Abstract
Anaplastic large T-cell lymphoma (ALTCL) is a rare subtype of non-Hodgkin T-cell lymphoma that occasionally occurs in the gastrointestinal tract of humans. Enteropathy-associated T-cell lymphoma (EATL) type 1 is the most common type of intestinal lymphoma in dogs, and ALTCL has not previously been reported in the intestinal tract of dogs. Thirteen dogs with intestinal masses diagnosed as intestinal lymphoma with anaplastic morphology were reviewed. Clinical data, including treatment protocols, were available for 11 cases. Immunohistochemistry for CD3, CD20, and CD30 was performed for all cases in addition to PCR for Antigen Receptor Rearrangements (PARR) for assessment of clonality. Eight (62%) of the cases presented with intestinal perforation, and all cases had 1 or more masses arising from the small intestine. Histologically, all cases were characterized by transmural infiltrates of large, CD3-positive and frequently CD30-positive cells. Neoplastic T cells had marked anisocytosis and anisokaryosis, prominent nucleoli, and occasionally indented to reniform nuclei. There was abundant necrosis and inflammation with occasional vascular invasion within neoplastic masses. All cases had a monoclonal T-cell receptor γ gene rearrangement. The median survival time was 5 days, with 1 dog surviving 2 years after the initial diagnosis. ALTCL can occur as an aggressive transmural lymphoma in the gastrointestinal tract of dogs and commonly causes intestinal perforation. ALTCL can be differentiated from EATL type 1 and might have implications for accurate prognostication and selection of therapeutic options in the future.
Keywords: dog, gastrointestinal, lymphoma, EATL, ALTCL, ALCL, immunohistochemistry
Anaplastic large T-cell lymphoma (ALTCL) is a rare subtype of non-Hodgkin’s T-cell lymphoma first described in humans in 1985.20 According to the most recent World Health Organization (WHO) classification, ALTCL is separated into a primary systemic and a primary cutaneous form with division of the systemic subtype into 2 distinct entities: anaplastic lymphoma kinase (ALK)–positive ALTCL and ALK-negative ALTCL.21 All subtypes are defined by the presence of atypical lymphoid cells with pleomorphic, horseshoe, or kidney-shaped nuclei and abundant cytoplasm (hallmark cells) and expression of the lymphocyte activation marker, CD30.24 ALTCL primarily affects lymph nodes with uncommon involvement of extranodal sites, including soft tissue, bone, lung, liver, and rarely the central nervous system or gastrointestinal tract.1,5,17 While both subtypes of systemic ALTCL may affect the intestine, involvement of the skin, liver, and gastrointestinal tract occurs more frequently in ALK-negative cases.18 ALTCL is poorly characterized in animal species with few case reports of ALTCL in dogs affecting the skin, subcutaneous tissue, and/or lymph nodes.15,23 ALTCL has not yet been described as a distinct entity in the intestine of any animal species.
Primary gastrointestinal lymphoma is uncommon in both humans and dogs, accounting for 10% to 15% and 5% to 7% of lymphoma cases, respectively.21,24 B-cell lymphoma is the predominant primary gastrointestinal lymphoma in humans, while most canine cases are classified as enteropathy-associated T-cell lymphoma (EATL) type 1. EATL type 1 is characterized by large lymphoid cells and is frequently associated with necrosis, transmural infiltration, and a rapid clinical course. In contrast, EATL type 2 is the most common intestinal lymphoma in cats but is uncommon in dogs and typically exhibits a more indolent behavior.11,23 Histologically, it is characterized by small- to intermediate-sized lymphoid cells that predominantly infiltrate the intestinal mucosa and rarely cause necrosis.11,23 In humans, EATL is strongly linked to inflammatory bowel disease. This connection, while suspected, has not been definitively proven in dogs.2
This study aims to describe the clinicopathologic features in a series of cases in dogs with intestinal T-cell lymphoma resembling ALTCL in humans.
Materials and Methods
Case Selection
Thirteen dogs, all of which were patients at different veterinary hospitals throughout the United States and had small intestinal biopsy samples submitted to Antech Diagnostics, were included in this study. Cases were selected based on a diagnosis of confirmed or suspected lymphoma and the histomorphologic features of neoplastic cells, including marked anaplasia and evidence of angioinvasion.
Tissue Processing
One or 2 resected segments of small intestine from each case were submitted to Antech Diagnostics. Cases originated from California, Kansas, Illinois, Massachusetts, New Jersey, and Washington between 2014 and 2017. Some of the cases also included a biopsy of liver, mesenteric lymph node, or spleen. Tissues were routinely sectioned and embedded in paraffin blocks upon receipt. Five microns in thickness sections of each block were routinely stained with hematoxylin and eosin and examined histologically. Special stains were performed for some cases in which marked inflammation was observed, including Grocott’s methenamine silver (GMS) stain, periodic acid–Schiff (PAS) stain, and Ziehl-Neelsen stain.
Immunohistochemistry
Immunohistochemistry for polyclonal rabbit anti-human CD3 (1:200; DAKO) and polyclonal rabbit anti-human CD20 (1:200; Thermo/Neomarkers) was performed on a Bond-Max automated system (Leica Microsystems, Bannockburn, IL) using a biotin-free, polymeric peroxidase-based detection kit with DAB chromagen and hematoxylin counterstain (Bond Polymer Refine Detection kit; Leica Microsystems) as previously described.6 Immunohistochemistry for monoclonal mouse anti-human CD30 (Ber-H2, 1:50; DAKO) was performed on a DAKO 48 immunostainer using a biotin-free, polymeric peroxidase-based detection kit with DAB chromagen and hematoxylin counterstain (FLEX Detection kit; DAKO).15 Cells with CD3, CD20, or CD30 membranous or cytoplasmic labeling were evaluated as positive. Positive controls consisted of sections of canine lymph node, and for negative controls, primary antibodies were replaced with buffer. The percentage of CD30 immunopositive cells was estimated by visual assessment of 1 representative slide per case by 1 pathologist (M.K.).
PCR for Antigen Receptor Rearrangements Testing
DNA extraction was performed on five 5-μm-thick (25 μm thickness total) serial sections from formalin-fixed, paraffin-embedded tissues. Rearrangements of the TCRγ and immunoglobulin heavy chain gene variable regions was assessed by amplifying the complementary determining region 3 (CDR3) as previously described.3,6
Results
Clinical History
Eight cases were spayed females and 5 were males (4 neutered). The mean age at the time of diagnosis was 11 years (range, 9–13 years; median, 11 years). Four cases were Pugs (31%), but no other breed was overrepresented (Table 1).
Table 1.
Clinical and Gross Pathologic Findings in 13 Dogs With Intestinal Anaplastic Large T-Cell Lymphoma.
| Dog No. | Breed | Sex | Age | Presenting Complaints | Gross Lesions | Survival Time |
|---|---|---|---|---|---|---|
| 1 | Blue Heeler | FS | 12 years | Vomiting, anorexia, fever | Small intestinal mass | 48 days |
| 2 | Rhodesian Ridgeback | MC | 10 years | Weight loss | Perforated jejunum, septic peritonitis | NA |
| 3 | Basset Hound | MC | 9 years | NA | Perforated jejunal mass, septic peritonitis | 2 years |
| 4 | Border Collie | MC | 12 years | Vomiting, fever | Perforated jejunum, septic peritonitis | 3 days |
| 5 | Staffordshire Terrier | FS | 10 years | Weight loss, vomiting | Jejunal masses, perforation, and septic peritonitis | 2 days |
| 6 | Poodle | FS | 11 years | Vomiting, fever | Perforated jejunum, septic peritonitis | 6 days |
| 7 | Pug | FS | 10 years | Dyspnea | Jejunal mass, perforation, and septic peritonitis | 2 days |
| 8 | Pug | FS | 11 years | Hyporexia | Jejunal and ileal masses | NA |
| 9 | Weimaraner | MC | 12 years | Weight loss, vomiting | Two jejunal masses | 34 days |
| 10 | Pug | FS | 13 years | Weight loss, hyporexia | Jejunal mass, liver mass | 5 days |
| 11 | Pug | M | 12 years | Seizures | Jejunal mass, hepatomegaly | 18 days |
| 12 | Greyhound | FS | 11 years | NA | Perforated jejunum, septic peritonitis | 3 days |
| 13 | Irish Terrier | FS | 10 years | Vomiting | Perforated jejunum, septic peritonitis | 64 days |
Abbreviations: FS, spayed female; M, intact male; MC, castrated male; NA, not applicable.
Eight cases presented with a small intestinal perforation and septic peritonitis diagnosed via cytology of abdominal fluid. The predominant clinical signs were vomiting, hyporexia or anorexia, pyrexia, and weight loss. One case presented for seizures, and a jejunal mass was discovered on abdominal ultrasonography. Seven cases had a single small intestinal mass. In 6 cases, these masses arose from the jejunum; the location of the mass for the remaining case was not reported. One case had 2 jejunal masses, and 2 cases had multiple masses throughout all segments of the small intestine. Two cases exhibited thickened jejunal and duodenal walls, respectively, with loss of defined layering on ultrasound. One case also had a single liver mass, and the liver and mesenteric lymph nodes of another case were enlarged and pale (Table 1). Concurrent reported conditions included diabetes mellitus (1 case), seizures (2 cases), dilated cardiomyopathy (1 case), unspecified heart disease (1 case), and leptospiral hepatopathy (1 case). One case had a history of a previously excised hepatocellular adenoma and inflammatory bowel disease, and another case had a history of pancreatitis and small intestinal rupture attributed to nonsteroidal anti-inflammatory drug (NSAID) use. One case had undergone a gastrotomy and enterotomy on separate occasions 10 years prior for unspecified reasons (Suppl. Table S1). All cases underwent an exploratory laparotomy followed by resection and anastomosis to remove the affected small intestine.
Treatment and Outcome
The mean survival time following diagnosis was 83 days (range, 2 days to 2 years; median, 5 days). Six cases were euthanized due to poor recovery from surgical excision of intestinal lesions (2–5 days). Two cases were presented 33 and 64 days after the initial surgery, respectively, with a second intestinal perforation. One of these cases died the following day while the other case was euthanized due to poor prognosis. Two cases were euthanized 18 to 48 days following diagnosis, but the reason was not specified. Only 1 case received chemotherapy and survived for 2 years following surgery (Table 1). Additional clinical information was not available for the remaining 2 cases.
Histologic Lesions
Lesions in all cases were characterized by transmural infiltrates of neoplastic round cells extending into the serosa and mesenteric adipose tissue (Fig. 1). Neoplastic masses frequently exhibited large areas of ischemic necrosis that ranged from multifocal (4 cases) to extensive and were frequently transmural (5 cases; Fig. 2). Masses were densely cellular and comprised large neoplastic lymphoid cells with indistinct cell margins and a small to abundant amount of eosinophilic cytoplasm. Nuclei were round to oval to occasionally indented or reniform with finely stippled chromatin and 1 to 2 prominent nucleoli. Neoplastic cells exhibited moderate to marked anisocytosis and anisokaryosis with occasional multinucleation (Fig. 3). The mitotic index was highly variable, ranging from 1 to 45 mitoses per 10 high-power (40×) fields with an average mitotic index of 11. Neoplastic infiltrates were also identified within the liver of 3 cases and the mesenteric lymph nodes of 1 case (Suppl. Table S1). Neoplastic infiltrates multifocally contained small numbers of large lymphoid cells with an eccentric, horseshoe-shaped to reniform nucleus, prominent nucleoli, abundant cytoplasm, and a variably distinct, eosinophilic Golgi region adjacent to the nucleus (hallmark cells; Fig. 4). There were 1 to 10 of these cells per ten 40× fields.
Figure 1. Anaplastic large T-cell lymphoma, jejunum, dog. Hematoxylin and eosin.
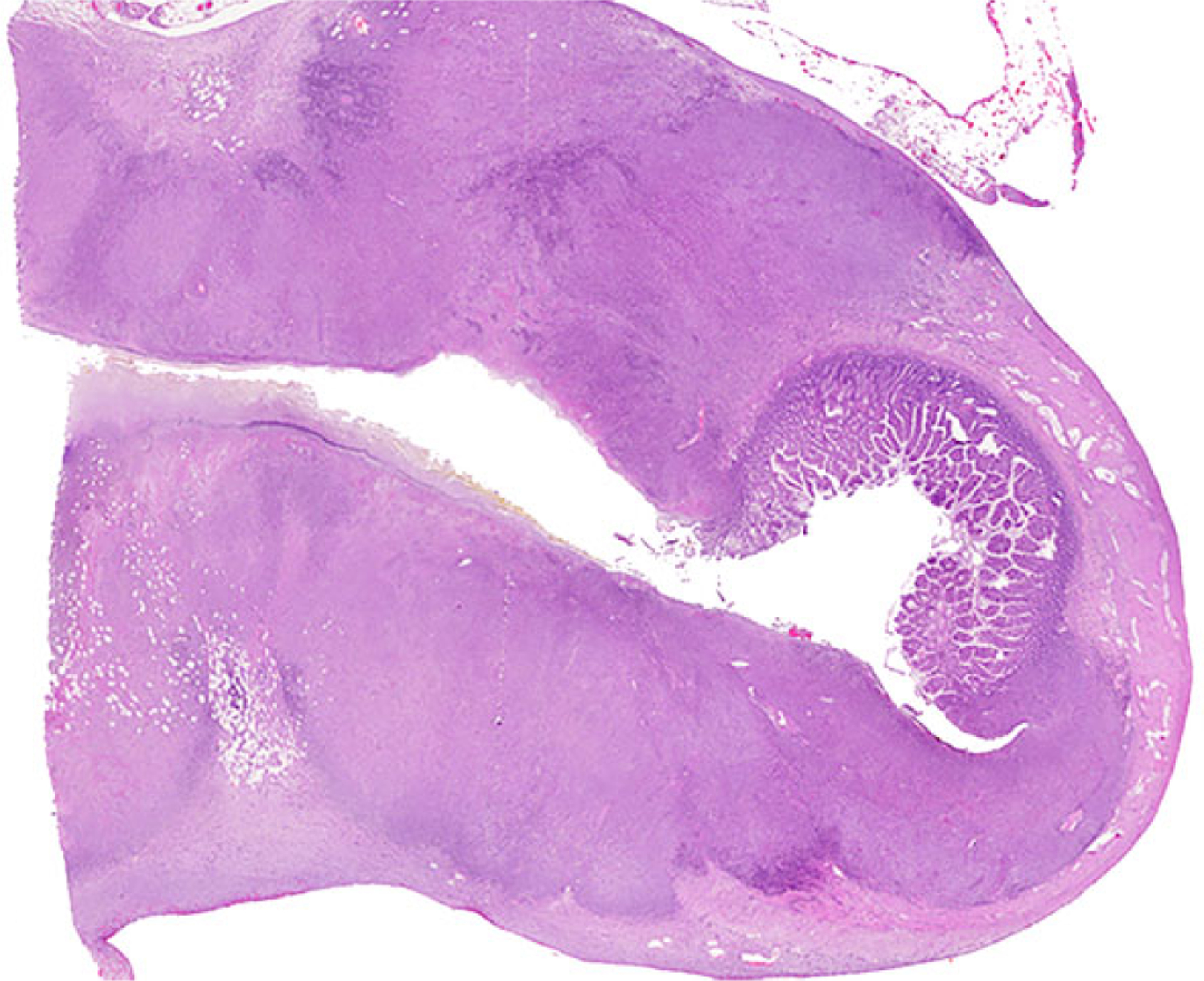
Dog No. 1. Transmural infiltrates of neoplastic lymphoid cells.
Figure 2. Anaplastic large T-cell lymphoma, jejunum, dog. Hematoxylin and eosin.
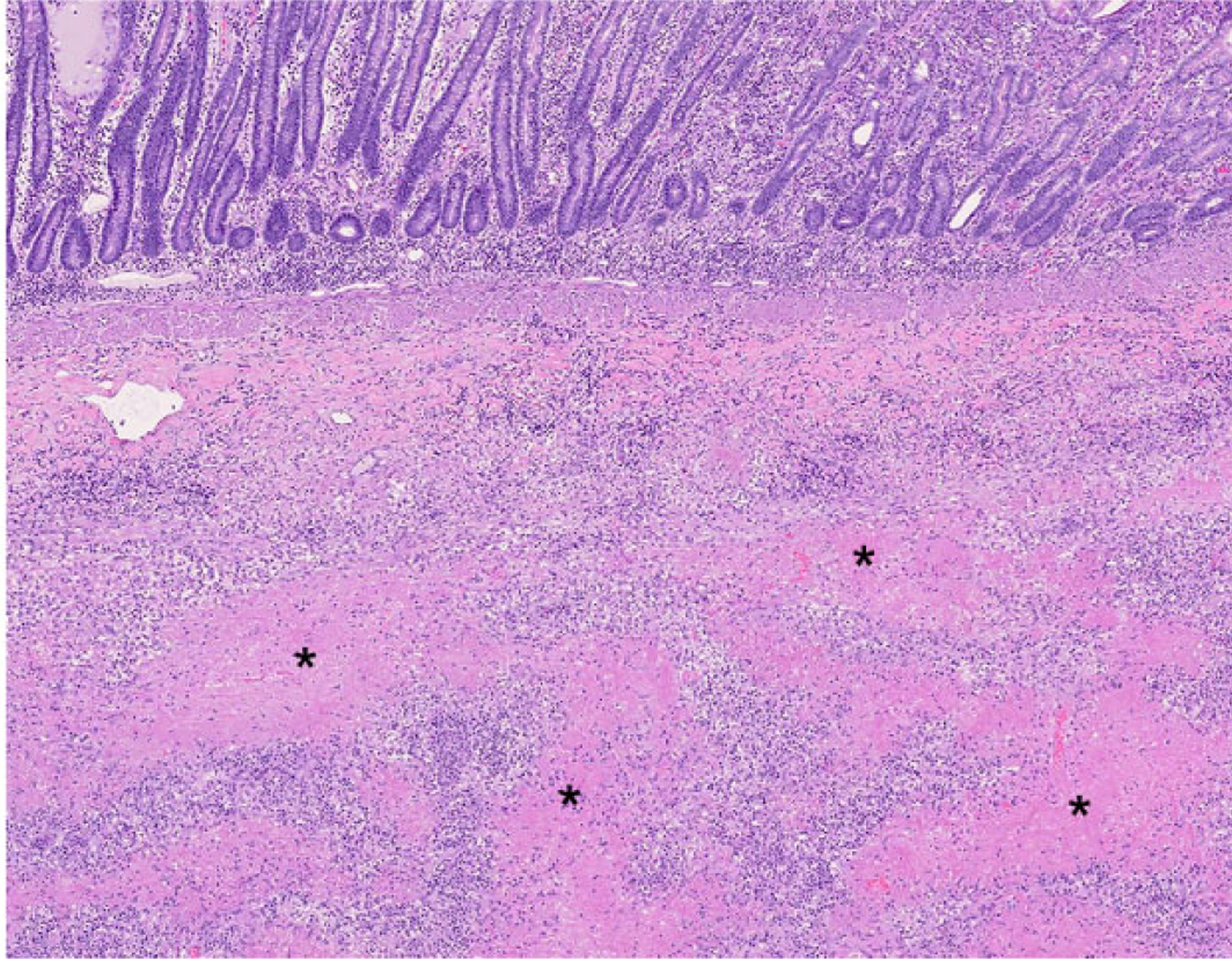
Dog No. 11. Multifocal to coalescing extensive areas of coagulative necrosis (asterisks) surrounded by neoplastic cells.
Figure 3. Anaplastic large T-cell lymphoma, jejunum, dog.
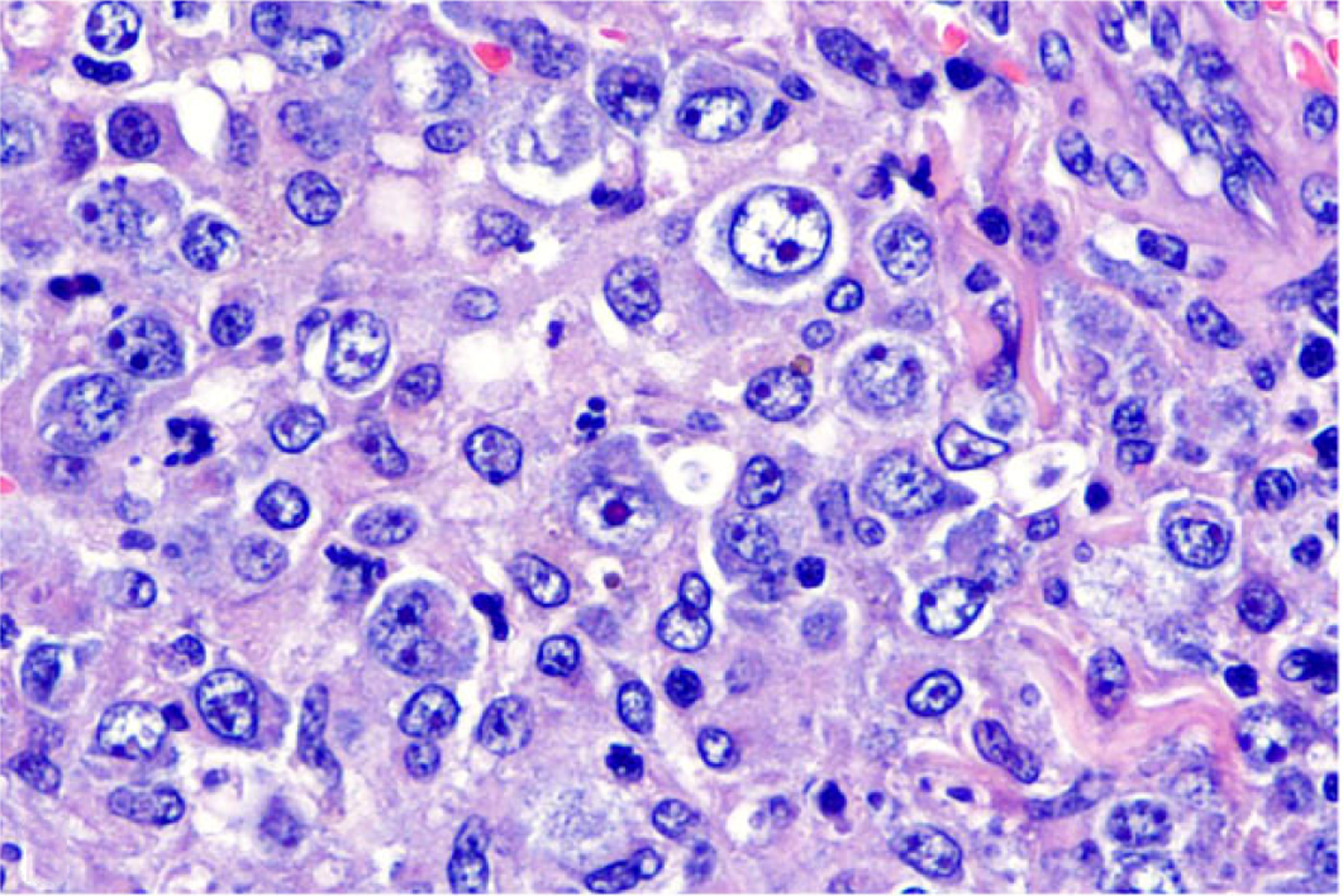
Dog No. 10. Large neoplastic cells with abundant eosinophilic cytoplasm and round to oval nuclei with 1 to 2 prominent nucleoli. Anisocytosis and anisokaryosis are moderate to marked. Hematoxylin and eosin (HE).
Figure 4. Anaplastic large T-cell lymphoma, jejunum, dog.
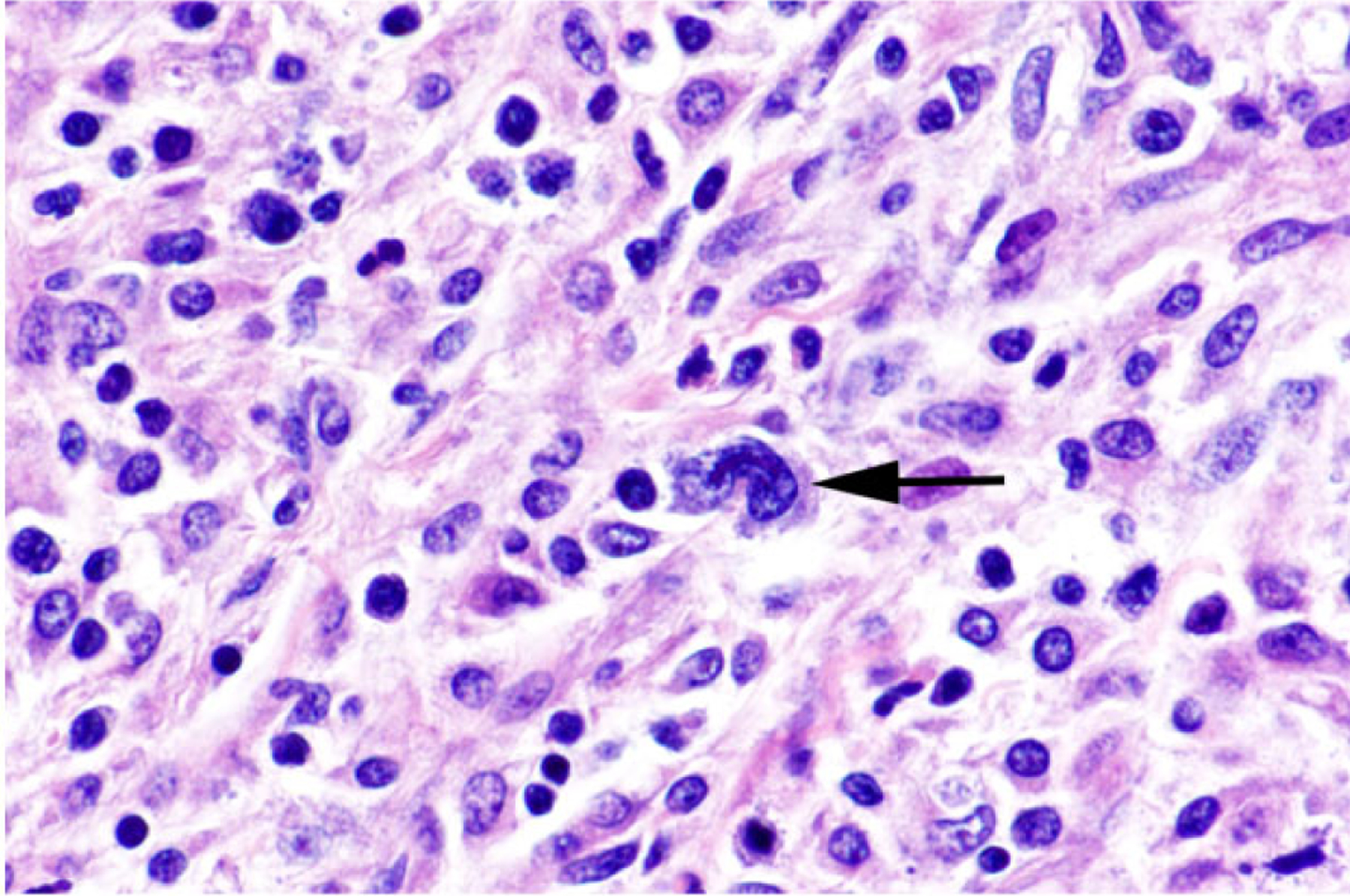
Dog No. 1. Occasional neoplastic cells are large (arrow) with an eccentric, horseshoe-shaped nucleus (hallmark cells). HE.
Commonly, neoplastic cell infiltrates resulted in ulceration (10 cases) and/or perforation of the intestine with abundant fibrinosuppurative inflammation and associated mixed bacteria and intestinal contents (4 cases; Suppl. Table S1). When the point of rupture was not captured in the histologic section, perforation was evidenced by fibrinosuppurative peritonitis (2 cases). Neoplastic masses were usually solitary, although 3 cases exhibited multiple disseminated masses (3 cases). In some cases, neoplastic cells were masked by dense inflammatory infiltrates that resulted in a primary inflammatory lesion being listed as a differential diagnosis (5 cases). Inflammatory infiltrates were mixed in all cases and included variable proportions of macrophages, neutrophils, lymphocytes, and plasma cells. Within foci of ischemic necrosis, neoplastic cells were commonly observed surrounding and invading blood vessels (6 cases; Fig. 5), frequently causing vascular wall necrosis. Extensive areas of granulation tissue and fibroplasia, usually adjacent to areas of ulceration, were also common. Only 1 dog exhibited evidence of inflammatory bowel disease, including multifocal lacteal and crypt dilation with filling of dilated crypts by eosinophilic proteinaceous material and few degenerate cells.
Figure 5. Anaplastic large T-cell lymphoma, jejunum, dog.
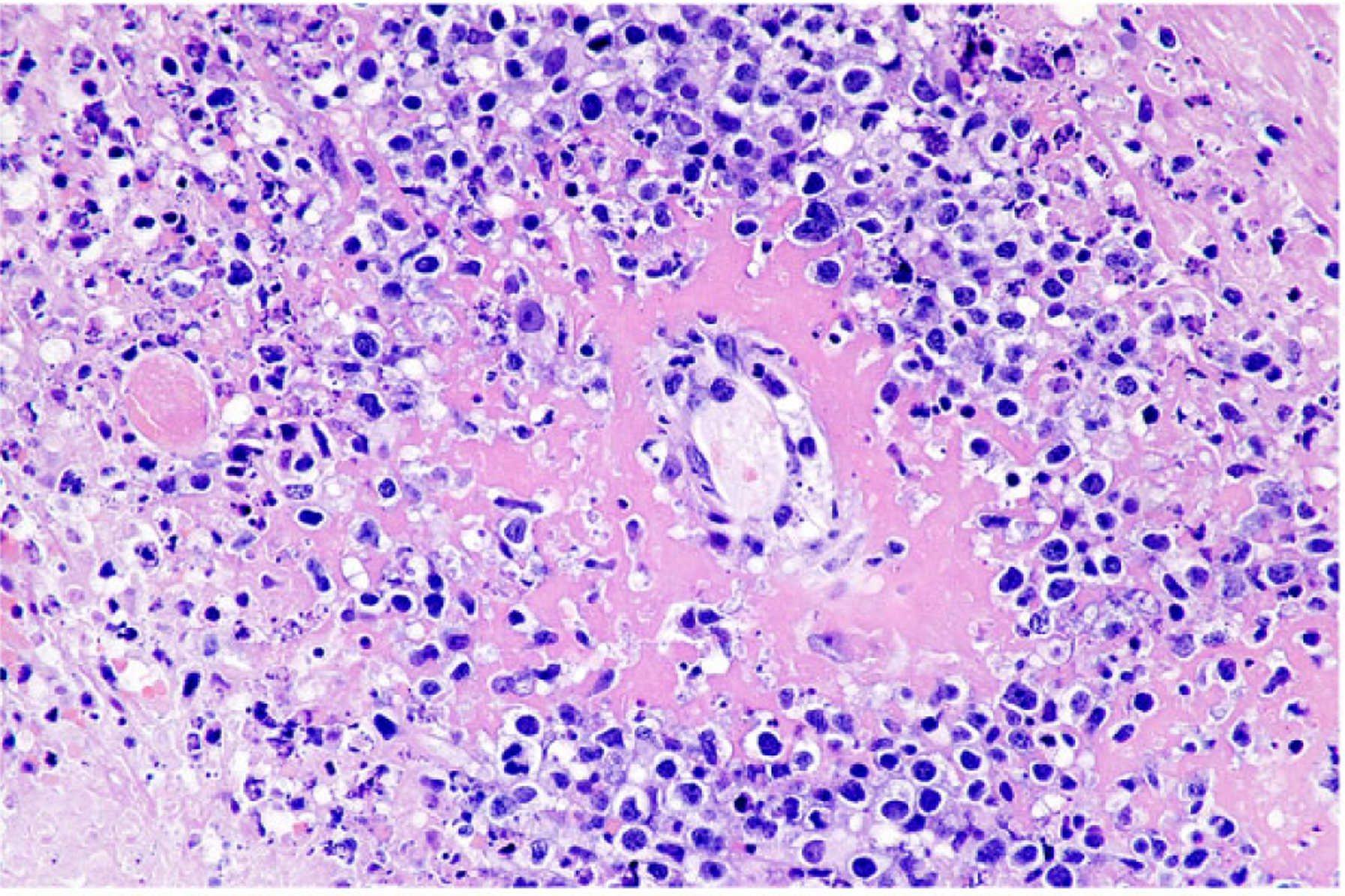
Dog No. 2. Neoplastic cells frequently surround and invade blood vessel walls, resulting in fibrinoid necrosis. HE.
Immunohistochemical Findings
Neoplastic lymphoid cells in all cases exhibited diffuse membranous or cytoplasmic immunoreactivity for CD3 (Fig. 6) while no neoplastic cells were immunoreactive for CD20. Intracytoplasmic immunopositivity for CD30 was observed in 50% to 100% of neoplastic cells, frequently in clusters and less commonly in diffuse sheets in all cases (Fig. 7). Small to moderately sized clusters of small lymphocytes throughout the lesion with strong membranous or cytoplasmic immunoreactivity for CD20 were observed in all cases and were consistent with inflammatory infiltrates (Fig. 8).
Figure 6. Anaplastic large T-cell lymphoma, jejunum, dog.
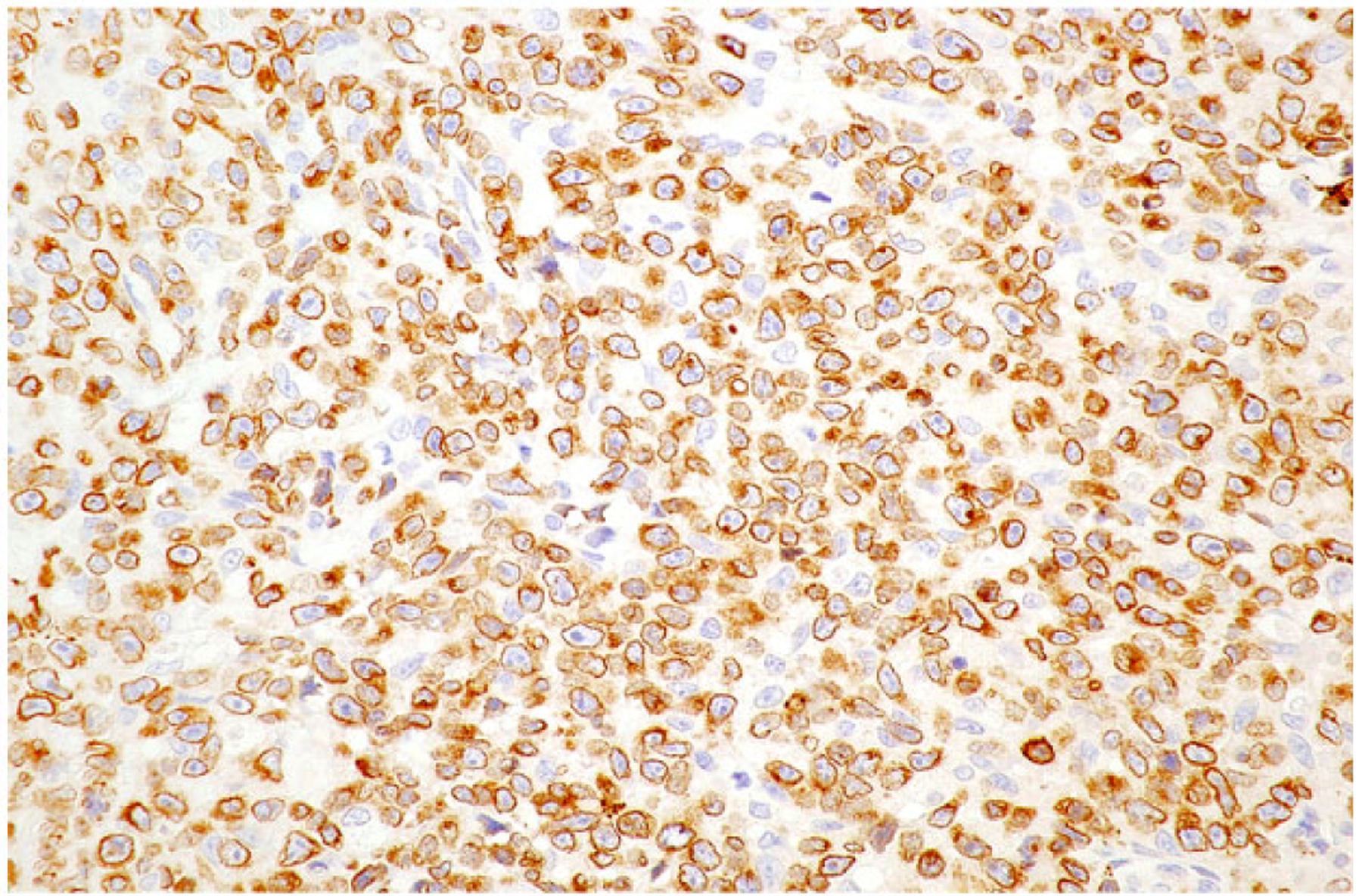
Dog No. 9. Neoplastic T cells exhibit strong membranous to cytoplasmic immunolabeling for CD3.
Figure 7. Anaplastic large T-cell lymphoma, jejunum, dog.
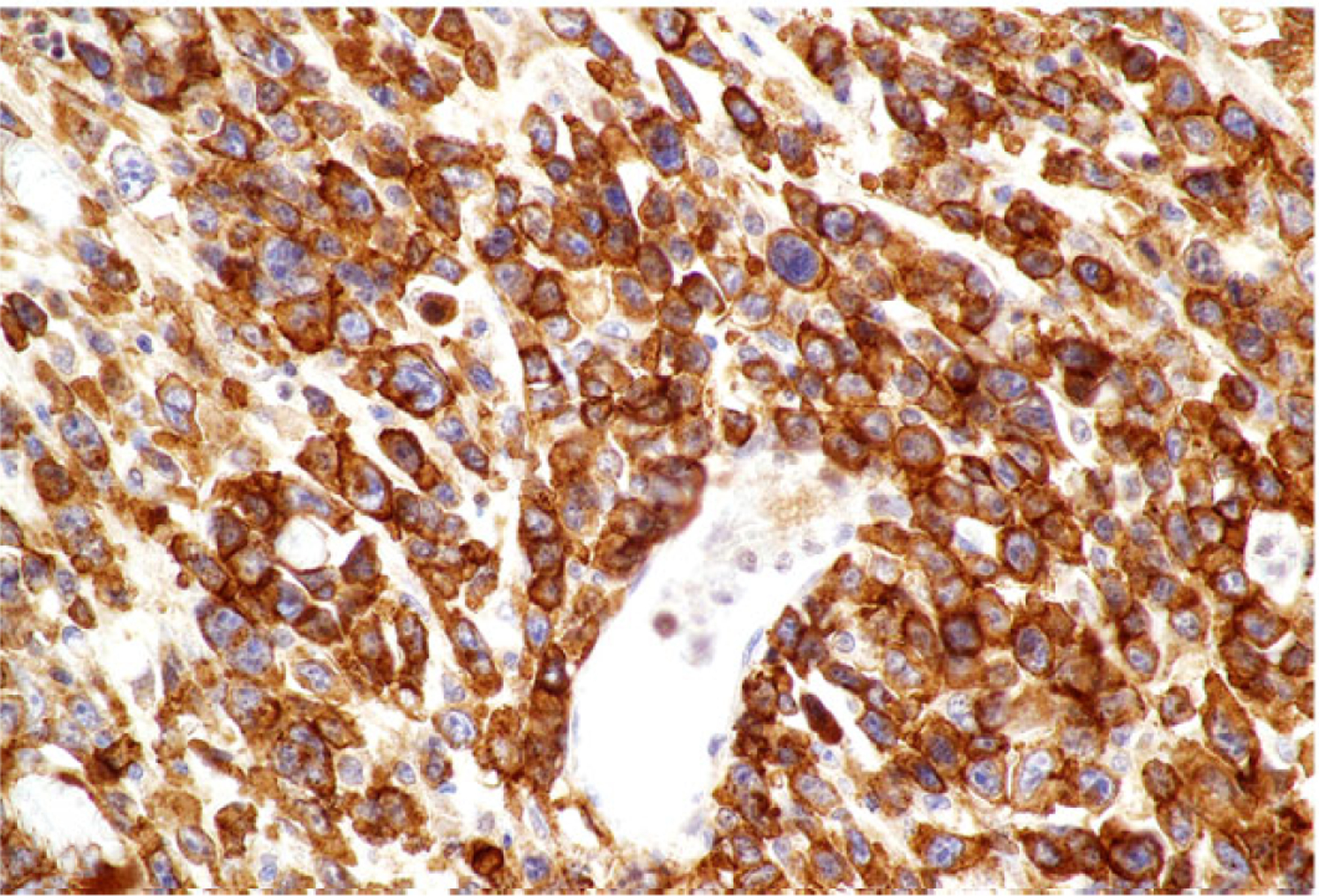
Dog No. 5. Neoplastic T cells with strong membranous to cytoplasmic immunolabeling for CD30.
Figure 8. Anaplastic large T-cell lymphoma, jejunum, dog.
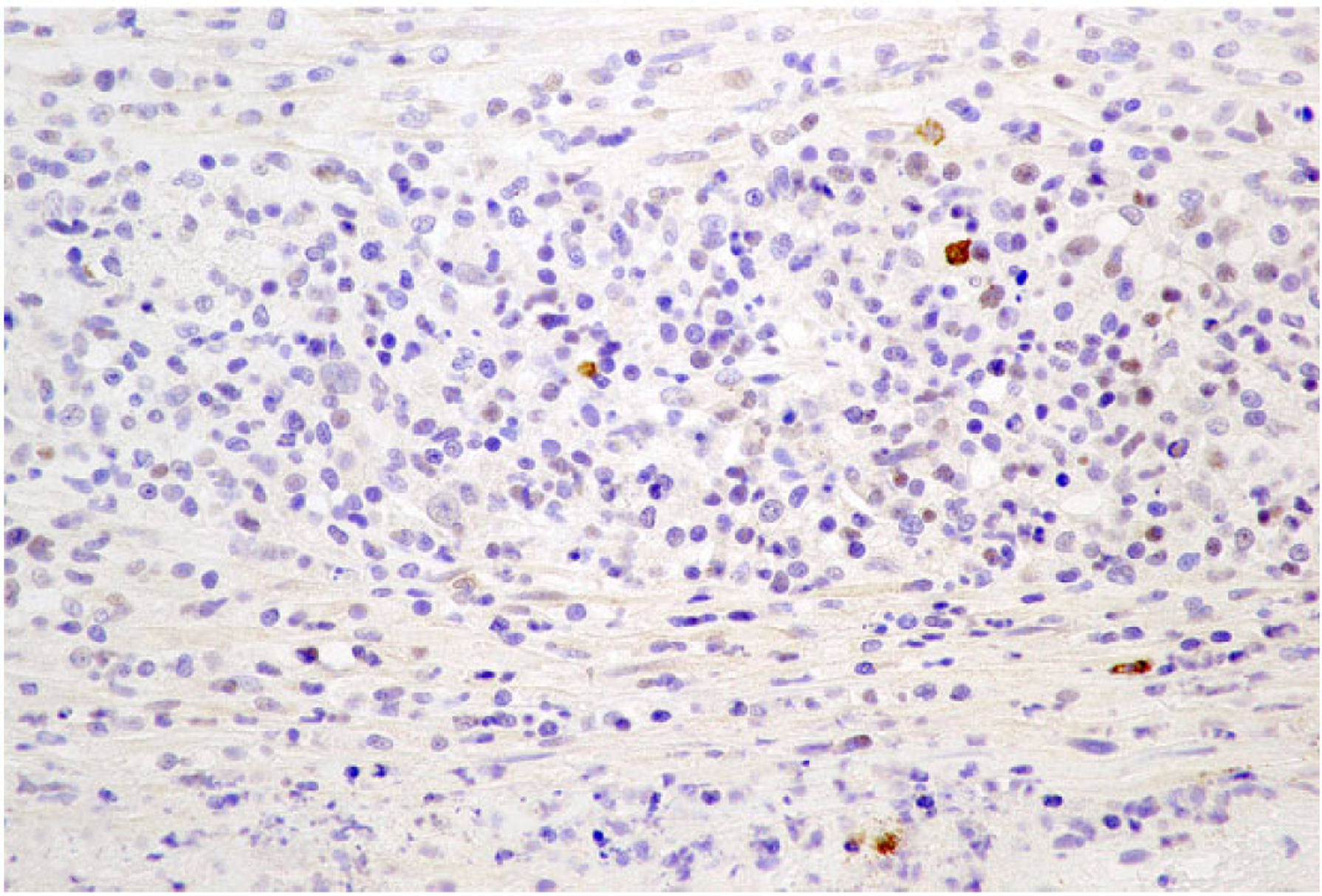
Dog No. 2. Rare, scattered cells, interpreted as reactive B lymphocytes, have strong membranous to intracytoplasmic immunolabeling for CD20.
PCR for Antigen Receptor Rearrangements Testing
Neoplastic masses from all cases had a monoclonal T-cell receptor γ gene rearrangement. Two cases also had a monoclonal rearrangement of the immunoglobulin heavy chain gene (Suppl. Table S1).
Discussion
This series characterizes a subtype of intestinal T-cell lymphoma in dogs that has not been previously described as an independent entity affecting the intestine and shares similar features with those described for ALTCL in humans. According to the WHO classification, ALTCL can be classified as primary cutaneous or systemic.21 The systemic form predominantly affects lymph nodes, but extranodal involvement is common, occurring in 60% of cases.23 The most common extranodal site is the skin followed by soft tissue, bone, lung, liver, and rarely the central nervous system or gastrointestinal tract.9 In dogs, ALTCL has been described as a rare form of highly aggressive, nonepitheliotropic cutaneous lymphoma in older dogs that begins as single or multiple skin nodules with rapid progression, in contrast to the more indolent course in human cutaneous ALTCL.24,25 Cutaneous forms have also been described in cats associated with injection sites.16 A nodal form has been reported sporadically in cats and young, large-breed dogs that typically develop generalized skin disease, lymphadenopathy, and dependent edema with rapid onset and progression.24 No dogs in this series exhibited cutaneous disease or generalized lymphadenopathy, but 3 cases had metastasis to the liver and 1 to the mesenteric lymph nodes. Although no necropsies were performed to assess for additional systemic involvement, the gastrointestinal tract is suspected to be the primary site of origin in all cases.
In humans, dogs, and cats, the histomorphologic diagnosis of ALTCL is based on the presence of cells with abundant cytoplasm and large, pleomorphic, and often horseshoe- to kidney-shaped nuclei (hallmark cells).24 Three histologic variants are described in humans, including the common, small cell, and lymphohistiocytic type.21 The common type is composed of sheets of hallmark cells while the latter 2 variants comprise a mixture of cells of various sizes or predominantly histiocytes, respectively, with admixed small numbers of hallmark cells. In this series, the number of cells with hallmark morphology was small, consistent with the small cell and lymphohistiocytic variants of ALTCL described in humans, although the predominance of large cells within the neoplasm is more in line with the common type.21 The presence of hallmark cells has recently been reported in a nodal case of ALTCL in a dog and 3 cases of injection site lymphoma in cats.15,16 An additional interesting histologic feature in this series, also described in cases of feline and human primary cutaneous ALTCL and cases of systemic canine ALTCL, is prominent angioinvasion and angiodestruction.12,13,23 Such vascular lesions aided in diagnosing lymphoma in cases where inflammatory cells far outnumbered neoplastic cells and may have been responsible for the ischemic changes seen in several of the cases of this report.
Most gastrointestinal T-cell lymphomas in both humans and dogs are classified as EATL type 1.6,7 Similarly to the cases reported here, EATL type 1 comprises large cells that often have transmural infiltration of the affected intestinal segment associated with necrosis and secondary inflammation.24 The distinction between EATL type 1 and ALTCL can therefore be difficult based on histomorphology alone. However, EATL type 1 derives its name from its close correlation with inflammatory bowel disease in humans, a connection that is suspected but as yet unproven in dogs.24 Diagnostic criteria of EATL type 1 in humans include signs of enteropathy, such as villous blunting, crypt hyperplasia, and intraepithelial lymphocytosis.2 In dogs, villous atrophy has been described as a consistent feature in cases of EATL type 1.6 In this series, only 1 case exhibited signs of inflammatory bowel disease, and villous atrophy was not noted in any of the cases. This, in addition to the striking anaplastic morphology of the neoplastic cells and the commonly observed vascular lesions, may allow for histomorphologic differentiation between ALTCL and EATL type 1 in the intestine of dogs.
The systemic form of ALTCL is further subdivided into 2 distinct subtypes, ALK-positive ALTCL and ALK-negative ALTCL. ALK is a tyrosine kinase that induces neoplastic transformation following translational fusion with an activating promoter.23 Despite identical histomorphologic appearances, prognosis differs significantly between the subtypes, with cases of ALK-positive ALTCL reaching a 5-year survival rate of nearly 80%, while the rate is only 48% in cases of ALK-negative ALTCL.21 ALK-positive ALTCLs occur most commonly in younger patients, frequently in children, while ALK-negative ALTCLs are more common in older patients.4 Unfortunately, the expression of ALK has not been studied in animals, and the possibility of chromosomal rearrangements in the ALK gene in cases of canine lymphoma remains to be investigated. Nevertheless, the poor survival time and older age of the dogs in this series correlate best with the ALK-negative subtype of humans. In addition, primary involvement of the gastrointestinal tract is more frequently reported with ALK-negative ALTCL.1,10,18
ALTCLs lacking ALK gene rearrangement are more frequently positive for CD3- than ALK-positive ALTCLs.18 All neoplasms in this series demonstrated diffuse immunopositivity for CD3, providing further correlation with the ALK-negative subtype. In humans, neoplastic cells also frequently express at least 1 cytotoxic marker, such as perforin, granzyme B, or TIA-1.24 Unfortunately, no commercial antibodies that cross-react with these antigens in dogs are available for use in paraffin-embedded sections. In reported cutaneous and nodal ALTCL in dogs, neoplastic cells have demonstrated diffuse immunopositivity for CD3, as seen in all dogs this case series.23 In human medicine, ALTCL is diagnosed through a combination of characteristic histomorphologic features and diffuse immunolabeling of neoplastic cells with CD30, a transmembrane cytokine receptor expressed on both activated T and B lymphocytes.19 The demonstration of CD30 expression in canine lymphoma is limited to a case report of pulmonary lymphomatoid granulomatosis, in which a subpopulation of cells expressed CD30, and a report of an ALCL of null cell phenotype in the lymph node of a dog.14,15 Neoplastic cells from all cases described here exhibited varying degrees of immunopositivity for CD30. However, despite the frequent use of CD30 immunohistochemistry for diagnosing ALTCL in humans, the differentiation between ALTCL and EATL type 1 can be challenging as up to 37.5% of EATL type 1 cases can express CD30 in a large portion of neoplastic cells.8 In such cases, histomorphology is used as the predominant determining factor in distinguishing ALTCL from EATL type 1.
The median survival time in this case series was a mere 5 days, with most cases being euthanized days to a few weeks following a second intestinal perforation or the development of complications associated with the first perforation. In humans, intestinal perforation is one of the main causes of death in intestinal T-cell lymphoma, resulting in death of up to 30% of patients.22 All dogs in this series presented with relatively acute clinical signs with death or euthanasia following shortly thereafter, indicating the rapidly progressive nature of the disease. The prognosis for intestinal ALTCL in dogs appears to be quite poor, similar to the prognosis of ALK-negative ALTCL in humans. Cases of cutaneous and ALK-positive systemic ALTCL in humans typically respond favorably to a combination of surgical resection, chemotherapy, and occasionally radiation therapy.21 Interestingly, 1 case survived 2 years following its initial diagnosis, during which it received the chemotherapeutic agent, lomustine, following surgical excision of the intestinal mass. This suggests the potential for canine intestinal ALTCL to respond to therapy. However, the low number of cases receiving chemotherapy in this series is insufficient to document such association. On the contrary, the short survival time of the remaining cases in the series indicates a poor prognosis with a rapid clinical course for canine intestinal ALTCL.
In both EATL type 1 and ALTCL, intense inflammatory cell infiltrates and/or large foci of necrosis may obscure the neoplastic cell population, resulting in a potential misdiagnosis of an inflammatory condition, rather than lymphoma, as occurred in several of the cases in this series.6,24 Full-thickness biopsies that allow assessment of the transmural infiltrate combined with numerous sections through different portions of the mass will aid in the diagnosis of ALTCL. As performed in several cases in this series, special stains can assist in ruling out an infectious etiology. In addition, PCR for Antigen Receptor Rearrangements (PARR) may be useful in supporting a diagnosis of lymphoma in severely inflamed cases. However, inflammatory lymphocytes might outnumber neoplastic lymphocytes and result in a polyclonal PARR result. All cases in this study had a monoclonal T-cell receptor γ gene rearrangement.
This case series describes primary intestinal ALTCL in dogs that are characterized by CD3- and CD30-positive T cells with clonal rearrangement of TCRG and a high degree of cellular anaplasia, the presence of hallmark cells, and common vasocentricity to allow differentiation from the much more common EATL type 1. Further investigation, including prospective studies, additional immunophenotyping, and genetic analysis, is needed to better understand the tumorigenesis and therapeutic options for intestinal ALTCL in dogs.
Supplementary Material
Acknowledgements
We thank the following clinics for providing follow-up information: Randall Road Animal Hospital, Crystal Lake, IL; Animal Specialty Group, Los Angeles, CA; VCA All-Care Animal Referral Center, Fountain Valley, CA; Southern California Veterinary Specialty Hospital, Irvine, CA; VCA Mission Animal Referral and Emergency Center, Mission, KS; Oradell Animal Hospital, Paramus, NJ; VCA South Shore Animal Hospital, South Weymouth, MA; Blue Pearl Veterinary Partners, Seattle, WA; Blue Pearl Veterinary Partners, Tacoma, WA; and Encina Veterinary Hospital, Walnut Creek, CA.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material for this article is available online.
References
- 1.Berge R, Oudejans J, Ossenkoppele G, et al. ALK expression in extranodal anaplastic large cell lymphoma favours systemic disease with (primary) nodal involvement and a good prognosis and occurs before dissemination. J Clin Pathol. 2000;53(6):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke JS. Lymphoproliferative disorders of the gastrointestinal tract: a review and pragmatic guide to diagnosis. Arch Pathol Lab Med. 2011;135(10): 1283–1297. [DOI] [PubMed] [Google Scholar]
- 3.Burnett RC, Vernau W, Modiano JF. Diagnosis of canine lymphoma neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol. 2003;40(1): 32–41. [DOI] [PubMed] [Google Scholar]
- 4.Cao Q, Liu F, Li S, et al. Primary rare anaplastic large cell lymphoma, ALK positive in small intestine: case report and review of the literature. Diagn Pathol. 2016;11(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey MJ, Medeiros LJ, Roepke JE, et al. Primary anaplastic large cell lymphoma of the small intestine. Am J Clin Pathol. 1999;112(5):696–701. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco V, Rodríguez-Bertos A, Rodríguez-Franco F, et al. Distinguishing intestinal lymphoma from inflammatory bowel disease in canine duodenal endoscopic biopsy samples. Vet Pathol. 2015;52(4):668–675. [DOI] [PubMed] [Google Scholar]
- 7.Coyle KA, Steinberg H. Characterization of lymphocytes in canine gastrointestinal lymphoma. Vet Pathol. 2004;41(2):141–146. [DOI] [PubMed] [Google Scholar]
- 8.Delabie J, Holte H, Vose JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the International Peripheral T-Cell Lymphoma Project. Blood. 2011;118(1):148–155. [DOI] [PubMed] [Google Scholar]
- 9.Falini B, Pileri S, Zinzani PL, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999;93(8):2697–2706. [PubMed] [Google Scholar]
- 10.Kiupel M, Smedley RC, Pfent C, et al. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet Pathol. 2011;48(1):212–222. [DOI] [PubMed] [Google Scholar]
- 11.Lee YY, Takata K, Wang RC, et al. Primary gastrointestinal anaplastic large cell lymphoma. Pathology. 2017;49(5):479–485. [DOI] [PubMed] [Google Scholar]
- 12.Massone C, El-Shabrawi-Caelen L, Kerl H, et al. The morphologic spectrum of primary cutaneous anaplastic large T-cell lymphoma: a histopathologic study on 66 biopsy specimens from 47 patients with report of rare variants. J Cutan Pathol. 2008;35(1):46–53. [DOI] [PubMed] [Google Scholar]
- 13.Nambudiri VE, Aboutalebi A, Granter SR, et al. Recurrent ALK-negative anaplastic large T-cell lymphoma presenting as necrotizing vasculitis. Am J Dermatopathol. 2013;35(4):512–516. [DOI] [PubMed] [Google Scholar]
- 14.Park HM, Hwang DN, Kang BT, et al. Pulmonary lymphomatoid granulomatosis in a dog: evidence of immunophenotypic diversity and relationship to human pulmonary lymphomatoid granulomatosis and pulmonary Hodgkin’s disease. Vet Pathol. 2007;44(6):921–923. [DOI] [PubMed] [Google Scholar]
- 15.Pittaway R, Wu Y, Sziadovits B, et al. Diagnosis of anaplastic large-cell lymphoma in a dog using CD30 immunohistochemistry. J Vet Diagn Invest. 2018; 30(3):455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roccabianca P, Avallone G, Rodriguez A, et al. Cutaneous lymphoma at injection sites: pathological, immunophenotypical, and molecular characterization in 17 cats. Vet Pathol. 2016;53(4):823–832. [DOI] [PubMed] [Google Scholar]
- 17.Sadiya N, Ghosh M. Primary ALK positive anaplastic large cell lymphoma of T-cell type of jejunum: report of a rare extranodal entity with review of literature. Arch Int Surg. 2014;4(1):50–53. [Google Scholar]
- 18.Savage KJ, Harris NL, Vose JM, et al. ALK– anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008; 111(12):5496–5504. [DOI] [PubMed] [Google Scholar]
- 19.Stein H, Foss HD, Dürkop H, et al. CD30*+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12): 3681–3695. [PubMed] [Google Scholar]
- 20.Stein H, Mason DY, Gerdes J, et al. The expression of Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848–858. [PubMed] [Google Scholar]
- 21.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008. [Google Scholar]
- 22.Vaidya R, Habermann TM, Donohue JH, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24(9): 2439–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valli VE. T-cell and NK-cell neoplasms. In: Veterinary Comparative Hematopathology. Ames, IA: Blackwell; 2007:339–345. [Google Scholar]
- 24.Valli VE, Bienzle D, Meuten DJ. Tumors of the hemolymphatic system. In: Tumors in Domestic Animals. 5th ed. Ames, IA: John Wiley; 2017:203–321. [Google Scholar]
- 25.Valli VE, Kiupel M, Bienzle D. Hematopoietic system. In: Maxie MG, ed. Jubb Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. St Louis, MO: Elsevier; 2016:230–232. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


