Abstract
Background:
the extent of medial meniscal extrusion (MME) that is associated with structural and symptomatic progression of knee osteoarthritis has not been defined yet.
Objective:
To investigate MRI-based thresholds of MME that are associated with structural progression of knee degenerative disease and symptoms over a period of 4 years.
Methods:
We studied 328 knees from 235 participants that were randomly selected from the Osteoarthritis Initiative Cohort. MME was quantified on coronal sections of intermediate-weighted sequences obtained at 3T. Knee pain and cartilage abnormalities were measured using the Western Ontario and McMaster Universities (WOMAC)-pain scale and the cartilage whole-organ magnetic resonance imaging score (WORMS). General estimating equations with logistic regression models were used to correlate baseline MME and changes in pain and cartilage damage. Receiver operating characteristic (ROC) analyses were performed to determine the area under the ROC curve (AUROC). Individual thresholds were determined by maximizing the product of sensitivity and specificity.
Results:
The AUROC for predicting progression of knee pain, medial compartment and medial tibial cartilage damage were 0.71, 0.70 and 0.72; the individual thresholds for MME were 2.5 mm, 2.7 mm and 2.8 mm, respectively. A single threshold of 2.5 mm was determined by maximizing the average of the product of sensitivity and specificity of the three outcome variables (knee pain progression, medial compartmental cartilage damage progression and medial tibial cartilage damage progression).
Conclusions:
Medial meniscal extrusion was associated with knee pain and cartilage damage progression over 4 years. A single threshold of 2.5 mm was found to be most useful for predicting knee pain, medial compartment and tibial cartilage damage progression over 4 years.
Clinical Impact:
This threshold could be used to standardize the diagnostic criteria of extrusion and to better characterize the risk for subsequent structural and symptomatic progression of knee osteoarthritis.
Keywords: Knee, Osteoarthritis, Meniscal extrusion, Cartilage
Introduction
The menisci of the knee are crescent-shaped wedges of fibrocartilage located on the tibial plateaus that have an essential role in distributing load, reducing friction, absorbing shock and increasing congruity of the knee joint [1]. Meniscal extrusion, which refers to displacement of the body of the meniscus beyond the outermost margin of the tibial plateau, may impair the normal mechanical function of the meniscus and is associated with osteoarthritis (OA)-related knee structural changes and symptoms [2-5]. An absolute extrusion distance of 3 mm is widely used as the threshold to define medial meniscal extrusion (MME) [6, 7]. In addition, magnetic resonance imaging (MRI)-based semi-quantitative grading systems have also been proposed for classifying extrusion [8, 9]. However, since the thresholds differ in various methods for assessing meniscal extrusion, the amount of meniscal displacement that could qualify as extrusion remains controversial.
Studies have confirmed the association of meniscal extrusion and cartilage destruction [2, 10-12]. Recently, Okada et al. [13] reported that for individuals without radiographic knee OA (KOA), 2.2 mm could be used as a MME threshold to predict KOA development within 4 years. A cross-sectional study by Svensson et al. [14] suggested that 4 mm may be a more optimal threshold than 3 mm since the former has better sensitivity and specificity in identifying individuals with respect to radiographic OA, bone marrow edema pattern and cartilage damage. Nevertheless, the extent of extrusion that is associated with progression of knee cartilage damage has not been defined yet. Given that cartilage damage is the structural hallmark of KOA [1], the MME threshold that predicts progression of cartilage damage needs to be determined.
Clinically, pain is the most common presentation of KOA and increased knee pain is considered a predictor of future physical functional limitation [15, 16]. However, how the degree of extrusion correlates with the severity and progression of knee pain is not well known. Recently, Hiroaki et al. [17] proposed 4.3 mm to be the ultrasound-based MME threshold related with the presence of knee pain, however, there is limited knowledge on MRI-based thresholds for severity and progression of knee pain.
A quantitative index related to the severity of structural changes and clinical symptoms could be used to standardize the diagnostic criteria of extrusion and to better characterize the risk for subsequent structural and symptomatic progression of knee OA. The purpose of this study was therefore to investigate MRI-based thresholds of MME that are associated with structural progression of knee degenerative disease and symptoms over a period of 4 years.
Materials and methods
Database and Subjects
Data for the current study was collected from the Osteoarthritis Initiative (OAI, https://oai.nih.gov), a longitudinal, multi-center cohort study in the United States of 4796 individuals with or at risk for symptomatic knee OA. The OAI is sponsored by the US National Institutes of Health (NIH), aiming to develop a large database to study the natural progression, risk factors and predictors of knee OA. The OAI was approved by the Institutional Review Board (IRB) of the OAI Coordinating Center at the University of California, San Francisco and the IRB of each site.
From the OAI, 346 knees of 246 participants with complete Western Ontario and McMaster Universities (WOMAC) knee pain scores and readable MRI images at baseline and the 48-month follow-up time point were randomly selected. To those 346 knees the following exclusion criteria were applied: 1) History of rheumatoid arthritis or other inflammatory arthropathy prior to baseline or between baseline and the 4-year time period, 2) history of knee surgery or trauma of the index knee prior to the 48-month time point and 3) knees that had meniscectomy. The resulting sample included 328 knees from 235 participants.
Knee Pain Scores
Knee pain severity was scored using the WOMAC pain subscale [18]. WOMAC-pain includes five items related with knee pain during different activities of daily life (during walking, using stairs, in bed, sitting or lying, and standing upright) over the previous 7 days. Each item is scored on a scale of 0-4 (0 = none, 4 = extreme pain). A sum score of the five WOMAC pain items was calculated to represent knee pain severity, with a possible sum score ranging from 0 to 20, where 0 indicates no pain and 20 represents the most severe pain. Based on previous studies a minimum clinically significant difference for knee pain progression was defined as an increase ≥ 9 points on a 0-100 normalized score from baseline to the 4-year time point based on previous epidemiological studies [19-22]. We dichotomized the patients into knee pain progression and no progression (patients with a WOMAC pain score increase less than 9 points on the 0-100 scale).
MR imaging protocol
MRI scans were performed at the four different clinical sites of the OAI with cross calibrated 3.0-T MR scanners (Magnetom Trio, Siemens, Erlangen, Germany) using quadrature transmit-receive coils (USA Instruments, Aurora, Ohio). The following sequences were used in this study: (a) a coronal 2D intermediate-weighted (IW) turbo spin-echo (TSE) sequence (repetition time [TR] / echo time [TE], 3700/29 msec; in plane spatial resolution, 0.365 × 0.456 mm2; section thickness, 3.0 mm); (b) a sagittal 2D IW TSE sequence with fat suppression (TR/TE, 3200/30 msec; in plane spatial resolution, 0.357 × 0.511 mm2; section thickness, 3.0 mm); and (c) a sagittal 3D dual-echo steady-state sequence (TR/TE, 16.3/4.7 msec; in plane spatial resolution, 0.365 × 0.456 mm2; section thickness, 0.7 mm). More details regarding the sequences are available in the OAI MR protocol [23].
MR image analysis
Baseline medial meniscal extrusion measurement
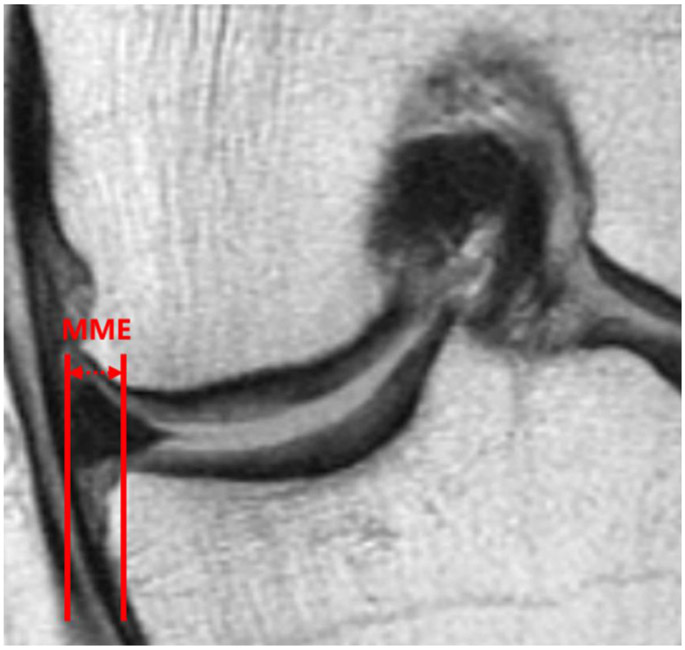
On the coronal 2D IW TSE sequence, the slice where the medial tibial spine had the greatest volume was selected for the extrusion measurement. Using the margin of the medial tibial plateau (excluding osteophytes) as the reference for assessment, MME was quantified in millimeters [2]: we drew two vertical lines in the image, one of which intersected the outer margin of the medial meniscus and the other intersected the edge of the medial tibial plateau at the point of transition from horizontal to vertical; the distance between the two vertical lines was defined as the measurement of MME as shown in Figure 1. Measurements were performed by a radiologist with 5 years of experience (Y.L.) and reproducibility of these measurements was obtained as outlined below.
Figure 1.
Medial meniscal extrusion (MME) measurement in the coronal plane using an IW TSE sequence demonstrating a medial extruded distance of 3.7 mm.
IW: intermediate-weighted. TSE: turbo spin-echo.
WORMS scoring
The UCSF modified semi-quantitative whole-organ magnetic resonance imaging score (WORMS) system was used to assess OA-related cartilage morphological abnormalities [24, 25]. Knee cartilage lesions were assessed in 6 regions (patella, trochlea, medial femur, medial tibia, lateral femur and lateral tibia) with an eight-point scale at baseline and the 4-year time point: 0 = normal cartilage, 1 = increased signal in fluid sensitive MR images or cartilage swelling, 2 = a partial thickness focal defect < 1 cm in greatest width, 2.5 = full thickness focal defect < 1 cm in greatest width, 3 = multiple areas of grade 2 lesions or a partial thickness lesion wider than 1 cm but < 75% of the region, 4 = diffuse (≥ 75% of the region) partial thickness lesion, 5 = multiple areas of grade 2.5 lesions or a full thickness lesion wider than 1 cm but < 75% of the region and 6 = diffuse (≥ 75% of the region) full thickness loss. Knee cartilage damage progression was evaluated for the whole knee, medial compartment, medial femur and medial tibia, respectively. Cartilage damage was dichotomized as progression (cartilage WORMS sum scores increased over 4 years) versus no progression. Medial meniscal lesions were graded in 3 regions (anterior/body/posterior) using the following 4-point scale: 0 = normal, 1 = intrasubstance signal, 2 = nondisplaced tear, 3 = displaced or complex tear, 4 = complete destruction/maceration. Medial meniscal injury (dichotomized) was defined as one or more of the 3 regions demonstrating a grade ≥ 2 lesion.
WORMS gradings of the MR images of all knees at baseline and the 48-month follow up time point were performed by a radiologist (Y.L. 5 years of experience) who was blinded to subject characteristics and clinical data. As is standard in epidemiological studies an adjudication was performed with a second board-certified musculoskeletal radiologist (T.M.L., with 24 years of experience) if cases were considered as borderline grades.
Reproducibility
Reproducibility for MME measurements was assessed in 20 randomly selected knees. Each knee was measured independently twice by two radiologists blinded to each other’s and their own previous measurements (Y.L. and S.C.F., 5 and 6 years of experience) on two separate occasions with at least 4 weeks in between those two readings. In order to calculate the intra- and inter-reader reproducibility of the WORMS grading, two radiologists (Y.L. and S.C.F., 5 and 6 years of experience) performed WORMS grading twice independently for 10 randomly selected knees. Intra-reader reproducibility and inter-reader reproducibility for MME measurements and WORMS grading were assessed by the intra-class correlation coefficients (ICCs) and Bland-Altman plots.
Statistical analysis
Statistical analyses were performed with STATA version 14 software (StataCorp LP, College Station, TX), using a two-sided, 0.05 level of significance. Generalized estimating equations with logistic regression models were used to assess the relationships between baseline meniscal extrusion values and changes in pain and cartilage damage progression over 4 years (dichotomized as progression versus no progression). The model was adjusted for age, sex, knee side, race, BMI, Kellgren & Lawrence (K&L) grades, and was then further adjusted for baseline meniscal injury. Receiver operating characteristic (ROC) analyses was performed to determine the area under the ROC curve (AUROC). Sensitivity, specificity, the percentage of correctly classified subjects (also known as accuracy) as well as positive and negative likelihood ratios were then calculated for each threshold. Individual optimal thresholds for MME was then determined using the method reported by Liu that maximizes the product of the sensitivity and specificity [26]. A single threshold across knee pain and cartilage damage progression outcomes was determined by choosing a threshold that maximizes the average of the product of sensitivity and specificity of the outcome variables.
Results
Our sample consisted of 170 right knees and 158 left knees (235 participants, 63.4% female). Mean baseline age and mean baseline body mass index (BMI) were 59.8 ± 8.0 years and 29.6 ± 4.8 kg/m2 (Table 1). Mean baseline medial extruded distance was 2.5 ± 1.4 mm, and 186 knees had an extrusion greater than 2 mm. After 4 years there were 70 knees that had changed K&L scores, and 258 knees stayed the same grades. Among the 70 knees, 33 knees progressed from K&L 0/1 to greater than 1.
Table 1:
Baseline characteristics of the study sample.
| Characteristics | n = 235 (328 knees) |
|---|---|
| Age, mean (SD) years | 59.8 (8.0) |
| Female [n (%)] | 149 (63.4%) |
| Body mass index, mean (SD) (kg/m2) | 29.6 (4.8) |
| Right knees [n (%)] | 170 (51.8%) |
| Knee Kellgren-Lawrence scores | |
| Grade 0 [n (%)] | 89 (27.1%) |
| Grade 1 [n (%)] | 102 (31.1%) |
| Grade 2 [n (%)] | 121 (36.9%) |
| Grade 3 [n (%)] | 15 (4.6%) |
| Grade 4 [n (%)] | 1 (0.3%) |
| Mean baseline WOMAC scores (0-100 scale) (SD) | 11.7 (16.4) |
| Mean MME (SD) (mm) | 2.5 (1.4) |
| 2 mm ≤ Knees with MME < 3 mm [n (%)] | 91 (27.7%) |
| 3 mm ≤ Knees with MME < 4 mm [n (%)] | 47 (14.3%) |
| Knees with MME ≥ 4 mm [n (%)] | 48 (14.6%) |
| Knees with pain progression [n (%)] | 60 (18.3%) |
| Knees with cartilage damage progression | |
| Whole knee [n (%)] | 224 (68.3%) |
| Medial compartment [n (%)] | 106 (32.3%) |
| Medial femur [n (%)] | 82 (25.0%) |
| Medial tibia [n (%)] | 50 (15.2%) |
MME: Medial meniscal extrusion. WOMAC scores: Western Ontario and McMaster Universities knee pain scores.
Reproducibility
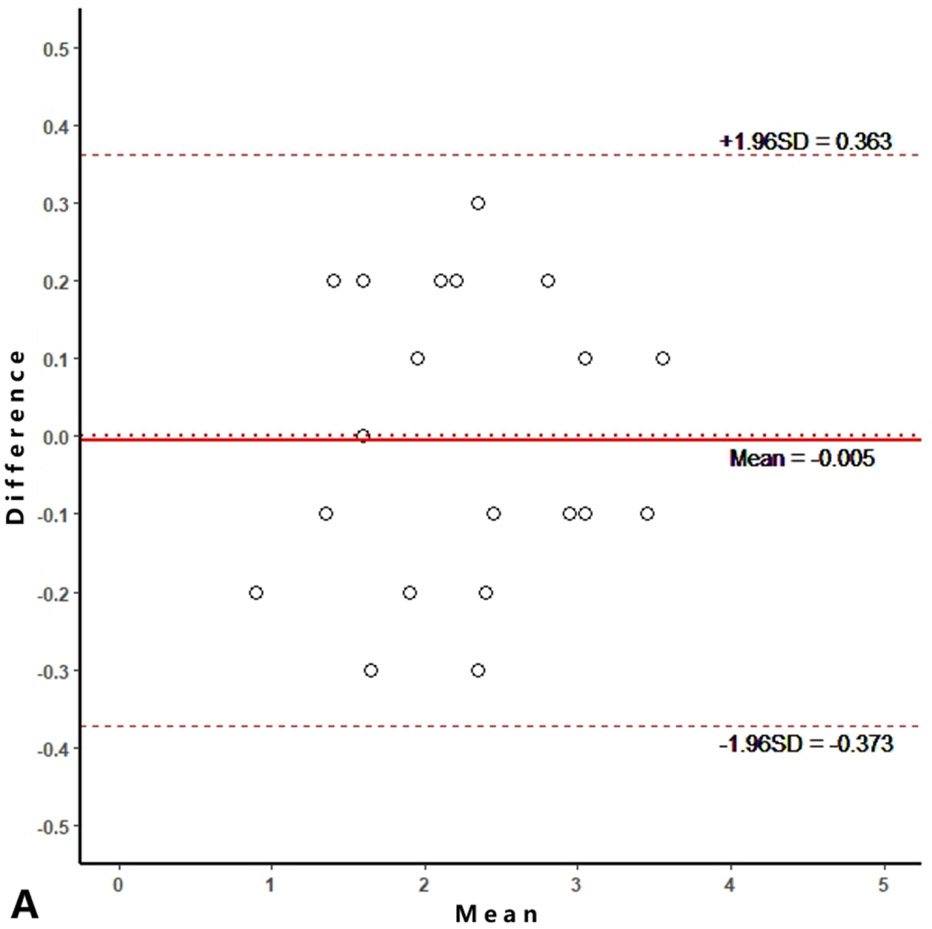
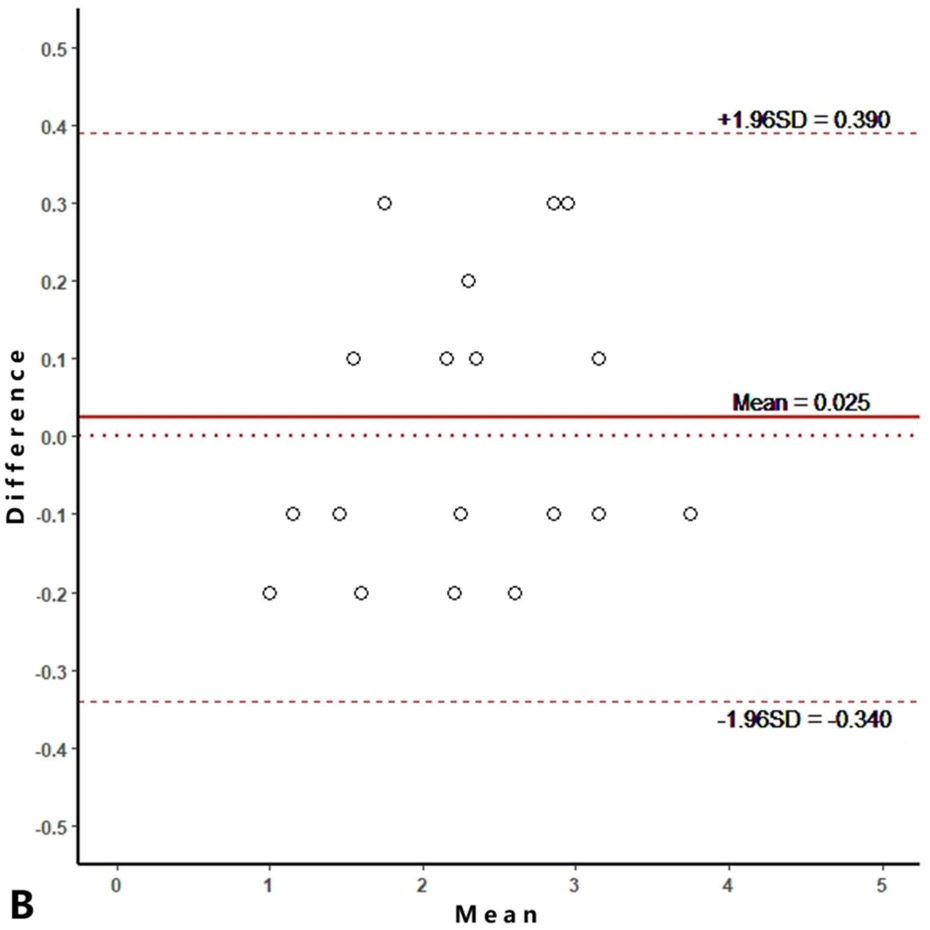
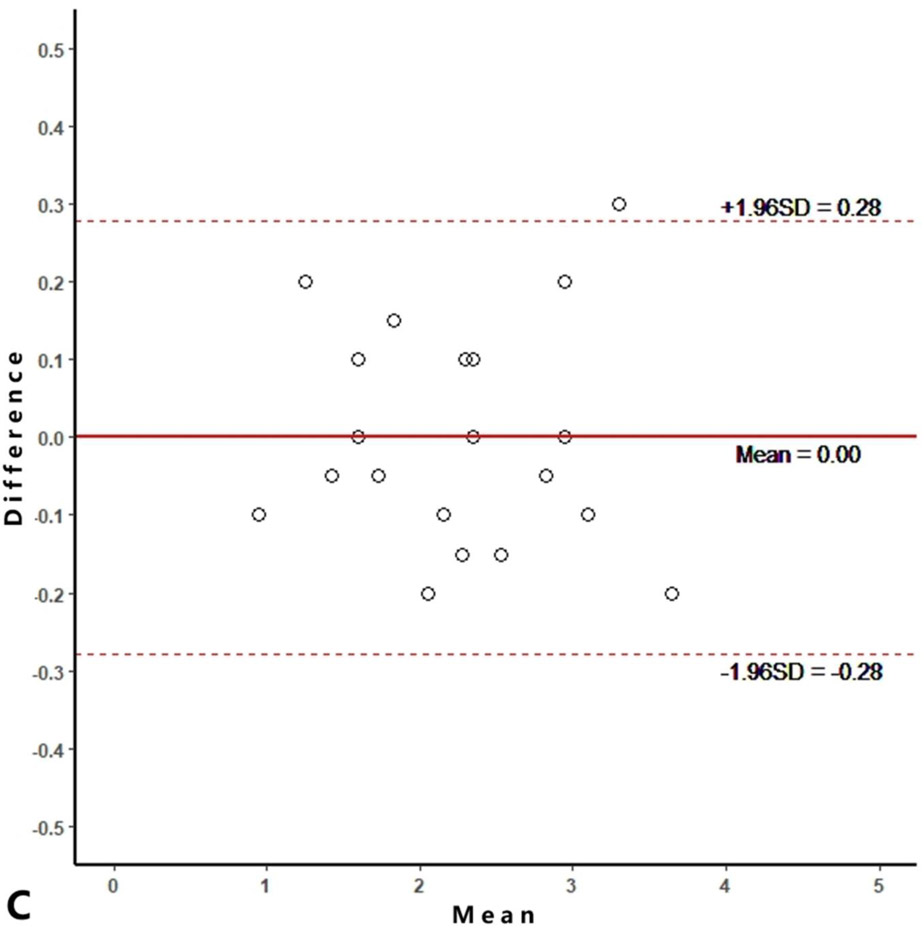
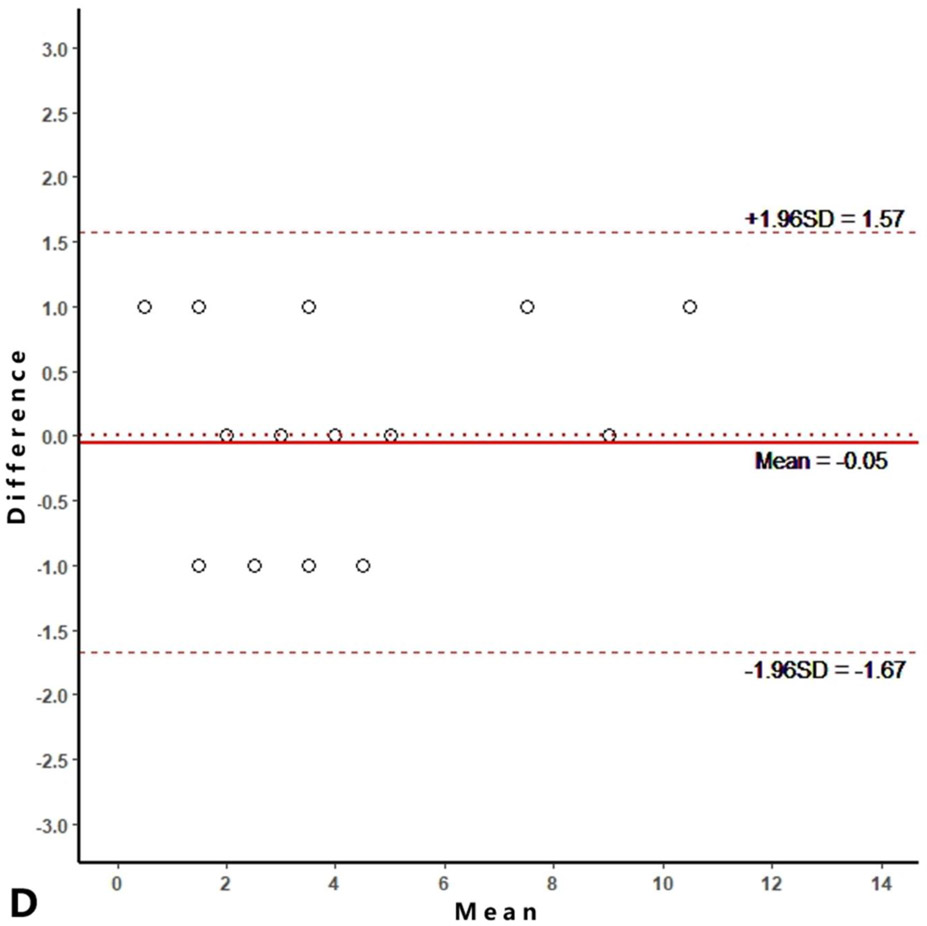
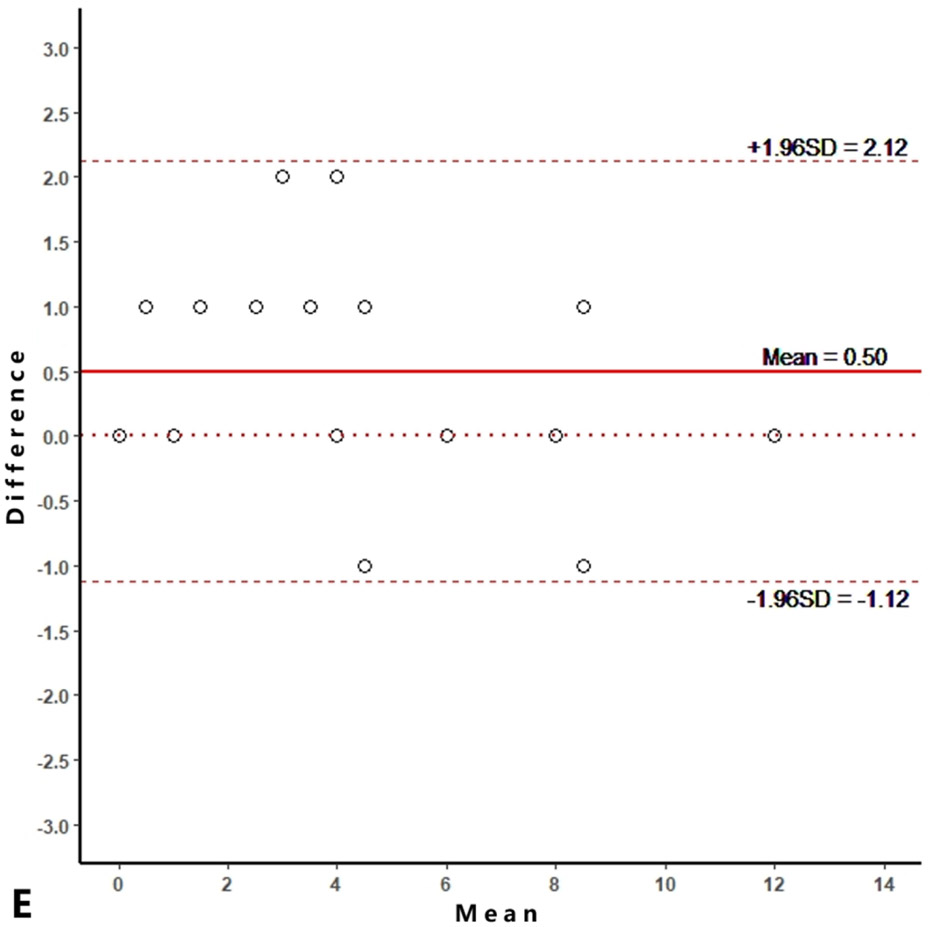
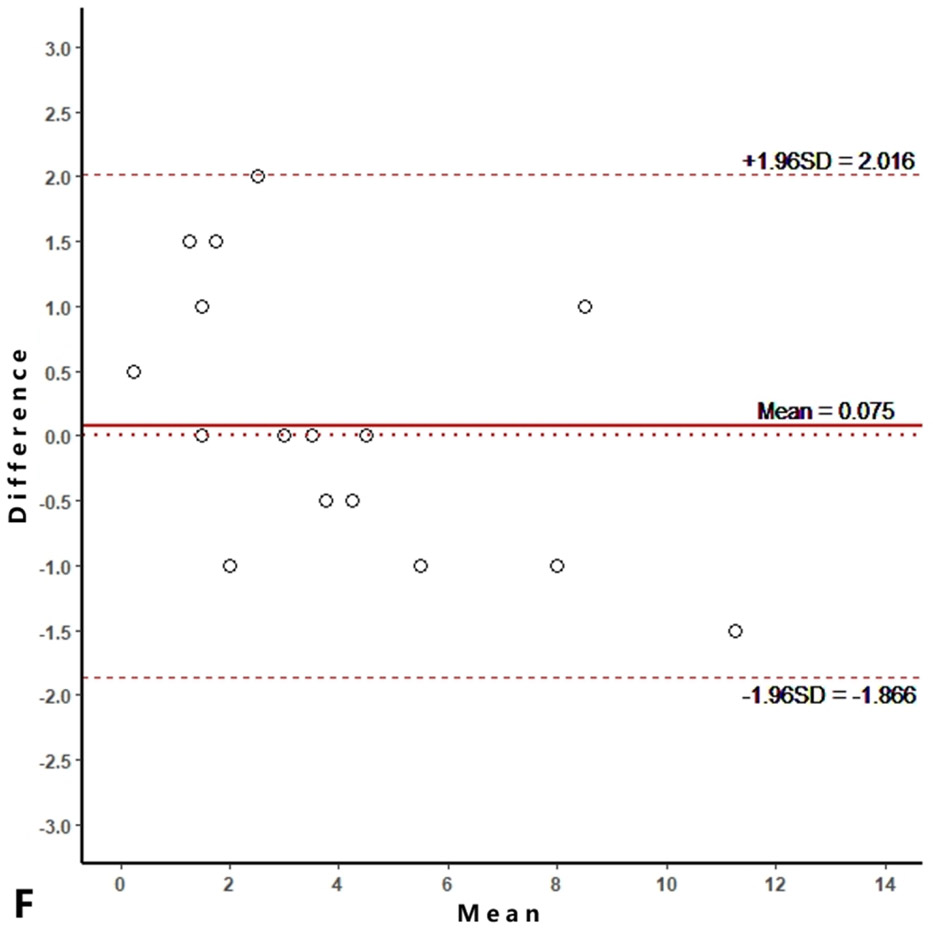
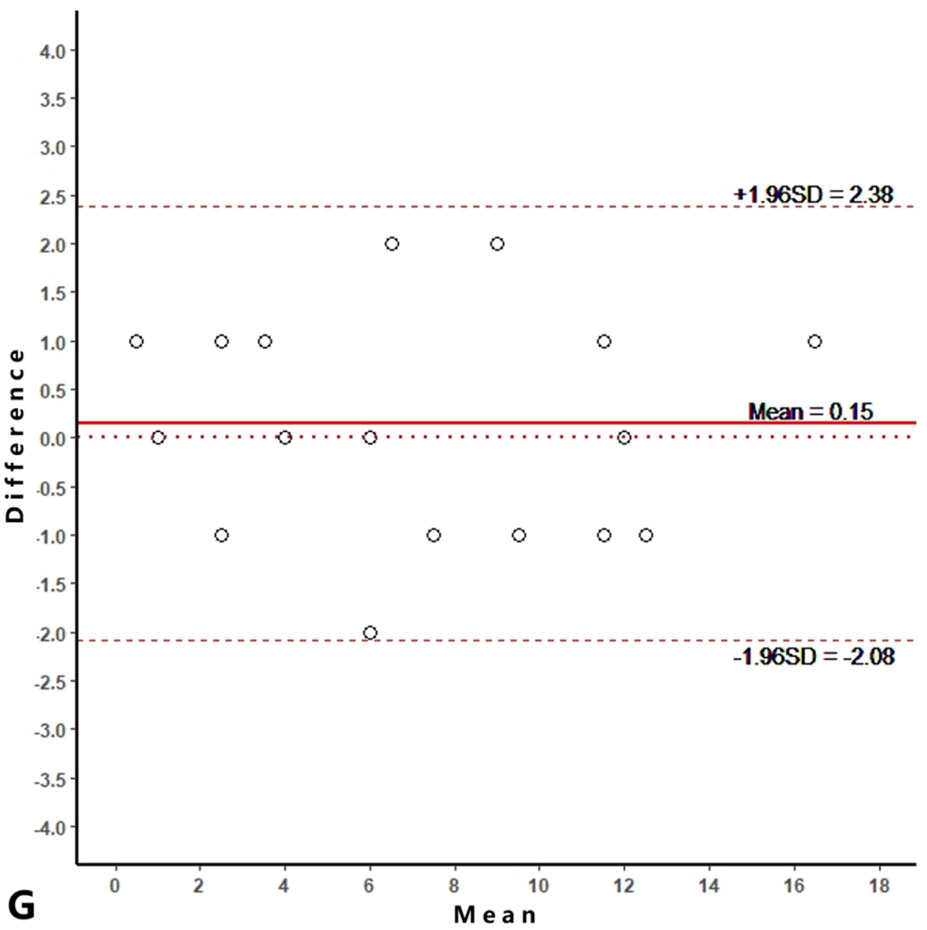
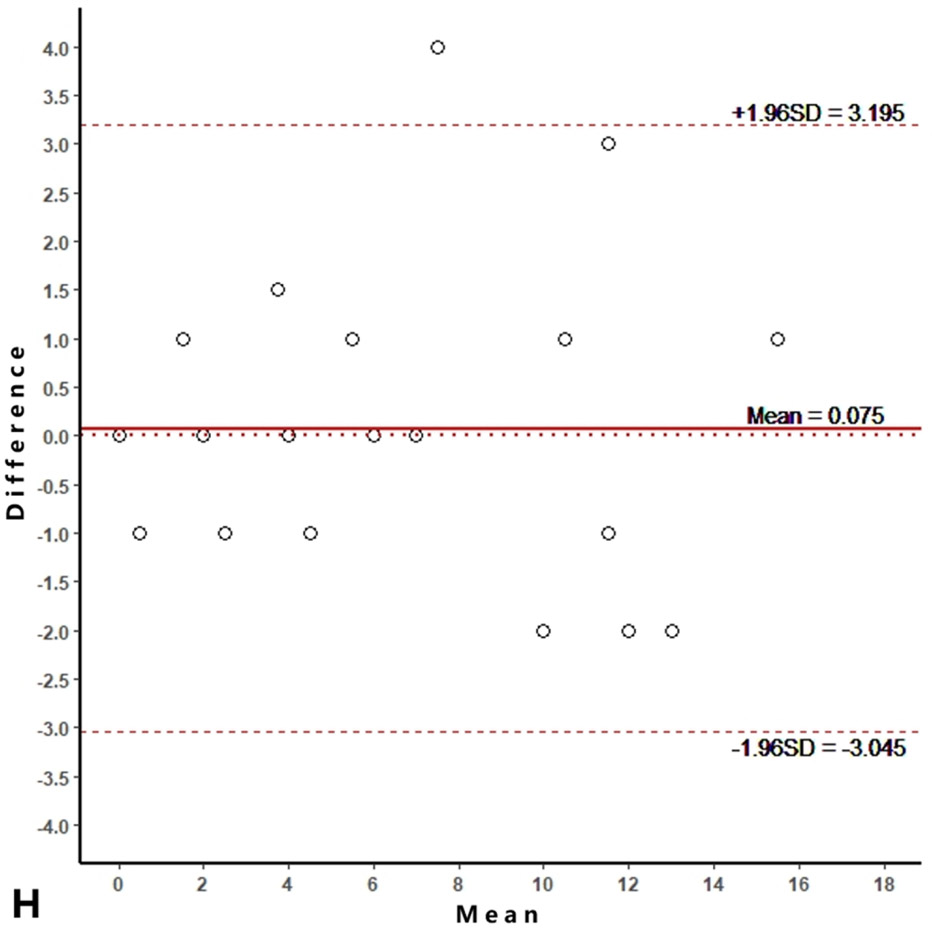
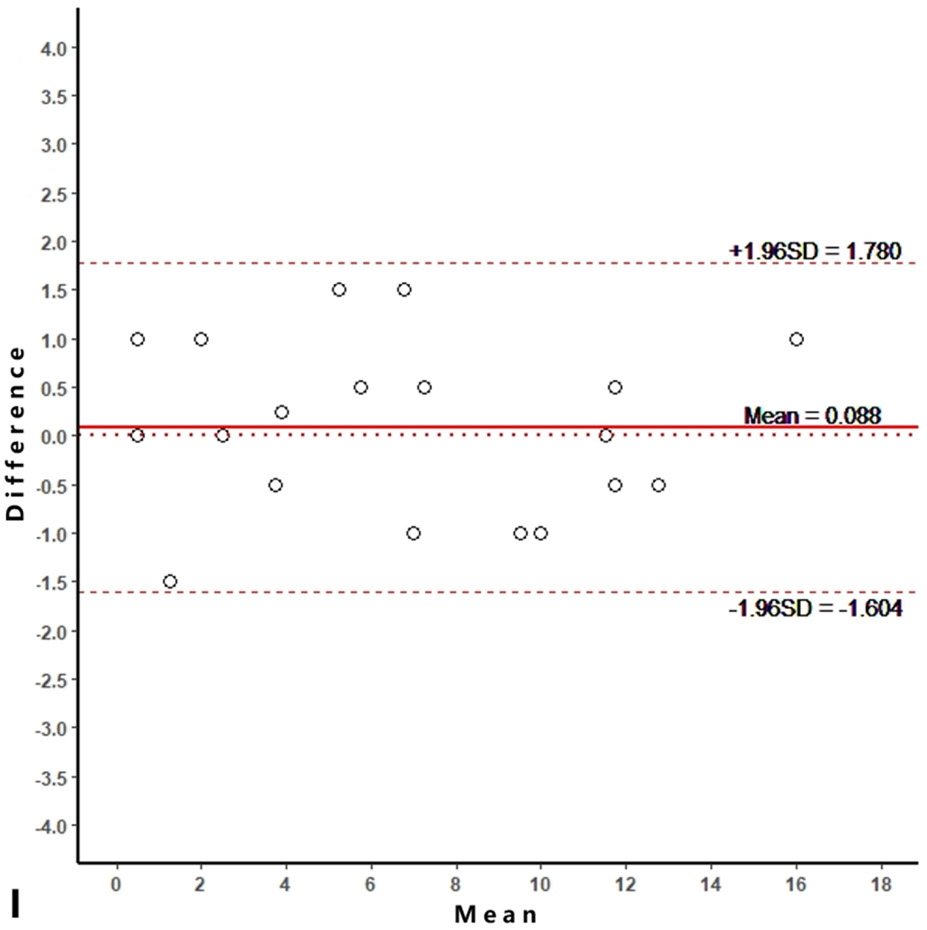
Intra-reader agreements (ICC values) for MME measurements were 0.967 and 0.968, and inter-reader agreement was 0.981. The intra-reader agreements were 0.953 and 0.967 for meniscus WORMS, and 0.983 and 0.971 for cartilage WORMS. ICCs for inter-reader agreement were 0.944 for meniscus WORMS, and 0.945 for cartilage WORMS. In the Bland-Altman plots of intra- and inter-reader assessments, most of the values ranged within a mean difference ± 1.96 SD (Figure 2).
Figure 2.
Bland-Altman plots of reproducibility for medial meniscal extrusion (MME) measurements, WORMS meniscus and WORMS cartilage. A, B: intra-reader agreement for MME measurements; C: inter-reader agreement for MME measurements; D, E: intra-reader agreement for WORMS meniscus; F: inter-reader agreement for WORMS meniscus; G, H: intra-reader agreement for WORMS cartilage; I: inter-reader agreement for WORMS cartilage.
Baseline pain scores and cartilage WORMS scores
At baseline, 149 knees had a WOMAC sum score (0-100 scale) of 0, 132 knees had a score of 1-25, 35 knees had a score of 26-50 and 12 knees had a score ≥ 51. Details for baseline knee cartilage WORMS scores were summarized in Supplementary Table 1.
Predictive ability of MME with respect to knee pain and cartilage damage progression
MME was shown to be a risk factor of knee pain progression over 4 years, with a statistically significant odds ratio of 1.33 (1.05, 1.68; 95% CI) (Table 2). The AUROC was 0.71 and the optimal threshold was determined to be 2.5 mm for which the sensitivity and specificity were 0.67 and 0.65.
Table 2:
Odds ratios, AUROCs and optimal thresholds (and corresponding sensitivity and specificity) for baseline MME in predicting knee pain and cartilage damage progression over 4 years. (Adjusted for age, sex, knee side, BMI, race, and K&L scores)
| Outcome Parameters | Odds Ratio (95% CI) |
P valuea |
AUROC | Thresholdb (mm) |
Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Pain progression | 1.33 (1.05, 1.68) | 0.016 | 0.71 | 2.5 | 0.67 | 0.65 |
| Cartilage damage progression | ||||||
| Whole knee | 1.13 (0.93, 1.36) | 0.209 | 0.62 | 2.5 | 0.46 | 0.69 |
| Medial compartment | 1.23 (1.01, 1.50) | 0.035 | 0.70 | 2.7 | 0.55 | 0.71 |
| Medial femur | 1.21 (0.99, 1.49) | 0.067 | 0.68 | 2.7 | 0.60 | 0.70 |
| Medial tibia | 1.28 (1.00, 1.63) | 0.048 | 0.72 | 2.8 | 0.58 | 0.69 |
P value for odds ratio, significant results are in italics (p value < 0.05).
Individual optimal Threshold. AUROC: Area Under the ROC curve.
MME was statistical significantly associated with medial compartmental and medial tibial cartilage damage progression, and the odds ratios were 1.23 (1.01, 1.50) and 1.28 (1.00, 1.63) (Table 2). However, MME did not show a statistically significant association with whole knee cartilage damage progression and medial femoral cartilage damage progression. In predicting medial compartmental cartilage damage progression, the AUROC was 0.70 and the optimal threshold was 2.7 mm. With this value the sensitivity and specificity were 0.55 and 0.71. Likewise, the AUROC for predicting medial tibial cartilage damage progression was 0.72, and optimal threshold, sensitivity and specificity were 2.8 mm, 0.58 and 0.69, respectively (Figure 3).
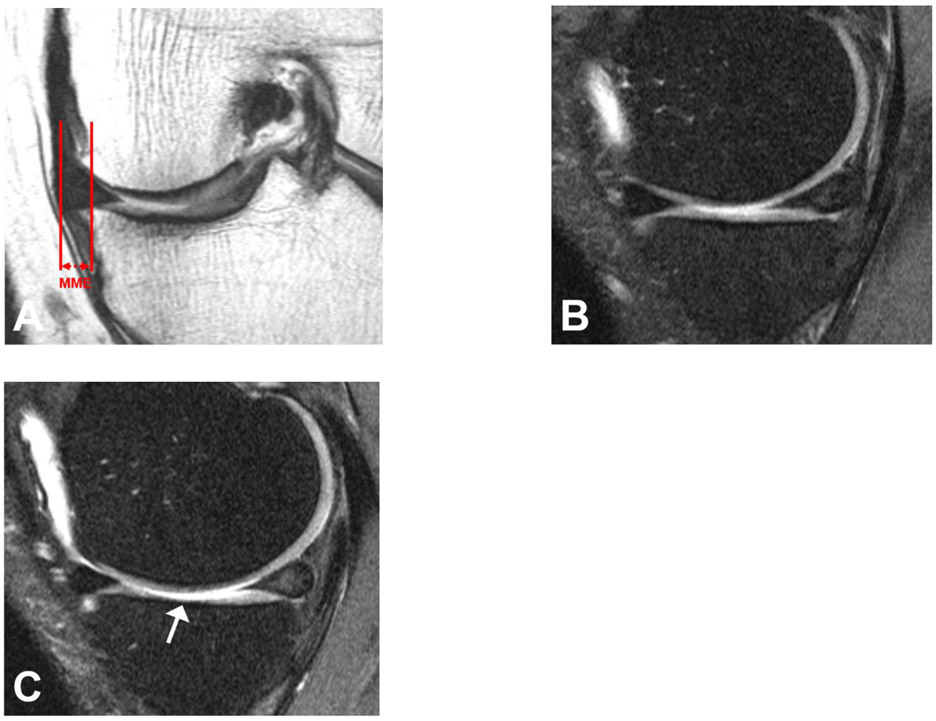
Figure 3.
MRI of the left knee of a 55-year old man at baseline and 4-year follow-up. At baseline, on the coronal IW TSE sequence medial meniscal extrusion (MME) measured 5.8 mm (a) and the sagittal fat-saturated IW TSE sequence demonstrated normal medial tibial cartilage (b). However, after 4 years the sagittal fat-saturated IW TSE sequence showed diffuse (<75%) high grade partial thickness medial tibial cartilage loss (WORMS grade 3) (arrow) (c).
A single threshold of 2.5 mm was determined by maximizing the average of the product of sensitivity and specificity of the three outcome variables (knee pain progression, medial compartmental cartilage damage progression and medial tibial cartilage damage progression). (Table 3). The sensitivity/specificity were 0.67/0.65 for predicting knee pain progression, 0.58/0.67 for medial compartmental damage progression and 0.62/0.63 for medial tibial cartilage damage progression.
Table 3.
Comparison of different thresholds in predicting knee pain and cartilage damage progression over 4 years.
| Threshold | Individual threshold | ||||||
|---|---|---|---|---|---|---|---|
| 2 mm | 3 mm | 4 mm | 2.5 mm | 2.7 mm | 2.8 mm | 2.5 mmc | |
| Pain progression | |||||||
| Sensitivity (Ratio a) | 0.75(1.12) | 0.47(0.70) | 0.28(0.42) | 0.67(1.00) | 0.67(1.00) | ||
| Specificity (Ratio) | 0.46(0.72) | 0.75(1.16) | 0.88(1.36) | 0.65(1.00) | 0.65(1.00) | ||
| Productb (Ratio) | 0.35(0.81) | 0.35(0.81) | 0.25(0.58) | 0.43(1.00) | 0.43(1.00) | ||
| Positive Likelihood (Ratio) | 1.40(0.74) | 1.84(0.98) | 2.37(1.26) | 1.88(1.00) | 1.88(1.00) | ||
| Negative Likelihood (Ratio) | 0.54(1.05) | 0.71(1.38) | 0.81(1.58) | 0.52(1.00) | 0.52(1.00) | ||
| Correctly Classified (Ratio) | 0.52(0.79) | 0.70(1.07) | 0.77(1.19) | 0.65(1.00) | 0.65(1.00) | ||
| Cartilage damage progression of medial compartment | |||||||
| Sensitivity (Ratio) | 0.69(1.25) | 0.42(0.78) | 0.24(0.45) | 0.55(1.00) | 0.58(1.05) | ||
| Specificity (Ratio) | 0.48(0.68) | 0.77(1.09) | 0.90(1.27) | 0.71(1.00) | 0.67(0.94) | ||
| Product (Ratio) | 0.33(0.85) | 0.33(0.84) | 0.21(0.57) | 0.39(1.00) | 0.38(0.99) | ||
| Positive Likelihood (Ratio) | 1.32(0.71) | 1.85(0.99) | 2.28(1.27) | 1.87(1.00) | 1.73(0.92) | ||
| Negative Likelihood (Ratio) | 0.65(1.02) | 0.75(1.17) | 0.85(1.31) | 0.64(1.00) | 0.64(0.99) | ||
| Correctly Classified (Ratio) | 0.55(0.83) | 0.66(1.00) | 0.68(1.05) | 0.66(1.00) | 0.64(0.97) | ||
| Cartilage damage progression of medial tibia | |||||||
| Sensitivity (Ratio) | 0.72(1.24) | 0.52(0.90) | 0.32(0.55) | 0.60(1.00) | 0.62(1.07) | ||
| Specificity (Ratio) | 0.45(0.65) | 0.75(1.08) | 0.88(1.28) | 0.69(1.00) | 0.63(0.91) | ||
| Product (Ratio) | 0.32(0.81) | 0.39(0.97) | 0.28(0.70) | 0.40(1.00) | 0.39(0.97) | ||
| Positive Likelihood (Ratio) | 1.31(0.70) | 2.07(1.10) | 2.70(1.44) | 1.87(1.00) | 1.66(0.88) | ||
| Negative Likelihood (Ratio) | 0.62(0.91) | 0.64(0.94) | 0.77(1.13) | 0.68(1.00) | 0.67(0.99) | ||
| Correctly Classified (Ratio) | 0.49(0.73) | 0.71(1.06) | 0.80(1.18) | 0.67(1.00) | 0.63(0.93) | ||
Ratio compared to individual optimal threshold.
Product of sensitivity and specificity.
The single threshold was determined by maximizing the average of the product of the sensitivity and specificity of the three outcome variables (knee pain progression, medial compartmental cartilage damage progression and medial tibial cartilage damage progression).
Comparison of different thresholds
The newly determined and three commonly used thresholds for MME were compared to evaluating the diagnostic ability in predicting knee pain and cartilage damage progression over 4 years. As shown in Table 3, both for predicting pain and cartilage damage progression, a 4 mm threshold had the highest specificity, positive likelihood, negative likelihood and the percentage of correctly classified subjects, while a 2 mm threshold had the highest sensitivity. Comparing with individual optimal thresholds, the products of sensitivity and specificity of 2 mm, 3 mm and 4 mm dropped by 15 ~ 43% depending on the outcome evaluated. Additionally, the threshold of 2.5 mm was very close to three individual optimal thresholds regarding all outcomes and parameters evaluated.
Further adjusting for meniscal injury
After adjusting for meniscal injury, the association between MME and pain progression continued to be significant, but the association between MME cartilage damage progression was no longer significant (Table 4). In addition, meniscal injury did not show a statistically significant association with knee pain progression (P = 0.204) but was significantly correlated with medial compartmental and medial tibial cartilage damage progression (P = 0.042 and 0.035, respectively).
Table 4:
Odds ratios, AUROCs and optimal thresholds (and corresponding sensitivity and specificity) for baseline MME in predicting knee pain and cartilage damage progression over 4 years. (Adjusted for age, sex, knee side, BMI, race, K&L socres and baseline meniscal injury)
| Outcome Parameters | Odds Ratio (95% CI) |
P valuea |
AUROC | Thresholdb (mm) |
Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Pain progression | 1.42 (1.10, 1.84) | 0.008 | 0.71 | 2.5 | 0.67 | 0.65 |
| Cartilage damage progression | ||||||
| Whole knee | 1.06 (0.86, 1.29) | 0.595 | 0.62 | 2.5 | 0.46 | 0.69 |
| Medial compartment | 1.13 (0.92, 1.39) | 0.248 | 0.70 | 2.7 | 0.55 | 0.71 |
| Medial femur | 1.15 (0.93, 1.44) | 0.194 | 0.68 | 2.7 | 0.60 | 0.70 |
| Medial tibia | 1.14 (0.88, 1.49) | 0.334 | 0.72 | 2.8 | 0.58 | 0.69 |
P value for odds ratio, significant results are in italics (p value < 0.05).
Individual optimal Threshold. AUROC: Area Under the ROC curve.
Discussion
Medial meniscal extrusion of 3 mm and greater has been suggested as an abnormal finding, but there is limited knowledge how this is correlated with articular cartilage degeneration and knee pain over 4 years. In the present study, MME was shown to be significantly associated with knee pain and cartilage damage progression. After adjusting for baseline meniscal injury, the association between MME and knee pain progression continued to be significant. Using the product method, 2.5 mm, 2.7 mm and 2.8 mm were the individual optimal thresholds to predict best the progression for knee pain, medial compartmental cartilage damage and medial tibial cartilage damage over 4 years, respectively. Furthermore, we determined a single threshold of 2.5 mm to best predict progression of knee pain and cartilage destruction.
Many studies [2, 3, 10-12, 27] have reported a significant association between MME and knee structural changes, but studies that focus on diagnostic thresholds for MME are relatively lacking. An absolute extrusion distance of 3 mm is widely used as the threshold for diagnosing MME. However, there was no clear evidence for this threshold when it was originally applied and published [6, 28]. Although based on the threshold of 3 mm some studies have found statistically significant results, it does not mean that in statistical terms 3 mm is the most accurate threshold. A diagnostic threshold should have a clear field/range of application, statistical evidence and clinical significance, which were reasons why meniscal extrusion thresholds have been increasingly discussed in recent years. In addition, it is worth noting that the measurement methods for MME differed in some early studies [6, 7, 29]. For example, in a cross-sectional study by Gale et al [29], the greatest distance from the most peripheral aspect of the meniscus to the border of the tibia was recorded as the extruded distance, while this method has been shown to overestimate maximal extrusion by Jones et al [30]. The above facts reminded us to re-consider the accuracy of the 3 mm threshold. Recently, Okada et al [13] reported that 2.2 mm is a MME threshold to predict radiographic KOA development. However, due to the inability of radiographs to directly visualize cartilage, radiographic progression of joint space narrowing (JSN) is neither a sensitive [31] nor a specific [32] measure of OA disease progression, especially in longitudinal studies [33]. This also reflected that the choice of outcome parameters was essential for the determination of threshold. By contrast, our study assessed MRI based progress of cartilage degeneration. As the structural hallmark of KOA [1], damaged cartilage is clearly more sensitive for the progression of KOA when compared with radiographic based JSN.
Pain from KOA is a key symptom in the decision to seek medical care and an important antecedent to disability [34]. Compared with using joint replacement as a primary endpoint, outcomes measures like KOOS (Knee injury and Osteoarthritis Outcome Score) and WOMAC scores have been used as proxies to evaluate disease severity based on patient clinical symptoms [27]. However, there is limited knowledge how MRI-based threshold relate to knee pain and other clinical symptoms. Recently, Wenger and colleagues [4] found that medial or lateral extrusion ≥ 3 mm was more frequent in painful than in painless knees. Using multivariable regression analyses, Klein et al [27] investigated the association between baseline meniscal extrusion and the 6-year change in KOOS knee pain score but failed to find a significant correlation between pain and extrusion. This may be due in part to a relatively simplified method to score MME, which only used 3 grades: 0 if absent, 1 if less than or equal to 50%, and 2 if greater than 50% meniscal extrusion.
Little is known about the extent of meniscus extrusion of middle-aged and elderly without radiographic tibiofemoral OA. A cross-sectional study by Svensson et al [35] reported that the mean absolute value of medial meniscal body extrusion for 718 individuals with K&L grade 0 were to be around 3 mm. In a subsequent cross-sectional study, Svensson et al [14] included 958 patients and found that 154 had radiographic KOA (K&L grade ≥ 2) and 426 patients had knee cartilage damage (WORMS grade ≥ 2), but only 68 patients had a MME ≥ 3 mm. The above two studies used a same well-characterized Framingham Community cohort containing 1039 individuals, which indicated that MRI-detectable knee cartilage damage may exist even in patients free from radiographic KOA with MME < 3 mm. In addition, based on a higher percentage of correctly classified persons with radiographic OA, bone marrow lesions and cartilage damage, Svensson et al [14] proposed 4 mm to suggest a “pathological” MME. However, this does not imply that 4 mm is the most optimal threshold with respect to sensitivity and specificity for longitudinal prediction of KOA symptoms and subsequent knee cartilage damage. As the present longitudinal study revealed, an evident decrease on the product of sensitivity and specificity of the 4 mm threshold was observed in predicting knee pain and progression of cartilage damage when compared with individual optimal thresholds. In contrast with previous papers, the newly determined threshold of 2.5 mm provided more comprehensive information, especially longitudinal information with clinical information (knee pain) and structural abnormalities (cartilage damage).
It was worth noting that the association between MME and medial compartmental and tibial cartilage damage progression were no longer significant after further adjusting for meniscal injury. However, meniscal injury was significantly correlated with medial compartmental and tibial cartilage damage progression. These findings suggest that MME may be a meniscal injury-depending cartilage damage predictor.
We acknowledge that our study has several limitations. First, we measured extrusion of the body of meniscus but did not assess extrusion in the anterior and posterior horn of the meniscus [9]. To date, there is still no consensus on the measurement method for extrusion in the anterior or posterior horn of the meniscus, which severely restricts the assessment. Second, knees in our study were scanned without loading, which may have resulted in an underestimation of the amount of meniscal extrusion [36]. Some ultrasound-based studies investigated meniscal extrusion on weight bearing knees and found it to be associated with development of pain, or incident OA [37-39], however, the additional requirements for hardware facilities make it difficult to apply weight-bearing MR imaging routinely in clinical practice.
In conclusion, our study provides individual optimized thresholds of medial meniscal extrusion that were associated with knee pain and cartilage damage progression over 4 years in subjects with or at risk for KOA. In addition, we found that a 2.5 mm threshold may be the best compromise with respect to longitudinal assessment of knee symptoms and structural degeneration for medial meniscal extrusion.
Supplementary Material
1. Funding
The study was supported by the Osteoarthritis Initiative, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262), with research conducted by the Osteoarthritis Initiative Study Investigators. The study was also funded in part by the Intramural Research Program of the National Institute on Aging, NIH. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. The analyses in this study were funded through the NIH/NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771 and P50-AR060752) and were also supported by the grants from National Natural Science Foundation of China (NSFC, No. 31630025 and 81930045).
Footnotes
Guarantor
The scientific guarantor of this publication is Thomas M. Link.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Gabby B. Joseph, PhD, kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained for this study.
Study subjects or cohorts overlap
The cohort has been reported in https://oai.nih.gov.
Methodology
Longitudinal, prospective cohort study with retrospective data analysis.
Diagnostic or prognostic study.
Multicenter study.
References
- 1.Englund M, Guermazi A, Lohmander LS. The Meniscus in Knee Osteoarthritis. Rheumatic Disease Clinics of North America 2009; 35:579–590 [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum 2006; 54:795–801 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wluka AE, Pelletier JP, et al. Meniscal extrusion predicts increases in subchondral bone marrow lesions and bone cysts and expansion of subchondral bone in osteoarthritic knees. Rheumatology (Oxford: ) 2010; 49:997–1004 [DOI] [PubMed] [Google Scholar]
- 4.Wenger A, Englund M, Wirth W, et al. Relationship of 3D meniscal morphology and position with knee pain in subjects with knee osteoarthritis: a pilot study. Eur Radiol 2012; 22:211–220 [DOI] [PubMed] [Google Scholar]
- 5.Swamy N, Wadhwa V, Bajaj G, Chhabra A, Pandey T. Medial meniscal extrusion: Detection, evaluation and clinical implications. Eur J Radiol 2018; 102:115–124 [DOI] [PubMed] [Google Scholar]
- 6.Rennie WJ, Finlay DB. Meniscal extrusion in young athletes: associated knee joint abnormalities. AJR Am J Roentgenol 2006; 186:791–794 [DOI] [PubMed] [Google Scholar]
- 7.Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol 2004; 33:569–574 [DOI] [PubMed] [Google Scholar]
- 8.Lynch JA, Roemer FW, Nevitt MC, et al. Comparison of BLOKS and WORMS scoring systems part I. Cross sectional comparison of methods to assess cartilage morphology, meniscal damage and bone marrow lesions on knee MRI: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2010; 18:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter DJ, Guermazi A, Lo GH, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 2011; 19:990–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis 2005; 64:556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding C, Martel-Pelletier J, Pelletier JP, et al. Knee meniscal extrusion in a largely non-osteoarthritic cohort: association with greater loss of cartilage volume. Arthritis Research & Therapy,9,2(2007-March-02) 2007; 9:R21–R21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Research & Therapy 2005; 8:R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada K, Yamaguchi S, Sato Y, et al. Comparison of meniscal extrusion and osteophyte formation at the intercondylar notch as a predictive biomarker for incidence of knee osteoarthritis-Data from the Osteoarthritis Initiative. Journal of Orthopaedic Science 2019; 24:121–127 [DOI] [PubMed] [Google Scholar]
- 14.Svensson F, Felson DT, Turkiewicz A, et al. Scrutinizing the cut-off for “pathological” meniscal body extrusion on knee MRI. European Radiology 2019; 29:2616–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisters MF, Veenhof C, van Dijk GM, Heymans MW, Twisk JW, Dekker J. The course of limitations in activities over 5 years in patients with knee and hip osteoarthritis with moderate functional limitations: risk factors for future functional decline. Osteoarthritis Cartilage 2012; 20:503–510 [DOI] [PubMed] [Google Scholar]
- 16.Heidari B Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian journal of internal medicine 2011; 2:205–212 [PMC free article] [PubMed] [Google Scholar]
- 17.Kijima H, Miyakoshi N, Kasukawa Y, et al. Cut-Off Value of Medial Meniscal Extrusion for Knee Pain. Adv Orthop 2017; 2017:6793026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology 1988; 15:1833–1840 [PubMed] [Google Scholar]
- 19.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum-Arthritis Care Res 2001; 45:384–391 [DOI] [PubMed] [Google Scholar]
- 20.Felix A, André A, Michel BA, Gerold S. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. Journal of Rheumatology 2002; 29:131–138 [PubMed] [Google Scholar]
- 21.Kemnitz J, Wirth W, Eckstein F, Ruhdorfer A, Culvenor AG. Longitudinal change in thigh muscle strength prior to and concurrent with symptomatic and radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2017; 25:1633–1640 [DOI] [PubMed] [Google Scholar]
- 22.Eckstein F, Collins JE, Nevitt MC, et al. Brief Report: Cartilage Thickness Change as an Imaging Biomarker of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis & Rheumatology 2015; 67:3184–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008; 16:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwaiger BJ, Mbapte Wamba J, Gersing AS, et al. Hyperintense signal alteration in the suprapatellar fat pad on MRI is associated with degeneration of the patellofemoral joint over 48 months: data from the Osteoarthritis Initiative. Skeletal Radiol 2018; 47:329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph GB, Nevitt MC, McCulloch CE, et al. Associations between molecular biomarkers and MR-based cartilage composition and knee joint morphology: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2018; 26:1070–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X Classification accuracy and cut point selection. Statistics in Medicine 2012; 31:2676–2686 [DOI] [PubMed] [Google Scholar]
- 27.Klein JS, Jose J, Baraga MG, Subhawong TK. Baseline Cartilage Thickness and Meniscus Extrusion Predict Longitudinal Cartilage Loss by Quantitative Magnetic Resonance Imaging: Data From the Osteoarthritis Initiative. J Comput Assist Tomogr 2016; 40:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa CR, Morrison WB, Carrino JA. Medial Meniscus Extrusion on Knee MRI: Is Extent Associated with Severity of Degeneration or Type of Tear? Am J Roentgenol 2004; 183:17–23 [DOI] [PubMed] [Google Scholar]
- 29.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage 1999; 7:526–532 [DOI] [PubMed] [Google Scholar]
- 30.Jones LD, Mellon SJ, Kruger N, Monk AP, Price AJ, Beard DJ. Medial meniscal extrusion: a validation study comparing different methods of assessment. Knee Surgery, Sports Traumatology, Arthroscopy 2018; 26:1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin S, LaValley MP, Guermazi A, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum 2005; 52:3152–3159 [DOI] [PubMed] [Google Scholar]
- 32.Hunter DJ, Zhang YQ, Tu X, et al. Change in joint space width: Hyaline articular cartilage loss or alteration in meniscus? Arthritis & Rheumatism 2006; 54:2488–2495 [DOI] [PubMed] [Google Scholar]
- 33.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Research & Therapy 2011; 13:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neogi T Structural correlates of pain in osteoarthritis. Clinical & Experimental Rheumatology 2017; 35 Suppl 107:75. [PubMed] [Google Scholar]
- 35.Svensson F, Felson DT, Zhang F, et al. Meniscal body extrusion and cartilage coverage in middle-aged and elderly without radiographic knee osteoarthritis. European Radiology 2019; 29:1848–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stehling C, Souza RB, Hellio Le Graverand MP, et al. Loading of the knee during 3.0T MRI is associated with significantly increased medial meniscus extrusion in mild and moderate osteoarthritis. Eur J Radiol 2012; 81:1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiba D, Maeda S, Sasaki E, et al. Meniscal extrusion seen on ultrasonography affects the development of radiographic knee osteoarthritis: a 3-year prospective cohort study. Clin Rheumatol 2017; 36:2557–2564 [DOI] [PubMed] [Google Scholar]
- 38.Chiba D, Sasaki E, Ota S, et al. US detection of medial meniscus extrusion can predict the risk of developing radiographic knee osteoarthritis: a 5-year cohort study. Eur Radiol 2020; 30:3996–4004 [DOI] [PubMed] [Google Scholar]
- 39.Kijima H, Miyakoshi N, Kasukawa Y, et al. Cut-Off Value of Medial Meniscal Extrusion for Knee Pain. Advances in Orthopedics 2017; 2017:6793026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.