Abstract
Sub-Tenon’s block (STB) is a good technique of local anaesthesia for many types of eye surgery. It has a relatively good risk profile, in that sight- and life-threatening complications appear to be extremely rare. STB has gained popularity in the last three decades, with refinements including different types of blunt metal cannula, plastic cannulae and ‘incisionless’ approaches. Usage of STB varies significantly across the globe. This narrative review documents the historical evolution of STB techniques, anatomical and physiological considerations, its utility and suitability, complications, explores the current practice and possible future applications.
Subject terms: Surgery, Eye diseases, Education
摘要
球筋膜下麻醉技术 (Sub-Tenon’s block, STB) 是许多眼科手术优选的局部麻醉技术。STB风险相对较低, 威胁视力和生命的并发症极为罕见。在过去的三十年里, STB不断发展并广泛应用, 其改进技术包括使用不同类型的钝性金属注射管、塑料注射管以及 “无切口”的方法。STB在全球的应用差异很大。这篇叙述性文献综述回顾了STB技术的历史演变、解剖学和生理学相关理论依据、实用性及适用性、相关并发症, 并探讨了STB当前的应用及未来展望。
Introduction
Sub-Tenon’s block (STB) anaesthesia was first described in 1884, but the technique has only become widespread since the 1990s. This narrative review documents the evolution of STB techniques, anatomical and physiological considerations, its utility and suitability, complications, explores current practice and possible future applications.
Methodology
This review is based upon previous teaching materials prepared by the authors. In addition, we conducted a literature review using Pubmed, Google Scholar and Medline, and Scopus using a combination of keywords: ‘sub-Tenon’, ‘anaesthesia’ and ‘block’ between January 1980 and May 2020. Other related articles were retrieved from the references of key articles. As a result, a total of 100 articles are included in this review.
Historical overview
Ophthalmic regional anaesthesia really began in 1884, when cocaine eye-drops transformed eye surgery [1]. By late 1884, various techniques of local anaesthesia had been described [2–4]. The first description of STB was Turnbull’s method, used for enucleation. Having opened Tenon’s capsule, cocaine drops were ‘allowed to flow into the cut’ and then ‘inserted as to flow down the blades’ of curved scissors, to reach the back of the globe [2, 3]. Almost simultaneously, Cocks described using an Anel (lacrimal) syringe ‘introducing the point of the syringe as far back as possible, keeping its point near the globe’ to inject cocaine to the sub-Tenon’s space, and along the rectus muscle sheaths [2, 4].
Various modifications of STB technique have emerged over the last century (Table 1) [5–24]. Renewed interest emerged in 1990s when Mein et al. described inserting a blunt cannula into the sub-Tenon’s space, after initial dissection, to deliver local anaesthetic (LA) solution [6, 7]. This approach was further popularized by Stevens [8, 9], Greenbaum [10, 11] and others. Further modifications include the use of different types of blunt metal cannula [12–16], sharp needle [17, 18], plastic cannulae [13, 19–21] and ‘incisionless’ approaches (using a blunt cannula, without dissection) [16, 22, 23]. Because of the inherent risks of sharp needles, this review will only consider blunt-cannula STB.
Table 1.
Major milestones in the development of the modern blunt-cannula sub-Tenon’s block (STB) techniques.
| Reference | Access to sub-Tenon space | Cannula type | Cannula design | Comments |
|---|---|---|---|---|
| Turnbull 1884 [2, 3] | By surgeon (after opening Tenon’s capsule) | No cannula. Drops ‘flow down the blades’ of scissors | N/A | Usually credited as first STB |
| Cocks 1884 [2, 4] | By surgeon (after opening Tenon’s capsule) | Anel (lacrimal) syringe | Blunt-ended metal cannula | First STB with cannula (published 2 weeks after Turnbull’s) |
| Swan 1956 [5] | Direct, via needle | Sharp needle | N/A | First description of needle STB (not discussed further in this paper) |
|
Mein and Woodcock 1990 [6] Hansen et al. 1990 [7] |
Sub-conjunctival anaesthesia, then limbal peritomy by surgeon | Metal cannula | Angled, 19G, blunt tip | First ‘modern’ STB, used for retinal surgery (which at the time would often include 360° peritomy) |
| Stevens 1992 [8] |
Scissors ‘nick’ after topical anaesthesia Inferonasal quadrant |
Metal ‘Southampton’ anterior chamber cannula | Angled, 19G, blunt tip | Describes the technique that has become established as ‘standard’ STB |
| Greenbaum 1992 [10, 11] | Incision at inferonasal or inferotemporal quadrant | Bespoke plastic STB cannula | 14G, 120 mm, blunt tip, end hole | Cannula was inserted 3–5 mm into the space |
| Stevens 1993 [9] | N/A | Metal cannula, designed specifically for STB. The first disposable metal STB cannula | Curved and flattened, 19G, 25 mm, blunt tip | The classic ‘Stevens type’ STB cannula. With the 1992 paper, describes the classic ‘Stevens technique’. |
| Fukasaku and Marron 1994 [24] | Incision | Metal cannula | 24G, 20 mm, blunt tip, curved | Superior rectus suture was placed to control eye position |
| Allman et al. 2008 [22] | Incisionless. The ‘less blunt’ cannula is pushed directly to sub-Tenon’s space | Eagle ‘Triport’ disposable metal cannula | Angled, 21G, 25 mm, blunt tip (but less blunt than Stevens type) | First description of ‘no-snip’ blunt cannula technique. |
| Chua et al. 2018[27] | Incisionless. Lacrimal dilator, or a commercially available bespoke plastic probe, to make initial hole in conjunctiva/Tenon’s capsule | Technique can be used with any STB cannula | N/A | Describes a simpler technique: ‘incision’ is made, but without using scissors. |
Table 1 summarises the highlights of the development in sub-Tenon’s block, and it is not exhaustive. Numerous authors have also described using other types of metal ophthalmic cannulae or plastic (intravenous type) cannulae for STB.
Since STB was re-introduced in the 1990s, most practitioners have used a blunt-ended cannula to place the anaesthetic behind the equator of the globe, via an inferonasal incision that is made using blunt-ended spring-scissors [8, 25, 26]. A major change has been the realization that scissors are not always necessary. Allman described a ‘no-snip’ STB technique using a ‘pencil point’ cannula [22]. Subsequently, others have described using a lacrimal dilator or commercially available plastic probe to make this initial incision [27]. In our experience, these ‘no-snip’ STBs may not be possible for those with thicker Tenon’s capsule (e.g. younger patients and those of African heritage).
The LA agent has also transitioned over the last century. Cocaine was abandoned for its adverse local and systemic side effects. Procaine hydrochloride was introduced in the 1900s, and later superseded by lignocaine (lidocaine) [28]. In recent years, most available LA agents have been used for STB including lidocaine, bupivacaine, levobupivacaine and ropivacaine.
Anatomical and physiological considerations
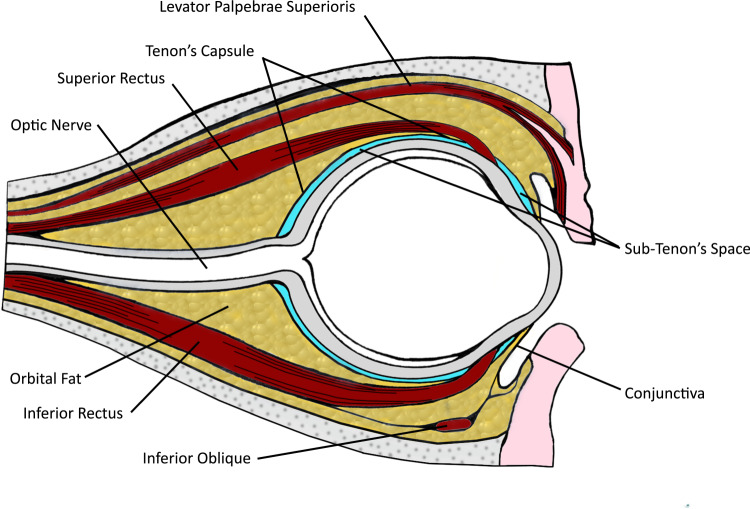
Tenon’s capsule is the fascial sheath that surrounds the globe and separates it from orbital fat (Fig. 1). Anteriorly, Tenon’s capsule merges with the conjunctiva just behind the limbus. Posteriorly, Tenon’s capsule fuses with the meninges around the optic nerve and sclera at the optic nerve exit. Close to the optic nerve, the sub-Tenon’s space is traversed by the short posterior ciliary arteries (SPCAs) (supplying optic nerve head and choroid) and the posterior ciliary nerves (serving sensation to most of the internal structures of the eyeball). Four vortex veins drain the choroidal circulation, and traverse the sub-Tenon’s space. The tendons of the six extraocular muscles pierce the sheath as they pass to their insertions on the globe. Hence, there is a potential space between the sclera and Tenon’s capsule [25, 29]. In practice, small volumes of LA in the posterior sub-Tenon’s space will give good analgesia, and larger volumes will spread around the extraocular muscle sheaths, giving akinesia.
Fig. 1. Sub-Tenon’s space.
Cross-sectional diagram of the eye, showing the sub-Tenon’s space surrounding the globe.
When accessing the sub-Tenon’s space, the practitioner should avoid damaging the rectus muscles. The inferonasal quadrant is most commonly used to access sub-Tenon’s space [25, 30], though superotemporal [24, 31], superonasal [31], inferotemporal [31–33] and four-quadrant approaches [6] have been described. The inferonasal approach is said to have a low likelihood of damage to vortex veins or other structures, and superonasal quadrant is potentially more hazardous due to the close proximity of vascular, neuronal and muscular contents in that area [25].
The sub-Tenon’s space is anatomically divided into anterior, equatorial and posterior sections. The equator can be thought of as the widest part of the globe, between cornea and macula. Anterior placement of the cannula may reduce the risk of potential trauma to structures behind the equator, but anterior STB has the disadvantage of higher likelihood of transient chemosis and discomfort to the patient [26]. In our experience, most practitioners perform a STB with the cannula behind the equator [25, 26, 30, 31], via an incision that is made around 5–6 mm posterior to the limbus. This distance is preferred because it is far enough back from the limbus for the conjunctiva (and attached Tenon’s) to be moveable when grasped with forceps, and also far enough forward for the Tenon’s layer to be relatively thin, thereby facilitating easy access to the sub-Tenon’s space with single snip, or a no-snip technique [22, 25–27, 30, 31].
The direction of gaze for STB is an important consideration. For an inferonasal approach, the patient is asked to look upwards and outwards (superolateral gaze). This makes access easier, but the practitioner should remember that such globe rotation may bring the optic nerve forward, rendering it vulnerable [34]. Smaller volumes STB often do not cause any significant rise in intra-ocular pressure (IOP) [35]. However larger volumes STB increase IOP [36] and the careful use of an ocular pressure-reducing device may be required [37].
Mechanism of action
Ultrasound [38] and magnetic resonance imaging [39] studies that have shown delivery of LA into the posterior sub-Tenon’s space create a lake of fluid circumferentially around the posterior aspect of the globe, directly blocking the ciliary nerves that provide sensory innervation to the globe (providing anaesthesia) [40]. Over the next 3–5 min, LA spreads along the extraocular muscle sheaths, diffuses into the intraconal space, and tracks forward to the fascial planes around the lids (providing akinesia of the globe and orbicularis oculi muscle) [38, 40]. The last akinetic muscle is often the superior oblique muscle, presumably because the muscle belly is further from the globe, and also the trochlear nerve is outside the muscle cone [29].
Smaller volumes of LA agent (e.g. 2 ml) usually provide excellent analgesia, but little or no akinesia. Larger volumes (e.g. ≥3–5 ml) may be needed if akinesia is required [41]. With the inferonasal quadrant approach for STB, muscles innervated by the oculomotor nerve may be blocked preferentially [42]. In practice, larger volumes of STB usually provide good analgesia and akinesia [42], including lid akinesia [43]—hence, a facial nerve block (with its added risks) is not necessary.
Suitability of STB
As a general principle, most patients or surgeries suitable for needle-based blocks would also be suitable for STB. However, there are some important considerations.
Patient factors
Active eye infections are a contraindication for STB [44]. Even in routine (non-infected) cases, 5% povidone-iodine should be instilled into the conjunctival fornix for at least 3 min prior to STB to minimize the risk of inoculating bacteria into the sub-Tenon space [45, 46].
Any process that results in scarring to the conjunctiva and/or Tenon’s capsule (e.g. chemical burns, ocular cicatricial pemphigoid (OCP), previous strabismus surgery or placement of a scleral explant or encircling band for retinal detachment [25]) may make STB difficult or impossible. This is because the Tenon’s capsule may become ‘stuck’ to the sclera, and attempted dissection for STB could cause scleral perforation [47]. Careful preoperative examination may identify an area free of scarring, allowing safe STB in a different quadrant. In OCP, it is best to avoid STB altogether, as STB could exacerbate the existing scarring with potential sight-threatening consequences.
Eyes with very thin sclera (e.g. previous scleritis) are less suitable for STB, due to the risk of scleral perforation [48]. Thin sclera can often be identified by its relative transparency (revealing dark bluish choroid underneath), and/or the sclera may bulge forward. If this thinning only affects part of the globe, STB could potentially be given via an unaffected quadrant. However, scleral thinning may not always be apparent [48], particularly if behind the equator. Thus, use of non-metal (plastic) and/or shorter/blunter cannulae may further minimize the risk of globe perforation.
STB is preferable to needle-based blocks in eyes that have a higher risk of globe perforation. Examples include deep-set eyes, highly myopic eyes, abnormal orbit, uncontrolled eye movements (e.g. nystagmus) or uncontrolled head movements. Photic sneezing (reflex sneezing in response to light) is common, and rarely these patients may have a reflex sneeze during an LA block [49, 50], particularly if intravenous sedatives have been used—blunt-cannula STB is also preferred for these patients.
Highly myopic globes are usually larger, longer, and may have thinner sclera and posterior staphyloma (bulging of the posterior sclera). Thus, myopic eyes are at increased risk of globe perforation with needle-based blocks [51, 52], Most posterior staphylomas are in the inferolateral position [51, 53], hence the standard inferonasal STB should be a preferred technique.
Use of anticoagulant and antithrombotic drugs might increase the risk of sight-threatening orbital haemorrhage, particularly if a sharp-needle block is used. However, the actual risk is low, and stopping anticoagulants may risk life-threatening thrombosis. In most cases it is advisable to continue these medications for ocular surgery [44]. Using STB, instead of a needle block, should reduce the risk of sight-threatening haemorrhage [54].
STB may have a special role in patients presenting with advanced multiple comorbidities for bilateral eye surgery where general anaesthesia and bilateral akinetic needle blocks may be undesirable. Staggered STB has been described for bilateral strabismus surgery [55].
Surgical factors
For many surgeons, STB is the default technique for most of their ocular surgeries. Operations requiring good akinesia (larger volume STB) may include cataract surgery [56], corneal surgery, vitreoretinal surgery [57] and glaucoma surgery [58]. Treatments to ablate the ciliary body and/or peripheral retina (e.g. cyclodiode laser to ciliary body, cryotherapy to retina) require good analgesia. For these cases, 2 mL of lidocaine 2% (small volume STB) usually provides excellent analgesia, whilst maintaining eye movement, allowing for a quicker and easier procedure [59]. Prolonged surgery is no barrier to using STB, as the surgeon can simply ‘top-up’ the local anaesthesia via the initial incision site or via a sub-Tenon catheter [19] intraoperatively.
STB may be less suitable for a few intra-ocular operations. Some glaucoma surgeons believe that any chemosis or sub-conjunctival haemorrhage resulting from STB might potentially enhance scarring of the conjunctiva, and this could increase the likelihood of failure in filtering surgery. However, there is very little clinical or scientific evidence to support or refute this claim, but it would be difficult to exclude a modest effect. Therefore, some surgeons prefer to avoid STB if there is the possibility that the eye may need glaucoma filtering surgery in future [60]. Penetrating injuries to the globe may be unsuitable for STB, and some are unsuitable for any type of local anaesthesia. Selected open globe injuries can be performed with a STB prior to, or during the procedure [61–63].
Additives to the LA
The volume of LA given may vary between practitioners: in our experience, the range is 1–10 ml, though most give between 2 and 5 ml. Smaller volume produces satisfactory anaesthesia but less akinesia [64]. Larger volumes give better, faster akinesia but more chemosis and potential for elevation of intra-ocular pressure [36]. Addition of hyaluronidase has been shown to significantly reduce median effective LA volume [65]. Adding a vasoconstrictor (e.g. adrenaline) is not recommended because of the risk of ocular ischaemia [25].
Other additives such as muscle relaxants, clonidine and opiates have been described for use with needle-based blocks, but their role in STB is unsubstantiated, thus their use is neither routine nor recommended [66].
Complications of STB
The complications of STB have been extensively reviewed, ranging from minor to sight- and life-threatening complications [67].
Minor complications
Sub-conjunctival haemorrhage usually occurs due to the tearing or damage to blood vessels during dissection and injection. The incidence varies and may approach 100% with shorter cannulae [68, 69] and in patients taking antithrombotics [69, 70]. Patients should be warned about this minor, transient complication [67].
Chemosis (swelling of the conjunctiva) occurs due to inappropriate forward spread of the LA. The incidence of chemosis is variable and depends on length of cannula, volume of LA, speed of injection and the nature of dissection or entry to the sub-Tenon’s space. A recent large study, using a flexible cannula for post-equatorial STB, reported 14.8% had chemosis that affected only 1 quadrant of the eye, and 4.5% of eyes had chemosis affecting 2 or more quadrants [20]. Chemosis usually resolves spontaneously or with applied pressure, and should not interfere with surgery. Glaucoma surgeons may wish to avoid the risk of sub-conjunctival haemorrhage or chemosis, as explained above [60].
Pain during STB administration has a reported incidence up to 44% of patients [9]. One study of 6000 patients reported 7% experiencing more than mild discomfort, and 7% of patients also complained of some discomfort during surgery [71]. Others reported similar findings [72]. In a recent large series, 93.2% experienced no or only mild discomfort during or after surgical procedure [20]. Randomised trials indicate that STB is well tolerated, and patient comfort compares well against needle-based blocks and topical anaesthesia [73–77].
Reflux of the LA during administration is common when the dissection of sub-Tenon’s capsule is incomplete and traction during injection may enlarge the initial dissection area. Loss of significant volume of LA agent may affect development of akinesia [41, 67, 78].
Sight- and life-threatening complications
Sight- and life-threatening complications of STB are extremely rare, with most being included in retrospective observational studies [56, 79] or case reports [67]. There have been no large prospective randomised controlled trials (RCTs) to look at this issue [56, 79]. There are a few published RCTs that have reported complication rates, but all have small sample sizes and were intended primarily to look at effectiveness. Thus, no RCT involving STB has been sufficiently powered to make any meaningful conclusion regarding the rate of serious complications. A large 1-year survey of complications of local anaesthesia included an estimated 180,000 STBs [56]. There was one sight-threatening complication (cilioretinal artery occlusion) and two life-threatening complications (one myocardial infarction, one anaphylaxis) though causation could not be established with certainty, and the degree of under-reporting could not be determined [56]. The Cochrane Collaboration’s review concluded that STB was a ‘safe method of providing anaesthesia for cataract surgery’ [73].
The actual types of sight- and life-threatening complications of STB are similar to those reported with needle-based blocks. Life-threatening complications include brainstem anaesthesia, seizures, ventricular fibrillation and death [67]. Sight-threatening complications include orbital and retrobulbar haemorrhages, scleral perforation, muscle paresis (especially the inferior and medial rectus muscles), optic neuropathy, accommodation defects, retinal and choroidal vascular occlusion and orbital swelling [67]. These rare complications continue to be reported as anecdotal cases. Clinical prospective observational audit data, and large studies with voluntary reporting, suggest that the incidence of serious sight-threatening complications with blunt-cannula STB is very low, and probably rather lower than with retrobulbar or peribulbar block [20, 56, 71, 79, 80].
The actual STB technique used may influence the likelihood of serious complications. A simple example is anterior ischaemic optic neuropathy (AION) or optic nerve head infarct associated with STB [67, 79, 81]. The optic nerve head is supplied by the SPCAs, which cross the sub-Tenon’s space, close to the optic nerve head. Thus, a very posterior placement of a metal cannula could potentially damage these small arteries, causing an AION. These SPCAs sometimes also supply the retinal circulation (usually as a cilioretinal artery), which explains the occurrence of some retinal infarcts with STB [56]. A long metal cannula could reach the optic nerve head itself [81]: these cannulae can be pushed through the anterior conjunctiva and Tenon’s capsule for the ‘no-snip’ STB technique, so it is likely that one could also pierce the coverings of the optic nerve, thereby causing brainstem anaesthesia [67, 79, 82, 83]. Over-deep initial dissection with scissors could also cause these problems [26]. Thus, it is recommended that practitioners should use minimal dissection, and avoid advancing the STB cannula to a ‘very posterior’ position [81]. Most teachers recommend that the cannula is placed just a little bit behind the equator, to minimize the risk of serious complications [63, 67]. Using a pointed device instead of scissors [22, 27] could also potentially damage muscles or even perforate the sclera, so these devices should also be used with caution.
Worldwide usage of STB
The usage of STB worldwide is difficult to ascertain. There is no robust data on the overall use of STB (or other anaesthesia modalities) for ophthalmic surgery in any country. A few national surveys have looked at anaesthesia for cataract surgery [84–92]. For some countries (Canada, Singapore and United Kingdom) [84, 88–91], repeated surveys have shown that practice has been changing significantly since the 1990s. Where serial surveys exist, they tend to show a move away from ‘sharp-needle’ LA block, with increasing use of STB and topical/intracameral anaesthesia (Table 2).
Table 2.
Summary of the use of sub-Tenon’s block to the total number of regional ophthalmic blocks used for cataract surgery (percentage, year of national survey).
| Country | Sub-Tenon’s block % (year of survey) |
|---|---|
| Canada |
31% (2010) [84] 61% (2018) [84] |
| Japan | 63% (1999) [85] |
| Korea | 42% (2012) [86] |
| New Zealand | 86% (2007) [87] |
| Singapore |
5% (2004) [88] 7% (2016) [89] |
| United Kingdom |
42% (2003) [79] 50.5% (2013) [56] |
| United States of America | 5% (2003) [92] |
Data should be interpreted with caution because of the inherent difficulty in getting a truly representative sample.
There appears to be significant worldwide variation (Table 2), though these results should be interpreted with caution: many did not survey a truly ‘representative sample’ of national practice, and practice is likely to have changed since publication. According to Table 2, STB is commonly used in some countries (e.g. Canada, Japan, New Zealand and United Kingdom [84, 87, 90, 91]) but infrequently in others (e.g. Singapore and USA [88, 89, 92]). We acknowledge that Table 2 may not reflect current practice.
Possible reasons for slow uptake of STB in some countries
Despite its superior safety profile [20, 67, 71], STB has not completely replaced needle-based blocks. There are many probable reasons why ophthalmic anaesthesia providers are slow to change from established techniques.
First, it is more expensive to perform a STB than a needle-based block. The cost of a disposable STB cannula is ~$3–5 (US dollars equivalent). Conjunctival forceps and spring-scissors are also needed. Reuseable cannulae are available, but this involves the initial expenditure, with additional ongoing costs of sterilization. Reusing cannula carries the risk of transmissible infection, if the lumen is not fully sterilized. Dedicated STB cannulae are not commercially available in some countries, though (as discussed above) other types of cannula can be used for STB.
Another barrier to progress is the question of who will perform the block. In many countries, it is normal for a non-ophthalmologist (e.g anaesthesiologist or nurse anaesthetist) to perform the anaesthetic block, using a needle. These clinicians may be unhappy with the idea of using scissors for a ‘surgical procedure’, and thus might ask the surgeon to perform the STB. This could mean that operating lists will be less efficient, because of more time taken per case. These concerns can be overcome with training, but many non-ophthalmologists may prefer using a ‘no-snip’ STB technique, as discussed above.
Another potential concern is a cosmetically imperfect appearance of the eye in the early post-operative period. While it is true that chemosis and/or sub-conjunctival haemorrhage is not uncommon with STB [67], the technique gives high rates of patient satisfaction [20, 41, 74–78]. Patients can be reassured that any cosmetic effect should be transient, that needle blocks can also cause similar effects, but that STB appears to have a significantly lower rate of serious complications than with needle-block techniques [56, 67, 79]. Some clinicians have suggested that a change from needle blocks to STB might deter patients from attending their private practice, but evidence from one large private provider suggests the opposite [20].
Teaching and training of STB
STB lends itself particularly well to training on models. In contrast, sharp-needle blocks have to be taught in relation to the complicated orbital geometry. This is of importance, since an animal-model’s orbital geometry does not align well with human anatomy. Excised eyes from a local abattoir facilitates sub-Tenon’s training by being easily obtainable, cheap and offering comparable ocular anatomy [93]. With the caveat to the trainee that most animal eyes have ‘tighter’ connective tissues than most aged patients, all STB techniques can be demonstrated and practiced easily. The microanatomy of the block—the LAs depot being placed between sclera and the conjunctival/Tenon’s double-layer—can be demonstrated by use of ultrasound on the model eye, which enhances understanding [15]. The authors sometimes use an even simpler model for training, made by wrapping some thin plastic around a cherry tomato or similar fruit: either can be very helpful for learning the ‘moves’ for STA.
The future of sub-Tenon anaesthesia
While topical/intracameral anaesthesia is becoming commonplace for cataract surgery [56], there will always be operations that need good akinesia, and/or good analgesia beyond the anterior segment of the globe. Vitreoretinal, glaucoma, corneal and cataract surgery will all include many cases that need either general anaesthesia (GA) or a good LA ‘block’. Recent evidence (e.g. Table 2) indicates that STB is gradually taking over from needle blocks, and in many cases is being used in place of GA.
For vitreoretinal and similar surgeries, STB has several obvious advantages when compared to GA. When compared to standard GA, STB can reduce the incidence of oculocardiac reflex, and reduce the need for intraoperative and post-operative opioids [94, 95]. Overall, changing from GA to STB should mean more efficient surgery with more operations done per session, less use of healthcare resources and higher patient satisfaction. There is evidence that this change from GA to STB is already taking place [96], and this trend is likely to continue.
Some surgeons use STB to assist with pain control during and after GA surgery. The use of preoperative STB in sevoflurane-anaesthetised patients undergoing buckle-surgery for retinal detachment demonstrated better intraoperative hemodynamic control, reduced need for sevoflurane, reduced opioid consumption and shorter time spent in recovery unit, when compared to placebo injection [97]. Using STB for post-operative pain control is helpful of GA or sedated patients, and may be particularly useful for children and the elderly [98, 99].
In cataract surgery, many more operations are now using topical and intracameral anaesthesia [56]. There will always be cataract cases that need a ‘block’ anaesthesia, and in many countries STB is now becoming the standard block (Table 2). In the United Kingdom, the 2017 National Guideline on cataract surgery states that patients should be offered either STB or topical (with or without intracameral) LA for cataract surgery, and that needle (peribulbar) block should only be considered if both STB and topical anaesthesia are contra-indicated [100]. With increasing evidence regarding the relative safety of STB, we predict that this technique will become even more widespread in the years to come.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koller C. On the use of cocaine for producing anaesthesia on the eye. Lancet. 1884;2:990–2. [Google Scholar]
- 2.Knapp H. Cocaine, it’s use in ophthalmic and general anesthesia. New York and London: Putnam & Sons, The Knickerbocker Press; 1885. p. 45.
- 3.Turnbull CS. The hydrochlorate of cocaine, a judicious opinion of its merits. Med Surg Rep. 1884;29:628–9. [Google Scholar]
- 4.Cocks DC. Cocaine as a local anaesthetic in enucleation of the eyeball. Med News. 1884:654–5.
- 5.Swan KC. New drugs and techniques for ocular anesthesia. Trans Am Acad Ophthalmol Otolaryngol. 1956;60:368–75. [PubMed] [Google Scholar]
- 6.Mein CE, Woodcock MG. Local anesthesia for vitreoretinal surgery. Retina. 1990;10:47–9. [PubMed] [Google Scholar]
- 7.Hansen EA, Mein CE, Mazzoli R. Ocular anesthesia for cataract surgery: a direct sub-Tenon’s approach. Ophthalmic Surg. 1990;21:696–9. [PubMed] [Google Scholar]
- 8.Stevens JD. A new local anesthesia technique for cataract extraction by one quadrant sub-Tenon’s infiltration. Br J Ophthalmol. 1992;76:670–4. doi: 10.1136/bjo.76.11.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens JD. Curved, sub-tenon cannula for local anesthesia. Ophthalmic Surg. 1993;24:121–2. [PubMed] [Google Scholar]
- 10.Greenbaum S. Parabulbar anesthesia. Am J Ophthalmol. 1992;114:776. doi: 10.1016/s0002-9394(14)74066-8. [DOI] [PubMed] [Google Scholar]
- 11.Kumar CM, Dodds C, McLure H, Chabria R. A comparison of three sub-Tenon’s cannulae. Eye. 2004;18:873–6. doi: 10.1038/sj.eye.6701332. [DOI] [PubMed] [Google Scholar]
- 12.McNeela BJ, Kumar CM. Sub-Tenon’s block with an ultrashort cannula. J Cataract Refract Surg. 2004;30:858–62. doi: 10.1016/S0886-3350(03)00555-8. [DOI] [PubMed] [Google Scholar]
- 13.Kumar CM, Dodds C. A disposable plastic sub-Tenon cannula. Anaesthesia. 2001;56:399–400. doi: 10.1046/j.1365-2044.2001.01976-48.x. [DOI] [PubMed] [Google Scholar]
- 14.Amin S, Minihan M, Lesnik-Oberstein S, Carr C. A new technique for delivering sub-Tenon’s anaesthesia in ophthalmic surgery. Br J Ophthalmol. 2002;86:119–20. doi: 10.1136/bjo.86.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar CM, McNeela BJ. Ultrasonic localization of anaesthetic fluid using sub-Tenon’s cannulae of three different lengths. Eye. 2003;17:1003–7. doi: 10.1038/sj.eye.6700501. [DOI] [PubMed] [Google Scholar]
- 16.El-Khayat A, Wakefield M, Boddy P, Prydal J. Fine cannula technique for sub-Tenon’s injection for ophthalmic anaesthesia. Eye. 2018;32:837–8. doi: 10.1038/eye.2017.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ripart J, Metge L, Prat-Pradal D, Lopez FM, Eledjam JJ. Medial canthus single-injection episcleral (sub-tenon anesthesia): computed tomography imaging. Anesth Analg. 1998;87:42–5. doi: 10.1097/00000539-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Rous SM. Simplified sub-Tenon’s anesthesia: miniblock with maxiblock effect. J Cataract Refract Surg. 1999;25:10–5. doi: 10.1016/s0886-3350(99)80005-4. [DOI] [PubMed] [Google Scholar]
- 19.Behndig A. Sub-Tenon’s anesthesia with a retained catheter in ocular surgery of longer duration. J Cataract Refract Surg. 1998;24:1307–9. doi: 10.1016/s0886-3350(98)80219-8. [DOI] [PubMed] [Google Scholar]
- 20.Lerch D, Venter JA, James AM, Pelouskova M, Collins BM, Schallhorn SC. Outcomes and adverse events of sub-Tenon’s anesthesia with the use of a flexible cannula in 35,850 refractive lens exchange/cataract procedures. Clin Ophthalmol. 2020;14:307–15. doi: 10.2147/OPTH.S234807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riad W, Ahmad N, Kumar CM. Comparison of metal and flexible sub-Tenon cannulas. J Cataract Refract Surg. 2012;38:1398–402. doi: 10.1016/j.jcrs.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Allman KG, Theron AD, Byles DB. A new technique of incisionless minimally invasive sub-Tenon’s anaesthesia. Anaesthesia. 2008;63:782–3. doi: 10.1111/j.1365-2044.2008.05592.x. [DOI] [PubMed] [Google Scholar]
- 23.Kumar CM, Seet E. Effective and cost-saving incisionless sub-Tenon’s block. Indian J Anaesth. 2017;61:84–5. doi: 10.4103/0019-5049.198394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukasaku H, Marron JA. Pinpoint anesthesia: a new approach to local ocular anesthesia. J Cataract Refract Surg. 1994;20:468–71. doi: 10.1016/s0886-3350(13)80186-1. [DOI] [PubMed] [Google Scholar]
- 25.Kumar CM, Dodds C. Sub-Tenon’s anesthesia. Ophthalmol Clin North Am. 2006;19:209–19. doi: 10.1016/j.ohc.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Guise P. Sub-Tenon’s anesthesia: an update. Local Reg Anesth. 2012;5:35–46. doi: 10.2147/LRA.S16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua A, Chua MJ, Kumar CM. Punctal dilator facilitates insertion of blunt cannula during sub-Tenon’s block. Anaesth Intensive Care. 2018;46:239–40. [PubMed] [Google Scholar]
- 28.Greenbaum S. Chapter 1: anesthesia for cataract surgery. In: Greenbaum S, editor. Ocular anesthesia. Philadephia: W.B. Saunders; 1997. p. 3–55.
- 29.Kumar CM. Anatomy of globe, orbit and its content. In: Jaichandran VV, Kumar CM, Jagadeesh V, editors. Principles and practice of ophthalmic anaesthesia. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2017. p. 3–14.
- 30.Vohra SB, Murray PI. Sub-tenon’s block: a national United Kingdom survey. Ophthalmic Surg Lasers Imaging. 2008;39:379–85. doi: 10.3928/15428877-20080901-16. [DOI] [PubMed] [Google Scholar]
- 31.Roman SJ, Chong Sit DA, Boureau CM, Auclin FX, Ullern MM. Sub-Tenon’s anaesthesia: an efficient and safe technique. Br J Ophthalmol. 1997;81:673–6. doi: 10.1136/bjo.81.8.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLure H, Bata S, Kumar C, Chabria R, Ahmed S, Williamson S. Inferonasal vs inferotemporal approach for sub-Tenon’s block: A-393. Eur J Anaesthesiol. 2005;22:104. [Google Scholar]
- 33.Bergman L, Berglin L, Algvere PV, Laurell CG, Stenkula S. Limbal sub-Tenon’s administration of retrobulbar anesthesia using a blunt irrigating cannula. Ophthalmic Surg Lasers. 1996;27:106–12. [PubMed] [Google Scholar]
- 34.Vohra SB. A review of directions of gaze during intraocular anesthetic blocks. Ophthalmic Surg Lasers Imaging. 2012;43:162–8. doi: 10.3928/15428877-20111215-02. [DOI] [PubMed] [Google Scholar]
- 35.Alwitry A, Koshy Z, Browning AC, Kiel W, Holden R. The effect of sub-Tenon’s anaesthesia on intraocular pressure. Eye. 2001;15:733–5. doi: 10.1038/eye.2001.239. [DOI] [PubMed] [Google Scholar]
- 36.Sohn HJ, Moon HS, Nam DH, Paik HJ. Effect of volume used in sub-Tenon’s anesthesia on efficacy and intraocular pressure in vitreoretinal surgery. Ophthalmologica. 2008;222:414–21. doi: 10.1159/000161556. [DOI] [PubMed] [Google Scholar]
- 37.Vallance JH, Patton N, Ferguson A, Bennett HG. Effect of the Honan intraocular pressure reducer in sub-Tenon’s anesthesia. J Cataract Refract Surg. 2004;30:433–6. doi: 10.1016/j.jcrs.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 38.Winder S, Walker SB, Atta HR. Ultrasonic localization of anesthetic fluid in sub-Tenon’s, peribulbar, and retrobulbar techniques. J Cataract Refract Surg. 1999;25:56–9. doi: 10.1016/s0886-3350(99)80011-x. [DOI] [PubMed] [Google Scholar]
- 39.Niemi-Murola L, Krootila K, Kivisaari R, Kangasmäki A, Kivisaari L, Maunuksela EL. Localization of local anesthetic solution by magnetic resonance imaging. Ophthalmology. 2004;111:342–7. doi: 10.1016/j.ophtha.2003.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Ripart J, Benbabaali M, Muller L. Sub-Tenon’s anesthesia. Ophthalmology. 2002;109:215–6. doi: 10.1016/s0161-6420(01)01000-4. [DOI] [PubMed] [Google Scholar]
- 41.Patton N, Malik TY, Aslam TM, Vallance JH. Effect of volume used in sub-Tenon’s anaesthesia on efficacy and intraocular pressure: a randomized clinical trial of 3 mL versus 5 mL. Clin Exp Ophthalmol. 2004;32:488–91. doi: 10.1111/j.1442-9071.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 42.Spiteri N, Sidaras G, Czanner G, Batterbury M, Kaye SB. Assessing the quality of ophthalmic anesthesia. J Clin Anesth. 2015;27:285–9. doi: 10.1016/j.jclinane.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Ripart J, Prat-Pradal D, Vivien B, Charavel P, Eledjam JJ. Medial canthus episcleral (sub-Tenon) anesthesia imaging. Clin Anat. 1998;11:390–5. doi: 10.1002/(SICI)1098-2353(1998)11:6<390::AID-CA4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 44.Kumar CM, Eke T, Dodds C, Deane JS, El-Hindy N, Johnston R, et al. Local anaesthesia for ophthalmic surgery. Joint guidelines from the Royal College of Anaesthetists and the Royal College of Ophthalmologists. 2012. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2012-SCI-247-Local-Anaesthesia-in-Ophthalmic-Surgery-2012.pdf. [DOI] [PMC free article] [PubMed]
- 45.Carrim ZI, Mackie G, Gallacher G, Wykes WN. The efficacy of 5% povidone-iodine for 3 min prior to cataract surgery. Eur J Ophthalmol. 2009;19:560–4. doi: 10.1177/112067210901900407. [DOI] [PubMed] [Google Scholar]
- 46.Koerner JC, George MJ, Meyer DR, Rosco MG, Habib MM. Povidone-iodine concentration and dosing in cataract surgery. Surv Ophthalmol. 2018;63:862–8. doi: 10.1016/j.survophthal.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Frieman BJ, Friedberg MA. Globe perforation associated with subtenon’s anesthesia. Am J Ophthalmol. 2001;131:520–1. doi: 10.1016/s0002-9394(00)00815-1. [DOI] [PubMed] [Google Scholar]
- 48.Faure C, Faure L, Billotte C. Globe perforation following no-needle sub-Tenon anesthesia. J Cataract Refract Surg. 2009;35:1471–2. doi: 10.1016/j.jcrs.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Kallio H, Paloheimo M, Maunuksela EL. Haemorrhage and risk factors associated with retrobulbar/peribulbar block: a prospective study in 1383 patients. Br J Anaesth. 2000;85:708–11. doi: 10.1093/bja/85.5.708. [DOI] [PubMed] [Google Scholar]
- 50.Abramson DC. Sudden unexpected sneezing during the insertion of peribulbar block under propofol sedation. Can J Anaesth. 1995;42:740–3. doi: 10.1007/BF03012675. [DOI] [PubMed] [Google Scholar]
- 51.Vohra SB, Good PA. Altered globe dimensions of axial myopia as risk factors for penetrating ocular injury during peribulbar anaesthesia. Br J Anaesth. 2000;85:242–5. doi: 10.1093/bja/85.2.242. [DOI] [PubMed] [Google Scholar]
- 52.Edge R, Navon S. Scleral perforation during retrobulbar and peribulbar anesthesia: risk factors and outcome in 50,000 consecutive injections. J Cataract Refract Surg. 1999;25:1237–44. doi: 10.1016/s0886-3350(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 53.Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology. 2014;121:1798–809. doi: 10.1016/j.ophtha.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 54.Kumar CM, Seet E. Stopping antithrombotics during regional anaesthesia and eye surgery: crying wolf? Br J Anaesth. 2017;118:154–8. doi: 10.1093/bja/aew404. [DOI] [PubMed] [Google Scholar]
- 55.Vohra SB. Staggered sub-Tenon’s blocks: a novel anesthetic approach for bilateral strabismus surgery. Ophthalmic Surg Lasers Imaging. 2012;43:241–6. doi: 10.3928/15428877-20120102-07. [DOI] [PubMed] [Google Scholar]
- 56.Lee RM, Thompson JR, Eke T. Severe adverse events associated with local anaesthesia in cataract surgery: 1 year national survey of practice and complications in the UK. Br J Ophthalmol. 2016;100:772–6. doi: 10.1136/bjophthalmol-2015-307060. [DOI] [PubMed] [Google Scholar]
- 57.Zheng D, Huang Z, Zhang G, Huang D, Lin G, Chen W. Incidence and impact factors of intraoperative loss of light perception under sub-Tenon’s anesthesia in patients with macular diseases. Eye. 2019;33:1784–90. doi: 10.1038/s41433-019-0491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guijarro-Oria FJ, Gutierrez-Diaz E, Dorado-Lopez-Rosado AM, Montero-Rodriguez M, Lago-Llinas MD, Mencia-Gutierrez E. Prospective study of cooperation and satisfaction in glaucoma surgery under sub-Tenon’s anesthesia. Arch Soc Esp Oftalmol. 2013;88:102–7. doi: 10.1016/j.oftal.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Tsatsos M, Burnett CA, Broadway DC, Eke T. Local anaesthesia for trans-scleral cyclodiode laser procedures: surgeon and patient satisfaction with sub-Tenon’s and peribulbar anaesthesia. Clin Exp Ophthalmol. 2011;39:472–3. doi: 10.1111/j.1442-9071.2010.02483.x. [DOI] [PubMed] [Google Scholar]
- 60.Eke T. Anaesthesia for glaucoma surgery. In: Jaichandran VV, Kumar CM, Jagadeesh V, editors. Principles and practice of ophthalmic anaesthesia. New Delhi: Jaypee Bros; 2017.
- 61.Scott IU, McCabe CM, Flynn HW, Lemus DR, Schiffman JC, Reynolds DS, et al. Local anesthesia with intravenous sedation for surgical repair of selected open globe injuries. Am J Ophthalmol. 2002;134:707–11. doi: 10.1016/s0002-9394(02)01692-6. [DOI] [PubMed] [Google Scholar]
- 62.Scott IU, Gayer S, Voo I, Flynn HW, Jr., Diniz JR, Venkatraman A. Regional anesthesia with monitored anesthesia care for surgical repair of selected open globe injuries. Ophthalmic Surg Lasers Imaging. 2005;36:122–8. [PubMed] [Google Scholar]
- 63.McClellan AJ, Daubert JJ, Relhan N, Tran KD, Flynn HW, Jr., Gayer S. Comparison of regional vs. general anesthesia for surgical repair of open-globe injuries at a University Referral Center. Ophthalmol Retin. 2017;1:188–91. doi: 10.1016/j.oret.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar CM. Low volume sub-Tenon’s block. Anaesthesia. 2007;62:970–1. doi: 10.1111/j.1365-2044.2007.05241.x. [DOI] [PubMed] [Google Scholar]
- 65.Schulenburg HE, Sri-Chandana C, Lyons G, Columb MO, McLure HA. Hyaluronidase reduces local anaesthetic volumes for sub-Tenon’s anaesthesia. Br J Anaesth. 2007;99:717–20. doi: 10.1093/bja/aem272. [DOI] [PubMed] [Google Scholar]
- 66.Kumar CM, Dodds C. Ophthalmic regional block. Ann Acad Med Singap. 2006;35:158–67. [PubMed] [Google Scholar]
- 67.Kumar CM, Eid H, Dodds C. Sub-Tenon’s anaesthesia: complications and their prevention. Eye. 2011;25:694–703. doi: 10.1038/eye.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar CM, Dodds C. Evaluation of the Greenbaum sub-Tenon’s block. Br J Anaesth. 2001;87:631–3. doi: 10.1093/bja/87.4.631. [DOI] [PubMed] [Google Scholar]
- 69.Kumar N, Jivan S, Thomas P, McLure H. Sub-Tenon’s anesthesia with aspirin, warfarin, and clopidogrel. J Cataract Refract Surg. 2006;32:1022–5. doi: 10.1016/j.jcrs.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 70.Benzimra JD, Johnston RL, Jaycock P, Galloway PH, Lambert G, Chung AK, et al. The Cataract National Dataset electronic multicentre audit of 55,567 operations: antiplatelet and anticoagulant medications. Eye. 2009;23:10–6. doi: 10.1038/sj.eye.6703069. [DOI] [PubMed] [Google Scholar]
- 71.Guise PA. Sub-Tenon anesthesia: a prospective study of 6,000 blocks. Anesthesiology. 2003;98:964–8. doi: 10.1097/00000542-200304000-00025. [DOI] [PubMed] [Google Scholar]
- 72.Malik A, Fletcher EC, Chong V, Dasan J. Local anesthesia for cataract surgery. J Cataract Refract Surg. 2010;36:133–52. doi: 10.1016/j.jcrs.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 73.Guay J, Sales K. Sub-Tenon’s anaesthesia versus topical anaesthesia for cataract surgery. Cochrane Database Syst Rev. 2015;8:Cd006291. doi: 10.1002/14651858.CD006291.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ryu JH, Kim M, Bahk JH, Do SH, Cheong IY, Kim YC. A comparison of retrobulbar block, sub-Tenon block, and topical anesthesia during cataract surgery. Eur J Ophthalmol. 2009;19:240–6. doi: 10.1177/112067210901900211. [DOI] [PubMed] [Google Scholar]
- 75.Khoo BK, Lim TH, Yong V. Sub-Tenon’s versus retrobulbar anesthesia for cataract surgery. Ophthalmic Surg Lasers. 1996;27:773–7. [PubMed] [Google Scholar]
- 76.Buys YM, Trope GE. Prospective study of sub-Tenon’s versus retrobulbar anesthesia for inpatient and day-surgery trabeculectomy. Ophthalmology. 1993;100:1585–9. doi: 10.1016/s0161-6420(93)31440-5. [DOI] [PubMed] [Google Scholar]
- 77.Lai MM, Lai JC, Lee WH, Huang JJ, Patel S, Ying HS, et al. Comparison of retrobulbar and sub-Tenon’s capsule injection of local anesthetic in vitreoretinal surgery. Ophthalmology. 2005;112:574–9. doi: 10.1016/j.ophtha.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 78.Patton N, Malik TY, Aslam TM. Sub-Tenon’s anesthetic administration for cataract surgery: how much stays in? Anesth Analg. 2005;101:1012–4. doi: 10.1213/01.ane.0000168448.05964.06. [DOI] [PubMed] [Google Scholar]
- 79.Eke T, Thompson JR. Serious complications of local anaesthesia for cataract surgery: a 1 year national survey in the United Kingdom. Br J Ophthalmol. 2007;91:470–5. doi: 10.1136/bjo.2006.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke JP, Plummer J. Adverse events associated with regional ophthalmic anaesthesia in an Australian teaching hospital. Anaesth Intensive Care. 2011;39:61–4. doi: 10.1177/0310057X1103900109. [DOI] [PubMed] [Google Scholar]
- 81.Kim SK, Andreoli CM, Rizzo JF, 3rd, Golden MA, Bradbury MJ. Optic neuropathy secondary to sub-tenon anesthetic injection in cataract surgery. Arch Ophthalmol. 2003;121:907–9. doi: 10.1001/archopht.121.6.907. [DOI] [PubMed] [Google Scholar]
- 82.Quantock CL, Goswami T. Death potentially secondary to sub-Tenon’s block. Anaesthesia. 2007;62:175–7. doi: 10.1111/j.1365-2044.2006.04894.x. [DOI] [PubMed] [Google Scholar]
- 83.Ruschen H, Bremner FD, Carr C. Complications after sub-Tenon’s eye block. Anesth Analg. 2003;96:273–7. doi: 10.1097/00000539-200301000-00054. [DOI] [PubMed] [Google Scholar]
- 84.Ong-Tone L. Practice patterns of Canadian ophthalmological society members in cataract surgery: 2017 survey. Can J Ophthalmol. 2018;53:1. doi: 10.1016/j.jcjo.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 85.Oshika T, Amano S, Araie M, Majima Y, Leaming DV. Current trends in cataract and refractive surgery in Japan: 1999 survey. Jpn J Ophthalmol. 2001;45:383–7. doi: 10.1016/s0021-5155(01)00333-1. [DOI] [PubMed] [Google Scholar]
- 86.Wi JM, Moon HS, Kim KH, Shyn KH. 2012 Survey of KSCRS and KOS member: current trends in cataract surgery in Korea. J Korean Ophthalmol Soc. 2015;56:1181–7. [Google Scholar]
- 87.Pick ZS, Leaming DV, Elder MJ. The fourth New Zealand cataract and refractive surgery survey: 2007. Clin Exp Ophthalmol. 2008;36:604–19. doi: 10.1111/j.1442-9071.2008.01869.x. [DOI] [PubMed] [Google Scholar]
- 88.Wagle AA, Wagle AM, Bacsal K, Tan CS, Chee SP, Au, et al. Practice preferences of ophthalmic anaesthesia for cataract surgery in Singapore. Singap Med J. 2007;48:287–90. [PubMed] [Google Scholar]
- 89.Tam YS, Kumar CM, Au Eong KG, Yip CC, Cheng J. Trends in cataract surgery technique and anaesthesia preferences in Singapore: a 2016 survey. Ann Acad Med Singap. 2018;47:390–3. [PubMed] [Google Scholar]
- 90.El-Hindy N, Johnston RL, Jaycock P, Eke T, Braga AJ, Tole DM, et al. The Cataract National Dataset Electronic Multi-centre Audit of 55,567 operations: anaesthetic techniques and complications. Eye. 2009;23:50–5. doi: 10.1038/sj.eye.6703031. [DOI] [PubMed] [Google Scholar]
- 91.Chandradeva K, Nangalia V, Hugkulstone CE. Role of the anaesthetist during cataract surgery under local anaesthesia in the UK: a national survey. Br J Anaesth. 2010;104:577–81. doi: 10.1093/bja/aeq056. [DOI] [PubMed] [Google Scholar]
- 92.Leaming DV. Practice styles and preferences of ASCRS members-2003 survey. J Cataract Refract Surg. 2004;30:892–900. doi: 10.1016/j.jcrs.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 93.Vohra SB. Equatorial sub-Tenon blocks: animal model training. Trends Anaesth Crit Care. 2015;5:141–5. [Google Scholar]
- 94.Chhabra A, Sinha R, Subramaniam R, Chandra P, Narang D, Garg SP. Comparison of sub-Tenon’s block with i.v. fentanyl for paediatric vitreoretinal surgery. Br J Anaesth. 2009;103:739–43. doi: 10.1093/bja/aep230. [DOI] [PubMed] [Google Scholar]
- 95.Farmery AD, Shlugman D, Rahman R, Rosen P. Sub-Tenon’s block reduces both intraoperative and postoperative analgesia requirement in vitreo-retinal surgery under general anaesthesia. Eur J Anaesthesiol. 2003;20:973–8. doi: 10.1017/s0265021503001571. [DOI] [PubMed] [Google Scholar]
- 96.Sallam AAB, Donachie PHJ, Williamson TH, Sparrow JM, Johnston RL. The Royal College of Ophthalmologists’ National Ophthalmology Database Study of vitreoretinal surgery: report 5, anaesthetic techniques. Br J Ophthalmol. 2016;100:246–52. doi: 10.1136/bjophthalmol-2014-306467. [DOI] [PubMed] [Google Scholar]
- 97.Bergman L, Backmark I, Ones H, von Euler C, Olivestedt G, Kvanta A, et al. Preoperative sub-Tenon’s capsule injection of ropivacaine in conjunction with general anesthesia in retinal detachment surgery. Ophthalmology. 2007;114:2055–60. doi: 10.1016/j.ophtha.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 98.Seo IS, Seong CR, Jung G, Park SJ, Kim SY, Kim MM. The effect of sub-Tenon lidocaine injection on emergence agitation after general anaesthesia in paediatric strabismus surgery. Eur J Anaesthesiol. 2011;28:334–9. doi: 10.1097/EJA.0b013e3283426ed6. [DOI] [PubMed] [Google Scholar]
- 99.Snir M, Bachar M, Katz J, Friling R, Weinberger D, Axer-Siegel R. Combined propofol sedation with sub-Tenon’s lidocaine/mercaine infusion for strabismus surgery in adults. Eye. 2007;21:1155–61. doi: 10.1038/sj.eye.6702426. [DOI] [PubMed] [Google Scholar]
- 100.National Institute for Health and Care Excellence. NICE Guideline NG77—cataract in adults: management. 2017. https://www.nice.org.uk/guidance/ng77/evidence/full-guideline-pdf-4655997901.