Abstract
Background
Despite significant progress with antiprogrammed cell death protein 1 (PD-1) therapy, a substantial fraction of metastatic melanoma patients show upfront therapy resistance. Biomarkers for outcome are missing and the association of baseline immune function and clinical outcome remains to be determined. We assessed the in vitro nonspecific stimulation of immune response at baseline and during anti-PD-1 therapy for metastatic melanoma.
Methods
Previously untreated metastatic melanoma patients received nivolumab and radiotherapy as part of the multicentric phase II trial NIRVANA (NCT02799901). The levels of Th1, Th2 and Th17 cytokines on in vitro non-specific stimulation of innate and adaptive immune cells were measured in patient sera before treatment, and at week 2 and week 6 after the beginning of the treatment, and correlated with tumorous response, progression-free survival (PFS) and occurrence of immune-related adverse events (irAEs). The results in melanoma patients were compared with those of a cohort of 9 sex and age-matched healthy donors.
Results
Seventeen patients were enrolled in this ancillary study. Median follow-up was 16 months (2.2–28.4). The 12-month PFS rate was 67.7%. The incidence of irAEs of any grade was 58.8%. Without in vitro stimulation no differences in cytokines levels were observed between responders and non-responders. On in vitro stimulation, metastatic patients had lower Th1 cytokine levels than healthy donors at baseline for tumor necrosis factor-α and interferon-γ (IFN-γ) (1136 pg/mL vs 5558 pg/mL, p<0.0001; and 3894 pg/mL vs 17 129 pg/mL, p=0.02, respectively). Responders exhibited increasing cytokine levels from baseline to week 6. Non-responders had lower interleukin 17A (IL-17A) levels at baseline than responders (7 pg/mL vs 32 pg/mL, p=0.03), and lower IFN-γ levels at week 6 (3.3 ng/mL vs 14.5 ng/mL, p=0.03). A lower level of IL-17A at week 2 and a lower level of IFN-γ at week 6 correlated with worse PFS (p=0.04 and p=0.04 respectively). At baseline, patients who developed irAEs had higher IL-6 levels (19.3 ng/mL vs 9.2 ng/mL, p=0.03) and higher IL-17A levels (52.5 pg/mL vs 2.5 pg/mL, p=0.009) than those without irAEs.
Conclusions
Our findings indicate that cytokine levels after in vitro non-specific stimulation could be a promising biomarker to predict the outcome of PD-1 inhibition therapy.
Keywords: immunity, cellular, immunocompetence, immunotherapy, programmed cell death 1 receptor, cytokines
Background
Immune checkpoint inhibitors (ICIs), based on the concept that cancer cells, can be targeted and eradicated by the immune system, have substantially improved clinical outcome in many types of cancer.1 For the treatment of melanoma, ICIs are represented by two major classes of monoclonal antibodies. The first one is targeted against the cytotoxic T-lymphocyte-associated antigen-4 (ipilimumab) and the second one against the programmed cell death protein 1 (PD-1; nivolumab or pembrolizumab) or its ligand (PD-L1). Despite significant progress with these agents, a substantial fraction of patients (~50% depending on therapeutic regimen) will not respond or will escape to ICI due to innate or acquired resistance.2 Moreover, severe immune-related adverse events (irAEs) that can sometimes be fatal can occur during immunotherapy.3 4 Therefore, biomarkers to predict the outcome of immunotherapy are strongly needed to guide individual treatment selection or sequence. To date, several studies aimed to identify biomarkers to predict outcome of ICI treatment, such as expression of PD-L1 within the tumor, tumor-infiltrating lymphocyte, T-cell receptor clonality, gene expression signature, peripheral blood markers and tumor mutational or neoantigen burden.5–9 Nevertheless, none of them can be considered as robust and reliable when selecting the patients who will benefit most from ICI treatment.
PD-1 blockade is believed to foster immune response by increasing Th1 and decreasing Th2 response,10 which may translate into an upheaval in cytokine concentrations such as Th1 pathway cytokines interferon-γ (IFN-γ) and interleukin 2 (IL-2), Th2 pathway cytokines IL-4 and IL-13, and Th17 pathway cytokine IL-17A.11 However, since a boosted immune system can backfire on non-cancerous cells, checkpoint inhibition exposes patients to an array of inflammatory irAEs involving various organs such as skin or the digestive tract.3 12 13 Patients who respond to ICI are more prone to irAEs compared with non-responders.14–17
Tarhini et al, demonstrated that baseline circulating IL-17A without in vitro stimulation predicts toxicity.18 But the capability of an individual’s immune system to respond to ICI and the factors that may predict the effectiveness of PD-1-blockade before treating patients remain to be determined. Functional tests provide a dynamic response and the individual’s ability to respond to immunological stimulation19 20 and has never been evaluated in patients treated with anti-PD-1 therapy.
The objective of this exploratory study was to assess the immune response, measured by a functional assay, of patients treated with anti-PD-1 antibodies for metastatic melanoma and to correlate these measures to the response to treatment and the occurrence of irAEs.
Patients, materials and methods
Patients, study design and treatment
NIRVANA is an ongoing multicentric phase 2 non-randomized prospective trial (ClinicalTrials.gov number NCT02799901) exploring the association of nivolumab (240 mg every 2 weeks until disease progression, intolerable toxicity, or withdrawal of consent) with multisite hypofractionned radiotherapy (performed on week 2 and week 4) in adults with previously untreated advanced melanoma (stage III unresectable or IV) without brain metastases. Written informed consent was obtained from all patients. The primary outcome is the overall survival. Biomarker assessment is a prespecified exploratory outcome of the trial and safety is one of the secondary endpoints.
Herein, blood specimens for correlative studies were planned at baseline (day 0), week 2 and week 6 from 17 patients included from the Department of Dermatology in Nice University Hospital. The cytokine profile was analyzed before and after non-specific stimulation of innate and adaptive immune system and then correlated with tumor response rate, progression-free survival (PFS) and incidence of irAEs. Baseline lymphocyte and monocyte counts were gathered to adjust the baseline cytokines levels on the respective amount of producing cells.
The results in melanoma patients were compared with those of a cohort of 9 age-matched healthy donors (2:1).
Response, survival and toxicity assessments
PFS was defined as the time from the start of nivolumab to the date of disease progression or death, whichever occurred first. Patients who were alive without disease progression were censored at the date of their last disease assessment. Overall survival was defined as the time from the start of nivolumab to death from any cause.
In terms of tumor response, patients were classified as either responders (complete response, partial response, stabilization) or non-responders.
Disease progression and tumor response were evaluated using the response evaluation criteria in solid tumors (V.1.1) by radiologists at each participating institution.21 In case of tumor progression, in order to exclude pseudoprogression, a second CT scan was performed 4 weeks after the last evaluation.
Data on irAE incidence were collected and graded according to the US National Cancer Institute common toxicity criteria for adverse events (V.4.0).
Blood collection and cytokine assay
Blood samples were taken to assess cytokine levels on day 0, week 2, week 6, and within 2 weeks of any progression. The 1 mL of whole blood was collected and stimulated with immune ligands (anti-CD3 as T-cells stimulant, and R848 as TLR 7/8 agonist to stimulate Natural Killer cells and foster T-cells’ response) on single lyophilized spheres (LyoSphere, Qiagen) within 8 hours from blood collection. Stimulated blood samples were incubated for 16 to 24 hours at 37°C and then centrifuged at 2000 to 3000 × g for 15 min to harvest the stimulated serum. Stimulated serum was stored at −20°C until the analysis and freeze-thaw cycles were minimized to preserve the quality of the samples. Serum levels of cytokines were measured either using the ProcartaPlex Immunoassay Kit (Luminex, ThermoFisher) or with custom-designed cartridges Ella (ProteinSimple). We measured 11 cytokines (IL-17A, IL-6, IL-1β, IFN-, IL-12p70, tumor necrosis factor-α (TNF-α), IL-10, IL-5, IL-4, IL-13 and GM-CSF) following the manufacturers’ instructions.22
Statistical analyses
For descriptive statistics, data are presented as mean and SD for continuous variables with Gaussian distribution, as median and IQR for continuous variables with non-Gaussian distribution, and as counts and percentages for categorical variables. The D’Agostino-Pearson normality test was used to determine if a variable had a Gaussian distribution or not. Groups of continuous values were compared by the Mann-Whitney test. Categorical variables were compared using χ2 test. Median follow-up was estimated with Schemper method. Event-time distributions were estimated with the use of the Kaplan-Meier method. Log-rank test was used to compare survival data. Comparisons were two tailed and statistical significance was set at p<0.05. To take into account the multiple testing problems, independent variables were sought with Spearman’s correlation method. Bonferroni correction was then applied to quantitative independent variables to adjust the statistical significance level for the comparisons of cytokines levels. Statistical analyses were performed using SPSS V.16.0 on Windows and GraphPad Prism V.7.0 (GraphPad Software).
Results
Patients’ characteristics and clinical outcomes
A total of 72 patients were included in the NIRVANA trial between April 2017 and June 2019. Eighteen patients were included in this ancillary study testing immune response, among them one was excluded due to positive hepatitis B virus serology according to the study’s non-inclusion criteria. Therefore, 17 patients were included in the current study. The median patient age was 72 years (IQR, 67.5–78.0), and a majority of the patients were male (n=13, 76.5%). Characteristics of patients are detailed in table 1. Over a median follow-up of 16 months (2.2–28.4), two patients died, yielding a 12-month overall survival rate of 83.3%. The tumor control rate was 64.7% (tumor stabilization (n=6), partial response (n=2) or complete response (n=3)). The remaining six patients (35.3%) presented immediate tumor progression. The 12-month PFS rate was 67.7%. The incidence of any grade of irAEs and grade 3–4 irAEs was 52.9% (n=9 patients) and 11.7% (n=2 patients), respectively.
Table 1.
Demographic, clinical characteristics and cytokines levels with in vitro stimulation, at baseline
| Characteristic | All (n=17) | Responders (n=11) | Non-responders (n=6) | P value |
| Median age (range) - years | 72 (53–88) | 73 (58–88) | 72 (53–81) | 0.84 |
| Sex (n) | ||||
| Male | 13 | 10 | 3 | 0.52 |
| Female | 4 | 1 | 3 | |
| ECOG-PS status (n (%)) | ||||
| 0 | 10 (59) | 8 | 2 | 0.28 |
| 1 | 5 (29) | 2 | 3 | |
| 2 | 2 (12) | 1 | 1 | |
| Metastasis stage (n (%)) | ||||
| M0, M1a, or M1b | 13 (76) | 8 | 5 | >0.99 |
| M1c | 4 (24) | 3 | 1 | |
| LDH (n) | ||||
| ≤ULN | 15 | 11 | 4 | 0.11 |
| >ULN | 2 | 0 | 2 | |
| BRAF status (n (%)) | ||||
| Mutation | 5 (29) | 2 | 3 | |
| No mutation | 12 (71) | 9 | 3 | 0.28 |
| Immune-related adverse events (n) | ||||
| Yes | 9 | 8 | 1 | |
| No | 8 | 3 | 6 | 0.04 |
| Last observation carried forward (days) | 540 (285; 735) | 600 (180; 750) | 360 (292; 810) | 0.94 |
| IL-1β/Monocytes (μg) | 6740 (2434; 12 572) | 6740 (1689; 20 266) | 5724 (2744; 8514) | 0.88 |
| TNF-α/Monocytes (μg) | 709 (261; 3373) | 1320 (537; 3899) | 2519 (371; 2519) | 0.29 |
| IL-6/Monocytes (μg) | 11857 (6321; 38 622) | 24280 (7170; 39 309) | 8529 (5827; 23 997) | 0.43 |
| IL-8/Monocytes (μg) | 4643 (3012; 10 859) | 3916 (3076; 12 219) | 5370 (2819; 13 909) | 0.66 |
| IFN-γ/Lymphocytes (μg) | 2639 (843; 11 479) | 3039 (941; 10 191) | 1656 (651; 11 864) | 0.86 |
| IL-12p70/Lymphocytes (μg) | 39 (15; 97) | 37 (16; 92) | 42 (14; 125) | 0.77 |
| IL-2/Lymphocytes (μg) | 60 (28; 129) | 70 (42; 137) | 34 (22; 98) | 0.25 |
| IL-17/Lymphocytes (μg) | 0.57 (0.54; 0.71) | 0.63 (0.56; 0.71) | 0.57 (0.40; 0.57) | 0.02* |
| IL-4/Lymphocytes (μg) | 21 (12; 51) | 25 (15; 63) | 17 (11; 109) | >0.99 |
| IL-5/Lymphocytes (μg) | 26 (19; 57) | 31 (22; 57) | 21 (18; 72) | 0.79 |
| IL-13/Lymphocytes (μg) | 18 (4; 78) | 20 (11; 76) | 8 (3; 93) | 0.67 |
Each result of baseline cytokines levels was divided by the baseline monocytes or lymphocytes counts.
*Tend to be stillstatistically significant with Bonferroni adjustment (p<0.02).
ECOG-PS, Eastern Cooperative Oncology Group - performance status; IFN, interferon; IL, interleukin; LDH, lactate dehydrogenase; TNF, tumor necrosis factor; ULN, Upper limit normal.
To validate our conditions, we first test on the seven first inclusions, the cytokine level with and without in vitro stimulation. Without in vitro stimulation of immune cells, the levels of cytokines were mostly undetectable at baseline and did not differ between patients whereas differences could be observed after in vitro stimulation (figure 1).
Figure 1.
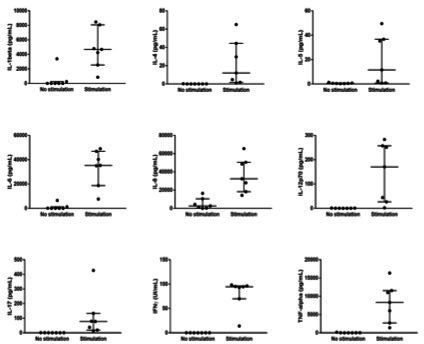
Effect of in vitro stimulation on the baseline levels of IL-1β, IL-4, IL-5, IL-6, IL-8, IL-12p70, IL-17, IFN-γ and TNF-α in the seven first patients included. before stimulation, cytokines are mostly undetectable. IFN-γ, interferon-γ; IL-8, interleukin 8; TNF-α, tumor necrosis factor-α.
We then compared inflammatory (IL-1 and IL-6) and Th1 (TNF-α and IFN-γ) baseline cytokine levels after in vitro stimulation from the 17 melanoma patients and 9 age and sex-matched healthy donors. Melanoma patients had significantly lower TNF-α, IFN-γ and IL-6 levels after in vitro stimulation compared with healthy donors (respectively p<0.0001, p=0.002, p=0.002, figure 2).
Figure 2.
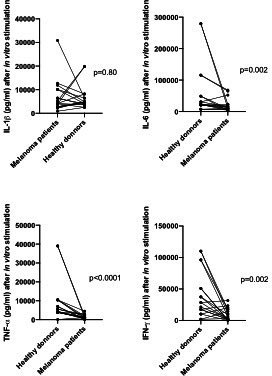
Comparison of baseline cytokines levels after stimulation in the 17 melanoma patients and in 16 age-matched healthy donors, showing that melanoma patients have significantly lower Th1 cytokines levels (TNF-α and IFN- γ). IFN-γ, interferon-γ; IL-16, interleukin 16; TNF-α, tumor necrosis factor-α.
Association between cytokines profiles after stimulation and PFS
We first explored the Th1 pathway: IFN-γ was significantly higher in responders compared with non-responders on week 6 (p=0.03, figure 3A), while IL-2 was not statistically different (p=0.13) between responders and non-responders (figure 3B). Moreover, PFS was significantly improved in patients with higher levels of IFN-γ at week 6 (p=0.04, figure 3C).
Figure 3.
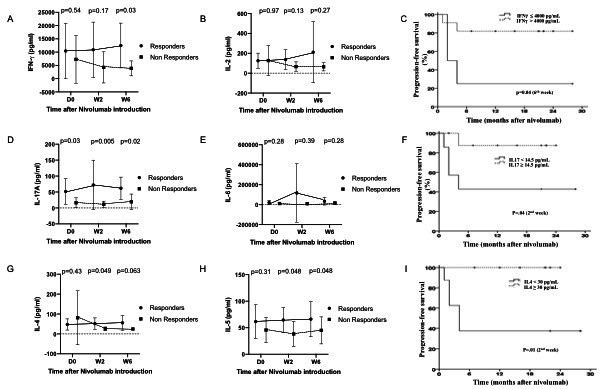
Cytokines levels of different immune pathways (Th1, Th2, Th17) after stimulation in responders and non-responders at baseline and during treatment with nivolumab (at week 2 and week 6). (A) IFN-γ levels in responders and non-responders at baseline and during treatment with nivolumab. IFN-γ levels become significantly higher in responders at week 6. (B) IL-2 levels in responders and non-responders at baseline and during treatment with nivolumab. Levels do not significantly differ between responders and non-responders. (C) Kaplan-Meier curves showing progression-free survival in patients with high and low IFN-γ levels at week 6. High IFN-γ levels at week 6 are associated with better progression free survival. (D) IL-17A levels in responders and non-responders at baseline and during treatment with nivolumab. IL-17A levels are significantly higher in responders at baseline as well as week 2 and week 6. (E) IL-6 levels in responders and non-responders at baseline and during treatment with nivolumab. Levels do not significantly differ between responders and non-responders. (F) Kaplan-Meier curves showing progression free survival in patients with high and low IL-17A levels at week 2. High IL-17A levels at week 2 are associated with better progression free survival. (G) IL-4 levels in responders and non-responders at baseline and during treatment with nivolumab. IL-4 levels become significantly higher in responders at week 2. (H) IL-5 levels in responders and non-responders at baseline and during treatment with nivolumab. IL-5 levels become significantly higher in responders at week 2. (I) Kaplan-Meier curves showing progression free survival in patients with high and low IL-4 levels at week 2. High IL-4 levels at week 2 are associated with better progression-free survival. IFN-γ, interferon-γ; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α.
We then explored Th17 pathway: IL-17A at baseline was significantly higher in responders compared with non-responders (p=0.03), as well as on week 2 (p=0.005) and week 6 (p=0.02, figure 3D), while IL-6 was not significantly different in responders compared with non-at each time point (figure 3E). PFS was significantly improved in patients with higher levels of IL-17A at week 2 (p=0.04, figure 3F). After adjustment on the baseline lymphocyte count, IL-17A was still significantly higher in responders compared with non-responders (p=0.02).
Cytokines from Th2 pathway also exhibited an increase during follow-up: IL-4 and IL-5 were slightly but significantly higher in responders compared with non-responders on week 2 (p=0.049 and p=0.048 respectively, figure 3G, H) and PFS was significantly improved in patients with higher levels of IL-4 at week 2 (p=0.01, figure 3I).
Clinical characteristics at baseline did not correlate with tumor response or PFS (table 1).
To adjust for multiple analysis problems, independence of variables was tested with Spearman’s method. All tested cytokines were correlated with IL-17A levels, whereas age and lactate dehydrogenase were independent (online supplemental file 1). Bonferroni’s correction was then applied to the statistical significance level, which yielded a corrected p at p<0.02 (table 1).
jitc-2021-002512supp001.pdf (934.8KB, pdf)
Baseline cytokine levels of IFN-γ, IL-17A and IL-4 did not significantly differ when complete and partial responders were compared with stable patients and non-responders (online supplemental file 2).
jitc-2021-002512supp002.pdf (48.8KB, pdf)
Association between cytokine profile after stimulation and irAEs
IrAEs were significantly more frequent in responders than non-responders (81.8% vs 16.7% respectively, p=0.04), and were also identified as a factor associated with improved prognosis for 1-year PFS (88.9% vs 38.1%, p=0.02). Patients that presented irAEs had significantly higher IL-6 levels before treatment with nivolumab on Day 0 than patients without irAEs (p=0.03), and the levels remained higher during the course of nivolumab treatment on week 2 (p=0.003) and week 6 (p=0.01, figure 4). The same observation was made with IL-17A levels (p=0.009 on day-0; p=0.04 on week 2; p=0.003 on week 6, figure 4), whereas IL-6 levels were comparable between responders and non-responders at each point (data not shown). IFN-γ levels in patients with irAEs were significantly higher on week 2 (p=0.018) and week 6 (p=0.01) but not on day 0 (p=0.25, figure 4).
Figure 4.
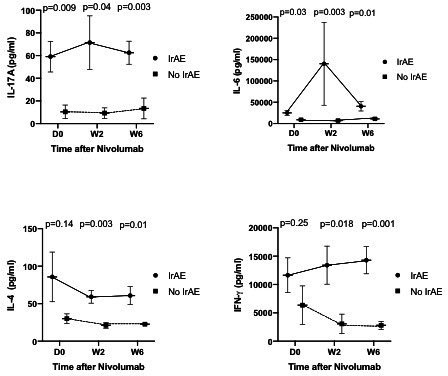
Cytokines (IL-17A, IL-6, IL-4, IFN-γ) levels after stimulation in patients with and without immune-related adverse events (irAE) at baseline and during treatment with nivolumab (at week 2 and week 6). IL-17A and IL-6 levels are consistently higher in patients with immune-related adverse events at each point, even at baseline. IL-4 and IFN-γ levels become both significantly higher in patients with immune related adverse events at week 2 and week 6. IFN-γ, interferon-γ; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α.
Discussion
Our exploratory results suggest that a functional assay measuring cytokines after non-specific stimulation of immune cells appears as a promising tool to identify patients that could benefit most of a treatment based on checkpoint inhibition and those at highest risk of irAEs. We have demonstrated in this cohort that patients with advanced melanoma at first line therapy are immunocompromised, with lower baseline Th1 cytokines levels (TNF-α and IFN-γ) compared with healthy donors. Furthermore, under treatment with ICI, responders exhibited a much stronger cytokine response than non-responders, reflecting successful activation of all inflammatory immune pathways (Th1, Th2, Th17). These findings confirm that response to ICI is in part determined by the preexisting ability of a patient’s immune system to react when stimulated.23 These results could only be observed after in vitro stimulation of immune cells.
The occurrence of irAEs was significantly higher in responders, and correlated with PFS, confirming the close pathophysiological link between tumor response and irAEs.14 Patients with irAEs had higher levels of IL-6, IL-17 and IFN-γ during treatment than the patients who did not present any irAEs. In fact, depending on the type of irAEs, Th1,24 Th225 as well as Th1726 27 pathways may be implicated in predisposed patients. Knowing more precisely which immune pathway is specifically activated when an irAEs occurs could allow for a more targeted approach to its management, while limiting the impact on tumor response.
Our results highlight the importance of all immune pathways in the response against cancer and more specifically the pivotal role of the Th17 pathway that is the only one predicting at baseline both tumor response and irAE occurrence in our cohort. Moreover, this result stood out even when the baseline IL-17A levels were related to the baseline lymphocyte counts. It has been demonstrated that IL-17A and/or Th17 cells may play a protective role in tumor immunity.11 28 In a case of skin and gastrointestinal irAEs induced by PD-1 inhibition with pembrolizumab, specific IL-17 blockade by secukinumab provided dramatic relief of the irAEs. Unfortunately, inhibition of the IL-17 pathway resulted in loss of ICI effectiveness and an upturn in tumor growth.26 As demonstrated in our cohort, this observation suggests that Th17 pathway is implicated in both the effectiveness of ICI and is the trigger of irAEs.
Our study has several limitations. This is a monocentric cohort of predominantly male patients. All patients were treated for melanoma, which hinders any hasty extrapolation to other types of cancer pending further dedicated studies. Patients also received radiotherapy with barely known specific effects on immunity.29 Multiple testing problems does not allow us to draw definite conclusions regarding the minimally significant variations of the cytokines belonging to the Th2 pathway and the study was not powered enough to find different baseline cytokine levels between responders, stable patients, and non-responders. Variation in cytokines levels after stimulation could partly reflect the concentration of lymphocytes and monocytes: this is why we correlated the cytokines levels with the lymphocyte or monocyte counts to mitigate this potential confounding factor. Furthermore, thresholds for cytokines levels were not defined a priori for PFS assessment. Our results on different cytokines levels are not independent and the size of the cohort is relatively limited precluding any multivariate analysis, which warrants further confirmation of our exploratory results in larger cohorts. This is likely why the classical prognosis factors of melanoma could not predict tumor response and PFS. Nevertheless, it is all the more striking that baseline IL-17A level reached significance in spite of a small number of subjects enrolled. This result must be confirmed on a validation cohort.
A functional immune assay underscores the implication of Th1, Th2 and Th17 pathways in the response to checkpoint inhibition and in the pathophysiology of irAEs. There was no significant variation of the cytokines levels before stimulation which shows the importance of the in vitro stimulation. The advantages of such assay are its simplicity and that it can be easily extended to other types of cancers as a ready to use tool using non-specific tumorous antigens for lymphocytic activation but detecting anergic lymphocytes thus identifying immunocompromised patients.
For the first time, we demonstrate a correlation between PFS, irAEs, and the Th17 immune pathway implicated in autoimmunity. These findings pave the way to an individualized tailored approach for the treatment of advanced malignancies: (1) combination of an anti-PD-1 with another ICI in case of poor immune reactivity/function; (2) restoration of immune function in immunocompromised patients before ICI and (3) identification of cytokine levels associated with tumorous response with limited irAEs. Further studies should confirm in larger cohorts the predictive values of IL-17A and other cytokines in the response to checkpoint inhibition and the occurrence of irAE.
Acknowledgments
We thank the patients who participated in this study, their families, and the staff members at the study sites who cared for them. We thank Nadège Parassol for useful comments. She received no compensation for this work.
Footnotes
AG and JD contributed equally.
HM and BS-P contributed equally.
Contributors: BS-P, JD and HM designed the study. AG, MC and VB carried out experiments. BS-P, AG, JD, HM and VB analysed and interpreted the data. BS-P, JD, KZ and LB performed statistical analysis. BS-P, AG, JD, HM, MC, CR-C, LT, AP-G and TP provided medical oversight. AG, BS-P, JD and HM drafted and revised the manuscript. All authors approved the final version of the manuscript.
Funding: This work was in part supported by Bristol-Myers Squibb and Cancéropôle Provence-Alpes-Côte d’Azur.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Study protocol was approved by French ethics committee (CPP PACA XI, n°2016-001381-29), the local ethic committee and participating institutions.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrova V, Arkhypov I, Weber R, et al. Modern aspects of immunotherapy with checkpoint inhibitors in melanoma. Int J Mol Sci 2020;21:2367. 10.3390/ijms21072367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med Overseas Ed 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 4.Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–75. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542–51. 10.1016/S1470-2045(16)30406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fares CM, Van Allen EM, Drake CG, et al. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book 2019;39:147–64. 10.1200/EDBK_240837 [DOI] [PubMed] [Google Scholar]
- 8.Gide TN, Wilmott JS, Scolyer RA, et al. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res 2018;24:1260–70. 10.1158/1078-0432.CCR-17-2267 [DOI] [PubMed] [Google Scholar]
- 9.Wood MA, Weeder BR, David JK, et al. Burden of tumor mutations, neoepitopes, and other variants are weak predictors of cancer immunotherapy response and overall survival. Genome Med 2020;12:33. 10.1186/s13073-020-00729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulos J, Carven GJ, van Boxtel SJ, et al. Pd-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother 2012;35:169–78. 10.1097/CJI.0b013e318247a4e7 [DOI] [PubMed] [Google Scholar]
- 11.Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol 2018;15:458–69. 10.1038/s41423-018-0004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davar D, Kirkwood JM. Pd-1 immune checkpoint inhibitors and immune-related adverse events: understanding the upside of the downside of checkpoint blockade. JAMA Oncol 2019;5:942–3. 10.1001/jamaoncol.2019.0413 [DOI] [PubMed] [Google Scholar]
- 13.Chang C-Y, Park H, Malone DC, et al. Immune checkpoint inhibitors and immune-related adverse events in patients with advanced melanoma: a systematic review and network meta-analysis. JAMA Netw Open 2020;3:e201611. 10.1001/jamanetworkopen.2020.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortellini A, Buti S, Agostinelli V, et al. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol 2019;46:362–71. 10.1053/j.seminoncol.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol 2020;6:519–27. 10.1001/jamaoncol.2019.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan L, Hwang SJE, Byth K, et al. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J Am Acad Dermatol 2020;82:311–6. 10.1016/j.jaad.2019.06.035 [DOI] [PubMed] [Google Scholar]
- 17.Verheijden RJ, May AM, Blank CU, et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and Anti-PD1-Treated patients in the Dutch melanoma treatment registry. Clin Cancer Res 2020;26:2268–74. 10.1158/1078-0432.CCR-19-3322 [DOI] [PubMed] [Google Scholar]
- 18.Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:39. 10.1186/s40425-015-0081-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cremoni M, Brglez V, Perez S, et al. Th17-Immune response in patients with membranous nephropathy is associated with thrombosis and relapses. Front Immunol 2020;11:574997. 10.3389/fimmu.2020.574997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with Chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol 2021;157:202–6. 10.1001/jamadermatol.2020.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22.Boyer-Suavet S, Cremoni M, Dupeyrat T, et al. Functional immune assay using interferon-gamma could predict infectious events in end-stage kidney disease. Clin Chim Acta 2020;502:287–92. 10.1016/j.cca.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki N, Kiyohara Y, Uhara H, et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci 2017;108:1022–31. 10.1111/cas.13226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshino K, Nakayama T, Ito A, et al. Severe colitis after PD-1 blockade with nivolumab in advanced melanoma patients: potential role of Th1-dominant immune response in immune-related adverse events: two case reports. BMC Cancer 2019;19:1019. 10.1186/s12885-019-6138-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka R, Okiyama N, Okune M, et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-α is a biomarker of nivolumab recativity. J Dermatol Sci 2017;86:71–3. 10.1016/j.jdermsci.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 26.Esfahani K, Miller WH. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med 2017;376:1989–91. 10.1056/NEJMc1703047 [DOI] [PubMed] [Google Scholar]
- 27.Callahan MK, Yang A, Tandon S, et al. Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. Journal of Clinical Oncology 2011;29:2505. 10.1200/jco.2011.29.15_suppl.2505 [DOI] [Google Scholar]
- 28.Kryczek I, Wei S, Szeliga W, et al. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 2009;114:357–9. 10.1182/blood-2008-09-177360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities Leveraging immunity for the next oncology practice. CA Cancer J Clin 2017;67:65–85. 10.3322/caac.21358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002512supp001.pdf (934.8KB, pdf)
jitc-2021-002512supp002.pdf (48.8KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data are available.


