Abstract
Acute kidney injury is a common complication in patients hospitalized with SARSCoV-2 (COVID-19), with prior studies implicating multiple potential mechanisms of injury. Although COVID-19 is often compared to other respiratory viral illnesses, few formal comparisons of these viruses on kidney health exist. In this retrospective cohort study, we compared the incidence, features, and outcomes of acute kidney injury among Veterans hospitalized with COVID-19 or influenza and adjusted for baseline conditions using weighted comparisons. A total of 3402 hospitalizations for COVID-19 and 3680 hospitalizations for influenza admitted between October 1, 2019 and May 31, 2020 across 127 Veterans Administration hospitals nationally were studied using the electronic medical record. Acute kidney injury occurred more frequently among those with COVID-19 compared to those with influenza (40.9% versus 29.4%, weighted analysis) and was more severe. Patients with COVID-19 were more likely to require mechanical ventilation and vasopressors and experienced higher mortality. Proteinuria and hematuria were frequent in both groups but more common in COVID-19. Recovery of kidney function was less common in patients with COVID-19 and acute kidney injury but was similar among survivors. Thus, findings from this study confirm that acute kidney injury is more common and severe among patients hospitalized with COVID-19 compared to influenza, a finding that may be driven largely by illness severity. Hence, the combined impact of these two illnesses on kidney health may be significant and have important implications for resource allocation.
Keywords: acute kidney injury, COVID-19, hematuria, influenza, proteinuria
Graphical abstract
see commentary on page 750
Acute kidney injury (AKI) is a well-recognized complication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease 2019 [COVID-19]),1, 2, 3, 4 occurring in one-third of hospitalized patients and up to three-quarters of critically ill patients,1 , 2 , 4 with associated in-hospital mortality rates of up to 50%.1 , 4 The reasons underlying the high AKI incidence rates and associated poor outcomes are not well understood. High rates of hematuria and proteinuria have also been observed in COVID-19.
The extent to which these findings differ from other severe viral respiratory illnesses is unknown. Although informal comparisons to influenza have been made, few direct comparisons have been performed. Literature on AKI in influenza suggests some risk factors common to both illnesses, such as those related to illness severity (e.g., critical illness and mechanical ventilation).5, 6, 7, 8 Similarly, elevated inflammatory markers have been observed in both influenza and COVID-19 and are often associated with AKI,5 , 8, 9, 10, 11, 12 whereas histopathologic data and clinical studies suggest ischemic injury as the predominant etiology of AKI in both illnesses.13, 14, 15, 16, 17, 18, 19 Further understanding of the relative and combined burden of AKI in these 2 illnesses is critical. We hypothesized that patients with COVID-19 would have higher rates and severity of AKI than similar patients hospitalized with influenza. To test this hypothesis, we compared the incidence, risk factors, clinical features, and recovery from AKI in a retrospective study of veteran patients hospitalized with either COVID-19 or influenza.
Methods
Study setting and design
We conducted a national retrospective cohort study of veterans, aged ≥18 years, who were admitted with COVID-19 or influenza between October 1, 2019, and May 31, 2020. Data were obtained from the electronic health record utilized by the Veterans Affairs (VA) Health Administration, which is composed of the Veterans Health Information and Technology Architecture and Computerized Patient Record System. This study was approved by the Institutional Review Board and the Research and Development Committee of the Tennessee Valley Healthcare System VA. The requirement for informed consent was waived because of the infeasibility of obtaining informed consent for a large national cohort.
Data collection
Data from October 1, 2018, to September 24, 2020, were collected from the Observational Medical Outcomes Partnership version 5 common data model transformation of the National Corporate Data Warehouse, which aggregates data from all VA facilities, and the VA COVID-19 Shared Data Resource.20, 21, 22 Complete hospitalization information was available for all patients. Baseline comorbidity data were obtained from available records up to the day of hospital admission. Inpatient conditions, vital signs, laboratory data, and exposures were obtained from records from admission through discharge. Kidney function and mortality outcomes were collected from VA laboratory data, administrative diagnosis and procedure codes, and VA vital status files. Diagnoses and procedures were defined using the International Classification of Diseases, Ninth Revision (ICD-9), and International Classification of Diseases, Tenth Revision (ICD-10), and Current Procedural Terminology codes (Supplementary Table S1). Laboratory tests were identified by Logical Observation Identifiers Names and Codes. Medications were obtained from outpatient VA pharmacy fill records and inpatient barcoded medication administration and categorized using the Anatomical Therapeutic Chemical classification and RxNorm.
Cohort exclusion criteria
Within the predefined study time frame and across 127 VA hospitals nationally, we identified 8454 hospitalizations that included a diagnosis of COVID-19 or influenza. Eligibility criteria included either a premorbid outpatient serum creatinine value and at least 1 inpatient serum creatinine value or at least 2 serum creatinine values in the absence of a premorbid baseline value. We applied several exclusion criteria to provide the 2 clinically relevant cohorts, as illustrated in Figure 1 . We excluded patients who underwent nephrectomy during the hospitalization and patients with a baseline estimated glomerular filtration rate (eGFR) <15 ml/min per 1.37 m2, kidney transplantation, or end-stage renal disease before index hospitalization. In patients who had >1 qualifying hospitalization during the study period, we restricted to the first qualifying hospitalization. Patients with both a positive COVID-19 and influenza test during the study period were excluded.
Figure 1.
Cohort selection flow diagram. Exclusion criteria applied to eligible hospitalizations to derive final study groups. COVID-19, coronavirus disease 2019; ESRD, end-stage renal disease.
Definitions
The primary exposures in this study were infection with COVID-19 or influenza. Two groups were defined: (i) patients with a positive COVID-19 test within 14 days before or during the hospitalization; and (ii) patients with a positive influenza A or influenza B test within 14 days before or during the hospitalization. Patients were diagnosed with COVID-19 or influenza by polymerase chain reaction–based or rapid antigen tests of nasopharyngeal, oropharyngeal, or respiratory specimens. The primary outcome in this study was AKI. AKI was defined using the peak in-hospital serum creatinine and staged using modified Kidney Disease: Improving Global Outcomes (KDIGO) creatinine-based criteria: stage 1, ≥0.3 mg/dl creatinine increase from baseline or creatinine 1.5 to 1.9 times baseline; stage 2, creatinine 2.0 to 2.9 times baseline; and stage 3, creatinine 3.0 times baseline or initiation of dialysis.23 To more accurately compare AKI staging and recovery, we preferred to anchor our definition to a known baseline creatinine, which was available in 84% of patients with COVID-19 and 92% with influenza. Secondary outcomes included hematuria, proteinuria, kidney replacement therapy, recovery from AKI, and death in hospital and at 90 days following peak serum creatinine. Recovery from AKI was defined by serum creatinine value within 20% of baseline serum creatinine, obtained within the following time frames: within 4, 30, and 90 days of peak serum creatinine.
Other definitions
Baseline serum creatinine to define AKI was the mean outpatient serum creatinine value 7 to 365 days before hospitalization.24 Among those without a known preadmission baseline, we used the lowest serum creatinine during hospitalization. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.25 Hematuria and proteinuria were defined as 1+ or higher red blood cells or protein on urine dipstick, respectively. Acute dialysis was defined using procedure codes (Supplementary Table S1). Death was ascertained through VA Vital Status File, which aggregates date of death from several locations.26
Covariates
Baseline comorbidities were ascertained in the preadmission time frame up to but not including the day of admission (Supplementary Table S1). Preadmission medication exposures were ascertained from 104 days before admission up to the admission date. In-hospital exposures included vital signs, laboratory results, medications, and mechanical ventilation.
Statistical approach
The primary analysis compared AKI incidence during hospitalization, mortality during hospitalization and within 90 days of peak serum creatinine, and AKI recovery occurring within 90 days of peak serum creatinine between patients hospitalized with COVID-19 or influenza. To account for measured confounding, an inverse probability of treatment weighted cohort was created that balanced the distributions of >50 observed covariates (Tables 1 and 2 ). The weights were calculated using the matching weight formula that creates covariate distributions similar to a 1-to-1 propensity score matched cohort.27 These weights are highly efficient, allowing the use all of the patients in the cohort while avoiding the potentially extreme weights of other methods. The propensity score used for the weighting was calculated via a logistic regression model predicting COVID-19 versus influenza. The model included 55 prehospitalization covariates, including major variables known to increase the risk for mortality, such as hypertension medications, history of congestive heart failure, proteinuria (dipstick), mean preadmission outpatient eGFR, and extensive comorbidities. To balance missingness patterns, missing values for preadmission body mass index (15.7%), preadmission temperature (6.7%), preadmission systolic blood pressure (6.2%), preadmission diastolic blood pressure (6.2%), preadmission pulse (6.2%), preadmission oxygen saturation (8.9%), and baseline eGFR (11.5%) were set to low leverage points (global means), and indicator variables for missingness were included in the propensity score model.28 This approach for balancing missingness patterns is preferred for a propensity score model, as opposed to a covariate-adjusted model of the outcome, where multiple imputation may be preferred. Covariate balance in the inverse probability of treatment weighted cohort was assessed using standardized mean differences (Tables 1 and 2). The weighted cohorts for patients hospitalized with COVID-19 or influenza who were (i) diagnosed with AKI or (ii) diagnosed with AKI and survived 90 days after peak were created using the same method. All analyses were conducted using R software version 4.0.2 (R Foundation for Statistical Computing).29 Confidence intervals and P values were calculated using the “survey” package in R to account for the inverse probability treatment weights. P values were calculated using χ2 tests with a continuity correction for categorical variables and 2-sample t tests for continuous variables.
Table 1.
Baseline characteristics of unweighted cohorts
| Demographics and comorbidities | Total (N = 7082) | No. nonmissing | COVID-19–positive (N = 3402) | No. nonmissing | Influenza-positive (N = 3680) | No. nonmissing | SMD |
|---|---|---|---|---|---|---|---|
| Age, yr, mean (SD) | 67.3 (13.5) | 7082 | 68.3 (13.9) | 3402 | 66.5 (13.1) | 3680 | 0.137 |
| Female, n (%) | 468 (7) | 7082 | 203 (6) | 3402 | 265 (7) | 3680 | 0.050 |
| Race, n (%) | 7082 | 3402 | 3680 | ||||
| Unknown | 485 (7) | 272 (8) | 213 (6) | 0.087 | |||
| African American | 2494 (35) | 1583 (47) | 911 (25) | 0.467 | |||
| Other | 151 (2) | 62 (2) | 89 (2) | 0.041 | |||
| White | 3952 (56) | 1485 (44) | 2467 (67) | 0.484 | |||
| Ethnicity, n (%) | 7082 | 3402 | 3680 | ||||
| Unknown | 205 (3) | 107 (3) | 98 (3) | 0.029 | |||
| Hispanic/Latino | 524 (7) | 292 (9) | 232 (6) | 0.087 | |||
| Not Hispanic/Latino | 6353 (90) | 3003 (88) | 3350 (91) | 0.091 | |||
| Diabetes mellitus, n (%) | 3581 (51) | 7082 | 1790 (53) | 3402 | 1791 (49) | 3680 | 0.079 |
| Hypertension, n (%) | 5915 (84) | 7082 | 2804 (82) | 3402 | 3111 (85) | 3680 | 0.057 |
| Coronary artery disease, n (%) | 3219 (45) | 7082 | 1434 (42) | 3402 | 1785 (49) | 3680 | 0.128 |
| Peripheral vascular disease, n (%) | 2336 (33) | 7082 | 1074 (32) | 3402 | 1262 (34) | 3680 | 0.058 |
| Heart failure, n (%) | 2220 (31) | 7082 | 938 (28) | 3402 | 1282 (35) | 3680 | 0.157 |
| Mild liver disease, n (%) | 1525 (22) | 7082 | 727 (21) | 3402 | 798 (22) | 3680 | 0.008 |
| Moderate to severe liver disease, n (%) | 194 (3) | 7082 | 90 (3) | 3402 | 104 (3) | 3680 | 0.011 |
| Chronic obstructive pulmonary disease, n (%) | 3944 (56) | 7082 | 1589 (47) | 3402 | 2355 (64) | 3680 | 0.353 |
| Cancer (ICD-9), n (%) | 1918 (27) | 7082 | 834 (25) | 3402 | 1084 (29) | 3680 | 0.111 |
| Cerebrovascular disease, n (%) | 2076 (29) | 7082 | 991 (29) | 3402 | 1085 (29) | 3680 | 0.008 |
| Myocardial infarction, n (%) | 1304 (18) | 7082 | 529 (16) | 3402 | 775 (21) | 3680 | 0.143 |
| Dementia, n (%) | 1020 (14) | 7082 | 649 (19) | 3402 | 371 (10) | 3680 | 0.257 |
| Hemiplegia or paraplegia, n (%) | 465 (7) | 7082 | 230 (7) | 3402 | 235 (6) | 3680 | 0.015 |
| HIV, n (%) | 148 (2) | 7082 | 64 (2) | 3402 | 84 (2) | 3680 | 0.028 |
| Peptic ulcer disease, n (%) | 585 (8) | 7082 | 241 (7) | 3402 | 344 (9) | 3680 | 0.083 |
| Rheumatic disease, n (%) | 301 (4) | 7082 | 127 (4) | 3402 | 174 (5) | 3680 | 0.049 |
| Metastatic solid tumor, n (%) | 376 (5) | 7082 | 148 (4) | 3402 | 228 (6) | 3680 | 0.083 |
| Baseline eGFR, ml/min per 1.73 m2, mean (SD) | 73.9 (22.5) | 6271 | 73.3 (23.2) | 2863 | 74.3 (22.0) | 3408 | 0.045 |
| Last preadmission BMI, kg/m2, mean (SD) | 30.2 (7.1) | 5972 | 30.6 (7.0) | 2735 | 29.9 (7.1) | 3237 | 0.090 |
| Last preadmission temperature, ° C, mean (SD) | 98.0 (0.9) | 6607 | 98.1 (0.9) | 3076 | 98.0 (0.8) | 3531 | 0.181 |
| Last preadmission SBP, mm Hg, mean (SD) | 131 (19) | 6641 | 132 (19) | 3093 | 131 (19) | 3548 | 0.052 |
| Last preadmission DBP, mm Hg, mean (SD)a | 76 (11) | 6640 | 76 (11) | 3092 | 76 (11) | 3548 | 0.045 |
| Last preadmission pulse, bpm, mean (SD) | 80 (15) | 6640 | 80 (15) | 3092 | 80 (15) | 3548 | 0.011 |
| Last preadmission O2 saturation, %, mean (SD) | 96.3 (2.6) | 6451 | 96.6 (2.3) | 2995 | 96.0 (2.8) | 3456 | 0.205 |
| Last preadmission protein dipstick, n (%) | 7082 | 3402 | 3680 | 0.235 | |||
| Not measured | 2994 (42) | 1641 (48) | 1353 (37) | ||||
| Negative or trace | 2838 (40) | 1211 (36) | 1627 (44) | ||||
| 1+ | 663 (9) | 285 (8) | 378 (10) | ||||
| ≥2+ | 587 (8) | 265 (8) | 322 (9) | ||||
| Last preadmission urine dipstick RBCs, n (%) | 7082 | 3402 | 3680 | 0.222 | |||
| Not measured | 3068 (43) | 1662 (49) | 1406 (38) | ||||
| Negative or trace | 3153 (45) | 1340 (39) | 1813 (49) | ||||
| 1+ | 497 (7) | 234 (7) | 263 (7) | ||||
| ≥2+ | 364 (5) | 166 (5) | 198 (5) | ||||
| Active ACE inhibitor user, n (%)b | 1515 (21) | 7082 | 625 (18) | 3402 | 890 (24) | 3680 | 0.142 |
| Active ARB user, n (%)b | 768 (11) | 7082 | 334 (10) | 3402 | 434 (12) | 3680 | 0.064 |
| Active CCB user, n (%)b | 1538 (22) | 7082 | 748 (22) | 3402 | 790 (21) | 3680 | 0.013 |
| Active α-blocker user, n (%)b | 221 (3) | 7082 | 83 (2) | 3402 | 138 (4) | 3680 | 0.076 |
| Active β-blocker user, n (%)b | 1959 (28) | 7082 | 709 (21) | 3402 | 1250 (34) | 3680 | 0.298 |
| Active K-sparing diuretic user, n (%)b | 312 (4) | 7082 | 126 (4) | 3402 | 186 (5) | 3680 | 0.066 |
| Active loop diuretic user, n (%)b | 893 (13) | 7082 | 296 (9) | 3402 | 597 (16) | 3680 | 0.229 |
| Active thiazide user, n (%)b | 891 (13) | 7082 | 450 (13) | 3402 | 441 (12) | 3680 | 0.037 |
| Active peripheral vasodilator user, n (%)b | 190 (3) | 7082 | 93 (3) | 3402 | 97 (3) | 3680 | 0.006 |
| Recent acyclovir fill, n (%)c | 98 (1) | 7082 | 26 (1) | 3402 | 72 (2) | 3680 | 0.103 |
| Recent azithromycin fill, n (%)c | 2098 (30) | 7082 | 775 (23) | 3402 | 1323 (36) | 3680 | 0.292 |
| Recent β-lactam fill, n (%)c | 512 (7) | 7082 | 212 (6) | 3402 | 300 (8) | 3680 | 0.074 |
| Recent cephalosporin fill, n (%)c | 344 (5) | 7082 | 127 (4) | 3402 | 217 (6) | 3680 | 0.101 |
| Recent hydroxychloroquine fill, n (%)c | 37 (1) | 7082 | 19 (1) | 3402 | 18 (0) | 3680 | 0.010 |
| Recent fluoroquinolone fill, n (%)c | 271 (4) | 7082 | 85 (2) | 3402 | 186 (5) | 3680 | 0.134 |
| Recent H2 blocker fill, n (%)c | 436 (6) | 7082 | 171 (5) | 3402 | 265 (7) | 3680 | 0.091 |
| Recent NSAID fill, n (%)c | 942 (13) | 7082 | 406 (12) | 3402 | 536 (15) | 3680 | 0.078 |
| Recent proton pump inhibitor fill, n (%)c | 1706 (24) | 7082 | 648 (19) | 3402 | 1058 (29) | 3680 | 0.229 |
| Recent steroid fill, n (%)c | 958 (14) | 7082 | 247 (7) | 3402 | 711 (19) | 3680 | 0.361 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; COVID-19, coronavirus disease 2019; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ICD-9, International Classification of Diseases, Ninth Revision; NSAID, nonsteroidal anti-inflammatory drug; RBC, red blood cell; SBP, systolic blood pressure; SMD, standardized mean difference.
SMDs are the absolute difference in means or percentages divided by an evenly weighted pooled SD or difference between groups in number of SDs. Bolded values denote SMD values >0.1, indicating imbalance between the groups.
DBP indicator variable for missingness not included in the logistic regression model because of collinearity with the SBP indicator variable for missingness.
Active user refers to pills on hand at admission.
Recent fill refers to fill within last 104 days.
Table 2.
Baseline characteristics of weighted cohorts
| Demographics and comorbidities | Total W = 4480 |
No. nonmissing | COVID-19–positive W = 2247 |
No. nonmissing | Influenza-positive W = 2233 |
No. nonmissing | SMD |
|---|---|---|---|---|---|---|---|
| Age, mean yr, (SD) | 66.9 (13.7) | 4480 | 66.9 (13.7) | 2247 | 66.9 (13.7) | 2233 | 0.002 |
| Female, n (%) | 289 (6) | 4480 | 145 (6) | 2247 | 144 (6) | 2233 | <0.001 |
| Race, n (%) | 4480 | 2247 | 2233 | ||||
| Unknown | 317 (7) | 159 (7) | 158 (7) | <0.001 | |||
| African American | 1601 (36) | 804 (36) | 798 (36) | 0.001 | |||
| Other | 105 (2) | 53 (2) | 52 (2) | 0.002 | |||
| White | 2456 (55) | 1231 (55) | 1226 (55) | 0.002 | |||
| Ethnicity, n (%) | 4480 | 2247 | 2233 | ||||
| Unknown | 132 (3) | 66 (3) | 65 (3) | 0.002 | |||
| Hispanic/Latino | 363 (8) | 183 (8) | 180 (8) | 0.003 | |||
| Not Hispanic/Latino | 3985 (89) | 1997 (89) | 1988 (89) | 0.004 | |||
| Diabetes mellitus, n (%) | 2299 (51) | 4480 | 1151 (51) | 2247 | 1148 (51) | 2233 | 0.003 |
| Hypertension, n (%) | 3731 (83) | 4480 | 1871 (83) | 2247 | 1861 (83) | 2233 | 0.002 |
| Coronary artery disease, n (%) | 2010 (45) | 4480 | 1003 (45) | 2247 | 1006 (45) | 2233 | 0.008 |
| Peripheral vascular disease, n (%) | 1452 (32) | 4480 | 726 (32) | 2247 | 726 (33) | 2233 | 0.004 |
| Heart failure, n (%) | 1383 (31) | 4480 | 694 (31) | 2247 | 689 (31) | 2233 | 0.001 |
| Mild liver disease, n (%) | 984 (22) | 4480 | 490 (22) | 2247 | 494 (22) | 2233 | 0.007 |
| Moderate to severe liver disease, n (%) | 122 (3) | 4480 | 61 (3) | 2247 | 61 (3) | 2233 | 0.002 |
| Chronic obstructive pulmonary disease, n (%) | 2437 (54) | 4480 | 1221 (54) | 2247 | 1216 (54) | 2233 | 0.002 |
| Cancer (ICD-9), n (%) | 1179 (26) | 4480 | 594 (26) | 2247 | 585 (26) | 2233 | 0.005 |
| Cerebrovascular disease, n (%) | 1281 (29) | 4480 | 645 (29) | 2247 | 636 (28) | 2233 | 0.005 |
| Myocardial infarction, n (%) | 793 (18) | 4480 | 396 (18) | 2247 | 397 (18) | 2233 | 0.004 |
| Dementia, n (%) | 633 (14) | 4480 | 322 (14) | 2247 | 311 (14) | 2233 | 0.013 |
| Hemiplegia or paraplegia, n (%) | 300 (7) | 4480 | 150 (7) | 2247 | 150 (7) | 2233 | 0.002 |
| HIV, n (%) | 102 (2) | 4480 | 50 (2) | 2247 | 51 (2) | 2233 | 0.004 |
| Peptic ulcer disease, n (%) | 353 (8) | 4480 | 179 (8) | 2247 | 174 (8) | 2233 | 0.006 |
| Rheumatic disease, n (%) | 196 (4) | 4480 | 97 (4) | 2247 | 99 (4) | 2233 | 0.006 |
| Metastatic solid tumor, n (%) | 230 (5) | 4480 | 115 (5) | 2247 | 115 (5) | 2233 | <0.001 |
| Baseline eGFR, ml/min per 1.73 m2, mean (SD) | 74.1 (22.7) | 3996 | 74.1 (22.7) | 2004 | 74.1 (22.7) | 1992 | 0.004 |
| Last preadmission BMI, kg/m2, mean (SD) | 30.4 (7.1) | 3815 | 30.4 (7.1) | 1914 | 30.4 (7.2) | 1901 | 0.001 |
| Last preadmission temperature, ° C, mean (SD) | 98.0 (0.9) | 4204 | 98.0 (0.8) | 2109 | 98.0 (0.9) | 2095 | 0.006 |
| Last preadmission SBP, mean (SD), mm Hg | 131 (19) | 4228 | 131 (19) | 2121 | 131 (19) | 2107 | 0.006 |
| Last preadmission DBP, mm Hg, mean (SD)a | 76 (11) | 4227 | 76 (11) | 2120 | 76 (11) | 2107 | 0.003 |
| Last preadmission pulse, bpm, mean (SD) | 80 (15) | 4228 | 80 (15) | 2121 | 80 (15) | 2107 | 0.002 |
| Last preadmission O2 saturation, %, mean (SD) | 96.4 (2.4) | 4099 | 96.4 (2.4) | 2057 | 96.4 (2.3) | 2042 | 0.006 |
| Last preadmission protein dipstick, n (%) | 4480 | 2247 | 2233 | 0.027 | |||
| Not measured | 1896 (42) | 957 (43) | 939 (42) | ||||
| Negative or trace | 1794 (40) | 887 (39) | 907 (41) | ||||
| 1+ | 408 (9) | 211 (9) | 197 (9) | ||||
| ≥2+ | 382 (9) | 192 (9) | 190 (9) | ||||
| Last preadmission urine dipstick RBCs, n (%) | 4480 | 2247 | 2233 | 0.012 | |||
| Not measured | 1938 (43) | 974 (43) | 964 (43) | ||||
| Negative or trace | 1976 (44) | 986 (44) | 990 (44) | ||||
| 1+ | 327 (7) | 167 (7) | 160 (7) | ||||
| ≥2+ | 238 (5) | 120 (5) | 118 (5) | ||||
| Active ACE inhibitor user, n (%)a | 942 (21) | 4480 | 472 (21) | 2247 | 470 (21) | 2233 | 0.002 |
| Active ARB user, n (%)b | 485 (11) | 4480 | 243 (11) | 2247 | 242 (11) | 2233 | <0.001 |
| Active CCB user, n (%)b | 965 (22) | 4480 | 486 (22) | 2247 | 478 (21) | 2233 | 0.006 |
| Active α-blocker user, n (%)b | 133 (3) | 4480 | 68 (3) | 2247 | 65 (3) | 2233 | 0.008 |
| Active β-blocker user, n (%)b | 1173 (26) | 4480 | 586 (26) | 2247 | 586 (26) | 2233 | 0.003 |
| Active K-sparing diuretic user, n (%)b | 187 (4) | 4480 | 94 (4) | 2247 | 93 (4) | 2233 | <0.001 |
| Active loop diuretic user, n (%)b | 506 (11) | 4480 | 252 (11) | 2247 | 254 (11) | 2233 | 0.006 |
| Active thiazide user, n (%)b | 567 (13) | 4480 | 282 (13) | 2247 | 285 (13) | 2233 | 0.006 |
| Active peripheral vasodilator user, n (%)b | 119 (3) | 4480 | 59 (3) | 2247 | 60 (3) | 2233 | 0.002 |
| Recent acyclovir fill, n (%)c | 45 (1) | 4480 | 23 (1) | 2247 | 22 (1) | 2233 | <0.001 |
| Recent azithromycin fill, n (%)c | 1254 (28) | 4480 | 627 (28) | 2247 | 627 (28) | 2233 | 0.003 |
| Recent β-lactam fill, n (%)c | 299 (7) | 4480 | 148 (7) | 2247 | 151 (7) | 2233 | 0.007 |
| Recent cephalosporin fill, n (%)c | 193 (4) | 4480 | 97 (4) | 2247 | 96 (4) | 2233 | 0.002 |
| Recent hydroxychloroquine fill, n (%)c | 26 (1) | 4480 | 13 (1) | 2247 | 13 (1) | 2233 | 0.003 |
| Recent fluoroquinolone fill, n (%)c | 139 (3) | 4480 | 69 (3) | 2247 | 69 (3) | 2233 | 0.001 |
| Recent H2 blocker fill, n (%)c | 267 (6) | 4480 | 134 (6) | 2247 | 133 (6) | 2233 | 0.001 |
| Recent NSAID fill, n (%)c | 596 (13) | 4480 | 297 (13) | 2247 | 299 (13) | 2233 | 0.005 |
| Recent proton pump inhibitor fill, n (%)c | 1023 (23) | 4480 | 510 (23) | 2247 | 513 (23) | 2233 | 0.006 |
| Recent steroid fill, n (%)c | 459 (10) | 4480 | 232 (10) | 2247 | 227 (10) | 2233 | 0.005 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; COVID-19, coronavirus disease 2019; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ICD-9, International Classification of Diseases, Ninth Revision; NSAID, nonsteroidal anti-inflammatory drug; SBP, systolic blood pressure; SMD, standardized mean difference; W, sum of the patient weights.
The W value can be thought of as the effective number of patients in the weighted cohort created by the matching weights. The entire cohort of 7082 patients was used with patients receiving weights greater than 0 and less than or equal to 1, yielding a sum of weights equal to 4480. SMDs are the absolute difference in means or percentage divided by an evenly weighted pooled SD or difference between groups in number of SDs. For the weighted cohort, all SMDs were <0.1, indicating good balance.
DBP indicator variable for missingness not included in the logistic regression model because of collinearity with the SBP indicator variable for missingness.
Active user refers to pills on hand at admission.
Recent fill refers to fill within last 104 days.
Results
We identified 7082 patients admitted to 127 VA hospitals between October 1, 2019, and May 31, 2020, who met eligibility criteria. Of these patients, 3402 had a diagnosis of COVID-19 and 3680 had a diagnosis of influenza. The distribution of hospitalization dates is shown in Figure 2 , with most influenza hospitalizations occurring between December 2019 and March 2020, and COVID-19 hospitalizations occurring between February and May 2020.
Figure 2.
Distribution of hospitalization dates. Number of hospitalizations for coronavirus disease 2019 (COVID-19) and influenza in each month of the study period. Blue columns denote hospitalizations for influenza. Red columns denote hospitalizations for COVID-19.
Patient characteristics
The unweighted descriptions are detailed in Table 1. Patients hospitalized with COVID-19 and influenza were similar in age. Compared with influenza, patients with COVID-19 were more often African American. The prevalence rates of preadmission diabetes and hypertension were similar between the groups, as was baseline eGFR. Patients with influenza more often had a history of congestive heart failure and chronic obstructive pulmonary disease. Following weighting, the 2 groups were well balanced across demographics and comorbid conditions (Table 2).
Inpatient characteristics
In the unweighted descriptions (Supplementary Tables S2A and B), the mean oxygen saturation trough was 88.6% in the COVID-19 group and 90.7% in the influenza group. Trough oxygen saturations <88% were observed in 26% of patients with COVID-19 and 14% of patients with influenza. Mechanical ventilation was required in 17% of patients with COVID-19 and 3% of those with influenza. Vasopressors were used in 13% of patients with COVID-19 and 2% of those with influenza. Mean peak lactic acid levels were 2.5 mmol/L in the COVID-19 group and 1.9 mmol/L in the influenza group. Mean peak white blood cell counts were 11.2 × 1000/mm3 in the COVID-19 group and 9.9 × 1000/mm3 in the influenza group.
After weighting (Table 3 ), patients with COVID-19 had lower mean oxygen saturation troughs (88.8% vs. 90.9%; P < 0.001) and more frequently had oxygen troughs of <88% (25% vs. 13%; P < 0.001). The COVID-19 group more often required mechanical ventilation (17% vs. 3%; P < 0.001) and vasopressors (13% vs. 1%; P < 0.001), and had higher peak lactic acid levels (2.3 mmol/L vs. 1.9 mmol/L; P < 0.001) and white blood cell counts (11.2 × 1000/mm3 vs. 9.7 × 1000/mm3; P < 0.001). More patients in the COVID-19 group received vancomycin (24% vs. 15%; P < 0.001), whereas the influenza group had more frequent use of steroids (18% vs. 30%; P < 0.001).
Table 3.
Inpatient characteristics of weighted cohorts
| Characteristics | COVID-19–positive W = 2247 |
No. nonmissing | Influenza-positive W = 2233 |
No. nonmissing | P value |
|---|---|---|---|---|---|
| Admission systolic blood pressure, mm Hg, mean (SD) | 132 (23) | 2224 | 136 (26) | 2230 | <0.001 |
| Systolic blood pressure trough, mm Hg, mean (SD) | 102 (16) | 2224 | 108 (17) | 2230 | <0.001 |
| Admission diastolic blood pressure, mm Hg, mean (SD) | 76 (13) | 2224 | 78 (14) | 2230 | <0.001 |
| Diastolic blood pressure trough, mm Hg, mean (SD) | 57 (11) | 2224 | 60 (11) | 2230 | <0.001 |
| Admission pulse, bpm, mean (SD) | 92 (19) | 2229 | 96 (20) | 2230 | <0.001 |
| Admission O2 saturation, %, mean (SD) | 94.4 (5.2) | 2182 | 94.8 (4.1) | 2209 | 0.009 |
| O2 saturation trough, %, mean (SD) | 88.8 (7.4) | 2182 | 90.9 (4.8) | 2209 | <0.001 |
| O2 saturation trough <88%, n (%) | 553 (25) | 2182 | 289 (13) | 2209 | <0.001 |
| Mechanical ventilation, n (%) | 376 (17) | 2247 | 66 (3) | 2233 | <0.001 |
| Lactic acid (peak), mmol/L, mean (SD) | 2.3 (2.8) | 1502 | 1.9 (1.4) | 1252 | <0.001 |
| WBC count (peak), 1000/mm3, mean (SD) | 11.2 (8.3) | 2242 | 9.7 (5.5) | 2232 | <0.001 |
| Proteinuria, n (%) | 2247 | 2233 | <0.001 | ||
| Not measured | 819 (36) | 873 (39) | |||
| Negative or trace | 474 (21) | 620 (28) | |||
| 1+ | 389 (17) | 359 (16) | |||
| ≥2+ | 564 (25) | 381 (17) | |||
| New-onset proteinuria, n (%) | 370 (42) | 887a | 254 (28) | 907a | <0.001 |
| Worsened preexisting proteinuria, n (%) | 117 (29) | 403b | 72 (19) | 387b | <0.001 |
| Hematuria, n (%) | 2247 | 2233 | 0.066 | ||
| Not measured | 859 (38) | 902 (40) | |||
| Negative or trace | 808 (36) | 780 (35) | |||
| 1+ | 261 (12) | 278 (12) | |||
| ≥2+ | 319 (14) | 273 (12) | |||
| New-onset hematuria, n (%) | 273 (28) | 986a | 207 (21) | 990a | <0.001 |
| Worsened preexisting hematuria, n (%) | 65 (23) | 287b | 55 (20) | 278b | 0.347 |
| Vasopressors, n (%) | 282 (13) | 2247 | 33 (1) | 2233 | <0.001 |
| NSAIDs, n (%) | 155 (7) | 2247 | 344 (15) | 2233 | <0.001 |
| Steroids, n (%) | 394 (18) | 2247 | 669 (30) | 2233 | <0.001 |
| Vancomycin, n (%) | 543 (24) | 2247 | 330 (15) | 2233 | <0.001 |
| β-Lactams, n (%) | 451 (20) | 2247 | 386 (17) | 2233 | 0.007 |
| Cephalosporins, n (%) | 1128 (50) | 2247 | 780 (35) | 2233 | <0.001 |
| Length of stay, d, mean (SD) | 14.7 (20.4) | 2247 | 5.5 (11.0) | 2233 | <0.001 |
COVID-19, coronavirus disease 2019; NSAID, nonsteroidal anti-inflammatory drug; W, sum of the patient weights; WBC, white blood cell.
The W value can be thought of as the effective number of patients in the weighted cohort created by the matching weights. The entire cohort of 7082 (2500 with acute kidney injury [AKI]) patients was used with patients receiving weights greater than 0 and less than or equal to 1, yielding a sum of weights equal to 4480 (1485 with AKI).
Denominator of patients without preexisting proteinuria (hematuria).
Denominator of patients with preexisting proteinuria (hematuria).
Kidney outcomes
AKI incidence and severity
In the unweighted descriptions (Supplementary Table S3), the incidence of AKI was 45% in patients with COVID-19 and 27% in those with influenza. In the COVID-19 group, the distribution of AKI was 59%, 15%, and 26% for stages 1, 2, and 3, respectively. The distribution in the influenza group was 82%, 11%, and 7% for stages 1, 2, and 3, respectively. Kidney replacement therapy was required in 12% of patients with AKI in COVID-19 and 2% of those with AKI in influenza.
After weighting (Table 4 ), the incidence of AKI was higher in COVID-19 than in influenza (41% vs. 29%; P < 0.001). The overall distribution of AKI severity was also higher in COVID-19 (60%, 15%, and 26% for stages 1, 2, and 3, respectively, in COVID-19 vs. 82%, 11%, and 6% for stages 1, 2, and 3, respectively, in influenza; P < 0.001). More patients in the COVID-19 group required kidney replacement therapy (13% vs. 2%; P < 0.001).
Table 4.
Renal and other outcomes among patients with AKI, weighted cohorts
| Characteristics | COVID-19–positive W = 742 |
No. nonmissing | Influenza-positive W = 743 |
No. nonmissing | P value |
|---|---|---|---|---|---|
| Stage of AKI, n (%)a | 742 | 743 | <0.001 | ||
| 1 | 442 (60) | 613 (82) | |||
| 2 | 110 (15) | 84 (11) | |||
| 3 | 190 (26) | 46 (6) | |||
| Acute dialysis, n (%) | 94 (13) | 742 | 12 (2) | 743 | <0.001 |
| Dialysis at discharge, n (%)b | 58 (8) | 742 | 8 (1) | 743 | <0.001 |
| Peak serum creatinine, mg/dl, mean (SD) | 3.10 (2.40) | 742 | 2.15 (1.13) | 743 | <0.001 |
| Discharge serum creatinine, mg/dl, mean (SD) | 2.05 (1.87) | 742 | 1.45 (0.85) | 743 | <0.001 |
| Death in hospital, n (%)c | 226 (30) | 742 | 26 (3) | 743 | <0.001 |
| Stage 1 | 74 (17) | 442 | 10 (2) | 613 | <0.001 |
| Stage 2 | 38 (35) | 110 | 7 (8) | 84 | <0.001 |
| Stage 3 | 113 (60) | 190 | 10 (21) | 46 | <0.001 |
| Death within 90 days of peak serum creatinine, n (%)c | 263 (35) | 742 | 70 (9) | 743 | <0.001 |
| Stage 1 AKI | 100 (23) | 442 | 46 (7) | 613 | <0.001 |
| Stage 2 AKI | 42 (38) | 110 | 12 (14) | 84 | <0.001 |
| Stage 3 AKI | 120 (63) | 190 | 12 (27) | 46 | <0.001 |
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; W, sum of the patient weights.
The W value can be thought of as the effective number of patients in the weighted cohort created by the matching weights. The entire cohort of 2500 patients with AKI was used with patients receiving weights greater than 0 and less than or equal to 1, yielding a sum of weights equal to 1485.
Kidney Disease: Improving Global Outcomes (KDIGO)–modified criteria.
Defined as receiving dialysis within 48 hours of discharge.
Mortality rates stratified by AKI stage and COVID-19 versus influenza diagnosis.
Hematuria and proteinuria
Among full cohort
In the unweighted descriptions, hematuria 2+ or higher on urine dipstick occurred in 16% of patients with COVID-19 and 11% of those with influenza (Supplementary Table S2A). New-onset hematuria, defined as 1+ or higher on urine dipstick and negative or trace blood on preadmission urine dipstick, was observed in 29% of patients with COVID-19 and 18% of patients with influenza. Worsening of preexisting hematuria was observed in 22% of patients with COVID-19 and 18% of patients with influenza. Proteinuria 2+ or higher occurred in 27% of patients with COVID-19 and 15% with influenza. New-onset proteinuria, defined as 1+ or higher on urine dipstick and negative or trace protein on preadmission urine dipstick, was observed in 44% of patients with COVID-19 and 26% of patients with influenza. Worsening proteinuria compared with preadmission baseline was observed in 29% of patients with COVID-19 and 17% of patients with influenza.
In the weighted comparisons (Table 3), proteinuria was more common in patients with COVID-19 compared with influenza. Proteinuria 2+ or higher was observed in 25% of the COVID-19 group compared with 17% of the influenza group (P < 0.001). Hematuria 2+ or higher occurred in 14% of patients with COVID-19 and 12% of patients with influenza (P = 0.066). Among those with prehospitalization data available, new-onset hematuria and proteinuria were both more common in COVID-19 (hematuria, 28% vs. 21% [P < 0.001]; and proteinuria, 42% vs. 28% [P < 0.001]). Worsening of baseline hematuria was more common in COVID-19; however, this difference was not statistically significant (23% vs. 20%; P = 0.347). Worsening of baseline proteinuria was more common in COVID-19 (29% vs. 19%; P < 0.001).
Among patients with AKI
In the unweighted descriptions of patients with AKI, hematuria 2+ or higher on urine dipstick occurred in 25% of patients with COVID-19 and 18% with influenza (Supplementary Table S2B). Proteinuria 2+ or higher occurred in 40% of patients with COVID-19 and 24% with influenza. After weighting, among patients with AKI (Table 5 ), hematuria 2+ or higher occurred more often in patients hospitalized with COVID-19 compared with influenza (24% vs. 19%; P = 0.001). Proteinuria 2+ or higher was more common in patients with COVID-19 compared with patients with influenza (39% vs. 26%; P < 0.001).
Table 5.
Inpatient characteristics of patients with AKI, weighted cohorts
| Characteristics | COVID-19–positive W = 742 |
No. nonmissing | Influenza-positive W = 743 |
No. nonmissing | P value |
|---|---|---|---|---|---|
| Admission systolic blood pressure, mm Hg, mean (SD) | 129 (25) | 731 | 130 (29) | 742 | 0.549 |
| Systolic blood pressure trough, mm Hg, mean (SD) | 99 (17) | 731 | 103 (17) | 742 | <0.001 |
| Admission diastolic blood pressure, mm Hg, mean (SD) | 74 (14) | 731 | 74 (15) | 742 | 0.998 |
| Diastolic blood pressure trough, mm Hg, mean (SD) | 55 (11) | 731 | 57 (11) | 742 | <0.001 |
| Admission pulse, bpm, mean (SD) | 93 (20) | 734 | 95 (21) | 742 | 0.144 |
| Admission O2 saturation, %, mean (SD) | 93.5 (6.6) | 717 | 94.7 (4.6) | 735 | <0.001 |
| O2 saturation trough, %, mean (SD) | 87.0 (8.6) | 717 | 90.5 (5.2) | 735 | <0.001 |
| O2 saturation trough <88%, n (%) | 253 (35) | 717 | 121 (16) | 735 | <0.001 |
| Mechanical ventilation, n (%) | 255 (34) | 742 | 44 (6) | 743 | <0.001 |
| Lactic acid (peak), mmol/L, mean (SD) | 2.9 (3.9) | 605 | 2.3 (1.8) | 488 | <0.001 |
| WBC count (peak), 1000/mm3, mean (SD) | 14.6 (9.7) | 741 | 10.9 (7.1) | 743 | <0.001 |
| Proteinuria, n (%) | 742 | 743 | <0.001 | ||
| Not measured | 155 (21) | 208 (28) | |||
| Negative or trace | 138 (19) | 193 (26) | |||
| 1+ | 162 (22) | 152 (20) | |||
| ≥2+ | 287 (39) | 190 (26) | |||
| New-onset proteinuria, n (%) | 158 (67) | 236a | 97 (39) | 252a | <0.001 |
| Worsened preexisting proteinuria, n (%) | 61 (35) | 176b | 40 (23) | 170b | 0.011 |
| Hematuria, n (%) | 742 | 743 | 0.001 | ||
| Not measured | 166 (22) | 222 (30) | |||
| Negative or trace | 284 (38) | 255 (34) | |||
| 1+ | 117 (16) | 122 (16) | |||
| ≥2+ | 176 (24) | 143 (19) | |||
| New-onset hematuria, n (%) | 149 (49) | 303a | 100 (32) | 314a | <0.001 |
| Worsened preexisting hematuria, n (%) | 36 (34) | 105b | 29 (30) | 100b | 0.434 |
| Vasopressors, n (%) | 199 (27) | 742 | 28 (4) | 743 | <0.001 |
| NSAIDs, n (%) | 33 (4) | 742 | 80 (11) | 743 | <0.001 |
| Steroids, n (%) | 192 (26) | 742 | 205 (28) | 743 | 0.391 |
| Vancomycin, n (%) | 305 (41) | 742 | 166 (22) | 743 | <0.001 |
| β-Lactams, n (%) | 225 (30) | 742 | 155 (21) | 743 | <0.001 |
| Cephalosporins, n (%) | 476 (64) | 742 | 317 (43) | 743 | <0.001 |
| Length of stay, d, mean (SD) | 19.6 (22.4) | 742 | 7.4 (13.2) | 743 | <0.001 |
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; NSAID, nonsteroidal anti-inflammatory drug; W, sum of the patient weights; WBC, white blood cell.
The W value can be thought of as the effective number of patients in the weighted cohort created by the matching weights. The entire cohort of 7082 (2500 with AKI) patients was used with patients receiving weights greater than 0 and less than or equal to 1, yielding a sum of weights equal to 4480 (1485 with AKI).
Denominator of patients without preexisting proteinuria (hematuria).
Denominator of patients with preexisting proteinuria (hematuria).
AKI-related outcomes
In-hospital and 90-day mortality
In the unweighted descriptions of patients with AKI, in-hospital mortality was 32% for patients with COVID-19 and 4% for patients with influenza (Supplementary Table S3). In-hospital mortality by AKI stage in the COVID-19 group was 18%, 36%, and 62% for stages 1, 2, and 3, respectively. In-hospital mortality in the influenza group was 2%, 8%, and 24% for stages 1, 2, and 3, respectively. Mortality at 90 days following peak serum creatinine was 38% in COVID-19 group and 10% in influenza group. Mortality at 90 days by AKI stage in the COVID-19 group was 25%, 40%, and 65% for stages 1, 2, and 3, respectively. Mortality at 90 days by AKI stage in the influenza group was 8%, 16%, and 32% for stages 1, 2, and 3, respectively.
The weighted comparisons (Table 4) showed that in-hospital mortality among those with AKI was higher in the COVID-19 group (30% vs. 3%; P < 0.001). Higher in-hospital mortality was seen with more severe stages of AKI in both groups, which was more pronounced in patients with COVID-19 versus influenza (17%, 35%, and 60% vs. 2%, 8%, and 21% mortality for stages 1, 2, and 3, respectively; all comparisons, P < 0.001). Similarly, mortality at 90 days after peak serum creatinine was higher in the COVID-19 group (35% vs. 9%; P < 0.001). Increasing mortality with AKI stage was observed in both groups, with 90-day mortality frequencies of 23%, 38%, and 63% (COVID-19 group) versus 7%, 14%, and 27% (influenza group) for stages 1, 2, and 3, respectively (all comparisons, P < 0.001).
Kidney recovery
Kidney recovery analysis was limited to patients who had a prehospital baseline creatinine, with peak serum creatinine occurring at least 90 days before the end of data acquisition, and those who did not require kidney replacement therapy during hospitalization. There were 3 patients in the COVID-19 group whose peak serum creatinine occurred <90 days before the end of data acquisition and were excluded from recovery analysis.
In the unweighted descriptions of those with AKI, the proportions of patients who remained on dialysis at time of discharge were 7% of patients with COVID-19 and 1% of patients with influenza (Supplementary Table S3). The proportions of patients who recovered to within 20% of baseline serum creatinine at 90 days after peak serum creatinine were 57% of the COVID-19 group and 82% of the influenza group (Supplementary Table S4A). Among survivors at 90 days, recovery from AKI was observed in 91% in the COVID-19 group and 91% in the influenza group (Supplemental Table S4B).
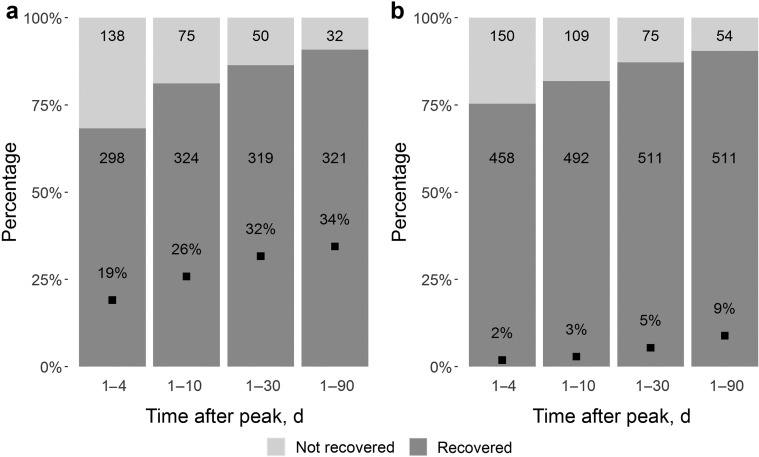
In the weighted comparisons of patients with AKI (Tables 4, 6, and 7 , Figure 3 , and Supplementary Figure S1), patients with COVID-19 had lower rates of recovery from AKI at 90 days following peak serum creatinine compared with patients with influenza (60% vs. 82%; P < 0.001). Among those who survived to 90 days following peak serum creatinine, the rates of recovery from AKI were similar (91% in COVID-19 group and 90% in influenza group; P = 0.494). Patients with COVID-19 more frequently remained on dialysis at discharge than patients with influenza (8% vs. 1%; P < 0.001).
Table 6.
Renal recovery in weighted cohort
| Variable | COVID-19–positive W = 539 |
No. nonmissing | Influenza-positive W = 619 |
No. nonmissing | P value |
|---|---|---|---|---|---|
| AKI recovery within 20% baseline serum creatinine within 90 days, n (%)a,b | 321 (60) | 539 | 511 (82) | 619 | <0.001 |
| Stage 1 | 260 (71) | 368 | 445 (86) | 518 | <0.001 |
| Stage 2 | 41 (46) | 89 | 50 (66) | 75 | <0.001 |
| Stage 3 without dialysis | 20 (25) | 82 | 16 (62) | 26 | <0.001 |
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; W, sum of the patient weights.
The W value can be thought of as the effective number of patients in the weighted cohort created by the matching weights. The entire cohort of 2500 patients with AKI was used with patients receiving weights greater than 0 and less than or equal to 1, yielding a sum of weights equal to 1485.
Restricted to patients with preadmission baseline serum creatinine, patients with peak serum creatinine occurring at least 90 days before end of data acquisition, and patients who did not require dialysis.
Recovery rates stratified by AKI stage and COVID-19 versus influenza diagnosis.
Table 7.
Renal recovery among survivors at 90 days after peak serum creatinine (weighted cohort)
| Variable | COVID-19–positive W = 441 |
No. nonmissing | Influenza-positive W = 478 |
No. nonmissing | P value |
|---|---|---|---|---|---|
| AKI recovery within 20% baseline serum creatinine within 90 days, n (%)a,b | 402 (91) | 441 | 430 (90) | 478 | 0.494 |
| Stage 1 | 325 (95) | 343 | 372 (92) | 404 | 0.111 |
| Stage 2 | 51 (79) | 65 | 42 (77) | 55 | 0.826 |
| Stage 3 without dialysis | 25 (77) | 33 | 15 (78) | 19 | 0.908 |
AKI, acute kidney injury; COVID-19, coronavirus disease 2019; W, sum of the patient weights.
The W value can be thought of as the effective number of patients in the weighted cohort created by the matching weights. The entire cohort of 1824 patients with AKI who survived 90 days after peak serum creatinine was used with patients receiving weights greater than 0 and less than or equal to 1, yielding a sum of weights equal to 1169.
Restricted to patients who survived 90 days after peak serum creatinine, patients with preadmission baseline serum creatinine, patients with peak serum creatinine occurring at least 90 days before end of data acquisition, and patients who did not require dialysis.
Recovery rates stratified by AKI stage and COVID-19 versus influenza diagnosis.
Figure 3.
Recovery from acute kidney injury (AKI) among survivors with (a) coronavirus disease 2019 (COVID-19) and (b) influenza. Proportion of patients achieving recovery from AKI of any stage (excluding patients who received acute dialysis or without a prehospital baseline creatinine) at 4, 10, 30, and 90 days following date of peak serum creatinine. Numbers in each column indicate absolute number of survivors (sum of the patient weights in the weighted cohort) at each time point in recovered (dark gray) and not recovered (light gray) groups. Square in each column denotes mortality rate. Note the higher mortality rates in the COVID-19 group and similar recovery among survivors.
Sensitivity analysis
To control for any potential seasonal, temporal, or surge effects in the early pandemic, we performed a sensitivity analysis that adjusted for admission date as a continuous variable with a 5-knot spline in the propensity score weighting. All covariates, including admission date, were balanced (all standardized mean differences, <0.1). This sensitivity analysis was consistent with the primary analysis, demonstrating robustness to temporal effects. Results from this analysis are detailed in Supplementary Table S5.
Discussion
In this study, we observed a higher incidence and severity of AKI in hospitalized veterans with COVID-19 compared with those with influenza, even after adjusting for baseline demographics and comorbidities. We also found that in-hospital mortality was higher and kidney recovery was lower among hospitalized patients with COVID-19 compared with influenza but similar among survivors. In addition, we found that proteinuria and hematuria occurred more frequently in COVID-19 but were common in both groups.
Prior studies have compared kidney outcomes in COVID-19 with other ill populations. Fisher et al. examined AKI in patients with COVID-19 compared with patients hospitalized in the same hospital 1 year prior. They identified risk factors shared between the groups, but also found that AKI was more common and severe with COVID-19.30 Our study extends on this work by focusing on a more specific control group to show that the incidence and severity of AKI in COVID-19 differ from those in patients with other severe viral respiratory illness, even after adjusting for premorbid demographics and disease. We found that AKI occurred more frequently and was more severe among those with COVID-19. The difference in AKI incidence persisted even after adjusting for baseline characteristics, suggesting that the higher rate of AKI in COVID-19 was not explained by comorbid conditions. We also observed that patients with COVID-19 were more likely to require mechanical ventilation and vasopressors and had higher in-hospital mortality than those with influenza, suggesting that higher severity of illness is likely an important contributor to these differences. These findings are consistent with a recent, smaller study that found similar rates of AKI in COVID-19 and influenza among critically ill patients with similar illness severity.31 It remains possible that the mechanisms of tubular injury in COVID-19 may be more varied and severe, reflecting more severe systemic inflammatory response, endothelial dysfunction, thrombotic microangiopathy, and direct viral invasion, although the latter remains controversial. A more diverse array of glomerular lesions has also been reported in COVID-19 compared with influenza.11 Last, a more severe presentation may accrue additional exposures that increase the risk for AKI. We observed that patients with COVID-19 received antibiotics more frequently than those with influenza, with nearly one-quarter receiving vancomycin.
Direct viral invasion of the kidneys has been hypothesized as a mechanism of AKI in COVID-19, with relatively high frequencies of proteinuria and microscopic hematuria,2 viral RNA, viral protein, and live virus detected in kidney tissue,19 , 32 , 33 as well as collapsing glomerulopathy and other lesions reported.16 , 32 , 34, 35, 36 Furthermore, a recent autopsy study correlated the presence of viral RNA in the kidney with clinical outcomes, including AKI.33 The degree to which the latter accounts for AKI in populations hospitalized with COVID-19 is unclear. We observed that hematuria and proteinuria were also common in influenza. The etiology of these findings may be multifactorial, including prevalent urine abnormalities in this comorbid VA population and possible effects of instrumentation (e.g., foley catheter insertion) during illness. Although these findings suggest that hematuria and proteinuria may not be unique to COVID-19, it remains possible that the higher rates could reflect a direct mechanism of injury due to the SARS-CoV-2 virus. A recent study in patients hospitalized with COVID-19 demonstrated evidence of proximal tubular dysfunction, including tubular proteinuria.37 Alternatively, the higher rates of hematuria and proteinuria observed in COVID-19 could suggest a similar mechanism of AKI in influenza, with a more severe pathophysiological course in COVID-19. Studies of proteinuria after AKI have demonstrated a dose-response relationship between AKI severity and subsequent proteinuria.38 , 39
Last, we found less recovery from AKI among those with COVID-19 compared with influenza, a difference likely driven by the higher mortality in COVID-19. Although this finding highlights the high mortality associated with AKI in COVID-19, it also provides some optimism for recovery in those who survive the illness, as approximately 90% of survivors of non–dialysis-requiring AKI recovered.
Our findings have important resource implications. We observed a substantial incidence of AKI in influenza, with one-quarter of patients experiencing AKI during hospitalization. It remains to be seen what the impact of mask wearing and social distancing will have on incidence of influenza in the current and future influenza seasons. Regardless, the potential added burden of AKI related to influenza in addition to AKI during hospitalization with COVID-19 could have downstream implications for resource utilization. Goldfarb et al. described their experience in the COVID-19 epicenter in the early months of the pandemic, including increased demand for kidney replacement therapy and decreased capacity to provide it, with COVID-19 affecting both their workforce (e.g., dialysis nurses) and dialysis supplies.40 Given the strain on the health system due to COVID-19, the overall increased burden of kidney disease and its resource allocation due to these illnesses will be important to project. The proportion of patients with AKI who remain dialysis dependent at discharge in our study suggests that this strain on resources may extend to outpatient dialysis centers. Another consideration is the possibility of coinfection with COVID-19 and influenza. Data from the early stages of the pandemic in China showed coinfection rates of COVID-19 with other respiratory viruses were as high as 25%.41
The strengths of our study include the use of national data, creatinine-based definitions of AKI, and rigorous attempts to adjust for baseline conditions that might influence AKI risk. Limitations include reduced generalizability to females, lack of histologic data, inability to meaningfully compare certain laboratory measurements, such as ferritin or lactate dehydrogenase, which were infrequently measured in the influenza group, and the possibility of residual confounding. The semiquantitative nature of urine dipstick measurements as well as potential differences in ascertainment and lack of reliable data on the relative use and timing of foley catheter placement between groups also need to be considered in interpreting the urinary findings. Assessment of recovery could have been impacted by ascertainment bias; however, we would suspect the longer length of stay and generally worse prognosis of COVID-19 patients to increase ascertainment in this group and potentially bias our results to the null. Last, we do not know the impact that evolving treatment of COVID-19 or admission thresholds may have had on AKI rates. One recent article examined secular trends of AKI in COVID-19 and showed a decrease in incidence of AKI over time, although it appears that severe AKI and need for dialysis remained substantial.42
In conclusion, we observed higher incidence and severity of AKI and higher mortality in veterans hospitalized with COVID-19 compared with influenza. These findings may be driven, at least in part, by severity of illness. Further studies to determine whether AKI in COVID-19 is due to a distinct pathophysiology are needed. In addition, among those who survived to 90 days, recovery from AKI was similarly high among patients infected with either illness. The relatively high incidence of AKI in both illnesses provides important information on the anticipated burden on the kidney health of veterans, and similarly ill populations, and their care providers.
Disclosure
EDS reports consulting for Akebia Therapeutics, Inc., in April 2019; honorarium for an invited education talk at the DaVita Annual Physician Leadership Conference in February 2019; royalties as an author for UptoDate, Inc.; and serves on the editorial board for the Clinical Journal of the American Society of Nephrology. All the other authors declared no competing interests.
Acknowledgments
This work was supported by the Veterans Affairs Health Services Research and Development (HSR&D) COVID-19 Rapid Response Project C19 20-214. BCB is supported by T32DK007569-32. Portions of the analytic work were supported by the Million Veteran Program grant SDR 18-194. EDS was also supported by the Vanderbilt O’Brien Kidney Center P30-DK114809 Clinical and Translational Research Core.
Author Contributions
EDS, MEM, SKP, AMP, RAG, AMH, SCS, JPA, and BCB designed the study; SKP, JD, AJV, and MEM collected the data; AMP, RAG, EDS, MEM, SKP, AMH, SCS, JPA, and BCB analyzed the data; AMP and AJV made the figures; BCB, EDS, AMP, RAG, SKP, MEM, and SCS drafted the initial manuscript, which was additionally revised by all authors. All authors approved the final version of the manuscript.
Footnotes
Table S1. Supplementary definitions.
Table S2A. Inpatient characteristics, unweighted cohort.
Table S2B. Inpatient characteristics of patients with AKI, unweighted cohort.
Table S3. Renal and other outcomes among patients with AKI, unweighted cohort.
Table S4A. Renal recovery overall, unweighted cohort.
Table S4B. Renal recovery among survivors (unweighted cohort).
Table S5. Sensitivity analysis of AKI incidence and severity adjusted for admission date.
Figure S1. Recovery from stage 2 or 3 AKI.
Supplementary Material
References
- 1.Mohamed M.M.B., Lukitsch I., Torres-Ortiz A.E. Acute kidney injury associated with Coronavirus Disease 2019 in urban New Orleans. Kidney 360. 2020;1:614–622. doi: 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J.R., Silberzweig J., Akchurin O. Characteristics of acute kidney injury in hospitalized COVID-19 patients in an urban academic medical center. Clin J Am Soc Nephrol. 2021;16:284–286. doi: 10.2215/CJN.07440520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan L., Chaudhary K., Saha A. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdulkader R.C., Ho Y.L., de Sousa Santos S. Characteristics of acute kidney injury in patients infected with the 2009 influenza A (H1N1) virus. Clin J Am Soc Nephrol. 2010;5:1916–1921. doi: 10.2215/CJN.00840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagshaw S.M., Sood M.M., Long J. Acute kidney injury among critically ill patients with pandemic H1N1 influenza A in Canada: cohort study. BMC Nephrol. 2013;14:123. doi: 10.1186/1471-2369-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indhumathi E., Krishna Makkena V., Mamidi V. Influenza A (H1N1) virus infection associated acute kidney injury – a study from a tertiary care center in South India. Saudi J Kidney Dis Transpl. 2020;31:759–766. doi: 10.4103/1319-2442.292309. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez A., Reyes L.F., Monclou J. Relationship between acute kidney injury and serum procalcitonin (PCT) concentration in critically ill patients with influenza infection. Med Intensiva. 2018;42:399–408. doi: 10.1016/j.medin.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Kute V.B., Godara S.M., Golpani K.R. High mortality in critically ill patients infected with 2009 pandemic influenza A (H1N1) with pneumonia and acute kidney injury. Saudi J Kidney Dis Transpl. 2011;22:83–89. [PubMed] [Google Scholar]
- 10.Crus-Lagunas A., Jimenez-Alvarez L., Ramirez G. Obesity and pro-inflammatory mediators are associated with acute kidney injury in patients with A/H1N1 influenza and acute respiratory distress syndrome. Exp Mol Pathol. 2014;97:453–457. doi: 10.1016/j.yexmp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Ng J.H., Bijol V., Sparks M.A. Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis. 2020;27:365–376. doi: 10.1053/j.ackd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the “cytokine storm” in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmona F., Carlotti A.P., Ramahlo L.N. Evidence of renal infection in fatal cases of 2009 pandemic influenza A (H1N1) Am J Clin Pathol. 2011;136:416–423. doi: 10.1309/AJCP1Y6LLHWSKYHW. [DOI] [PubMed] [Google Scholar]
- 14.Sevignani G., Soares M.F., Marques G.L. Acute kidney injury in patients infected by H1N1: clinical histological correlation in a series of cases. J Bras Nephrol. 2013;35:185–190. doi: 10.5935/0101-2800.20130030. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P., Uppal N.N., Wanchoo R. COVID-19-associated kidney injury: a case series of kidney biospy findings. J Am Soc Nephrol. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudose S., Batal I., Santoriello D. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2021;31:1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golmai P., Larsen C.P., DeVita M.V. Histopathological and ultrastructural findings in postmortem kidney biospy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31:1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoriello D., Khairallah P., Bomback A.S. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fihn S.D., Francis J., Clancy C. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood) 2014;33:1203–1211. doi: 10.1377/hlthaff.2014.0054. [DOI] [PubMed] [Google Scholar]
- 21.Lynch K.E., Deppen S.A., DuVall S.L. Incrementally transforming electronic medical records into the observational medical outcomes partnership common data model: a multidimensional quality assurance approach. Appl Clin Inform. 2019;10:794–803. doi: 10.1055/s-0039-1697598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scehnet J., DuVall S. VA informatics and computing infrastructure: VA COVID-19 shared data resource update. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3834-notes.pdf Available at:
- 23.Kellum J.A., Lameire N., KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siew E.D., Ikizler T.A., Matheny M.E. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7:712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohn M.W., Arnold N., Maynard C. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L., Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. 2013;9:215–234. doi: 10.1515/ijb-2012-0030. [DOI] [PubMed] [Google Scholar]
- 28.D’Agostino R.B., Jr., Rubin D.B. Estimating and using propensity scores with partially missing data. J Am Stat Assoc. 2000;95:749. [Google Scholar]
- 29.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:7–18. [Google Scholar]
- 30.Fisher M., Neugarten J., Bellin E. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31:2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobb N.L., Sathe N.A., Duan K.I. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc. 2021;18:632–640. doi: 10.1513/AnnalsATS.202007-805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kissling S., Rotman S., Gerber C. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun F., Lutgehetmann M., Pfefferle S. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396:597–598. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velez J.C.Q., Caza T., Larsen C.P. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol. 2020;16:565–567. doi: 10.1038/s41581-020-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen C.P., Bourne T.D., Wilson J.D. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H., Larsen C.P., Hernandez-Arroyo C.F. AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol. 2020;31:1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werion A., Belkhir L., Perrot M. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98:1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parr S.K., Matheny M.E., Abdel-Kader K. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int. 2018;93:460–469. doi: 10.1016/j.kint.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu C.Y., Chinchilli V.M., Coca S. Post-acute kidney injury proteinuria and subsequent kidney disease progression: the Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Study. JAMA Intern Med. 2020;180:402–410. doi: 10.1001/jamainternmed.2019.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldfarb D.S., Benstein J.A., Zhdanova O. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15:880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng Y., Luo R., Wang X. The incidence, risk factors, and prognosis of acute kidney injury in adult patients with Coronavirus Disease 2019. Clin J Am Soc Nephrol. 2020;15:1394–1402. doi: 10.2215/CJN.04650420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowe B., Cai M., Xie Y. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16:14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.