Abstract
Background
Head and neck squamous cell carcinomas (HNSCCs) are common malignancies caused by carcinogens, including tobacco and alcohol, or infection with human papillomavirus (HPV). Immune checkpoint inhibitors targeting the programmed cell death 1 (PD-1) pathway are effective against unresectable recurrent/metastatic HNSCC. Here, we explored the safety and efficacy of anti-PD-1 therapy in at-risk resectable HPV-positive and HPV-negative HNSCC in the neoadjuvant setting.
Methods
The phase I/II CheckMate 358 trial in virus-associated cancers assessed neoadjuvant nivolumab in patients with previously untreated, resectable HPV-positive or HPV-negative HNSCC. Patients received nivolumab 240 mg intravenously on days 1 and 15, with surgery planned by day 29. Safety/tolerability (primary endpoint) was assessed by monitoring adverse events (AEs) and surgical delays. Radiographic response was measured before surgery using RECIST v1.1, adapted for a single post-nivolumab evaluation. Pathologic specimens were examined for treatment response using immune-based criteria.
Results
From November 2015 to December 2017, 52 patients with AJCC (seventh edition) stage III–IV resectable HNSCC received neoadjuvant nivolumab (26 HPV-positive, 26 HPV-negative). Any-grade treatment-related AEs (TRAEs) occurred in 19 patients (73.1%) and 14 patients (53.8%) in the HPV-positive and HPV-negative cohorts, respectively; grade 3–4 TRAEs occurred in five (19.2%) and three patients (11.5%), respectively. No patient had a protocol-defined TRAE-related surgical delay (>4 weeks). Thirty-eight patients were reported as undergoing complete surgical resection, 10 had a planned post-nivolumab biopsy instead of definitive surgery due to a protocol misinterpretation, and four did not undergo surgery or biopsy, including two with tumor progression. Radiographic response rates in 49 evaluable patients were 12.0% and 8.3% in the HPV-positive and HPV-negative cohorts, respectively. There were no complete pathologic responses by site or central review in operated patients. Among 17 centrally evaluable HPV-positive tumors, one (5.9%) achieved major pathological response and three (17.6%) achieved partial pathologic response (pPR); among 17 centrally evaluable HPV-negative tumors, one (5.9%) achieved pPR.
Conclusions
Neoadjuvant nivolumab was generally safe and induced pathologic regressions in HPV-positive (23.5%) and HPV-negative (5.9%) tumors. Combinatorial neoadjuvant treatment regimens, and continued postoperative therapy for high-risk tumors, are warranted in future trials to enhance the efficacy of this approach.
Trial registration number
ClinicalTrials.gov NCT02488759; https://clinicaltrials.gov/ct2/show/NCT02488759.
Keywords: clinical trials as topic, head and neck neoplasms, immunotherapy
Background
Head and neck squamous cell carcinomas (HNSCCs) are among the most common malignancies worldwide.1 Tobacco use and alcohol consumption are well-known primary risk factors for HNSCC.2 3 More recently, infection with human papillomavirus (HPV) has been identified as an increasingly significant risk factor for oropharyngeal cancer (OPC), with an estimated prevalence of >70% in OPC in North America.4 5 HPV-negative and HPV-positive HNSCCs are genetically distinct: HPV-negative HNSCC is characterized by genetic complexity, nearly universal TP53 mutations, frequent alterations in cell cycle regulator oncogenes, and overexpression of epidermal growth factor receptor (EGFR); in contrast, HPV-positive HNSCC is associated with wild-type TP53, activating PIK3CA mutations, lower EGFR expression, and upregulation of the tumor suppressor protein p16, which is widely used as a surrogate marker of HPV infection in OPC.6–8
Likely as a result of these genetic and potentially immunologic differences, HPV-positive tumors have been associated with improved prognosis and better treatment response.9–11 Moreover, as smoking and HPV infection are not mutually exclusive risk factors, tobacco exposure is associated with a worse prognosis even in patients with HPV-positive HNSCC.12 13 Although HPV-negative and HPV-positive HNSCCs have distinct genetic profiles,7 there is conflicting evidence on whether their respective overall tumor mutational burdens (TMBs) differ substantially.14–16 Due to the distinct etiologic and prognostic differences based on HPV status, we hypothesized that enrollment and analysis of these two different subgroups might yield insights into genetic, transcriptomic, and immunologic characteristics of pathologic and clinical response.
Immune checkpoint inhibitors targeting interactions of programmed cell death 1 (PD-1) with programmed cell death ligand 1 (PD-L1) have emerged as effective treatments for some patients with recurrent or metastatic HNSCC, with improved efficacy and safety versus standard-of-care regimens.17–21 In studies of anti-PD-1 (nivolumab, pembrolizumab) or anti-PD-L1 (durvalumab) monotherapy for recurrent/metastatic HNSCC, objective response rates (ORRs) ranged from 13.3% to 17.9%, but observed benefits over standard regimens were most pronounced for overall survival (OS), which was generally doubled at 12 months.17–21 Subgroup analyses showed that ORR benefit with immune checkpoint inhibitors was generally greater in patients having tumors with higher PD-L1 expression levels17–21; however, with nivolumab, OS benefit was seen regardless of PD-L1 status.18 Similarly, ORR was higher in HPV-positive disease, but OS benefit was observed regardless of tumor HPV status.11 Since response kinetics differ based on PD-L1 and HPV status, on-treatment tissue analyses paired with functional anatomic imaging would be valuable.22
Because of the benefits observed with anti-PD-(L)1 therapy in the recurrent/metastatic setting, there is increased interest in investigating immunotherapy earlier in the course of HNSCC. Preoperative targeting of micrometastatic disease using neoadjuvant immunotherapy might prevent local or distant disease recurrence in patients with resectable advanced HNSCC, and potentially reduce the extent of surgical resection.23 Although neoadjuvant anti-PD-(L)1 therapies have been explored in other tumor types,24–27 information in HNSCC is limited. Three small trials, all conducted at one or two centers, with two focused exclusively on HPV-negative HNSCC and one on OPC (86% HPV-positive), have shown encouraging early results.22 28 29 Here, we report results from the CheckMate 358 trial, which is the first to assess the safety and potential efficacy of neoadjuvant anti-PD-1 in both HPV-positive and HPV-negative HNSCC cohorts.
Methods
Study design, patients, and treatment
CheckMate 358 is a global multicenter, multicohort phase I/II trial of nivolumab (alone or in combination regimens) in recurrent/metastatic or neoadjuvant settings for patients with virus-associated malignancies. Two neoadjuvant HNSCC cohorts, including 52 patients with HPV-positive or HPV-negative tumors, were enrolled at 10 sites in the USA and Europe. Patients were ≥18 years of age with newly diagnosed, pathologically confirmed squamous cell or undifferentiated carcinoma of the oral cavity, pharynx, and/or larynx, and an Eastern Cooperative Oncology Group performance status of 0–1. Tumors were amenable to pretreatment biopsy and deemed surgically resectable, with a requirement for ≥T1 primary lesions and ≥N1 nodal disease.30 Patients with active brain metastases, prior malignancy within ≤3 years, autoimmune disorders, conditions requiring systemic immunosuppressive treatment, or prior exposure to antitumor vaccines or T-cell modulating drugs were excluded.
Before enrollment, tumor HPV status was determined at study sites or centrally in all patients using p16INK4a (p16) immunohistochemistry (IHC, n=46), HPV in situ hybridization (n=4), or PCR (n=2). p16 IHC was deemed positive if >70% of tumor cells showed strong and diffuse nuclear and cytoplasmic staining.31 Tested samples were derived from the primary tumor or a lymph node metastasis.
Patients received nivolumab 240 mg intravenously on days 1 and 15, with surgery scheduled on day 29 (±7 days). Administration of the second nivolumab dose could be delayed ≤7 days. Postoperative (adjuvant) treatment was allowed at the investigator’s discretion if nivolumab-related toxicities had resolved to grade ≤1. For unresectable recurrent/metastatic disease occurring ≤12 months postoperatively or the end of adjuvant therapy (whichever is later), medically eligible patients could resume on-study nivolumab treatment every 2 weeks for ≤2 years.
Study endpoints
The primary endpoint was safety/tolerability of neoadjuvant nivolumab, evaluated by the incidence of treatment-related adverse events (TRAEs) and surgical delays (protocol-defined as a TRAE-related delay >4 weeks from scheduled surgery date). Exploratory endpoints included pathologic response (assessed by study investigators (site review), or by a head and neck pathologist (SIC) from UPMC with independent subset review by a pathologist (JMT) from the Sidney Kimmel Comprehensive Cancer Center (central review)), radiographic response (change in sum of diameters of target lesions measured once before surgery using adapted Response Evaluation Criteria in Solid Tumors (RECIST) v1.1),32 recurrence-free survival (RFS, time from surgery to recurrence per investigator or death from any cause, whichever occurred first), OS (time from first nivolumab dose to date last known alive or death), and immunologic changes in blood and tumor.
Response assessment, event schedules, survival monitoring, and follow-up were conducted as previously described for the neoadjuvant Merkel cell carcinoma cohort of CheckMate 358.27 AEs were assessed per National Cancer Institute Common Terminology Criteria for Adverse Events v4 by system organ class and MedDRA preferred terms.33 The CheckMate 358 trial included a separate neoadjuvant cohort for patients with HPV-positive gynecologic cancers, in which neoadjuvant nivolumab could be followed by biopsy and definitive standard-of-care chemoradiation rather than surgical resection, at the discretion of the investigator. This was misinterpreted by two investigators to pertain to the neoadjuvant HNSCC cohorts as well, leading to approximately 20% of patients receiving planned post-nivolumab tumor biopsies instead of curative-intent surgical resection.
Pathologic response assessment and PD-L1 immunohistochemistry
Site pathology reviews categorized patients as achieving pathologic complete response (pCR) or non-pCR. Central review categorized patients as achieving pCR (no residual viable tumor (RVT) on H&E evaluation of completely resected tumor specimens including all sampled lymph nodes), major pathologic response (MPR, ≤10% RVT), or pathologic partial response (pPR, >10% to 50% RVT),34 based on immune-related pathologic response criteria.35 36 Tumors undergoing planned biopsy without complete resection, due to a misinterpretation of the protocol, were also evaluated for pathologic response using major pathologic response on biopsy (MPRbx) criteria.37 MPRbx criteria were developed to assess early on-treatment tumor biopsies from patients with advanced unresectable melanoma receiving immune checkpoint blockade therapy, and correlated significantly with OS.
Tumor PD-L1 expression was evaluated in recent archival or fresh pretreatment biopsies using automated IHC (PD-L1 IHC 28-8 pharmDx assay; Dako, an Agilent Technologies, Inc. company, Santa Clara, CA, USA). PD-L1-positive tumors had ≥1% of tumor cells with cell surface expression at any intensity. PD-L1 expression by tumor cells and/or tumor-associated immune cells was determined using a combined positive score (CPS; number of PD-L1-positive cells (tumor cells, lymphocytes, macrophages) /total number of viable tumor cells×100).
Whole-genome sequencing and gene expression profiling
Whole-genome sequencing (Illumina, San Diego, CA, USA) was used to assess TMB, defined as the total number of missense mutations per exome within a tumor sample relative to normal host tissue. Tumor and normal tissue samples were sequenced at a minimum of 50× and 25× coverage, respectively. A minimum of 20× coverage was achieved for ≥80% of target bases. Missense somatic mutations were counted if they were called by both Strelka2 (https://github.com/Illumina/strelka) and TNscope (https://github.com/Sentieon/sentieon-google-genomics). Gene expression was profiled by RNA sequencing (RNAseq) using pretreatment tumor samples. The samples were sequenced to a minimum depth of 85 million paired end reads with 50 base pair read length. Sequencing quality was assessed using the Picard QC tool kit (V.1.14, http://broadinstitute.github.io/picard/). Samples were required to have ≥85% of total reads aligned to the genome. Inflammation gene signature scores were derived using single-sample gene set enrichment analysis for a signature comprising 13 genes: CCL2, CCL3, CCL4, CD8A, CXCL9, CXCL10, GZMK, HLA-DMA, HLA-DMB, HLA-DOA, HLA-DOB, ICOS, and IRF1.38 Immune checkpoint gene expression (PDCD1 (PD-1), CD274 (PD-L1), and LAG3) was also assessed.
Statistical analysis
Enrollment of ≥42 evaluable patients was planned (≥21 patients each for the HPV-positive and HPV-negative cohorts) based on estimation precision of safety events and pathologic response rates (online supplemental methods 1), with “evaluable” patients defined as those with available paired pre-treatment (screening) and post-treatment (day 29) tumor samples. Safety was summarized in treated patients using descriptive statistics. Pathologic and radiographic responses were evaluated only in patients receiving ≥1 nivolumab dose with relevant baseline and/or post-baseline assessments. Kaplan-Meier techniques were used to estimate RFS and OS (online supplemental methods 2).39 Methods for reporting of median RFS and OS, associated RFS and OS rates, and corresponding 95% CIs have been previously described.27
jitc-2021-002568supp001.pdf (549.2KB, pdf)
jitc-2021-002568supp002.pdf (1.3MB, pdf)
Results
Patient, tumor, and treatment characteristics
Between November 2015 and December 2017, 52 patients with HNSCC received neoadjuvant nivolumab in CheckMate 358; 26 patients had HPV-positive and 26 had HPV-negative disease. Database lock occurred on July 31, 2020; median (range) follow-up was 38.2 months (18.8 to 52.5) and 27.9 months (1.0 to 53.9) in the HPV-positive and HPV-negative cohorts, respectively. Median age was 62.5 and 59.5 years, respectively (table 1).
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic | HPV-positive (n=26) |
HPV-negative (n=26) |
| Age, years, median (range) | 62.5 (34–82) | 59.5 (42–85) |
| Sex* | ||
| Male | 21 (80.8) | 17 (65.4) |
| Female | 5 (19.2) | 9 (34.6) |
| Region* | ||
| USA/Canada | 16 (61.5) | 18 (69.2) |
| Europe | 10 (38.5) | 8 (30.8) |
| Race | ||
| White | 23 (88.5) | 24 (92.3) |
| Black or African American | 1 (3.8) | 0 (0.0) |
| Other/not reported | 2 (7.7) | 2 (7.7) |
| AJCC (seventh edition) stage at study entry30 | ||
| III | 4 (15.4)† | 3 (11.5)† |
| IV | 22 (84.6) | 23 (88.5)‡ |
| IVA§ | 21 (80.8) | 22 (84.6) |
| IVB§ | 1 (3.8) | 0 (0.0) |
| ECOG performance status* | ||
| 0 | 20 (76.9) | 16 (61.5) |
| 1 | 6 (23.1) | 10 (38.5) |
| Smoking status¶ | ||
| Never | 5 (19.2) | 9 (34.6) |
| Former** | 17 (65.4) | 6 (23.1) |
| Current | 4 (15.4) | 11 (42.3) |
| Tumor cell PD-L1 expression | ||
| Quantifiable | 21 (80.8) | 23 (88.5) |
| ≥1%†† | 18 (85.7) | 18 (78.3) |
| <1%†† | 3 (14.3) | 5 (21.7) |
| Not quantifiable | 5 (19.2) | 3 (11.5) |
| PD-L1 CPS | ||
| Quantifiable | 21 (80.8) | 23 (88.5) |
| ≥1†† | 19 (90.5) | 19 (82.6) |
| <1†† | 2 (9.5) | 4 (17.4) |
| ≥10†† | 14 (66.7) | 13 (56.5) |
| <10†† | 7 (33.3) | 10 (43.5) |
| Not quantifiable | 5 (19.2) | 3 (11.5) |
| Tumor site | ||
| Oral cavity | 1 (3.8) | 16 (61.5) |
| Hypopharynx | 0 (0.0) | 3 (11.5) |
| Oropharynx | 24 (92.3) | 4 (15.4) |
| Larynx | 1 (3.8) | 3 (11.5) |
| Time from initial diagnosis to study entry | ||
| ≤1 year | 26 (100.0) | 25 (96.2) |
| >1 year | 0 (0.0) | 1 (3.8) |
Data are presented as n (%) unless indicated otherwise.
*95% CIs for proportions with respective characteristics overlapped between the HPV-positive and HPV-negative subgroups, indicating no significant difference.
†Additional substaging information for these patients was not available at the time of database lock.
‡Additional substaging information for one patient with HPV-negative stage IV disease was not available at the time of database lock.
§For tumors of the oral cavity, hypopharynx, oropharynx, and larynx, stage IVA represents moderately advanced local/regional disease (T1–T3/N2/M0 or T4a/N0–N2/M0) and stage IVB represents very advanced local/regional disease (any T/N3/M0 or T4b/any N/M0).30
¶95% CIs for proportions of never and current smokers overlapped between the HPV-positive and HPV-negative subgroups, indicating no significant difference. CIs for proportions of former smokers did not overlap in the HPV-positive (44.3% to 82.8%) and HPV-negative (9.0% to 43.6%) subgroups, indicating a significant difference.
**Patients who stopped smoking at any time before protocol enrollment.
††Percentage presented as a proportion of quantifiable patients for each threshold indicated.
AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; PD-L1, programmed cell death ligand 1.
Most patients had American Joint Committee on Cancer (AJCC, seventh edition) stage IV disease30 at enrollment (84.6% and 88.5%, respectively). Among patients with quantifiable PD-L1 IHC data, 18 of 21 (85.7%) and 18 of 23 (78.3%) had tumor cell expression ≥1%, and 19 of 21 (90.5%) and 19 of 23 (82.6%) had CPS ≥1, in the HPV-positive and HPV-negative cohorts, respectively. As expected, anatomic distribution of tumor sites differed, with most HPV-positive tumors located in the oropharynx and most HPV-negative tumors in the oral cavity.
All patients in the HPV-positive cohort and 25 of 26 patients in the HPV-negative cohort received both planned doses of nivolumab. One patient in the HPV-negative cohort discontinued nivolumab after only one dose because of grade 3 treatment-related rash but proceeded to surgery (online supplemental figure S1).
Safety
All 52 treated patients were assessed for safety of neoadjuvant nivolumab. Any-grade TRAEs occurring between the first dose of neoadjuvant nivolumab and 100 days after the last dose were reported in 19 patients (73.1%) in the HPV-positive and in 14 patients (53.8%) in the HPV-negative cohorts (online supplemental tables S1 and S2); the most common any-grade event was fatigue, occurring in six (23.1%) and five (19.2%) patients, respectively. Grade 3–4 TRAEs were reported for five (19.2%) and three patients (11.5%), respectively; two (7.7%) and four (15.4%) had treatment-related serious AEs. No patient in either cohort discontinued nivolumab due to a TRAE, and there were no treatment-related deaths. Any-grade select TRAEs (AEs with potential immunologic cause) occurred in four (15.4%) and five patients (19.2%) in the HPV-positive and HPV-negative cohorts, respectively; one grade 3–4 event occurred in each cohort (gastrointestinal (colitis) in the HPV-positive cohort and skin-related (maculo-papular rash) in the HPV-negative cohort; online supplemental tables S1 and S2).
Among the 52 treated patients, 38 were reported at the time of the database lock as having undergone complete surgical resection (18 in the HPV-positive cohort and 20 in the HPV-negative cohort; online supplemental figure S1). However, one patient initially reported as undergoing surgery in the HPV-negative cohort was subsequently found to have received a planned post-nivolumab biopsy instead, due to a misinterpretation of the study protocol. For the same reason (a protocol misinterpretation), 10 additional patients (eight in the HPV-positive cohort and two in the HPV-negative cohort) also received a planned post-nivolumab biopsy instead of surgery. The remaining four patients, all with HPV-negative tumors, did not undergo surgery or biopsy due to non-treatment-related multiple organ dysfunction syndrome (n=1), consent withdrawal before surgery (n=1), or rapid tumor progression (n=2).
Among the 18 patients with HPV-positive tumors who underwent surgery, median interval (range) between first nivolumab dose and surgery was 4.4 weeks (3.6 to 6.0). In the 20 patients with HPV-negative tumors who were reported at database lock to have undergone surgery, median interval (range) between first nivolumab dose and surgery was 4.0 weeks (3.0 to 5.3). One patient in each cohort had surgery delayed >7 days but ≤4 weeks for administrative reasons (surgery performed on days 42 and 37 in patients with HPV-positive or HPV-negative tumors, respectively). No patient had a protocol-defined TRAE-related surgical delay (>4 weeks).
There was one on-study death in the HPV-positive cohort due to disease progression and 13 on-study deaths in the HPV-negative cohort, eight because of disease progression and five due to AEs unrelated to neoadjuvant nivolumab or protocol surgery (online supplemental table S3).
Radiographic tumor response
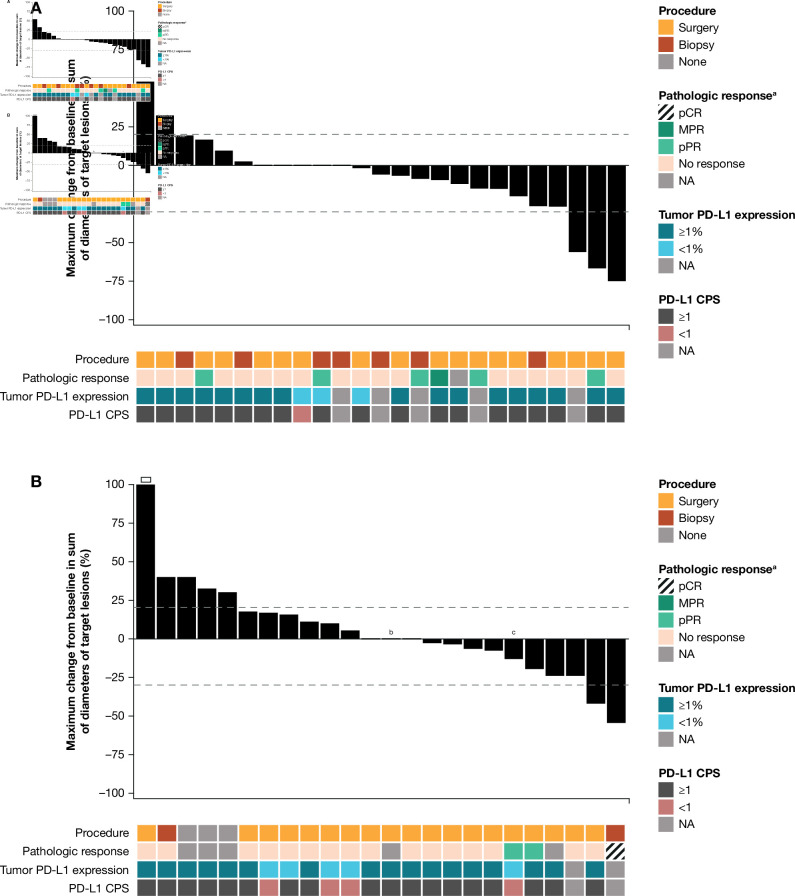
Twenty-five and 24 patients with HPV-positive or HPV-negative tumors, respectively, were evaluable for radiographic response. Fourteen of 25 patients (56.0%) with HPV-positive HNSCC showed any radiographic tumor reduction from baseline, and three (12.0%) had reductions ≥30% in alignment with RECIST v1.1 (figure 1A); median change in tumor burden from baseline was –5.9% (range, –75% to +54%). Ten of 24 patients (41.7%) with HPV-negative HNSCC showed any radiographic tumor reduction from baseline, and two (8.3%) had reductions ≥30% in alignment with RECIST v1.1 (figure 1B); median change in tumor burden from baseline was 0.0% (range, –55% to +112%). Radiographic tumor reduction did not appear to be associated with pathologic response or PD-L1 status (online supplemental tables S4 and S5).
Figure 1.
Characteristics of treatment response. Change from baseline in the sum of target lesion diameters according to adapted RECIST v1.1 in evaluable patients in the (A) HPV-positive (n=25) and (B) HPV-negative (n=24) cohorts. Dashed horizontal lines indicate 30% target lesion reduction (consistent with a partial response in the absence of new lesions) and 20% increase (consistent with progressive disease). An open square indicates truncation of percent change at +100%. Note that radiographic responses were measured using adapted RECIST v1.1 comprising a single on-treatment imaging scan before surgery, with no confirmatory scan performed. Detailed per-patient data are provided in online supplemental tables S4 and S5. CPS, combined positive score; HPV, human papillomavirus; MPR, major pathologic response; NA, not available; pCR, pathological complete response; pPR, pathologic partial response; PD-L1, programmed cell death ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors. aBased on central pathology review. bPatient received only one neoadjuvant dose of nivolumab. cFollowing database lock, patient was found to have received planned post-nivolumab biopsy instead of complete surgical resection.
Pathologic tumor response
Overall, 37 patients who underwent surgery were evaluated for pathologic response by site review (18 in the HPV-positive cohort and 19 in the HPV-negative cohort) and 34 were evaluated by central review (17 in the HPV-positive cohort and 17 in the HPV-negative cohort); these evaluations excluded the patient who was reported at database lock as receiving surgery but was later found to have received a post-nivolumab biopsy instead (online supplemental tables S4 and S5). No patient achieved a pCR by either site or central review. Central review also evaluated MPR and pPR. Among the 17 HPV-positive tumors evaluated by central review, one (5.9%) achieved a MPR and three (17.6%) achieved a pPR, yielding a MPR+pPR rate of 23.5%. Among the 17 HPV-negative tumors evaluated by central review, one (5.9%) achieved a pPR.
To evaluate potential pathologic tumor regression in the 11 patients who were surgical candidates but underwent a planned tumor biopsy instead due to a protocol misinterpretation (including one who was reported at database lock as receiving surgery but was later found to have received a biopsy), a central pathologic review was conducted according to immune-based pathological response criteria for tumor biopsies (MPRbx).37 This revealed two pPRs among eight HPV-positive tumors and one pCR and one pPR among three HPV-negative tumors (online supplemental tables S4 and S5). Among the nine patients with pathologic responses by total resection specimen or MPRbx criteria across the two cohorts, five had PD-L1 CPS ≥1, one had CPS <1, and three were not assessable for CPS.
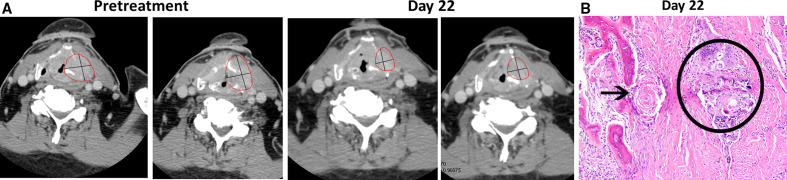
Figure 2 presents a patient with HPV-negative HNSCC responding to neoadjuvant nivolumab therapy.
Figure 2.
Tumor regression in a 76-year-old white man with stage IVA HPV-negative HNSCC (T4aN0M0; patient no. 37 per online supplemental table S5). The tumor was PD-L1 positive (tumor PD-L1 ≥1% and CPS >1) and originated in the larynx. (A) Evidence of primary tumor regression on CT scans (red circles) after receipt of two doses of nivolumab preoperatively. The tumor measured 51×44 mm at baseline and 41×27 mm at day 22. (B) On day 37, the patient underwent surgery as originally planned (total laryngectomy with bilateral neck dissection, ypT4aN0), revealing a pathologic partial response (pPR) by central pathology review. Representative H&E staining of primary tumor specimen (final magnification ×200) illustrating treatment response. Keratin granuloma (within black circle are multinucleated giant cells surrounding acellular keratin and microcalcifications) surrounded by dense fibrosis. Black arrow points to a keratin granuloma adjacent to the thyroid cartilage with osseous metaplasia. This area shows no viable squamous cell carcinoma. Adjuvant radiotherapy was administered to the primary tumor site as standard-of-care at the investigator’s discretion. At 3.9 years of follow-up, this patient remains alive and tumor-free per the investigator. CPS, combined positive score; HPV, human papillomavirus; PD-L1, programmed cell death ligand 1.
Recurrence-free and overall survival
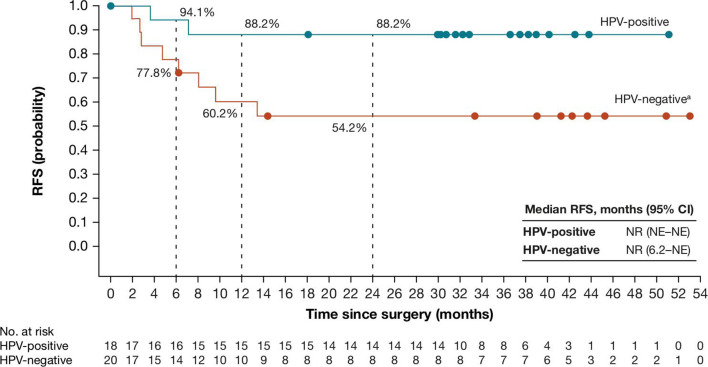
Median RFS (95% CI) for 38 patients who were reported at database lock to have undergone surgery was not reached in either cohort (figure 3). At 24 months postoperatively, RFS rates (95% CI) were 88.2% (60.6 to 96.9) and 54.2% (28.8 to 73.9) for the HPV-positive and HPV-negative cohorts, respectively. Two and five postoperative recurrences were reported in these cohorts, respectively.
Figure 3.
Recurrence-free survival in patients with HNSCC who were reported as undergoing surgery in the HPV-positive (n=18) and HPV-negative (n=20) cohorts. Median follow-up for patients from date of surgery was 33.1 months (range, 18.2–51.4) and 36.3 months (range, 1.9–53.3), respectively. In the HPV-positive cohort, there were two tumor recurrences. In the HPV-negative cohort, there were five tumor recurrences and three deaths from disease progression (n=1) or adverse events unrelated to neoadjuvant nivolumab or protocol surgery (n=2). HPV, human papillomavirus; NE, not estimable; NR, not reached; RFS, recurrence-free survival. aAfter database lock, one patient in the HPV-negative cohort was found to have received a planned post-nivolumab biopsy instead of complete surgical resection; RFS for this patient was 14.5 months.
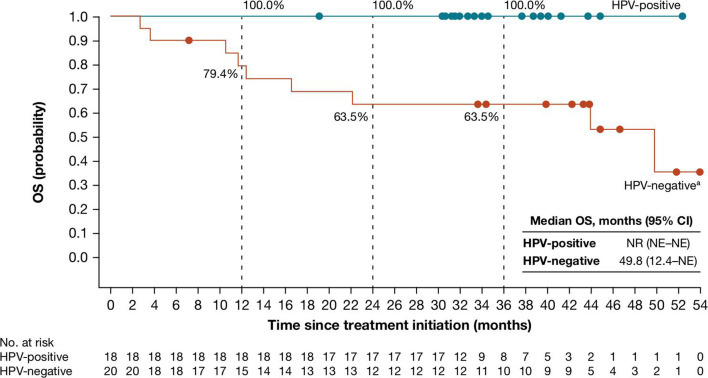
Median OS (95% CI) for 38 patients who were reported at database lock to have undergone surgery was not reached (not estimable–not estimable) in the HPV-positive cohort and 49.8 months (12.4–not estimable) in the HPV-negative cohort (figure 4); 36 months after treatment initiation, OS rates (95% CI) were 100.0% (100.0 to 100.0) and 63.5% (38.3 to 80.7), respectively. OS outcomes for all 52 treated patients are shown in online supplemental figure S2.
Figure 4.
Overall survival in patients with HNSCC who were reported as undergoing surgery in the HPV-positive (n=18) and HPV-negative (n=20) cohorts. Median follow-up for these cohorts was 34.3 months (range, 19.1 to 52.3) and 37.1 months (range, 2.7 to 53.9), respectively. In the HPV-positive cohort, there were no on-study deaths. In the HPV-negative cohort, there were nine on-study deaths from disease progression (n=6) or adverse events unrelated to neoadjuvant nivolumab or protocol surgery (n=3). HPV, human papillomavirus; NE, not estimable; NR, not reached; OS, overall survival. aAfter database lock, one patient in the HPV-negative cohort was found to have received a planned post-nivolumab biopsy instead of complete surgical resection; OS for this patient was 49.8 months.
Subsequent anticancer therapies
At database lock, 18 of 26 patients (69.2%) in the HPV-positive and 20 of 26 (76.9%) in the HPV-negative cohorts had received anticancer therapy after neoadjuvant nivolumab (online supplemental figure S3). Twenty-five of the 38 patients (65.8%) who were reported at database lock to have undergone surgery received subsequent therapies, of whom 23 had planned adjuvant therapies (standard-of-care radiotherapy and/or systemic therapy). Two patients in the HPV-negative cohort received on-study post-recurrence nivolumab.
Whole genome sequencing analyses
Nineteen patients across both cohorts had pretreatment tumor specimens suitable for whole genome sequencing; at database lock, 17 had both HPV status and pathologic response by central review, and two had HPV status only. Median TMB in patients with HPV-positive and HPV-negative tumors was 27 and 71, respectively; none of six patients (0.0%) with HPV-positive tumors had TMB >100, versus six of 13 (46.2%) with HPV-negative tumors (online supplemental figure S4A). Associations between TMB and smoking status or pathologic response are shown in online supplemental figures S4A, B. Because there were only two pathologic responders among patients with available tumor specimens, no conclusions could be drawn regarding associations between pathologic response and TMB.
Gene expression profiling (RNAseq) analyses
Twenty-one patients across both cohorts had evaluable RNAseq data. Inflammation gene signature scores, T-cell signature, CD8A gene expression, and B-cell and dendritic cell signatures were higher in HPV-positive tumors (online supplemental figure S5). CD56 gene expression and M2 macrophage signature were slightly lower in HPV-positive tumors. Regulatory T-cell signature expression was similar between the groups. Expression of the immune checkpoints PDCD1 (PD-1) and LAG3 appeared higher in HPV-positive versus HPV-negative tumors (online supplemental figure S6). Thus, HPV-positive tumors displayed a more highly inflammatory microenvironment, with enhanced expression of immune checkpoints likely reflecting cellular activation.40 41 Because there were only two pathologic responders among patients with available tumor specimens (as shown in online supplemental figures S5 and S6), no conclusions could be drawn regarding associations of pathologic response with gene expression profiles.
Discussion
PD-1 pathway inhibitors are effective against some unresectable recurrent/metastatic HPV-positive and HPV-negative HNSCCs,18–21 encouraging the investigation of neoadjuvant strategies to improve outcomes for patients with this disease. Furthermore, an operative delay of 3–4 weeks has previously been shown to be safe in HNSCC.42–44 The rationale for neoadjuvant immune checkpoint inhibition in at-risk resectable tumors is supported by an emerging body of clinical evidence across various cancer types.24–27 45 In addition to the potential for generating enhanced and sustained antitumor immunity and clearance of micrometastases in treatment-naïve patients, neoadjuvant anti-PD-(L)1 offers the possibility of pre-surgical tumor down-staging, evaluation of pathologic response as a potential predictor of long-term outcomes, and availability of sufficient on-treatment tissues for in-depth biomarker and mechanistic studies.45 However, this must be balanced with the possibility of undue surgical delay, particularly in patients experiencing TRAEs and/or tumor progression during the treatment “window.”
In CheckMate 358, patients with resectable stage III–IV HNSCC received nivolumab for ~4 weeks preoperatively. There were no unexpected TRAE-related surgical delays, consistent with previously published reports of various neoadjuvant regimens of anti-PD-(L)1 with or without anti-CTLA-4 in HNSCC.22 28 29 However, in the current study, four of 26 (15%) patients with HPV-negative tumors did not undergo surgery due to consent withdrawal, disease progression, or unrelated death. Furthermore, due to a protocol misinterpretation by some investigators, approximately 20% of patients did not undergo curative-intent post-nivolumab surgical resection but had a tumor biopsy instead (see supplementary figure S1 and tables S4 and S5). This reduced the number of specimens eligible for conventional pathologic response determination, although innovative MPRbx criteria were applied to evaluate pathologic changes in these specimens. Notably, in patients undergoing curative-intent resection, none achieved a pCR; one MPR and three pPRs were observed in the HPV-positive cohort (23.5% pathologic response rate among centrally evaluated cases), and only one pPR was observed in the HPV-negative cohort (5.9%). Although case numbers are small, this suggests that HPV-positive tumors display an early and more frequent response to nivolumab monotherapy than HPV-negative tumors, which was also observed clinically in the CheckMate 141 study of nivolumab for recurrent/metastatic HNSCC.17 18 However, the lack of pCRs in our study in HNSCC contrasts with studies in other cancers showing substantial pCR rates, some of which employed longer neoadjuvant treatment intervals.45 For instance, a trial of nivolumab therapy for 4 weeks preoperatively in non-small-cell lung cancer yielded a pCR rate of 10%24; nivolumab administered for 8 weeks preoperatively in melanoma yielded a pCR rate of 25%26; and pembrolizumab given for 9 weeks before surgery for muscle-invasive bladder cancer mediated pCRs in 42% of patients.25
Three other reports of neoadjuvant immune checkpoint blockade in HNSCC have recently been published.22 28 29 Relatively small numbers of patients (n=14 to n=36) received anti-PD-(L)1 monotherapy at one or two medical centers in each study, for 2–8 weeks before surgery; in addition, two of these trials included a randomized combination therapy arm with anti-CTLA-4 agents. Each trial focused on either HPV-negative22 28 or HPV-positive tumors,29 and median follow-up ranged from 14 to 22 months. Two studies employed a study-specific “pathologic tumor response” scoring system based on tumor necrosis and histiocytic reaction,22 28 and all three recorded pathologic response separately in the primary tumor or lymph node metastases. In contrast, the current study treated 52 patients at 10 medical centers, including equally sized cohorts of patients with HPV-positive or HPV-negative HNSCC who were followed for a median of 38 and 28 months, respectively. Furthermore, we used widely accepted methods for scoring immune-mediated pathologic response in the primary tumor and all sampled lymph nodes,35 36 based on percent residual viable tumor within a defined tumor regression bed. While such methodologic variations make cross-study comparisons difficult, a paucity of substantial pathologic responses after anti-PD-(L)1 monotherapy (i.e., ≤50% residual viable tumor, assessing all resected tissues) is evident across these trials.
In general, neoadjuvant studies of immune checkpoint blockade in other cancer types have shown pathologic response exceeding radiographic response. However, in the current study in HNSCC, both the RECIST radiographic response rate and the pathologic response rate were low. This is consistent with evidence that the tumor microenvironment of HNSCC may be particularly immunosuppressive.40 41 46 These results suggest that combinatorial neoadjuvant therapies are needed to mediate more robust HNSCC regression during the pre-surgical window. The combination of nivolumab plus ipilimumab yielded numerically higher rates of pathologic and radiographic response than nivolumab monotherapy in one study,22 although durvalumab plus tremelimumab did not appear to provide an advantage over durvalumab alone in another trial.29 Ongoing and future HNSCC trials will leverage additional neoadjuvant immunotherapy combinations based on the biology of this disease. Such regimens may include chemoradiotherapy, EGFR inhibition (cetuximab), inflammatory agonists (TLR, STING, etc), natural killer cell targeting agents, and other checkpoint inhibitors such as anti-TIM-3 and anti-LAG-3.23 47
While pathologic response may provide an early surrogate endpoint for efficacy, the clinical success of neoadjuvant immunotherapy is ultimately measured by RFS and OS. In our trial, there were few pathologic responders, and there was no clear association between pathologic response and RFS or OS (online supplemental tables S4 and S5). Twenty-four months post-surgery, RFS rates were 88% vs 54% for the HPV-positive and HPV-negative cohorts, respectively. We observed similarly divergent results associated with HPV status for OS. These substantial survival differences may not be surprising in previously untreated, locally advanced disease. For instance, in a study of chemoradiation for stage III–IV locally advanced oropharyngeal SCC, the 3-year progression-free survival rates were 73.7% vs 43.4%, and the 3-year OS rates were 82.4% vs 57.1%, for patients with HPV-positive vs HPV-negative tumors, respectively.9 Similar differences based on HPV status were observed for RFS and OS in another study of patients with oropharyngeal SCC undergoing surgery.48 Differences based on HPV status have also been observed for ORR using anti-PD-(L)1 therapy in recurrent/metastatic HNSCC.9 11 18 49 Such differences may relate to the more highly immune-reactive microenvironment found in HPV-positive HNSCC containing strongly immunogenic tumor-associated viral neoantigens.40 46 Thus, in view of the modest pathologic responses seen in CheckMate 358, it is unclear how the brief period of neoadjuvant nivolumab monotherapy in HNSCC (which has ~15% ORR in recurrent/metastatic disease) may have contributed to the observed long-term clinical outcomes.
Clearly, more effective treatment strategies for HNSCC are needed. In addition to evaluating combinatorial neoadjuvant therapies as mentioned previously, more effective application of post-surgical therapies should be considered in efforts to optimize survival outcomes in HNSCC. For instance, Uppaluri et al treated patients with high-risk HPV-negative tumors (positive surgical resection margins or extranodal extension after receiving neoadjuvant pembrolizumab) with adjuvant chemoradiotherapy plus pembrolizumab on-study.28 With this adaptive treatment strategy, only 17% of high-risk tumors relapsed within a year, suggesting an improvement over historical data. These results imply that more aggressive neoadjuvant/adjuvant immunotherapy regimens are warranted for HPV-negative HNSCC, consistent with the generally more aggressive approach taken when treating this HNSCC subtype. Multimodal treatment approaches in earlier stages of disease, integrating neoadjuvant and adjuvant strategies, will likely be needed to achieve further advances in immunotherapy for patients with HNSCC.
Acknowledgments
We thank the patients and their families for making this study possible, and the clinical study teams who participated in the study. We would like to acknowledge Luc G. T. Morris, MD, Memorial Sloan Kettering Cancer Center (MSKCC), New York, New York, USA, for accruing patients, overseeing the clinical care of study patients at MSKCC, coordinating specimens for pathological and correlative endpoints, and advocating for the trial and its rationale in multidisciplinary tumor conference discussions. We also gratefully acknowledge the clinical efforts and expertise of Umamaheswar Duvvuri, Seungwon Kim, and Jonas Johnson at the UPMC Hillman Cancer Center, and Wayne Koch, Carole Fakhry, Hyunseok Kang, Sarah Sagorsky, and Trish Brothers from the Johns Hopkins University School of Medicine and Kimmel Cancer Center, as well as the Biospecimen Operations leadership of Dharmesh Patel (Bristol Myers Squibb), who was integral in managing tumor specimens and related pathological reviews. We also thank Dako, an Agilent Technologies, Inc. company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; and Bristol Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Company Ltd. (Osaka, Japan). Professional medical writing and editorial assistance were provided by Richard Daniel, PhD, of Parexel, funded by Bristol Myers Squibb.
Footnotes
Twitter: @robertferrismd
Presented at: Presented in part at the European Society for Medical Oncology 2017 Congress, Madrid, Spain, September 8–12, 2017.
Correction notice: This paper has been revised since first published to update 'Acknowledgements'.
Contributors: Study conception: RLF, SLT. Data collection: RLF, WCS, RL, AG, UMM, CK, WS, CHH, LAD, HG, SIC, JMT, JES, JL, BL. Data analysis and interpretation: all authors.
Funding: The CheckMate 358 study is supported by Bristol Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Company Ltd. (Osaka, Japan). RLF participation was supported by R01 CA206517-02 and P50 CA097190-13.
Competing interests: RLF reports consulting or advisory from Bristol Myers Squibb (BMS), MedImmune, Merck, Lilly, Pfizer, Amgen, EMD Serono, PPD, Bain Capital Life Sciences, GlaxoSmithKline, Iovance Biotherapeutics, Numab Therapeutics AG, Oncorus, Ono Pharmaceutical, Regeneron, Novasenta, Aduro Biotech, MacroGenics, Nanobiotix, Torque Therapeutics, Lifescience Dynamics, Sanofi, and Zymeworks, Inc; and research funding from BMS, MedImmune, Merck, Tesaro, Novasenta, VentiRx, and AstraZeneca/MedImmune. WCS reports consulting from BMS and Regeneron. RL reports personal and institutional research funding from BMS. AG has nothing to disclose. UMM reports consulting and advisory from MSD Oncology, Roche, BMS, and Celgene; and travel accommodations from BMS, Celgene, Amgen, and Pierre Fabre. CK has nothing to disclose. WS reports honoraria from BMS and Array BioPharma; consulting or advisory from BMS, Novartis, Regeneron, ION Pharma, and Merck; research funding from Novartis, Merck, and Genentech; and institutional research funding from BMS. CHC reports consulting or advisory fees from BMS, CUE Biopharma, Ignyta, Mirati Therapeutics, and Sanofi; research funding from BMS, Ignyta, Lilly, Regeneron, IRX Therapeutics, and Lion Biotechnologies; and travel accommodation expenses from Mirati Therapeutics. LAD reports institutional expert input forum payments from MSD BV Netherlands; and institutional speaker fee payment from BMS. HG has nothing to disclose. SIC has nothing to disclose. LV has nothing to disclose. JMT reports consulting and advisory from BMS, MedImmune, Merck, Compugen, and Akoya Biosciences, and stock options from Akoya Biosciences. JES has nothing to disclose. JL reports employment and stock ownership from BMS. BL reports employment and stock ownership from BMS. TC reports employment and stock ownership from BMS. AB reports employment and stock ownership from BMS. SLT reports consulting or advisory from Five Prime Therapeutics, Immunocore, and Merck; travel accommodations from Five Prime Therapeutics, Merck, and BMS; research funding from BMS; stock ownership by her spouse in Tizona Therapeutics, DNAtrix, RAPT, WindMIL, Dragonfly Therapeutics, Ervaxx, Trieza Therapeutics, and Dracen Pharmaceuticals; consulting or advisory by her spouse in DNAtrix, RAPT, WindMIL, Dragonfly Therapeutics, Ervaxx, Amgen, AstraZeneca, Immunomic Therapeutics, Janssen Oncology, and Dynavax Technologies; royalties by her spouse in WindMIL, Immunomic Therapeutics, Arbor Pharmaceuticals, BMS, and NexImmune; and research funding by her spouse from Compugen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Bristol Myers Squibb’s policy on data sharing may be found online (https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol was approved by an institutional review board or independent ethics committee at each site before activation. The study was conducted in accordance with Good Clinical Practice guidelines as defined by the International Conference on Harmonisation, and in accordance with ethical principles of the European Union Directive and the US Code of Federal Regulations. All patients provided written informed consent in accordance with the Declaration of Helsinki.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021. 10.3322/caac.21660. [Epub ahead of print: 04 Feb 2021]. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48:3282–7. [PubMed] [Google Scholar]
- 3.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 2007;99:777–89. 10.1093/jnci/djk179 [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–301. 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Zumsteg ZS. A snapshot of the evolving epidemiology of oropharynx cancers. Cancer 2018;124:2893–6. 10.1002/cncr.31383 [DOI] [PubMed] [Google Scholar]
- 6.Begum S, Gillison ML, Nicol TL, et al. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 2007;13:1186–91. 10.1158/1078-0432.CCR-06-1690 [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Network . Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576–82. 10.1038/nature14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saada-Bouzid E, Peyrade F, Guigay J. Molecular genetics of head and neck squamous cell carcinoma. Curr Opin Oncol 2019;31:131–7. 10.1097/CCO.0000000000000536 [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant AK, Sojourner EJ, Vitzthum LK, et al. Prognostic role of p16 in nonoropharyngeal head and neck cancer. J Natl Cancer Inst 2018;110:1393–9. 10.1093/jnci/djy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCusker MG, Orkoulas-Razis D, Mehra R. Potential of pembrolizumab in metastatic or recurrent head and neck cancer: evidence to date. Onco Targets Ther 2020;13:3047–59. 10.2147/OTT.S196252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol 2012;30:2102–11. 10.1200/JCO.2011.38.4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen SY, Massa S, Mazul AL, et al. The association of smoking and outcomes in HPV-positive oropharyngeal cancer: a systematic review. Am J Otolaryngol 2020;41:102592. 10.1016/j.amjoto.2020.102592 [DOI] [PubMed] [Google Scholar]
- 14.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011;333:1157–60. 10.1126/science.1208130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbison RA, Kubik M, Konnick EQ, et al. The mutational landscape of recurrent versus nonrecurrent human papillomavirus-related oropharyngeal cancer. JCI Insight 2018;3:e99327. 10.1172/jci.insight.99327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Sun H, Zeng Q, et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep 2019;9:13404. 10.1038/s41598-019-49771-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. 10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 20.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 21.Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: Eagle, a randomized, open-label phase III study. Ann Oncol 2020;31:942–50. 10.1016/j.annonc.2020.04.001 [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld JD, Hanna GJ, Jo VY, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol 2020;6:1563–70. 10.1001/jamaoncol.2020.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stafford M, Kaczmar J. The neoadjuvant paradigm reinvigorated: a review of pre-surgical immunotherapy in HNSCC. Cancers Head Neck 2020;5:4. 10.1186/s41199-020-00052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–86. 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol 2018;36:3353–60. 10.1200/JCO.18.01148 [DOI] [PubMed] [Google Scholar]
- 26.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 2018;24:1649–54. 10.1038/s41591-018-0197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topalian SL, Bhatia S, Amin A, et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 trial. J Clin Oncol 2020;38:2476–87. 10.1200/JCO.20.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uppaluri R, Campbell KM, Egloff AM, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter, phase II trial. Clin Cancer Res 2020;26:5140–52. 10.1158/1078-0432.CCR-20-1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrarotto R, Bell D, Rubin ML, et al. Impact of neoadjuvant durvalumab with or without tremelimumab on CD8+ tumor lymphocyte density, safety, and efficacy in patients with oropharynx cancer: CIAO trial results. Clin Cancer Res 2020;26:3211–9. 10.1158/1078-0432.CCR-19-3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edge SB, Byrd DR, Compton CC. AJCC cancer staging manual. 7th edn. New York, NY: Springer, 2010. [Google Scholar]
- 31.El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck 2012;34:459–61. 10.1002/hed.21974 [DOI] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute . National Cancer Institute: common terminology criteria for adverse events (CTCAE) version 4.0. Available: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 34.Tetzlaff MT, Messina JL, Stein JE, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol 2018;29:1861–8. 10.1093/annonc/mdy226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853–60. 10.1093/annonc/mdy218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein JE, Lipson EJ, Cottrell TR, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade. Clin Cancer Res 2020;26:545–51. 10.1158/1078-0432.CCR-19-2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein JE, Soni A, Danilova L, et al. Major pathologic response on biopsy (MPRbx) in patients with advanced melanoma treated with anti-PD-1: evidence for an early, on-therapy biomarker of response. Ann Oncol 2019;30:589–96. 10.1093/annonc/mdz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015;523:231–5. 10.1038/nature14404 [DOI] [PubMed] [Google Scholar]
- 39.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 40.Cillo AR, Kürten CHL, Tabib T, et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity 2020;52:183–99. 10.1016/j.immuni.2019.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Succaria F, Kvistborg P, Stein JE, et al. Characterization of the tumor immune microenvironment in human papillomavirus-positive and -negative head and neck squamous cell carcinomas. Cancer Immunol Immunother 2021;70:1227–37. 10.1007/s00262-020-02747-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jie H-B, Schuler PJ, Lee SC, et al. CTLA-4⁺ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res 2015;75:2200–10. 10.1158/0008-5472.CAN-14-2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jie H-B, Srivastava RM, Argiris A, et al. Increased PD-1+ and TIM-3+ TILs during cetuximab therapy inversely correlate with response in head and neck cancer patients. Cancer Immunol Res 2017;5:408–16. 10.1158/2326-6066.CIR-16-0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kansy BA, Shayan G, Jie H-B, et al. T cell receptor richness in peripheral blood increases after cetuximab therapy and correlates with therapeutic response. Oncoimmunology 2018;7:e1494112. 10.1080/2162402X.2018.1494112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020;367. 10.1126/science.aax0182. [Epub ahead of print: 31 Jan 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013;73:1733–41. 10.1158/0008-5472.CAN-12-2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen EEW, Bell RB, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer 2019;7:184. 10.1186/s40425-019-0662-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 2006;24:5630–6. 10.1200/JCO.2005.04.6136 [DOI] [PubMed] [Google Scholar]
- 49.Zandberg DP, Algazi AP, Jimeno A, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 2019;107:142–52. 10.1016/j.ejca.2018.11.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002568supp001.pdf (549.2KB, pdf)
jitc-2021-002568supp002.pdf (1.3MB, pdf)
Data Availability Statement
Bristol Myers Squibb’s policy on data sharing may be found online (https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html).