Abstract
Background: Smaller studies suggest lower morbidity and mortality associated with coronavirus disease 2019 (COVID-19) in women. Our aim is to assess the impact of female sex on outcomes in a large cohort of patients hospitalized with COVID-19.
Materials and Methods: This is a retrospective observational cohort study of 10,630 adult patients hospitalized with a confirmed COVID-19 polymerase chain reaction between March 1, 2020 and April 27, 2020, with follow-up conducted through June 4, 2020. Logistic regression was used to examine the relationship between sex and the primary outcomes, including length of stay, admission to intensive care unit (ICU), need for mechanical ventilation, pressor requirement, and all-cause mortality as well as major adverse events and in-hospital COVID-19 treatments.
Results: In the multivariable analysis, women had 27% lower odds of in-hospital mortality (odds ratio [OR] = 0.73, 95% confidence interval [CI] 0.66–0.81; p < 0.001), 24% lower odds of ICU admission (OR = 0.76, 95% CI 0.69–0.84; p < 0.001), 26% lower odds of mechanical ventilation (OR = 0.74, 95% CI 0.66–0.82; p < 0.001), and 25% lower odds of vasopressor requirement (OR = 0.75, 95% CI 0.67–0.84; p < 0.001). Women had 34% less odds of having acute cardiac injury (OR = 0.66, 95% CI 0.59–0.74; p < 0.001; n = 7,289), 16% less odds of acute kidney injury (OR = 0.84, 95% CI 0.76–0.92; p < 0.001; n = 9,840), and 27% less odds of venous thromboembolism (OR = 0.73, 95% CI 0.56–0.96; p < 0.02; c-statistic 0.85, n = 9,407).
Conclusions: Female sex is associated with lower odds of in-hospital outcomes, major adverse events, and all-cause mortality. There may be protective mechanisms inherent to female sex, which explain differences in COVID-19 outcomes.
Keywords: COVID-19, sex, gender, hospital outcomes, intensive care, mortality
Introduction
As an early epicenter of the coronavirus disease 2019 (COVID-19) pandemic in the United States, New York State has felt a significant impact from COVID-19 with over 500,000 cases; New York City accounting for over half the state's cases with over 728,000 reported cases and nearly 35,000 deaths due to COVID-19 as of October 25, 2020.1,2 Despite this overwhelming volume, our understanding about the progression, pathophysiology, clinical presentations, and optimal treatment regimens remain limited. Complications of COVID-19 include, but are not limited to, acute respiratory distress syndrome, cardiac injury, acute kidney injury (AKI), venous and arterial thromboembolic events, neurological and neuropsychiatric complications, multisystem organ failure, shock, and death.
Studies from China demonstrate that the prevalence of COVID-19 is similar among males and females, however, a higher proportion of critically ill patients are male with higher mortality rates.3,4 One study from Italy reported a male:female mortality ratio of 3:1, while other countries have shown a mortality ratio of 2:1.5 Proposed mechanisms for sex-based differences include hormonal and estrogen-specific effects, immunological states, immune response pathways, prevalence of baseline comorbidities, differential health behaviors, and prevalence of smoking.6,7 Although sex-specific research is essential to delineate pathways responsible for differences in risk, to our knowledge, there is no large-scale study focusing on sex-based differences in COVID-19-associated hospital outcomes.
With one of the largest data sets of hospitalized COVID-19 patients, our health care network provides a unique opportunity to examine sex differences on a large scale. The current study examines the impact of female and male sex on differences on in-hospital outcomes, major adverse events, and treatment regimens to find sex-specific targets that may ameliorate risk.
Materials and Methods
Study design, setting, and population
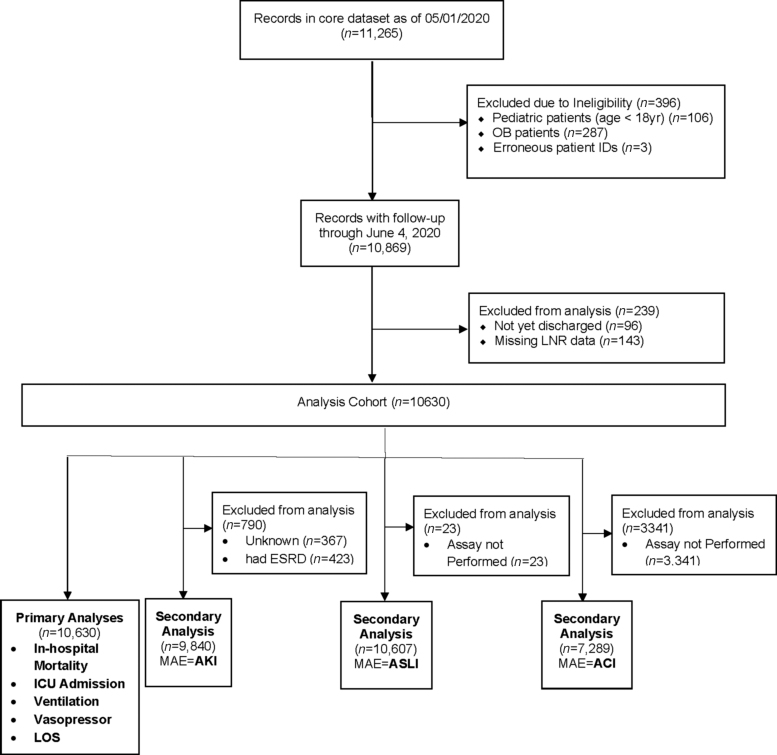
This was a retrospective observational cohort study of a large New York Health System. Eligible patients included adults aged 18 years and older who tested positive by polymerase chain reaction testing of a nasopharyngeal sample for COVID-19 and were hospitalized in 1 of 13 acute care hospitals from March 1, 2020 to April 27, 2020. Follow-up was conducted through June 4, 2020 (Fig. 1).
FIG. 1.
Analysis consort flow diagram. Inclusion and exclusion criteria applied to 11,265 COVID-19 inpatients at a single health system admitted from March 1, 2020 and April 27, 2020 with follow-up conducted through June 4, 2020.
Patients who were transferred between hospitals within the health system were merged and treated as one encounter. Those transferred to hospitals outside of the health system were considered discharged. Patients were considered discharged whether discharged dead or discharged alive. For patients who had multiple qualifying hospital admissions, we included the first. Data were obtained from the enterprise inpatient electronic health record (EHR, Sunrise Clinical Manager; Invision, Allscripts, Chicago, IL). Patients were identified as male or female based on the recorded sex in the EHR.
The focus of this study was on adult patients who were hospitalized for acute complications due to COVID. Obstetric patients with a positive COVID diagnosis were admitted to the hospital for delivery of a newborn, and not for acute COVID-related complications. Accordingly, patients admitted to inpatient obstetric services were excluded. In addition, patients were excluded if they were younger than 18 years of age at time of admission or had erroneous patient identification numbers.
Individual-level informed consent was not obtained given the retrospective nature of the analysis. The Institutional Review Board of Northwell Health approved the study protocol before the commencement of the study.
We collected data on patient demographics, comorbidities, home and hospital medications, baseline laboratory results, and intensive care unit (ICU) admission. Start of hospital care was defined as earliest date of presentation to emergency department or direct admission to the hospital. We used patient-reported race and ethnicity and categorized patients into one of five racial/ethnic groups: White, Hispanic or Latino, Black, Asian, Other, and Unknown/Declined. Anyone who did not select Hispanic/Latino was classified according to race group. We categorized patients by English, Spanish, or other language. Insurance was categorized as commercial, Medicaid, Medicare, self-pay, or other. Patients were stratified into three age groups: 18–50, 51–74, or ≥75 years old (Table 1).
Table 1.
Baseline Demographics
| All (n = 10,630), n (%) | Female (n = 4,290), n (40.4%) | Male (n = 6,340), n (59.6%) | p | ||
|---|---|---|---|---|---|
| Age | |||||
| 18–50 | 2,019 (19.0) | 663 (15.5) | 1,356 (21.4) | <0.001 | |
| 51–74 | 5,454 (51.3) | 2,054 (47.9) | 3,400 (53.6) | ||
| 75+ | 3,157 (29.7) | 1,573 (36.7) | 1,584 (25.0) | ||
| Age at admit, mean (SD) | 64.9 (16.1) | 67.3 (16.6) | 63.2 (15.5) | <0.001 | |
| Age at admit, median | 65.0 | 68.0 | 64.0 | ||
| Age at admit, IQR (range) | 54–77 (18–107) | 57–80 (18–107) | 53–74 (18–100) | ||
| BMI | |||||
| Normal weight | 1,994 (18.8) | 834 (19.4) | 1,160 (18.3) | <0.001 | |
| Underweight | 178 (1.7) | 96 (2.2) | 82 (1.3) | ||
| Preobesity | 2,974 (28.0) | 998 (23.3) | 1,976 (31.2) | ||
| Obesity class I | 1,775 (16.7) | 682 (15.9) | 1,093 (17.2) | ||
| Obesity class II | 801 (7.5) | 365 (8.5) | 436 (6.9) | ||
| Obesity class III | 619 (5.8) | 324 (7.6) | 295 (4.7) | ||
| Unknown | 2,289 (21.5) | 991 (23.0) | 1,298 (20.5) | ||
| Ethnicity/Race | |||||
| White | 3,610 (34.0) | 1,500 (35.0) | 2,110 (33.3) | <0.001 | |
| Hispanic or Latino | 2,246 (21.1) | 805 (18.8) | 1,441 (22.7) | ||
| Asian | 908 (8.5) | 325 (7.6) | 583 (9.2) | ||
| Black | 2,221 (20.9) | 1,059 (24.7) | 1,162 (18.3) | ||
| Other | 1,184 (11.1) | 444 (10.4) | 740 (11.7) | ||
| Unknown | 461 (4.3) | 157 (3.7) | 304 (4.8) | ||
| Language | |||||
| English | 8,521 (80.2) | 3,478 (81.1) | 5,043 (79.5) | 0.003 | |
| Spanish | 1,325 (12.5) | 479 (11.2) | 846 (13.3) | ||
| Other | 784 (7.4) | 333 (7.8) | 451 (7.1) | ||
| Insurance | |||||
| Commercial | 3,187 (30.0) | 1,136 (26.5) | 2,051 (32.4) | <0.001 | |
| Medicaid | 2,175 (20.5) | 794 (18.5) | 1,381 (21.8) | ||
| Medicare | 5,010 (47.1) | 2,278 (53.1) | 2,732 (43.1) | ||
| Other | 126 (1.2) | 49 (1.1) | 77 (1.2) | ||
| Self-pay | 132 (1.2) | 33 (0.8) | 99 (1.6) | ||
| Medications | |||||
| Unknowna | 916 (8.6) | 308 (7.2) | 608 (9.6) | ||
| Beta blocker | 2,913 (27.4) | 1,236 (28.8) | 1,677 (26.4) | <0.001 | |
| ACE inhibitor | 1,335 (12.6) | 496 (11.6) | 839 (13.2) | <0.001 | |
| ARB | 1,712 (16.1) | 761 (17.7) | 951 (15.0) | <0.001 | |
| Statin | 3,811 (35.9) | 1,584 (36.9) | 2,227 (35.1) | <0.001 | |
| Antiplatelet and/or anticoagulation | 3,268 (30.7) | 1,333 (31.1) | 1,935 (30.5) | <0.001 | |
| Comorbidities | |||||
| Hypertension | 6,428 (60.5) | 2,742 (63.9) | 3,686 (58.1) | <0.001 | |
| Diabetes mellitus | 3,914 (36.8) | 1,554 (36.2) | 2,360 (37.2) | 0.29 | |
| Coronary artery disease | 1,417 (13.3) | 429 (10.0) | 988 (15.6) | <0.001 | |
| Heart Failure | 916 (8.6) | 394 (9.2) | 522 (8.2) | 0.09 | |
| Peripheral vascular disease | 273 (2.6) | 111 (2.6) | 162 (2.6) | 0.92 | |
| COPD | 666 (6.3) | 288 (6.7) | 378 (6.00) | 0.12 | |
| Asthma | 885 (8.3) | 524 (12.2) | 361 (5.7) | <0.001 | |
| CKD | 509 (4.8) | 180 (4.2) | 329 (5.2) | 0.02 | |
| ESRD | 423 (4.0) | 162 (3.8) | 261 (4.1) | 0.38 | |
| Chronic liver disease | 292 (2.8) | 99 (2.3) | 193 (3.0) | 0.02 | |
| Cancer | 818 (7.7) | 380 (8.9) | 438 (6.9) | <0.001 | |
| Tobacco use | |||||
| Active | 252 (2.4) | 63 (1.5) | 189 (3.0) | <0.001 | |
| Former or smoker (current status unknown) | 1,860 (17.5) | 447 (10.4) | 1,055 (16.6) | ||
| Never | 7,862 (74.0) | 3,383 (78.9) | 4,479 (70.7) | ||
| Unknown | 656 (6.2) | 260 (6.1) | 396 (6.3) | ||
| CCI | |||||
| 0 | 908 (8.5) | 301 (7.0) | 607 (9.6) | <0.001 | |
| 1–2 | 2,208 (20.8) | 732 (17.1) | 1,476 (23.3) | ||
| 3–4 | 2,496 (23.5) | 992 (23.1) | 1,504 (23.7) | ||
| 5+ | 5,018 (47.2) | 2,265 (52.8) | 2,753 (43.4) | ||
| CCI, mean (SD) | 4.8 (3.5) | 5.2 (3.5) | 4.5 (3.5) | <0.001 | |
| CCI, median | 4 | 5 | 4 | ||
| CCI, IQR (range) | 2–7 (0–23) | 3–7 (0–22) | 2–7 (0–23) | ||
Patients who died or were discharged alive who were not missing LNR.
Frequencies and percentages of unknown status were the same for all medications, and were included in the chi-square tests.
ACE inhibitor, angiotensin converting enzyme-inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; IQR, interquartile range; LNR, lymphocyte-to-neutrophil ratio; SD, standard deviation.
Comorbidities were identified by International Statistical Classification of Disease and Related Health Problems, 10th Revision (ICD-10) coding and shown in Table 1. We calculated the Charlson Comorbidity Index (CCI) as a measure of total comorbidity burden.8,9 Smoking history was categorized as active smoker, former smoker, smoker (current status unknown), never a smoker, or unknown. Body mass index (BMI) was categorized as normal weight BMI 18.5–24.9, underweight BMI <18.5, preobesity BMI 25.0–29.9, obesity class I BMI 30.0–34.9, obesity class II 35.0–39.9, or obesity class III BMI ≥40.
Baseline laboratory results were obtained within 48 hours of the time of admission. Baseline troponin levels were identified as tests drawn nearest to the date and time of admission that were within 72 hours before admission up to 48 hours after, and on or before date and time of discharge. Baseline laboratories were categorized into groups as shown in Table 2. For the in-hospital troponin, alanine transaminase (ALT) and aspartate aminotransferase (AST) laboratory assays, results were screened to identify peak levels from blood samples that were drawn between 72 hours before admission and time of discharge.
Table 2.
Baseline Laboratory Markers by Sex
| All (n = 10,630), n (%) | Female (n = 4,290), n (40.4%) | Male (n = 6,340), n (59.6%) | p | |
|---|---|---|---|---|
| ALT, U/L | ||||
| Normal | 4,842 (45.6) | 1,956 (45.6) | 2,886 (45.5) | 0.004 |
| Mildly elevated (>1 to ≤4 × ULNa) | 5,161 (48.5) | 2,074 (48.3) | 3,087 (48.7) | |
| Moderately elevated (>4 to ≤10 × ULN) | 493 (4.6) | 185 (4.3) | 308 (4.9) | |
| Severely elevated (>10 × ULN) | 84 (0.8) | 48 (1.1) | 36 (0.6) | |
| Assay not performed | 50 (0.5) | 27 (0.6) | 23 (0.4) | |
| AST, U/L | ||||
| Normal | 4,276 (40.2) | 2,070 (48.3) | 2,206 (34.8) | <0.001 |
| Mildly elevated (>1 to ≤4 × ULNb) | 5,769 (54.3) | 2,031 (47.3) | 3,738 (59.0) | |
| Moderately elevated (>4 to ≤10 × ULN) | 446 (4.2) | 123 (2.9) | 323 (5.1) | |
| Severely elevated (>10 × ULN) | 89 (0.8) | 42 (1.0) | 47 (0.7) | |
| Assay not performed | 50 (0.5) | 24 (0.6) | 26 (0.4) | |
| D-dimer, ng/mL | ||||
| Normalc | 4,920 (46.3) | 1,946 (45.4) | 2,974 (46.9) | 0.27 |
| Elevated | 504 (4.7) | 199 (4.6) | 305 (4.8) | |
| Severely elevated | 1,256 (11.8) | 503 (11.7) | 753 (11.9) | |
| Unknown | 3,950 (37.2) | 1,642 (38.3) | 2,308 (36.4) | |
| CRP, mg/dL | ||||
| Normald | 128 (1.2) | 77 (1.8) | 51 (0.8) | <0.001 |
| Elevated | 8,210 (77.2) | 3,208 (74.8) | 5,002 (78.9) | |
| Unknown | 2,292 (21.6) | 1,005 (23.4) | 1,287 (20.3) | |
| Ferritin, ng/mL | ||||
| Normale | 1,936 (18.2) | 1,186 (27.7) | 750 (11.8) | <0.001 |
| Elevated | 6,207 (58.4) | 2,038 (47.5) | 4,169 (65.8) | |
| Unknown | 2,487 (23.4) | 1,066 (24.9) | 1,421 (22.4) | |
| Troponinf | ||||
| Normal (≤1 × URLg) | 4,833 (45.5) | 1,966 (45.8) | 2,867 (45.2) | <0.001 |
| Mildly elevated (>1 to ≤3 × URL) | 1,136 (10.7) | 423 (9.9) | 713 (11.3) | |
| Severely elevated (>3 × URL) | 1,318 (12.4) | 468 (10.9) | 850 (13.4) | |
| Assay not performed | 3,343 (31.4) | 1,433 (33.4) | 1,910 (30.1) | |
ALT ULN = 25 U/L for women, and 35 U/L for men.
AST ULN = 40 U/L for both women and men.
D-dimer ULN = 230 ng/mL D-dimer units.
CRP ULN = 0.40 mg/dL.
Ferritin ULN = 400 ng/mL.
Troponin values included in analysis include Troponin I, Troponin T, and high sensitivity Troponin T.
Troponin URL as defined in Supplementary Table S1.
ALT, alanine transaminase; AST, aspartate transaminase; CRP, C-reactive protein; ULN, upper limit of normal; URL, upper reference limit.
The following COVID-19 treatment and research protocol medications, including steroids, hydroxychloroquine, tocilizumab, remdesivir, anakinra, and sarilumab were examined. Home medications were determined from home medication reconciliation. A patient was considered to have been admitted to the ICU if there was a recorded date or time of ICU level of care. This date or time was identified as the earliest of start of vasopressors, ventilation, or admission to a named ICU. Length of stay (LOS) was defined as the total number of days spent in the hospital.
As the secondary in-hospital outcomes, acute cardiac injury (ACI), AKI, acute severe liver injury (ASLI), and venous thromboembolism (VTE) were also binary, their relationship to sex were analyzed in the same way as the primary outcome. Secondary outcomes were analyzed using data as available. Since the number of troponin unknowns was large, it was initially included as an outcome by using a trichotomous multinomial logistic regression model. This model was compared to a binary logistic regression using normal and mildly/severely elevated, excluding the unknowns. Since the results of this latter model were nearly identical to the former (i.e., the odds ratios [OR] for sex and covariates), only the latter is reported.
ACI was defined using four unique troponin assays as defined in Supplementary Table S1. Ratios were calculated as troponin test values divided by the upper reference limit corresponding to the assay used. For the analysis of the outcome, patients were identified as having ACI if their in-hospital peak troponin ratios were >1, that is, their troponin levels were categorized as mildly elevated or severely elevated. Of note, 69% of our total subject population of 10, 630 had troponin drawn, per health system COVID-19 protocol recommendations to draw cardiac enzymes on admission, specifically Troponin T or Troponin I, from patients with COVID-19 and cardiovascular disease (CVD) with CVD defined as essential hypertension, diabetes mellitus, coronary artery disease, and congestive heart failure. In addition, troponin sampling was recommended in CVD patients experiencing clinical decompensation, deterioration, or if there was evidence of cardiac injury. Patients who did not have troponin level data were ultimately excluded from the analysis, after determining that such exclusion did not appreciably alter the results when compared to a model that included the missing data (see Statistical Methods section above).
AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria.10,11 Baseline serum creatinine and adjudication of AKI was automatically calculated from a prebuilt operational algorithm, based upon KDIGO AKI criteria and the United Kingdom National Health Service AKI algorithm, which has been previously described.11 Patients with end-stage renal disease or who did not have baseline and/or follow-up test results were excluded from the analysis of the outcome, in-hospital AKI.
ASLI was defined based on ALT and AST assays. Baseline ALT and AST levels with their respective upper limit of normal (ULN) are reported in Table 2. For the analysis of the outcome, in-hospital ASLI, patients were identified as having ASLI if their peak in-hospital ALT or AST levels were >10 × ULN. Patients who were missing ALT and AST level data were excluded from the analysis.
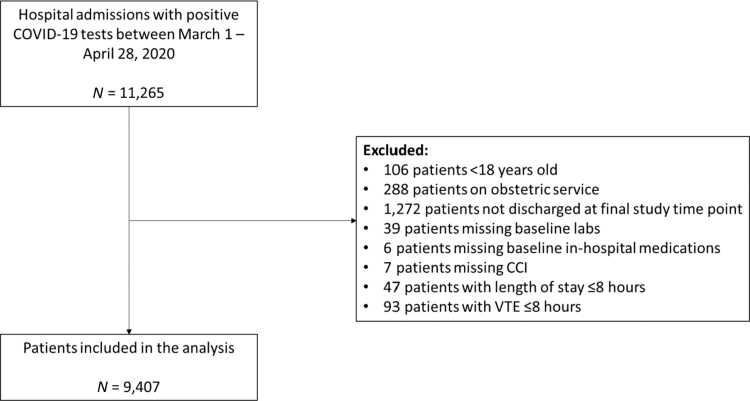
Since VTE is a key component of COVID-19 morbidity and mortality, data were obtained from a separate radiology database in the health system, which included patients with an outcome (discharge or death) on April 30, 2020 (shortly before the current data set) totaling 9,407 patients as opposed to 10,630 in the current analysis (Fig. 2). Deep vein thrombosis was defined as the visualization of deep vein incompressibility (where compression could be performed) or the appearance of echogenic material filling the vein lumen with altered color/spectral Doppler findings as visualized on complete duplex ultrasound or limited point of care ultrasound.12 Diagnosis of pulmonary embolism was confirmed by the appearance of filling defects on computed tomography pulmonary angiography.
FIG. 2.
VTE consort flow diagram. Inclusion and exclusion criteria applied to data obtained from a separate VTE outcomes radiology database in a single health system, including 11,265 COVID-19 inpatients admitted between March 1, 2020 and April 28, 2020 with follow-up conducted through April 30, 2020. CCI, Charlson Comorbidity Index; VTE, venous thromboembolism.
Statistical methods
Descriptive statistics are reported for baseline variables stratified by sex. Continuous variables are reported as means and standard deviations, medians, and interquartile ranges. Categorical variables are reported as frequencies and percentages. Two sample t-tests were used to test for association between sex and continuous variables. Chi-square tests or Fisher's exact tests were used to test for association between sex and categorical variables. “Unknown” and “Missing” were considered as one combined category, “Unknown.” For lymphocyte-to-neutrophil ratio (LNR), the percentage of records with missing values was less than 10%, and these records with missing values were excluded from all analyses.
Logistic regression was used to examine the relationship between sex and the primary outcomes, adjusting for baseline demographic, clinical, and laboratory factors. Backward elimination was used to select the final model. The candidate variables for inclusion in the model were sex, age, ethnicity/race, insurance, BMI, CCI, LNR, D-dimer, C-reactive protein (CRP), ferritin, and antiplatelet/anticoagulant with defined groups listed above. These “candidate variables” were used in the backward elimination model for each of the outcomes. The interaction between sex and age group was also examined because it was postulated that the effect of sex might depend on age. Results from logistic regression models were reported as OR, 95% confidence intervals (CI), p-values, c-statistics [(c); where the c-statistic is the area under the ROC curve].
Multiple linear regression, with backward elimination, was used to examine the relationship between sex and LOS after natural log transformation of LOS, adjusting for baseline demographic, clinical, and laboratory factors. The candidate variables for inclusion in the model were as listed above, in addition to the interaction between sex and age group.
All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC). A p-value <0.05 was considered statistically significant.
Results
After applying exclusion criteria to the overall cohort, 10,630 patients met study criteria with 40.4% being female, 34.0% white, 21.1% Hispanic, 20.9% black, and 8.5% Asian. Of the patients, 51.3% were aged 51–74 years, and 29.7% were 75 years of age or older. Obesity and preobesity were noted in 30.0% and 28.0%, respectively, and a CCI of 5 or greater was noted in 47.2% (Table 1).
Age distribution was associated with sex (p < 0.001), with more men in the 51–74 age group, and more women in the 75+ group. BMI distribution was associated with sex (p < 0.001), with more men with preobesity and class I obesity, and more women with class II obesity or class III obesity. CCI was associated with sex (p < 0.001), with women more likely to have a CCI of 5 or greater. Hypertension, asthma, and cancer were more commonly noted in women, while CAD, CKD, and chronic liver disease were more commonly noted in men. Smoking status was associated with sex (p < 0.001), with active and prior tobacco use or smoker (current status unknown) more commonly noted in men as shown in Table 1.
With respect to outpatient medications, women were more commonly on beta blockers, ARBs, statins, and antiplatelet and/or anticoagulation therapy, while men were more commonly on angiotensin converting enzyme inhibitors as shown in Table 1. At baseline, median neutrophil count and creatinine were higher in men while median lymphocyte count and LNR were higher in women than men, p < 0.001 (Supplementary Table S2).
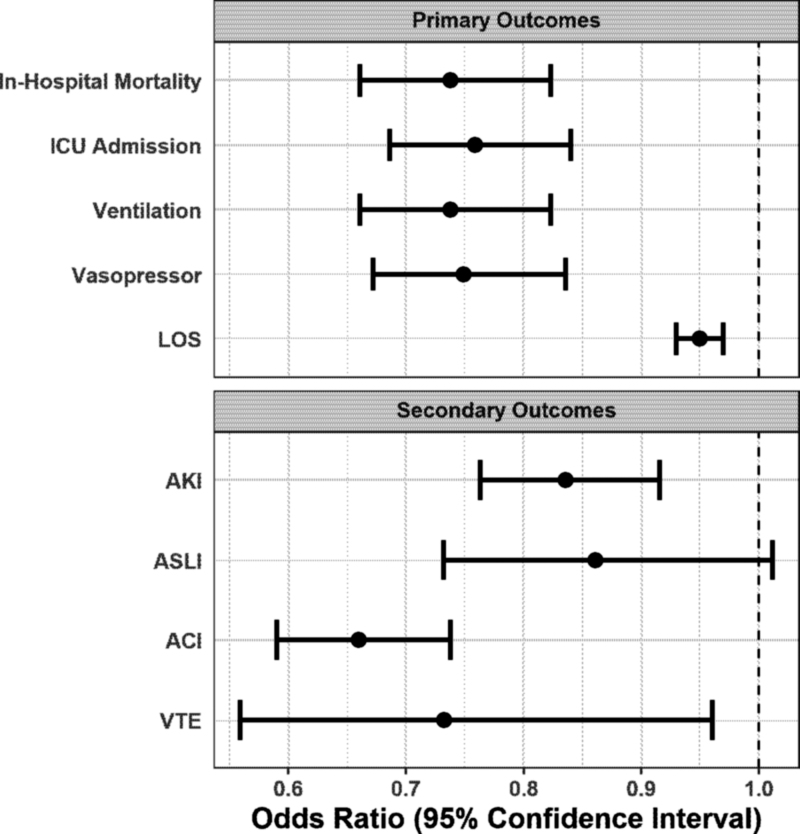
With respect to the primary outcomes, after controlling for age, ethnicity/race, insurance [included in final models for in-hospital mortality and LOS only], BMI, CCI, LNR, D-dimer, CRP, ferritin, and antiplatelet/anticoagulation, women had 27% lower odds of in-hospital mortality (OR = 0.73, 95% CI 0.66–0.81; p < 0.001), 24% lower odds of being admitted to the ICU (OR = 0.76, 95% CI 0.69–0.84; p < 0.001), 26% lower odds of being placed on mechanical ventilation (OR = 0.74, 95% CI 0.66–0.82; p < 0.001), and 25% lower odds of requiring vasopressors (OR = 0.75, 95% CI 0.67–0.84; p < 0.001), as shown in Table 3, Figure 3 and Supplementary Table S5. Examination of the relationship between sex and LOS demonstrated females to have lower LOS compared to males (adjusted geometric mean 4.1% ± 9% vs. 4.3% ± 9%, p = 0.01) as shown in Supplementary Table S3.
Table 3.
Multivariable Predictors of In-Hospital Mortality, Intensive Care Unit Admission, Ventilation, and Vasopressor Requirement in Comparison to Sex
| In-hospital mortalitya |
ICU admission |
Ventilation |
Vasopressor |
|||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Gender | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.73 | 0.66–0.81 | 0.76 | 0.69–0.84 | 0.74 | 0.66–0.82 | 0.75 | (0.67–0.84) |
| c-Statistic | 0.77 | 0.67 | 0.70 | 0.70 | ||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | ||||
Statistically significant variables controlled for include age, ethnicity/race (for in-hospital mortality only), insurance, BMI, CCI, LNR, D-dimer, CRP, ferritin, antiplatelet/anticoagulation.
CI, confidence interval; ICU, intensive care unit.
FIG. 3.
Forest plot of primary outcomes and major adverse events. Forest Plot showing the odds ratio for primary outcomes, after controlling for age, ethnicity/race, insurance [included in final models for in-hospital mortality and LOS only], BMI, CCI, LNR, D-dimer, CRP, ferritin and antiplatelet/anticoagulation in 10,630 COVID-19 inpatients meeting eligibility criteria at a single health system admitted from March 1, 2020 and April 27, 2020 with follow-up conducted through June 4, 2020. ACI, acute cardiac injury; AKI, acute kidney injury; ASLI, acute severe liver injury; BMI, body mass index; CRP, C-reactive protein; ICU, intensive care unit; LNR, lymphocyte-to-neutrophil ratio; LOS, length of stay.
With respect to major adverse events, as shown in Figure 3 and Table 4, unadjusted logistic regression analysis shows women to have 24% lower odds of having ACI (OR = 0.76, 95% CI 0.69–0.84; p < 0.001; c = 0.53; n = 7,289) and 34% lower odds after multivariable analysis (OR = 0.66, 95% CI 0.59–0.74; p < 0.001; c = 0.76; n = 7,289). AKI findings were similar to ACI. Unadjusted logistic regression analysis shows women to have 14% lower odds of having AKI (OR = 0.86, 95% CI 0.79–0.93; p < 0.001; c = 0.52) and 16% lower odds after multivariable analysis (OR = 0.84, 95% CI 0.76–0.92; p < 0.001; c = 0.70; n = 9,840). With respect to the major adverse event of ASLI, unadjusted logistic regression analysis shows women to have 29% lower odds of having ASLI compared to men (OR = 0.71, 95% CI 0.61–0.83; p < 0.001; c = 0.54), with no statistically significant difference noted after multivariable analysis (OR = 0.86, 95% CI 0.73–1.01; p = 0.07; c = 0.64; n = 10,607). In addition, the VTE rate was significantly higher in men compared to women (3.3% vs. 2.3%, p = 0.003). Unadjusted logistic regression analysis shows women to have 32% lower odds of developing VTE compared to men (OR = 0.68, 95% CI 0.53–0.88; p = 0.004; c = 0.54) and 27% lower odds after multivariable analysis (OR = 0.73, 95% CI 0.56–0.96; p < 0.03; c = 0.85; n = 9,407).
Table 4.
Secondary Analysis Results of Major Adverse Events in Comparison to Sex
| No.of females with outcome/total females (%) | No. of males with outcome/total males (%) | Odds ratio (female vs. male) | 95% CI | c-Statistic | p-Value (logistic model)a | Odds ratio (female vs. male) | 95% CI | c-Statistic | p-Value (logistic model)a | |
|---|---|---|---|---|---|---|---|---|---|---|
| AKI, n = 9,840b | 1,469/3,970 (37.0) | 2,384/5,870 (40.6) | 0.86 | 0.79–0.93 | 0.52 | <0.001 | 0.84 | 0.76–0.92 | 0.70 | <0.001 |
| ASLI, n = 1,0607c | 254/4,280 (5.9) | 518/6,327 (8.2) | 0.71 | 0.61–0.83 | 0.54 | <0.001 | 0.86 | 0.73–1.01 | 0.64 | 0.07 |
| ACI, n = 7,289d | 1,072/2,858 (37.5) | 1,954/4,431 (44.1) | 0.76 | 0.69–0.84 | 0.53 | <0.001 | 0.66 | 0.59–0.74 | 0.76 | <0.001 |
| VTE, n = 9,407e | 88/3,827 (2.3) | 186/5,580 (3.3) | 0.68 | 0.53–0.88 | 0.54 | 0.004 | 0.73 | 0.56–0.96 | 0.85 | <0.03f |
Statistically significant variables controlled for include age, BMI, CCI, ethnicity/race, insurance, D-dimer, CRP, ferritin, antiplatelet/anticoagulation, LNR.
Excluded n = 790 (7.4%) from AKI analysis if their status was missing, unknown or they had baseline ESRD.
Excluded n = 23 (0.22%) from ASLI analysis for whom assay was not performed.
Excluded n = 3,341 (31%) from ACI analysis for whom troponin assay was not performed.
Excluded n = 1,858 (16%) from VTE analysis for those missing baseline laboratories, in-hospital medications, CCI, <18 years old, obstetric patients, LOS ≤8 hours, VTE ≤8 hours, or not discharged at final study time point.
Statistically significant variables controlled for include history of VTE, COPD, steroid use within 48 hours, immunosuppressant use within 48 hours, azithromycin use within 48 hours, HCQ use within 48 hours, Famotidine use within 48 hours, D-dimer, prior ICU stay, and anticoagulation used within 24 hours.
ACI, acute cardiac injury; AKI, acute kidney injury; ASLI, acute severe liver injury; LOS, length of stay; VTE, venous thromboembolism.
Inpatient COVID-19 drug regimens included steroids, hydroxychloroquine, tocilizumab, remdesivir, anakinra, and sarilumab. Of the drug regimens examined, the maximum number of drugs that any single patient received was four. In general, men received more treatments (p < 0.001) with 43.9% receiving two or more regimens compared to 36.0% in women (Supplementary Tables S4 and S6). In all the analyses, we looked at the interaction between age and sex to determine if the effect of sex depended on age. In all of the analyses, no interaction was observed, and therefore, all models included only main effects of age and sex.
Discussion
This comprehensive analysis is the largest study to date that directly assesses the impact of sex on COVID-19 outcomes. Our study strongly demonstrates female sex to be associated with lower odds of in-hospital outcomes, major adverse events, and all-cause mortality compared to male sex after controlling for confounding variables.
In our study, males were younger, had higher prevalence of CAD, CKD, chronic liver disease, history of smoking, and lower prevalence of class II and III obesity, whereas females had higher prevalence of hypertension, asthma, and cancer. Similarly, the TriNetX Network, a global federated health research multinational registry of COVID-19 patients, showed a high prevalence of comorbidities in men, however, this could not entirely explain the higher all-cause mortality noted in men compared to women (8.1% vs. 4.6%).13
Older age, smoking status, and a high CCI have been shown to be associated with poor prognoses for severe and critical COVID-19 cases,14 with elevated CCI associated with an increased risk of severe COVID-19 morbidity and mortality when controlled for age and sex.15 In contrast to these aforementioned study findings, our large scale analysis demonstrates that despite controlling for older age and higher mean CCIs in women, their odds of developing primary in-hospital outcomes, including all-cause mortality and major adverse events, were still significantly lower than men. It remains unclear whether the increase in adverse events, such as AKI, ACI, and VTE, noted in men in our study was simply a marker of more severe COVID illness or if the adverse events themselves are the mechanistic link to worse outcomes. Mechanisms such as increased thrombogenicity and severe microvascular endothelial injury, which have been shown to be associated with procoagulant state in COVID-19 infection,16 could account for some of the adverse events noted more frequently in men, however, could not be assessed adequately in this study.
It has been proposed that females, compared to males, are less susceptible to viral infections due to differences in innate immunity, steroid hormones, and factors related to sex chromosomes.17 One potential explanation is that immune regulatory genes encoded by the X-chromosome cause lower viral load levels and decreased inflammation.17 In females, the biallelic expression pattern over many X-chromosome genes may contribute to greater risk of autoimmune disease; however, there may be a protective effect that is beneficial from an infectious disease standpoint, influencing response to viral infections.18
Sex chromosome genes and sex hormones influence the varied immune response between the sexes, including activation of endothelial estrogen receptors which increases nitric oxide and decreases reactive oxygen species (ROS), protecting the vascular system from vasoconstriction, inflammation, and ROS production.6,18–20 In a study of SARS-CoV in mice, male mice underwent gonadectomy, which did not impact disease outcomes; however, female mice treated with estrogen receptor antagonist therapy or oophorectomy demonstrated increased mortality to SARS-CoV, suggesting female sex hormone protective mechanism.21 Although estrogen is presumed to be protective in women in the premenopausal years, there was no worsening of outcomes noted in older women in our study.
Although outside the scope of our current analysis, it is worth mentioning the intersection between sex and gender. Social norms and behaviors have been postulated to be potential gender-based behavior influencers contributing to differences in COVID-19 outcomes between men and women. Women demonstrate higher regard for practicing protective behaviors such as hand washing and mask wearing,7,22 while behaviors more prevalent in men and associated to male gender, such as smoking and alcohol consumption, are linked to comorbidities associated with adverse outcomes in COVID-19.23,24 Although our study had significantly more men presently smoking or with history of smoking, smoking was accounted for in our multivariable analysis with women repeatedly shown to fare better than their male counterparts in terms of primary outcomes and major adverse events.
This study has several limitations. Given the high acuity and volume of patients presenting to the hospitals, documentation may have been limited or incomplete. Data were collected from the EHR, thereby impeding detailed medical history review. Testing and treatment were inconsistent as they were based on clinical judgment in a rapidly changing environment with limited knowledge of best practices.
Conclusions
This retrospective observational cohort study of a large New York Health System showed that female sex is associated with lower odds of in-hospital outcomes, major adverse events, including AKI, ACI, and VTE, and all-cause mortality as compared to males. The differing outcomes between males and females infected with COVID-19 may be due to protective factors inherent to female sex and protective behaviors typically associated with female gender. These findings should not lead to the assumption that women require less clinical concern or attention when presenting with COVID-19. In addition, educating men and women on preventative behavior measures that mitigate risk of contraction and spread of respiratory illnesses such as COVID-19 is imperative. For a more comprehensive understanding of female sex and the association with better outcomes and fewer major adverse events, additional genetic, immunologic, hormonal, and behavioral science analysis is warranted to address this complex question.
Supplementary Material
Acknowledgments
We acknowledge the contributions of the Northwell Health COVID-19 Research Consortium. We also acknowledge and honor all of our Northwell team members who consistently put themselves in harm's way during the COVID-19 pandemic. We dedicate this article to them, as their vital contribution to knowledge about COVID-19 and sacrifices on the behalf of patients made it possible.
Authors' Contributions
A.T.: Conceptualization, Writing—Original Draft, Writing—Review and Editing.
E.G.: Conceptualization, Writing—Original Draft, Writing—Review and Editing, Supervision, Project Administration.
J.C.: Methodology, Software, Validation, Data Curation, Writing—Review and Editing.
J.S.H.: Methodology, Software, Validation, Data Curation, Writing—Review and Editing.
S.R.: Conceptualization; Writing—Review and Editing.
N.K.: Methodology, Software, Validation.
M.L.: Methodology, Software, Validation, Writing—Review and Editing.
C.W.: Writing—Review and Editing.
D.M.: Methodology, Writing—Original Draft.
S.K.S.: Writing—Original Draft; Writing—Review and Editing
D.B.: Writing—Review and Editing.
M.A.B.: Writing—Original Draft.
A.C.S.: Writing—Review and Editing.
R.-M.B.: Conceptualization, Writing—Original Draft, Writing—Review and Editing, Supervision, Project Administration.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by grants numbered: R24AG064191 from the National Institute on Aging of the National Institutes of Health R01LM012836 from the National Library of Medicine of the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication. The views expressed in this article are those of the authors and do not represent the views of the National Institutes of Health, the U.S. Department of Health and Human Services, or any other government entity.
Supplementary Material
References
- 1. COVID-19 United States Cases by County. Johns Hopkins Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu/us-map Accessed December9, 2020
- 2. New York Covid Map and Case Count—The New York Times. Available at: https://www.nytimes.com/interactive/2020/us/new-york-coronavirus-cases.html Accessed December9, 2020
- 3. Gao Q, Hu Y, Dai Z, Xiao F, Wang J, Wu J. The epidemiological characteristics of 2019 novel coronavirus diseases (COVID-19) in Jingmen, Hubei, China. Medicine (Baltimore) 2020;99:e20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 2020;8:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Stadio A, Ricci G, Greco A, de Vincentiis M, Ralli M. Mortality rate and gender differences in COVID-19 patients dying in Italy: A comparison with other countries. Eur Rev Med Pharmacol Sci 2020;24:4066–4067 [DOI] [PubMed] [Google Scholar]
- 6. Takahashi T, Ellingson MK, Wong P, et al. . Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020;588:315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walter LA, McGregor AJ. Sex- and gender-specific observations and implications for COVID-19. West J Emerg Med 2020;21:507–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 9. Quan H, Sundararajan V, Halfon P, et al. . Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO). Acute Kidney Injury Workgroup KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138 [Google Scholar]
- 11. Hirsch JS, Ng JH, Ross DW, et al. . Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020;98:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Needleman L, Cronan JJ, Lilly MP, et al. . Ultrasound for lower extremity deep venous thrombosis: Multidisciplinary recommendations from the Society of Radiologists in Ultrasound consensus conference. Circulation 2018;137:1505–1515 [DOI] [PubMed] [Google Scholar]
- 13. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID-19: Insights from a multinational registry. Mayo Clin Proc 2020;95:1613–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou W, Qin X, Hu X, Lu Y, Pan J. Prognosis models for severe and critical COVID-19 based on the Charlson and Elixhauser comorbidity indices. Int J Med Sci 2020;17:2257–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christensen, D.M., Strange, J.E., Gislason, G.. et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID-19 patients. J Gen Intern Med 2020;35:2801–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evans PC, Ed Rainger G, Mason JC, et al. . Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res 2020;115:2177–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: Clinical response to viral infection. J Biol Regul Homeost Agents 2020;34:339–343 [DOI] [PubMed] [Google Scholar]
- 18. Sharma G, Volgman AS, Michos ED. Sex differences in mortality from COVID-19 pandemic: Are men vulnerable and women protected? JACC Case Rep 2020;2:1407–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson DP, Huber SA, Moussawi M, et al. . Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ 2011;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol-Heart Circ Physiol 2018;315:H1569–H1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol 2017;198:4046–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Capraro V, Barcelo H.. The effect of messaging and gender on intentions to wear a face covering to slow down cOVID-19 Transmission. PsyArXiv 2020. [Epub ahead of print]; DOI: 10.31234/osf.io/tg7vz [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The Lancet. The gendered dimensions of COVID-19. Lancet 2020;395:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruggieri A, Gagliardi MC. Gender differences in COVID-19: Some open questions. Ital J Gend-Specif Med 2020;6:33219 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.