal outflow of CSF occurs at the expense of cranial CSF absorption [53]. Under normal CSF pressure conditions in sheep, up to a quarter of the total CSF clearance occurs through the spinal lymphatics, [54] with the majority of this clearance occurring through cranial pathways that either shunt or pass thought the brain parenchyma.
It is noteworthy that the entry of fluid, immune cells, macromolecules and waste products occur at the “initial” lymphatics that line all organs, including the meningeal linings of the brain. These lymphatic capillaries lack a basal lamina and are uniquely adapted for the entry of excess fluid and macromolecules.[55] The initial lymphatics drain into the collecting lymphatics, which are comprised of mini-segments termed “lymphangions” that actively pump lymph and create suction or negative pressures upstream within the initial lymphatics. While there have not been lymphangions found in dorsal meningeal lymphatics, Anh and colleagues recently uncovered lymphangions in the basal meningeal lymphatics, suggesting yet another mechanism for CSF outflow convection.[38] We observed that lymphangion activity was repressed during proinflammatory conditions and a mouse model of Alzheimer’s,[56, 57] suggesting that impaired convective drainage would occur with neuroinflammation or disease. Similar results were reported in a transgenic model of Alzheimer’s.[58] These disease-induced alterations in lymphatic function within the CNS are important consideration in nanomedicine drug delivery. Because of the technical difficulties of identifying lymphatic vessels and lymph nodes and the previously unrecognized importance of how they can mediate drug distribution in brain, few studies assess lymphatic clearance mechanisms or study whether the lymphatic and glymphatic CSF pathways can be exploited to enhance exposure and retention of colloids in the CSF. This will be an important area of future investigation.
Taken these data in sum, the lymphatics are the primary outflow conduit for CSF, and constitute a system-wide functional unidirectional vascular system that removes excess fluid and waste products and delivers them through lymph nodes to the venous circulation for immune surveillance, maintenance of fluid homeostasis, and waste processing. Because venous reabsorption is an unlikely major route of CSF outflow from the brain, CSF outflow today is thought to occur predominantly to the peripheral lymphatics. As a result, the flow of drugs administered into the CSF likely transit through the lymphatics and associated lymph nodes and ultimately to the hemovascular system.
4. Drug Administration to the CSF
4.1. Possible routes of IT administration
The blood brain barrier (BBB) and blood spinal cord barrier (BSCB) are comprised of endothelial cells possessing tight junctions and efflux pumps. These barriers collectively prevent the parenchymal passage of all but a fraction of small, uncharged molecules.[59–61] Delivery of drugs via parenteral routes of administration typically results in low CNS concentrations, which has been a severe impediment to the development of new therapies to treat CNS disease. One of the most common approaches to bypass the challenges of parenteral delivery is convection enhanced delivery (CED). CED is an approach by which active agents are infused directly into the parenchyma under a positive pressure gradient, which serves to dilate the extracellular space and enhance the volume of distribution. Although CED has been shown to be effective in preclinical models, challenges remain for widespread clinical application.[62] Importantly, CED cannot be used to treat disseminated disease within the CNS and would not be expected to be useful for disease affecting the surfaces of the brain and spinal cord, such as leptomeningeal metastasis.
Intrathecal (IT) injection is a promising alternative approach for bypassing the BBB and BSCB. By definition, IT injection refers to administration of a substance directly into the thecal sac that contains CSF. Common clinical use of the term intrathecal is often intended to refer only to intrathecal lumbar (IT-L) injection. In fact, IT delivery can also be achieved through intracisternal magna (ICM) or intracerebroventricular (ICV) injection. For the purposes of this review, IT will not be presumed to refer to lumbar injection only. We will specify the subcategory of IT injection (IT-L, ICM, or ICV) where it is known. We will use the encompassing term “IT” when the exact location of injection was not been defined by authors of an original paper, or when we intend to refer to all three potential IT injection locations.
4.2. IT administration in preclinical models
IT administration of active molecules can be challenging in small animals, given the relatively small size of the SAS. The lateral ventricles are a common site for IT administration, and they can be reached singly or in tandem with the aid of a surgical stereotax in rodents. Advantages of ICV administration compared to other routes include the relative consistency of the surgical procedure and well-understood approaches for cannulation.[63] Disadvantages include damage produced by passing the needle through healthy brain, the potential for backflow, asymmetry when only one ventricle is infused, and difficulty performing repeat infusions without the aid of a catheter. The cisterna magna, which can be accessed in small animals by retracting muscles through an incision at the back of the neck, may be a useful alternative. Advantages of ICM administration include being able to reach CSF without disturbing the brain parenchyma and a large target volume (that is also compliant), which enables comparatively larger infusion volumes to be administered safely. Disadvantages include damage to the neck muscle, difficulty performing repeat infusions without the aid of a catheter (cannulation of the cisterna magna has been described, although it is a less commonly used approach [64]), and the development of transient pressure gradients from a large injection volume could disrupt structures and impact convective deliveries. The subarachnoid space of the lumbar spinal cord is another possible route of IT administration in small animals. Advantages of IT-L administration include the speed of the procedure, an absence of surgical recovery time, and the ease with which multiple infusions can be performed, either through separate injections or via cannulation.[65] The major disadvantage of IT-L administration is the technical challenge of achieving an IT infusion (versus an intraspinal or epidural infusion), particularly in mice. Regardless of the site of administration, osmotic minipumps can be attached to cannulae to facilitate longer dosing schemes for substances that are expected to be stable at body temperature.
4.3. IT administration in clinical application
In humans, IT drug administration is typically performed via IT-L or ICV infusion. The lumbar cistern can be accessed via lumbar puncture, which is straightforward in the majority of patients. Disadvantages of lumbar puncture include the fact that the procedure is painful, so sedation is required in most children. It can also be technically difficult to perform lumbar puncture in some adults, including those who are obese or have abnormal spine anatomy. To enable repeated or continuous IT-L infusions, a lumbar catheter can be surgically placed into the lumbar cistern and connected to a pump, which is typically implanted subcutaneously in the abdomen. The most common clinical uses of such pumps are to administer narcotics for patients with chronic pain or baclofen for patients with spasticity. The most common ICV site in humans is the lateral ventricle of the brain. ICV drug administration to the lateral ventricles can be achieved via surgical implantation of a ventricular catheter that is connected to a ventricular access device (also called an Ommaya reservoir). Ventricular access devices can also be connected to catheters placed in the fourth ventricle or tumor resection cavity. ICV injection in humans has the disadvantage of needing to surgically access the lateral ventricles via a catheter that must be placed blindly through the cerebral cortex. Even with a frameless stereotax, it can be difficult to ensure that all of the catheter’s holes are within the ventricle instead of the parenchyma. This is important, because if some of the catheter’s holes are within the brain parenchyma, infusions can cause leukoencephalopathy. Instillation of a catheter directly into the 4th ventricle is an alternative that circumvents some of these issues. We have developed a neurosurgical technique at our institution that enables safe catheter placement in the 4th ventricle of children undergoing surgical resection of posterior fossa tumors.[66–68] This approach has the advantage of enabling direct visualization without traversing brain parenchyma, preventing damage to the brain and ensuring catheter exit ports are well-exposed to CSF. In the context of tumor resection, this also avoids additional surgery for catheter placement.
5. Nanomedicine in the CSF
IT delivery of nanomedicine is not a recent concept. The earliest description of utilizing a carrier system to redirect drug distribution following IT administration was in 1978, when Kimelberg and colleagues demonstrated that liposome-encapsulated methotrexate achieved better tissue penetration and slower peripheral clearance than free methotrexate when administered via ICV (lateral) injection in cynomolgus monkeys.[69] Since this first report, IT delivery of nanomedicine has received growing preclinical and clinical interest, increasing from only two papers prior to 1990 to over 40 papers in the past decade. Several IT nanomedicines have reached clinical trial or been clinically approved for use in humans.
Our approach for reviewing this field involved PubMed searches for routes of administration (intrathecal, intraventricular, intracerebroventricular, intracisternal) and key terms related to carrier types (nanoparticle, microparticle, liposome, cyclodextrin, micelle, dendrimer, gold, polystyrene, and iron oxide). Secondary searches to include broader delivery terms (CSF, nanomedicine, nanocarrier, particle, and colloid) did not yield additional papers. Our discussion focuses on primary articles with reference to review articles where appropriate. Epidural delivery was excluded as a central focus but included where crossover with intrathecal discussion was particularly relevant. Manuscripts in which adjuvant molecules but not the nanomedicine itself were delivered intrathecally were excluded. Novel therapeutics injected by the IT route but not including a carrier (e.g., Spinraza®, which is a clinically used oligonucleotide for IT administration [70]) were also excluded.
Reports of IT nanomedicine are summarized in Table 2 (an expanded version detailing biophysical properties, when reported, can be found in Supplementary Data). Taken in sum, it is evident that investigators have utilized a variety of biomaterial platforms (polymers, lipids, metals, and other) and active payloads (small molecules, proteins, genes, and imaging agents) for both therapeutic and investigative purposes. Therapeutic development is clustered around several major themes, including pain, neurovascular disease and neurotrauma, neurodegeneration, and cancer metastasis. Preclinically, IT therapies have been utilized extensively for gene delivery to both treat and model human disease, with lesser focus on small molecule delivery. These gene therapies have not yet reached clinical application. Although most investigations have developed IT approaches for a therapeutic goal, a number of key papers focus on imaging or contribute to the basic science understanding of how colloidal systems move within the subarachnoid space in a disease agnostic fashion. Collectively, existing reports highlight a myriad of potential avenues for future investigation to define structure-function relationships for nanomedicine within the SAS.
Table 2.
Summary of preclinical IT nanomedicine
| Com position | Payload | Location | Species | Application | Reference | |
|---|---|---|---|---|---|---|
| Hyperbranched Polymer | ||||||
| PAMAM | SA | Rabbit | Neuroinflammation1 | Dai, et al. 2010 | [129] | |
| PAMAM | ICV, SA | Mouse | None | Albertazzi, et al. 2013 | [127] | |
| PL | ICV | Rat | Neurodegeneration | Neelov, et al. 2013 | [131] | |
| PAMAM | Triamcinolone | Not spec. | Mouse | Pain | Kim, et al. 2017 | [128] |
| Linear Polymer | ||||||
| PCL-SS-P[(GMA-TEPA)-st-OEGMA] | pDNA | ICV | Mouse | None | Choi, et al. 2015 | [142] |
| PEI | pDNA | ICV | Mouse | None | Goula, et al. 1997 | [135] |
| PEI | pDNA | ICV | Mouse | None | Lemkine, et al. 2002 | [136] |
| PEI | siRNA | ICV | Mouse | Alzheimer’s | Helmschrodt, et al. 2017 | [149] |
| PEI (NL4–10K) | pDNA | IT-L | Rat | None | Zeng, et al. 2007 | [89] |
| PEI-CD | pDNA | IT-L | Rat | None | Tang, et al. 2006 | [88] |
| PEI-PEG | siRNA | ICV | Mouse | Neurodegeneration | Shyam, et al. 2015 | [152] |
| PEI-PEG | pDNA | IT-L | Rat | None | Shi, et al. 2003 | [85] |
| PEI-PEG | pDNA | IT-L | Rat | None | Tang, et al. 2003 | [86] |
| PEI-PEG, DOSPA / DOPE | pDNA | IT-L | Rat | Neuropathy | Wang, et al. 2005 | [87] |
| PEI-PEG (Tet1) | pDNA | ICV | Mouse | None | Kwon, et al. 2010 | [137] |
| Lipid | ||||||
| PC | IT-L | Rat | None | Umbrain, et al. 1997 | [72] | |
| PC | IT-L | Rat | Pain | Yanez, et al. 1995 | [109] | |
| DEPG, DPPG | Bupivacaine | IT-L, EP | Dog | Pain | Joshi, et al. 2015 | [94] |
| DMPC | BCNU | ICM | Rat | Cancer | Kitamura, et al. 1996 | [77] |
| DMPC | Neostigmine | IT-L | Mouse | Pain | Grant, et al. 2002 | [100] |
| DMPC | Morphine | IT-L | Mouse | Pain | Grant, et al. 1995 | [97] |
| DPPC | Morphine | IT-L | Rat | Pain | Nishiyama, et al. 2000 | [96] |
| DPPC | Alfentanil | IT-L | Rat | Pain | Bernards, et al. 1992 | [98] |
| DPPC | Alfentanil | IT-L | Rat | Pain | Wallace, et al. 1994 | [99] |
| DPPG | Cytarabine | ICV | Rat | Cancer | Kim, et al. 1987 | [75] |
| DPPG | Cytarabine | ICV | Primate | None | Kim, et al. 1993 | [76] |
| DSPC, DSPE-PEG | GABA | ICV | Rat | Behavior | Vaz, et al. 2015 | [159] |
| DSPG, PC | Amphotericin-B | IT-L | Mouse | Neuroimmunity | Gazzoni, et al. 2012 | [168] |
| DSPG, PC | Amphotericin-B | IT-L | Mouse | Neuroimmunity | Capilla, et al. 2013 | [167] |
| Not spec. | L-690, 330 | ICV | Mouse | Behavior | Shtein, et al. 2013 | [161] |
| Not Spec. | Ciprofloxacin, Minocycline | IT-L | Rat | Pain | Valdes, et al. 2018 | [95] |
| PC | Fasudil | ICM | Rat | Neurovascular | Takanashi, et al. 2001 | [80] |
| PC | Fasudil | ICM | Dog | Neurovascular | Takanashi, et al. 2001 | [81] |
| PC | Fasudil | ICM | Rat | Neurovascular | Takanashi, et al. 2001 | [83] |
| Not Spec. | Clodronate | ICM | Rabbit | Neuroimmunity | Trostdorf, et al. 1999 | [165] |
| PC | Dolichol | ICV | Rat | None | Jankowski, et al. 1992 | [132] |
| PC | Gadodiamide | ICV | Rat | Imaging | Rodriguez, et al. 2010 | [133] |
| PC | Methotrexate | ICV | Primate | Cancer | Kimelberg, et al. 1978 | [69] |
| PC | Clodronate | ICV | Rat | Neuroimmunity | Polfliet, et al. 2001 | [164] |
| PC | Clodronate | ICV | Rat | Neuroimmunity | Polfliet, et al. 2001 | [163] |
| PC, PG | Clodronate | IT-L | Rat | Neuroimmune | Sun, et al. 2018 | [166] |
| DC-Chol | cDNA | ICV | Rat | Brain Injury | Zhou, et al. 1999 | [92] |
| DOTAP or DOPC / silica | pDNA | IT-L | Rat | Pain | Dengler, et al. 2013 | [117] |
| DOTAP | pDNA | ICM | Rat | Neurovascular | Cao, et al. 2002 | [93] |
| DOTIM | pDNA | IT-L, ICV | Mice | None | Meuli-Simmen, et al. 1999 | [144] |
| DOTIM-Chol | cDNA | ICV | Rat | None | Thorsell, et al. 1999 | [139] |
| DOSPA/DOPE | pDNA | IT-L | Rat | Pain | Yao, et al. 2002 | [118] |
| DOSPA/DOPE | pDNA | IT-L | Rat | Pain | Yao, et al. 2002 | [119] |
| DOSPA/DOPE, DOTMA | pDNA | ICV | Rat | None | Thorsell, et al. 1996 | [140] |
| DOSPA/DOPE, PEI-PEG | pDNA | IT-L | Rat | Neuropathy | Wang, et al. 2005 | [87] |
| DSPC-PEG | mRNA | ICV | Mouse | Neurodegeneration | Nabhan, et al. 2016 | [91] |
| MLRI | mRNA | ICV | Rat | None | Anderson, et al. 2003 | [150] |
| MLRI | mRNA, pDNA | ICV, ICM | Rat | None | Zou, et al. 2010 | [138] |
| MLRI | pDNA | ICM | Rat | None | Hauck, et al. 2008 | [148] |
| Not Spec. (HJV) | pDNA | ICV | Primate | None | Hagihara, et al. 2000 | [143] |
| PC, DOPE, Sph, DC-Chol | ASO | IT-L | Rat | Pain | Noda, et al. 2003 | [120] |
| PC, PS*** | ASO | ICV | Rat | None | Ogo, et al. 1994 | [141] |
| PC (HJV) | ASO | ICV | Rat | Sexual dimorphism1 | Suzuki, et al. 2000 | [160] |
| PEG-PAsp(DET) | pDNA | ICM | Mouse | None | Uchida, et al. 2013 | [169] |
| pHgMelb HK10 (Melittin) | pDNA | ICV | Mouse | None | Schellinger, et al. 2013 | [146] |
| PS, PC (HJV) | pDNA | ICM | Mouse | Cancer | Mabuchi, et al. 1997 | [78] |
| Various lipids | pDNA | ICV | Mouse | None | Akita, et al. 2015 | [134] |
| Metal | ||||||
| Iron Oxide | ICV | Rat, Mouse | Neurodevelopment | Sumner, et al. 2009 | [121] | |
| Iron Oxide | ICV | Mouse | Neurodevelopment | Zhong, et al. 2015 | [122] | |
| Iron Oxide (Au) | IT-L | In vitro | None | Lueshen, et al. 2014 | [218] | |
| Iron oxide (Citrate) | ICV | Mouse | Neurodegeneration | Mundt, et al. 2009 | [130] | |
| Iron oxide (Dextran) | ICV | Rat | None | Muldoon, et al. 2004 | [123] | |
| Iron oxide (Doxorubicin) | Doxorubicin | IT-L | Mice | Cancer | Kheirkhah, et al. 2018 | [219] |
| Iron Oxide (Ketolorac) | Ketolorac | IT-L | Mouse | Pain | Wu, et al. 2018 | [113] |
| Iron oxide (DNA) | DNA | ICV | Mice | Receptor Imaging | Liu, et al. 2009 | [124] |
| Iron oxide (PEI-PEG, Tat) | DNA | IT-L | Rat | None | Song, et al. 2010 | [125] |
| Silica | ||||||
| ORMOSIL | pDNA | ICV | Mouse | None | Bharali, et al. 2005 | [151] |
| ORMOSIL | pDNA | ICV | Mouse | Neurodegeneration | Klejbor, et al. 2007 | [153] |
| DOTAP or DOPC / silica | pDNA | IT-L | Rat | Pain | Dengler, et al. 2013 | [117] |
| Solid Polymer (synthetic) | ||||||
| PS | ICM | Mouse | None | Householder, et al. 2019 | [73] | |
| PS | ICM | Mouse | Neurovascular | Mestre, et al. 2018 | [27] | |
| PLGA | Nimodipine | ICM, ICV | Rat, dog | Neurovascular | Hanggi, et al. 2016 | [79] |
| PLGA | Ropivacaine | IT-L, EP | Sheep | Pain | Ratajczak-Enselme, et al. 2009 | [108] |
| PLGA | Baclofen | IT-L | Rabbit | Pain | Lagarce, et al. 2005 | [101] |
| PLGA | Curcumin | IT-L | Mouse | Pain | Reretti, et al. 2017 | [114] |
| PLGA | Neuroregulin-1 | IT-L | Mouse | Neurotrauma | Santhosh, et al. 2017 | [84] |
| PLGA | Hydromorphone, ketamine | IT-L | Rat | Pain | Han, et al. 2015 | [102] |
| PLGA | Bupivacaine | IT | Rat | Pain | Curley, et al. 1996 | [103] |
| PLGA, PLA | Bupivacaine | Not spec. | Rabbit | None | Le Guevello, et al. 1993 | [104] |
| PLA | Bupivacaine | IT-L | Rabbit | Pain | Estebe, et al. 1995 | [105] |
| PLA | Bupivacaine | IT-L | Rabbit | Pain | Le Corre, et al. 1995 | [106] |
| PLA | Fentanyl | IT-L | Rat | Pain | Okuda, et al. 1999 | [241] |
| PLGA | BDNF | IT-L | Dog | Pain | Tan, et al. 2013 | [90] |
| PLGA | pDNA | IT-L | Rat | Pain | Soderquist, et al. 2010 | [115] |
| PPAs | pDNA | IT-L | Rat | None | Wang, et al. 2004 | [145] |
| Solid Polymer (natural) | ||||||
| Alginate / chitosan | Bupivacaine | N.S. | Rabbit | Pain | Cereda, et al. 2018 | [110] |
| Tyrosine-derived polymer | Ketoprofen | IT-L | Rat | Pain | Spofford, et al. 2009 | [112] |
| Chitosan | siRNA | IT-L | Rat | Pain | Cai, et al. 2009 | [116] |
| Sugar | ||||||
| HP-β-CD | ICV | Mouse | Neurometabolism | Camargo, et al. 2001 | [197] | |
| β-CD | Various steroids | IT-L | Rat | Pain | Svensson, et al. 2013 | [157] |
| CD | Various steroids | ICV | Rat | Pain | Frye, et al. 1996 | [158] |
| HP-β-CD | Pregnanolone | ICV | Sheep1’ | Behavior | Hirst, et al. 2000 | [155] |
| HP-β-CD | Testosterone | ICV | Rat, Hamster | Behavior | Wood, et al. 2004 | [154] |
| HP-β-CD | Various fatty acids | ICV | Rat | Behavior | Schw inkendorf, et al. 2010 | [156] |
| M-β-CD | Bupivacaine | IT-L | Rat | Pain | Karashima, et al. 2007 | [111] |
| Quantum Dots | ||||||
| Quantum Dots | ICV | Rat | Imaging | Varela, et al. 2016 | [126] | |
Lipids
DC-Chol: dimethylaminoethane-(carboamoyl)cholesterol
DEPG: dielaidoyl phosphatidylglycerol
DMPC: dimyristoyl phosphatidylcholine
DOPC: dioleoyl phosphatidylethanolamine
DOTAP:1,2-dioleoyl-3-trimethylammoniumpropane DOTIM: 1-[2-(9-(Z)-octadecenoyloxy)ethyl]-2-(8-(Z)-heptadecenyl)-3- (hydroxyethyl)imidazolinium chloride
DOTMA: trimethyl[2,3-(dioleyloxy)propyl]ammonium Chloride
DOSPA: 2,3-dioleyloxy-N-[2(sperminecarboxamido) ethyl]-N,N-dimethyl-1-propanaminium trifluoroacetate
DOPE: dioleoyl phosphatidylethanolamine
DPPC: dipalmitoyl phosphatidylcholine
DPPG: dipalmitoyl phosphatidylglycerol
DSPC: diasteroyl phosphatidylcholine
DSPE: diasteroyl phosphatidylethanolamine
DSPG: diasteroyl phosphatidylglycerol
MLRI: dissymmetric Myristoyl (14:0) and Lauroyl (12:1) Rosenthal Inhibitor-substituted compound formed from the tetraalkylammonium glycerol-based DORI
PC: phosphatidylcholine
PG: phosphatidylglycerol
Polymers
β-CD: beta-cyclodextrin
CD: cyclodextrin
HP-β-CD: hydroxypropyl-beta-cyclodextrin
M-β-CD: maltosyl-beta-cyclodextrin
PAMAM: polyamidoamine
PASP(DET): poly[N9-[N-(2-aminoethyl)-2-aminoethyl]aspartamide]
PEI: polyethylenimine PEG: polyethylene glycol
pHgMelbHK10: poly[(HPMA-g-Melittin)-b-(HPMA-MaAhxK10)]
PL: polylysine
PLA: poly(lactic acid)
PLGA: poly(lactic-co-glycolic acid)
PPA: polyphosphoramidates
Notation
/ blended
- covalently linked
( ) surface modified
Footnotes
Neonatal
Fetal
Our discussion of preclinical papers will begin by discussing papers that develop or characterize colloidal systems in a disease-agnostic fashion. We will next address development of IT administered nanomedicine by intended delivery site within the CNS: delivery to CSF exposed surfaces, loco-regional delivery to the spinal cord, delivery to the parenchyma, and delivery to cells of the CSF. Clinical papers are organized according to each of the nanomedicine therapeutics that have reached clinical trial or been approved for use in humans: cytarabine loaded liposomes for treatment of cancer, drug-empty hydroxypropyl-beta-cyclodextrin (2HßCD) for treatment of neurodegeneration, bupivacaine loaded liposomes for control of pain, liposome encapsulated fasidul for anesthesia, and PLGA encapsulated nimodipine in neurotrauma.
5.1. Preclinical IT nanomedicine delivery
5.1.1. Delivery to CSF-exposed surfaces
The surface area of the brain is large. In humans, the cerebral cortex alone possesses a surface area of approximately 2000 cm2, which is more than four times the surface area of a rodent brain if it were scaled by brain volume (cortical folding in higher order species accounts for this difference).[71] IT administered colloids would therefore be expected to gain ready access to surfaces of the CNS, including pia mater, arachnoid mater, and blood vessels within the SAS, as well as to parenchymal tissues close to the surfaces of the brain and spinal cord that could be accessed by diffusion or local exchange of fluid. Preclinical development of IT nanomedicine delivery to CSF exposed surfaces focuses on cancer metastasis, neurotrauma, and neurovascular disease. Equal attention has been paid to delivery of small and large molecules, including proteins and genes, utilizing lipid, polymeric, and metal carriers.
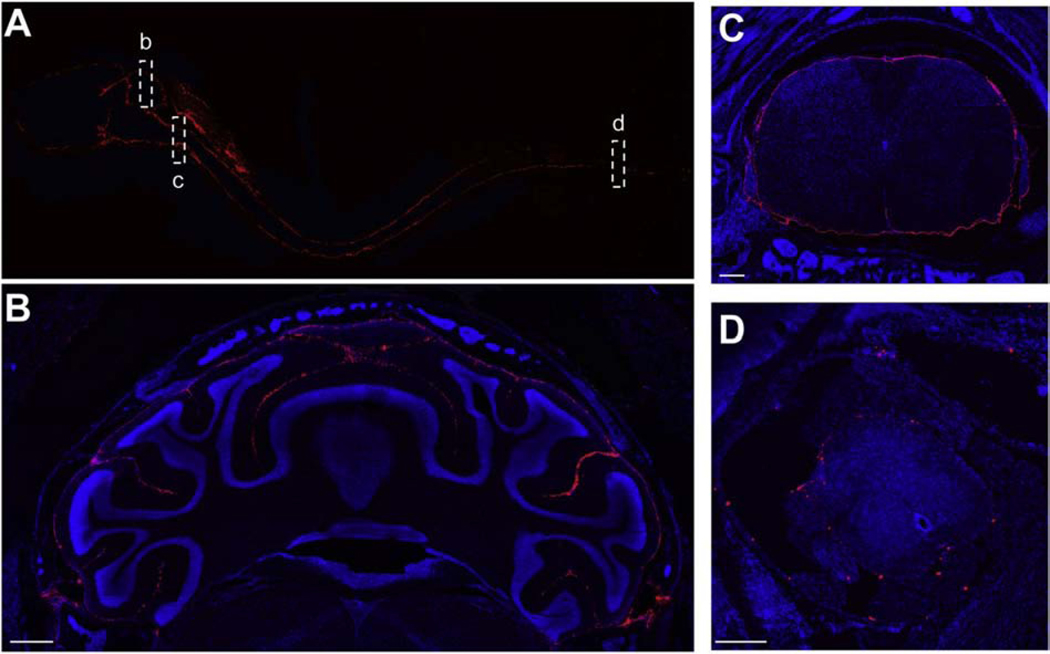
It is clear that even large colloids are mobile within the SAS when administered IT. In early work, Umbrain and colleagues examined the distribution of 99mTc-labeled multilamellar lipid vesicles (MLV) and small unilamellar lipid vesicles (SUV) administered by IT-L injection in healthy rats.[72] Both MLV and SUV distributed rapidly, reaching the entire neuraxis within minutes of injection. SUV, possessing a diameter of ~50nm, were observed to clear from thoracic spinal cord and head regions. MLV, with a volumetric median diameter of ~3.5um, cleared more slowly and were observed to redistribute from the spinal cord to the head over time. Recently, our group performed detailed characterization of the fate of fluorescent, polystyrene nanoparticles and microparticles in the subarachnoid space following ICM delivery to healthy mice (Figure 3).[73] Both nanoparticles and microparticles distributed rapidly across all surfaces of the brain and spinal cord and were well-retained in the SAS. Microparticles achieved surprising access to distal regions of the SAS, although their number density was sparse in comparison to nanoparticles, suggesting less opportunity for cellular interactions. Nanoparticles reached relatively stable patterns of distribution within 2 hours and persisted for at least three weeks after ICM administration. Nanoparticles were preferentially located on ventral surfaces compared to dorsal surfaces, did not penetrate the parenchyma, and were concentrated reproducibly in specific anatomical regions, including the cribiform plate, supracerebellar cistern, and pituitary recess. Intravital microscopy studies performed by Mestre and colleagues utilized similar polystyrene nanoparticles to directly measure CSF flow velocity within the perivascular space of arteries penetrating the pia mater in mice.[27] Their data demonstrate substantial mixing of perivascular fluid and CSF, which was driven by arterial pulsation and reduced in hypertension, emphasizing the role of peripheral circulation in CSF dynamics. Importantly, performing these measurements in live animals showed that the cross-sectional area of the perivascular space was 10-fold greater in living animals than in fixed tissues, an observation that solidifies the ability of IT administered colloids to deliver payloads to CSF-exposed tissues (and that also heightens the prospect of achieving parenchymal delivery of IT administered colloids, which will be discussed below). In still other studies, there is evidence that the fate of IT administered colloids follows the movement of CSF. Intraparenchymal injection of micellular (~20 nm) of lamotrigine resulted in greater distribution in the brain when compared to free drug and was accompanied by clearance that occurred predominantly through the cervical lymphatics.[74]
Figure 3. Mobility of nanoparticle systems in the SAS.
Solid, 100nm, fluorescently labeled polystyrene nanoparticles distribute throughout the neuroaxis to reach all CSF-exposed surfaces of the brain and spinal cord 2 hours following ICM administration in mice. (A) Delivery of nanoparticles is detected in all regions of the intact decalcified neuroaxis. Insets highlight extensive nanoparticle distribution in (B) sulci of the cerebellum, (C) cervical spinal cord, and (D) sacral spinal cord. Blue is DAPI and red is the stably labeled nanoparticle. Scale bar is 500um in (B) and 200um in (C) and (D). Figure reproduced from Householder, et al.[73]
Drugs that have been delivered from nanocarriers are observed to possess much longer half-lives within the CNS compared to free form. Early work focused on delivery of cytotoxic agents for cancer metastatsis, with payloads of interest including methotrexate,[69] cytarabine (also known as cytosine arabinoside, or Ara C),[75, 76] and 1,3-bis(2-chloroethyl)-2-nitrosourea (BCNU).[77] Cytarabine reached cytotoxic levels of drug in the CSF for several weeks after infusion when delivered from multilamellar liposomes, which highlights the potential utility of nanomedicine for sustaining payload delivery within the SAS. This approach was subsequently translated into a clinical formulation, DepoCyt®, which will be discussed in section 5.2. Improved drug delivery was also observed for methotrexate loaded liposomes in primates [69] and BCNU loaded liposomes in rodents, which were shown to be effective at treating meningeal gliomatosis in rodents.[77] Meninengeal gliomatosis was also effectively treated by ICM administration of liposomes loaded plasmid DNA followed by treatment with the antiviral agent ganciclovir.[78] Preclinical data on actual treatment of cancer metastasis in the SAS are otherwise sparse.
Additional examples of efficacy following IT administration can be found in neurovascular disease and neurotrauma. Microparticles composed of PLGA and encapsulating the calcium channel blocker nimodipine were engineered for attenuation of vasospasm following subarachnoid hemorrhage.[79] Nimodipine concentration in CSF was 1000x higher for IT compared to IV administration, and plasma PK was improved for both ICV and ICM injection. Nimodipine was detected in plasma for 21 days following IT injection in canines, emphasizing the ability of the SAS to serve as a sustained release depot for encapsulated small molecules. CSF delivery was slightly elevated for ICM versus ICV injection. These results could suggest differential clearance of either nimodipine or carrier as a function of IT injection location, although the data were too noisy for these differences to be considered statistically significant. Treatments were generally tolerated, barring a relatively mild granulomatous foreign body type reaction that was observed in the cerebral ventricles and subarachnoid space following carrier injection. Disruption of the ependymal lining and resulting inflammation was dose-dependent, reversible, and appeared to be worse for ICV compared to ICM administration. It is possible that reduced mechanical compliance of the lateral ventricles enhanced any deleterious effects of the injection for ventricular compared to cisternal injection, although this was not directly studied. Notably, a version of nimodipine loaded PLGA (EG-1962) was further developed for clinical application, which will be discussed below. The vasodilator, fasudil, is another drug that has been engineered for IT delivery. Fasudil was encapsulated within hydrogenated soybean phosphatidylcholine (HSPC) to treat vasospasm associated with subarachnoid hemorrhage. [80, 81]. Liposomal fasudil was demonstrated to be neuroprotective, confirming the potential utility of this approach to improve outcomes (which has been reviewed elsewhere[82]). Efficacy of liposomal fasudil was also observed in ischemia.[83] PLGA has also been developed for delivery of neuroregulin-1 in spinal cord injury.[84]
Given that CSF is known to clear through dorsal root ganglia (DRG), they are a logical target for IT delivery. Wang and colleagues implemented a significant series of studies to robustly establish the ability of synthetic and biological particles to mediate gene delivery to DRG, demonstrating the effective use of polyethylenimine (PEI), lipofectamine 2000, cyclodextrin-PEI conjugates, adeno-associated viral vectors (AAv), and baculovirus vectors by the IT route in rodents. [85–89] Early work showed that IT-L injection of PEI/DNA complexes induced 40-fold higher transgene expression than IT-L administration of naked DNA.[85] Transgene expression was detectable at low levels for 8 weeks following a single injection, however, repeat dosing experiments revealed that PEI/DNA complexes (and not PEI or DNA individually) produced cellular apoptosis that was associated with substantial attenuation of transgene expression with subsequent doses. Attachment of poly(ethylene glycol) (PEG) eliminated the attenuation of transgene expression and improved biocompatibility of the system. A separate study explored in detail the role of PEG engraftment for enhancing gene delivery from PEI/DNA complexes.[86] The presence of PEG alone was sufficient to induce dramatic enhancements in transgene expression in vivo, although the actual degree of PEG engraftment did not appear to have a significant influence on expression levels. Lower PEG engraftment produced other potentially favorable nanoparticle properties, including reduced size, decreased aggregation, and decreased interactions with serum protein. Next, synthetic (PEI, liposomal) and biological (AAv, baculovirus) carriers were loaded with DNA encoding either luciferase or nerve growth factor (NGF). These constructs were administered via IT-L injection, with the goal of comparing efficacy of the different systems and enhancing regeneration of transected sciatic nerves in rats.[87] All carriers were observed to either localize with or induce gene expression in DRG. PEI/DNA produced slightly higher transgene expression than lipofectamine, yielding luciferase expression levels that were comparable to both viral systems. Delivery of PEI/DNA complexes encoding NGF ultimately improved peripheral nerve regeneration. Finally, development of a biodegradable cyclodextrin-PEI yielded possible improvements in biocompatibility with equivalent transgene expression when compared to nondegradable PEI following IT-L administration.[88] PLGA particles loaded with brain derived neurotrophic factor (BDNF) were also shown to promote repair and function of DRG neurons in a canine model of cauda equina syndrome.[90]
Liposomal gene delivery to DRG has been studied in Friedreich’s Ataxia,[91] where is was demonstrated that ICV administration in mice could achieve effective gene expression across the brain. Although the spinal cord was not imaged, it is interesting to note in the original report that signal is clearly detectable along the back and shoulders of mice, including in non-CNS tissues. Whether this represents local movement of liposomes through DRG into innervated muscle or systemic clearance to circulation and reuptake in peripheral tissues remains to be determined. Gene delivery to CSF exposed surfaces has also been shown to be effective in rodent models of traumatic brain injury (TBI) and ischemia.[92, 93]
5.1.2. Locoregional delivery
IT or epidural injection of medicine for locoregional control of pain or induction of anesthesia is an approach that has been used successfully in humans since the late 1800s. Much of this success has been extended to incorporate nanomedicine, with the expectation that encapsulation will prolong or heighten locoregional exposure of payload in the CNS while minimizing undesired off-target effects. Extensive preclinical data support this expectation. Lipid or polymer encapsulated small molecules, including bupivacaine, morphine, alfentanil, ketamine, and ketoprofen, have been shown to possess longer CSF half-lives, reduced toxicity, and heightened efficacy compared to free drug counterparts when administered through the IT-L route. IV and ICM administration are, unsurprisingly, less common for control of pain. Locoregional delivery via IT administration has also proved useful for delivery of gene therapeutics.
Differentiated pharmacokinetics and favorable toxicity profiles have been observed for several liposome encapsulated opioids. This was demonstrated rigorously by Joshi and colleagues for liposomal delivery of bupivacaine in canines.[94] Bupivacaine possessed a much longer half-life when delivered from liposomes compared to free form via either epidural or IT routes. There was also a lower incidence of bupivacaine-associated toxicity, even when a higher dose of liposomal bupivacaine was administered compared to free bupivacaine. Interestingly, co-administration of liposomal bupivacaine with lidocaine and epinephrine produced a higher incidence of toxicity, which the authors speculate is due to the known ability of lidocaine to produce premature release of bupivacaine from liposomes. The introduction of toxicity via specific interaction of an adjuvant treatment with the carrier itself has not been broadly reported in the field of drug delivery and merits further study. In other studies, Valdes and colleagues demonstrated that minocycline and ciprofloxacin loaded solid lipid nanoparticles (SLNs) were effective at controlling pain in a model of autoimmune induced pain (arthritis), which they proposed was due to direct action on resident glia.[95] Liposomal morphine,[96, 97] liposomal alfentanil,[98, 99] liposomal neostigmine,[100] and PLGA systems loaded with baclofen, ketamine, hydromorphone, and bupivacaine [101–106] have also been developed for IT administration. These studies provide consistent evidence for improved PK, reduced toxicity, and prolonged efficacy when molecules for pain control are delivered in encapsulated versus free form.
Some evidence suggests that composition of the carrier plays a major role in governing analgesic and anesthetic effects following IT drug delivery. Whether this is due solely to the differential release of encapsulated material or also to differential distribution of the carrier as a function of composition is not yet clear. For example, Nishiyama, et al., studied antinocioceptive efficacy of morphine delivered by isomeric phospholipids, either L-dipalmitoylphosphatidylcholine (L-DPPC) or D-dipalmitoylphosphatidylcholine (D-DPPC), compared to freely administered morphine.[96] Duration of antinocioceptive efficacy was longer for both L-DPPC and D-DPPC encapsulated morphine when compared to freely administered morphine. Interestingly, greater activity and decreased toxicity were observed for D-DPPC compared to L-DPPC encapsulated morphine. These differences were speculated to be a function of differential hydrolysis (i.e., slower degradation of D-DPPC compared to L-DPPC), although further studies would be needed to directly probe drug release in vivo.
Specific injection location even within a lumbar region is likely to be a significant parameter governing nanomedicine distribution and activity following IT administration. Epidurally administered molecules must diffuse through meningeal layers to reach the CSF; thus, intrathecal administration of analgesics and anesthetics is generally expected to be more potent than epidural administration.[107] Indeed, entrapment of liposomes within the epidural space corresponding to a lower free bupivacaine compared to IT administration in canines.[94] Differential plasma PK for carrier payload was also observed when ropivacaine loaded PLGA was administered by IT-L versus epidural injection in sheep.[108]
Encapsulation of analgesic and anesthetics within nanocarriers cannot be predicted to be uniformly beneficial across all possible measures of drug performance. For example, liposomal alfentanil produced an increase in the duration of spinal antinocioception, but it did not alter the peak intensity or timing of analgesic effects when compared to freely administered alfentanil.[98] Bupivacaine achieved effective motor blockade when it was delivered from a fast-releasing formulation, but it was not efficacious when delivered from a more slowly releasing formulation (which produced effectively lower bioavailability).[105] Significantly, pain-related carrier toxicity termed touch-evoked agitation was observed for IT administered phospholipid emulsions and liposomes.[109] The degree of rodent vocalization upon touch as well as spontaneous immobility behavior was speculated to represent an allodynia response. The intensity of touch-evoked agitation corresponded to lipid composition of the substance injected, and it was observed for both emulsion and liposomal formulations. Touch-evoked agitation appeared to be related to phase transition temperature of the constituent lipid, and it could be blocked via administration of a phospholipase inhibitor. These studies thus suggested that the toxicity was produced via differential exposure of the CNS to a hydrolysis product of the injected phospholipids.
The majority of intrathecal anesthetic and analgesic nanomedicines have utilized lipid carriers, presumably due to the expectation of easier clinical translation compared to more experimental or recently developed materials. However, other potential carriers have also been tried. Both alginate/chitosan composite nanoparticles[110] and maltosyl-beta-cyclodextrin[111] yielded improved delivery and efficacy of bupivacaine. Efficacy has also been observed for ketoprofen delivered from tyrosine-derived polymer microparticles,[112], ketorolac conjugated superparamagnetic iron oxide nanoparticles,[113] and curcumin loaded PLGA. [114]
Gene delivery for treatment of pain includes the use of PLGA,[115] chitosan complexes,[116] lipid-silica protocells,[117] and liposomes.[118–120] Each of these reports emphasize the general feasibility of locoregional drug delivery via IT administration of nanomedicine carrying large payloads, although carrier composition appears to play an important role in mediating the spatial distribution of gene expression following IT delivery. DNA contained in lipid-silica protocell nanoparticles composed of DOTAP were localized near the lumbar injection site both initially and long after IT-L administration, whereas DNA contained in lipid-silica protocol nanoparticles composed of DOPC were observed to experience substantial redistribution over time, reaching a high concentration at caudal locations in the spinal cord 8 weeks after IT-L administration in rodents.[117] DNA delivery to the brain was observed for DOPC but not DOTAP lipid-silica protocell nanoparticles. These data suggest better mobility for DOPC compared to DOTAP, although whether this is due to differences in CSF clearance, tissue penetration, or transfection efficiency in vivo remains to be determined. In all cases, protocell nanoparticle distribution was confined to the subarachnoid space, pia mater, and arachnoid mater; no parenchyma penetration was observed. PLGA microparticles were similarly confined to the SAS in rodents.[115]
5.1.3. Delivery to the parenchyma
Historically, the CSF was often viewed as being produced within the ventricles, contained in the SAS, and cleared directly to the periphery. IT administered molecules were presumed to remain confined to the ventricles and SAS, or, at most, to reach only superficial surfaces of the brain and spinal cord. However, deepened understanding of dynamic CSF exchange with perivascular, interstitial, and lymphatic fluids leaves open the possibility that either colloidal carrier or payload could move with CSF into the parenchyma. Here, we will address the question of whether IT administered colloids can traffic to deep parenchymal target and overview the use of IT nanomedicine in disease models that are expected to impact parenchyma targets. It is important to note that although the diseases and processes addressed in this section affect deeper tissue regions of the brain or spinal cord, it is possible that some of the therapeutic effects are achieved via unknown mechanisms involving delivery to superficial tissue targets or premature release of the payload.
Whether IT administered colloids reach deep parenchymal structures in significant quantities remains an open question that likely depends both on the properties of the colloid and the definition of “significant.” Sumner and colleagues showed that ICV administered, micron-sized particles of iron oxide particles could be found in the rostral migratory stream of rats one week after administration, although the mechanism of delivery is presumed to be cellular in origin.[121] Particles were observed to specifically labeled 30% of neural progenitor cells in the subventricular zone. Because co-administration of an anti-mitotic agent eliminated MRI signal far from the site of administration, distal hypointense MRI signals were concluded to be due to migration of labeled progenitors, not freely moving particles. Labeling of neural progenitor cells was also achieved by anti-CD15 modified superparamagnetic iron oxide nanoparticles in rats.[122] Both studies concluded that iron oxide particles did not reach the parenchyma freely, achieving parenchymal delivery only via movement of cells that had internalized the colloidal systems. Similarly, Muldoon and colleagues administered 20nm dextran coated iron oxide via ICV injection to demonstrate effective ventricular distribution and clearance of particles to deep cervical lymph nodes and found no evidence for parenchymal delivery in rats.[123] Contrasting data can be found in other reports. Liu and colleagues observed significant parenchymal penetration of similarly sized, oligonucleotide modified iron oxide following ICV delivery in mice.[124] Sparse parenchymal delivery of 120nm, targeted, DNA-complexed iron oxide was also observed in the spinal cord following IT-L administration in rats.[125] Liposome constituents in an equimolar ratio to encapsulated drug (suggesting that the carrier could be intact) were detected in deep parenchymal targets in primates.[69]
Rigorous evidence supports the expectation that ultra-small colloids (i.e., sub 10nm particles) can traffic from CSF to the parenchymal tissue near to CSF, though precise distribution depends on properties of the colloid. Quantum dots were definitively observed in periventricular tissue following ICV administration in rats, providing relatively definitive evidence that ultra-small colloids can traffic at least several hundred microns into tissue.[126] Importantly, the spatial distribution of these quantum dots suggested movement beyond perivascular spaces and into the parenchyma. Some data suggest that surface properties may play an important role in this process. In mice, ultra-small G4 dendrimers were shown to be capable of bypassing the ependymal barrier following IT administrations, whereas ultra-small G4-C12 dendrimers remained enriched at the ependymal surface and did not enter the parenchyma.[127] Following direct injection of these same particle systems into the parenchyma, G4 dendrimers were observed to diffuse while G4-G12 dendrimers did not. Thus, the mechanism blocking passage of G4–12 dendrimers across the ependymal surface was suggested to be related to restricted diffusion (i.e., binding). In other studies, CSF administered dendrimers were demonstrated to localize with microglia in the parenchyma of the brain and spinal cord in mice and rabbits,[128, 129] which raises the important point that some parenchymal delivery could be due to cell internalization and trafficking even for very small colloids. Substantial delivery of citrate-coated iron oxide (VSOP-C184, which has an effective diameter of ~9nm) was observed in periventricular tissue following ICV administration in mice.[130] Although VSOP-C184 was not detected in deep parenchymal targets, the study authors raise detection sensitivity and short study duration as significant confounders that could have prevented detection of a parenchymal signal.
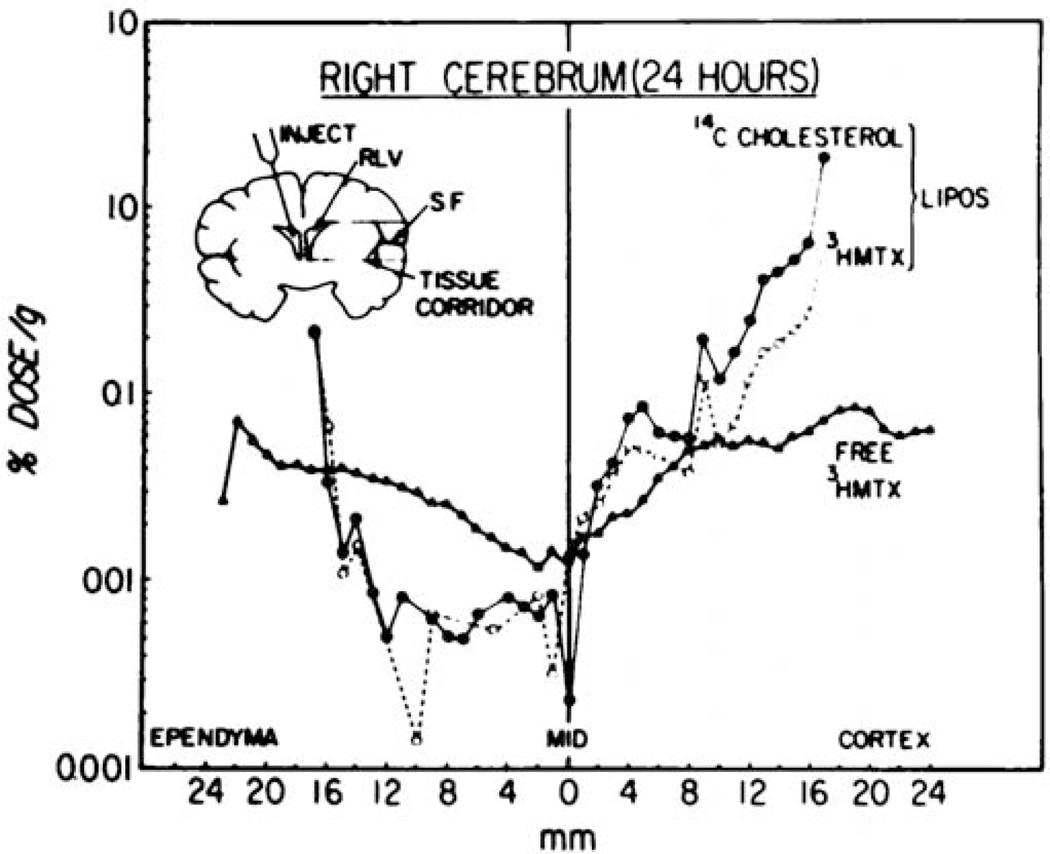
A variety of reports confirm that colloid payloads, including lipids, small molecules, and genes, do reach deep parenchymal targets, often to a surprising extent. Kimelberg and colleagues reported the first distribution results from IT experiments, measuring regional concentration of both liposomes and encapsulated methotrexate following ICV administration in cynomolgus monkeys.[69] These fascinating and rigorous data demonstrate that freely administered methotrexate achieved a more uniform distribution between ependymal and cortical surfaces compared to liposome encapsulated methotrexate, although the absolute concentration of methotrexate at CSF-exposed surfaces was higher for liposome delivery compared to administration of methotrexate in free form (Figure 4). This surface enrichment suggests a more sustained presence of methotrexate in the CSF when delivered from liposomes compared to free form. Dual radiolabeling enabled direct measurement of both payload and carrier concentration in individual subjects. Methotrexate and liposome constituents were detected in a 1:1 ratio at early times, with methotrexate concentration increasing relative to lipid concentration after 24 hours, which suggests eventual clearance of the lipid carrier. Interestingly, methotrexate and liposome constituents reached a 1:1 ratio close to the ventricular surface (near to the injection site), whereas methotrexate was observed at a lower concentration than liposome constituents along the cortical surface (far from the injection site). It is interesting to speculate that these differences could be due to premature release of methotrexate as liposomes traverse from the ventricles to the SAS of the cortical surface. Parenchymal delivery of a colloidal carrier was also reported for poly(lysine) dendrimers, which were shown to distribute throughout the cortex and hippocampus following ICV administration in rats.[131] Encapsulated payloads were observed to distribute rapidly throughout the neuroaxis for both dolichol loaded liposomes and gadodiamide loaded phospholipid bicosomes.[132, 133] Parenchymal delivery of a small, hydrophobic carbocyanine dye (1,1’-Dioctadecyl-3,3,3’,3’-Tetramethylindodicarbocyanine, 4-Chlorobenzenesulfonate, DiD) was observed to be a function of lipid composition for solid nanoparticles delivered ICV to mice.[134] Because DiD was not covalently bound to the carrier, it is difficult to ascertain whether the different distributions are due to differential mobility of the carrier or differential release of the payload from the carrier prior to tissue entry.
Figure 4. Differential fate of payload and carrier.
Free methotrexate and liposome encapsulated methotrexate experience distinct distributions within the brain following ICV administration in nonhuman primates. Cholesterol was labeled with 14C and methotrexate was labeled with 3H for quantitative scintography. A 0.5 × 0.8cm “tissue corridor” was sliced into 0.5 or 1mm sections to generate an effective line profile drug or carrier distribution from the ependymal to cortical surfaces. Methotrexate and cholesterol share a similar distribution in tissues close to the injection site, with more distal locations suggesting some release and clearance. These data are described in greater depth in the text of Section 5.1.3. Figure reproduced from Kimelberg, et al.[69]
Some of the compelling evidence for parenchymal delivery of nanomedicine encapsulated payloads comes from the realm of gene therapy. Cortical and periventricular transfection, including within the subventricular zone, has been reported across a wide range of studies for IT administered PEI complexes, polyplexes, and liposomes.[135–142] When DNA encoding lacZ was loaded into liposomes and delivered ICM to rhesus macaque monkeys, gene expression within the parenchyma was widespread across the entire CNS, particularly in tissues near to CSF but also including deep targets in the hippocampus and dentate nucleus.[143] Gene delivery reached 30–60% transfection efficiency in some regions. Transfection efficiency and overall distribution were remarkably higher for ICM administration when directly compared to CED for the same system. Meuli-Simmen and colleagues demonstrated that widespread, infusion volume-dependent gene transfection could be achieved via both ICM and IT-L routes; an infusion volume of 20uL was determined to be optimal compared to 5, 10, or 50uL, with transfection persisting for several weeks after administration of liposomes.[144] These studies highlight that injection protocol is a significant factor governing efficacy following IT administration and further establish a. possible role for nanomedicine delivery to deep parenchymal targets via the IT route.
Surface properties are also expected to be important following IT nanomedicine delivery. Polyplex charge was observed mediate periventricular gene delivery via the ICV route.[142] Gene delivery in the spinal cord was observed to be a function of charge density, with a direct, enhancing influence of primary amino groups.[145] Some work has addressed the development of cellular or intracellular targeting. For example, bulk transfection of the brain has also been reported for ICV administration of N-(2-hydroxypropyl)methacrylamide (HPMA)-oligolysine copolymers that were modified with the bee venom-derived, membrane-lytic peptide melittin.[146] Other cationic polymer systems engineered for endosomal escape have demonstrated efficient gene delivery to bulk brain following ICV administration.[147] Direct comparison of CED with ICM injection established that transfection was dramatically improved by the ICM route.[148]
Recent studies conducted by Helmschrodt and colleagues demonstrate impressive delivery of fluorescently labeled siRNA and effective gene silencing to deep parenchymal targets for a PEI carrier administered ICV to mice; [149] these data demonstrate impressive penetration of fluorescently labeled siRNA into the parenchyma (Figure 5). Widespread parenchymal delivery has also been reported for mRNA loaded liposomes, which were shown to protect their payload from degradation in CSF.[150] Parenchymal transfection was also clearly demonstrated following administration of 30nm organically modified silica (ORMOSIL) via ICV. [151] Interestingly, gene transfection efficiency was observed to be a function of shape in vivo for PEI-PEG micelles, with rod-shaped particles outperforming spherical and worm-shaped particles.[152] Collectively, these works affirm a role for IT nanomedicine delivery to the parenchyma and raise a variety of new engineering opportunities, including consideration of surface charge, shape, and attachment of targeting molecules to colloidal carriers.
Figure 5. Parenchymal delivery of nanoscale colloids.
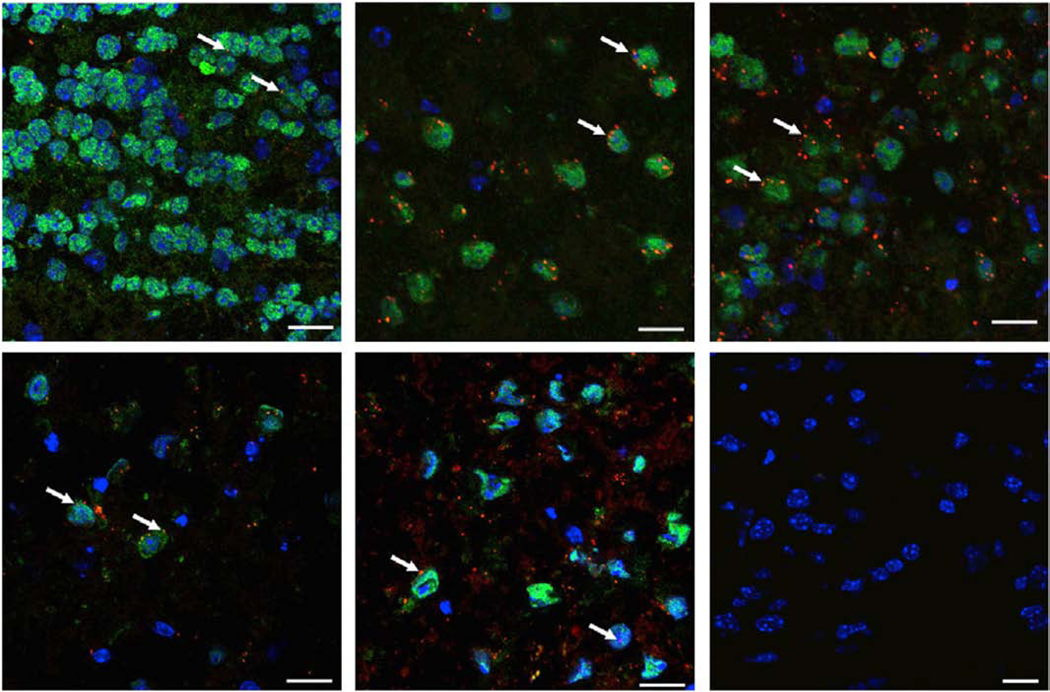
siRNA/PEI complexes achieve widespread parenchymal penetration in multiple regions of the CNS following ICV administration in mice. Blue is DAPI, green is NeuN, and red is Alexa 647-labeled siRNA complexed with PEI. White arrowheads highlight PEI-siRNA granules. Negative control shows absence of signal following administration of unlabeled PEI-siRNA. Scale bar is 20um. Figure reproduced and adapted slightly from Helmschrodt, et al.[149]
IT nanomedicine has been useful for the treatment of a variety of diseases that affect deep parenchymal targets. PEI-PEG micelles were used to deliver siRNA targeting BACE and APP via ICV administration in a murine model of Alzheimer’s.[152] Even though higher N/P ratios (10 and 20) were more effective in vitro, significant toxicity was observed following ICV administration that prevented further study. In contrast, infusion of micelles with a lower N/P ratio (5) was observed to be tolerated. These micelles were determined to be biocompatible even after a week of infusion into the ventricles, and gene knockdown was observed in periventricular, brainstem, and spinal cord tissues. In other studies, organically modified silican nanoparticles (ORMOSIL) were used to deliver genes via ICV administration to generate a model of Huntington’s Disease.[153] Abnormal pathology and inflammation was observed throughout the brain, and this was accompanied by the predicted motor impairments.
Both liposomes and cyclodextrin based systems have also been developed for relatively nonspecific purposes in behavior. These studies merit review but do not directly address the question of carrier localization, which is an important limitation. Efficacy in behavioral models has been demonstrated for IT delivery of carrier encapsulated small molecules, including cyclodextrin based systems for delivery of testosterone, pregnanolone, estrogen, other steroids, and fatty acids,[154–158] and liposomes for small molecules and genes.[159–161] These studies support the expectation that molecules delivered from IT administered carriers could impact processes regulated by parenchymal targets, but additional work will be needed to establish the mechanisms by which these effects were achieved.
5.1.4. Delivery within the CSF
Immune cells within the CNS help maintain the BBB, regulate host response to pathogens, and form a major component of inflammation and tissue repair following injury. Not surprisingly, the CSF serves as an important medium through which resident and peripheral immune cells maintain neuroimmunity. Viral, bacterial, and fungal infections can occur within any compartment of the CNS, although they most commonly affect CSF-exposed surfaces such as the leptomeninges. Thus, delivery of active agents to the CSF to control infection or modulate the immune system is a highly relevant approach that has been studied extensively for delivery of freely administered compounds to control infectious disease. Use of nanomedicine for this purpose is less common, which could be because freely administered agents are relatively effective. Clodronate is a first-generation bisphosphonate drug that induces apoptosis in phagocytic cells. Once encapsulated in liposomes, it is highly effective at achieving macrophage depletion.[162] Although clodronate liposomes are most commonly administered by parenteral routes, they have been shown to be effective at depleting CNS macrophages when administered by IT-L, ICV, and ICM injection.[163–166] Polfliet and colleagues observed both perivascular and meningeal depletion of macrophages following ICV injection, and ICV administration enabled more specific and longer lived depletion of macrophages in the CNS compared to the periphery.[163] Antibiotic treatment of infection in the CSF has also been achieved. IT administration of liposomal amphotericine B was reported to be effective at treating both meningeal cryptococcosis and Cryptococcus neoformans infection.[167, 168] In both of these studies, IT administration was more effective at controlling infection than IV administration. Cationic polymers have also been demonstrated to sustain CSF gene expression in the CSF following ICM administration.[169] Interestingly, nanomicelles formed from the cationic polymers were shown to be more effective at maintaining gene expression than when linear polymers were complexed directly with pDNA.
5.2. Clinical IT nanomedicine delivery
5.2.1. Liposomal Cytarabine (DepoCyt®)
Cytarabine is an antimetabolic cancer agent that is metabolized and incorporated into cellular DNA during S phase, producing cell apoptosis by inhibition of DNA synthesis. It is used in many cancer applications, often as a high dose IT therapeutic for the treatment of CNS neoplasms.[170] Encapsulation of cytarabine into multi-lamellar liposomes produces a foam-like nanomaterial capable of sustaining cytarabine release in physiological environments.[171] This system, known as DepoCyt® and often referred to as liposomal cytarabine, was approved by the FDA in 1999 for IT treatment of Lymphomatous Meningitis via IT administration. IT DepoCyt® has been shown to be effective across a variety of cancer types in both prophylactic and therapeutic settings, and cytarabine pharmacokinetics are improved when the drug is delivered via from DepoCyt® instead of as a free drug via the IT route. Cytotoxic levels of cytarabine can be maintained in the CSF for up to 7–14 days following ICM injection and close to 7 days following IT lumbar injection of DepoCyt®.[172–175]. By comparison, the concentration of freely administered cytarabine falls below cytotoxic levels less than 24 hours after CSF administration.[171] The half-life of liposomal cytarabine in the CSF has been measured to be 43 hours [174, 175] which is significantly longer than the reported half-life of 3.4 hours for freely administered cytarabine.[176] This extended release profile allows lower maximal concentrations of drug exposure while prolonging the potential S-phase targeted cytotoxicity of cytarabine, lowering the number of drug injections necessary.[170, 177] It is important to note, as a multilamellar liposome, DepoCyt® possesses a foam-like structure whose primary function is to serve as a depot for sustained drug release; it has not been specifically engineered for mobility within the SAS or parenchyma.
Liposomal encapsulation of cytarabine appears to produce distinct toxicities compared to free drug. The most common toxicities reported following IT DepoCyt® include reversible, low-moderate grade headaches, nausea, and vomiting. Chemical arachnoiditis has been recorded as a rare but severe side effect among pediatric and adult populations but was found to be mitigated by the concomitant use of corticosteroids (e.g. dexamethasone).[172, 178–183] A single case of ventriculomeningitis leading to death after DepoCyt® treatment occurred, which is the most severe treatment-related adverse event reported.[184] This event may have been related to the utilization of a lower than recommended steroid dose. Another rare but serious adverse event observed was cauda equina syndrome, which causes symptoms such as lower extremity paralysis and incontinence. [185–190] The frequency of severe side effects from liposomal cytarabine could increase with increasing number of injections,[191] while there were conflicting reports as to whether mild-moderate side effects occurred dependently or independently of cumulative dose number[182, 191] Recent studies comparing outcomes of IT DepoCyt® with free drug IT chemotherapies (methotrexate or Triple Intrathecal Therapy) suggest that phrophylactic treatment with liposomal cytarabine results in similar or possibly reduced relapse rates and improved survival compared to free drug IT chemotherapies,[192, 193] whereas liposomal cytarabine as first-line treatment shows only similar outcomes to free drug IT chemotherapies.[194, 195] Taken in sum, clinical studies demonstrate that DepoCyt® enhances the pharmacokinetic profile of cytarabine, resulting in similar outcomes compared to free drug when administered by the IT route. DepoCyt® was discontinued for unspecified manufacturing problems in 2017. Ultimately, the most significant advantage of DepoCyt® was not improved efficacy but the reduced number of IT injections required to achieve efficacy.
5.2.2. Hydroxypropyl-ß-Cyclodextrin (HP-ß-CD)
Cyclodextrins are cyclic oligosaccharides with a hydrophilic exterior that are able to solubilize small, hydrophobic molecules within their hydrophobic core. Cyclodextrins do not provide controlled release but do modify the expected bioavailability and distribution of solubilized agents. Cyclodextrins can also exert biological effects in absence of drug payload. In humans, treatment with HP-β-CD has been observed to redistribute cholesterol in the CNS, as seen by mild to significant increases in plasma and CSF levels of 24-(S)-hydroxycholesterol post-administration, a cholesterol made almost exclusively in the CNS.[196–199] HP-β-CD has been used clinically to treat Niemann-Pick Type C disease, where it has been shown robustly to stabilize progression and often improve neurologic symptoms in pediatric populations.[198–201] In the single adult study utilizing HP-β-CD for the treatment of Niemann Pick’s, no adverse events were observed in one patient, while the other twice experienced chemical arachnoiditis after administration. [202] No therapeutic benefit was observed in these adults. Adverse effects due to HP-β-CD among pediatric patients included headache, fatigue, unsteadiness, and in almost all patients, ototoxicity. Three out of four pediatric studies reported that 100% of patients experienced some degree of ototoxicity, which appeared to be dose-dependent and resulted in mid- to high-frequency hearing loss. Importantly, the beneficial stabilization and even improvement of Niemann Pick’s by HP-β-CD outweighed the hearing loss, as these ototoxic events in children can be mitigated by the use of hearing aids.
5.2.3. Liposomal Bupivacaine (EXPAREL®)
Bupivacaine is a local anesthetic in the amino amide family that works by reversibly blocking neuronal Na+ channels, decreasing sensory neural output and deadening pain.[203] It provides one of the most effective and longest actions upon its target out of any of the other - caine anesthetics.[204] Liposomal Bupivacaine (EXPAREL®) is a formulation of Bupivacaine encapsulated in DepoFoam Multivesicular liposome technology, an approach that is comparable to DepoCyt®, discussed above. EXPAREL® is approved by the FDA for postoperative pain management after hemorrhoidectomies and bunionectomies.[205] While analgesic lifetime varies widely by surgical location and by route of administration, liposomal bupivacaine has been shown in a generalizable fashion to possess superior extended release profiles over the free drug.[205] One clinical study showed extended analgesic actions of epidurally-administered liposomal bupivacaine at 6.25 hours post-surgery as opposed to 3.2 hours post-surgery with the use of free bupivacaine.[206] Patients undergoing abdominal aortic surgery experienced pain relief of 10.6 hours upon use of the liposomal formulation, which was much longer than the average pain relief of 2.4 hours upon use of free bupivacaine. This increase in analgesia lifetime can decrease the need for opioids to manage patients’ postoperative pain.[205–207] The most common side effects from liposomal bupivacaine are nausea, vomiting, constipation, pyrexia, dizziness, and headache. More severe cardiac side effects have also been reported. These have been shown to be similar in type and severity to the side effects and toxicities of free bupivacaine.[205, 208]
5.2.4. PLGA encapsulated Nimodipine (EG-1962)
Nimodipine is a small, lipophilic compound that is poorly water souble. As a calcium channel blocker, it is potently capable of reducing vasospasm and FDA-approved for the treatment of subarachnoid hemorrhage.[79] Building off of favorable preclinical studies, nimodipine loaded PLGA microparticles produced by Edge Therapeutics, or EG-1962, have undergone clinical testing for IT administration. The Nimodipine Microparticles to Enhance Recovery While Reducing Toxicity After Subarachnoid Hemorrhage (NEWTON) and NEWTON-2 studies studied tolerability, safety, and efficacy of EG-1962 in aneurysmal subarachnoid hemorrhage.[209–212] Phase 1/2a results demonstrated safety and efficacy via reduction in delayed cerebral ischemia. However, the trials were recently terminated following the data monitoring committee (DMC) determination that the trial was unlikely to meet its primary endpoint.
6. Summary and future directions
This review focuses on the use of colloidal nanomedicine for IT drug delivery. In Section 3, we discuss current understanding of CSF distribution within the CNS, which involves circulation through the SAS, exchange with interstitial fluid, and outflow pathways that include XXX. Published data described in Section 5.1.1 support the expectation that both colloids and encapsulated molecules can move through the SAS to reach cellular targets and influence disease processes that are near to the surfaces of the brain and spinal cord. Compared to freely administered molecules, IT administered nanomedicines are well-retained within the CNS, and encapsulated payloads experience slower clearance and mixing within the CSF, which can enhance tissue exposure (Sections 5.1.1 and 5.1.2). Size, surface properties, and shape have all been observed to influence the fate of CNS colloids (Section 5.1.3).
Encapsulation of small molecules within colloidal delivery systems offers a number of advantages. The majority of the IT delivery systems described here report improvements in drug pharmacokinetics within the CNS, reduced toxicity, and enhanced efficacy. These benefits hold true across a multitude of disease models that affect different tissue targets within the CNS. Some of the greatest successes in preclinical studies are with gene delivery, where there is robust evidence that IT administered therapeutics can induce or suppress gene expression across multiple tissues within the CNS and in tissues that are far from the injection site or located within the parenchyma. IT gene therapies have not yet made it into the clinic, however. Translational and clinical work (Section 5.2) has primarily focused on the development of nanomedicine for treatment of pain, neurodegeneration, and cancer. The available clinical data supports safety of these approaches, although dramatic improvements in therapeutic outcome remain to be seen.
The available evidence suggests that molecules clear from the CNS through routes that are shared with CSF following IT administration. This includes movement along perineural and perivascular spaces, lymphatics, and the nasal mucosa. The question of whether colloidal carriers can reach deep parenchymal targets following IT administration is addressed in Section 5.1.3, and it is clear that the field has not matured sufficiently to resolve this question in a truly generalizable manner. The evidence described to-date suggests that ultra-small colloids, hyperbranched polymers, linear polymers, complexed genes, and low molecular weight carrier constituents can enter the parenchyma. In contrast, solid nanoparticles and microparticles do not appear to be as capable of moving from CSF into the parenchyma. Even within similar classes of agents, some of the localization reports reach differing conclusions regarding parenchymal fate. Presumably these distinct conclusions reflect both differing capability of different kinds of nanomedicine as well as differing techniques utilized to track delivery. For example, although size has been identified as a possible determinant of parenchymal fate, the role of particle deformability has not yet been addressed. Considering that the extracellular space in the brain is estimated to be 40–60nm in width,[213] and that particles as large as 80nm have been demonstrated to diffuse through the brain parenchyma when binding is low,[214] we expect that some colloids should be capable of moving from the CSF into or through parenchymal tissues. Given the problem of detection sensitivity, it is more likely that positive results represent true positives, although the actual quantity delivered may be relatively low. Thus, one major conclusion from this review is that additional work will be necessary to develop a more comprehensive understanding of how to best engineer colloids for deep parenchymal delivery.
Two important points of discussion arise for interpretation of original research in this field. First, some studies that purport parenchymal delivery of the colloid are in fact tracking the payload, not the carrier itself. We have demonstrated that conflation of carrier and payload fate can lead to misinterpretation of drug delivery mechanisms.[215] Second, internalization of colloids by mobile cells (e.g., progenitor or resident immune cells, either in the CSF or close to CSF-exposed surfaces) or degradation/instability of the particle are alternative mechanisms by which covalently labeled materials could reach deep parenchymal targets. These alternative explanations are difficult to exclude for many of the systems here using conventional approaches for tracking delivery of the constituent materials. Advanced imaging techniques such as fluorescence resonance energy transfer (FRET) that enable determination of whether colloidal systems remain intact could be useful for mechanistic work that would untangle the distinct delivery mechanisms for carrier versus payload. Given known artifacts for fixation of tissues (and the technical challenge of actually accessing the CNS, undisturbed, through bone), in situ and live imaging techniques may be particularly informative approaches for tracking carrier delivery.[216]
Toward the goal of developing systems that can reach deep parenchymal targets, noninvasive stimuli such as magnetic fields or ultrasound may be useful. External magnetic fields have already been shown to enable manipulation of the cellular level distribution of ferromagnetic materials in tissue.[217] IT magnetic targeting was demonstrated in a tissue mimic of the human spine, whereby nanoparticles were observed to accumulate at an almost 10x higher concentration in the region of interest following activation of the magnetic field.[218] Song and colleagues developed cationic iron oxide / DNA complexes that were surface modified with the cell penetrating peptide Tat for IT-L delivery in rats. [125] Application of a magnetic field following complex administration produced a shift in gene expression to tissues far from the injection location. Other reports of magnetic manipulation of colloids in the CSF include delivery to both superficial targets and deep parenchymal tissue, including for therapeutic purposes.[113, 219] Imaging guided focused ultrasound (FUS), administered in combination with administration of microbubbles, is one approach for achieving disruption of the BBB and enhanced mixing of fluids, which would be expected to impact colloid distribution following IT administration. FUS was recently shown to enable significantly increased parenchymal transport of polymeric nanoparticles in the living brain.[220] Tan and colleagues demonstrated that application of FUS with microbubbles could enhance significantly enhance gene delivery to the subventricular zone following ICV administration of polyplexes.
Another opportunity for enhancing tissue distribution for IT drug delivery could be found in manipulation of underlying physiological processes that govern CSF movement. Recent studies show that the volume of interstitial space within the parenchyma is increased 40–60% during sleep, which reduces resistance to convective flow and enhances delivery and clearance of macromolecules.[221] Hyperosmolar infusion has also been shown to enhance the distribution of IT administered antibodies.[222] Additional work is needed to determine whether manipulation of physiology could be used to improve efficacy of IT nanomedicine.
Although IT administration of nanomedicine is an approach that has been used in various contexts for several decades, work remains to robustly define the properties that will enable an IT administered nanomedicine to reach specific tissue targets in the CNS. We can identify several gaps that represent opportunities to advance our understanding of how to engineer nanomedicine for IT drug delivery. First, comparison of the fate or efficacy of multiple carrier within a single experimental setting is very rare. Such comparisons are useful for determining what differences in distribution or activity are due to measurement approach versus the properties of the colloid itself. We suggest that the field as a whole would benefit from direct comparison of different carrier systems. Second, rigorous study of IT drug delivery requires that careful attention be paid to the possibly different fates of the carrier and payload. Understanding how carriers release their payloads in vivo, once exposed to cells and tissues (versus simplified controlled release assays), is already an important consideration for other routes of administration, and we predict it will be important here, as well. Third, several opportunities already exist to manipulate the fate of IT administered nanomedicine or to adapt so-called “smart” polymer systems to enable stimuli triggered release. Because our choices for how to best engineer nanomedicine for IT delivery rely on relatively incomplete knowledge of how CSF flows, mixes, and clears in the CNS, we suggest that better understanding of the anatomy and physiology of the SAS will be a critical foundation upon which this field can progress.
Ultimately, the research reports described here highlight the burgeoning field of IT nanomedicine for CNS drug delivery. There remain many important avenues for future investigation, but it is clear that this approach holds great promise for the treatment of human disease.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calias P, et al. , Intrathecal delivery of protein therapeutics to the brain: a critical reassessment. Pharmacol Ther, 2014. 144(2): p. 114–22. [DOI] [PubMed] [Google Scholar]
- 2.Papisov MI, Belov VV, and Gannon KS, Physiology of the intrathecal bolus: the leptomeningeal route for macromolecule and particle delivery to CNS. Mol Pharm, 2013. 10(5): p. 1522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf DA, et al. , Dynamic dual-isotope molecular imaging elucidates principles for optimizing intrathecal drug delivery. JCI Insight, 2016. 1(2): p. e85311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardridge WM, Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS, 2011. 8(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papisov MI, et al. , Investigation of intrathecal transport of NPT002, a prospective therapeutic based on phage M13, in nonhuman primates. Drug Deliv Transl Res, 2012. 2(3): p. 210–21. [DOI] [PubMed] [Google Scholar]
- 6.Iliff JJ, et al. , A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med, 2012. 4(147): p. 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toma A, 60 - Hydrocephalus in Adults, in Principles of Neurological Surgery (Fourth Edition), Ellenbogen RG, et al. , Editors. 2018, Content Repository Only!: Philadelphia. p. 822–831.e1. [Google Scholar]
- 8.Snyder JM, et al. , 20 - Nervous System, in Comparative Anatomy and Histology (Second Edition), Treuting PM, Dintzis SM, and Montine KS, Editors. 2018, Academic Press: San Diego. p. 403–444. [Google Scholar]
- 9.Reina MA, et al. , Electron microscopy and the expansion of regional anesthesia knowledge. Techniques in Regional Anesthesia and Pain Management, 2002. 6(4): p. 165–171. [Google Scholar]
- 10.Ghannam JY and Al Kharazi KA, Neuroanatomy, Cranial Meninges, in StatPearls. 2019: Treasure Island (FL). [PubMed] [Google Scholar]
- 11.Kayalioglu G, Chapter 3 - The Vertebral Column and Spinal Meninges, in The Spinal Cord, Watson C, Paxinos G, and Kayalioglu G, Editors. 2009, Academic Press: San Diego. p. 17–36. [Google Scholar]
- 12.Haines DE, Chapter 7 - The Meninges, in Fundamental Neuroscience for Basic and Clinical Applications (Fifth Edition), Haines DE and Mihailoff GA, Editors. 2018, Elsevier. p. 107–121.e1. [Google Scholar]
- 13.Reina MA, et al. , [Structure of the arachnoid layer of the human spinal meninges: a barrier that regulates dural sac permeability]. Rev Esp Anestesiol Reanim, 2010. 57(8): p. 486–92. [DOI] [PubMed] [Google Scholar]
- 14.Mortazavi MM, et al. , Subarachnoid Trabeculae: A Comprehensive Review of Their Embryology, Histology, Morphology, and Surgical Significance. World Neurosurg, 2018. 111: p. 279–290. [DOI] [PubMed] [Google Scholar]
- 15.Brinker T, et al. , A new look at cerebrospinal fluid circulation. Fluids Barriers CNS, 2014. 11: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal MB and Pollay M, The secretion of cerebrospinal fluid. Exp Eye Res, 1977. 25 Suppl: p. 127–48. [DOI] [PubMed] [Google Scholar]
- 17.Spector R, Robert Snodgrass S, and Johanson CE, A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol, 2015. 273: p. 57–68. [DOI] [PubMed] [Google Scholar]
- 18.Weed LH, Studies on Cerebro-Spinal Fluid. No. II : The Theories of Drainage of Cerebro-Spinal Fluid with an Analysis of the Methods of Investigation. J Med Res, 1914. 31(1): p. 21–49. [PMC free article] [PubMed] [Google Scholar]
- 19.Milhorat TH, et al. , Cerebrospinal fluid production by the choroid plexus and brain. Science, 1971. 173(3994): p. 330–2. [DOI] [PubMed] [Google Scholar]
- 20.Kittisupamongkol W, Cerebrospinal fluid production rate. Clin Exp Ophthalmol, 2009. 37(8): p. 827; author reply 827–8. [DOI] [PubMed] [Google Scholar]
- 21.Damkier HH, Brown PD, and Praetorius J, Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev, 2013. 93(4): p. 1847–92. [DOI] [PubMed] [Google Scholar]
- 22.Bulat M. Dynamics and Statics of the Cerebrospinal Fluid: The Classical and a New Hypothesis. 1993. Springer Berlin Heidelberg. [Google Scholar]
- 23.Sánchez AL, et al. , On the bulk motion of the cerebrospinal fluid in the spinal canal. Journal of Fluid Mechanics, 2018. 841(25): p. 203–227. [Google Scholar]
- 24.Oreskovic D. and Klarica M, The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev, 2010. 64(2): p. 241–62. [DOI] [PubMed] [Google Scholar]
- 25.Tangen KM, et al. , Computational and In Vitro Experimental Investigation of Intrathecal Drug Distribution: Parametric Study of the Effect of Injection Volume, Cerebrospinal Fluid Pulsatility, and Drug Uptake. Anesth Analg, 2017. 124(5): p. 1686–1696. [DOI] [PubMed] [Google Scholar]
- 26.Tangen KM, et al. , CNS wide simulation of flow resistance and drug transport due to spinal microanatomy. J Biomech, 2015. 48(10): p. 2144–54. [DOI] [PubMed] [Google Scholar]
- 27.Mestre H, et al. , Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun, 2018. 9(1): p. 4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon S, Velasquez FC, and Sevick-Muraca EM, Near-infrared fluorescence lymphatic imaging in vascular endothelial growth factor-C overexpressing murine melanoma. Biomed Opt Express, 2018. 9(10): p. 4631–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siyahhan B, et al. , Flow induced by ependymal cilia dominates near-wall cerebrospinal fluid dynamics in the lateral ventricles. J R Soc Interface, 2014. 11(94): p. 20131189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worthington WC Jr. and Cathcart RS 3rd, Ependymal cilia: distribution and activity in the adult human brain. Science, 1963. 139(3551): p. 221–2. [DOI] [PubMed] [Google Scholar]
- 31.Nakada T, Virchow-Robin space and aquaporin-4: new insights on an old friend. Croat Med J, 2014. 55(4): p. 328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oreskovic D, Rados M, and Klarica M, Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience, 2017. 354: p. 69–87. [DOI] [PubMed] [Google Scholar]
- 33.Smith AJ, Jin BJ, and Verkman AS, Muddying the water in brain edema? Trends Neurosci, 2015. 38(6): p. 331–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon MJ and Iliff JJ, Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta, 2016. 1862(3): p. 442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weller RO, et al. , Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol, 2009. 117(1): p. 1–14. [DOI] [PubMed] [Google Scholar]
- 36.Wagner HJ, Pilgrim C, and Brandl J, Penetration and removal of horseradish peroxidase injected into the cerebrospinal fluid: role of cerebral perivascular spaces, endothelium and microglia. Acta Neuropathol, 1974. 27(4): p. 299–315. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RA, Lymphatics of blood vessels. Lymphology, 1969. 2(2): p. 44–56. [PubMed] [Google Scholar]
- 38.Zhang ET, Inman CB, and Weller RO, Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat, 1990. 170: p. 111–23. [PMC free article] [PubMed] [Google Scholar]
- 39.Engelhardt B, et al. , Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol, 2016. 132(3): p. 317–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davson H, The Cerebrospinal Fluid, in Handbook of Neurochemistry: Volume II: Structural Neurochemistry, A. Lajtha, Editor. 1969, Springer US: Boston, MA. p. 23–48. [Google Scholar]
- 41.Sakka L, Coll G, and Chazal J, Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis, 2011. 128(6): p. 309–16. [DOI] [PubMed] [Google Scholar]
- 42.Levick JR and Michel CC, Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res, 2010. 87(2): p. 198–210. [DOI] [PubMed] [Google Scholar]
- 43.Kwon S, et al. , Fluorescence imaging of lymphatic outflow of cerebrospinal fluid in mice. J Immunol Methods, 2017. 449: p. 37–43. [DOI] [PubMed] [Google Scholar]
- 44.Ma Q, et al. , Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun, 2017. 8(1): p. 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kida S, Pantazis A, and Weller RO, CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol, 1993. 19(6): p. 480–8. [DOI] [PubMed] [Google Scholar]
- 46.Louveau A, et al. , Structural and functional features of central nervous system lymphatic vessels. Nature, 2015. 523(7560): p. 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aspelund A, et al. , A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med, 2015. 212(7): p. 991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston M, et al. , Subarachnoid injection of Microfil reveals connections between cerebrospinal fluid and nasal lymphatics in the non-human primate. Neuropathol Appl Neurobiol, 2005. 31(6): p. 632–40. [DOI] [PubMed] [Google Scholar]
- 49.Johnston M, et al. , Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res, 2004. 1(1): p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn JH, et al. , Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature, 2019. 572(7767): p. 62–66. [DOI] [PubMed] [Google Scholar]
- 51.Dedrick RL, Interspecies scaling of regional drug delivery. J Pharm Sci, 1986. 75(11): p. 1047–52. [DOI] [PubMed] [Google Scholar]
- 52.Bulat M. and Klarica M, Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res Rev, 2011. 65(2): p. 99–112. [DOI] [PubMed] [Google Scholar]
- 53.Luedemann W, et al. , Spinal cerebrospinal fluid pathways and their significance for the compensation of kaolin-hydrocephalus. Acta Neurochir Suppl, 2002. 81: p. 271–3. [DOI] [PubMed] [Google Scholar]
- 54.Bozanovic-Sosic R, Mollanji R, and Johnston MG, Spinal and cranial contributions to total cerebrospinal fluid transport. Am J Physiol Regul Integr Comp Physiol, 2001. 281(3): p. R909–16. [DOI] [PubMed] [Google Scholar]
- 55.Pepper MS and Skobe M, Lymphatic endothelium: morphological, molecular and functional properties. J Cell Biol, 2003. 163(2): p. 209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aldrich MB and Sevick-Muraca EM, Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine, 2013. 64(1): p. 362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwon S, et al. , Impaired Peripheral Lymphatic Function and Cerebrospinal Fluid Outflow in a Mouse Model of Alzheimer’s Disease. J Alzheimers Dis, 2019. 69(2): p. 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng W, et al. , Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis, 2016. 93: p. 215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daneman R. and Prat A, The blood-brain barrier. Cold Spring Harb Perspect Biol, 2015. 7(1): p. a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pardridge WM, The blood-brain barrier: bottleneck in brain drug development. NeuroRx, 2005. 2(1): p. 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garg T, et al. , Current strategies for targeted delivery of bio-active drug molecules in the treatment of brain tumor. J Drug Target, 2015. 23(10): p. 865–87. [DOI] [PubMed] [Google Scholar]
- 62.Mehta AM, Sonabend AM, and Bruce JN, Convection-Enhanced Delivery. Neurotherapeutics, 2017. 14(2): p. 358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeVos SL and Miller TM, Direct intraventricular delivery of drugs to the rodent central nervous system. J Vis Exp, 2013(75): p. e50326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xavier ALR, et al. , Cannula Implantation into the Cisterna Magna of Rodents. J Vis Exp, 2018(135). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ineichen BV, et al. , Direct, long-term intrathecal application of therapeutics to the rodent CNS. Nat Protoc, 2017. 12(1): p. 104–131. [DOI] [PubMed] [Google Scholar]
- 66.Sandberg DI, et al. , Infusion of 5-Azacytidine (5-AZA) into the fourth ventricle or resection cavity in children with recurrent posterior Fossa Ependymoma: a pilot clinical trial. J Neurooncol, 2019. 141(2): p. 449–457. [DOI] [PubMed] [Google Scholar]
- 67.Sandberg DI, et al. , Methotrexate administration directly into the fourth ventricle in children with malignant fourth ventricular brain tumors: a pilot clinical trial. J Neurooncol, 2015. 125(1): p. 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandberg DI and Kerr ML, Ventricular access device placement in the fourth ventricle to treat malignant fourth ventricle brain tumors: technical note. Childs Nerv Syst, 2016. 32(4): p. 703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimelberg HK, et al. , Distribution of free and liposome-entrapped [3H]methotrexate in the central nervous system after intracerebroventricular injection in a primate. Cancer Res, 1978. 38(3): p. 706–12. [PubMed] [Google Scholar]
- 70.Prakash V, Spinraza-a rare disease success story. Gene Ther, 2017. 24(9): p. 497. [DOI] [PubMed] [Google Scholar]
- 71.Toro R, et al. , Brain size and folding of the human cerebral cortex. Cereb Cortex, 2008. 18(10): p. 2352–7. [DOI] [PubMed] [Google Scholar]
- 72.Umbrain V, et al. , Scintigraphic visualization of intrathecal liposome biodistribution. Acta Anaesthesiol Scand, 1997. 41(1 Pt 1): p. 25–34. [DOI] [PubMed] [Google Scholar]
- 73.Householder KT, et al. , Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice. Sci Rep, 2019. 9(1): p. 12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan J, et al. , Lymphatic clearance is the main drainage route of lamotrigine-loaded micelles following delivery to the brain. J Pharm Pharmacol, 2019. 71(10): p. 1488–1496. [DOI] [PubMed] [Google Scholar]
- 75.Kim S, et al. , Multivesicular liposomes containing 1-beta-D-arabinofuranosylcytosine for slow-release intrathecal therapy. Cancer Res, 1987. 47(15): p. 3935–7. [PubMed] [Google Scholar]
- 76.Kim S, et al. , Prolongation of drug exposure in cerebrospinal fluid by encapsulation into DepoFoam. Cancer Res, 1993. 53(7): p. 1596–8. [PubMed] [Google Scholar]
- 77.Kitamura I, et al. , Intrathecal chemotherapy with 1,3-bis(2-chloroethyl)-1-nitrosourea encapsulated into hybrid liposomes for meningeal gliomatosis: an experimental study. Cancer Res, 1996. 56(17): p. 3986–92. [PubMed] [Google Scholar]
- 78.Mabuchi E, et al. , Gene delivery by HVJ-liposome in the experimental gene therapy of murine glioma. Gene Ther, 1997. 4(8): p. 768–72. [DOI] [PubMed] [Google Scholar]
- 79.Hanggi D, et al. , A Site-Specific, Sustained-Release Drug Delivery System for Aneurysmal Subarachnoid Hemorrhage. Neurotherapeutics, 2016. 13(2): p. 439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takanashi Y, et al. , Intrathecal application with liposome-entrapped Fasudil for cerebral vasospasm following subarachnoid hemorrhage in rats. J Clin Neurosci, 2001. 8(6): p. 557–61. [DOI] [PubMed] [Google Scholar]
- 81.Takanashi Y, et al. , Efficacy of intrathecal liposomal fasudil for experimental cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery, 2001. 48(4): p. 894–900; discussion 900–1. [DOI] [PubMed] [Google Scholar]
- 82.Ishida T, Takanashi Y, and Kiwada H, Safe and efficient drug delivery system with liposomes for intrathecal application of an antivasospastic drug, fasudil. Biol Pharm Bull, 2006. 29(3): p. 397–402. [DOI] [PubMed] [Google Scholar]
- 83.Takanashi Y, et al. , Neuroprotection by intrathecal application of liposome-entrapped fasudil in a rat model of ischemia. Neurol Med Chir (Tokyo), 2001. 41(3): p. 107–13; discussion 113–4. [DOI] [PubMed] [Google Scholar]
- 84.Santhosh KT, Alizadeh A, and Karimi-Abdolrezaee S, Design and optimization of PLGA microparticles for controlled and local delivery of Neuregulin-1 in traumatic spinal cord injury. J Control Release, 2017. 261: p. 147–162. [DOI] [PubMed] [Google Scholar]
- 85.Shi L, et al. , Repeated intrathecal administration of plasmid DNA complexed with polyethylene glycol-grafted polyethylenimine led to prolonged transgene expression in the spinal cord. Gene Ther, 2003. 10(14): p. 1179–88. [DOI] [PubMed] [Google Scholar]
- 86.Tang GP, et al. , Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials, 2003. 24(13): p. 2351–62. [DOI] [PubMed] [Google Scholar]
- 87.Wang X, et al. , Gene transfer to dorsal root ganglia by intrathecal injection: effects on regeneration of peripheral nerves. Mol Ther, 2005. 12(2): p. 314–20. [DOI] [PubMed] [Google Scholar]
- 88.Tang GP, et al. , Low molecular weight polyethylenimines linked by beta-cyclodextrin for gene transfer into the nervous system. J Gene Med, 2006. 8(6): p. 736–44. [DOI] [PubMed] [Google Scholar]
- 89.Zeng J, Wang X, and Wang S, Self-assembled ternary complexes of plasmid DNA, low molecular weight polyethylenimine and targeting peptide for nonviral gene delivery into neurons. Biomaterials, 2007. 28(7): p. 1443–51. [DOI] [PubMed] [Google Scholar]
- 90.Tan J, et al. , Changes in compressed neurons from dogs with acute and severe cauda equina constrictions following intrathecal injection of brain-derived neurotrophic factor-conjugated polymer nanoparticles. Neural Regen Res, 2013. 8(3): p. 233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nabhan JF, et al. , Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Sci Rep, 2016. 6: p. 20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zou LL, et al. , Liposome-mediated NGF gene transfection following neuronal injury: potential therapeutic applications. Gene Ther, 1999. 6(6): p. 994–1005. [DOI] [PubMed] [Google Scholar]
- 93.Cao YJ, Shibata T, and Rainov NG, Liposome-mediated transfer of the bcl-2 gene results in neuroprotection after in vivo transient focal cerebral ischemia in an animal model. Gene Ther, 2002. 9(6): p. 415–9. [DOI] [PubMed] [Google Scholar]
- 94.Joshi GP, Patou G, and Kharitonov V, The safety of liposome bupivacaine following various routes of administration in animals. J Pain Res, 2015. 8: p. 781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valdes C, et al. , Antinociceptive antibiotics-loaded into solid lipid nanoparticles of prolonged release: Measuring pharmacological efficiency and time span on chronic monoarthritis rats. PLoS One, 2018. 13(4): p. e0187473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishiyama T, et al. , The effects of intrathecal morphine encapsulated in L- and D-dipalmitoylphosphatidyl choline liposomes on acute nociception in rats. Anesth Analg, 2000. 91(2): p. 423–8. [DOI] [PubMed] [Google Scholar]
- 97.Grant GJ, et al. , Intrathecal administration of liposomal morphine in a mouse model. Anesth Analg, 1995. 81(3): p. 514–8. [DOI] [PubMed] [Google Scholar]
- 98.Bernards CM, et al. , Liposome encapsulation prolongs alfentanil spinal analgesia and alters systemic redistribution in the rat. Anesthesiology, 1992. 77(3): p. 529–35. [DOI] [PubMed] [Google Scholar]
- 99.Wallace MS, et al. , Antinociception and side effects of liposome-encapsulated alfentanil after spinal delivery in rats. Anesth Analg, 1994. 79(4): p. 778–86. [DOI] [PubMed] [Google Scholar]
- 100.Grant GJ, Piskoun B, and Bansinath M, Intrathecal administration of liposomal neostigmine prolongs analgesia in mice. Acta Anaesthesiol Scand, 2002. 46(1): p. 90–4. [DOI] [PubMed] [Google Scholar]
- 101.Lagarce F, et al. , Baclofen-loaded microspheres: preparation and efficacy testing in a new rabbit model. Eur J Pharm Biopharm, 2005. 59(3): p. 449–59. [DOI] [PubMed] [Google Scholar]
- 102.Han FY, et al. , Novel polymeric bioerodable microparticles for prolonged-release intrathecal delivery of analgesic agents for relief of intractable cancer-related pain. J Pharm Sci, 2015. 104(7): p. 2334–44. [DOI] [PubMed] [Google Scholar]
- 103.Curley J, et al. , Prolonged regional nerve blockade. Injectable biodegradable bupivacaine/polyester microspheres. Anesthesiology, 1996. 84(6): p. 1401–10. [DOI] [PubMed] [Google Scholar]
- 104.Le Guevello P, et al. , High-performance liquid chromatographic determination of bupivacaine in plasma samples for biopharmaceutical studies and application to seven other local anaesthetics. J Chromatogr, 1993. 622(2): p. 284–90. [DOI] [PubMed] [Google Scholar]
- 105.Estebe JP, et al. , Prolongation of spinal anesthesia with bupivacaine-loaded (DL-lactide) microspheres. Anesth Analg, 1995. 81(1): p. 99–103. [DOI] [PubMed] [Google Scholar]
- 106.Le Corre P, et al. , Spinal controlled delivery of bupivacaine from DL-lactic acid oligomer microspheres. J Pharm Sci, 1995. 84(1): p. 75–8. [DOI] [PubMed] [Google Scholar]
- 107.Bujedo BM, Santos SG, and Azpiazu AU, A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag, 2012. 8(3): p. 177–92. [DOI] [PubMed] [Google Scholar]
- 108.Ratajczak-Enselme M, et al. , Epidural, intrathecal and plasma pharmacokinetic study of epidural ropivacaine in PLGA-microspheres in sheep model. Eur J Pharm Biopharm, 2009. 72(1): p. 54–61. [DOI] [PubMed] [Google Scholar]
- 109.Yanez AM, et al. , Touch-evoked agitation produced by spinally administered phospholipid emulsion and liposomes in rats. Structure-activity relation. Anesthesiology, 1995. 82(5): p. 1189–98. [DOI] [PubMed] [Google Scholar]
- 110.Cereda CMS, et al. , Bupivacaine in alginate and chitosan nanoparticles: an in vivo evaluation of efficacy, pharmacokinetics, and local toxicity. J Pain Res, 2018. 11: p. 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karashima K, et al. , Prolongation of intrathecal and sciatic nerve blocks using a complex of levobupivacaine with maltosyl-beta-cyclodextrin in rats. Anesth Analg, 2007. 104(5): p. 1121–8, tables of contents. [DOI] [PubMed] [Google Scholar]
- 112.Spofford CM, et al. , Ketoprofen produces modality-specific inhibition of pain behaviors in rats after plantar incision. Anesth Analg, 2009. 109(6): p. 1992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu PC, et al. , Magnetic field distribution modulation of intrathecal delivered ketorolac iron-oxide nanoparticle conjugates produce excellent analgesia for chronic inflammatory pain. J Nanobiotechnology, 2018. 16(1): p. 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pieretti S, et al. , “Curcumin-loaded Poly (d, l-lactide-co-glycolide) nanovesicles induce antinociceptive effects and reduce pronociceptive cytokine and BDNF release in spinal cord after acute administration in mice”. Colloids Surf B Biointerfaces, 2017. 158: p. 379–386. [DOI] [PubMed] [Google Scholar]
- 115.Soderquist RG, et al. , Release of plasmid DNA-encoding IL-10 from PLGA microparticles facilitates long-term reversal of neuropathic pain following a single intrathecal administration. Pharm Res, 2010. 27(5): p. 841–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cai YQ, et al. , Role of M2, M3, and M4 muscarinic receptor subtypes in the spinal cholinergic control of nociception revealed using siRNA in rats. J Neurochem, 2009. 111(4): p. 1000–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dengler EC, et al. , Mesoporous silica-supported lipid bilayers (protocells) for DNA cargo delivery to the spinal cord. J Control Release, 2013. 168(2): p. 209–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao MZ, et al. , Interleukin-2 gene therapy of chronic neuropathic pain. Neuroscience, 2002. 112(2): p. 409–16. [DOI] [PubMed] [Google Scholar]
- 119.Yao MZ, et al. , Interleukin-2 gene has superior antinociceptive effects when delivered intrathecally. Neuroreport, 2002. 13(6): p. 791–4. [DOI] [PubMed] [Google Scholar]
- 120.Noda K, et al. , Antisense knockdown of spinal-mGluR1 reduces the sustained phase of formalin-induced nociceptive responses. Brain Res, 2003. 987(2): p. 194–200. [DOI] [PubMed] [Google Scholar]
- 121.Sumner JP, et al. , In vivo labeling of adult neural progenitors for MRI with micron sized particles of iron oxide: quantification of labeled cell phenotype. Neuroimage, 2009. 44(3): p. 671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhong XM, et al. , In Vivo Targeted Magnetic Resonance Imaging of Endogenous Neural Stem Cells in the Adult Rodent Brain. Biomed Res Int, 2015. 2015: p. 131054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muldoon LL, et al. , Trafficking of superparamagnetic iron oxide particles (Combidex) from brain to lymph nodes in the rat. Neuropathol Appl Neurobiol, 2004. 30(1): p. 70–9. [DOI] [PubMed] [Google Scholar]
- 124.Liu CH, et al. , DNA-based MRI probes for specific detection of chronic exposure to amphetamine in living brains. J Neurosci, 2009. 29(34): p. 10663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song HP, et al. , Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials, 2010. 31(4): p. 769–78. [DOI] [PubMed] [Google Scholar]
- 126.Varela JA, et al. , Targeting neurotransmitter receptors with nanoparticles in vivo allows single-molecule tracking in acute brain slices. Nat Commun, 2016. 7: p. 10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Albertazzi L, et al. , In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol Pharm, 2013. 10(1): p. 249–60. [DOI] [PubMed] [Google Scholar]
- 128.Kim H, et al. , Polyamidoamine dendrimer-conjugated triamcinolone acetonide attenuates nerve injury-induced spinal cord microglia activation and mechanical allodynia. Mol Pain, 2017. 13: p. 1744806917697006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dai H, et al. , Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine (Lond), 2010. 5(9): p. 1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mundt AP, et al. , Targeting activated microglia in Alzheimer’s pathology by intraventricular delivery of a phagocytosable MRI contrast agent in APP23 transgenic mice. Neuroimage, 2009. 46(2): p. 367–72. [DOI] [PubMed] [Google Scholar]
- 131.Neelov IM, et al. , Molecular properties of lysine dendrimers and their interactions with Abeta-peptides and neuronal cells. Curr Med Chem, 2013. 20(1): p. 134–43. [PubMed] [Google Scholar]
- 132.Jankowski W. and Strosznajder J, Uptake and subcellular distribution of intraventricularly injected [1–3H]dolichol in rat brain. Acta Biochim Pol, 1992. 39(2): p. 215–22. [PubMed] [Google Scholar]
- 133.Rodriguez G, et al. , Bicosomes: bicelles in dilute systems. Biophys J, 2010. 99(2): p. 480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Akita H, et al. , Effect of hydrophobic scaffold on the cellular uptake and gene transfection activities of DNA-encapsulating liposomal nanoparticles via intracerebroventricular administration. Int J Pharm, 2015. 490(1–2): p. 142–5. [DOI] [PubMed] [Google Scholar]
- 135.Goula D, et al. , Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther, 1998. 5(5): p. 712–7. [DOI] [PubMed] [Google Scholar]
- 136.Lemkine GF, et al. , Preferential transfection of adult mouse neural stem cells and their immediate progeny in vivo with polyethylenimine. Mol Cell Neurosci, 2002. 19(2): p. 165–74. [DOI] [PubMed] [Google Scholar]
- 137.Kwon EJ, et al. , Targeted nonviral delivery vehicles to neural progenitor cells in the mouse subventricular zone. Biomaterials, 2010. 31(8): p. 2417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zou S, et al. , Lipid-mediated delivery of RNA is more efficient than delivery of DNA in non-dividing cells. Int J Pharm, 2010. 389(1–2): p. 232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thorsell A, Fox E, and Heilig M, Lipid mediated gene delivery in the adult rat brain: quantitative analysis of expression. Neurochem Int, 1999. 35(1): p. 65–71. [DOI] [PubMed] [Google Scholar]
- 140.Thorsell A, Blomqvist AG, and Heilig M, Cationic lipid-mediated delivery and expression of prepro-neuropeptide Y cDNA after intraventricular administration in rat: feasibility and limitations. Regul Pept, 1996. 61(3): p. 205–11. [DOI] [PubMed] [Google Scholar]
- 141.Ogo H, et al. , Modulation of substance P/neurokinin-1 receptor in human astrocytoma cells by antisense oligodeoxynucleotides. Gen Pharmacol, 1994. 25(6): p. 1131–5. [DOI] [PubMed] [Google Scholar]
- 142.Choi JL, et al. , Guanidinylated block copolymers for gene transfer: A comparison with amine-based materials for in vitro and in vivo gene transfer efficiency. Biomaterials, 2015. 54: p. 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hagihara Y, et al. , Widespread gene transfection into the central nervous system of primates. Gene Ther, 2000. 7(9): p. 759–63. [DOI] [PubMed] [Google Scholar]
- 144.Meuli-Simmen C, et al. , Gene expression along the cerebral-spinal axis after regional gene delivery. Hum Gene Ther, 1999. 10(16): p. 2689–700. [DOI] [PubMed] [Google Scholar]
- 145.Wang J, et al. , Polyphosphoramidate gene carriers: effect of charge group on gene transfer efficiency. Gene Ther, 2004. 11(12): p. 1001–10. [DOI] [PubMed] [Google Scholar]
- 146.Schellinger JG, et al. , Melittin-grafted HPMA-oligolysine based copolymers for gene delivery. Biomaterials, 2013. 34(9): p. 2318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wei H, et al. , Dual responsive, stabilized nanoparticles for efficient in vivo plasmid delivery. Angew Chem Int Ed Engl, 2013. 52(20): p. 5377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hauck ES, et al. , Whole animal in vivo imaging after transient, nonviral gene delivery to the rat central nervous system. Mol Ther, 2008. 16(11): p. 1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Helmschrodt C, et al. , Polyethylenimine Nanoparticle-Mediated siRNA Delivery to Reduce alpha-Synuclein Expression in a Model of Parkinson’s Disease. Mol Ther Nucleic Acids, 2017. 9: p. 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anderson DM, et al. , Stability of mRNA/cationic lipid lipoplexes in human and rat cerebrospinal fluid: methods and evidence for nonviral mRNA gene delivery to the central nervous system. Hum Gene Ther, 2003. 14(3): p. 191–202. [DOI] [PubMed] [Google Scholar]
- 151.Bharali DJ, et al. , Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci U S A, 2005. 102(32): p. 11539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shyam R, et al. , Intraventricular Delivery of siRNA Nanoparticles to the Central Nervous System. Mol Ther Nucleic Acids, 2015. 4: p. e242. [DOI] [PubMed] [Google Scholar]
- 153.Klejbor I, et al. , ORMOSIL nanoparticles as a non-viral gene delivery vector for modeling polyglutamine induced brain pathology. J Neurosci Methods, 2007. 165(2): p. 230–43. [DOI] [PubMed] [Google Scholar]
- 154.Wood RI, et al. , Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology (Berl), 2004. 171(3): p. 298–305. [DOI] [PubMed] [Google Scholar]
- 155.Hirst JJ, Egodagamage KC, and Walker DW, Effect of a neuroactive steroid infused into the cerebral ventricles of fetal sheep in utero using small infusion volumes. J Neurosci Methods, 2000. 97(1): p. 37–44. [DOI] [PubMed] [Google Scholar]
- 156.Schwinkendorf DR, et al. , Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int J Obes (Lond), 2011. 35(3): p. 336–44. [DOI] [PubMed] [Google Scholar]
- 157.Svensson E, et al. , Intrathecal neurosteroids and a neurosteroid antagonist: effects on inflammation-evoked thermal hyperalgesia and tactile allodynia. Neurosci Lett, 2013. 548: p. 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Frye CA and Duncan JE, Estradiol benzoate potentiates neuroactive steroids’ effects on pain sensitivity. Pharmacol Biochem Behav, 1996. 53(1): p. 27–32. [DOI] [PubMed] [Google Scholar]
- 159.Vaz GC, et al. , Cardiovascular and behavioral effects produced by administration of liposome-entrapped GABA into the rat central nervous system. Neuroscience, 2015. 285: p. 60–9. [DOI] [PubMed] [Google Scholar]
- 160.Suzuki M, et al. , Suppression of copulatory behavior by intracerebroventricular infusion of antisense oligodeoxynucleotide of granulin in neonatal male rats. Physiol Behav, 2000. 68(5): p. 707–13. [DOI] [PubMed] [Google Scholar]
- 161.Shtein L, et al. , The inositol monophosphatase inhibitor L-690,330 affects pilocarpine-behavior and the forced swim test. Psychopharmacology (Berl), 2013. 227(3): p. 503–8. [DOI] [PubMed] [Google Scholar]
- 162.van Rooijen N. and Hendrikx E, Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol, 2010. 605: p. 189–203. [DOI] [PubMed] [Google Scholar]
- 163.Polfliet MM, et al. , Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J Immunol, 2001. 167(8): p. 4644–50. [DOI] [PubMed] [Google Scholar]
- 164.Polfliet MM, et al. , A method for the selective depletion of perivascular and meningeal macrophages in the central nervous system. J Neuroimmunol, 2001. 116(2): p. 188–95. [DOI] [PubMed] [Google Scholar]
- 165.Trostdorf F, et al. , Reduction of meningeal macrophages does not decrease migration of granulocytes into the CSF and brain parenchyma in experimental pneumococcal meningitis. J Neuroimmunol, 1999. 99(2): p. 205–10. [DOI] [PubMed] [Google Scholar]
- 166.Sun ML, et al. , Lappaconitine, a C18-diterpenoid alkaloid, exhibits antihypersensitivity in chronic pain through stimulation of spinal dynorphin A expression. Psychopharmacology (Berl), 2018. 235(9): p. 2559–2571. [DOI] [PubMed] [Google Scholar]
- 167.Capilla J, et al. , Efficacy of intrathecal liposomal amphotericin B plus oral posaconazole in the treatment of acute meningeal cryptococcosis in a murine model. Int J Antimicrob Agents, 2013. 42(3): p. 282–3. [DOI] [PubMed] [Google Scholar]
- 168.Gazzoni AF, et al. , Efficacy of intrathecal administration of liposomal amphotericin B combined with voriconazole in a murine model of cryptococcal meningitis. Int J Antimicrob Agents, 2012. 39(3): p. 223–7. [DOI] [PubMed] [Google Scholar]
- 169.Uchida S, et al. , In vivo messenger RNA introduction into the central nervous system using polyplex nanomicelle. PLoS One, 2013. 8(2): p. e56220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moreno L, et al. , [Liposomal cytarabine for the treatment of leptomeningeal dissemination of central nervous system tumours in children and adolescents]. An Pediatr (Barc), 2016. 85(5): p. 274 e1–274 e8. [DOI] [PubMed] [Google Scholar]
- 171.Bhojwani D. and Pui CH, Intrathecal liposomal cytarabine: more friend than foe? Leuk Lymphoma, 2008. 49(8): p. 1427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bomgaars L, et al. , Phase I trial of intrathecal liposomal cytarabine in children with neoplastic meningitis. J Clin Oncol, 2004. 22(19): p. 3916–21. [DOI] [PubMed] [Google Scholar]
- 173.Phuphanich S, et al. , A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J Neurooncol, 2007. 81(2): p. 201–8. [DOI] [PubMed] [Google Scholar]
- 174.Peyrl A, et al. , Pharmacokinetics and safety of intrathecal liposomal cytarabine in children aged <3 years. Clin Pharmacokinet, 2009. 48(4): p. 265–71. [DOI] [PubMed] [Google Scholar]
- 175.Peyrl A, et al. , Pharmacokinetics and toxicity of intrathecal liposomal cytarabine in children and adolescents following age-adapted dosing. Clin Pharmacokinet, 2014. 53(2): p. 165–73. [DOI] [PubMed] [Google Scholar]
- 176.Zimm S, et al. , Cytosine arabinoside cerebrospinal fluid kinetics. Clin Pharmacol Ther, 1984. 35(6): p. 826–30. [DOI] [PubMed] [Google Scholar]
- 177.Spina M, et al. , Phase 2 study of intrathecal, long-acting liposomal cytarabine in the prophylaxis of lymphomatous meningitis in human immunodeficiency virus-related non-Hodgkin lymphoma. Cancer, 2010. 116(6): p. 1495–501. [DOI] [PubMed] [Google Scholar]
- 178.Benesch M, et al. , Safety and toxicity of intrathecal liposomal cytarabine (Depocyte) in children and adolescents with recurrent or refractory brain tumors: a multi-institutional retrospective study. Anticancer Drugs, 2009. 20(9): p. 794–9. [DOI] [PubMed] [Google Scholar]
- 179.Duarte RF, et al. , Clinical experience using intrathecal liposomal cytarabine to manage neoplastic meningitis in three patients with acute promyelocytic leukemia. Leuk Res, 2011. 35(7): p. e128–30. [DOI] [PubMed] [Google Scholar]
- 180.Nygaard R. and Kivivuori SM, Treatment for recurrent medulloblastoma with intrathecal liposomal cytarabine and systemic metronomic combination therapy. Anticancer Drugs, 2012. 23(3): p. 342–6. [DOI] [PubMed] [Google Scholar]
- 181.Gaviani P, et al. , Liposomal cytarabine in neoplastic meningitis from primary brain tumors: a single institutional experience. Neurol Sci, 2013. 34(12): p. 2151–7. [DOI] [PubMed] [Google Scholar]
- 182.Jurczak W, et al. , Liposomal cytarabine in the prophylaxis and treatment of CNS lymphoma: toxicity analysis in a retrospective case series study conducted at Polish Lymphoma Research Group Centers. Med Oncol, 2015. 32(4): p. 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Partap S, et al. , Liposomal cytarabine for central nervous system embryonal tumors in children and young adults. J Neurooncol, 2011. 103(3): p. 561–6. [DOI] [PubMed] [Google Scholar]
- 184.Butto A, et al. , Fulminant chemical ventriculomeningitis following intrathecal liposomal cytarabine administration. J Clin Neurosci, 2011. 18(10): p. 1417–8. [DOI] [PubMed] [Google Scholar]
- 185.Jabbour E, et al. , Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood, 2007. 109(8): p. 3214–8. [DOI] [PubMed] [Google Scholar]
- 186.Sommer C, et al. , Neuroophthalmological side effects following intrathecal administration of liposomal cytarabine for central nervous system prophylaxis in three adolescents with acute myeloid leukaemia. Ann Hematol, 2008. 87(11): p. 887–90. [DOI] [PubMed] [Google Scholar]
- 187.Hilgendorf I, et al. , Neurological complications after intrathecal liposomal cytarabine application in patients after allogeneic haematopoietic stem cell transplantation. Ann Hematol, 2008. 87(12): p. 1009–12. [DOI] [PubMed] [Google Scholar]
- 188.Ostermann K, et al. , Neurologic complications after intrathecal liposomal cytarabine in combination with systemic polychemotherapy in primary CNS lymphoma. J Neurooncol, 2011. 103(3): p. 635–40. [DOI] [PubMed] [Google Scholar]
- 189.Gallego Perez-Larraya J, et al. , Neurologic complications of intrathecal liposomal cytarabine administered prophylactically to patients with non-Hodgkin lymphoma. J Neurooncol, 2011. 103(3): p. 603–9. [DOI] [PubMed] [Google Scholar]
- 190.Jahn F, et al. , Safety and Efficacy of Liposomal Cytarabine in the Treatment of Neoplastic Meningitis. Oncology, 2015. 89(3): p. 137–42. [DOI] [PubMed] [Google Scholar]
- 191.Gokbuget N, et al. , Liposomal cytarabine is effective and tolerable in the treatment of central nervous system relapse of acute lymphoblastic leukemia and very aggressive lymphoma. Haematologica, 2011. 96(2): p. 238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bassan R, et al. , Randomized trial of radiation-free central nervous system prophylaxis comparing intrathecal triple therapy with liposomal cytarabine in acute lymphoblastic leukemia. Haematologica, 2015. 100(6): p. 786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Garcia-Recio M, et al. , Analysis of the role of intratecal liposomal cytarabine in the prophylaxis and treatment of central nervous system lymphomatosis: The Balearic Lymphoma Group experience. PLoS One, 2017. 12(6): p. e0179595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Niwinska A, Rudnicka H, and Murawska M, Breast cancer leptomeningeal metastasis: the results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin Breast Cancer, 2015. 15(1): p. 66–72. [DOI] [PubMed] [Google Scholar]
- 195.Levinsen M, et al. , Efficacy and Toxicity of Intrathecal Liposomal Cytarabine in First-line Therapy of Childhood Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol, 2016. 38(8): p. 602–609. [DOI] [PubMed] [Google Scholar]
- 196.Megias-Vericat JE, et al. , Early experience with compassionate use of 2 hydroxypropyl-beta-cyclodextrin for Niemann-Pick type C disease: review of initial published cases. Neurol Sci, 2017. 38(5): p. 727–743. [DOI] [PubMed] [Google Scholar]
- 197.Camargo F, et al. , Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci, 2001. 70(2): p. 131–42. [DOI] [PubMed] [Google Scholar]
- 198.Ory DS, et al. , Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet, 2017. 390(10104): p. 1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maarup TJ, et al. , Intrathecal 2-hydroxypropyl-beta-cyclodextrin in a single patient with Niemann-Pick C1. Mol Genet Metab, 2015. 116(1–2): p. 75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Berry-Kravis E, et al. , Long-Term Treatment of Niemann-Pick Type C1 Disease With Intrathecal 2-Hydroxypropyl-beta-Cyclodextrin. Pediatr Neurol, 2018. 80: p. 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Matsuo M, et al. , Effects of intracerebroventricular administration of 2-hydroxypropylbeta-cyclodextrin in a patient with Niemann-Pick Type C disease. Mol Genet Metab Rep, 2014. 1: p. 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Garcia-Robles AA, et al. , Use of 2 hydroxypropyl-beta-cyclodextrin therapy in two adult Niemann Pick Type C patients. J Neurol Sci, 2016. 366: p. 65–67. [DOI] [PubMed] [Google Scholar]
- 203.Dullenkopf A. and Borgeat A, [Local anesthetics. Differences and similarities in the “-cains”]. Anaesthesist, 2003. 52(4): p. 329–40. [DOI] [PubMed] [Google Scholar]
- 204.Covino BG, Pharmacology of local anaesthetic agents. Br J Anaesth, 1986. 58(7): p. 701–16. [DOI] [PubMed] [Google Scholar]
- 205.Chahar P. and Cummings KC 3rd, Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res, 2012. 5: p. 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Boogaerts JG, et al. , Epidural administration of liposome-associated bupivacaine for the management of postsurgical pain: a first study. J Clin Anesth, 1994. 6(4): p. 315–20. [DOI] [PubMed] [Google Scholar]
- 207.Smoot JD, et al. , The efficacy and safety of DepoFoam bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammaplasty: a randomized, double-blind, active-control study. Aesthet Surg J, 2012. 32(1): p. 69–76. [DOI] [PubMed] [Google Scholar]
- 208.Bergese SD, et al. , Bupivacaine extended-release liposome injection exhibits a favorable cardiac safety profile. Reg Anesth Pain Med, 2012. 37(2): p. 145–51. [DOI] [PubMed] [Google Scholar]
- 209.Hanggi D, et al. , Clinical Trial Protocol: Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study Comparing EG-1962 to Standard of Care Oral Nimodipine in Adults with Aneurysmal Subarachnoid Hemorrhage [NEWTON-2 (Nimodipine Microparticles to Enhance Recovery While Reducing TOxicity After SubarachNoid Hemorrhage)]. Neurocrit Care, 2019. 30(1): p. 88–97. [DOI] [PubMed] [Google Scholar]
- 210.Hanggi D, et al. , NEWTON: Nimodipine Microparticles to Enhance Recovery While Reducing Toxicity After Subarachnoid Hemorrhage. Neurocrit Care, 2015. 23(2): p. 274–84. [DOI] [PubMed] [Google Scholar]
- 211.Hanggi D, et al. , Randomized, Open-Label, Phase 1/2a Study to Determine the Maximum Tolerated Dose of Intraventricular Sustained Release Nimodipine for Subarachnoid Hemorrhage (NEWTON [Nimodipine Microparticles to Enhance Recovery While Reducing Toxicity After Subarachnoid Hemorrhage]). Stroke, 2017. 48(1): p. 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Etminan N, et al. , Intrathecal application of the nimodipine slow-release microparticle system eg-1962 for prevention of delayed cerebral ischemia and improvement of outcome after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl, 2015. 120: p. 281–6. [DOI] [PubMed] [Google Scholar]
- 213.Thorne RG and Nicholson C, In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A, 2006. 103(14): p. 5567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mastorakos P, et al. , Highly PEGylated DNA Nanoparticles Provide Uniform and Widespread Gene Transfer in the Brain. Adv Healthc Mater, 2015. 4(7): p. 1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cook RL, et al. , A critical evaluation of drug delivery from ligand modified nanoparticles: Confounding small molecule distribution and efficacy in the central nervous system. J Control Release, 2015. 220(Pt A): p. 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Papisov MI, et al. , Delivery of proteins to CNS as seen and measured by positron emission tomography. Drug Deliv Transl Res, 2012. 2(3): p. 201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Klostergaard J. and Seeney CE, Magnetic nanovectors for drug delivery. Nanomedicine, 2012. 8 Suppl 1: p. S37–50. [DOI] [PubMed] [Google Scholar]
- 218.Lueshen E, et al. , Intrathecal magnetic drug targeting using gold-coated magnetite nanoparticles in a human spine model. Nanomedicine (Lond), 2014. 9(8): p. 1155–69. [DOI] [PubMed] [Google Scholar]
- 219.Kheirkhah P, et al. , Magnetic Drug Targeting: A Novel Treatment for Intramedullary Spinal Cord Tumors. Sci Rep, 2018. 8(1): p. 11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hersh DS, et al. , MR-guided transcranial focused ultrasound safely enhances interstitial dispersion of large polymeric nanoparticles in the living brain. PLoS One, 2018. 13(2): p. e0192240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Xie L, et al. , Sleep drives metabolite clearance from the adult brain. Science, 2013. 342(6156): p. 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pizzo ME, et al. , Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol, 2018. 596(3): p. 445–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Johanson CE, et al. , Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res, 2008. 5: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pardridge WM, CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv, 2016. 13(7): p. 963–75. [DOI] [PubMed] [Google Scholar]
- 225.Vuillemenot BR, et al. , Safety Evaluation of CNS Administered Biologics-Study Design, Data Interpretation, and Translation to the Clinic. Toxicol Sci, 2016. 152(1): p. 3–9. [DOI] [PubMed] [Google Scholar]
- 226.Baudrie V, et al. , Determination of cerebrospinal fluid production rate using a push-pull perfusion procedure in the conscious rat. Fundam Clin Pharmacol, 1990. 4(3): p. 269–74. [DOI] [PubMed] [Google Scholar]
- 227.Chiu C, et al. , Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS, 2012. 9(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang Q, et al. , Monoclonal antibody exposure in rat and cynomolgus monkey cerebrospinal fluid following systemic administration. Fluids Barriers CNS, 2018. 15(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang YF, et al. , Cerebrospinal fluid may mediate CNS ischemic injury. Cerebrospinal Fluid Res, 2005. 2: p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Welch K, Secretion of Cerebrospinal Fluid by Choroid Plexus of the Rabbit. Am J Physiol, 1963. 205: p. 617–24. [DOI] [PubMed] [Google Scholar]
- 231.Thomas WB, Hydrocephalus in dogs and cats. Vet Clin North Am Small Anim Pract, 2010. 40(1): p. 143–59. [DOI] [PubMed] [Google Scholar]
- 232.Lorenzo AV, Hammerstad JP, and Cutler RW, Cerebrospinal fluid formation and absorption and transport of iodide and sulfate from the spinal subarachnoid space. J Neurol Sci, 1970. 10(3): p. 247–58. [DOI] [PubMed] [Google Scholar]
- 233.Boulton M, et al. , Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol, 1998. 274(1): p. R88–96. [DOI] [PubMed] [Google Scholar]
- 234.Chen RL, et al. , Age-related changes in choroid plexus and blood-cerebrospinal fluid barrier function in the sheep. Exp Gerontol, 2009. 44(4): p. 289–96. [DOI] [PubMed] [Google Scholar]
- 235.Chen CP, Chen RL, and Preston JE, The influence of cerebrospinal fluid turnover on age-related changes in cerebrospinal fluid protein concentrations. Neurosci Lett, 2010. 476(3): p. 138–41. [DOI] [PubMed] [Google Scholar]
- 236.Chen CP, Chen RL, and Preston JE, The influence of ageing in the cerebrospinal fluid concentrations of proteins that are derived from the choroid plexus, brain, and plasma. Exp Gerontol, 2012. 47(4): p. 323–8. [DOI] [PubMed] [Google Scholar]
- 237.Curran RE, et al. , Cerebrospinal fluid production rates determined by simultaneous albumin and inulin perfusion. Exp Neurol, 1970. 29(3): p. 546–53. [DOI] [PubMed] [Google Scholar]
- 238.Hodel J, et al. , Imaging of the entire cerebrospinal fluid volume with a multistation 3D SPACE MR sequence: feasibility study in patients with hydrocephalus. Eur Radiol, 2013. 23(6): p. 1450–8. [DOI] [PubMed] [Google Scholar]
- 239.Chazen JL, et al. , Automated segmentation of MR imaging to determine normative central nervous system cerebrospinal fluid volumes in healthy volunteers. Clin Imaging, 2017. 43: p. 132–135. [DOI] [PubMed] [Google Scholar]
- 240.Toma M. and Nguyen PDH, Fluid-structure interaction analysis of cerebrospinal fluid with a comprehensive head model subject to a rapid acceleration and deceleration. Brain Inj, 2018. 32(12): p. 1576–1584. [DOI] [PubMed] [Google Scholar]
- 241.Okuda T, et al. , [Prolonged antinociceptive effect of poly (DL-lactic acid)-fentanyl composites after their intrathecal injection in rats]. Masui, 1999. 48(2): p. 141–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.