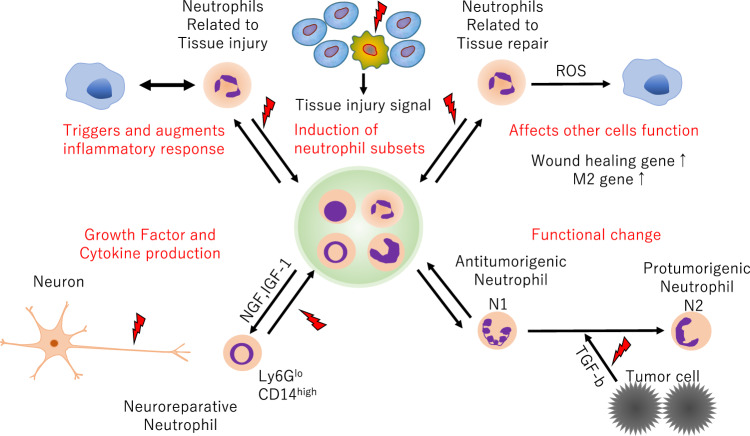
Several recent studies have shown that diverse neutrophil subsets exhibit distinct phenotypic and functional properties in terms of disease onset and progression.1,2 In a study published in Nature Immunology, Sas et al.3 discovered a new neutrophil subset that promotes neuronal survival and axonal regeneration in the central nervous system (CNS). In an immune-driven CNS axon regeneration model (crush injury of the optic nerve by intraocular injection of zymosan), the authors observed a unique granulocyte subset that had characteristics of immature neutrophils and neuroprotective properties and that drove CNS axon regeneration in vivo, in part via secretion of a cocktail of growth factors (Fig. 1). This pro-regenerative neutrophil subset promoted repair in the optic nerve and spinal cord, demonstrating the relevance of this subset across CNS compartments and neuronal populations. These findings related to the phenotypes of beneficial neutrophil subsets in neuroregeneration could ultimately lead to the development of new immunotherapies that reverse CNS damage and restore lost neurological function in a variety of diseases.
Fig. 1.
Functional diversity of neutrophil subsets. Neutrophils play pivotal roles in both tissue injury and repair
Neutrophils are generated from the bone marrow and circulate in the peripheral blood, protecting the host from viruses, bacteria, and other sources of infection. When infection or tissue damage occurs, neutrophils immediately migrate to the relevant site, inducing an inflammatory reaction by secreting various cytokines and chemokines and eliminating the source of infection by proteases and oxides. Although tightly controlled migration and activation of neutrophils is essential for infection defense, neutrophils that cause an overcontrolled inflammatory response exacerbate tissue damage.4
Neutrophils have a shorter lifespan than other immune cells, such as macrophages, and are present in a large pool in the bone marrow and spleen; thus, their sole function is thought to be triggering inflammation in the early stage of the immune response. However, research has recently shown that bone marrow-derived leukocytes, especially macrophages, exhibit marked transcriptional and functional plasticity in response to environmental stimuli and that they adapt to stimuli derived from tissues and pathological conditions to establish a diverse subset.5 For example, macrophages in the lungs, intestine, and spleen adopt features that are needed for surfactant clearance, immune tolerance, and iron recycling, respectively.1,6 This suggests that there may also be diverse subset of neutrophils, and in fact, neutrophils are involved in the pathogenesis and repair of many tissues and the induction of macrophage subsets according to pathological findings. For example, in acute liver injury, neutrophil-derived reactive oxygen species induce the establishment of a tissue repair subset of macrophages, and neutrophil-produced factors such as matrix metalloproteinase and fibronectin are involved in tissue repair.1,7 Experiments have shown that depletion of neutrophils delays the repair and functional recovery of the nervous system and lungs.7 Furthermore, transforming growth factor β can induce functionally different subtypes of neutrophils in cancer.8 These results indicate that neutrophils are involved not only in the early stages of the immune response but also in the subsequent exacerbation of and recovery from the disease.1,3 In addition, recovered leukocytes but not activated leukocytes have been shown to improve the prognosis of neurological disorders.9,10 However, the stimuli and transcriptional changes that cause changes in neutrophil subtypes and the subtypes that contribute to tissue repair and neuroprotection remain unclear.
Sas et al.3 used an immune-driven CNS axon regeneration model induced by crush injury of the murine optic nerve, which resulted in extensive neuronal loss and axonal transection that could be mitigated by intraocular injection of zymosan, a fungal cell wall extract. Similar to other CNS axons, such as white matter tracts in the brain and spinal cord, transected axons in the optic nerve fail to undergo long-distance regeneration. In this model, injection of zymosan induced the innate immune response through pattern recognition receptors, leading to both vitreal inflammation and proregenerative responses.3 Analysis of the cellular composition of infiltrates that accumulated in the posterior chamber of the eye following intraocular zymosan injection and optic nerve crush injury showed that neutrophils were the major subset of infiltrating leukocytes. Additionally, blockade of this population by C-X-C motif chemokine receptor 2 antiserum accelerated neuroregeneration and mobilization of Ly6GloCD14hi immature neutrophils, which have ring-shaped nuclei, to the site of injury. Furthermore, transcriptomic analysis by single-cell RNA sequencing revealed that Arg1, Mrc1, Itga6, Tgfb1, Igf1, and Ccl5 transcripts were enriched in the immature Ly6Glo neutrophil subset that was expanded in the cohort treated with α-C-X-C motif chemokine receptor 2. Thus, zymosan-induced Ly6Glo neutrophils have characteristics of alternatively activated myeloid cells. Conditioned culture medium harvested from Ly6Glo neutrophils exhibited neurite growth-promoting effects, and these effects were heat-sensitive, suggesting that proteins were responsible for this activity. A multiplexed antibody array assay identified nerve growth factor and insulin-like growth factor 1 as candidate growth factors, and neutralization of either nerve growth factor or insulin-like growth factor 1 with antagonistic antibodies mitigated neuronal regeneration in vitro. Therefore, these Ly6Glo neutrophils promoted neuronal survival and axonal regeneration in part by secreting growth factors such as nerve growth factor and insulin-like growth factor 1. In addition, adoptive transfer experiments directly confirmed that Ly6Glo neutrophils conferred neuroprotective and proregenerative effects in two CNS compartments: the optic nerve and spinal cord. Finally, the authors investigated the neuroregenerative capacity of human myeloid cells with characteristics of immature neutrophils. Similar to zymosan-induced Ly6Glo murine neutrophils, the HL-60 human promyelocytic leukemia cell line showed immature and alternatively activated characteristics. Moreover, the HL-60 cell line showed neuroprotective and proregenerative functions, suggesting the existence of human neuroregenerative neutrophils.
The pathological features of many CNS disorders are axonal transection and neuronal death, and neurons in the CNS (including the retina, optic nerve, brain, and brain stem) have long been considered nonregenerative. Therefore, the findings reported by Sas et al.3 increase opportunities for developing novel neuroprotective immunotherapies that might alleviate and reverse neurological deficits. Their findings may also lead to the development of novel strategies for promoting recovery from degenerative neurological diseases, such as amyotrophic lateral sclerosis and multiple sclerosis, as well as from damage caused by traumatic brain and spinal injuries and stroke.
This study also raises several new questions about neutrophil subsets. First, are the Ly6Glo regenerative immature neutrophils identified in this study the same as or different from immature neutrophils in the bone marrow (e.g., promyelocytes and myelocytes)? Second, are regenerative and growth factor-producing capacities common features of immature neutrophils, or are tissue-derived factors crucial for differentiation of this proregenerative neutrophil subset? Third, do age, sex, or other factors affect the abundance of these Ly6Glo regenerative immature neutrophils? Finally, does this subset or other neutrophil subsets exert beneficial functions in other diseases?
Although the investigation of neutrophil diversity has just begun and further research is needed, this study opens a new door and provides a new research paradigm. As mentioned earlier, neutrophils play critical roles not only in tissue repair but also in protecting against infection and inducing inflammation. This exquisite balance between inflammation and tissue repair can lead to disease pathogenesis, making targeted therapies challenging because neutrophils exert both beneficial and harmful effects even when they are known to be involved in disease. To solve this problem, it is essential to elucidate how neutrophils are functionally stimulated at the site of disease or injury to acquire new subtypes. Furthermore, extrapolation to humans and functional proof are needed before therapeutic application can be considered. The surface markers and functions of immune cells, including neutrophils, are different between humans and mice, making it very difficult to find a human counterpart for the new subset found in mice. In addition, even if a morphological and surface markers of a new subset can be identified, whether this subset contributes to pathological improvement and repair of the injured site in humans in the same manner as in mice is difficult to confirm. However, single-cell analysis and CRISPR/Cas9 gene knockout systems have become great tools for solving such problems, and many researchers worldwide are currently working toward a solution. Furthermore, the immune response and cell population involved in disease pathogenesis change dramatically with time, and inappropriate timing might cause unexpected adverse effects rather than beneficial outcomes. In other words, to obtain the maximum effect, it is of utmost importance to grasp the whole picture of the pathological condition and provide treatment at the time during which the new subset is involved. Many mouse models are in use worldwide, and although it is possible to grasp the whole picture over time, it is not easy to collect clinical specimens from humans; additionally, it is difficult to elucidate the dynamic movement of immune cells. Overcoming these challenges will help identify a subset of neutrophils and allow precise control of specific subtypes without affecting essential neutrophil functions such as inflammation and maintenance of homeostasis. We hope that in the near future, we will be able to control diverse neutrophil functions and subtypes, which was previously thought to be impossible, allowing us to treat diseases for which no effective remedies currently exist.
Acknowledgements
We thank Angela Morben, DVM, ELS, from Edanz Group (https://en-author-services.edanz.com/ac) for editing the English text of a draft of this manuscript. This work was supported by AMED (grant number JP20fk0108129), GSK (grant number A-32), and the Japan Intractable Diseases (Nanbyo) Research Foundation (grant number 2020B02).
Competing interests
The authors declare no competing interests.
References
- 1.Yang W, et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019;10:1076. doi: 10.1038/s41467-019-09046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballesteros I, et al. Co-option of neutrophil fates by tissue environments. Cell. 2020;183:1282–1297.e18. doi: 10.1016/j.cell.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Sas AR, et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 2020;21:1496–1505. doi: 10.1038/s41590-020-00813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 5.Aurora AB, Olson EN. Immune modulation of stem cells and regeneration. Cell Stem Cell. 2014;15:14–25. doi: 10.1016/j.stem.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavin Y, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017;169:750–765.e17. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blazquez-Prieto J, et al. Impaired lung repair during neutropenia can be reverted by matrix metalloproteinase-9. Thorax. 2018;73:321–330. doi: 10.1136/thoraxjnl-2017-210105. [DOI] [PubMed] [Google Scholar]
- 8.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giles DA, et al. Myeloid cell plasticity in the evolution of central nervous system autoimmunity. Ann. Neurol. 2018;83:131–141. doi: 10.1002/ana.25128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jassam YN, et al. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]