Abstract
Stroke produces a powerful inflammatory cascade in the brain, but also a suppression of the peripheral immune system, which is also called stroke-induced immunosuppression (SIIS). The main processes that lead to SIIS are a shift from a lymphocyte phenotype T-helper (Th) 1 to a Th2 phenotype, a decrease of the lymphocyte counts and NK cells in the blood and spleen, and an impairment of the defense mechanisms of neutrophils and monocytes. The direct clinical consequence of SIIS in stroke patients is an increased susceptibility to stroke-associated infections, which is enhanced by clinical factors like dysphagia. Among these infections, stroke-associated pneumonia (SAP) is the one that accounts for the highest impact on stroke outcome, so research is focused on its early diagnosis and prevention. Biomarkers indicating modifications in SIIS pathways could have an important role in the early prediction of SAP, but currently, there are no individual biomarkers or panels of biomarkers that are accurate enough to be translated to clinical practice. Similarly, there is still no efficient therapy to prevent the onset of SAP, and clinical trials testing prophylactic antibiotic treatment and β-blockers have failed. However, local immunomodulation could open up a new research opportunity to find a preventive therapy for SAP. Recent studies have focused on the pulmonary immune changes that could be caused by stroke similarly to other acquired brain injuries. Some of the traits observed in animal models of stroke include lung edema and inflammation, as well as inflammation of the bronchoalveolar lavage fluid.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-021-02177-0.
Keywords: Stroke, Immunosuppression, Biomarkers, Inflammation, Infection, Pneumonia
Background
Stroke-induced immunosuppression (SIIS) is a set of processes that lead to a peripheral suppression of the immune system after the occurrence of stroke. One of the main and direct consequences of this SIIS is that it makes stroke patients more susceptible to bacterial infections. Stroke-associated infections (SAIs) represent one of the major complications post-stroke, which worsens the functional outcome of patients and increases their mortality rates [1]. Approximately 30% (24–36%) of patients develop infections after stroke, with pneumonia and urinary tract infections (UTI) being the most common forms, both having a frequency of 10% [2].
Among these infections, stroke-associated pneumonia (SAP) is usually the most acute type of SAI and has the worst impact on functional outcome [3]. It increases mortality for up to 1 year, prolongs hospital stays, and worsens the functional outcome at discharge [4]. The clinical definition of pneumonia after stroke has differed in many studies in both the terminology and the diagnosis of the complication [5]. To address this issue, the Pneumonia in Stroke Consensus (PISCES) Group proposed the term SAP, to encompass all terms referring to lower respiratory tract infections in stroke patients within the first 7 days after stroke onset [6]. In the same publication, new diagnostic criteria were proposed based on the criteria for the definition of healthcare-associated infection from the Centers for Disease Control and Prevention (CDC) of the United States of America, which were the most used criteria by that point [7]. However, SAP assessment is still challenging, especially due to the limited role of chest radiography. For this, chest computed tomography has been proposed as a complement to PISCES criteria in the screening of SAP in stroke patients. In a small cohort, thorax high-resolution computed tomography (THRCT) was able to differentiate between bronchopneumonia and other low respiratory tract infections in SAP patients, demonstrating a high accuracy in the diagnosis of SAP [8]. More recently, Kishore et al. [9] have also addressed this issue, but they have observed that conventional chest X-rays have limited accuracy for the diagnostic of SAP when compared with THRCT. Similarly, they found in their work some discrepancies between PISCES criteria and THRCT. Larger studies, including perhaps serial imaging, are needed in this field, to validate whether PISCES criteria and THRCT might be combined for the diagnosis of SAP.
Nowadays, the clinical strategies against SAP are based on wide-spectrum antibiotics once an infection is diagnosed through clinical criteria, along with prevention by dysphagia screening in stroke units. Recently, the PISCES consortia launched a recommendation for a standardized approach to antibiotic therapy in post-stroke pneumonia [10]. Nonetheless, early treatment before the development of clinical signs could prevent the onset of SAP or ameliorate its consequences. This would have benefits for patients and saving also a great number of resources for health care systems [11]. Several clinical trials have explored this idea through the administration of prophylactic antibiotics to acute stroke patients. However, the meta-analysis performed by Vermeij et al. [12] showed that preventive antibiotics neither reduce the risk of pneumonia nor the risk of death or poor outcome after stroke. The treatment did reduce the occurrence of overall infections and UTIs, but did not show any effect regarding the occurrence of SAP. These results were translated into recommendations against the routine use of prophylactic antibiotics in stroke guidelines.
In addition, antibiotics can have both protective and detrimental effects on the central nervous system (CNS) and could have modulatory effects on the immune system. The administration of antibiotics affects human bacterial communities and may lead to a dysbiosis of intestinal microbiota, which could remain altered for 6 months after antibiotic therapy [13]. Studies in mice living in germ-free conditions showed the importance of gut microbiota in the development and function of the CNS. These germ-free mice developed brain diseases that were replicated in antibiotic-depleted microbiota animals [14]. In experimental stroke, it has been observed that the depletion of gut microbiota by antibiotics worsens the outcome of the ischemic mice, decreasing their survival, and developing acute colitis [15]. However, Benakis et al. found that antibiotic-induced alterations in the gut microbiota decreased ischemic injury in the brain. Concretely, dysbiosis reduced the trafficking of effector T cells from the gut to leptomeninges after stroke. In the brain, these cells enhance neuroinflammation [16]. Further studies are needed to elucidate the effects and interaction of antibiotics with stroke, to determine the impact of the antibiotic treatment in the brain lesion in SAI patients.
The failure of these clinical trials could make the stroke units rethink therapeutic strategies for SAP prevention or early treatment. Diverse reasons might be responsible for the lack of success of these trials, such as the inclusion of patients with a relatively low risk for infection, the wrong choice of the antibiotic regime, or the time-window for patient inclusion. But another plausible explanation is that despite being useful for SAP treatment, antibiotics might not be effective in the prevention of SAP, thus opening a new opportunity for alternative preventive measures, with SIIS as an alternative therapeutic target.
Along these lines, immunomodulation of the peripheral immune system has also been explored as an alternative therapy for the prevention of these infections, but to a lesser extent than preventive antibiotics and mostly in experimental stroke. Developing new immunomodulating preventive therapies against the development of SAP will require a deep knowledge of the mechanisms that lead to SIIS and the resulting susceptibility of stroke patients to bacterial infections.
Hence, this review intends to present those mechanisms implicated in SIIS, focusing on which clinical implications these pathways could have, as the finding of biomarkers capable of predicting the onset of SAI and SAP and the use of immunomodulatory therapies (either systemic or local) to prevent them.
Cellular and molecular mechanisms of stroke-induced immunosuppression
After the occurrence of stroke, an activation of the inflammatory cascade due to the release of damage-associated molecular patterns (DAMPs) by the injured and dead cells causes a neuroinflammatory state in the brain. The release of inflammatory mediators, together with oxidative stress and other factors, increases the permeability of the brain-blood barrier (BBB), which in turn facilitates leukocyte infiltration into the brain [17].
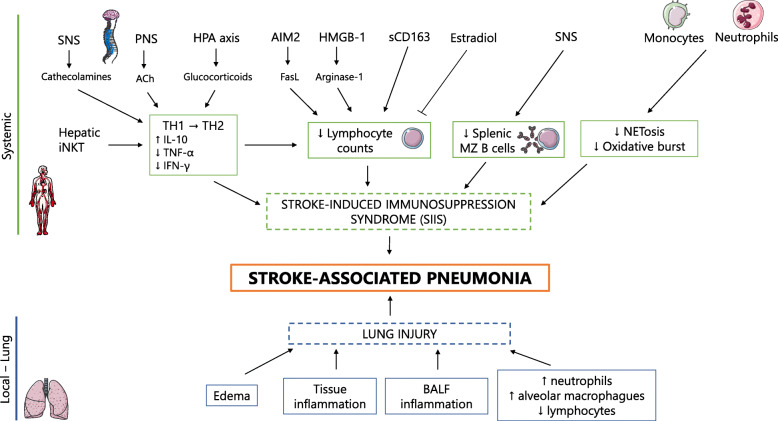
Conversely, focusing on the peripheral immune system, immunosuppression has been reported early within the first hours after stroke. One of the main processes of SIIS is the shift of the T cell response from a T-helper (Th) 1 response, characterized by the secretion of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), to a T-helper 2 (Th2) anti-inflammatory response, with the secretion of interleukin (IL)-10 and IL-4 among others. An increased ratio of Th2-type cytokines over Th1-type cytokines characterizes this shift. Another characteristic trait of SIIS is the lymphocyte and splenic impairment, which consists mainly of lymphocytopenia in blood, spleen, and lymph nodes [18]. The molecular and cellular processes that drive to SIIS are described in this chapter, and summarized in Fig. 1.
Fig. 1.
The interplay of local and systemic processes leading to pneumonia in stroke patients. Stroke-associated pneumonia is influenced by systemic and local mechanisms. Locally, there are pulmonary alterations due to stroke itself and the inflammatory processes that develop. BALF inflammation, lung inflammation, and edema seem to be the principal alterations, although there are some discrepancies between studies. On a systemic level, various represented processes lead to 3 main alterations that cause systemic immunosuppression after stroke: an increase of the T-helper (Th) 2/Th1 cytokine ratio; a reduction of the lymphocyte counts in the spleen, thymus, and blood; and a decrease of the antimicrobial defense mechanisms of neutrophils and monocytes. SNS sympathetic nervous system, iNKT invariant natural killer T cells, PNS parasympathetic nervous system, ACh acetylcholine, HPA hypothalamic–pituitary–adrenal, HMGB-1 high motility group box-1, sCD163 soluble cluster of differentiation 163, MZ marginal zone, FasL Fas ligand, BALF bronchoalveolar lavage fluid. Parts of this figure were supported by Servier Medical Art with permission under the Creative Commons Attribution 3.0 Unported License
Various studies have reported a decrease in the production of IFN-γ and the secretion of TNF-α in blood samples from mice whom underwent a middle cerebral artery occlusion (MCAO), as well as an increase of the circulating levels of IL-10 [18, 19]. IL-10 is a well-known anti-inflammatory cytokine that inhibits the production of TNF-α and IFN-γ [20]. A time-dependent increase in IL-10 as well as a decrease in TNF-α/IL-10 ratio was observed in stroke patients developing infection [18]. Jiang et al. [21] observed the same pattern of circulating cytokine expression in human stroke patients. In the same study, they were able to locate this shift in the pattern of circulating cytokine expression, which could occur between days 1 and 3 after stroke.
As mentioned earlier, the shift from Th1 to Th2 is associated with a reduction in lymphocyte counts at the spleen, blood, and thymus. The decrease was observed in MCAO mice 12 h after surgery, and it is due to enhanced apoptosis in these organs [18]. Spleen atrophy characterized by a drastic loss of splenocytes due to apoptosis was observed at 4 days in experimental stroke [22]. In line with this, Yan and Zhang [19] observed a contraction of the spleen volume in MCAO rats beginning at 12 h after the occlusion. This reduction of the splenic volume has also been observed in humans [23, 24].
Although susceptibility to infection after stroke could be eased by SIIS, the latter may represent an evolved mechanism to prevent immune reactions, mainly autoreactive immune responses against CNS molecular components by infiltrating lymphocytes. The efferent neuronal pathways through CNS regulates immune system are presented below.
SIIS is mediated by the autonomic nervous system
The shift from the Th1 response to the Th2 response seems to be led by the autonomic nervous system (ANS), especially the sympathetic nervous system (SNS). Catecholamines like epinephrine and norepinephrine are SNS mediators that are secreted when the SNS is activated. They act through β-adrenergic receptors of immune cells, suppressing Th1 activities and cellular immune responses through the inhibition of IFN-γ secretion and the stimulation of IL-10 production by immune cells, among other processes [25].
After MCAO surgery in mice, higher levels of metanephrine (MN) and normetanephrine (NMN) (metabolites of epinephrine and norepinephrine, respectively) were observed from 6 h after surgery until 2 weeks. Treating MCAO mice with the beta-blocker propranolol blocks the catecholaminergic receptors and partially inhibits the activation of the SNS in these animals. The effects of this blockade in the circulation were an increase of IFN-γ levels; a decrease of IL-10, MN, and NMN; and an increase of the splenic volume [19]. The inhibition of the SNS through propranolol has also been proven to reduce lymphocyte dysfunction and bacterial infections, as well as improve the survival of MCAO mice [18], thus supporting the relevance of the SNS in SIIS.
β-arrestin2 (ARRB2) has been studied to elucidate the endogenous factors and pathways of the SNS that are involved in SIIS. ARRB2 protein is involved in multiple pathways. Wang et al. [26] found elevated splenic levels of ARRB2 in MCAO rats and a correlation of the protein with sympathetic activity. They also observed dysfunction of splenic monocytes, which was reversed with ARRB2 deficiency in the same manner as propranolol. Hence, these results suggest ARRB2 as an effector of the SNS for the mediation of SIIS.
In fact, ARRB2 seems to be mediating a loss of splenic marginal zone B cells in MCAO animals. McCulloch et al. [27] demonstrated that stroke induces a substantial loss of splenic B cells, especially in those from the marginal B zone. This cell loss is causing impairment to some immune functions, such as those related with the early defense against bacterial microorganisms, and this is associated with spontaneous lung infections. The treatment with propranolol reversed these impairments and reduced the susceptibility to lung infections in MCAO mice.
It is well known that the activity of the hypothalamic-pituitary-adrenal (HPA) axis increases after stroke, with an early increase in the concentrations of cortisol [28, 29]. In a similar manner to catecholamines, glucocorticoids stimulate the secretion of anti-inflammatory cytokines. Prass et al. [18] observed that the inhibition of the HPA axis through the glucocorticoid receptor blocker RU486 prevented lymphopenia, lymphocyte apoptosis, and monocyte dysfunction. However, it did not reduce bacterial infections, unlike propranolol treatment. These results reveal a role of the HPA axis in SIIS, but perhaps not as pivotal as the SNS.
The other division of the ANS, the parasympathetic nervous system (PNS), also seems to play a part in SIIS. The vagus nerve and acetylcholine (ACh) suppress the secretion of proinflammatory cytokines through the binding of acetylcholine to the α7 nicotinic acetylcholine receptor (α7nAChR) in activated macrophages. Specifically, the inhibition of TNF-α production in spleen is driven by ACh-producing T cells [30]. Engel et al. [31] observed a rapid increase of parasympathetic activity in experimental stroke mice through an analysis of heart rate variability and blood pressure. Moreover, MCAO surgery was performed on two types of mice: wild-type (WT) mice who suffered a vagotomy and α7nAChR knockout (KO) mice. Both cases showed a decrease in the bacterial load of the lungs when compared with MCAO WT mice on day 3 after ischemia.
These results suggest that increased parasympathetic activity plays some role in the increased susceptibility to bacterial infections after stroke, possibly through the suppression of innate immune responses of the lungs due to the expression of α7nAChR in activated lung macrophages and alveolar epithelial cells. The results obtained by Lafargue et al. [32] are in line with this hypothesis. They observed attenuation of the effect of stroke in lung injury and mortality when α7nAChR was pharmacologically inhibited or genetically depleted in MCAO mice with pneumonia. Conversely, pretreatment with an α7nAChR activator before MCAO surgery increased lung injury.
Other immunomediators of SIIS apart from ANS
The Th2/Th1 cytokine ratio is also favored by the hepatic invariant natural killer T (iNKT) cells, producing greater amounts of IL-10, but not IFN-γ. The direct immunomodulation of iNKT cells through the administration of a specific activator (α-galactosylceramide) in MCAO mice reduced stroke-induced neutrophil pulmonary influx, lung edema, and infections. This reduction was observed similarly in MCAO animals treated with propranolol [33].
A decrease in the lymphocyte proliferation also seems to cause the reduction of lymphocyte counts and could be partially explained by a release of arginase I (ArgI) from neutrophils. The enzyme ArgI is constitutively expressed in neutrophil granules, and its main function is to metabolize l-arginine into l-ornithine and urea. Higher levels of this enzyme were found in the blood of MCAO mice on day 4 after ischemia, and the supplementation of l-arginine in vitro in splenic T cells in the same animals restored T cell proliferation and increased IFN-γ production [34]. In humans, ARG1 mRNA expression and ArgI serum activity correlate with neutrophil-to-lymphocyte ratio (NLR) and stroke severity in stroke patients [35]. These two clinical parameters are associated with SAI [36, 37], thus suggesting a role of ArgI in immune dysfunction.
The release of ArgI could be mediated by high mobility group box 1 (HMGB1), a well-known DAMP, via the receptor of advanced glycation end-products (RAGE). Liesz et al. [38] found that this pathway seems to stimulate the production of myeloid-derived suppressor cells (MDSCs), which could include neutrophils. They also observed that mice with RAGE deficiency after MCAO surgery had reduced splenic lymphocyte apoptosis and increased activated T cell counts. These results suggest that HMGB1 could stimulate the release of ArgI by MDSCs, in turn causing a decrease in leukocyte production.
Soluble CD163 (sCD163) is another molecule that seems to be implicated in the reduction of lymphocyte counts. CD163 is a receptor expressed in the membrane of monocytes and macrophages and is shed through matrix metalloproteinase ADAM17 into sCD163. O’Connell et al. [39] reported higher blood levels of sCD163 and ADAM17 in stroke patients, which negatively correlated with post-stroke lymphocyte counts. In vitro, the authors related the elevations of sCD163 with the suppression of lymphocyte proliferation.
Intense research is being conducted on new signaling pathways implicated in SIIS. Last year, a role of the cluster of differentiation (CD) 200-CD200R1 signaling axis in SIIS was identified. CD200R1 is an inhibitory immune receptor that is expressed in myeloid cells, and it has been observed that CD200R1-KO MCAO mice developed more spontaneous bacterial lung infections than WT MCAO mice, as well as lymphocytopenia and worse functional outcomes, among other effects [40]. These findings suggest that CD200-CD200R1 could be a therapeutic target for immunomodulating preventive therapies of SAI due to its possible protective role against them.
Another mechanism that seems to be implicated in the reduction of lymphocyte counts through the increase of T cell apoptosis is the activation of the AIM2 inflammasome-driven signaling cascade in monocytes [41]. This inflammasome is activated by sensing cell-free DNA, and through the secretion of IL-1β, induces the expression of FasL in monocytes, leading to the T cell apoptosis. Inhibiting this pathway, Roth et al. have observed a reduction of bacterial infections after experimental stroke and an increase of the T cell survival.
Innate immune cells are also modified after stroke
Apart from lymphocytes, splenic NK cells have also been reported to undergo atrophy and a reduction in the count after stroke, and this process is directly led by SNS and HPA [24]. In the same study, the administration of propranolol and RU486 significantly increased the NK-cell-mediated immune response against post-stroke pneumonia, which improved the mortality rates and reduced the bacterial burden in MCAO mice infected with Listeria monocytogenes (LM).
Some functions of the peripheral innate immune cells are altered after stroke, and they could also contribute to SIIS. The mechanisms related to bacterial killing, like oxidative burst and NETosis, are significantly impaired in the monocytes and neutrophils of stroke patients. This impairment was observed upon admission and normalized after day 5 for NETosis. In contrast, the inhibition of the oxidative burst persisted until day 7 [42].
Recently, it has also been described a reduced expression of CD11b in the neutrophils of stroke patients when compared with healthy controls. CD11b recognizes and binds to opsonized bacteria and facilitates the internalization of pathogens, among other functions, and it is considered a leukocyte activation marker [43]. However, in this same study, van Gemmeren et al. did not found an impairment of the neutrophil oxidative burst of stroke patients. The authors ascribe this finding to the small sample size.
Regarding monocytes, Li et al. [44] found overexpression of microRNA-4445 in this leukocyte type. This overexpression seems to be implicated in SIIS, as it causes a suppression of the tumor necrosis factor receptor-associated 4 (TRAF4)/inhibitor of kappa B alpha (IκBα)/nuclear factor kappa B (NF-κB) signaling pathway, activating, in turn, the expression of anti-inflammatory cytokines in monocytes.
Sex hormones play a part in SIIS
Differences between sexes in stroke have been observed in many studies, and male sex is a risk factor for its incidence and outcome. The difference between sexes seems to diminish with menopause among women, thus suggesting a neuroprotective role of endogenous estrogens against stroke. Male sex has been also described as a risk factor for SAP [45].
For this reason, experimental stroke studies and the study of SIIS are almost always performed with males. However, Zang et al. [46] performed a novel study on SIIS in female mice and described a protective role of estrogens (concretely, estradiol (E2)) in the peripheral immunosuppression of MCAO mice, which improved splenocyte counts. Furthermore, a similar protective role to that of E2 was observed for an agonist of G1: a synthetic agonist of the membrane estrogen receptor G protein-coupled receptor 30. This suggests a potential treatment for SIIS.
Regarding male sexual hormones, Dziennis et al. [47] reported an exacerbation of the peripheral immunosuppression in castrated MCAO mice that were treated with dihydrotestosterone (DHT) replacement. The authors described the role of DHT as a peripheral immunosuppressor following stroke.
Altogether, these antagonistic roles of the sex hormones in immunosuppression could be involved in the fact that male patients have a higher risk of suffering SAP. However, it is also needed to take into account that most women who undergo a stroke are post-menopausal, so these differences among sexes could be lower than expected.
Clinical implications (1): the use of cellular and molecular changes as biomarkers for the prediction of stroke-associated infections
As mentioned in the “Background” section, there is a lack of preventive therapies for SAI and SAP in clinical practice. The patient selection could be one of the reasons for the failure of the clinical trials with prophylactic antibiotics. Nowadays, apart from dysphagia screening, there are no routine measures to identify patients who have the highest risk of experiencing SAI in clinical practice. The ability to select such patients could represent an improvement in the experimental design of future clinical trials, either with the administration of preventive antibiotics or through immunomodulation. For this reason, research on biomarkers for the prediction of SAI development has been active in the last decade. Different cell and molecular changes related to the mentioned pathways have been explored and could act as predictive biomarkers for SAI or SAP (table S1).
Cell counts
As a result of leukocyte recruitment to the brain, immunological changes can be observed in circulating immune cells, and systemic inflammation is observed after stroke. In the first hours following stroke, there is an exponential increase in the peripheral neutrophil count and a decrease in the circulating levels of lymphocytes [48]. Due to these changes in peripheral blood cells, different leukocyte counts are being studied as biomarkers in stroke research, such as white blood cell (WBC) count and, recently, the NLR. The leukocyte counts and ratios are easily detectable and they make the early determination a possibility, which means they could potentially be useful biomarkers.
WBC count was explored as a predictor of SAI, SAP, and UTI, and the results indicated it could be an independent predictor of these complications in acute stroke patients [49]. Higher levels of circulating NK cells within the first hours after stroke were observed in patients who developed SAI in a study with 59 participants [50]. NLR is a well-known marker of systemic inflammation. Higher levels of NLR have been found in SAP patients, and there seems to be an association between NLR levels and the severity of SAP [37].
Monocyte to high-density lipoprotein (HDL) cholesterol ratio (MHR) has emerged as a novel inflammation marker. In a recent study, Sun et al. [51] showed the value of MHR and monocyte count as predictors of SAP at admission in a study with more than 800 SAI patients.
Clinical routine and nutritional-related molecules
Blood biomarkers that are measured routinely in most patients in clinical practice are also good candidates for SAI prediction due to their accessibility, in a similar manner to leukocyte counts. In non-diabetic stroke patients, hyperglycemia upon admission was found to be independently associated with SAI, SAP, and UTI, as well as a poor functional outcome at 3 months after the stroke episode [52]. Rodriguez-Sanz et al. [53] showed an inverse correlation between HDL cholesterol levels and the development of SAI. After establishing a cut-off point for SAI prediction (38.5 mg/dl), higher HDL was significantly associated with a lower risk of developing SAI.
Biomarkers related to nutritional status have also been explored. Serum albumin levels were identified as an independent predictor of SAP in a cohort of 705 patients [54]. Furthermore, lower levels of serum prealbumin were associated with a greater risk of SAI in a smaller cohort of 104 ischemic stroke patients [55]. Huang et al. [56] recently found an association between lower levels of vitamin D and SAP. In this study, patients were divided into 3 categories according to their vitamin D status: deficiency (< 25 nmol/L), insufficiency (25–50 nmol/L), and sufficiency (> 50 nmol/L). Patients with vitamin D deficiency had a higher incidence of SAP than patients with vitamin D insufficiency and sufficiency. Moreover, when adjusting by cofounders, vitamin D remained an independent predictor of SAP.
Acute-phase reactants
C-reactive protein (CRP) has been widely used in clinical practice as an acute-phase marker, which is why it has been also studied deep in the field of SAI. Fluri et al. [49] investigated the role of CRP and other markers in SAI in more than 300 patients. They found that CRP is an independent predictor of SAI, both alone and in combination with clinical factors and other markers. Our group reviewed the studies related to CRP and SAI to date in a systematic review. CRP was observed to be an independent predictor of SAI, and there was an optimal time window between 24 and 48 h after stroke [57].
Serum amyloid A (SAA) is another acute phase-protein that has been associated with the prediction of SAI in a small cohort of patients [58] and was validated as an independent predictor in a recent study with two new cohorts with more than 250 participants each [59]. An association of SAA with SAP was observed in a different study, but in this case, the association was in combination with another marker, the soluble urokinase-type plasminogen activator receptor (suPAR) [60]. When cut-off points were established, the combination of these two markers predicted SAP with high specificity and sensitivity at 48 h after stroke onset. Mid-regional pro-adrenomedullin (MR-proADM) was also associated with SAP but at an earlier time than the SAA/suPAR combination (24 h after stroke).
The relationship of suPAR and MR-proADM with SAI was first explored by Bustamante et al. [61] when evaluating various classical sepsis biomarkers as predictors of SAI. suPAR and MR-proADM were both found to be independent predictors of SAI but in two different cohorts with different time points for blood collection. MR-proADM was found to be an early predictor of SAI in a cohort of 78 patients (based on blood collection at the first 6 h after stroke onset). On the other hand, suPAR was found to be a predictor of SAI in a different cohort, where the time point of the collection was at 24 h after stroke.
Fluri et al. [49] explored procalcitonin (PCT) and copeptin jointly with WBC, and each one by itself was found to be an independent predictor of SAI, SAP, and UTI. Their combination, along with CRP and clinical variables, improved the prediction of infection in stroke patients. Recently, PCT and copeptin were studied as SAP predictors along with other biomarkers by pooling data from two clinical trials (PREDICT [62] and STRAWINSKI [63]). Of all the studied biomarkers, the results showed that only PCT and copeptin were independent predictors of SAP when adjusting for clinical variables such as dysphagia, chronic obstructive pulmonary disease (COPD), hypercholesterolemia, and the National Institute of Health Stroke Scale (NIHSS) at admission [64]. In contrast, at earlier time points, Hu et al. [65] did not find PCT to be useful for the prediction of SAP, only when the clinical suspicion of SAP is already high. In the STRAWINSKI clinical trial, PCT was used as a guide for prophylactic antibiotic therapy. The clinical trial was negative, and PCT was not a predictor of SAI in the study population [63].
Cytokines and immune-related molecules
Cytokines are important immune mediators in the post-stroke response, so they have been studied as predictors of SAI, including pro-inflammatory IL-6 and anti-inflammatory IL-10. Worthmann et al. [66] studied both, along with lipopolysaccharide-binding protein (LBP) and CRP. The four proteins were measured at different time points in 56 stroke patients, and all of them were associated with SAI incidence at most of the time points, but only CRP and IL-10 were independent predictors of SAI at the earliest time point (6 h after stroke onset). In a different study, IL-6 was an independent predictor of SAI in a cohort of 82 stroke patients and was also associated with mortality [67]. In a meta-analysis, Bustamante et al. [68] reported an independent association of IL-6 with the overcoming of SAI, but the additional predictive value over clinical predictors of SAI was modest.
IL-10 has also been evaluated as a predictor of SAI in other studies. Chamorro et al. [69] reported that IL-10 and circulating monocytes are independent predictors of SAI in 110 stroke patients, and there were higher levels of IL-6 in patients who developed an infection. In line with these results, Ashour et al. [36] reported that IL-10 is an independent predictor of SAI at admission in a study on 60 patients after establishing a cut-off point. Salat et al. [70] explored various cytokines in SAI patients and assessed the role of IL-10, IL-13, and IFN-γ as independent predictors of SAI.
Another study also explored IFN-γ jointly with microRNA-21 (miRNA-21) in a cohort of 54 patients. miRNA-21 was demonstrated to be related to IFN-γ deficiency when it is increased. A significant increase in miRNA-21 levels and a significant decrease in IFN-γ were observed in the peripheral blood of patients who developed SAI, and there was a correlation between both molecules [71]. Another studied cytokine in this field is IL-1 receptor antagonist (IL-1ra). IL1-ra was studied along with IL-10, cortisol, and lymphocyte count, and only IL-1-ra was independently associated with the risk of infection in 112 stroke patients [72].
The expression of human leucocyte antigen-DR (HLA-DR) on peripheral monocytes has also been studied as a predictor of SAI and SAP. In the PREDICT study, HLA-DR was found as an independent predictor of SAP in the presence of dysphagia on day 1 after stroke [62]. Similar results were found by Zhang et al. [73] but focusing on SAI. Even though there was an increase of expression in SAI patients in comparison with non-SAI patients on days 4 and 6 after stroke and not earlier, the authors reported a predictive value of HLA-DR for the risk of infection after a stroke on days 1 and 2.
Others
The ANS seems to have a role in immunosuppression after stroke, as mentioned in the “Clinical implications (1): the use of cellular and molecular changes as biomarkers for the prediction of stroke-associated infections” section. Heart rate variability (HRV) reflects the activity of this system, so the possible role of HRV in the prediction of SAI was explored by Günther et al. [74]. They found that HRV indices were able to predict the development of SAI before the onset of symptoms.
Suda et al. [75] investigated whether thyroid hormone levels at admission could be associated with the incidence of SAI. They found that lower levels of free triiodothyronine (FT3) were associated with an increased risk of developing SAI. This association remained significant after adjusting by clinical cofounders when establishing a cut-off point for FT3.
Limitations and future perspectives in biomarkers’ studies
Even though multiple candidates have been studied as possible biomarkers for the prediction of SAI, SAP, or UTI development in stroke patients, as presented in this section, none of them have been proven to have enough predictive value to be used in clinical practice. This could be due to some limitations of these studies. First, only a few studies evaluated the additional predictive value of the biomarkers for the prediction of SAI or SAP. Second, some discrepancies among studies have been observed, perhaps due to the variability between them. The blood collection time might represent one of the most important issues. Usually, patients develop SAP within the first 2–3 days after stroke, so an early detection in the first hours is needed to predict the onset of the infection and to be able to develop therapies to prevent them. Furthermore, most of the biomarker studies have not been evaluated regarding a gold standard test for SAP diagnosis, as there is a lack of a gold standard test for the clinical diagnosis of SAP, and therefore, its diagnosis might differ between studies, hindering its reproducibility. For this reason, it is important to phenotype well all the patients, whether with chest tomography or other tools that could be developed in future studies.
Although none of the presented candidates had enough statistical power by itself to be translated to clinical practice, combining biomarkers with high sensitivity and high specificity could open up new possibilities for researchers. Future studies on SAI and SAP biomarkers should combine the discovery and study of new candidates with the screening of complementary candidates to find a combination with the highest specificity and sensitivity. Hence, more research needs to be done in this field to find a biomarker or combination thereof that could be used to make decisions for the administration of preventive therapies. Multicentric cohorts collecting blood at early time-points and following standardized criteria for pneumonia diagnosis are perhaps the desirable study design.
Clinical implications (2): moving immunomodulation towards brain recovery and protection against infections
Prophylactic antibiotic therapy does not seem to affect the functional outcomes or the mortality rate in stroke patients [76]. In previous sections, we hypothesized various reasons why antibiotic prophylaxis could not be effective for the prevention of SAP. Another possible explanation for this failure could be that the depletion of part of the gut microbiota by the antibiotics could be favoring the growth of more resistant and aggressive bacterial strains. This, in turn, could be promoting the appearance of pneumonia in stroke patients, not only because of the aspiration of digestive content but also due to the translocation of the gut microbiota to the lung, as has been suggested in experimental studies [77]. This translocation and the subsequent dissemination are mainly due to an impaired intestinal permeability after stroke. Thus, the need for alternative preventive therapies for SAI and SAP is vital, and intense research is being done in this field.
The “Cellular and molecular mechanisms of stroke-induced immunosuppression” section of this review presented the known pathways implicated in SIIS. Along with other clinical factors such as dysphagia, invasive procedures, and comorbidities, SIIS increases the susceptibility to infections in stroke patients [78]. This immunosuppressive response could be attenuated by modulating one or more of the mechanisms of SIIS at the acute phase of stroke, thus reducing the susceptibility of the patients to infection and decreasing the mortality of stroke patients derived from this complication.
In experimental stroke, immunomodulation has been explored as prophylactic therapy for SAI and SAP (Table 1). Several studies have studied the action of the β-adrenoreceptor blockers propranolol and 6-hydroxydopamine (6-OHDA) in experimental rodent models of stroke. They observed that the inhibition of the SNS reduced bacterial infection of the lungs and partially reestablished some of the mechanisms affected by SIIS [18, 19, 33, 79]. The blockade of the HPA axis has also been explored through the administration of the glucocorticoid receptor blocker RU486, which seemed to restore leukocyte counts but did not reduce bacterial lung infections in MCAO rodents [18, 33]. These studies were discussed in detail in the “Cellular and molecular mechanisms of stroke-induced immunosuppression” section.
Table 1.
Immunomodulating therapies to prevent stroke-associated pneumonia and infections
| Mechanism of action | Reference | Drug | Time of administration | Type of study | Major findings |
|---|---|---|---|---|---|
| Inhibition of the SNS | Prass et al. 2003 [18] | Propanolol | Immediately before and also 4 and 8 h after MCAO. | Experimental (MCAO mice) | Prevention of lymphocyte apoptosis, lymphopenia, monocytic deactivation and changes in lymphocyte cytokine production; prevention of bacteremia and pneumonia; ↑ survival rates |
| Wong et al. 2011 [33] | Propanolol and 6-OHDA | 24 h after MCAO | Experimental (MCAO mice) | Reversion of the iNKT cell phenotype induced by MCAO; ↑ survival rates; ↓ bacterial load in blood, lungs, liver, and spleen | |
| Yan and Zhang 2014 [19] | Propanolol | Immediately before and also 4 and 8 h after MCAO. | Experimental (MCAO mice) | ↓ serum levels of MN, NMN and IL-10; ↑ pro-inflammatory cytokines; ↑ spleen volume | |
| Deng et al. 2016 [79] | 6-OHDA | 3 days before MCAO | Experimental (MCAO rats) | Reversion of the expression of MHC class II; ↑ TNF-a and IFN-γ levels in LPS-stimulated macrophages in vitro; ↓ NF-κB activation; ↑ β-arrestin2 expression | |
| Sykora et al 2015 [80] | β1-selective BBs, nonselective BBs | Before and after stroke | Clinical | ↓ frequency of pneumonia; association of post-stroke BB treatment with mortality | |
| Maier et al. 2015 [81] | BBs (mainly metoprolol and bisoprolol) | Before and after stroke | Clinical | No differences in the risk of pneumonia; ↓ mortality. | |
| Maier et al. 2018 [82] | BBs | Before and after stroke | Clinical | No differences in the rates of pneumonia nor mortality | |
| Inhibition of the HPA axis | Prass et al. 2003 [18] | RU486 | 24 h, 5 h, and immediately before MCAO | Experimental (MCAO mice) | Prevention of lymphocyte apoptosis, lymphopenia, and monocytic deactivation |
| Immunomodulation of iNKT cells | Wong et al. 2011 [33] | α-GalCer | 24 h after MCAO | Experimental (MCAO mice) | ↑ systemic levels of IFN-γ; ↓ stroke-induced neutrophil pulmonary influx and lung edema; ↓ bacterial load in blood, lungs, liver and spleen |
| Inhibition of CD147 | Jin et al. 2019 [83] | CD147 antibody | 4 h after MCAO | Experimental (MCAO mice) | ↓ lung damage; ↓ lung leukocyte infiltration; ↓ plasma and lung IL-17A |
| Inhibition of PTEN | Guan et al. 2013 [84] | Bvp | 24 h after MCAO | Experimental (MCAO mice) | ↓ bacterial loads in lung of bpv-treated mice; restoration of akt activation in the lung; ↓ mortality |
| GM-CSF | Dames et al. 2018 [85] | Recombinant mGM-CSF | 6, 30, and 54 h after MCAO | Experimental (MCAO mice) | ↑ leukocyte counts in lung; ↑ WBC count; ↑ long-term outcome |
Experimental and clinical studies are represented in this table. In the Major findings column, all the results are referred to the patients or animals treated with the immunomodulator agent in comparison with their respective non-treated controls. MCAO middle cerebral artery occlusion, NA non-annotated, 6-OHDA 6-hydroxydopamine, iNKT invariant natural killer T cells, NM metanephrine, NMN normetanephrine, IL-10 interleukin-10, MHC major histocompatibility complex, TNF-α tumor necrosis factor-α, IFN-γ interferon-γ, LBP lipopolysaccharide binding protein, BB beta blocker, α-GalCer α-Galactosylceramide, Bvp bisperoxovanadium, GM-CSF granulocyte-macrophage colony-stimulating factor
It is worth mentioning that β-adrenoreceptor antagonists as propranolol seem to be neuroprotective, reducing infarct volumes and improving neurological scores when being administered after MCAO surgery [86]. Thus, β-blockers therapy after stroke could at the same time attenuate brain injury and prevent suffering infections.
However, the action of β-blockers in the reduction of SAI and SAP in humans has been explored in various studies with different degrees of success. In a retrospective study, Sykora et al. [80] reported a reduction in stroke mortality and SAP ratio in stroke patients under β-blocker therapy, along with a reduction of the SAP ratio in patients with pre-stroke β-blocker therapy. However, Maier et al. [81] performed a prospective clinical trial and did not observe differences in the ratio of SAP between patients receiving the post-stroke β-blocker therapy and those that did not. The effect of β-blockers on major stroke patients was also studied, and they did not reduce the incidence of SAP or SAI in this type of patient [82].
Experimental studies have explored other immunomodulation strategies as a preventive therapy for SAP. The inhibition of CD147 seems to reduce the lungs’ susceptibility to bacterial infections in MCAO mice. The protein CD147 is expressed broadly in many leukocyte subtypes and is involved in some immune processes like T cell activation and Th17 cell differentiation. By inhibiting CD147, Jin et al. [83] observed attenuation of lung damage, increased IFN-γ levels in the lungs, and the modulation of leukocyte subpopulation changes in the lungs of MCAO mice. Another immunomodulatory therapy involving the inhibition of PTEN seemed to reduce bacterial lung infections and mortality in MCAO mice, possibly through the restoration of the PI3K/Akt cascade in the lungs [84]. Systemic treatment with the pluripotent cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) was also explored as an immunomodulating therapy for the prevention of bacterial infections in experimental stroke. This treatment did not reduce the bacterial burden or mortality in MCAO mice, but it did augment antibacterial immune responses and improved the long-term neurological outcome [85].
There has been a lack of success in clinical trials on β-blockers, and there have been discrepancies among them. Furthermore, no other immunomodulation therapy has reached a human-based study phase. Thus, new approaches are needed to find therapeutic targets. Thus far, immunomodulation strategies regarding SAP and SAI prevention have been systemic, with a possible detrimental effect on the brain or other side effects. A local immunomodulation approach could be a new horizon for the discovery of new preventive therapies for SAP.
The lung as a possible target for new immunomodulatory therapies
One of these new possible targets could be the local lung defenses. The brain-lung crosstalk after acute brain injury is well known, in which the brain and lungs interact closely and bidirectionally. The occurrence of major pulmonary injuries has been observed after acute brain injury. In the case of stroke, patients can suffer other types of pulmonary injuries apart from pneumonia, such as acute lung injury (ALI). An ALI incidence of 22% has been reported in acute stroke patients [87], although the clinical phenotype is mild when compared with other forms of acute brain injury, such as severe traumatic brain injury (TBI) and subarachnoid hemorrhage. Pneumonia, ALI, acute respiratory distress syndrome, and neurogenic pulmonary edema have also been reported after these two conditions [88]. After TBI, a systemic immunosuppression similar to SIIS occurs, which increases the risk of nosocomial infections [89], along with other factors in these neurocritical patients, such as mechanical ventilation. Another reason why the lung could be a target for the local immunomodulation is that its epithelial permeability could be altered by stroke, similarly to the gut epithelium [90].
The mechanisms underlying this interaction between the brain and lungs are still not well understood, although a “double hit model” has been proposed. After an acute brain lesion, the first “hit” involves the secretion of pro-inflammatory cytokines in the brain, the alteration of the BBB permeability, and the activation of the CNS. This causes several systemic alterations that result in local damage to the peripheral organs, such as the lungs, and make them more susceptible to bacterial infections. In turn, the organ damage could exacerbate brain damage [91].
A new approach in the research of preventive therapies for SAP could be derived from the exploration of the cellular and molecular modifications in the lungs after stroke that also contributes to the susceptibility to pneumonia. In a hypothetical scenario, the possible lung damage could be prevented through local immunomodulation, hence decreasing the susceptibility of stroke patients to SAP without hampering neurorestoration in the brain after stroke.
Samary et al. [92] studied this issue in a rat model of focal ischemia. Lung edema and inflammation were observed in ischemic rats, as well as ultrastructural changes in the lung parenchyma. Regarding cytokine levels, they observed increased expressions of TNF-α and IL-6 in the brain and plasma, as well as higher TNF-α levels in the bronchoalveolar lavage fluid (BALF). Furthermore, the phagocytic capability of alveolar macrophages was decreased in stroke animals, along with alterations in different ventilatory parameters, such as the respiratory rate and volume tidal.
In a different study performed in MCAO mice, Austin et al. [93] observed lung inflammation in the animals, but not ALI. They observed BALF inflammation, but they did not observe pulmonary edema or the same pro-inflammatory cytokine expression profile as Samary et al. (2018).
Farris et al. [94] also explored the lung in MCAO mice, and they found alterations in the immune cell niche in the lungs of the ischemic animals, with a decrease of lymphocytes and an increase of alveolar macrophages and neutrophils. The production of multiple chemokines was reduced, including CCL3, CCL20, and CCL22 among others.
These three studies demonstrate the existence of pulmonary alterations after ischemic stroke, but there is some controversy between them. Thus, more research is needed to elucidate the extent of this lung damage and the mechanisms underlying it. In addition, whether the extension and nature of this lung damage is related with the occurrence of lung infections remains to be proved.
Conclusions
Despite the recent advances in acute stroke treatment with reperfusion therapies, SAI and particularly pneumonia represent some of the major complications after stroke, which have a high impact on patient outcomes and health care systems. Due to the failure of clinical trials on preventive antibiotics, attractive pathways for researchers and clinicians against this complication involve the prediction and early detection with biomarkers and preventive interventions with immunomodulators. However, the rapidly changing clinical scenario of stroke patients creates new challenges for such research.
Despite the undoubted clinical benefits, the generalization of mechanical thrombectomy exposes hyperacute stroke patients to situations of high risk for aspiration, such as long transfers and prolonged interventions. Therefore, the need to move to very early interventions for both risk stratification and preventive measures points to the identification of earlier signals of the impaired immune response to identify both biomarkers and therapeutic targets. From a therapeutic point of view, researchers also have to consider the potential side-effects on the brain of enhancing systemic immunity. In this sense, the lungs arise as a potential therapeutic target to be explored in the next years, particularly the lung damage caused by brain ischemia, as well as the mechanisms and pathways that are involved.
Supplementary Information
Additional file 1: Table S1. Studied biomarkers for the prediction of SAI, SAP or UTI. This table summarizes the biomarkers presented in section 4 alphabetically ordered.
Acknowledgments
Not applicable.
Abbreviations
- 6-OHDA
6-Hydroxydopamine
- ALI
Acute lung injury
- ANS
Autonomic nervous system
- ArgI
Arginase-1
- ARRB2
β-arrestin2
- BALF
Bronchoalveolar lavage fluid
- BBB
Blood-brain barrier
- CD
Cluster of differentiation
- CNS
Central nervous system
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- DAMPs
Damage-associated molecular pattern
- DHT
Dihydrotestosterone
- FT3
Free triiodothyronine
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HDL
High-density lipoprotein
- HLA-DR
Human leucocyte antigen-DR
- HMGB1
High mobility group box 1
- HPA
Hypothalamic-pituitary-adrenal
- HRV
Heart rate variability
- IFN-γ
Interferon-γ
- IL
Interleukin
- iNKT
Hepatic invariant natural killer T
- MCAO
Middle cerebral artery occlusion
- MHR
Monocyte to HDL cholesterol ratio
- miRNA-21
MicroRNA-21
- MN
Metanephrine
- MR-proADM
Mid-regional pro-adrenomedullin
- NF-κB
Nuclear factor kappa B
- NIHSS
National Institute of Health Stroke Scale
- NLR
Neutrophil-to-lymphocyte ratio
- NMN
Normetanephrine
- PCT
Procalcitonin
- PISCES
Pnemonia In Stroke Consensus
- PNS
Parasympathetic nervous system
- RAGE
Receptor of advanced glycation end-products
- SAA
Serum amyloid A
- SAI
Stroke-associated infection
- SAP
Stroke-associated pneumonia
- SIIS
Stroke-induced immunosuppression
- SNS
Sympathetic nervous system
- suPAR
Soluble urokinase-type plasminogen activator receptor
- TBI
Traumatic brain injury
- Th
T-helper
- THRCT
Thorax high resolution computed tomography
- TNF-α
Tumor necrosis factor-α
- TRAF4
Tumor necrosis factor receptor-associated 4
- UTI
Urinary tract infection
- WBC
White blood cell
- α7nAChR
α7 nicotinic acetylcholine receptor
Authors’ contributions
AB, JM, and JF defined the research of interest and were involved in topic selection. JF carried out the literature review and wrote the first draft of the manuscript. AB, JM, and FMM reviewed the manuscript and made contributions for improvement. All authors read and approved the final manuscript.
Funding
This project received funding from Instituto de Salud Carlos III (ISCIII) [PI17/02130], co-financed by the European Regional Development Fund (FEDER), and from Fundació La Marató de TV3 [201706]. Neurovascular Research Laboratory takes part into the Spanish stroke research network INVICTUS+ [RD16/0019/0021].
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Suda S, Aoki J, Shimoyama T, Suzuki K, Sakamoto Y, Katano T, Okubo S, Nito C, Nishiyama Y, Mishina M, Kimura K. Stroke-associated infection independently predicts 3-month poor functional outcome and mortality. J Neurol. 2018;265(2):370–375. doi: 10.1007/s00415-017-8714-6. [DOI] [PubMed] [Google Scholar]
- 2.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11(1):110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustamante A, Giralt D, García-Berrocoso T, Rubiera M, Álvarez-Sabín J, Molina C, Serena J, Montaner J. The impact of post-stroke complications on in-hospital mortality depends on stroke severity. Eur Stroke J. 2017;2(1):54–63. doi: 10.1177/2396987316681872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teh WH, Smith CJ, Barlas RS, Wood AD, Bettencourt-Silva JH, Clark AB, Metcalf AK, Bowles KM, Potter JF, Myint PK. Impact of stroke-associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurol Scand. 2018;138(4):293–300. doi: 10.1111/ane.12956. [DOI] [PubMed] [Google Scholar]
- 5.Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, Di Napoli M, et al. How is pneumonia diagnosed in clinical stroke research? Stroke. 2015;46(5):1202–1209. doi: 10.1161/STROKEAHA.114.007843. [DOI] [PubMed] [Google Scholar]
- 6.Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, di Napoli M, Kalra L, Langhorne P, Montaner J, Roffe C, Rudd AG, Tyrrell PJ, van de Beek D, Woodhead M, Meisel A. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus Group. Stroke. 2015;46(8):2335–2340. doi: 10.1161/STROKEAHA.115.009617. [DOI] [PubMed] [Google Scholar]
- 7.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Zapata-Arriaza E, Serrano-Gotarredona P, Navarro-Herrero S, Moniche F, Pardo-Galiana B, Pallisa E, Vega-Salvatierra Á, Mancha F, Escudero-Martínez I, Bustamante A, Montaner J. Chest computed tomography findings and validation of clinical criteria of stroke associated pneumonia. J Stroke. 2019;21(2):217–219. doi: 10.5853/jos.2018.03251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishore AK, Devaraj A, Vail A, Ward K, Thomas PG, Sen D, Procter A, Win M, James N, Roffe C, Meisel A, Woodhead M, Smith CJ. Use of pulmonary computed tomography for evaluating suspected stroke-associated pneumonia. J Stroke Cerebrovasc Dis. 2021;30(6):105757. doi: 10.1016/j.jstrokecerebrovasdis.2021.105757. [DOI] [PubMed] [Google Scholar]
- 10.Kishore AK, Jeans AR, Garau J, Bustamante A, Kalra L, Langhorne P, Chamorro A, Urra X, Katan M, Napoli MD, Westendorp W, Nederkoorn PJ, van de Beek D, Roffe C, Woodhead M, Montaner J, Meisel A, Smith CJ. Antibiotic treatment for pneumonia complicating stroke: recommendations from the pneumonia in stroke consensus (PISCES) group. Eur Stroke J. 2019;4(4):318–328. doi: 10.1177/2396987319851335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali AN, Howe J, Majid A, Redgrave J, Pownall S, Abdelhafiz AH. The economic cost of stroke-associated pneumonia in a UK setting. Top Stroke Rehabil. 2018;25(3):214–223. doi: 10.1080/10749357.2017.1398482. [DOI] [PubMed] [Google Scholar]
- 12.Vermeij JD, Westendorp WF, Dippel DWJ, van de Beek D, Nederkoorn PJ. Antibiotic therapy for preventing infections in people with acute stroke. Cochrane Database Syst Rev. 2018;1:CD008530. doi: 10.1002/14651858.CD008530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dethlefsen L, Huse SM, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erny D, De Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, Dames C, Kershaw O, Gruber AD, Curato C, Oyama N, Meisel C, Meisel A, Dirnagl U. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke. 2016;47(5):1354–1363. doi: 10.1161/STROKEAHA.115.011800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2015;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iadecola C, Anrathner J. The immunology of stroke: from mechanisms to translation. Nat Med. 2012;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell Type 1-like immunostimulation. J Exp Med. 2003;198(5):725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan FL, Zhang JH. Role of the sympathetic nervous system and spleen in experimental stroke-induced immunodepression. Med Sci Monit. 2014;20:2489–2496. doi: 10.12659/MSM.890844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore KW, Malefyt RDW, Robert L, Garra AO. Interleukin-10 and the interleukin-10 receptor. Mol Cell Biol. 2001;1:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C, Kong W, Wang Y, Ziai W, Yang Q, Zuo F, Li F, Wang Y, Xu H, Li Q, Yang J, Lu H, Zhang J, Wang J. Changes in the cellular immune system and circulating inflammatory markers of stroke patients. Oncotarget. 2017;8(2):3553–3567. doi: 10.18632/oncotarget.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176(11):6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 23.Chiu NL, Kaiser B, Nguyen YV, Welbourne S, Lall C, Cramer SC. The volume of the spleen and its correlates after acute stroke. J Stroke Cerebrovasc Dis. 2016;25(12):2958–2961. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Jin WN, Liu Y, Shi K, Sun H, Zhang F, Zhang C, Gonzales RJ, Sheth KN, la Cava A, Shi FD. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity. 2017;46(3):474–487. doi: 10.1016/j.immuni.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev United States. 2000;52:595–638. [PubMed] [Google Scholar]
- 26.Wang H, Deng QW, Peng AN, Xing FL, Zuo L, Li S, et al. Β-Arrestin2 functions as a key regulator in the sympathetic-triggered immunodepression after stroke. J Neuroinflammation. 2018;15(1):1–11. doi: 10.1186/s12950-017-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCulloch L, Smith CJ, McColl BW. Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nat Commun. 2017;8:1–15. doi: 10.1038/s41467-016-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emsley HCA, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, Hallenbeck JM, del Zoppo G, Rothwell NJ, Tyrrell PJ, Hopkins SJ. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139(1-2):93–101. doi: 10.1016/S0165-5728(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 29.Franceschini R, Tenconi GL, Zoppoli F, Barreca T. Endocrine abnormalities and outcome of ischaemic stroke. Biomed Pharmacother. 2001;55(8):458–465. doi: 10.1016/S0753-3322(01)00086-5. [DOI] [PubMed] [Google Scholar]
- 30.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engel O, Akyüz L, Da Costa Goncalves AC, Winek K, Dames C, Thielke M, et al. Cholinergic pathway suppresses pulmonary innate immunity facilitating pneumonia after stroke. Stroke. 2015;46(11):3232–3240. doi: 10.1161/STROKEAHA.115.008989. [DOI] [PubMed] [Google Scholar]
- 32.Lafargue M, Xu L, Carlès M, Serve E, Anjum N, Iles KE, Xiong X, Giffard R, Pittet JF. Stroke-induced activation of the α7 nicotinic receptor increases Pseudomonas aeruginosa lung injury. FASEB J. 2012;26(7):2919–2929. doi: 10.1096/fj.11-197384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong CHY, Jenne CN, Lee W-Y, Léger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334(6052):101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 34.Sippel TR, Shimizu T, Strnad F, Traystman RJ, Herson PS, Waziri A. Arginase I release from activated neutrophils induces peripheral immunosuppression in a murine model of stroke. J Cereb Blood Flow Metab. 2015;35(10):1657–1663. doi: 10.1038/jcbfm.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrone AB, O’Connell GC, Regier MD, Chantler PD, Simpkins JW, Barr TL. The role of arginase 1 in post-stroke immunosuppression and ischemic stroke severity. Transl Stroke Res. 2016;7(2):103–110. doi: 10.1007/s12975-015-0431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashour W, Al-Anwar AD, Kamel AE, Aidaros MA. Predictors of early infection in cerebral ischemic stroke. J Med Life. 2016;9(2):163–169. [PMC free article] [PubMed] [Google Scholar]
- 37.Nam KW, Kim TJ, Lee JS, Kwon HM, Lee YS, Ko SB, Yoon BW. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. 2018;49(8):1886–1892. doi: 10.1161/STROKEAHA.118.021228. [DOI] [PubMed] [Google Scholar]
- 38.Liesz A, Dalpke A, Mracsko E, Antoine DJ, Roth S, Zhou W, Yang H, Na SY, Akhisaroglu M, Fleming T, Eigenbrod T, Nawroth PP, Tracey KJ, Veltkamp R. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;35(2):583–598. doi: 10.1523/JNEUROSCI.2439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connell GC, Tennant CS, Lucke-Wold N, Kabbani Y, Tarabishy AR, Chantler PD, et al. Monocyte-lymphocyte cross-communication via soluble CD163 directly links innate immune system activation and adaptive immune system suppression following ischemic stroke. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritzel RM, Al Mamun A, Crapser J, Verma R, Patel AR, Knight BE, et al. CD200-CD200R1 inhibitory signaling prevents spontaneous bacterial infection and promotes resolution of neuroinflammation and recovery after stroke. J Neuroinflammation. 2019;16:1–16. doi: 10.1186/s12974-019-1426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth S, Cao J, Singh V, Tiedt S, Hundeshagen G, Li T, et al. Post-injury immunosuppression and secondary infections are caused by an AIM2 inflammasome-driven signaling cascade. Immunity. 2021;54:648–659.e8. doi: 10.1016/j.immuni.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Ruhnau J, Schulze K, Gaida B, Langner S, Kessler C, Broker B, et al. Stroke alters respiratory burst in neutrophils and monocytes. Stroke. 2014;45(3):794–800. doi: 10.1161/STROKEAHA.113.003342. [DOI] [PubMed] [Google Scholar]
- 43.van Gemmeren T, Schuppner R, Grosse GM, Fering J, Gabriel MM, Huber R, Worthmann H, Lichtinghagen R, Weissenborn K. Early post-stroke infections are associated with an impaired function of neutrophil granulocytes. J Clin Med. 2020;9(3):872. doi: 10.3390/jcm9030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Lu G, Wang D, He JL, Zuo L, Wang H, Gu ZT, Zhou JS, Yan FL, Deng QW. MicroRNA-4443 regulates monocyte activation by targeting tumor necrosis factor receptor associated factor 4 in stroke-induced immunosuppression. Eur J Neurol. 2020;27(8):1625–1637. doi: 10.1111/ene.14282. [DOI] [PubMed] [Google Scholar]
- 45.Zapata-Arriaza E, Moniche F, Blanca P-G, Bustamante A, Escudero-Martínez I, Uclés O, Ollero-Ortiz Á, Sánchez-García JA, Gamero MÁ, Quesada Á, Vidal de Francisco D, Romera M, de la Cruz C, Sanz G, Montaner J. External validation of the ISAN, A2DS2, and AIS-APS scores for predicting stroke-associated pneumonia. J Stroke Cerebrovasc Dis. 2018;27(3):673–676. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 46.Zang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, et al. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol. 2010;184(8):4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dziennis S, Akiyoshi K, Subramanian S, Offner H, Hurn PD. Role of dihydrotestosterone in post-stroke peripheral immunosuppression after cerebral ischemia. Brain Behav Immun. 2011;25(4):685–695. doi: 10.1016/j.bbi.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gill D, Sivakumaran P, Aravind A, Tank A, Dosh R, Veltkamp R. Temporal trends in the levels of peripherally circulating leukocyte subtypes in the hours after ischemic stroke. J Stroke Cerebrovasc Dis. 2018;27(1):198–202. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Fluri F, Morgenthaler NG, Mueller B, Christ-Crain M, Katan M. Copeptin, Procalcitonin and routine inflammatory markers-predictors of infection after stroke. PLoS One. 2012;7:e48309. doi: 10.1371/journal.pone.0048309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Raedt S, De Vos A, Van Binst AM, De Waele M, Coomans D, Buyl R, et al. High natural killer cell number might identify stroke patients at risk of developing infections. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e71. doi: 10.1212/NXI.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y, Lu J, Zheng D, Qian J, Zhang H, Xing D, et al. Predictive value of monocyte to HDL cholesterol ratio for stroke-associated pneumonia in patients with acute ischemic stroke. Acta Neurol Belg. 2020:7. [DOI] [PubMed]
- 52.Zonneveld TP, Nederkoorn PJ, Westendorp WF, Brouwer MC, van De Beek D, Kruyt ND. Hyperglycemia predicts poststroke infections in acute ischemic stroke. Neurology. 2017;88(15):1415–1421. doi: 10.1212/WNL.0000000000003811. [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez-Sanz A, Fuentes B, Martínez-Sánchez P, Prefasi D, Martínez-Martínez M, Correas E, Díez-Tejedor E. High-density lipoprotein: a novel marker for risk of in-hospital infection in acute ischemic stroke patients? Cerebrovasc Dis. 2013;35(3):291–297. doi: 10.1159/000347077. [DOI] [PubMed] [Google Scholar]
- 54.Dziedzic T, Pera J, Klimkowicz A, Turaj W, Slowik A, Rog TM, Szczudlik A. Serum albumin level and nosocomial pneumonia in stroke patients. Eur J Neurol. 2006;13(3):299–301. doi: 10.1111/j.1468-1331.2006.01210.x. [DOI] [PubMed] [Google Scholar]
- 55.Ye S, Lin SP, Wu K, Fan Y, Xu M. Serum prealbumin is a predictive biomarker for stroke-associated infection after an ischemic stroke. Int J Neurosci. 2017;127(7):601–605. doi: 10.1080/00207454.2016.1218874. [DOI] [PubMed] [Google Scholar]
- 56.Huang GQ, Cheng HR, Wu YM, Cheng QQ, Wang YM, Fu JL, Zhou HX, Wang Z. Reduced vitamin D levels are associated with stroke-associated pneumonia in patients with acute ischemic stroke. Clin Interv Aging. 2019;14:2305–2314. doi: 10.2147/CIA.S230255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bustamante A, Vilar-Bergua A, Guettier S, Sánchez-Poblet J, García-Berrocoso T, Giralt D, Fluri F, Topakian R, Worthmann H, Hug A, Molnar T, Waje-Andreassen U, Katan M, Smith CJ, Montaner J. C-reactive protein in the detection of post-stroke infections: systematic review and individual participant data analysis. J Neurochem. 2017;141(2):305–314. doi: 10.1111/jnc.13973. [DOI] [PubMed] [Google Scholar]
- 58.Azurmendi L, Lapierre-Fetaud V, Schneider J, Montaner J, Katan M, Sanchez JC. Proteomic discovery and verification of serum amyloid A as a predictor marker of patients at risk of post-stroke infection: a pilot study. Clin Proteomics. 2017:14–27. [DOI] [PMC free article] [PubMed]
- 59.Schweizer J, Bustamante A, Lapierre-Fétaud V, Faura J, Scherrer N, Azurmendi Gil L, Fluri F, Schütz V, Luft A, Boned S, Sanchez JC, Montaner J, Katan M. SAA (serum amyloid A): a novel predictor of stroke-associated infections. Stroke. 2020;51(12):3523–3530. doi: 10.1161/STROKEAHA.120.030064. [DOI] [PubMed] [Google Scholar]
- 60.Zapata-Arriaza E, Mancha F, Bustamante A, Moniche F, Pardo-Galiana B, Serrano-Gotarredona P, Navarro-Herrero S, Pallisa E, Faura J, Vega-Salvatierra Á, Penalba A, Escudero-Martínez I, Ramos-Herrero VD, Azurmendi L, Charles Sanchez J, Montaner J. Biomarkers predictive value for early diagnosis of stroke-associated pneumonia. Ann Clin Transl Neurol. 2019;6(9):1882–1887. doi: 10.1002/acn3.50849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bustamante A, García-Berrocoso T, Penalba A, Giralt D, Simats A, Muchada M, Zapata E, Rubiera M, Montaner J. Sepsis biomarkers reprofiling to predict stroke-associated infections. J Neuroimmunol. 2017;312:19–23. doi: 10.1016/j.jneuroim.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann S, Harms H, Ulm L, Nabavi DG, Mackert BM, Schmehl I, Jungehulsing GJ, Montaner J, Bustamante A, Hermans M, Hamilton F, Göhler J, Malzahn U, Malsch C, Heuschmann PU, Meisel C, Meisel A, on behalf of the PREDICT Investigators Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia – The PREDICT study. J Cereb Blood Flow Metab. 2017;37(12):3671–3682. doi: 10.1177/0271678X16671964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulm L, Hoffmann S, Nabavi D, Hermans M, Mackert BM, Hamilton F, et al. The randomized controlled STRAWINSKI trial: Procalcitonin-guided antibiotic therapy after stroke. Front Neurol. 2017;8:1–10. doi: 10.3389/fneur.2017.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hotter B, Hoffmann S, Ulm L, Montaner J, Bustamante A, Meisel C, Meisel A. Inflammatory and stress markers predicting pneumonia, outcome, and etiology in patients with stroke: Biomarkers for predicting pneumonia, functional outcome, and death after stroke. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e692. doi: 10.1212/NXI.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hug A, Mürle B, Dalpke A, Zorn M, Liesz A, Veltkamp R. Usefulness of serum procalcitonin levels for the early diagnosis of stroke-associated respiratory tract infections. Neurocrit Care. 2011;14(3):416–422. doi: 10.1007/s12028-009-9325-6. [DOI] [PubMed] [Google Scholar]
- 66.Worthmann H, Tryc AB, Dirks M, Schuppner R, Brand K, Klawonn F, et al. Lipopolysaccharide binding protein, interleukin-10, interleukin-6 and C-reactive protein blood levels in acute ischemic stroke patients with post-stroke infection. J Neuroinflammation. 2015;12:1–9. doi: 10.1186/s12974-014-0231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwan J, Horsfield G, Bryant T, Gawne-Cain M, Durward G, Byrne CD, Englyst NA. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp Gerontol. 2013;48(9):960–965. doi: 10.1016/j.exger.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Bustamante A, Sobrino T, Giralt D, García-Berrocoso T, Llombart V, Ugarriza I, Espadaler M, Rodríguez N, Sudlow C, Castellanos M, Smith CJ, Rodríguez-Yánez M, Waje-Andreassen U, Tanne D, Oto J, Barber M, Worthmann H, Wartenberg KE, Becker KJ, Chakraborty B, Oh SH, Whiteley WN, Castillo J, Montaner J. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: a systematic review and meta-analysis. J Neuroimmunol. 2014;274(1-2):215–224. doi: 10.1016/j.jneuroim.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Chamorro Á, Amaro S, Vargas M, Obach V, Cervera Á, Torres F, Planas AM. Interleukin 10, monocytes and increased risk of early infection in ischaemic stroke. J Neurol Neurosurg Psychiatry. 2006;77(11):1279–1281. doi: 10.1136/jnnp.2006.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salat D, Penalba A, García-Berrocoso T, Campos-Martorell M, Flores A, Pagola J, Bustamante A, Quintana M, Giralt D, Molina C, Alvarez-Sabín J, Montaner J. Immunological biomarkers improve the accuracy of clinical risk models of infection in the acute phase of ischemic stroke. Cerebrovasc Dis. 2013;35(3):220–227. doi: 10.1159/000346591. [DOI] [PubMed] [Google Scholar]
- 71.Lin SP, Ye S, Chen XH, Jiang HL, Mao HF, Chen MT, Ma QJ, Long Y, Fan Y, Lin PY. Increased expression of microRNA-21 in peripheral blood mediates the down-regulation of IFN-γ and increases the prevalence of stroke-associated infection. J Neurol Sci. 2016;366:235–239. doi: 10.1016/j.jns.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 72.Tanzi P, Cain K, Kalil A, Zierath D, Savos A, Gee JM, Shibata D, Hadwin J, Carter K, Becker K. Post-stroke infection: a role for IL-1ra? Neurocrit Care. 2011;14(2):244–252. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang DP, Yan FL, Xu HQ, Zhu YX, Yin Y, Lu HQ. A decrease of human leucocyte antigen-DR expression on monocytes in peripheral blood predicts stroke-associated infection in critically-ill patients with acute stroke. Eur J Neurol. 2009;16(4):498–505. doi: 10.1111/j.1468-1331.2008.02512.x. [DOI] [PubMed] [Google Scholar]
- 74.Günther A, Salzmann I, Nowack S, Schwab M, Surber R, Hoyer H, Witte OW, Hoyer D. Heart rate variability - a potential early marker of sub-acute post-stroke infections. Acta Neurol Scand. 2012;126(3):189–196. doi: 10.1111/j.1600-0404.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 75.Suda S, Aoki J, Shimoyama T, Suzuki K, Sakamoto Y, Katano T, Okubo S, Nito C, Nishiyama Y, Mishina M, Kimura K. Low free triiodothyronine at admission predicts poststroke infection. J Stroke Cerebrovasc Dis. 2018;27(2):397–403. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 76.Badve MS, Zhou Z, Anderson CS, Hackett ML. Effectiveness and safety of antibiotics for preventing pneumonia and improving outcome after acute stroke: systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2018;27(11):3137–3147. doi: 10.1016/j.jstrokecerebrovasdis.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Stanley D, Mason LJ, MacKin KE, Srikhanta YN, Lyras D, Prakash MD, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22(11):1277–1284. doi: 10.1038/nm.4194. [DOI] [PubMed] [Google Scholar]
- 78.Liu DD, Chu SF, Chen C, Yang PF, Chen NH, He X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP) Neurochem Int. 2018;114:42–54. doi: 10.1016/j.neuint.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Deng QW, Yang H, Yan FL, Wang H, Xing FL, Zuo L, Zhang HQ. Blocking sympathetic nervous system reverses partially stroke-induced immunosuppression but does not aggravate functional outcome after experimental stroke in rats. Neurochem Res. 2016;41(8):1877–1886. doi: 10.1007/s11064-016-1899-8. [DOI] [PubMed] [Google Scholar]
- 80.Sykora M, Siarnik P, Diedler J. β-Blockers, pneumonia, and outcome after ischemic stroke. Stroke. 2015;46(5):1269–1274. doi: 10.1161/STROKEAHA.114.008260. [DOI] [PubMed] [Google Scholar]
- 81.Maier IL, Karch A, Mikolajczyk R, Bähr M, Liman J. Effect of beta-blocker therapy on the risk of infections and death after acute stroke—a historical cohort study. PLoS One. 2015;10:1–10. doi: 10.1371/journal.pone.0116836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maier IL, Becker JC, Leyhe JR, Schnieder M, Behme D, Psychogios MN, et al. Influence of beta-blocker therapy on the risk of infections and death in patients at high risk for stroke induced immunodepression. PLoS One. 2018;13:1–11. doi: 10.1371/journal.pone.0196174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jin R, Liu S, Wang M, Zhong W, Li G. Inhibition of CD147 attenuates stroke-associated pneumonia through modulating lung immune response in mice. Front Neurol. 2019;10:1–15. doi: 10.3389/fneur.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guan YT, Mao LL, Jia J, Dong CS, Zhou XM, Zheng SL, Liu H, Kong HM, Zhen XC, Cheng J. Postischemic administration of a potent PTEN inhibitor reduces spontaneous lung infection following experimental stroke. CNS Neurosci Ther. 2013;19(12):990–993. doi: 10.1111/cns.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dames C, Winek K, Beckers Y, Engel O, Meisel A, Meisel C. Immunomodulatory treatment with systemic GM-CSF augments pulmonary immune responses and improves neurological outcome after experimental stroke. J Neuroimmunol. 2018;321:144–149. doi: 10.1016/j.jneuroim.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 86.Goyagi T, Horiguchi T, Nishikawa T, Tobe Y. Post-treatment with selective β1 adrenoceptor antagonists provides neuroprotection against transient focal ischemia in rats. Brain Res Netherlands. 2010;1343:213–217. doi: 10.1016/j.brainres.2010.04.079. [DOI] [PubMed] [Google Scholar]
- 87.Bai W, Li W, Ning YL, Li P, Zhao Y, Yang N, et al. Blood glutamate levels are closely related to acute lung injury and prognosis after stroke. Front Neurol. 2018;8:1–11. doi: 10.3389/fneur.2017.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mrozek S, Constantin J-M, Geeraerts T. Brain-lung crosstalk: implications for neurocritical care patients. World J Crit Care Med. 2015;4(3):163–178. doi: 10.5492/wjccm.v4.i3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu PJ, Pittet JF, Kerby JD, Bosarge PL, Wagener BM. Acute brain trauma, lung injury, and pneumonia: more than just altered mental status and decreased airway protection. Am J Phys Lung Cell Mol Phys. 2017;313(1):L1–15. doi: 10.1152/ajplung.00485.2016. [DOI] [PubMed] [Google Scholar]
- 90.Wen SW, Wong CHY. An unexplored brain-gut microbiota axis in stroke. Gut Microbes. 2017;8(6):601–606. doi: 10.1080/19490976.2017.1344809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care. 2009;11(3):417–426. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 92.Samary CS, Ramos AB, Maia LA, Rocha NN, Santos CL, Magalhães RF, et al. Focal ischemic stroke leads to lung injury and reduces alveolar macrophage phagocytic capability in rats. Crit Care Critical Care. 2018;22:1–11. doi: 10.1186/s13054-018-2164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Austin V, Ku JM, Miller AA, Vlahos R. Ischaemic stroke in mice induces lung inflammation but not acute lung injury. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-40392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farris BY, Monaghan KL, Zheng W, Amend CD, Hu H, Ammer AG, Coad JE, Ren X, Wan ECK. Ischemic stroke alters immune cell niche and chemokine profile in mice independent of spontaneous bacterial infection. Immunity, Inflamm Dis. 2019;7(4):326–341. doi: 10.1002/iid3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Studied biomarkers for the prediction of SAI, SAP or UTI. This table summarizes the biomarkers presented in section 4 alphabetically ordered.
Data Availability Statement
Not applicable.