Abstract
In this unprecedented crisis of severe acute respiratory syndrome coronavirus 2 and its associated coronavirus disease 2019 (COVID-19), polymerase chain reaction and then serological testing platforms have been massively developed to face the important screening demand. Polymerase chain reaction and serological testing platforms are not the only actors impacted by the crisis, transfusion services are facing important difficulties. A positive direct antiglobulin test is frequently observed for patients encountering COVID-19. Patients with severe symptoms may develop anaemia and become good candidates for blood transfusions. The interpretation of a positive direct antiglobulin test for patients recently transfused and suffering from COVID-19 is complex. The differentiation between COVID-19 induced antibodies and possible associated transfusion alloantibodies is therefore crucial. In this context, the elution technique incorporated in an appropriate decision-making process plays its full role. This intricate topic is presented through a case report followed by literature review and finally decision-making process for COVID-19 patients necessitating red blood cells administration.
Keywords: Direct antiglobulin test, COVID-19, SARS-CoV-2, Transfusion, Elution
Abbreviations
- Ab
Antibody
- Ag
Antigen
- AHG
Anti-Human Globulin
- AIHA
Autoimmune Haemolytic Anaemia
- CAS
Cold Agglutinin Syndrome
- COVID-19
Coronavirus Disease 2019
- CRP
C-Reactive Protein
- DAT
Direct Antiglobulin Test
- ECMO
Extracorporeal Membrane Oxygenation
- IAHA
Immuno-Allergic Haemolytic Anaemia
- ICU
Intensive Care Unit
- IAS
Irregular Antibody Screening
- IAT
Indirect Antiglobulin Test
- Nl
normal
- PCR
Polymerase Chain Reaction
- RBCs
Red Blood Cells
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SOP
Standard Operating Procedure
1. Introduction: case report
1.1. Presentation
A 43-year-old man was hospitalised for hepatorenal syndrome. The patient was confirmed positive to SARS-CoV-2 at the admission by PCR testing. In the first weeks, the patient received oxygen supplementation (2L per day) and presented only minor COVID-19-related symptoms: dyspnoea on exertion, lack of appetite and asthenia. During his stay, the patient faced many cirrhosis complications such as spontaneous bacterial peritonitis, urinary tract infection, terlipressin-induced ischemic skin necrosis with associated haemorrhage, oesophagitis, polyneuropathy, hyponatremia, coagulation disorders.
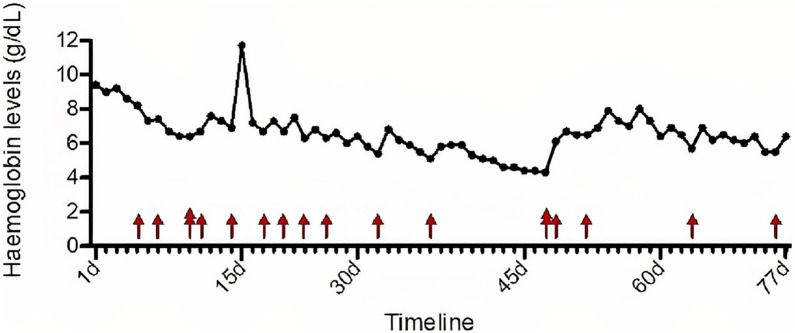
The patient presented aregenerative macrocytic anaemia requiring regular blood transfusions (see Fig. 1 ). The anaemia's origin was multifactorial: chronic inflammatory syndrome (CRP level fluctuating between 22,4 mg/L and 124,4 mg/L), hypersplenism secondary to portal hypertension, previous alcohol consumption, malnutrition, vitamin B12 and folate deficiency, impaired coagulation, acute renal impairment, liver cirrhosis and bleeding upon testicular wound. There was no argument for a manifest haemolysis and it was therefore not retained as a possible cause of anaemia (see Table 1 ). The positive DAT was not associated with the presence of warm autoantibodies by the clinicians/laboratory (see Table 2 for the timeline). The blood smears did not show significative number of schistocytes but showed high number of target cells and significant number of knizocytes along with the formation of few rouleaux and a striking poikilocytosis (see Fig. 2 ). Knizocytes, rouleaux formation and poikilocytosis have been recently associated with COVID-19 [1]. Target cells are frequently observed in liver diseases. These red blood cell morphological features were not observed anymore after COVID-19 episode (see Fig. 2). LDH and indirect bilirubin levels were elevated but constantly decreasing and were most likely due to severe hepatic impairment. Reticulocytes were very low (from 2 to 4 *103/μL with Nl 30–100 *103/μL)) but should be assessed carefully considering patient's acute renal impairment. After injection of darbepoetin alfa 100 μg/0,5 ml, reticulocyte count increased to 191*103/μL and then lessened back to normal values. Haptoglobin levels were measured twice with normal to slightly increased values (1,91 g/L and 2,03 g/L with Nl 0,30–2,00 g/L). However, the haptoglobin measurements should be interpreted with caution because of hepatic impairment and inflammatory status that respectively decrease and upregulate haptoglobin production. Haemoglobinuria and bilirubinuria trends varied from positive to negative but should again be analysed carefully considering the acute renal impairment with acute tubular necrosis. Bone marrow aspirate examination did not reveal erythroid regeneration in response to anaemia and showed important inflammation features. Increase of proerythroblasts in the bone marrow is normally observed in response to anaemia as they are highly sensitive to erythropoietin secreted by the kidney under hypoxic conditions. However, our patient suffered from acute renal impairment leading to diminished EPO secretion.
Fig. 1.
Time follow-up of haemoglobin levels and administration of red blood cells.
Table 1.
Haemolysis parameters.
| Parameter | Observation | Argument for Haemolysis |
|---|---|---|
| Reticulocyte | From 2 to 4 *103/μL (Nl: 30–100 *103/μL) | Should be elevated but acute renal impairment may diminish EPO levels |
| Haptoglobin | 1,91 g/L and 2,03 g/L (Nl: 0,30–2,00 g/L) | Should be collapsed but hepatic impairment decreases haptoglobin synthetizing function while inflammatory syndrome has upregulated effect on haptoglobin level |
| LDH | Elevated but constantly decreasing | Should be increased but levels lessen correlated to hepatic function improvement |
| Bilirubin | Elevated but constantly decreasing | Should be increased but levels lessen correlated to hepatic function improvement |
| Haemoglobinuria and bilirubinuria | Not significant | May be observed in severe haemolysis |
| Blood smear | No significative number of schistocytes | Generally positive in AIHA |
| DAT | Positive | Positive in AIHA |
EPO: Erythropoietin/DAT: Direct Antiglobulin Test/AIHA: Auto-Immune Haemolytic Anaemia.
Table 2.
Testing performed by the hospital blood transfusion service.
| Days since hospitalisation | Polyvalent DAT | IgG DAT | C3d DAT | IAS | Plasma antibody screen | Elution antibody screen |
|---|---|---|---|---|---|---|
| 25 days | + | + | + | – | NA | Not specific in IAT |
| 31 days | + | + | + | + | Not specific in IAT | Anti-Jka + non specific reaction |
| 36 days | + | + | + | + | Anti-K and anti-Jka in IAT | NP |
| 44 days | NP | NP | NP | + | Anti-K and anti-Jka in IAT | NA |
| 47 days | + | + | + | + | Anti-K and anti-Jka in IAT and ficine | NP |
| 55 days | + | + | – | + | Anti-K and anti-Jka in IAT | Anti-K and anti-Jka in IAT |
| 58 days | + | + | – | + | Anti-K and anti-Jka with dosage effect in IAT and ficine | Anti-K and anti-Jka in IAT |
| 65 days | NP | + | – | + | Anti-K in IAT | NP |
DAT: Direct Antiglobulin Test/IAS: Irregular Antibody Screening/IAT: Indirect Antiglobulin Test/NA: Not Applicable/NP: Not Performed.
Fig. 2.
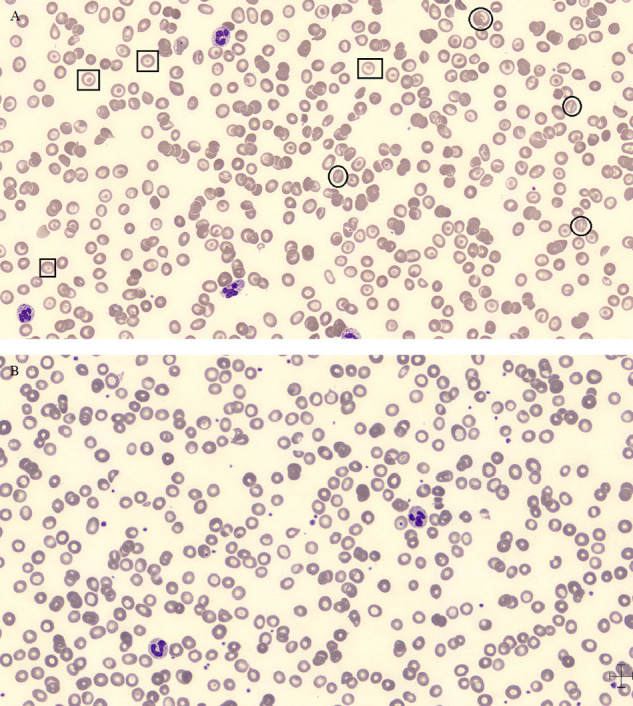
Blood smear features during and after COVID-19 episode. A. Blood smear features during COVID-19 episode. Squares = Target cells; Circles = Knizocytes. B. Normalisation of blood smear features after resolution of COVID-19 episode.
1.2. Testing performed at the hospital blood transfusion service
ABO group control and irregular antibody screening (IAS) were performed before any transfusion of red blood cells (RBCs). IAS had already been tested negative 15 times before first evidence of antibody. A few days before patient's first positive IAS, DAT was performed to investigate the origin of chronic anaemia as the cause was still not obvious for clinicians. The DAT was mixed positive with IgG and C3d specificity. At that time either a positive DAT in a context of AIHA or a post-transfusion alloantibody was suspected but there was no significant manifestation of haemolysis. An acid elution process was performed but the detected immunoglobulins showed neither specificity nor panagglutination. Six days later, IAS and DAT were tested again. This was the first positive IAS for this patient and an antibody screen was performed both with plasma and eluate. The indirect antiglobulin test (IAT) was non-specific with the plasma but an anti-Jka alloantibody was discovered in the eluate. The elution process allowed the hospital blood transfusion service to consider the presence of a transfusion alloantibody and therefore to select Jka negative cross-matched RBCs for patient's safety. The IAT with the plasma revealed four days later the anti-Jka alloantibody but also the appearance of an anti-K alloantibody (see Table 2).
This case report triggers the importance of performing an elution process for poly-transfused patients when an IgG-type DAT is evidenced. The appearance of an alloantibody in the plasma may be delayed if all antibodies are fixed upon their respective antigen at the surface of the RBC membrane. The elution process is a high-burden working process and an appropriate decision-making algorithm is therefore important to limit the situations where it is effectively applicable. It is even more important in this current context in which the patients infected by SARS-COV-2 often present a positive DAT and are prone to multiple transfusions.
2. Discussion
2.1. DAT basics
About seventy-five years ago, the Direct Antiglobulin Test (DAT) was described by R. Coombs who had the innovative idea to use a second antibody, a rabbit AHG, to amplify the agglutination between RBCs sensitized by antibodies [2]. This laboratory testing is now widely used and offers valuable information in many differential diagnosis. The DAT has been slightly redesigned over time. It is still performed by traditional tube agglutination which is the reference method but new more sensitive testing methods now exist such as gel microcolumn method [3], [4], [5], solid-phase method or more recently flow cytometry [6], [7], [8]. Nowadays, the DAT consists of testing patient's RBCs to investigate the presence of immunoglobulins or complement proteins at the membrane's surface. The test is performed firstly using polyspecific reagents (recognizing both immunoglobulin and complement fragments) and subsequently with monospecific reagents if the polyspecific reagent shows RBCs agglutination [9], [10]. Main situations resulting in positive DAT are Autoimmune Haemolytic Anaemia (AIHA), Immunoallergic Haemolytic Anaemia (IAHA), Alloimmune-mediated Haemolytic Transfusion Reaction, and Haemolytic disease of the foetus/newborn [9], [10], [11]. Recently, correlation between COVID-19 and positive DAT has been established.
2.2. Positive DAT in COVID-19 patients
Sensitisation of RBCs by immunoglobulins or complement proteins has been reported several times in different infectious contexts, such as viral and bacterial infections. Infection by Mycoplasma pneumoniae has been frequently associated with autoimmune haemolytic anaemia induced by cold agglutinins that fix complement with anti-I and anti-i specificity [12], [13], [14], [15]. This phenomenon, also called Cold Agglutinin Syndrome (CAS), is a secondary AIHA [16]. Infectious mononucleosis by Epstein-Barr virus has also been frequently associated with CAS [17], [18], [19], [20], [21]. Similarly, several authors have reported the presence of positive DAT consequently to other infectious agents such as Cytomegalovirus [22], [23], [24], [25], Plasmodium [26], [27], [28], B19 parvovirus [29], human immunodeficiency virus-1 [30], [31], [32], Brucella [33], [34], [35], [36], adenovirus [37], Influenza A [38], [39], Varicella virus [40], [41], [42], Rubella virus [43], [44], [45], [46], Legionella pneumophila [47], [48] and Chlamydia pneumoniae [49], [50].
Positive DAT has recently been observed among SARS-CoV-2 infected patients. Case reports associating SARS-CoV-2 infection with development of secondary AIHA are continuously increasing in the literature. Some authors report the appearance of secondary AIHA with a positive DAT showing complement specificity (CAS) [51], [52], [53]. Other papers report a mixed-type AIHA [54], [55], [56], [57], [58]. Finally, one case report describes secondary pure-IgG DAT in post-COVID-19 AIHA [59]. The presence of other pathogens responsible of respiratory infections had been excluded in most case reports, supporting the relationship between the SARS-CoV-2 infection and secondary AIHA [51], [53], [54], [55], [58], [59]. This has been described both in adult and paediatric populations. A case series of seven patients showed positive DAT in all cases, 2 for IgG, 2 for C3d and 3 were mixed type. The authors concluded that anti-erythrocyte antibodies were warm antibodies in 4 cases and cold agglutinins in 3 cases [60]. Platton et al. collected blood samples of 20 patients hospitalised in intensive care units confirmed for SARS-CoV-2 infection by PCR and 20 patients hospitalised in intensive care units negative for SARS-CoV-2 infection. They observed a positive DAT in 80% of infected patients against 35% in non-infected patients. In the infected-patient group, the DAT was always IgG-positive except in one, which was positive for C3d. Eluates didn’t show any specificity when tested by IAT [61]. One larger scale study has been published by Berzuini et al. studying 113 patients infected by SARS-CoV-2. They found 52 of 113 (46%) patients who presented a positive DAT using a microcolumn screening assay, with 88% of IgG specificity. Four patients were tested positive for both IgG and C3d (8%) while only 2 patients were positive for C3d. Positive DAT was confirmed either by a second agglutination technique or by flow cytometry. As demonstrated by Platton et al., the eluates of DAT-positive patients did not react against panel of reagent RBCs [62]. Results from our hospital internal study among 225 SARS-CoV-2 confirmed patients (99 from ICU and 126 in classic hospitalisation wards (CHW)) showed a positive rate for DAT of 44% [63]. The positivity rate was significantly higher in the ICU patient group (56% positivity) against 35% in other units. A total of 58,8% of positive DAT showed IgG-specificity, 32,4% a mixed IgG-C3d specificity while only 8,8% were C3d specific. Same conclusion was drawn for eluates, which were not specific against panel of reagent RBCs.
Results of all these case reports and studies demonstrate a high percentage of positive DAT among patients with COVID-19. The case reports with demonstrated clinical and biological haemolysis tend to demonstrate rather C3d or mixed-type DAT. However, the number of patients studied is very limited. In larger scale studies, most COVID-19 patients show IgG-specific DAT and in a lesser extent mixed IgG-C3d specificity. Clinical or biological manifestations of AIHA are not always observed in these substantial studies.
Warm and cold AIHA associated with SARS-CoV-2 infection occur after the beginning of the infection and seem to correlate with the cytokine storm [59]. Underlying mechanisms behind positive DAT among COVID-19 patients are currently not well understood. Several hypotheses have been proposed such as molecular mimicry [64], [65], hyperinflammation induced by cytokine storm or alteration of the RBCs membrane with exposition of cryptic antigens [66], [67]. Bastard et al. recently discovered auto-Abs against type I interferon among patients with life-threatening COVID-19 pneumonia [68].
2.3. COVID-19, haemoglobin drop, transfusion need, alloimmunisation and positive DAT
Lower haemoglobin levels have been demonstrated among patients infected by SARS-CoV-2. Algassim et al. found significantly lower haemoglobin levels in SARS-CoV-2 infected patients admitted in ICU compared to those in CHW. Drop in haemoglobin levels is associated with poorer prognosis. One hundred sixty-three patients out of 250 (65%) were anaemic in ICU against 111 out of 257 (43%) in CHW [69]. Lower haemoglobin levels for severe COVID-19 have been highlighted in many other studies [70], [71], [72], [73]. A meta-analysis about anaemia in COVID-19 collected data from 40,450 patients. The mean haemoglobin level was 12,97 g/dL but significantly lower levels were observed in severe COVID-19 cases [74]. Berzuini et al. reported that 44 of 113 COVID-19 patients received at least one blood transfusion with significantly higher percentage among patients presenting a positive DAT [62]. In other studies, the percentage of COVID-19 patients needing blood transfusion varies between 6,2 and 11,1% [75], [76], [77], [78]. Blood transfusion needs for COVID-19 patients are related to extracorporeal membrane oxygenation (ECMO) and coagulation dysfunction [77].
Multiple transfusions and positive DAT among COVID-19 patients complicate immune-hematologic investigations for hospital blood transfusion services. Some situations justify the need to exclude the presence of underlying alloantibodies when DAT is positive. For example, further investigation is required when the patient presents post-transfusion haemolysis signs or in case of insufficient post-transfusion increment of haemoglobin levels. The Kidd (Jk) blood group may generate difficult situations as illustrated by our case report [79]. The elution process is appropriate in the sense that it eluates the potential alloantibodies coated on RBCs while COVID-19 associated antibodies don’t give any RBC antigen specificity on commercial panels after elution [61], [62]. COVID-19 autoantibodies are probably attached with low affinity at the RBC membrane and they may be removed during washing step of the elution process. Berzuini et al. also observed that eluates from COVID-19 patients did not react against commercial panel RBCs while they reacted against RBCs prepared from DAT-negative COVID-19 patients. The hyperinflammation state during the disease probably modifies the RBC membrane with exposure of cryptic antigens allowing the binding of complement proteins or immunoglobulins [62]. A decision-making process should be elaborated in hospital blood transfusion services for COVID-19 patients presenting a positive DAT and needing blood transfusion.
2.4. Elaboration of a decision-making process for DAT-positive COVID-19 patients needing RBCs administration
The algorithm should include DAT-positive COVID-19 patients that will need immediately or in the near future administration of red blood cells because of post-transfusion haemolysis risks. The transfusion timeline may be adequate as a first step in the algorithm. Post-transfusion alloantibodies usually appear 2 to 3 days after the transfusion and elution process is thus not necessary if RBCs have been transfused for less than 3 days. The potential alloantibodies are first entirely fixed on RBCs. They are not circulant yet in the plasma/serum and elution process may therefore be justified. We assume that any transfusion alloantibody will appear in the plasma or serum of the patient after 30 days, which renders the elution process worthless after that timeframe. Serum investigation must always be conducted in parallel. IAT should be performed after a positive IAS.
In addition to the timeline, the high-burden load of elution process should be taken into consideration when designing the decision-making process. This why clinical and biological haemolysis parameters along with post-transfusion haemoglobin level monitoring are further steps to be considered into the decision-making process before conducting elution techniques. These criteria should be discussed with clinicians and appropriate follow-up of patients should be in place. When the algorithm triggers the start of the elution process, the result should be documented for future consideration if other transfusions are required. If the elution process results in the identification of a transfusion antibody, hospital blood transfusion services should take appropriate measures such as respect of patient RhK phenotype and serologic cross-match between donor red blood cells and patient eluate/plasma.
There are similarities between the plasma of AIHA patients and SARS-CoV 2 patients. Both can present a positive DAT and may be polytransfused. Furthermore, it has been proven that patients with AIHA are at risk of developing transfusion alloantibody even when antigen-matched units are selected [80]. Autoantibodies and alloantibodies coexist in about 1/3 of patients with AIHA [81]. Including the AIHA on this decision-making process would be a potential improvement. However, AIHA management is not exactly similar. The autoantibodies present in AIHA have often panagglutinin specificity after elution process while COVID-19 autoantibodies are removed and don’t give any agglutination when eluate is tested. Auto-adsorption is often required to get rid of the autoantibodies in AIHA in order to determine the presence and the specificity of potential alloantibodies [82], [83].
3. Conclusion
Tailored decision-making process can be implemented in hospital blood transfusion services to help with the management of polytransfused COVID-19 patients presenting a positive DAT. Adequate management process should be in place to avoid severe haemolytic transfusion reactions. This paper aims to help blood transfusion services in their investigations and therefore to improve the quality of patients treatment.
Authorship contributions
J.C. was the first author and the designer of decision-making processes. A.B., P.S., M.v.D. and V.D. contributed to the design of the decision-making process and reviewed the manuscript structure and contents. L.C. and B.D. contributed to the review of the clinical aspects of the paper.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We thank the blood transfusion hospital staff from our institution for their assistance with decision-making process elaboration and daily application.
References
- 1.Berzuini A., Bianco C., Migliorini A.C., Maggioni M., Valenti L., et al. Red blood cell morphology in patients with COVID-19-related anaemia. Blood Transfus. 2021;19:34–36. doi: 10.2450/2020.0242-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coombs R.R.A., Mourant A.E., Race R.R. A new test for the detection of weak and incomplete Rh agglutinins. Br J Exp Pathol. 1945;26:255–266. [PMC free article] [PubMed] [Google Scholar]
- 3.Novaretti M.C., Jens E., Pagliarini T., Bonifacio S.L., Dorlhiac-Llacer P.E., et al. Comparison of conventional tube test technique and gel microcolumn assay for direct antiglobulin test: a large study. J Clin Lab Anal. 2004;18:255–258. doi: 10.1002/jcla.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S.S., Chaudhary R., Khetan D. A comparison of conventional tube test and gel technique in evaluation of direct antiglobulin test. Hematology. 2007;12:175–178. doi: 10.1080/10245330601111862. [DOI] [PubMed] [Google Scholar]
- 5.Dittmar K., Procter J.L., Cipolone K., Njoroge J.M., Miller J., et al. Comparison of DATs using traditional tube agglutination to gel column and affinity column procedures. Transfusion. 2001;41:1258–1262. doi: 10.1046/j.1537-2995.2001.41101258.x. [DOI] [PubMed] [Google Scholar]
- 6.Stroncek D.F., Njoroge J.M., Procter J.L., Childs R.W., Miller J. A preliminary comparison of flow cytometry and tube agglutination assays in detecting red blood cell-associated C3d. Transfus Med. 2003;13:35–41. doi: 10.1046/j.1365-3148.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 7.Alzate M.A., Manrique L.G., Bolaños N.I., Duarte M., Coral-Alvarado P., et al. Simultaneous detection of IgG, IgM, IgA complexes and C3d attached to erythrocytes by flow cytometry. Int J Lab Hematol. 2015;37:382–389. doi: 10.1111/ijlh.12297. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary R., Das S.S., Gupta R., Khetan D. Application of flow cytometry in detection of red-cell-bound IgG in Coombs-negative AIHA. Hematology. 2006;11:295–300. doi: 10.1080/10245330600915958. [DOI] [PubMed] [Google Scholar]
- 9.Parker V., Tormey C.A. The direct antiglobulin test: indications, interpretation, and pitfalls. Arch Pathol Lab Med. 2017;141:305–310. doi: 10.5858/arpa.2015-0444-RS. [DOI] [PubMed] [Google Scholar]
- 10.Zantek N.D., Koepsell S.A., Tharp D.R., Jr., Cohn C.S. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707–709. doi: 10.1002/ajh.23218. [DOI] [PubMed] [Google Scholar]
- 11.Theis S.R., Hashmi M.F. StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC; Treasure Island (FL): 2020. Coombs Test. StatPearls. [Google Scholar]
- 12.Stein B., DeCredico N., Hillman L. Evaluation of the Direct Antiglobulin Test (DAT) in the Setting of Mycoplasma pneumoniae Infection. JAMA. 2018;319:1377–1378. doi: 10.1001/jama.2018.1969. [DOI] [PubMed] [Google Scholar]
- 13.Fischer B.G., Baduashvili A., Evans A.T. Cold Agglutinins in Mycoplasma Infection. JAMA. 2018;320:1038–1039. doi: 10.1001/jama.2018.9249. [DOI] [PubMed] [Google Scholar]
- 14.Khan F.Y., Yassin M.A. Mycoplasma pneumoniae associated with severe autoimmune hemolytic anemia: case report and literature review. Braz J Infect Dis. 2009;13:77–79. doi: 10.1590/s1413-86702009000100018. [DOI] [PubMed] [Google Scholar]
- 15.Han X., He B., Wang F. [Mycoplasma pneumonia associated with hemolytic anemia: case report and literature review] Zhonghua Jie He He Hu Xi Za Zhi. 2011;34:832–836. [PubMed] [Google Scholar]
- 16.Berentsen S., Randen U., Tjønnfjord G.E. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 2015;29:455–471. doi: 10.1016/j.hoc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Wentworth P., Bate L.R. Acute hemolytic anemia secondary to infectious mononucleosis. Can Med Assoc J. 1980;123:482–486. [PMC free article] [PubMed] [Google Scholar]
- 18.Gurol F. Acute hemolytic anemia complicating infectious mononucleosis. Mich Med. 1966;65:22–24. [PubMed] [Google Scholar]
- 19.Mantadakis E., Chatzimichael E., Kontekaki E., Panopoulou M., Martinis G., et al. EBV-related Cold Agglutinin Disease Presenting With Conjugated Hyperbilirubinemia: A Pediatric Case Report and Mini Review. J Pediatr Hematol Oncol. 2019;41:324–327. doi: 10.1097/MPH.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 20.Fadeyi E.A., Simmons J.H., Jones M.R., Palavecino E.L., Pomper G.J. Fatal autoimmune hemolytic anemia due to immunoglobulin g autoantibody exacerbated by epstein-barr virus. Lab Med. 2015;46:55–59. doi: 10.1309/LM9OWRF64OGQODEA. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins W.J., Koster H.G., Marsh W.L., Carter R.L. Infectious mononucleosis: an unsuspected source on anti-I. Br J Haematol. 1965;11:480–483. doi: 10.1111/j.1365-2141.1965.tb06611.x. [DOI] [PubMed] [Google Scholar]
- 22.Salloum E., Lundberg W.B. Hemolytic anemia with positive direct antiglobulin test secondary to spontaneous cytomegalovirus infection in healthy adults. Acta Haematol. 1994;92:39–41. doi: 10.1159/000204136. [DOI] [PubMed] [Google Scholar]
- 23.Khalifeh H.K., Mourad Y.M., Chamoun C.T. Infantile Cytomegalovirus-Associated Severe Warm Autoimmune Hemolytic Anemia: A Case Report. Children (Basel) 2017, 94;4 doi: 10.3390/children4110094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berlin B.S., Chandler R., Green D. Anti-“i” antibody and hemolytic anemia associated with spontaneous cytomegalovirus mononucleosis. Am J Clin Pathol. 1977;67:459–461. doi: 10.1093/ajcp/67.5.459. [DOI] [PubMed] [Google Scholar]
- 25.Murray J.C., Bernini J.C., Bijou H.L., Rossmann S.N., Mahoney D.H., Jr., et al. Infantile cytomegalovirus-associated autoimmune hemolytic anemia. J Pediatr Hematol Oncol. 2001;23:318–320. doi: 10.1097/00043426-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Facer C.A. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. II. Specificity of erythrocyte-bound IgG. Clin Exp Immunol. 1980;39:279–288. [PMC free article] [PubMed] [Google Scholar]
- 27.Abdalla S., Weatherall D.J. The direct antiglobulin test in P. falciparum malaria. Br J Haematol. 1982;51:415–425. doi: 10.1111/j.1365-2141.1982.tb02797.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson A.S., Delisca G., Booth G.S. Warm autoimmune hemolytic anemia secondary to Plasmodium ovale infection: a case report and review of the literature. Transfus Apher Sci. 2013;49:571–573. doi: 10.1016/j.transci.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Iaguzhinskaia O.E., Fevraleva I.S., Elizhbaeva M.A., Levina A.A., Semenova G.M., et al. [Secondary autoimmune hemolytic anemia as a result of B19 parvovirus persistence in immunodeficient patients] Ter Arkh. 2011;83:62–68. [PubMed] [Google Scholar]
- 30.Koduri P.R., Singa P., Nikolinakos P. Autoimmune hemolytic anemia in patients infected with human immunodeficiency virus-1. Am J Hematol. 2002;70:174–176. doi: 10.1002/ajh.10096. [DOI] [PubMed] [Google Scholar]
- 31.Telen M.J., Roberts K.B., Bartlett J.A. HIV-associated autoimmune hemolytic anemia: report of a case and review of the literature. J Acquir Immune Defic Syndr (1988) 1990;3:933–937. [PubMed] [Google Scholar]
- 32.Saif M.W. HIV-associated autoimmune hemolytic anemia: an update. AIDS Patient Care STDS. 2001;15:217–224. doi: 10.1089/10872910151133783. [DOI] [PubMed] [Google Scholar]
- 33.Sari I., Kocyigit I., Altuntas F., Kaynar L., Eser B. An unusual case of acute brucellosis presenting with Coombs-positive autoimmune hemolytic anemia. Intern Med. 2008;47:1043–1045. doi: 10.2169/internalmedicine.47.1000. [DOI] [PubMed] [Google Scholar]
- 34.Meena D.S., Sonwal V.S., Rohila A.K., Meena V. Acute Brucellosis Presenting as an Autoimmune Hemolytic Anemia. Case Rep Infect Dis. 2018;2018:1030382. doi: 10.1155/2018/1030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eskazan A.E., Dal M.S., Kaya S., Dal T., Ayyildiz O., et al. Two cases of autoimmune hemolytic anemia secondary to brucellosis: a review of hemolytic disorders in patients with brucellosis. Intern Med. 2014;53:1153–1158. doi: 10.2169/internalmedicine.53.0936. [DOI] [PubMed] [Google Scholar]
- 36.Mak W.W., Adrian M.M., Ahlam K. Brucellosis-induced autoimmune haemolytic anaemia (AIHA) Med J Malaysia. 2019;74(5):344–443. [PubMed] [Google Scholar]
- 37.Mori T., Yamada Y., Aisa Y., Uemura T., Ishida A., et al. Cold agglutinin disease associated with adenovirus infection after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2005;36:263–264. doi: 10.1038/sj.bmt.1705029. [DOI] [PubMed] [Google Scholar]
- 38.Shizuma T. [A case of autoimmune hemolytic anemia caused by type A influenza infection in a patient with alcoholic liver cirrhosis] Kansenshogaku Zasshi. 2010;84:296–299. doi: 10.11150/kansenshogakuzasshi.84.296. [DOI] [PubMed] [Google Scholar]
- 39.Schoindre Y., Bollée G., Dumont M.D., Lesavre P., Servais A. Cold agglutinin syndrome associated with a 2009 influenza A H1N1 infection. Am J Med. 2011;124:e1–e2. doi: 10.1016/j.amjmed.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Kumar K.J., Kumar H.C., Manjunath V.G., Arun V. Autoimmune Hemolytic Anemia due to Varicella Infection. Iran J Pediatr. 2013;23:491–492. [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchis Cervera J., Carbonell Uberos F. Autoimmune hemolytic anemia with anti-DC specificity following a primary infection by Varicella virus. Haematologica. 1997;82:508–509. [PubMed] [Google Scholar]
- 42.Johnson A.M. Cold agglutinin disease after chickenpox. Am J Clin Pathol. 1992;98:271–272. doi: 10.1093/ajcp/98.2.271b. [DOI] [PubMed] [Google Scholar]
- 43.König A.L., Schabel A., Sugg U., Brand U., Roelcke D. Autoimmune hemolytic anemia caused by IgG lambda-monotypic cold agglutinins of anti-Pr specificity after rubella infection. Transfusion. 2001;41:488–492. doi: 10.1046/j.1537-2995.2001.41040488.x. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal N., Naithani R., Mahapatra M. Rubella infection with autoimmune hemolytic anemia. Indian J Pediatr. 2007;74:495–496. doi: 10.1007/s12098-007-0085-z. [DOI] [PubMed] [Google Scholar]
- 45.König A.L., Schabel A., Sugg U., Brand U., Roelcke D. [Autoimmune hemolytic anemia caused by cold agglutinins of the anti-Pr specificity after rubella infection] Beitr Infusionsther Transfusionsmed. 1996;33:26–29. [PubMed] [Google Scholar]
- 46.Brody M., Kreysel H.W. [Cold agglutinin syndrome after rubella infection] Kinderarztl Prax. 1992;60:134–136. [PubMed] [Google Scholar]
- 47.Durrance R.J., Das Gracas F., Sivamurthy S., Singh B.B. Legionella-Induced Autoimmune Hemolytic Anemia: A Delayed and Unexpected Complication. J Hematol. 2019;8:44–45. doi: 10.14740/jh487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strikas R., Seifert M.R., Lentino J.R. Autoimmune hemolytic anemia and Legionella pneumophila pneumonia. Ann Intern Med. 1983;99:345. doi: 10.7326/0003-4819-99-3-345. [DOI] [PubMed] [Google Scholar]
- 49.Belda J., Romero A., Caliz A. [Chlamydia pneumoniae infection associated with autoimmune hemolytic anemia due to warm antibodies] Arch Bronconeumol. 1996;32:251–252. doi: 10.1016/s0300-2896(15)30772-9. [DOI] [PubMed] [Google Scholar]
- 50.Berentsen S., Tjønnfjord G.E. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107–115. doi: 10.1016/j.blre.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Capes A., Bailly S., Hantson P., Gerard L., Laterre P.F. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol. 2020;99:1679–1680. doi: 10.1007/s00277-020-04137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jawed M., Hart E., Saeed M. Haemolytic anaemia: a consequence of COVID-19. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-238118. e238118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patil N.R., Herc E.S., Girgis M. Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection. Hematol Oncol Stem Cell Ther. 2020;20:30113–30116. doi: 10.1016/j.hemonc.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez C., Kim J., Pandey A., Huang T., DeLoughery T.G. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190:31–32. doi: 10.1111/bjh.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wahlster L., Weichert-Leahey N., Trissal M., Grace R.F., Sankaran V.G. COVID-19 presenting with autoimmune hemolytic anemia in the setting of underlying immune dysregulation. Pediatr Blood Cancer. 2020;67:e28382. doi: 10.1002/pbc.28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenzweig J.D., McThenia S.S., Kaicker S. SARS-CoV-2 infection in two pediatric patients with immune cytopenias: a single institution experience during the pandemic. Pediatr Blood Cancer. 2020;67:e28503. doi: 10.1002/pbc.28503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huscenot T., Galland J., Ouvrat M., Rossignol M., Mouly S., et al. SARS-CoV-2-associated cold agglutinin disease: a report of two cases. Ann Hematol. 2020;99:1943–1944. doi: 10.1007/s00277-020-04129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zagorski E., Pawar T., Rahimian S., Forman D. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID-19) Br J Haematol. 2020;190:e183–e184. doi: 10.1111/bjh.16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vega Hernández P., Borges Rivas Y., Ortega Sánchez E., Marqués Cabrero A., Remedios Mateo L., et al. Autoimmune Hemolytic Anemia in a Pediatric Patient With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Pediatr Infect Dis J. 2020;39:e288. doi: 10.1097/INF.0000000000002809. [DOI] [PubMed] [Google Scholar]
- 60.Lazarian G., Quinquenel A., Bellal M., Siavellis J., Jacquy C., et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190:29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Platton S., Mendes N., Booth C., Lancut J., Lee K., et al. Positive direct antiglobulin tests in patients with COVID-19. Transfusion. 2020;61:333–334. doi: 10.1111/trf.16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berzuini A., Bianco C., Paccapelo C., Bertolini F., Gregato G., et al. Red cell-bound antibodies and transfusion requirements in hospitalized patients with COVID-19. Blood. 2020;136:766–768. doi: 10.1182/blood.2020006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brochier A., Cabo J., Guerrieri C., Belkhir L., Laterre P.-F., et al. Autoimmune hemolytic anemia in COVID-19 patients, the « transmissible » direct Coombs test. J Hematol Clin Res. 2021;5:004–008. [Google Scholar]
- 64.Angileri F., Légaré S., Marino Gammazza A., Conway de Macario E., Macario A.J.L., et al. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br J Haematol. 2020;190:e92–e93. doi: 10.1111/bjh.16883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angileri F., Legare S., Marino Gammazza A., Conway de Macario E., Jl Macario A., et al. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun Rev. 2020;19:102591. doi: 10.1016/j.autrev.2020.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hendrickson J.E., Tormey C.A. COVID-19 and the Coombs test. Blood. 2020;136:655–656. doi: 10.1182/blood.2020007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berentsen S. New Insights in the Pathogenesis and Therapy of Cold Agglutinin-Mediated Autoimmune Hemolytic Anemia. Front Immunol. 2020;11:590. doi: 10.3389/fimmu.2020.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Algassim A.A., Elghazaly A.A., Alnahdi A.S., Mohammed-Rahim O.M., Alanazi A.G., et al. Prognostic significance of hemoglobin level and autoimmune hemolytic anemia in SARS-CoV-2 infection. Ann Hematol. 2021;100:37–43. doi: 10.1007/s00277-020-04256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mudatsir M., Fajar J.K., Wulandari L., Soegiarto G., Ilmawan M., et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Res. 2020;9:1107. doi: 10.12688/f1000research.26186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu G., Wang J. Dynamic changes in routine blood parameters of a severe COVID-19 case. Clin Chim Acta. 2020;508:98–102. doi: 10.1016/j.cca.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 73.Ghahramani S., Tabrizi R., Lankarani K.B., Kashani S.M.A., Rezaei S., et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25:30. doi: 10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taneri P.E., Gómez-Ochoa S.A., Llanaj E., Raguindin P.F., Rojas L.Z., et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(8):763–773. doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Velázquez-Kennedy K., Luna A., Sánchez-Tornero A., Jiménez-Chillón C., Jiménez-Martín A., et al. Transfusion support in COVID-19 patients: Impact on hospital blood component supply during the outbreak. Transfusion. 2020;61:361–367. doi: 10.1111/trf.16171. [DOI] [PubMed] [Google Scholar]
- 76.Pagano M.B., Cataife G., Fertrin K.Y., Gernsheimer T., Hess J.R., et al. Blood use and transfusion needs at a large health care system in Washington state during the SARS-CoV-2 pandemic. Transfusion. 2020;60:2859–2866. doi: 10.1111/trf.16051. [DOI] [PubMed] [Google Scholar]
- 77.Cai X., Ren M., Chen F., Li L., Lei H., et al. Blood transfusion during the COVID-19 outbreak. Blood Transfus. 2020;18:79–82. doi: 10.2450/2020.0076-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barriteau C.M., Bochey P., Lindholm P.F., Hartman K., Sumugod R., et al. Blood transfusion utilization in hospitalized COVID-19 patients. Transfusion. 2020;60:1919–1923. doi: 10.1111/trf.15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lawicki S., Covin R.B., Powers A.A. The Kidd (JK) Blood Group System. Transfus Med Rev. 2017;31:165–172. doi: 10.1016/j.tmrv.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Delaney M., Apelseth T.O., Bonet Bub C., Cohn C.S., Dunbar N.M., et al. Red-blood-cell alloimmunization and prophylactic antigen matching for transfusion in patients with warm autoantibodies. Vox Sang. 2020;115:515–524. doi: 10.1111/vox.12914. [DOI] [PubMed] [Google Scholar]
- 81.Jager U., Barcellini W., Broome C.M., Gertz M.A., Hill A., et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648. doi: 10.1016/j.blre.2019.100648. [DOI] [PubMed] [Google Scholar]
- 82.El Dewi D.M., Metwally T. Adsorption Technique in Pre-Transfusion Testing For Patients with Warm Type Autoimmune Hemolytic Anemia. Egypt J Immunol. 2017;24:47–51. [PubMed] [Google Scholar]
- 83.James P., Rowe G.P., Tozzo G.G. Elucidation of alloantibodies in autoimmune haemolytic anaemia. Vox Sang. 1988;54:167–171. doi: 10.1111/j.1423-0410.1988.tb03893.x. [DOI] [PubMed] [Google Scholar]