Summary
Background:
Metabolic syndrome in adolescence has been associated with adverse cardiometabolic outcomes in adulthood. Preliminary data suggest that boys may have worsened metabolic syndrome components compared to girls. Yet, little is known about the physical health of military dependents, a potentially at-risk population.
Objective:
Examine sex differences in metabolic syndrome components in a sample of adolescent military dependents.
Methods:
Participants were adolescents (N = 139; 14.4 ± 1.6 years; 45.3% male; 41.0% non-Hispanic White, 19.4% non-Hispanic Black; BMI-z: 1.9 ± 0.4) at-risk for adult obesity and binge-eating disorder due to an age- and sex-adjusted BMI ≥85th percentile and loss-of-control eating and/or elevated anxiety. A multivariate analysis of covariance was conducted to compare objectively measured metabolic syndrome components across boys and girls. Covariates were age, race, loss-of-control eating status, anxiety symptoms, and BMI-z.
Results:
Metabolic syndrome components differed by sex (P = .01). Boys had higher systolic blood pressure (P = .049), lower high-density lipoprotein cholesterol (P = .01), and higher glucose (P = .001) than girls. Waist circumference, diastolic blood pressure, and triglycerides did not differ between boys and girls (P > .05).
Conclusions:
Future research should prospectively examine these relationships into adulthood. If the current findings are supported, prevention programs should consider targeting cardiometabolic health particularly among male adolescent military dependents.
Keywords: adolescents, metabolic syndrome, military, obesity, overweight, sex differences
Metabolic syndrome (MetS) is a cluster of risk factors for adverse cardiometabolic outcomes, such as type 2 diabetes and cardiovascular disease.1 MetS is diagnosed if at least three of the following five criteria are met: (a) central obesity, (b) elevated systolic and/or diastolic blood pressure, (c) low high-density lipoprotein cholesterol (HDL-C), (d) elevated triglycerides, and (e) elevated fasting glucose.1 The prevalence of MetS in adolescents in the United States is approximately 8.6%,2 and notably, worsened MetS components in adolescence are associated with an increased risk of adverse cardiometabolic outcomes in adulthood. e.g.,3
The majority of data comes from civilian samples and suggests that boys may have worsened MetS components compared to their female peers. In a representative sample of adolescents in the United States, descriptive data showed that more boys met diagnostic criteria for at least one MetS component than girls (55.5% vs 45.5%, respectively). In addition, boys had a higher prevalence of meeting full MetS criteria than girls (10.8% vs 6.1%, respectively), although this difference did not reach significance.2 Similarly, a cross-sectional study of treatment-seeking adolescents with severe obesity found that, compared to girls, boys had significantly higher triglycerides and systolic blood pressure, as well as lower HDL-C.4 In another sample of children and adolescents across all weight strata, boys had higher waist circumference, fasting glucose, and systolic blood pressure.5 However, the data are not entirely consistent. For example, one study found that girls were more likely to have multiple metabolic risk factors than boys, although interpreting this finding is complicated given that analyses did not adjust for body composition.6 In another sample of youths, waist circumference and systolic blood pressure were more frequently abnormal in boys than girls, but there were no significant differences in dysglycemia.7 Moreover, insulin resistance, HDL-C, and triglycerides were more frequently abnormal in girls than boys.7 However, these effects varied across pubertal stages.7 Taken together, findings from civilian samples suggest that adolescent boys may have worsened metabolic syndrome components than girls. However, given some contradictory results, future research is needed to examine sex differences in high-risk populations to inform future prevention and intervention efforts. Notably, sex differences in MetS components have not been examined in the children of service members (military-dependent children), a population that may be particularly vulnerable given their exposure to unique stressors such as frequent relocations and parental deployment.8 Indeed, MetS may be uniquely associated with stress. A recent meta-analysis of 30 studies in adults found that participants categorized as high-stress had a 45% higher chance of having MetS than adults in the low-stress groups.9 Stress may impact MetS through several different mechanisms, including behavioral (eg, decreased physical activity, poor diet quality) and physiological (eg, glucocorticoid resistance) pathways.9
Among adolescents in the United States, the most recent comparison suggests that military dependents have slightly lower rates of overweight/obesity than the general population. Among military dependents aged 12 to 17 years, an estimated 29.9% had overweight/obesity, compared to an estimated 33.6% (95% confidence interval: 30.9–36.5) of adolescents aged 12 to 19 years for the general population.10,11 Data also suggest high rates of disordered eating in military dependents,12 which have been associated with worsened cardiometabolic health e.g.,13 as well as a potentially exacerbated presentation compared to civilian youth.14 Contrasting with civilian data showing that adolescent girls are more likely to experience disordered eating than their male counterparts e.g.,15 few sex differences have been observed among adolescent military dependents.16 Lastly, the military is primarily comprised of males,17 and individuals who serve are more likely to have had a family member in the military,18 further emphasizing that adolescent dependents, particularly boys, are an important group to examine to ensure military readiness. Therefore, the objective of this study is to examine sex differences in MetS components of adolescent military-dependent boys and girls at-risk for adult obesity and eating disorders. We hypothesized that, after adjusting for body mass index and other relevant covariates, boys would present with worsened MetS components compared to girls.
1 |. METHODS
1.1 |. Participants and Procedure
Participants were a convenience sample of TRICARE-eligible (a health care program for United States service members and their families) male and female adolescent military dependents who completed baseline assessments prior to participating in an obesity and binge-eating disorder prevention trial (ClinicalTrials.gov identifier: NCT02671292). Eligible youth were identified by the Defense Enrollment Eligibility Reporting System and recruited through direct mailings, referrals from providers who care for adolescents with TRICARE benefits, and flyers posted, with permission, on military bases and listservs. Recruitment efforts were targeted towards parents of adolescents in the greater Washington, DC metropolitan area. Adolescents and parents/guardians provided written assent and consent, respectively. The study protocol was approved by the Uniformed Services University of the Health Sciences Institutional Review Board and the Fort Belvoir Community Hospital Research Office.
Adolescents were eligible if they were 12 to 17 years at the start of the study, had a BMI at or above the 85th percentile adjusted for age and sex,19 and were English-speaking. Youth were deemed at high-risk for adult obesity and binge-eating disorder based on either reports of at least one episode of loss-of-control eating during the previous 3 months or elevated anxiety symptoms.20,21 Adolescents were excluded if they had a major medical or psychiatric condition, weight loss during the past 3 months for any reason exceeding 3% of body weight, or if they self-reported current or recent pregnancy, regular use of prescription medications that affect appetite or body weight (unless weight stable for at least 3 months), or current involvement in psychotherapy or a structured weight loss program. Participants and their parents were assessed at the Uniformed Services University’s Developmental Research Laboratory on Eating and Weight Behaviors or the Family Medicine or Pediatric Clinics at Fort Belvoir Community Hospital.
1.2 |. Measures
1.2.1 |. Body Composition
Height and fasting weight were measured with clothes on and shoes removed to calculate BMI. BMI SD scores (BMI-z), accounting for age and sex, were then computed based on the Centers for Disease Control and Prevention standards.19 In a large study of youth, BMI-z was a stronger predictor of percentage body fat as measured by dual X-ray absorptiometry than BMI;22 therefore, it was used as the primary measure of adiposity in this study.
1.2.2 |. Metabolic syndrome components
Waist circumference (cm) was measured in triplicate at the iliac crest with a tension tape measure. Systolic and diastolic blood pressure (mmHg) were measured with the Life Source UA-789 Digital Blood Pressure Monitor (A&D Medical, San Jose, CA) while participants sat upright. High-density lipoprotein cholesterol (HDL-C; mg/dL), triglycerides (mg/dL), and glucose (mg/dL) were measured from fasting blood samples collected by trained phlebotomists. Blood drawn at both sites were analyzed on Cobas 6000 c 501 systems (Roche Diagnostics, Indianapolis, IN) using standard analytic procedures.23
1.2.3 |. Loss-of-Control eating
The Eating Disorder Examination interview v.14 OD/C.224 was administered by trained interviewers to assess the presence of loss-of-control eating episodes in the past 3 months. This semi-structured interview has shown excellent inter-rater reliability in adolescents.25
1.2.4 |. Anxiety
The trait subscale of the State-Trait Anxiety Inventory for Children was used to establish the anxiety eligibility criterion, with a score ≥ 32 indicating elevated anxiety.26 This questionnaire is a 20-item self-report measure of trait anxiety that has shown very good psychometric properties.26 In this sample, the trait subscale demonstrated acceptable internal consistency (Cronbach’s α = .77).
1.2.5 |. Depressive symptoms
Current depressive symptoms were measured with the widely used, valid, and reliable Beck Depression Inventory-II.27 Scores range from 0 to 63; higher scores indicate greater depression. The Beck Depression Inventory has demonstrated high internal consistency and factorial validity in assessing self-reported depression in adolescents.28 In this sample, the questionnaire demonstrated good internal consistency (Cronbach’s α = .84).
1.2.6 |. Perceived stress
Stress was assessed with the Perceived Stress Scale,29 a 14-item self-report measure of the extent to which participants perceive experiencing psychological stress in response to general life events that have occurred over the past month. The 14 items are averaged to create a total score; higher scores indicate greater perceived stress. The questionnaire has been validated in both community and laboratory samples.29 In this sample, the measure demonstrated acceptable internal consistency (Cronbach’s α = .76).
1.3 |. Data analytic plan
All analyses were conducted using IBM SPSS 25.0 (IBM Corp., Armonk, NY). Data were screened for outliers and normality. Across all variables, extreme but plausible outliers (defined a priori as at least three standard deviations from the mean; <1.5% of data points) were recoded to that threshold in order to retain these cases but minimize their influence on findings.30 Participant characteristics by sex were examined using independent samples t-tests or chi-square tests, as appropriate. To compare whether there were differences in the frequency of meeting MetS criteria for boys and girls, we used cut-off values commonly used in previous studies e.g.13 MetS was considered present if participants met at least three of the following criteria: abdominal obesity (≥90th percentile for age and sex), impaired fasting glucose (≥100 mg/dL), high triglycerides (≥90th percentile for age and sex), low HDL-cholesterol (≤10th percentile for age and sex), and high blood pressure (≥90th percentile for age, sex, and height).31–33
For the primary analyses, metabolic functioning was examined continuously due to the lack of consensus for clinical cut-offs for these components in youth.34 A multivariate analysis of covariance (MANCOVA) was conducted with sex (boys vs girls) as the independent variable and MetS components (waist circumference, systolic blood pressure, diastolic blood pressure, HDL-C, triglycerides, and glucose) as the dependent variables. Covariates were age in years (continuous), race (coded as 0 = non-Hispanic White or 1 = other), loss-of-control eating status (coded as 0 = absent and 1 = present), anxiety symptoms (continuous), and BMI-z (continuous). Analyses were repeated adjusting for perceived stress and depressive symptoms, given their potential influence on MetS components.35,36 All primary analyses were repeated, adjusting for BMI instead of BMI-z,to ensure that the pattern of findings remained similar regardless of the measure of adiposity used as a covariate. Lastly, to ensure that group differences in body composition did not influence findings, as a secondary analysis, the primary MANCOVA was repeated in a subset of the sample of boys and girls, matched on BMI-z; this MANCOVA did not include BMI-z as a covariate. All tests were two-tailed and findings were considered significant when P-values were less than .05. Eleven participants (7.9%) were missing perceived stress scores and were therefore not included in the model additionally adjusting for perceived stress and depressive symptoms.
2 |. RESULTS
2.1 |. Participant characteristics
One-hundred and thirty-nine adolescents (14.4 ± 1.5 years; 41.0% non-Hispanic White, 19.4% non-Hispanic Black; BMI-z: 1.9 ± 0.4) were studied. Seventeen (12.2%) of participants met criteria for MetS. Participant characteristics by sex for the sample are shown in Table 1. Boys were significantly younger (P = .01), had higher BMI-z (P = .001) and BMI percentile (P = .01), and reported fewer depressive symptoms (P < .001) and less perceived stress (P = .02). Boys had a higher waist circumference (P = .01), lower HDL-C (P = .001), higher glucose (P < .001), and were more likely to meet criteria for MetS (P = .01). No other characteristic significantly differed by sex (Ps > .05).
TABLE 1.
Participant characteristics by sex for entire sample (N = 139)
| Boys (n = 63) | Girls (n = 76) | p | |
|---|---|---|---|
| Age (years) | 13.9 (1.4) | 14.8 (1.5) | .01* |
| Race, n (%) | .69 | ||
| Non-Hispanic White | 27 (42.9) | 30 (39.5) | |
| Non-Hispanic Black | 11 (17.5) | 16 (21.1) | |
| Hispanic | 13 (20.6) | 15 (19.7) | |
| Other/unknown | 12 (19.0) | 15 (19.7) | |
| Visit location, n (%) | .54 | ||
| Fort Belvoir | 50 (79.4) | 57 (75.0) | |
| Uniformed Services University | 13 (20.6) | 19 (25.0) | |
| BMI | 30.2 (4.8) | 30.3 (3.8) | .94 |
| BMI-z | 2.1 (0.4) | 1.8 (0.3) | .001* |
| BMI percentile | 97.2 (2.5) | 96.0 (3.0) | .01* |
| Loss-of-control eatinga, n (%) | 39 (61.9) | 44 (57.9) | .63 |
| Anxiety | 37.7 (5.3) | 39.5 (6.5) | .07 |
| Depressive symptoms | 9.7 (5.3) | 14.9 (7.8) | <.001* |
| Perceived stress | 24.9 (6.8) | 27.7 (6.4) | .02* |
| Waist circumference (cm) | 100.1 (11.8) | 95.0 (10.9) | .01* |
| Systolic blood pressure (mmHg) | 120.6 (13.6) | 116.4 (11.5) | .05 |
| Diastolic blood pressure (mmHg) | 73.6 (10.3) | 72.9 (8.0) | .64 |
| HDL-C (mg/dL) | 47.8 (9.2) | 54.1 (11.7) | .001* |
| Triglycerides (mg/dL) | 101.6 (53.3) | 85.8 (44.4) | .06 |
| Glucose (mg/dL) | 90.0 (6.2) | 86.0 (5.8) | <.001* |
| MetSb, n (%) | .01 | ||
| Presence | 13 (20.6) | 4 (5.3) | |
| Absence | 50 (79.4) | 72 (94.7) |
Abbreviations: BMI-z, body mass index adjusted for age and sex; HDL-C, high-density lipoprotein cholesterol.
Notes: Data presented as M (SD) unless otherwise noted.
Loss-of-control eating presence in the past 3 months.
Metabolic syndrome (MetS) was defined as meeting three of the following five criteria: abdominal obesity (≥90th percentile for age and sex), impaired fasting glucose (≥100 mg/dL), high triglycerides (≥90th percentile for age and sex), low HDL-C (≤10th percentile for age and sex), and high blood pressure (≥90th percentile for age, sex, and height).
Group differences are significant at P < .05 for independent samples t-tests or Chi-square analyses, as appropriate.
2.2 |. Primary analyses
In the total sample, adjusting for BMI-z, there was a significant association between sex and MetS components, Pillai’s Trace = 0.13, F (6,127) = 3.24, P = .01. Univariate analyses indicated that boys had higher systolic blood pressure (boys: 120.9 ± 1.6 vs girls: 116.3 ± 1.5; P = .049), lower HDL-C (boys: 48.6 ± 1.3 vs girls: 53.5 ± 1.2; P = .01) and higher glucose (boys: 89.9 ± 0.8 vs girls: 86.2 ± 0.7; P = .001) than girls. No other component significantly differed by sex (Ps > .05). All univariate analyses are shown in Figure 1 and Table 2. When analyses were repeated, adjusted for perceived stress and depressive symptoms, the main effect of sex remained significant, Pillai’s Trace = 0.15, F(6,114) = 3.32, P = .01, and findings remained consistent such that boys had lower HDL-C (P = .01) and higher glucose (P < .001) than girls. With the additional covariates in the model, the relationship between sex and systolic blood pressure became nonsignificant (P = .07). When analyses were repeated using BMI as a covariate instead of BMI-z, the pattern of findings remained similar.
FIGURE 1.
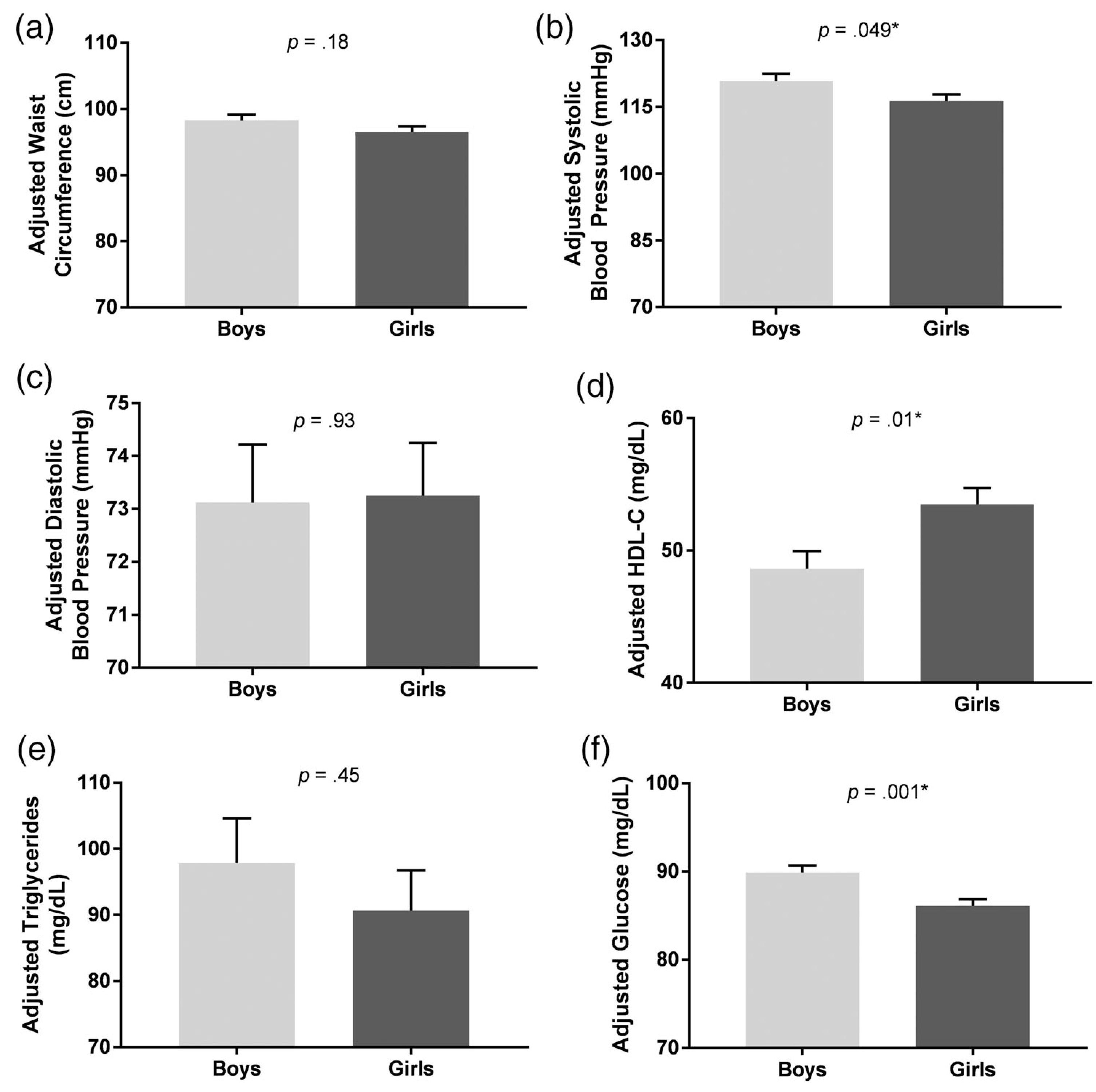
Associations of sex with waist circumference, systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol (HDL-C), triglycerides, and glucose, adjusting for age, race, loss-of-control eating status, anxiety, and BMI-z. Compared to girls, boys had (A) no difference in waist circumference, P = .18, (B) higher systolic blood pressure, P = .049, (C) no difference in diastolic blood pressure, P = .93, (D) lower HDL-C, P = .01, (E) no difference in triglycerides, P = .45, and (F) higher glucose, P = .001
TABLE 2.
Univariate analyses from significant (P = .01) MANCOVA in entire sample
| Boys (n = 67) (M ± SE) | Girls (n = 82) (M ± SE) | F | P | |
|---|---|---|---|---|
| Waist circumference (cm) | 98.27 ± 0.90 | 96.55 ± 0.82 | 1.84 | .18 |
| Systolic blood pressure (mmHg) | 120.87 ± 1.63 | 116.32 ± 1.48 | 3.94 | .049* |
| Diastolic blood pressure (mmHg) | 73.12 ± 1.10 | 73.26 ± 0.99 | 0.01 | .93 |
| HDL-C (mg/dL) | 48.62 ± 1.34 | 53.50 ± 1.21 | 6.69 | .01* |
| Triglycerides (mg/dL) | 97.82 ± 6.76 | 90.64 ± 6.11 | 0.57 | .45 |
| Glucose (mg/dL) | 89.89 ± 0.80 | 86.17 ± 0.72 | 11.37 | .001* |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; MANCOVA, multivariate analysis of covariance.
Notes: Adjusted for age, race, loss-of-control eating status, anxiety, and BMI-z.
Significant at P < .05.
2.3 |. Secondary analyses
A total of 116 participants (14.2 ± 1.5 years; 50% female; 39.7% non-Hispanic White, 20.7% non-Hispanic Black) drawn from the larger dataset were matched on BMI-z (boys: 2.0 ± 0.4 vs girls: 2.0 ± 0.2; P > .99). In this subsample, boys were significantly younger (P = .01), reported less anxiety (P = .02) and depressive symptoms (P < .001), less-perceived stress (P = .04), and had higher glucose (P = .003). No other characteristic significantly differed by sex (ps > .05).
When the MANCOVA was repeated in this subsample matched on BMI-z, the association between sex and MetS components remained significant, Pillai’s Trace = 0.16, F(6,103) = 3.24, P = .01. Similar to the primary analyses that did not adjust for perceived stress and depressive symptoms, univariate analyses indicated that boys had higher systolic blood pressure (boys: 121.3 ± 1.7 vs girls: 115.2 ± 1.7; P = .02) and glucose (boys: 89.8 ± 0.8 vs girls: 86.0 ± 0.8; P = .002), and lower HDL-C (boys: 48.1 ± 1.3 vs girls: 52.0 ± 1.3; P = .04) than girls. No other component significantly differed by sex (Ps > .05). All univariate tests for the BMI-z-matched cohorts are shown in Table 3.
TABLE 3.
Univariate analyses from significant (P = .01) MANCOVA in BMI-z matched subsample (n = 116)
| Boys (n = 58) (M ± SE) | Girls (n = 58) (M ± SE) | F | p | |
|---|---|---|---|---|
| Waist circumference (cm) | 98.93 ± 0.92 | 97.27 ± 0.93 | 1.53 | .22 |
| Systolic blood pressure (mmHg) | 121.29 ± 1.72 | 115.18 ± 1.74 | 5.94 | .02* |
| Diastolic blood pressure (mmHg) | 73.28 ± 1.17 | 73.46 ± 1.18 | 0.01 | .92 |
| HDL-C (mg/dL) | 48.08 ± 1.31 | 51.99 ± 1.32 | 4.21 | .04* |
| Triglycerides (mg/dL) | 102.16 ± 7.31 | 91.37 ± 7.37 | 1.03 | .31 |
| Glucose (mg/dL) | 89.77 ± 0.83 | 85.95 ± 0.83 | 10.09 | .002* |
Abbreviations: BMI-z, body mass index adjusted for age and sex; HDL-C, high-density lipoprotein cholesterol; MANCOVA, multivariate analysis of covariance.
Notes: Adjusted for age, race, loss-of-control eating status, and anxiety.
Significant at P < .05.
3 |. DISCUSSION
Adjusting for BMI-z, we found that adolescent military dependent boys had worsened MetS components compared with their female peers, including systolic blood pressure, HDL-C, and glucose. A similar pattern remained in analyses additionally adjusting for depressive symptoms and perceived stress, such that boys had lower HDL-C and higher glucose than girls. Lastly, within a subsample matched on BMI-z, the pattern of findings persisted, such that boys had worsened systolic blood pressure, glucose, and HDL-C than girls, supporting the notion that findings were not solely due to sex differences in BMI-z. These findings suggest that at an equivalent BMI, male adolescent military dependents may present with some worsened components of MetS than female adolescent military dependents.
These findings parallel the majority of previous research on sex differences in MetS components among civilian youth.2,4,5 There are several potential explanations for these observed sex differences. Lifestyle factors may partially explain these differences, as a previous study in treatment-seeking adolescents with severe obesity found that boys reported more screen time, higher intake of sugar-sweetened beverages and soda, and lower intake of vegetables compared to girls.4 Physiological differences may also explain worsened MetS components in boys vs girls. For example, males tend to have more visceral adiposity in the abdomen and are less sensitive to insulin action than females.37 Long-term testosterone exposure may also promote blood pressure elevation in boys, particularly in the presence of increased intra-abdominal adiposity.38 In comparison, female sex hormones such as estrogen may confer protective cardiometabolic effects for females, such as increased HDL-C.39 However, these potential lifestyle and physiological mechanisms were not examined in the current study. Notably, given that girls in the current sample reported more stress and depressive symptoms than boys, it might have been expected that girls would have had higher—or at least equivalent—MetS values. As this was not the case, future research should directly examine the behavioral, physiological and psychological mediators between sex and MetS components in adolescent military dependents.
The current findings may be particularly relevant for the military health system. It is estimated that approximately 30% of military dependents have overweight or obesity,40 and about 20% report disordered eating,12 with a potentially exacerbated severity compared to their civilian counterparts.14 Excess weight and obesity and associated comorbidities in military family beneficiaries is estimated to cost the TRICARE program over a billion dollars per year.41 As such, it is important to develop targeted interventions for military dependents to prevent and ameliorate the adverse physical outcomes associated with disordered eating and high weight. Notably, puberty appears to be a more critical period for a persistent increase in some blood pressure for boys than girls, potentially due to differences in testosterone.42 Systolic blood pressure is also a stronger predictor of cardiovascular risk than diastolic blood pressure43; therefore, the finding that boys had higher systolic blood pressure than their female counterparts suggests the potential need for prevention programs in adolescent military dependent boys.
Importantly, high weight and associated adverse physical outcomes may also significantly impact future military readiness.44 Compared to their civilian peers, military dependents are more likely to serve in the military as adults,18 and approximately four in five active duty military members are men.17 Excess weight is the leading cause of medical rejection from the military.45 In addition, the development of obesity-related comorbidities and/or eating disorders also precludes individuals from serving in the military. Therefore, adolescent military-dependent boys represent a particularly important population for prevention and/or early intervention to ensure that future military readiness is not impacted. In civilian samples, preliminary research suggests family-based lifestyle interventions46 or diet and exercise interventions47 may improve MetS components in youth with high weight. In another study, the remission of loss-of-control eating following obesity prevention programs was associated with improved glucose, HDL-C, and triglycerides at 6-month follow-up,48 suggesting that targeting loss-of-control eating may improve some MetS components. The effectiveness of these interventions, as well as other psychological and behavioral interventions, should be examined in adolescent military dependents.
Strengths of this study include the direct measurement of MetS components in a potentially vulnerable population. In addition, analyses adjusted for relevant covariates, such as BMI and psychopathology. However, certain limitations should be noted. First, we did not have a measurement of adiposity, such as dual-energy X-ray absorptiometry. However, previous research has shown a high correlation (r = .78) between age- and sex-adjusted BMI scores and adiposity as measured by dual-energy X-ray absorptiometry in youth.49 The study did not collect information on the family history of overweight/obesity or MetS, both of which may be important variables to consider when examining sex differences in MetS components. This study also did not have additional measures of cardiometabolic health, such as inflammatory markers or presence of non-alcoholic fatty liver disease, which would potentially have provided more insight into physiological differences between boys and girls. We also did not have a measure of pubertal status, which can differentially impact cardiometabolic outcomes across boys and girls,7 or relevant physiological data such as estrogen and testosterone concentrations.38,39 Therefore, it is unknown if our findings would have changed after adjusting for these potential covariates. Importantly, this analysis was cross-sectional, so temporality and causality of the observed relationships cannot be determined. Participants who used prescription medications that may impact appetite or body weight were included (albeit only if they were weight stable for at least 3 months), which potentially may have impacted our findings. Future research should examine the impact of medication status on the relationship between sex and metabolic syndrome components in adolescent military dependents.
Another consideration is that this sample consisted of adolescents with overweight/obesity, but without significant obesity-related medical comorbidities, who volunteered for a randomized, controlled interventional trial. Therefore, findings may not generalize to adolescents with more severe pathophysiology or to those not interested in an interventional study. However, examining differences in MetS components among adolescents prior to the development of clinical conditions is important for targeted prevention. While the degree of sex differences in MetS components in adolescence may be somewhat small with regard to clinical significance, these differences may point to increased risk of adverse clinical outcomes in the future. For example, previous research has shown that even a small increase within the non-clinical range of fasting glucose may increase the risk of developing type 2 diabetes in healthy young adults.50 Future research should address these limitations by prospectively examining sex differences in cardiometabolic health in military dependents across pubertal development and into adulthood. It would also be important to examine potential psychological, behavioural, and physiological mechanisms explaining these sex differences. If mechanisms are elucidated and sex differences are supported prospectively, developing targeted approaches to prevent worsening cardiometabolic health may be warranted.
In conclusion, after adjusting for body mass index, male adolescent military dependents had worsened metabolic syndrome components compared to their female counterparts. These findings are particularly relevant given that adverse physical outcomes in adolescent military dependents may significantly impact military health system costs as well as future military readiness. Research is required to prospectively examine mediators and moderators of the relationship between sex and cardiometabolic health to inform development and application of prevention and intervention programs to mitigate adverse cardiometabolic health outcomes.
ACKNOWLEDGEMENTS
L.M.S., M.T.K., M.K.H.N., and J.L. contributed to study design; all authors contributed to data collection; L.M.S. conducted data analysis and interpretation, completed the literature search, generated the figures and together with M.T.K. and M.K.H.N. wrote the first draft of the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [grant number 1R01DK104115–01 to MTK] and the Defense Health Agency [number MED 83–10180 to MH]. JAY is supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number ZIA-HD-00641]. The funding sources had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of Uniformed Services University of the Health Sciences or the United States Department of Defense.
Funding information
Defense Health Agency, Grant/Award Number: MED 83–10180; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: ZIA-HD-00641; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/ Award Number: 1R01DK104115–01
Abbreviations:
- HDL-C
high-density lipoprotein cholesterol
- BMI-z
BMI SD score, adjusted for age and sex
- MANCOVA
multivariate analysis of covariance
- MetS
metabolic syndrome
REFERENCES
- 1.Grundy SM, Brewer HBJ, Cleeman JI, Smith SCJ, Lenfant C. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/American heart association conference on scientific issues related to definition. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 2.Johnson WD, Kroon JJM, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: national health and nutrition examination survey (NHANES), 2001–2006. Arch Pediatr Adolesc Med. 2009;163(4):371–377. [DOI] [PubMed] [Google Scholar]
- 3.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton lipid research clinics follow-up study. Pediatrics. 2007;120 (2):340–345. [DOI] [PubMed] [Google Scholar]
- 4.Barstad LH, Juliusson PB, Johnson LK, et al. Gender-related differences in cardiometabolic risk factors and lifestyle behaviors in treatment-seeking adolescents with severe obesity. BMC Pediatr. 2018;18(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calcaterra V, Larizza D, De Silvestri A, et al. Gender-based differences in the clustering of metabolic syndrome factors in children and adolescents. J Pediatr Endocrinol Metab. 2020;33:279–288. [DOI] [PubMed] [Google Scholar]
- 6.Guimaraes RF, Silva MPD, Mazzardo O, et al. Metabolic risk factors clustering among adolescents: a comparison between sex, age and socioeconomic status. Cien Saude Colet. 2019;24(2):545–552. [DOI] [PubMed] [Google Scholar]
- 7.Guzzetti C, Ibba A, Casula L, Pilia S, Casano S, Loche S. Cardiovascular risk factors in children and adolescents with obesity: sex-related differences and effect of puberty. Front Endocrinol. 2019;10:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park N. Military children and families: strengths and challenges during peace and war. Am Psychol. 2011;66(1):65–72. [DOI] [PubMed] [Google Scholar]
- 9.Kuo WC, Bratzke LC, Oakley LD, Kuo F, Wang H, Brown RL. The association between psychological stress and metabolic syndrome: a systematic review and meta-analysis. Obes Rev. 2019;20(11):1651–1664. [DOI] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eilerman PA, Herzog CM, Luce BK, et al. A comparison of obesity prevalence: military health system and United States populations, 2009–2012. Mil Med. 2014;179(5):462–470. [DOI] [PubMed] [Google Scholar]
- 12.Waasdorp CE, Caboot JB, Robinson CA, Abraham AA, Adelman WP. Screening military dependent adolescent females for disordered eating. Mil Med. 2007;172(9):962–967. [DOI] [PubMed] [Google Scholar]
- 13.Tanofsky-Kraff M, Shomaker LB, Stern EA, et al. Children’s binge eating and development of metabolic syndrome. Int J Obes. 2012;36(7): 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schvey NA, Sbrocco T, Stephens M, et al. Comparison of overweight and obese military-dependent and civilian adolescent girls with loss-of-control eating. Int J Eat Disord. 2015;48(6):790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micali N, Ploubidis G, De Stavola B, Simonoff E, Treasure J. Frequency and patterns of eating disorder symptoms in early adolescence. Adolesc Health. 2014;54(5):574–581. [DOI] [PubMed] [Google Scholar]
- 16.Quattlebaum M, Burke NL, Higgins Neyland MK, et al. Sex differences in eating related behaviors and psychopathology among adolescent military dependents at risk for adult obesity and eating disorders. Eat Behav. 2019;33:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Office of the Under Secretary for Personnel and Readiness. Population Representation in the Military Services; 2016. https://www.dmdc.osd.mil/appj/dwp/dwp_reports.jsp.
- 18.Kleykamp MA. College, jobs, or the military? Enlistment during a time of war. Soc Sci Q. 2006;87(2):272–290. [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vit Health Stat. 2002; 246:1–190. [PubMed] [Google Scholar]
- 20.Tanofsky-Kraff M, Shomaker LB, Wilfley DE, et al. Excess weight gain prevention in adolescents: three-year outcome following a randomized controlled trial. J Consult Clin Psychol. 2017;85(3):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonneville KR, Horton NJ, Micali N, et al. Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: does loss of control matter? JAMA Pediatr. 2013;167(2):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heo M, Wylie-Rosett J, Pietrobelli A, Kabat GC, Rohan TE, Faith MS. US pediatric population-level associations of DXA-measured percentage of body fat with four BMI metrics with cutoffs. Int J Obes. 2014; 38(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gammeren AJ, van Gool N, de Groot MJ, Cobbaert CM. Analytical performance evaluation of the Cobas 6000 analyzer - special emphasis on trueness verification. Clin Chem Lab Med. 2008;46(6): 863–871. [DOI] [PubMed] [Google Scholar]
- 24.Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn C, Wilson G, eds. Binge Eating: Nature, Assessment, and Treatment. 12th ed. New York, NY: Guilford Press; 1993:317–360. [Google Scholar]
- 25.Tanofsky-Kraff M, Shomaker LB, Wilfley DE, et al. Targeted prevention of excess weight gain and eating disorders in high-risk adolescent girls: a randomized controlled trial. Am J Clin Nutr. 2014;100(4):1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spielberger G. Lushene, Vagg, Jacobs. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- 27.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 28.Wang YP, Gorenstein C. Psychometric properties of the Beck depression inventory-II: a comprehensive review. Braz J Psychiatry. 2013;35 (4):416–431. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 30.Osborne JW, Overbay A. The power of outliers (and why researchers should ALWAYS check for them). Res Eval. 2004;9:1–8. [Google Scholar]
- 31.Hickman TB, Briefel RR, Carroll MD, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: data from the third National Health and nutrition examination survey. Prev Med. 1998;27:879–890. [DOI] [PubMed] [Google Scholar]
- 32.National Heart, Lung, and Blood Institute. Update on the 1987 task force report on high blood pressure in children and adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on hypertension control in children and adolescents. Pediatrics. 1996;98:649–658. [PubMed] [Google Scholar]
- 33.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145(4):439–444. [DOI] [PubMed] [Google Scholar]
- 34.Agudelo GM, Bedoya G, Estrada A, Patiño FA, Muñoz AM, Velásquez CM. Variations in the prevalence of metabolic syndrome in adolescents according to different criteria used for diagnosis: which definition should be chosen for this age group? Metab Syndr Relat Disord. 2014;12(4):202–209. [DOI] [PubMed] [Google Scholar]
- 35.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. [DOI] [PubMed] [Google Scholar]
- 36.Shomaker LB, Tanofsky-Kraff M, Stern EA, et al. Longitudinal study of depressive symptoms and progression of insulin resistance in youth at risk for adult obesity. Diabetes Care. 2011;34(11):2458–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varlamov O, Bethea CL, Roberts CTJ. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol. 2015;5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syme C, Abrahamowicz M, Leonard GT, et al. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: sex differences and underlying mechanisms. Arch Pediatr Adolesc Med. 2008;162(5):453–461. [DOI] [PubMed] [Google Scholar]
- 39.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–1666. [DOI] [PubMed] [Google Scholar]
- 40.Bagchi A, Bencio K, Kim J, Lee M, Schone E. Health Care Survey of DoD Beneficiaries 2007 Annual Report. Washington, DC: Mathematica Policy Research, Inc; 2007. [Google Scholar]
- 41.Dall TM, Zhang Y, Chen YJ, et al. Cost associated with being overweight and with obesity, high alcohol consumption, and tobacco use within the military health system’s TRICARE prime-enrolled population. Am J Health Promot. 2007;22(2):120–139. [DOI] [PubMed] [Google Scholar]
- 42.Shankar RR, Eckert GJ, Saha C, Tu W, Pratt JH. The change in blood pressure during pubertal growth. J Clin Endocrinol Metab. 2005;90(1): 163–167. [DOI] [PubMed] [Google Scholar]
- 43.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51(4):1142–1148. [DOI] [PubMed] [Google Scholar]
- 44.Tanofsky-Kraff M, Sbrocco T, Theim KR, et al. Obesity and the US military family. Obesity (Silver Spring). 2013;21(11):2205–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Research Council. Introduction. In: Sackett PR, Mavor AS, eds. Assessing Fitness for Military Enlistment: Physical, Medical, and Mental Health Standards. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 46.Monzavi R, Dreimane D, Geffner ME, et al. Improvement in risk factors for metabolic syndrome and insulin resistance in overweight youth who are treated with lifestyle intervention. Pediatrics. 2006; 117(6):e1111–e1118. [DOI] [PubMed] [Google Scholar]
- 47.Ho M, Garnett SP, Baur LA, et al. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167(8):759–768. [DOI] [PubMed] [Google Scholar]
- 48.Shank LM, Tanofsky-Kraff M, Radin RM, et al. Remission of loss of control eating and changes in components of the metabolic syndrome. Int J Eat Disord. 2018;51(6):565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wohlfahrt-Veje C, Tinggaard J, Winther K, et al. Body fat throughout childhood in 2647 healthy Danish children: agreement of BMI, waist circumference, skinfolds with dual X-ray absorptiometry. Eur J Clin Nutr. 2014;68(6):664–670. [DOI] [PubMed] [Google Scholar]
- 50.Tirosh A, Shai I, Tekes-Manova D, et al. Normal fasting plasma glucose levels and type 2 diabetes in Young men. N Eng J Med. 2005; 353:1454–1462. [DOI] [PubMed] [Google Scholar]


