Abstract
Objectives
To evaluate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hospitalization and fatality rates in residents of homeless shelters run by Samusocial of Paris.
Methods
We conducted a retrospective serological study between July and August 2020 on all residents and staff members of three homeless shelters run by Samusocial of Paris: two centres providing healthcare accommodation (HCA) and one a women's dormitory. We included all adults present in the shelters or who died of a proven SARS-CoV-2 infection during the first wave (March–May). SARS-CoV-2 antibodies were detected in serum samples using the SARS-CoV-2 IgG Architect (Abbott) test. Any participant with a positive PCR or serology was defined as a confirmed SARS-CoV-2 case.
Results
We included 100 residents and 83 staff members. The confirmed SARS-CoV-2 rate by PCR or serology was 72/100 (72.0%) for residents and 17/83 (20.5%) for staff members. Women accommodated in the dormitory had the highest infection rate (90.6%). The hospitalization rate in residents was 17/72 (23.6%) and the death rate 4/72 (5.6%). All hospitalizations and deaths occurred among HCA residents. Among the residents of HCA shelters, 34/68 (50%) presented at least two comorbidity factors associated with being at high risk for severe SARS-CoV-2 infection.
Conclusion
The SARS-CoV-2 infection rate was high in residents of these homeless shelters (10.6% seroprevalence in the Île-de-France region during the first wave). Severe SARS-CoV-2 infection was highly associated with the prevalence of comorbidities. This population should be considered as a priority in vaccination campaigns and in access to individual housing units when at risk.
Keywords: Homeless, Homeless shelters, Hospitalization, Mortality, SARS-CoV-2, Vaccination
Introduction
A new coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) was identified in Wuhan, China, at the end of December 2019. The first patient was diagnosed in France on 24 January 2020 [1]. After a rapid spread, a national lockdown was implemented in France on 17th March 2020.
Homeless populations are particularly vulnerable to health issues and experience higher rates of illness and death compared with the general population; they have a 17.5- to 30-year reduction in life expectancy [2]. Despite their high vulnerability, homeless people have been neglected by research during the current SARS-CoV-2 pandemic. Some studies show a high prevalence of SARS-CoV-2 infection among the homeless [3,4] whose comorbidities [5] put them at high risk for severe SARS-CoV-2 infection [6].
We conducted a study in three homeless shelters to estimate the SARS-CoV-2 infection attack rate (IAR), and we studied hospitalization and mortality rates among residents and staff members.
Methods
Description of centres
Samusocial of Paris is a non-profit organization providing medical and/or social assistance to people experiencing homelessness and social exclusion. Healthcare accommodation (HCA) provides accommodation and medical attention to vulnerable individuals. Residents share meals, bathrooms and showers. We conducted this study in two HCA centres (HCA-1 and HCA-2).
The Women's Shelter is a 40-bed (dormitory) drop-in day centre and overnight shelter for single women facing homelessness. The bathrooms, showers and meals are collective. There are no windows to ventilate the rooms.
Study design
All adults (≥18 years old) present in the shelters, or who died of a proven SARS-CoV-2 infection between 1st March and 31st May 2020 (cluster dates), were eligible for inclusion following informed consent. Participants who had left the centres at inclusion were contacted and offered participation. Residents who had survived and were not able to give consent (not present at inclusion, with guardianship or curatorship) were excluded. From July to August 2020, we performed a retrospective serological study among residents and staff members of these shelters. The management of outbreaks and data collection are detailed in the Supplementary Material.
Case definition
A confirmed SARS-CoV-2 infection was defined as follows: any resident or staff member with a positive PCR test between 1st March and 31st May 2020 or with a positive serology. Death and hospitalization were taken into account only if they were due to SARS-CoV-2 infection and occurred between 1st March and 31st May.
Laboratory procedures
Serological tests were carried out between July and August 2020, using the Abbott immunoassay (Abbott, Maidenhead, UK). Antibody levels ≥1.40 were considered positive, 0.50–1.39 equivocal (following Abbott-Diagnostics Product Information Letter PI1060-2020) or <0.5 negative. In this study, equivocal results were considered as positive.
Statistical analysis
Statistical analysis was performed using R software. We expressed descriptive data as median (with interquartile range, IQR) for continuous variables and number (%) for categorical variables. We estimated univariate odds ratios associated with hospitalization using logistic regression. More details are available in the Supplementary Material.
Ethical considerations
The Ethics Commission of CPP Sud-Est approved this study (No. 2020-059B), which is registered on ClinicalTrials.gov (NCT04470648).
Results
Population characteristics
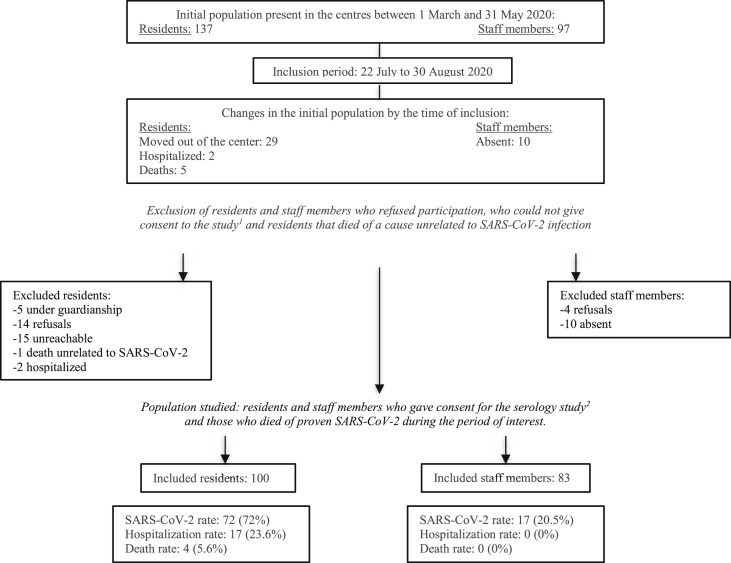
From 22nd July to 30th August 2020, we included 100 residents (72.9%) and 83 staff members (85.6%) present in the centres between 1st March and 31st May (Fig. 1 , Supplementary Material). The characteristics of the 183 participants are detailed in Table 1 .
Fig. 1.
Flow chart. 1 Under guardianship, had left centre and were unreachable or were hospitalized. 2 Residents who had left the centre by the time of inclusion were contacted and offered participation in the study.
Table 1.
Main characteristics of residents in healthcare accommodation (HCA) or the dormitory and of staff members of all centres
|
Residents |
Staff members |
||||
|---|---|---|---|---|---|
| HCA 1&2 | Dormitory | p | Total [95%CI] | Total | |
| Total population | 68 | 32 | 83 | ||
| Age (years), median [IQR] | 55 [38; 60] | 43 [33; 60] | 0.0001 | 54 [37; 64] | 43 [32; 54] |
| Male sex, n (%) | 65 (95.6%) | 0 (0%) | 4.6E-23 | 65 (65%) | 28 (33.7%) |
| Addictive behaviours: | 36 (52.9%) | 7 (21.9%) | 0.0046 | 43 (43%) | 22 (26.5%) |
| Alcohol abuse n (%) | 22 (32.4%) | 6 (18.8%) | 0.23 | 28 (28%) | 0 |
| Active chronic smoking n (%) | 31 (45.6%) | 4 (12.5%) | 0.0014 | 35 (35%) | 22 (26.5%) |
| Substance abuse n (%) | 6 (8.8%) | 0 (0%) | 0.17 | 6 (6%) | 0 |
| Medical conditions: | |||||
| COPD n (%) | 9 (13.2%) | 1 (3.1%) | 0.16 | 10 (10%) | 1 (1.2%) |
| Heart conditionsan (%) | 11 (16.2%) | 0 (0%) | 0.015 | 11 (11%) | 0 |
| Chronic kidney disease n (%) | 7 (10.3%) | 0 (0%) | 0.093 | 7 (7%) | 0 |
| Obesity BMI ≥30 n (%) | 11 (16.2%) | 3 (9.4%) | 0.54 | 12 (12%) | 0 |
| Type 2 diabetes mellitus n (%) | 18 (26.5%) | 0 (0%) | 0.0005 | 18 (18%) | 3 (3.6%) |
| Cancer n (%) | 5 (7.4%) | 0 (0%) | 0.17 | 5 (5%) | 0 |
| Hypertension n (%) | 12 (26.5%) | 2 (6.3%) | 0.215 | 20 (20%) | 6 (7.2%) |
| Cerebrovascular disease n (%) | 2 (2.9%) | 0 (0%) | 1 | 2 (2%) | 0 |
| Liver disease n (%) | 3 (5.9%) | 0 (0%) | 0.549 | 4 (4%) | 0 |
| Pregnancy n (%) | 0 (0%) | 2 (6.3%) | 0.100 | 2 (2%) | 0 |
| Other n (%)b | 28 (41.2%) | 4 (12.5%) | / | 32 (32%) | 4 (48.2%) |
| ≥2 risk factors for severe SARS-CoV-2c | 34 (50.0 %) | 4 (12.5%) | 0.0003 | 38 (38%) | 0 |
| Symptomatic n (%)d | 18 (58.1%) | 15 (51.7%) | 0.67 | 43 (59.7%) | 16 (94,1%) |
| Isolated n (%) | 46 (67.6%) | 25 (78.1%) | 0.35 | 71 (71%) | NA |
| Positive SARS-CoV-2 PCR n (%) | 14 (30.9%) | 8 (25%) | 0.615 | 29/97 (29.9%) | 7/60 (11.6%) |
| Positive SARS-CoV-2 serology, n (%) | 39/64 (60.9%) | 28 (87.5%) | 0.009 | 67/96 (69.8%) | 15/81 (18.5%) |
| Infection attack rate | 43 (63.2%) | 29 (90.6%) | 0.0042 | 72 (72%) [62.0; 80.3] | 17 (20.5%) |
| Hospitalization ratee | 17 (25%) | 0 | 0.001 | 17 (23.6%) [14.7; 35.3] | 0 (0%) |
| Infection fatality ratee | 4 (5.9%) | 0 | 0.302 | 4 (5.6%) [1.8; 14.3] | 0 (0%) |
COPD, chronic obstructive pulmonary disease.
Such as heart failure, coronary artery disease, or cardiomyopathy.
Psychiatric issues (17), active tuberculosis (7), HIV (4) and autoimmune diseases (4).
Proven factors according to the US Centers for Disease Control: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
Among the infected.
Hospitalizations and deaths were taken into account only if they were linked to SARS-CoV-2 infection.
Infection attack rates among residents and staff members
The overall IAR was 72.0% (n = 72, 95%CI 62.0–80.3) including 29/97 (29.9%) positive PCR tests and 67/96 (69.8%) positive serologies. IAR was higher in the dormitory centre compared to the HCA (90.6% and 63.2%, respectively; p 0.0042) (Table 1). Most of the suggestive symptoms and confirmed cases occurred between 8th March and 15th April. The median time from suggestive symptoms or positive PCR testing and serology was 124 days (IQR 93–128).
Staff members had a total of 17 SARS-CoV-2 infections and an IAR of 20.5%.
Hospitalization and infection fatality rates among residents and staff members
Of 72 SARS-CoV-2-infected residents, 17 were hospitalized and four died, leading to a hospitalization rate of 23.6% (95%CI 14.7–35.3) and an infection fatality rate of 5.6% (95%CI 1.8–14.3) (Table 1). Twelve (70.6%) of the 17 hospitalized residents needed oxygen therapy. Two patients (11.7%) were transferred to intensive care units. All deaths and hospitalizations due to coronavirus disease 2019 (COVID-19) occurred among residents of the HCA. Residents of the dormitory had fewer risk factors for severe infection than those in the HCA (p 0.0003) (Table 1).
The main characteristics of positive HCA residents (hospitalized and non-hospitalized) are detailed in Table 2 . In HCA, patients over 65 years old and with more than two risk factors were more often hospitalized (Table 2, OR 12.74; IQR 2.38–68.26 and OR 7.86; IQR 1.75–35.23, respectively). No staff members were hospitalized.
Table 2.
Main characteristics of residents tested positive in healthcare centres (hospitalized and non-hospitalized), with associated odds ratios estimated by conditional logistic regression
| Hospitalized | Non-hospitalized | Odds ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Total | 17 | 26 | ||
| Age ≥65 years, n (%) | 11 (64.7%) | 4 (15.4%) | 12.74 (2.38–68.26) | 0.000606 |
| Male, n (%) | 17 (100%) | 25 (96.2%) | – | – |
| Addictive behaviours, n (%): | 11 (64.7%) | 12 (46.1%) | 2.1 (0.58–7.6) | 0.254 |
| Alcohol abuse, n (%) | 8 (47.1%) | 8 (30.7%) | 1.93 (0.53–7.06) | 0.316 |
| Active chronic smoking, n (%) | 9 (52.9%) | 11 (42.3%) | 1.45 (0.41–5.09) | 0.561 |
| Substance abuse, n (%) | 1 (5.9%) | 3 (11.5%) | 0.45 (0.04–4.84) | 0.488 |
| Medical conditions: | ||||
| COPD n (%) | 4 (23.5%) | 2 (7.7%) | 4.55 (0.65–31.8) | 0.109 |
| Heart conditions n (%) | 8 (47.1%) | 1 (3.8%) | 16.75 (1.89–148.44) | 0.00162 |
| Chronic kidney disease n (%) | 4 (23.5%) | 1 (3.8%) | 12.84 (1.05–156.72) | 0.0222 |
| Obesity BMI ≥30 n (%) | 5 (29.4%) | 2 (7.7%) | 0.56 (0.11–2.84) | 0.48 |
| Type 2 diabetes mellitus n (%) | 8 (47.1%) | 7 (26.9%) | 2.96 (0.74–11.82) | 0.114 |
| Cancer n (%) | 1 (5.9%) | 2 (7.7%) | 0.51 (0.04–6.07) | 0.585 |
| ≥2 risk factors for severe SARS-CoV-2 | 14 (82.4%) | 8 (30.7%) | 7.86 (1.75–35.23) | 0.0032 |
| Initial room occupation: | ||||
| Single room | 2 (11.8%) | 0 (0%) | Ref | |
| Double room | 6 (35.3%) | 10 (38.5%) | 0.41 (0.1–1.71) | 0.216 |
| Triple room | 8 (47.1%) | 14 (53.8%) | 1.1 (0.3–4.02) | 0.89 |
| Quadruple room | 1 (5.9%) | 2 (7.7%) | 3.46 (0.18–67.64) | 0.422 |
COPD, chronic obstructive pulmonary disease.
Discussion
We found an IAR of 72% during the first wave of the COVID-19 outbreak among residents of homeless shelters. In the dormitory, the IAR was 90.6%. Seroprevalence among residents in our study was higher (69.8% for all residents, 87.5% for dormitory residents) than in the general population during the first wave in the same region (Île-de-France: 10.6%) [7]. Another serological study showed similar results, with a seroprevalence up to 88.7% in overcrowded housing [4]. Moreover, in our study, staff members had an IAR of 20.5%, which is consistent with the range of 4–45% reported in another study in this population [8].
The findings of our study are consistent with those of previous studies showing that homeless populations are at high risk for severe infection and death [5]. The hospitalization rate was 23.6% and the infection fatality rate 5.6%, which is notably higher than in the general population in France (3.6% and 0.7%, respectively) [9] or in other developed countries [10]. One study found that the estimated hospitalization incidence for SARS-CoV-2 was three times higher in homeless populations than in the general population [11].
Our study has several limitations. First, the participation rate was low (70%), as we lost over 30% of the initial population, which limits the interpretation of the findings. Second, we lacked statistical power to perform multivariate analysis due to the small sample size and heterogeneity of the groups. Finally, we were likely to miss infections since: (a) PCR tests were done mostly once during the cluster for the residents (for some at a time distant from their infection), and (b) there were gaps between clusters and serology samplings. To limit these drawbacks, we combined positive PCR tests and positive serology to calculate IAR. Although the serological test used has an excellent specificity (99.3%) and sensitivity (96%) [12], the delay may have led to fading of antibody response and therefore to an underestimation of infections.
Our study is in line with that of Roederer et al. who showed that overcrowded housing conditions constitute risks during an infectious disease outbreak [4]. Although these findings should be balanced against the risk of sleeping in the streets, both studies support the need to draw up broad person-centred approaches to prevent COVID-19 hospitalizations and deaths, as shown in the study by Lewer et al. [13].
In conclusion, we found high levels of infection in homeless people, especially those living in overcrowded conditions. Homeless people often carry risk factors of severe forms of SARS-CoV-2 and are more likely to be hospitalized and to die. Our findings show that these high-risk populations should be prioritized in vaccination campaigns.
Authors contributions
All authors contributed to the study conception and design. MH oversaw the inclusion of participants, collected the blood samples, and extracted the data from the different databases. AC performed all the statistical analyses. MH, AR, APC, SR, MWT, CC and YY analysed the data. MH wrote the manuscript. AR, APC, CC and YY supervised the study. All authors have read and approved the final manuscript.
Transparency declaration
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The work was sponsored by Inserm, a public non-profit agency, under the leadership of Professor Yazdan Yazdanpanah.
Acknowledgments
The authors would like to express their gratitude to Clothilde Legouge, Nathalie Meier, Marie Bordat, Marion Quach Hong, Françoise Kenfer and all of the Samusocial team for their valuable help.
Editor: J. Rodriguez-Baño
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.05.039.
Ethical approval
This study is part of clinical trial C20-50 sponsored by Inserm. It was granted approval by the local Ethics Committee or “Comité de Protection des Personnes” on 17/07/2020 (No. 2020-059 B), and registered in a public trials registry (CT 2050). All study participants gave their informed, written consent, in line with French legal guidelines.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romaszko J., Cymes I., Dragańska E., Kuchta R., Glińska-Lewczuk K. Mortality among the homeless: causes and meteorological relationships. PLOS ONE. 2017;12 doi: 10.1371/journal.pone.0189938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imbert E., Kinley P.M., Scarborough A., Cawley C., Sankaran M., Cox S.N., et al. Coronavirus disease 2019 outbreak in a San Francisco homeless shelter. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roederer T., Mollo B., Vincent C., Nikolay B., Llosa A.E., Nesbitt R., et al. Seroprevalence and risk factors of exposure to COVID-19 in homeless people in Paris, France: a cross-sectional study. Lancet Public Health. 2021:1–3. doi: 10.1016/S2468-2667(21)00001-3. S2468-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazel S., Geddes J.R., Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529–1540. doi: 10.1016/S0140-6736(14)61132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SPF. COVID-19: point épidémiologique. 2020. https://maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-9-juillet-2020 Available from. [Google Scholar]
- 8.Chen Y., Tong X., Wang J., Huang W., Yin S., Huang R., et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020;81:420–426. doi: 10.1016/j.jinf.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-19: mathematical model indicates that between 3% and 7% of French people have been infected. Institut Pasteur; 2020. https://www.pasteur.fr/en/research-journal/press-documents/covid-19-mathematical-model-indicates-between-3-and-7-french-people-have-been-infected Available from: [Google Scholar]
- 10.Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G., et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrooyen L., Delforge M., Lebout F., Vanbaelen T., Lecompte A., Dauby N. Homeless people hospitalized with COVID-19 in Brussels. Clin Microbiol Infect. 2021;27:151–152. doi: 10.1016/j.cmi.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turbett S.E., Anahtar M., Dighe A.S., Beltran W.G., Miller T., Scott H., et al. Evaluation of three commercial SARS-CoV-2 serologic assays and their performance in two-test algorithms. J Clin Microbiol. 2020;59 doi: 10.1128/JCM.01892-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewer D., Braithwaite I., Bullock M., Eyre M.T., White P.J., Aldridge R.W., et al. COVID-19 among people experiencing homelessness in England: a modelling study. Lancet Respir Med. 2020;8:1181–1191. doi: 10.1016/S2213-2600(20)30396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.