Abstract
Evidence suggests that the 7-repeat variant of a 48 base pair variable number tandem repeat polymorphism in the dopamine receptor D4 (DRD4) gene may be associated with the development of attention problems. A parallel literature suggests that genes linked to dopaminergic functioning may be associated with differential sensitivity to context, such that the direction of the genetic effect is hypothesized to vary across environmental experience. Guided by these literatures, we used data from the NICHD Study of Early Child Care and Youth Development to consider (a) whether individual differences in children’s inattention problems across middle childhood are predicted by gene–environment interactions between the DRD4 gene 7-repeat polymorphism and children’s experiences of maternal sensitivity across infancy and early childhood and (b) the degree to which such interactions are consistent with the differential-sensitivity model. Largely consistent with the hypothesized model, gene–environment interactions indicated that, in the context of insensitive early maternal care, the DRD4 7-repeat polymorphism was associated with higher levels of inattention. Although somewhat less consistently, there was also evidence that, in the context of highly sensitive care, the 7-repeat polymorphism was associated with lower levels of inattention. Overall, the magnitude of the absolute genetic effect increased over time, as children’s inattention trajectories diverged.
Growing evidence suggests that genetic polymorphisms implicated in dopaminergic functioning may play a role in children’s development of attention problems. In particular, the 7-repeat variant of a variable number tandem repeat (VNTR) polymorphism in the dopamine receptor D4 (DRD4) gene has been linked with higher levels of attention problems, across samples (see Faraone et al., 2005; Gizer, Ficks, & Waldman, 2009; Thapar, Langley, Asherson, & Gill, 2007) and multiple points in development (El-Faddagh, Laught, Maras, Vöhringer, & Schmidt, 2004; Schmidt, Fox, Perez-Edgar, Hu, & Hamer, 2001).
Theoretical work and accumulating evidence from related phenotypes suggest, however, that individual differences in children’s attention-problem development may also be explained by gene–environment interaction (G × E) processes between dopamine-related genes, such as DRD4, and children’s caregiving experiences. Some have proposed that genetic factors and/or early transactional processes between genes and experience may lead to differential sensitivity to context, such that some children may be more strongly influenced by their experiences than others (Belsky, 1997; Belsky & Pluess, 2009; Boyce & Ellis, 2005). Those with genetic factors linked to heightened sensitivity are posited to benefit most from highly supportive contexts yet also be the most adversely impacted by deleterious contexts. In other words, contrary to genetic main effects considered typically in genetic studies of attention problems, the direction of the genetic effect may vary across high- and low-quality environmental experiences.
Informed by the differential-sensitivity model, we considered in the present study whether interactional processes between the DRD4 7-repeat polymorphism and children’s experiences of maternal sensitivity in infancy and early childhood are predictive of children’s trajectories of inattentive behavior across middle childhood.
Attention-Problem Development
Attention problems affect a substantial number of children. Based on nationally representative parent reports, approximately 8% of school-aged children are diagnosed with attention-deficit/hyperactivity disorder (Center for Disease Control & Prevention, 2005). Given that this likely represents only children at the upper tail of a continuous attention-problem distribution (Levy, Hay, McStephen, Wood, & Waldman, 1997; Thapar, Langley, O’Donovan, & Owen, 2006), even larger numbers of children experience subclinical levels of attention problems that likely affect their social and academic development. Attention problems are predictive of social and academic challenges in nonclinical samples (Duncan et al., 2007; NICHD Early Child Care Research Network [ECCRN], 2009).
Longitudinal work examining intraindividual stability/growth in children’s (nonclinical) attention problems over time suggests substantial interindividual variability in children’s attention-problem growth trajectories across the elementary school years (Berry & Willett, 2009; Fleming, Harachi, Cortes, Abbott, & Catalano, 2004; Friedman et al., 2007). The processes explaining individual differences in children’s attention-problem trajectories are, however, somewhat less clear.
Genetic Differences and Attention Problems
Part of the variation in children’s attention-problem trajectories is likely explained by genetic differences. Heritability estimates tend to be substantial (~0.60–0.90; Kahn & Faraone, 2006; Thapar et al., 2007) and may grow in magnitude into the elementary school years (Deater-Deckard, Petrill, Thompson, & DeThorne, 2005). Further, much of the temporal rank-order stability (i.e., cross-time covariance) in children’s attention-problem levels can be explained by genetic factors (Kuntsi, Rijsdijk, Ronald, Asherson, & Plomin, 2005; Price et al., 2005). Findings from the molecular-genetic literature suggest that candidate genes implicated in the way catecholamines (e.g., dopamine and norepinephrine) function in the brain may account for part of these broad-scale genetic effects (see Faraone et al., 2005; Thapar et al., 2007).
In particular, one polymorphism within the DRD4 gene, a 48 base pair (bp) VNTR in exon III, has shown some of the most consistent associations with children’s attention problems (see Gizer et al., 2009). The DRD4 48-bp VNTR is highly variable (2–11 repeats); the 4-repeat is the most common, followed by the 7-repeat and the 2-repeat (Chang, Kidd, Livak, Pakstis, & Kidd, 1996). Dopamine D4 receptors are highly expressed in the prefrontal cortex (Meador-Woodruff et al., 1996; Oak, Oldenhof, Hubert, & Van Tol, 2000), and the 7-repeat marker has been implicated in reduced D4 receptor expression (Schoots & Van Tol, 2003).
Given its potential link to prefrontal functioning, and in turn executive cognitive processing, the DRD4 7-repeat has been proposed to play a role in the development of attention problems, as well attention-related aspects of temperament. Meta-analyses of case-control and family-based studies suggest that the DRD4 7-repeat is associated with clinical-level attention problems (Gizer et al., 2009; Li, Sham, Owen, & He, 2006). Some evidence also suggests that the DRD4 7-repeat may be associated with dimensional measures of attention problems (Schmidt et al., 2001) and attention-related aspects of temperament (Auerbach, Benjamin, Farry, Gellar, & Ebstein, 2001) in normative samples.
G × E Interaction: Differential Sensitivity to Context
Beyond genetic main effects, there is increasing interest in the role of G × E interactions in children’s development of attention problems (Thapar et al., 2007). In particular, multiple theoretical models suggest that both the magnitude and direction of genetic effects on attention-related phenotypes may vary as a function of children’s early experiences (Belsky, 1997; Belsky & Pluess, 2009; Boyce & Ellis, 2005). Genetic factors and early G × E processes have been proposed to lead some children to be more malleable or sensitive to their experiences than others, in a “for better and for worse” manner (Belsky & Pluess, 2009). In supportive environmental conditions, those with genetic variants or G × E processes linked to heightened sensitivity are theorized to reap the largest developmental benefits from this support. In the context of nonsupportive and/or actively deleterious environmental conditions, those with heightened environmental sensitivity are theorized to be the most adversely affected. The parallel environmental effect is predicted to be negligible for those without genetic variants linked to environmental supportive or nonsupportive sensitivity. In statistical terms, this is represented by a “crossover” G × E interaction (see Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Belsky & Pluess, 2009). In substantive terms, the degree to which the more versus less sensitive genetic variant serves as a developmental “risk” or “advantage” is conditional on experience.
Although G × E studies of children’s attention problems tend to be limited to interactions with prenatal experiences (see Ficks & Waldman, 2009), evidence from studies of related phenotypes suggests that G × E processes between the DRD4 7-repeat and children’s early parental caregiving experiences may play an important role in the development of attention problems. A growing literature suggests that children’s caregiving experiences are predictive of executive cognitive abilities, such as inhibitory control, thought to partially underlie the broad attention-problem phenotype (Barkley, 1997; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Across multiple samples and measures, children who experience higher quality parent–child interactions have been found to show more effective cognitive and behavioral inhibitory control (Belsky, Fearon, & Bell, 2007; Kochanska, Murray, & Harlan, 2000; Li-Grinning, 2007; NICHD ECCRN, 2003).
The differential-sensitivity model, however, posits that this relation may differ as a function of genetic and/or early G × E processes (Belsky & Pluess, 2009; Boyce & Ellis, 2005). The magnitude of the parenting effect on children’s development of attention problems is hypothesized to be stronger for those with a DRD4 7-repeat allele, compared to those without a 7-repeat allele. In parallel, the degree to which the 7-repeat is associated with adverse, benign, or beneficial effects on children’s attention-problem development is hypothesized to be conditional on their parenting experiences. Those with a 7-repeat allele are expected to show the highest levels of attention problems under conditions of low-quality early parenting, yet the lowest levels of attention problems under conditions of high-quality parenting.
There is some evidence consistent with these predictions for related phenotypes (for recent meta-analytic results testing a wider range of early experiences, see Bakermans-Kranenburg & van IJzendoorn, 2011). For example, the association between the DRD4 7-repeat and sensation seeking in toddlerhood has been shown to be moderated by children’s experiences of maternal sensitivity in infancy (Sheese, Voekler, Rothbart, & Posner, 2007). High levels of maternal sensitivity were associated with lower levels of sensation seeking for children with a DRD4 7-repeat allele but not for children without a DRD4 7-repeat.
Similar crossover G × E interactions have been shown for children’s externalizing behaviors. Bakermans-Kranenburg and van IJzendoorn (2006) found that the relation between the DRD4 7-repeat allele and early childhood externalizing problems was conditional on maternal sensitivity in infancy. Compared with children homozygous for the 4-repeat allele, those with the 7-repeat showed substantially higher levels of externalizing problems when their mothers were rated as low sensitivity in infancy. Although it did not quite reach traditional levels of statistical significance, of children rated as having highly sensitive maternal care infancy, the 7-repeat allele was (descriptively) associated with the lowest levels of externalizing. In subsequent work with a selected sample of high-externalizing 2- and 3-year-old children, this same research group found that the effects of a randomized controlled trial testing the impact of a positive parenting intervention extended only to children with a DRD4 7-repeat allele (Baker-mans-Kranenburg, van IJzendoorn, Pijlman, Mesman, & Juffer, 2008).
G × E Interaction and Attention Problems Over Time
In addition to the potential role of these G × E processes in the development of attention problems in early childhood, there is reason to suspect that similar G × E processes may be related to the degree to which children’s attention problem trajectories diverge over time. Transactional models of developmental (Ford & Lerner, 1992; Gottlieb, 1991; Magnusson, 1988; Masten & Cicchetti, 2010; Sameroff, 1983) posit that children’s early abilities have cascading effects on their long-term trajectories. Early skills set the stage for self-organizing, transactional processes between children and their experiences over time. The differential-sensitivity model highlights the notion that these transactional processes may be particularly strong for those with genetic and environmental factors that lead some children to be more sensitive to their experiences than others.
Children experiencing low-quality care across infancy and early childhood, particularly those with a DRD4 7-repeat, may show early attentional struggles prior to school entry. In turn, these early attention problems may have cascading effects on children’s later attentional trajectories. Children with higher levels of attention-related problems tend to have more difficulty engaging in classroom activities (Brock, Rimm-Kaufman, Nathanson, & Grimm, 2009; Li-Grinning, 2007) and lower levels of academic success (Brock et al., 2007; Duncan et al., 2007). As these children fall further and further behind, their abilities to successfully regulate their attention in the classroom may increasingly diminish. This may be particularly the case across the elementary school years, when normative changes in the complexity of classroom learning tasks may increasingly tax children’s attentional abilities.
Children with attention problems may also actively select or evoke experiences that undermine their attentional abilities over time. Higher levels of attention problems, including primarily inattentive behaviors (Pope, Bierman, & Mumma, 1991), are predictive of peer problems (Hoza et al., 2005; Murray-Close et al., 2010) as well as heightened levels of teacher-child conflict (Berry & Willett, 2009). In turn, such experiences may serve as stressors that lead to increases in children’s attention-problem levels over time. Some evidence suggests that high levels of teacher–child conflict are in turn associated with increases in children’s attention problems across the elementary school years (Berry & Willett, 2009). The differential-sensitivity model suggests that the effects of such transactional processes on children’s attention-problem trajectories will be stronger for those with a DRD4 7-repeat. The model predicts inversed effects for DRD4 7-repeat children who tend to experience high-quality early caregiving. On average, these children may show the most effective early attention skills, as well as the types of experiences that support the development of subsequent attentional skills over time.
Taken together, the magnitude of the (conditional) relation between children’s early parenting experiences and their attention problems may increase over time for those with a DRD4 7-repeat. In the context of low-quality early parenting, 7-repeat children may tend to show increases in their attention problems, whereas, in the context of high-quality parenting, those with the same 7-repeat genotype may maintain their low levels or show decreases in their attention problems over time. For children without a DRD4 7-repeat, any initial effect of early parenting on children’s attention problems may remain stable or fade over time.
The Present Study
Our aim was to consider the role of interactional processes between children’s early caregiving experiences and the DRD4 7-repeat polymorphism in children’s development of attention problems, specifically, their levels of classroom inattention, in middle childhood. Our first goal was to consider whether the DRD4 7-repeat polymorphism was conditionally related to children’s nonclinical inattention levels, as a function of their maternal caregiving experiences across infancy and early childhood, and whether the effects were consistent with the differential-sensitivity model. A second goal was to test whether these G × E processes predicted divergences in children’s inattention trajectories from prekindergarten (4.5 years old) through fifth grade.
Method
Participants
In the current study, we examined longitudinal data from Phases I, II, and III of the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development, a longitudinal study of 1,364 children (50.1% male) and their families in 10 sites across the United States. Potential participants were recruited originally from among 8,986 mothers who gave birth within a 24-hr sampling period in 31 hospitals across the sites. Participants were selected such that they represent demographically the catchment area from which they were recruited (for a review, see NICHD ECCRN, 1997).
Eight of the original 10 sites (711 children) chose to participate in the genetics protocol of the study. This subsample is not nationally representative, yet it does show some sociodemographic diversity. Approximately, 83% of children are Caucasian, 10% are African American, 6% are Latino, and 2% are other ethnicities. During the children’s early infancy, approximately 8% of mothers had less than a high school education, 20% had a high school education, 57% had between 2 and 4 years of college, and 16% had postgraduate educations 1 month after the child was born. Approximately 28% of the children were from low-income families (i.e., mean income to needs ratio between infancy and prekindergarten ≤ 2).
Missing data
Within the subsample of children participating in the genetics protocol, missing data ranged from 0% to 30.5%, the latter representing children without teacher ratings of inattention in prekindergarten. Seventy-six children could not be genotyped owing to low-quality DNA samples. In an attempt to adjust for biases introduced by missing data, we used full-information maximum likelihood (FIML) estimation methods. FIML estimation helps to adjust for these biases, under the assumption that missing data is “missing at random”; that is, missingness is conditional on observed variables that are included in the model and, after adjusting for these variables, are not conditional on unobserved values of the variables with missing data (Allison, 2003). We included several control covariates that provide information about the variables of substantive interest, as well as missingness, as effects in the models (Allison, 2003). In addition, we used the Extra DV approach (Graham, 2003) to include several auxiliary variables that were of less substantive interest to our research questions yet were correlated with the substantive variables in the model. In the context of FIML, auxiliary variables can help to reduce biases due to missing data (Collins, Schafer, & Kam, 2001; Graham, 2003). Descriptions of the auxiliary variables are provided in Appendix A. The fitted parameter estimates for the auxiliary variables are not included in the path diagrams or tables provided below for visual clarity. They are available from the first author, upon request.
Procedures
Demographic information, including maternal education and maternal age at birth, as well as maternal depression ratings, were collected when the child was 1 month old. Other demographic measures, such as family income to needs ratio, were collected at each of the data collection periods used in the present study (i.e., 6, 15, 24, 54 months [prekindergarten] and first, third, and fifth grades). Mothers were administered a commonly used personality questionnaire when the child was 6 months old. Maternal sensitivity across infancy and early childhood was observed and rated during a mother–child interaction task at 4 times over this span (6, 15, 24, and 36 months). The DNA protocol was conducted when children were approximately 15 years old. Buccal mucosa cells were collected with cotton swabs by the subject, and DNA extraction was based on adaptations to the procedure outlined by Freeman and colleagues (2003).
Measures
Common-item inattention scales.
We used three items from the Teacher Report Form (Achenbach, 1991) to compute the common-item inattention scales. Teachers rated the degree to which specific inattention-related behaviors were representative of the child’s behavior on a 3-point Likert-type scale (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). Based on face validity, we selected and summed three items addressing child inattention: fails to finish things he/she starts; cannot concentrate/pay attention for long; and inattentive, easily distracted. The wording of one item at the prekindergarten assessment varied slightly from that of the other grades. We included this item in the common-item scale, despite the slight wording difference, as we concluded that the item tapped the same child behaviors.1 Internal-consistency reliability was acceptable for this scale at each grade (α = 0.83 to 0.87).
Inattention composite scales.
We computed composite inattention scales in prekindergarten and first, third, and fifth grades, using constructs developed by Kim, Deater-Deckard, Mullineaux, and Allen (2010). Item and scale selection was based on face validity. As above, three items were selected from the Teacher Report Form. In first, third, and fifth grades, an item from the teacher-rated Social Skills Rating System (Gresham & Elliott, 1990) was also used in the composite score. In third and fifth grades, teachers also completed portions of the Disruptive Behaviors Disorders Rating Scale (Pelham, Gnagny, Greenslade, & Milich, 1992). Three items from the inattentive scale were selected. In addition, independent observers rated children’s behaviors in the classroom, using the Observational Ratings of the Caregiving Environment (NICHD ECCRN, 1996) system in prekindergarten and the Classroom Observation System (National Center for Early Development and Learning, 1997) in third and fifth grades. During the prekindergarten classroom observations, global ratings of child behaviors indicative of paying attention to objects and classroom activities were completed. Interrater reliability based upon repeated-measures analysis of variance (ANOVA) was 0.88. In third and fifth grades, independent observers captured discrete child behaviors and interactions reflecting off-task inappropriate behavior, off-task unoccupied behavior, spaced out/disengaged, and paying attention. Interrater reliability estimates based upon repeated-measures ANOVA ranged from 0.70 to 0.99. Both the questionnaire and the observational measures were scaled in a Likert-type manner; items were reflected when appropriate. To create the composite score for each assessment period, the respective individual indicators described above were standardized and averaged. The internal-consistency reliability estimates of the composite scales used in the present analyses were acceptable at each of the four grades (α = 0.80–0.89). Higher scores indicate higher levels of inattention. Prior work using confirmatory factor analytic methods indicated that the composite measure fit the data well (see Kim et al., 2010).
Time.
Consistent with latent growth modeling methods (Bollen & Curran, 2005), we included a time variable in the model implicitly by fixing the factor loadings for the growth rate factor to be scaled in years, centered on the prekindergarten assessment period.
Genotype.
Genotyping of the DRD4 7-repeat allele was based on modification of the methods described by Anchordoquy et al. (2003). It was modified as follows: 1 × Taq Gold Buffer, 2.25 mM final concentration of MgCl2, 10% DMSO, 0.2 mM deoxyribonucleotide triphosphates, 0.1 mM deazo guano-sine-5′-triphosphate, 0.75 mM primers, 40 ng of DNA, and 1 U of Taq Gold (Applied Biosystems, Foster City, CA) in a volume of 12 μL. The primer sequences are 5′-6-FAMGCGAC TACGTGGTCTACTCG-3′ and reverse, 5′-AGGACCCTCATGGCCTTG-3′. One microliter was removed and placed in a 96-well plate with 10 μL of formamide containing LIZ-500 standard (Applied Biosystems, Foster City, CA). The plate was run using a fragment analysis protocol in the 3730XL DNA Analyzer (Applied Biosystems). Fragments were analyzed using Genemapper software (Applied Biosystems) with polymerase chain reaction products of 379, 427, 475 (43), 523, 571, 619 (73), 667, 715, 763, and 811 bp.
Based on prior work and a limited representation of DRD4 7-repeat homozygotes, genotype was dummy coded to indicate children as either hetero- or homozygous for the DRD4 7-repeat allele (1; DRD4 7+) or having no 7-repeat allele (0; DRD4 7−). Exact tests were conducted to test Hardy–Weinberg equilibrium (HWE) for the main alleles (7-repeat and 4-repeat) using the GENEPOP program (Raymond & Rousset, 1995). Given the diverse and geographically stratified nature of the sample, there was a somewhat unsurprising indication that the frequency of the 7+ genotype varied across research site (χ2 = 14.75, df = 7, p = .04). The 7+ genotype ranged from 17% to 39% across sites. Because differences in race and geographic location can reflect different population admixtures that can lead to divergences from HWE, we stratified the exact tests by research site and conducted them only for Caucasian children. There was no deviation from HWE at any research site (α = 0.05) in the stratified analyses. There was, however, some indication of disequilibrium, prior to stratifying for site (p = .001), owing to an underrepresentation of heterozygotes. This can reflect population admixtures and other substantive explanations (e.g., nonrandom mating and protective alleles) that are accounted for in the stratified tests above (Hartl & Clark, 1997). However, it could also reflect potential genotyping errors. The distribution of the 7+ frequency across the research site was quite similar to the distribution of the 7+ frequencies shown across the 18 (European/American) samples considered recently in a meta-analysis of DRD4 and personality (i.e., between 11% and 40%; Munafo, Yalcin, Willis-Owen, & Flint, 2008), suggesting that genotyping error was likely rather minimal. Given this, and recent simulation work indicating that genotyping error may reduce statistical power but has rather minimal effects on Type I error (Fardo, Becker, Bertram, Tanzi, & Lange, 2009; Yong Zou & Donner, 2006), we felt it appropriate to use these data, despite the divergence from HWE in the unstratified analyses. As discussed below, research site and race were included as statistical controls, along with several other covariates.
Early maternal sensitivity.
Observational measures of maternal sensitivity were conducted when children were approximately 6, 15, 24, and 36 months old (see NICHD ECCRN, 1999, for review). At all time points mother–child interactions were videotaped during a 15-min semistructured play procedure. At the 6-month in-home assessment, mothers were asked to play with their children as they would typically, for 7 min. In the remaining time, mothers and children were provided with a standardized set of toys/objects to play with (or not). At the 15- (home), 24- (laboratory), and 36-month (laboratory) assessments, mothers and children were presented with three boxes, each containing a set of developmentally appropriate books/toys, and were asked to play as they would typically. No other directions were given than to go through the boxes in a particular order. Blind raters coded the videotaped interactions for maternal sensitivity. The 6-, 15-, and 24-month sensitivity scores were based on a composite score of maternal sensitivity to nondistress, positive regard, and intrusiveness (reversed). Each was rated on a 1 to 4 scale and then summed to create the composite. Higher ratings indicate higher levels of maternal sensitivity. The 36-month composite was adjusted for developmental appropriateness and included ratings of maternal supportive presence, respect for autonomy, and hostility (reversed). Each was rated on a 1 to 7 scale and summed to create the composite. Approximately 20% of the videotapes were coded by two raters at each assessment period. Interrater reliability estimates based upon repeated-measures ANOVA (Winer, 1971) ranged from 0.83 to 0.87. Internal-consistency reliability (Cronbach α) for the maternal-sensitivity scales ranged from 0.70 to 0.81. In the present study, we adopted a composite representing average maternal sensitivity across infancy and early childhood by standardizing each scale and averaging the standardized scores across the four measures. The scale was centered on the grand mean. Although this approach obscures intraindividual stability/change, longitudinal measurement variability made growth modeling approaches inappropriate.
Control covariates
Time-varying maternal sensitivity.
To consider specifically the role of maternal sensitivity across infancy and early childhood, we controlled for the time-varying effects of maternal sensitivity on children’s inattention trajectories between prekindergarten and fifth grade. We also tested whether these time-varying effects interacted with genotype. Additional observational measures of maternal sensitivity were conducted when children were approximately 4.5 years old (i.e., prekindergarten) and again in first, third, and fifth grades. Similar to the earlier sensitivity measures, mother–child interactions were videotaped during developmentally appropriate semistructured play tasks in the laboratory (for detailed descriptions of the tasks, see https://secc.rti.org/manuals.cfm?P=3). At each age, the videotapes were coded by blind raters. Although the tasks differed slightly over time, maternal behaviors were coded on a 7-point scale similar to the 36-month sensitivity composite. A global sensitivity scale was computed by summing across three dimensions: maternal supportive presence, respect for autonomy, and hostility (reversed). Higher scores indicate more sensitive maternal behavior. Based on repeated-measures ANOVA methods, interrater reliability was acceptable at each time point, ranging from 0.84 to 0.91. Internal-consistency reliability was also acceptable (α = 0.78–0.82). Each composite score was standardized in the present analyses.
Early and time-varying income to needs ratio.
Annual family income was reported across the eight time points of interest in the present study (i.e., 6, 15, 24, 36, and 54 months and first, third, and fifth grades). At each time point, family income to needs ratio was calculated by dividing family income by the US Census Bureau poverty threshold for the appropriate family size. We created an early income variable by averaging family income to needs from 6 to 36 months. Income in the subsequent years was considered as a time-varying covariate.
Maternal prenatal smoking.
Because prior work has shown an indication that prenatal maternal smoking may moderate the association between the DRD4 7-repeat marker and children’s attention problems (see Ficks & Waldman, 2009), and the potential relation between maternal sensitivity and maternal prenatal smoking, we tested both the main effect and the potential moderating effect of maternal prenatal smoking. Maternal prenatal smoking was measured retrospectively when the child was approximately 24 months old. The variable was dummy coded to represent mothers who smoked at any time during the pregnancy.
Maternal personality.
Maternal genotype was unavailable; however, as a crude proxy, we controlled for several indicators of maternal personality, in an attempt to adjust for passive gene–environment correlation. At 6 months, maternal personality traits were measured using the agreeableness, extra-version, and neuroticism subscales of the NEO Five-Factor Inventory (Costa & McCrae, 1985) and the NEO Personality Inventory scales (Costa & McCrae, 1985). The agreeableness subscale was taken from the NEO Five-Factor Inventory; mothers rated the degree to which items tapping trust, modesty, compliance, altruism, straightforwardness, and tender-mindedness were reflective of their personalities. Higher scores indicate higher levels of compassion and cooperation. The remaining subscales were taken from the NEO Personality Inventory. On the extraversion subscale, mothers rated the degree to which items measuring the quantity and intensity of interpersonal interactions, activity level, need for stimulation, and capacity for joy reflected their personalities. Higher scores indicate more sociable, active, and person-oriented personalities. The neuroticism subscale comprises items reflecting adjustment and emotional instability, proneness to psychological distress, unrealistic ideas, and/or maladaptive coping responses. Higher scores on this subscale indicate higher levels of worrying and anxiety. Internal-consistency reliability estimates were within the acceptable range (α = 0.76–0.92).
Maternal depression.
At 1 month of age, maternal depression was measured using self-reports on the Center for Epidemiological Studies Depression Scale (Radloff, 1977). Mothers report the frequency of symptoms of depression over the previous week using a 4-point Likert-type rating, with higher scores representing more frequent symptoms. Items were summed; higher scores indicate higher levels of depression. The Center for Epidemiological Studies Depression Scale reports acceptable internal-consistency reliability (α = 0.85 in nonclinical samples).
Maternal vocabulary.
Maternal receptive vocabulary was measured when the child was approximately 36 months old using the Peabody Picture Vocabulary Test—III (Dunn & Dunn, 1981). Higher scores are indicative of broader maternal vocabulary. This commonly used measure reports reasonable split-half reliability (0.80–0.83).
Maternal education.
Mothers reported the number of years that they had attended school when the child was 1 month old.
Site.
Site dummy variables were included to adjust for biases due to research site.
Child covariates.
We included child gender and ethnicity as control covariates. Boys were coded as 1. Because the within-ethnicity group sizes were too small for the Latino, Asian, and other designations, we created a dummy variable to represent ethnicity as African American versus all other races.
Analytic plan
We adopted two complementary analytic strategies to take advantage of the respective strengths of each. Initially, we addressed our research questions using the longitudinal common-item inattention scale. This allowed us to consider the degree to which children’s inattention growth trajectories diverged conditionally, as a function of an interaction between genotype and early maternal sensitivity. We fitted taxonomies of latent growth models (Bollen & Curran, 2005), using the robust maximum likelihood estimator available in the Mplus 5.21 (Muthén & Muthén, 2009). Model constraints were used to test the simple slopes of statistically significant interactions. All model comparisons were based on Satorra–Bentler (S-B; Satorra & Bentler, 2001) adjusted likelihood-ratio tests.
It was impossible to create identical longitudinal composite scales across measures and raters, because the relevant measures were not administered consistently over time. Thus, we could not model growth using these composites. Instead, at each time point (i.e., four sets of models), we regressed the inattention composite on genotype, early maternal sensitivity, and an interaction term representing the G × E interaction. We included ratings of temporally concurrent maternal sensitivity and income, as well as the identical time-invariant controls from the latent growth models, as controls in these models.
Results
Preliminary analyses
As expected, the frequency of the 7-repeat allele was lower than that of the 4-repeat allele. Approximately 16% of the sample was heterozygous for the 7-repeat, and 4% were homozygous for the 7-repeat. Of those without the DRD4 7-repeat, the majority of children were either homozygous (64%) or heterozygous (26%) for the 4-repeat allele, with the remaining children having comparatively lower frequency 2-, 3-, 5-, 6-, and 8-repeat alleles.
There were no differences in the frequency of the 7-repeat allele between African American and non-African American children (χ2 = 1.24, df = 1, p = ns), nor were there differences across gender (χ2 = 0.24, df = 1, p = ns). There were, however, indications of gene–environment correlation (rGE). As noted above, there were significant differences across sites. Although the correlations only reached marginal levels of statistical significance, on average, DRD4 7+ children tended to experience more sensitive care and have more extraverted mothers (r = .07, p = .07 and r = .07, p = .08, respectively).
Both the inattention composite scales and the common-item inattention scales showed moderate to strong rank-order stability (Table 1). The longitudinal means for the common-item scale, as well as a visual examination of empirical growth plots, suggested that there was a slightly positive, curvilinear growth function, on average. However, the plots also suggested notable between-child heterogeneity in children’s growth rates.
Table 1.
Cross-time zero-order correlations for the common-item inattention scale and inattention composite scales
| Common-Item Inattention Scale | Inattention Composite Scale | |||||||
|---|---|---|---|---|---|---|---|---|
| 1. Pre-K | 2. 1st Grade | 3. 3rd Grade | 4. 5th Grade | 1. Pre-K | 2. 1st Grade | 3. 3rd Grade | 4. 5th Grade | |
| 1 | 1.00 | 1.00 | ||||||
| 2 | .36 | 1.00 | .40 | 1.00 | ||||
| 3 | .29 | .54 | 1.00 | .29 | .53 | 1.00 | ||
| 4 | .30 | .51 | .56 | 1.00 | .31 | .48 | .59 | 1.00 |
Note: All correlations are statistically significant at p < .001.
A taxonomy of unconditional latent growth models indicated that, on average, children’s inattention trajectories were best represented by a quadratic growth function. In absolute terms, the quadratic function fit the data well (χ2 = 7.95, df = 4, p = .05; comparative fit index = 0.99, root mean square error of approximation = 0.04). It also provided a significantly better fit than a linear functional form (ΔS-B χ2 = 48.32, df = 1, p < .001). On average, children showed slightly positive instantaneous rates of linear change (αlinear_slope = 0.25, p < .001), but the positive rate of change decayed over time (αquadratic_slope = −0.03, p < .001). There was statistically significant variation in children’s prekindergarten inattention levels (ψpre-K = 1.71, p < .001) as well as their rates of linear change (ψlinear_slope = 0.05, p < .001). The rate of quadratic change did not vary randomly across children and was fixed to 0 for parsimony. Children’s prekindergarten inattention levels were uncorrelated with their linear inattention growth rates (ψpre-K;linear_slope = −0.04, p = .17).
G × E interaction and children’s inattention trajectories
The full-covariate model (i.e., includes all control covariates) provided a reasonable fit to the data (χ2 = 152.41, df = 89, p < .001; comparative fit index = 0.98, root mean square error of approximation = 0.02; Figure 1, Table 2, and Table 3). Controlling for the family, child, and maternal covariates, higher levels of maternal sensitivity were associated with lower levels of classroom inattention in prekindergarten (γ = −0.36, p = .001). Contrary to our hypothesis, this relation was not significantly moderated by genotype (γ = −0.003, p = .99), nor was there a main effect for genotype when the interaction term was constrained to 0. There was, however, a statistically significant G × E interaction predicting children’s linear inattention growth rates (γ = −0.15, p < .001). Given that maternal sensitivity was mean centered, the (conditional) estimated effect of genotype displayed in Table 2a indicates the DRD4 7+ genotype was associated with more positive in attention growth rates for children experiencing average levels of maternal sensitivity across infancy and early childhood (γ = 0.07, p = .03). The time-varying relations between maternal sensitivity in middle childhood and inattention, as well as the respective relations between prenatal smoking and prekindergarten inattention and inattention growth, were not moderated by genotype.
Figure 1.
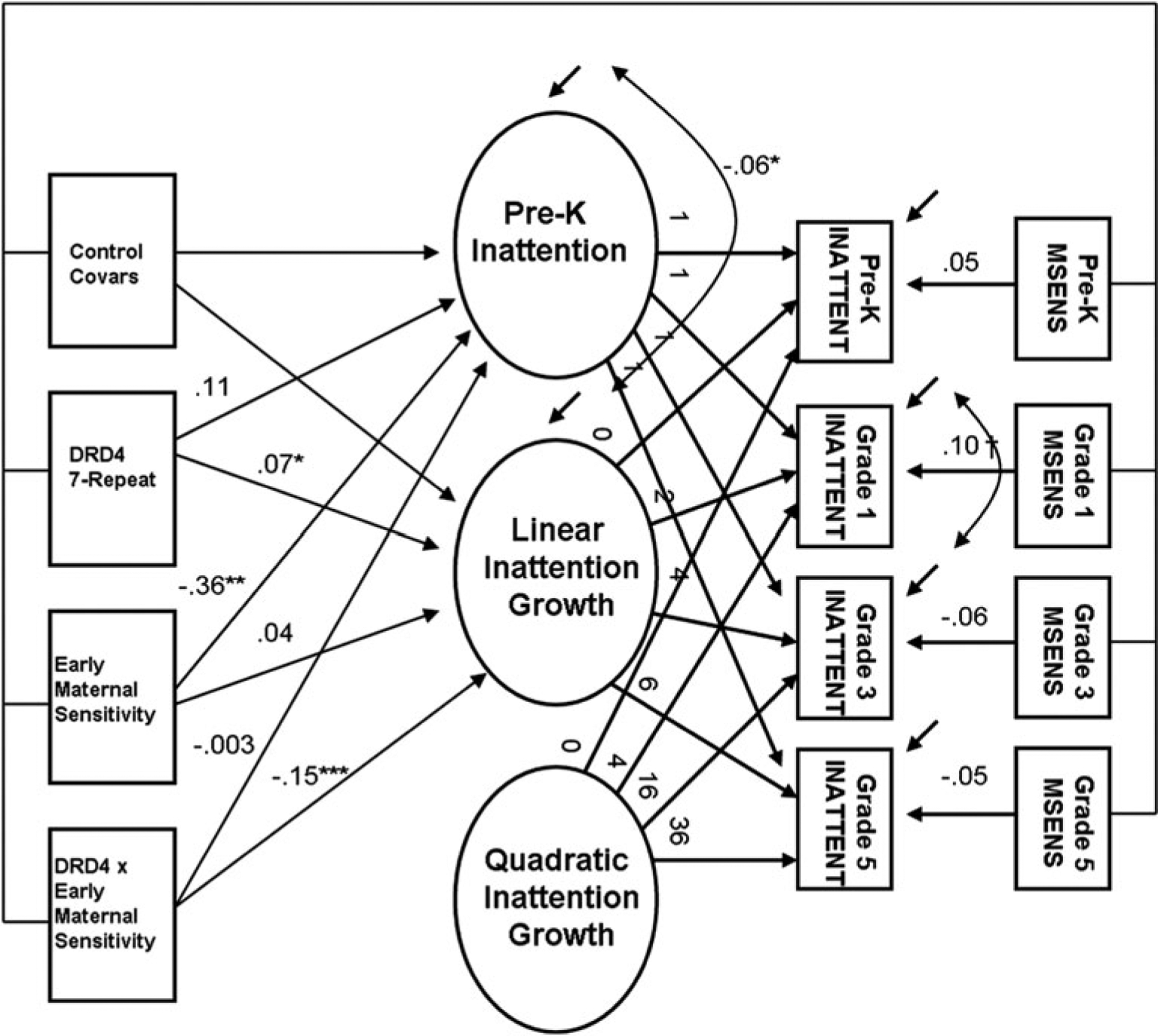
A path diagram and select fitted parameters representing a conditional latent growth model testing whether children’s inattention trajectories between prekindergarten and fifth grade are predicted by gene–environment interactions between dopamine receptor D4 7-repeat genotype and early maternal sensitivity. Each latent factor includes an intercept. The quadratic factor did not vary randomly across children and was fixed at zero for parsimony. INATTENT, time-varying teacher-rated inattention; MSENS, time-varying observed maternal sensitivity. The fitted parameters for the control and auxiliary variables are not included in the model for visual clarity. †p < .10. *p < .05. **p < .01. ***p < .001.
Table 2.
Fitted regression coefficients from a conditional latent growth model testing whether children’s inattention trajectories are predicted by G × E interactions between DRD4 7-repeat genotype and early maternal sensitivity
| Pre-K Inattention γ |
Linear Inattention Growth Rate γ |
|
|---|---|---|
| Substantive covariates | ||
| Intercept | 1.19*** | 0.41*** |
| DRD4 7+ | 0.11 | 0.07* |
| Average early maternal sensitivity | −0.36** | 0.04 |
| DRD4 7+* early maternal sensitivity | −0.003 | −0.15*** |
| Control covariates | ||
| African American | 0.02 | 0.11*** |
| Male | 0.40*** | 0.10*** |
| Early income | 0.01 | −0.01 |
| Maternal vocabulary | −0.01 | 0.00 |
| Maternal education | −0.06* | −0.003 |
| Maternal extroversion | −0.01 | 0.02 |
| Maternal neuroticism | 0.001 | −0.002 |
| Maternal agreeableness | −0.002 | −0.002 |
| Maternal depression | 0.003 | 0.000 |
| Maternal prenatal smoking | 0.09 | 0.03 |
| Between-child residual variance | 0.90*** | 0.04*** |
| R2 | .25 | .20 |
Note: The fitted parameters for the site dummy variables were included in the model but are not included for visual clarity. The time-varying covariates are not included here for visual clarity; they are provided in Table 3. The following auxiliary variables (see Graham, 2003) were also included as dependent variables in the model: maternal age at birth, maternal-rated temperament at 6 months (difficulty), maternal-rated prekindergarten temperament (inhibitory control, attention focusing, activity level). G × E, Gene × Environment; γ, unstandardized fitted regression parameter; DRD4, dopamine receptor D4.
p < .05.
p < .01.
p < .001.
Table 3.
Fitted parameters representing the time-varying effects of maternal sensitivity and income/needs ratio on children’s inattention levels
| Time Specific Inattention | Pre-K γ |
1st Grade γ |
3rd Grade γ |
5th Grade γ |
|---|---|---|---|---|
| Contemporaneous maternal sensitivity | 0.05 | 0.10† | −0.06 | −0.05 |
| Contemporaneous income | −0.003 | −0.05** | −0.02 | 0.001 |
| Within-child residual variance | 1.19*** | 1.90*** | 2.11*** | 0.81*** |
Note: γ, unstandardized fitted regression parameter.
p < .10.
p < .01.
p < .001.
Based on the parameter estimates from the final model, we provide the fitted inattention trajectories for prototypical children with high (i.e., mean + 1 SD) and low (i.e., mean − 1 SD) levels of early maternal sensitivity, conditional on genotype, in Figure 2. The effects for all other covariates in the model are held at their respective means.
Figure 2.

The fitted inattention growth trajectories between prekindergarten and fifth grade for prototypical dopamine receptor D4 (DRD4) 7+ and DRD4 7− children, conditional on high (i.e., mean + 1 SD) and low (i.e., mean − 1 SD) early sensitivity ratings. TRF, Teacher Report Form.
As illustrated by the fitted values on the y intercept in Figure 2, on average, children with lower levels of early maternal sensitivity tended to have higher levels of inattention as prekindergarteners, regardless of genotype. In contrast, the fitted growth trajectories illustrate a crossover G × E interaction. For those with the DRD4 7+ genotype, low levels of early maternal sensitivity were associated with increases in children’s inattention levels over time (i.e., solid trajectory, with marker), an approximate 0.66 SD2 increase between prekindergarten and fifth grade. Children with the DRD4 7− genotype (i.e., solid trajectory, nonmarker) who experienced equally low levels of early maternal sensitivity showed negligible decreases in their inattention levels over this same period (−0.04 SD). That is, holding low sensitivity levels constant, the DRD4 7+ genotype was associated higher levels of inattention.
Further, the magnitude of the conditional genetic effect increased over time. Planned comparisons indicated that the estimated conditional genetic effect for low maternal sensitivity (i.e., solid trajectory, marker versus nonmarker) approached statistical significance by first grade (0.31 SD, p =.07). By fifth grade, prototypical low-sensitivity, DRD4 7+ children were estimated to show inattention levels that were, on average, about 0.77 SD higher than DRD4 7− children with equally low levels of early maternal sensitivity.
For children with high levels of early maternal sensitivity, the direction of the conditional genetic effect was inversed (i.e., dashed trajectories, marker versus nonmarker), and the magnitude of the effect was comparatively smaller. Regardless of genotype, those with high levels of early maternal sensitivity showed slight increases in their inattention levels through fifth grade. However, on average, the conditional inattention growth rates were more positive for DRD4 7-repeat children (0.33 SD) than for DRD4 7+ children, who comparatively maintained their low inattention levels over time (0.13 SD). Holding children’s high levels of maternal sensitivity constant, the DRD4 7+ was associated with lower levels of inattention. The conditional genetic effect for high maternal-sensitivity children, as operationalized as a standard deviation above the mean, did not reach statistical significance at any point within this age range. This effect did, however, approach statistical significance at somewhat higher levels of maternal sensitivity (e.g., mean + 1.5 SD), by third grade (ΔS-B χ2 = 3.77, df = 1, p =.05).
The parallel interpretation of the interaction indicates that the long-term association between early maternal sensitivity and inattention is much stronger for children with the DRD4 7+ genotype. Although prototypical children with lower levels of early maternal sensitivity tended to have higher levels of inattention as prekindergarteners (regardless of genotype), these differences were no longer present by fifth grade for DRD4 7− children (i.e., trajectories without markers), on average. In contrast, the prekindergarten inattention differential between children with high and low levels in early maternal sensitivity more than doubled by fifth grade for children with the DRD4 7+ genotype.
G × E: Inattention across measures, raters, and time
In an additional set of analyses, we considered a similar set of questions using multimeasure (all grades), multirater (all grades, but first) composite measures of children’s inattention levels and maximum likelihood estimated, multiple regression models. The final, full-covariate models are presented in Table 4.
Table 4.
Fitted coefficients from (full information maximum likelihood) multiple regression models testing the moderating role of early maternal sensitivity on the relation between the DRD4 7-repeat genotype and composite measures of inattention across middle childhood
| Inattention Composite | ||||
|---|---|---|---|---|
| Pre-K B |
1st Grade B |
3rd Grade B |
5th Grade B |
|
| Substantive covariates | ||||
| Intercept | 0.04 | 0.01 | 0.01 | 0.00 |
| DRD4 7+ | 0.04 | 0.11 | 0.08 | 0.14† |
| Average early maternal sensitivity | −0.11† | −0.08 | −0.03 | −0.03 |
| DRD4 7+* early maternal sensitivity | −0.01 | −0.29* | −0.19† | −0.24* |
| Control covariates | ||||
| Contemporaneous maternal sensitivity | −0.02 | 0.07* | −0.11*** | −0.06* |
| African American | −0.02 | 0.22† | 0.22* | 0.37*** |
| Male | 0.24*** | 0.29 | 0.27*** | 0.32*** |
| Early income | 0.02 | −0.02 | −0.01 | −0.03* |
| Contemporaneous income | −0.01 | −0.01 | 0.00 | 0.00 |
| Maternal education | −0.03† | −0.02 | −0.04*** | −0.02 |
| Maternal vocabulary | −0.002 | −0.01* | −0.001 | 0.000 |
| Maternal extroversion | −0.004 | −0.01 | −0.004 | −0.003 |
| Maternal neuroticism | 0.004 | −0.01† | −0.01 | −0.004 |
| Maternal agreeableness | 0.004 | −0.01 | −0.01 | −0.01 |
| Maternal depression | 0.003 | 0.01 | 0.003 | 0.004 |
| Maternal prenatal smoking | 0.11 | 0.09 | 0.03 | 0.10 |
| R2 | .11 | .17 | .19 | .25 |
Note: Fitted parameters for the site dummy variables were included in the model but are not included in the table for visual clarity. The following “auxiliary variables” (see Graham, 2003) were also included as dependent variables in the model: maternal age at birth, maternal-rated temperament at 6 months (difficulty), maternal-rated prekindergarten temperament (inhibitory control, attention focusing, activity level). B, unstandardized fitted regression parameter; DRD4, dopamine receptor D4.
p < .10.
p < .05.
p < .01.
p < .001.
There was no main effect of genotype on children’s prekindergarten inattention composite scores. There was a statistically marginal main effect for early maternal sensitivity (here, conditional on DRD4 7− children; B = −0.11, p = .08); however, neither of the respective relations between early or contemporaneous maternal sensitivity and inattention was moderated by genotype.
The regression model for children’s first-grade inattention composite scores indicated a statistically significant G × E interaction (B = −0.29, p = .01, ΔR2 =.01). As shown in Figure 3a, there was a significant negative association between early maternal sensitivity and first-grade inattention for DRD4 7+ children (i.e., dashed slope; BDRD4_7+ = −0.36; p < .001). This corresponds to a standardized conditional association of −0.33. In contrast, there was no such association for children with the DRD4 7− genotype (i.e., solid slope; BDRD4_7− = −0.08, p = .17).
Figure 3.
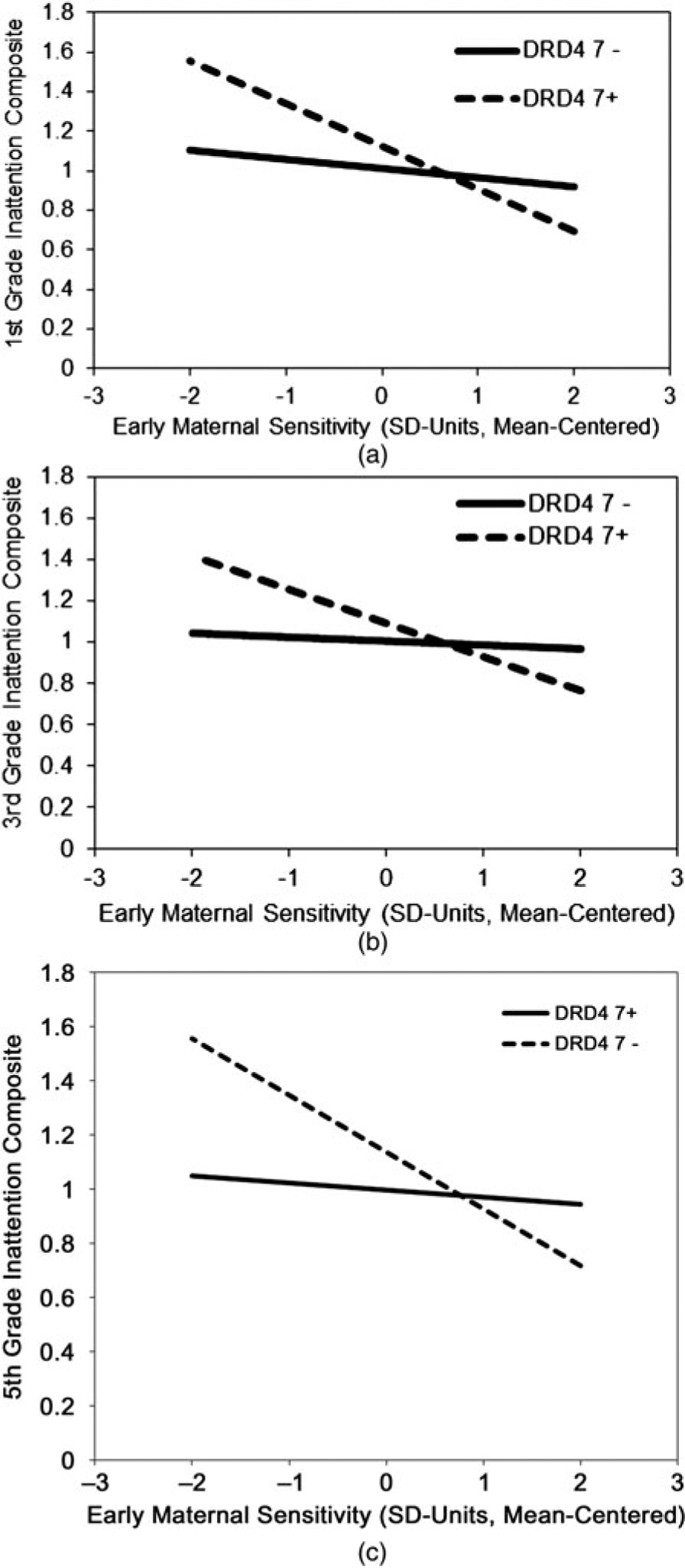
The associations between (a) early maternal sensitivity and first-grade inattention levels, conditional on genotype; (b) early maternal sensitivity and third-grade inattention levels, conditional on genotype; and (c) early maternal sensitivity and fifth-grade inattention levels, conditional on genotype. A constant of 1 was added to the predicted inattention scores to make them positive. Although the fitted relations are based on interactions between unstandardized covariates, maternal sensitivity is displayed in standard deviation units for clarity. DRD4, dopamine receptor D4.
A crossover pattern was also evident. Tests of the simple slopes indicated that, on average, the DRD4 7+ genotype was associated with significantly higher levels of inattention (~0.38 SD) when children experienced low levels of early maternal sensitivity (i.e., mean − 1 SD; BLOSENS = 0.33, p = .01). The conditional genetic effect reversed direction for children who experienced high levels of early maternal sensitivity. Children with the DRD4 7+ genotype and early maternal sensitivity ratings that were one standard deviation above the mean had first-grade inattention scores that were (descriptively) lower, on average, than those of their DRD4 7− peers with equally high levels of maternal sensitivity. This conditional genetic only approached statistical significance at rather high levels of maternal sensitivity (e.g., mean + 2 SD). Higher levels of sensitivity in first-grade were (counterintuitively) associated with higher levels of first-grade inattention (B = 0.07, p = .04). This relation was not moderated by genotype.
A similar, albeit statistically marginal, G × E interaction effect was evident for children’s third-grade inattention composite scores (Table 4; B = −0.19, p =.05; ΔR2 = 0.01). As above, tests of the simple slopes indicated that a statistically significant conditional effect of early maternal sensitivity emerged only for children with the DRD4 7+ genotype. For DRD4 7+ children, higher levels of early maternal sensitivity were associated with lower inattention composite scores (Figure 3b; BDRD4_7+ = −0.21, p < .01). This corresponded to a conditional standardized association of approximately −0.22. For DRD4 7− children, there was no association between early maternal sensitivity and third-grade inattention (BDRD4_7− = −0.03, p = .61). The conditional genetic effect illustrated a similar crossover pattern. Tests of the simple slopes indicated that the DRD4 7+ genotype was associated with heightened levels of inattention in the context of low levels of early maternal sensitivity (i.e., mean − 1 SD; BLOSENS = 0.23, p = .04). Although the conditional genetic effect appeared to reverse direction at higher levels of early maternal sensitivity, this simple slope did not reach statistical significance, even at very high levels of the maternal-sensitivity distribution. Contemporaneous maternal sensitivity was negatively associated with children’s third-grade inattention scores (B = −0.11, p < .001); however, this relation was not moderated by genotype.
Finally, a similar G × E interaction was noted for the fifth-grade inattention composite (Table 4; B = −0.24, p < .01; ΔR2 = 0.01). Planned comparisons indicated that the conditional effect of early maternal sensitivity extended only to DRD4 7+ children (Figure 3c; BDRD4_7+ = −0.27, p < .001). This conditional association corresponded to a standardized association of approximately −0.28. Early maternal sensitivity was unrelated to children’s fifth-grade inattention scores for children with the DRD4 7− genotype (BDRD4_7− = −0.03, p = .47). It is interesting that, unlike prior grades, the (conditional) genetic effect displayed in Table 4 (B = 0.14, p = .07) indicated that the DRD4 7+ genotype was marginally associated with higher fifth-grade inattention scores for children with average levels of maternal sensitivity across infancy and early childhood. Based on this finding, we conducted follow-up analyses of this same effect in the common-item growth model, by recentering the growth intercept at later points in time. At average levels of maternal sensitivity, the DRD4 7+ genotype was associated with higher inattention levels at marginal levels of statistical significance by first grade (B = 0.24, p =.08) and was statistically significant by third grade (B = 0.38, p = .04).
At the descriptive level, the direction of the conditional genetic effect varied as a function of children’s early maternal sensitivity ratings. For children with low levels of early maternal sensitivity (i.e., mean − 1 SD), the DRD4 7+ genotype was associated with comparatively higher inattention levels (BLOSENS = 0.33, p < .01). This corresponds to a conditional standardized association of approximately 0.43. Inversely, the DRD4 7+ genotype was associated with lower inattention levels for children in the context of high levels of early maternal sensitivity. However, again, the simple slopes indicated that the conditional effect only approached statistical significance at very high levels of early maternal sensitivity. Higher levels of contemporaneous maternal sensitivity were associated with lower levels of fifth-grade inattention (B = −0.04, p = .04). This relation was not moderated by genotype.
Discussion
Growing evidence suggests that the DRD4 7-repeat polymorphism may play a role in the development attention problems (Faraone et al., 2005; Gizer et al., 2009; Li et al., 2006). A parallel literature suggests that some genetic effects may be better represented by G × E interactions with children’s early caregiving experiences. Emerging work suggests that some G × E interactions may be indicative of differential sensitivity to context, such that some children are more sensitive to their environmental experiences than others, in a “for better and for worse” manner (Belsky & Pluess, 2009). The present findings may provide some support for the potential role of G × E processes in children’s development of inattention problems in middle childhood.
We found that the relation between the DRD4 genotype and children’s inattention problems was moderated by their early levels of maternal sensitivity. There was some indication of a crossover interaction, such that the magnitude and direction of the genetic effect varied across levels of maternal sensitivity. With the exception of teacher’s inattention ratings in prekindergarten, the DRD4 7+ genotype was associated with the highest levels of inattention when children grew up in the context of low levels of maternal sensitivity across infancy and early childhood. In contrast, there was some, albeit mixed, indication that the DRD4 7+ genotype was associated with the lowest levels of inattention when these children grew up in the context of high levels of early maternal sensitivity. The results from the latent growth models indicated that this conditionally “beneficial” effect of the DRD4 7+ genotype emerged by third grade for those with maternal-sensitivity ratings that were 1.5 SD above the mean. This relation grew more substantial by fifth grade. The results from the multiple-measure/multiple-rater models showed the same descriptive trend, but it only approached traditional levels of statistical significance at the upper tail of the maternal-sensitivity distribution and, in one case, never reached statistical significance.
The crossover effects are consistent with a growing body of work suggesting that genes implicated in the functioning of catecholamines, such as dopamine, may be associated with heighted sensitivity to the effects of experience (see Belsky & Pluess, 2009). The DRD4 7-repeat has been shown to moderate the respective relations between early maternal sensitivity and the development of sensation seeking (Sheese et al., 2007) and externalizing problems (Bakermans-Kranenburg et al., 2006, 2008), such that those with the DRD4 7+ genotype show the most optimal developmental outcomes in the context of highly supportive environments but the least optimal outcomes in the context of unsupportive environments. The present findings extend these similar trends to children’s levels of nonclinical inattention problems. Further, given that children’s later maternal-sensitivity levels in middle childhood showed somewhat inconsistent associations with their contemporaneous inattention levels and did not appear to interact with genotype, these results suggest that maternal sensitivity in infancy and early childhood may be particularly important.
Note that the absolute magnitude of the conditional genetic effect differed across low and high levels of early maternal sensitivity. In the context of low early maternal sensitivity, the adverse effect of the DRD4 7+ was notably larger than was the beneficial effect of the DRD4 7+ in the context of high maternal sensitivity. In the latter case, the results from the analyses of the composite inattention measures indicated that the beneficial effects were more inconsistent and tended to be restricted to the high positive tail of the maternal-sensitivity distribution. This asymmetry is consistent with prior studies of related phenotypes (e.g., Bakermans-Kranenburg & van IJzendoorn, 2006). It could be that environmental stressors have a comparatively stronger impact on these outcomes than do environmental supports for DRD4 7+ children. Others have proposed such a curvilinear relation between parenting and development (Scarr, 1992).
Asymmetrical findings across supportive and adverse environments could also reflect the restricted range of the outcome measure (Belsky et al., 2009). Although the measure of maternal sensitivity used in the present study is thought to reflect a wide range of emotionally negative and positive adult–child interactions, our outcome measures were largely restricted to problematic child behavior. The weaker and more inconsistent findings for the beneficial effect of high maternal sensitivity for DRD4 7+ children could be explained by the fact that these children tended to approach the floor of the measure. Future work with measures that tap attention skills as well as problems may begin to address this question more fully.
The comparatively more inconsistent beneficial effect of the DRD4 7+ genotype for those with high ratings of maternal sensitivity could also reflect processes that are more consistent with diathesis–stress models of psychopathology than with differential sensitivity per se. The DRD4 7-repeat variant may serve as a risk factor for attention problems that becomes phenotypically expressed in the context environment risk, with no beneficial effects in the context of environmental supports. That is, DRD4 7+ children may be more sensitive to their environments—for worse, but not for better.
The answer ultimately remains unclear in the present data. Given the descriptive trends of the crossover interactions from the composite measures of inattention, the fact that results from the growth models did show evidence of the crossover in the mid- to late elementary school years, and the consistency of the present findings with prior studies considering related outcomes (see Bakermans-Kraneburg & van IJzendoorn, 2011), there is at least some indication that the present findings may be consistent with the differential-sensitivity framework.
It is interesting that, to some degree, these findings are also consistent with prior work considering the main effects of the DRD4 7+ genotype on inattention (Faraone et al., 2005; Gizer et al., 2009; Li et al., 2006). Although the findings differed slightly across the common-scale and composite measures of inattention, there was an indication of a positive relation between the DRD4 7+ genotype and inattention in the later elementary school years for children with average levels of maternal sensitivity. That is, in the context of early parenting that is neither particularly sensitive nor particularly insensitive, which is the vast majority of the cases, the DRD4 7+ genotype was associated with higher levels of inattention. However, the present findings also suggest that genetic effects may be obscured when such moderating effects are not modeled explicitly.
The present findings also indicated that the magnitude of the absolute (conditional) genetic effects increased over time. Regardless of genotype, children with lower levels of early maternal sensitivity tended to have higher levels of prekindergarten inattention. However, the G × E interaction predicted the degree to which these early inattention differences diverged versus converged over time. In the context of low maternal sensitivity, on average, DRD4 7+ children showed notable increases in their initially high inattention levels through fifth grade. In contrast, in the context of high early maternal sensitivity, DRD4 7+ children tended to largely maintain their initially low inattention levels over time. For children with the DRD4 7− genotype, prekindergarten inattention differences associated with early maternal sensitivity tended to fade over time as their inattention trajectories converged. As a function of these varying trajectories, the absolute conditional genetic effect increased over time.
This developmental increase in the (conditional) genetic effect is consistent with some findings from the quantitative behavioral genetic literature. Deater-Deckard and colleagues (2005) found that the heritability of observer-/teacher-rated task persistence was negligible at the end of early childhood but increased substantially across middle childhood. In a meta-analysis of twin studies of intelligence and personality, McCartney, Harris, and Bernieri (1990) found similar heritability increases for intelligence and aspects of temperament.
The developmental mechanisms underlying these varying inattention trajectories remain unclear. Regardless of genotype, low levels early maternal sensitivity may lead to higher levels of inattention at the end of early childhood. In turn, entering school with higher levels of inattention problems may be associated with experiential stressors, such as struggles with one’s teachers (Berry & Willett, 2009) and peers (Hoza et al., 2005; Murray-Close et al., 2010), that exacerbate children’s inattention problems over time. Inversely, children who enter school with fewer inattention problems may tend to have the types experiences that support the maintenance of these skills over time. The differential-sensitivity model suggests that such cascading effects, for better and for worse, will vary as a function of genetic or G × E processes that affect children’s sensitivity to experience. Consistent with the present findings, the inattention trajectories for DRD4 7+ children might be hypothesized to diverge over time, whereas early inattention differences might be expected to fade for those with a DRD4 7-repeat. Of course, the true mediating processes underlying the G × E effect on children’s inattention trajectories remain empirical questions to be tested directly.
Limitations and future directions
There are limitations to this study that could affect the interpretation of the findings. Given the correlational nature of the design, it is impossible to establish whether genetic, environmental, or interactional effects between the two are causal factors underlying individual differences in children’s inattention trajectories. We attempted to account statistically for the potential confounding effects of rGE by including several maternal and demographic covariates in model; however, it remains possible that unobserved or unobservable variables, including passive gene–environment correlation (which we could not control), may account for the reported G × E effects. Further, although interactive effects for the DRD4 7-repeat are beginning to show similar results across samples, it remains unclear whether the 7-repeat is the causal genetic locus. The DRD4 7-repeat may be in linkage disequilibrium with other polymorphisms that explain the present G × E interaction in similar substantive terms or as a spurious effect due to the unobserved markers’ role in causing both parenting behaviors and children’s inattention trajectories.
In addition, we selected teacher and observer ratings of children’s inattention because prior work suggests comparatively stronger phenotypic stability, stronger relations with neuropsychological measures thought to reflect attention problems, and weaker impacts of contrast effects compared to parental reports (see Thapar et al., 2006). This analytic choice, however, limited the prekindergarten inattention data to children attending 10 or more hours of nonparental care at the age of 4.5 years old. The majority of children in the sample (~75%) were in nonparental care at this age. Further, we accounted for several covariates associated with selection into nonparental care in the model. However, it is possible that unobserved variables associated with selection into care may bias the reported estimates.
Conclusion
Increasing attention is being paid to G × E interactions in children’s development. In particular, theoretical work suggests that some G × E effects may be explained by differential sensitivity to context, such that genetic factors may lead some children’s developmental trajectories to be more influenced by experience than others. Although mixed, our findings were somewhat consistent with the differential-sensitivity model. A significant G × E interaction between the DRD4 7-repeat polymorphism and children’s experiences of maternal sensitivity across infancy and early childhood indicated that, in the context of highly sensitive care, the DRD4 7-repeat was associated with the most adaptive inattention trajectories. In contrast, the same genotype was associated with the least adaptive trajectories in the context of highly insensitive care. The magnitude of the absolute (conditional) genetic effect increased over time.
Acknowledgments
This work was supported in part by Grant HD25451 from the National Institute of Child Health and Human Development (NICHD) Study of Early Child Care and Youth Development (to K.M.). It was also supported by a Julius B. Richmond Fellowship (Harvard University Center on the Developing Child), a Graduate Student Research Grant (Harvard University Mind/Brain/ Behavior Initiative), and postdoctoral research support provided (to D.B.) by Dr. Clancy Blair (NIH 5R01HD051502 and New York University, Institute of Human Development and Social Change). We thank Dr. Blair for his insightful reviews of prior versions of this manuscript. DNA extraction and genotyping was performed at the Genome Core Facility in the Huck Institutes for Life Sciences at Pennsylvania State University under the direction of Dr. Deborah S. Grove, Director for genetic analysis. Finally, this research would not have been possible without the inspired work of the NICHD Early Child Care Research Network and research staff, who designed and conducted the overall study, or without the dedicated children, families, and teachers who participated.
Appendix A
The following is a description of the auxiliary variables included in the model.
Maternal age at birth
This is the age (years) of mothers when they gave birth to study child.
Infant temperament
Mothers rated their children for temperament using a modified version of the Revised Infant Temperament Questionnaire (Carey & McDevitt, 1978) when the child was 6 months old. The global temperament composite scale provides a rating of infant difficulty, with higher scores indicative of more difficult infants. Internal-consistency reliability estimates for this scale are acceptable (α = 0.81).
Prekindergarten temperament
Mothers rated children on three related dimensions of temperament when their children were approximately 54 months old, using the Child Behavior Questionnaire (Rothbart, Ahadir, Hershey, & Fisher, 2001). The inhibitory control subscale measures the child’s ability to plan and inhibit approach responses when instructed or in novel contexts. The attention focusing subscale taps the child’s ability to maintain focus on a task, and the activity level subscale measures the child’s gross motor activity (i.e., rate and extent of locomotion). Higher scores on each of the subscales represent higher levels of each of these dimensions. Although internal reliability consistency was slightly lower for the activity level subscale (α = 0.70), the internal reliability estimates were within the acceptable range (α = 0.70–0.75).
Footnotes
We fitted the same models discussed below, using scales which included only the two remaining items. The substantive interpretations of the results were virtually identical.
The standard deviation used in these calculations is based on the root of estimated variance of the intercept from an unconditional growth model, with time centered at fifth grade. We used the estimated fifth-grade variance, because it was the largest variance and thus provides comparatively more conservative estimates than when time is centered earlier in development.
References
- Achenbach TM (1991). Integrative guide for the 1991 CBCL/4–18, YSR, and TRF profiles. Burlington, VT: University of Vermont, Department of Psychiatry. [Google Scholar]
- Allison PD (2003). Missing data techniques for structural equation modeling. Journal of Abnormal Psychology, 112, 545–557. [DOI] [PubMed] [Google Scholar]
- Anchordoquy HC (2003). Genotyping of three candidate genes after whole-genome preamplification of DNA collected from buccal cells. Behavior Genetics, 33, 73–78. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Benjamin J, Faroy M, Geller V, & Ebstein R (2001). DRD4 related to infant attention and information processing: A developmental link to ADHD? Psychiatric Genetics, 11, 31–35. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, & van IJzendoorn MH (2006). Gene–environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology, 48, 406–409. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Pijlman FT, Mesman J, & Juffer F (2008). Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology, 44, 293–300. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, & van IJzendoorn MH (2011). Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology, 23, 39–52. [DOI] [PubMed] [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121, 65–94. [DOI] [PubMed] [Google Scholar]
- Belsky J (1997). Variation in susceptibility to rearing influences: An evolutionary argument. Psychological Inquiry, 8, 182–186. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science, 16, 300–304. [Google Scholar]
- Belsky J, Fearon RMP, & Bell B (2007). Parenting, attention and externalizing problems: Testing mediation longitudinally, repeatedly and reciprocally. Journal of Child Psychology and Psychiatry and Allied Disciplines, 48, 1233–1242. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, & Williams R (2009). Vulnerability genes or plasticity genes? Molecular Psychiatry, 14, 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). The nature (and nurture?) of plasticity in early human development. Perspectives in Psychological Science, 4, 345–351. [DOI] [PubMed] [Google Scholar]
- Berry D, & Willett JB (2009, April). Pre-kindergarten attention problems, teacher–child conflict, and achievement in late elementary school: A child-by-environment mediational growth model. Poster presented at the Biennial Conference of the Society for Research in Child Development Denver, CO. [Google Scholar]
- Bollen KA, & Curran PJ (2005). Latent curve models: A structural equation perspective. New York: Wiley. [Google Scholar]
- Boyce WT, & Ellis BJ (2005). Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17, 271–301. [DOI] [PubMed] [Google Scholar]
- Brock LL, Rimm-Kaufman SE, Nathanson L, & Grimm NA (2009). The contributions of “hot” and “cool” executive function to children’s academic achievement, learning-related behaviors, and engagement in kindergarten. Early Research Quarterly, 24, 337–349. [Google Scholar]
- Carey WB, & McDevitt SC (1978). Revision of the infant temperament questionnaire. Pediatrics, 61, 735–739. [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2005). Mental health in the United States: Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder—United States, 2003. Morbidity and Mortality Weekly Report, 54, 842–847. [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, & Kidd KK (1996). The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Human Genetics, 98, 91–101. [DOI] [PubMed] [Google Scholar]
- Collins LM, Schafer JL, & Kam CM (2001). A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods, 6, 330–351. [PubMed] [Google Scholar]
- Costa PT, & McCrae RR (1985). The NEO personality inventory manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Deater-Deckard K, Petrill SA, Thompson L, & DeThorne L (2005). A cross-sectional–behavioral genetic analysis of task persistence in the transition to middle childhood. Developmental Science, 8, F21–F26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Dowsett CJ, Claessens A, Magnuson K, Huston AC, Klebanov P, et al. (2007). School readiness and later achievement. Developmental Psychology, 43, 1428–1446. [DOI] [PubMed] [Google Scholar]
- Dunn LM, & Dunn LM (1981). Peabody Picture Vocabulary Test—Revised. Circle Pines, MN: American Guidance Service. [Google Scholar]
- El-Faddagh M, Laucht M, Maras A, Vöhringer L, & Schmidt MH (2004). Association of dopamine D4 receptor (DRD4) gene with attention-deficit/hyperactivity disorder (ADHD) in a high-risk community sample: A longitudinal study from birth to 11 years of age. Journal of Neural Transmission, 111, 883–889. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. (2005). Molecular genetics of attention-deficit/ hyperactivity disorder. Biological Psychiatry, 57, 1313–1323. [DOI] [PubMed] [Google Scholar]
- Fardo DW, Becker KD, Bertram L, Tanzi RE, & Lange C (2009). Recovering unused information in genome-wide association studies: The benefit of analyzing SNPs out of Hardy–Weinberg equilibrium. European Journal of Human Genetics, 17, 1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficks CA, & Waldman ID (2009). Gene–environment interactions in attention-deficit/hyperactivity disorder. Current Psychiatry Report, 11, 387–392. [DOI] [PubMed] [Google Scholar]
- Fleming CB, Harachi TW, Cortes RC, Abbott RD, & Catalano RF (2004). Level and change in reading scores and attention problems during elementary school as predictors of problem behavior in middle school. Journal of Emotional and Behavioral Disorders, 12, 130–144. [Google Scholar]
- Ford DH, & Lerner RM (1992). Developmental systems theory: An integrative approach. Newbury Park, CA: Sage. [Google Scholar]
- Freeman B, Smith N, Curtis C, Huckett L, Mill J, & Craig IW (2003). DNA from buccal swabs recruited by mail: Evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behavior Genetics, 33, 67–72. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, et al. (2007). Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychological Science, 18, 893–900. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, & Waldman ID (2009). Candidate gene studies of ADHD: A meta-analytic review. Human Genetics, 126, 51–90. [DOI] [PubMed] [Google Scholar]
- Gottlieb G (1991). Experiential canalization of behavioral development: Theory. Developmental Psychology, 27, 4–13. [Google Scholar]
- Graham JW (2003). Adding missing-data relevant variables to FIML-based structural equation models. Structural Equation Modeling: A Multidisciplinary Journal, 10, 80–100. [Google Scholar]
- Gresham FM, & Elliott SN (1990). The social skills rating system. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Hartl DL, & Clark AG (1997). Principles of population genetics. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Hoza B, Mrug S, Gerdes AC, Hinshaw SP, Bukowski WM, Gold JA, et al. (2005). What aspects of peer relationships are impaired in children with ADHD? Journal of Consulting and Clinical Psychology, 73, 411–423. [DOI] [PubMed] [Google Scholar]
- Khan SA, & Faraone SV (2006). The genetics of ADHD: A literature review of 2005. Current Psychiatry Reports, 8, 393–397. [DOI] [PubMed] [Google Scholar]
- Kim J, Deater-Deckard K, Mullineaux PY, & Allen B (2010). Longitudinal studies of anger and attention span: Context and informant effects. Journal of Personality, 78, 419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, & Harlan ET (2000). Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology, 36, 220–232. [PubMed] [Google Scholar]
- Kuntsi J, Rijsdijk F, Ronald A, Asherson P, & Plomin R (2005). Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biological Psychiatry, 57, 647–654. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, & Waldman ID (1997). Attention deficit hyperactivity disorder: A category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 737–744. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, & He L (2006). Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder. Human Molecular Genetics, 15, 2276–2284. [DOI] [PubMed] [Google Scholar]
- Li-Grining CP (2007). Effortful control among low-income preschoolers in three cities: Stability, change, and individual differences. Developmental Psychology, 43, 208–221. [DOI] [PubMed] [Google Scholar]
- Magnusson D (1988). Individual development from an interactional perspective: A longitudinal study. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Masten AS, & Cicchetti D (2010). Developmental cascades. Development and Psychopathology, 22, 491–495. [DOI] [PubMed] [Google Scholar]
- McCartney K, Harris MJ, & Bernieri E (1990). Growing up and growing apart: A developmental meta-analysis of twin studies. Psychological Bulletin, 107, 226–237. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, & Watson SJ (1996). Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology, 15, 17–29. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Yalcin B, Willis-Owen SA, & Flint J (2008). Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: Meta-analysis and new data. Biological Psychiatry, 63, 197–206. [DOI] [PubMed] [Google Scholar]
- Murray-Close D, Hoza B, Hinshaw SP, Arnold LE, Swanson J, Jensen PS, et al. (2010). Developmental processes in peer problems of children with attention-deficit/hyperactivity disorder in the Multimodal Treatment Study of Children With ADHD: Developmental cascades and vicious cycles. Development and Psychopathology, 22, 785–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2009). Mplus statistical analysis with latent variables: User’s guide. Los Angeles: Author. [Google Scholar]
- National Center for Early Development and Learning. (1997). Classroom Observation System–Kindergarten. Charlottesville, VA: University of Virginia Press. [Google Scholar]
- NICHD Early Child Care Research Network. (1996). Characteristics of infant child care: Factors contributing to positive caregiving. Early Childhood Research Quarterly, 11, 296–306. [Google Scholar]
- NICHD Early Child Care Research Network. (1997). The effects of infant child care on infant–mother attachment security: Results of the NICHD Study of Early Child Care. Child Development, 68, 860–879. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. (1999). Child care and mother– child interaction in the first three years of life. Developmental Psychology, 35, 1399–1413. [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. (2003). Do children’s attention processes mediate the link between family predictors and school readiness? Developmental Psychology, 39, 581–593. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. (2009). Family–peer linkages: The mediational role of attentional processes. Social Development, 18, 875–895. [Google Scholar]
- Oak JN, Oldenhof J, & Van Tol HHM (2000). The dopamine D4 receptor: One decade of research. European Journal of Pharmacology, 405, 303–327. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy E, Greenslade KE, & Milich R (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 31, 210–218. [DOI] [PubMed] [Google Scholar]
- Pope AW, Bierman KL, & Mumma GH (1991). Aggression, hyperactivity and inattention–immaturity: Behavior dimensions associated with peer rejection in elementary school boys. Developmental Psychology, 27, 663–671. [Google Scholar]
- Price TS, Simonoff E, Asherson P, Curran S, Kuntsi J, Waldman I, et al. (2005). Continuity and change in preschool ADHD symptoms: Longitudinal genetic analysis with contrast effects. Behavior Genetics, 35, 121–132. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. [Google Scholar]
- Raymond M, & Rousset F (1995). GENEPOP (version 1.2): A population genetics software for exact test and ecumenicism. Journal of Heredity, 86, 248–249. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, & Fisher P (2001). Investigations of temperament at 3–7 years: The Children’s Behavior Questionnaire. Child Development, 72, 1394–1408. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ (1983). Developmental systems: Contexts and evolution. In Mussen PH(Series Ed.) & Kessen W (Ed.), Handbook of child psychology: Vol. 1. History, theory, and methods (4th ed., pp. 237–294). New York: Wiley. [Google Scholar]
- Satorra A, & Bentler PM (2001). A scaled difference chi-square test statistic for moment structure analysis. Psychometrika, 66, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S (1992). Developmental theories for the 1990s: Development and individual differences. Child Development, 63, 1–19. [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hu S, & Hamer DH (2001). Association of DRD4 with attention problems in normal childhood development. Psychiatric Genetics, 11, 25–29. [DOI] [PubMed] [Google Scholar]
- Schoots O, & Van Tol HHM (2003). The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics Journal, 3, 343–348. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, & Posner MI (2007). Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology, 19, 1039–1046. [DOI] [PubMed] [Google Scholar]
- Thapar A, Langley K, Asherson P, & Gill M (2007). Gene–environment interplay in attention-deficit hyperactivity disorder and the importance of a developmental perspective. British Journal of Psychiatry, 190, 1–3. [DOI] [PubMed] [Google Scholar]
- Thapar A, Langley K, O’Donovan M, & Owen M (2006). Refining the attention deficit hyperactivity disorder phenotype for molecular genetic studies. Molecular Psychiatry, 11, 714–720. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57, 1336–1346. [DOI] [PubMed] [Google Scholar]
- Winer BJ (1971). Statistical principles in experimental design (2nd ed.). New York: McGraw–Hill. [Google Scholar]
- Yong Zou G, & Donner A (2006). The merits of testing Hardy–Weinberg equilibrium in the analysis of unmatched case-control data: A cautionary note. Annals of Human Genetics, 70, 923–933. [DOI] [PubMed] [Google Scholar]


