Abstract
A 57-year-old man with lung cancer, previously treated with the programmed death-1 inhibitor pembrolizumab, was evaluated for liver injury and acute pancreatitis. Serum IgG4 levels were not elevated. Contrast-enhanced CT showed pancreatic swelling, contrast unevenness in the liver and thickening of the common bile duct and gall bladder. Magnetic resonance cholangial pancreatography revealed beads in the left intrahepatic bile duct and localised narrowing of the head and body of the central pancreatic duct. Endoscopic ultrasound-guided fine-needle and liver needle biopsy showed CD8+ and CD4+ T lymphocyte aggregates, whereas immunostaining revealed greater infiltration by CD8+ cells than CD4+ cells. IgG4-related disease was ruled out based on serum and pathological findings. The patient simultaneously presented with immune-related adverse events, autoimmune pancreatitis-like features and sclerosing cholangitis, which were ameliorated by steroid therapy. CD8+ lymphocytes were the dominant infiltrating cells in autoimmune pancreatitis and sclerosing cholangitis.
Keywords: oncology, pancreas and biliary tract, liver disease
Background
Immune checkpoint inhibitors are known to sustain T-cell activation by inhibiting the activity of immune checkpoint molecules, including cytotoxic T-lymphocyte-associated protein 4, programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1), which typically act as brakes in immune cells; therefore, their administration can enhance anticancer effects by provoking the immune system to combat the disease.1
Although the results of a meta-analysis using advanced solid tumours revealed that patients treated with immune checkpoint inhibitors were less likely to develop severe adverse events than those administered chemotherapy,2 immune-related adverse events (irAEs) are unavoidable challenges in clinical treatment.3 However, irAEs are unpredictable and can variously affect different target organs.4
Pembrolizumab is a humanised monoclonal IgG4 antibody against PD-1, which was approved by the US Food and Drug Administration in 2015 to treat PD-L1-positive nonsmall cell lung cancer. The indications for its use in other cancers are continuously expanding.
Herein, we present a case of a patient with lung cancer who had been treated with pembrolizumab and was simultaneously affected by autoimmune pancreatitis (AIP)-like features and sclerosing cholangitis (SC). We further examined the status of the patient’s pancreatic tissue to explore the possibility of this rare irAE.
Case presentation
A 57-year-old man underwent surgery for lung cancer in February 2020. Examination of an excised specimen revealed the presence of polymorphic carcinoma. The patient did not receive adjuvant chemotherapy and consequently relapsed, experiencing multiple metastases in the bone and right adrenal gland, which were confirmed in June 2020.
On 3 July 2020, the patient was treated with carboplatin, nab-paclitaxel and pembrolizumab combination therapy every 4 weeks. On 14 August 2020, a diagnosis of liver injury was made based on the following liver function results: aspartate transaminase, 45 U/L; alanine transaminase, 84 U/L; alkaline phosphatase 490 U/L and gamma-glutamyl transpeptidase, 91 U/L.
Pembrolizumab was continually administered based on assessment of the liver injury as grade 1 according to the Common Terminology Criteria for Adverse Events V.5.0. However, the patient reported epigastric pain on 24 August 2020, and we found elevated levels of serum amylase (332 U/L) on 28 August 2020. Fever developed on 7 September 2020, and his epigastric pain continued.
Investigations
On 11 September 2020, a blood test revealed aggressive liver injury based on the following remarkable elevations in serum levels: aspartate aminotransferase, 147 U/L; alanine aminotransferase, 406 U/L; alkaline phosphatase 1330 U/L; gamma-glutamyl transpeptidase, 1290 U/L and amylase, 1034 U/L.
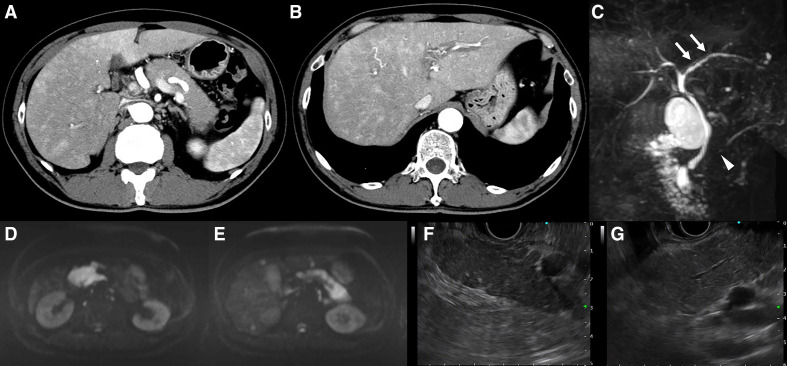
The C reactive protein levels were elevated at 10.77 mg/dL, whereas the white cell count was normal. Although the levels of antinuclear antibodies were elevated (1:80), other autoimmune markers, including serum IgG4, were negative (table 1). Contrast-enhanced CT showed swelling of the entire pancreas (resembling a sausage), without an increase in the surrounding adipose tissue, and low absorption in the early phase contrast CT. The finding of a capsule-like rim was ambiguous (figure 1A). We observed contrast unevenness in the liver and thickening of the common bile duct and gall bladder (figure 1B). Magnetic resonance cholangial pancreatography revealed the appearance of beads in the left intrahepatic bile duct and localised narrowing of the head and body of the central pancreatic duct (figure 1C). The appearance of beads in the left intrahepatic bile duct was confirmed via endoscopic retrograde cholangiography. Diffusion-weighted imaging revealed a remarkably high signal in the entire pancreas (figure 1D, E). Endoscopic ultrasound (EUS) showed swelling of the pancreas accompanied by lobularity with honeycombing, hyperechoic foci without shadowing in the pancreatic parenchyma (figure 1F) and hyperechoic main pancreatic duct margins in the main pancreatic duct (figure 1G). These findings resembled those typically observed during early chronic pancreatitis. We suspected the onset of AIP-like symptoms and SC as irAEs related to pembrolizumab. As the severity of irAE was grade 3, the patient required hospitalisation for close observation with examination and appropriate treatment.
Table 1.
Laboratory data on admission
| WBC | 4.1×109/L | PT-INR | 0.99 |
| RBC | 4.33×1012/L | APTT | 29.6 s |
| Hb | 125g/L | IgG | 746 mg/dL |
| Ht | 36.10% | IgM | 75 mg/dL |
| Plt | 187×109/L | IgA | 147 mg/dL |
| AST | 147 IU/L | IgG4 | 19.4 mg/dL |
| ALT | 406 IU/L | ANA | 1:80 |
| LDH | 246 IU/L | ASMA | <20 |
| T-Bil | 1.1 mg/dL | AMA | <20 |
| ALP | 1330 IU/ L | anti-M2 | <1.5 |
| GGT | 1290 IU/L | HBsAg | (-) |
| Amylase | 1034 IU/L | anti-HCV Ab | (-) |
| Lipase | 860 IU/L | CEA | 1.5 ng/mL |
| Trypsin | 11800 ng/mL | CA19-9 | 43.7 IU/mL |
| TP | 5.8 g/dL | ||
| Alb | 3.3 g/dL | ||
| CRP | 10.77 mg/dL | ||
| UN | 11.3 mg/dL | ||
| Cre | 1.00 mg/dL | ||
| Na | 130 mEq/L | ||
| K | 4.3 mEq/L | ||
| Cl | 97 mEq/L |
anti-HCV Ab, anti-hepatitis C virus antibody; Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, anti-mitochondrial antibodies; ANA, antinuclear antibodies; APTT, activated partial thromboplastin time; ASMA, anti-smooth muscle antibody; AST, aspartate aminotransferase; CA19-9, cancer antigen 19–9; CEA, carcinoembryonic antigen; Cre, creatinine; CRP, C reactive protein; GGT, γ-gamma-glutamyl transpeptidase; Hb, haemoglobin; HBsAg, hepatitis B surface antigen; Ht, hematocrit; LDH, lactate dehydrogenase; Plt, platelet count; PT-INR, prothrombin time and international normalized ratio; RBC, red blood cells; T-bil, total bilirubin; TP, total protein; UN, urea nitrogen; WBC, white blood cells.
Figure 1.
Findings of radiological imaging: contrast-enhanced computed tomography (CECT), magnetic resonance cholangial pancreatography (MRCP) and endoscopic ultrasonography (EUS). (A) CECT. Sausage-like swelling of the whole pancreas, characterised by a lack of increase in the surrounding adipose tissue and low absorption in the early phase of contrast. No capsule-like rim was observed. (B) Liver parenchyma showing strong staining around the bile duct in the early phase of contrast, suggesting cholangitis. This observation was more intense in the right liver lobe than in the left. A periportal colour sign was also recognised. (C) MRCP. Appearance of beads in the left intrahepatic bile duct (arrow) and localised narrowing in the head and the body of the main pancreatic duct (arrowhead). (D, E) Examination of the whole pancreas revealing a remarkably high signal in diffusion weighted imaging. (F) EUS. Pancreatic swelling accompanied by lobularity with honeycombing, and hyperechoic foci without shadowing in the pancreatic parenchyma. (G) Hyperechoic main pancreatic duct margin in the main pancreatic duct.
Treatment
We administered 120 mg/day methylprednisolone and 200 mg/day nafamostat mesylate to treat the pancreatitis. Although the blood test results revealed decreased serum levels of liver enzymes and amylase, we also performed tissue examinations to confirm irAEs as this was an atypical case. Thus, EUS-guided fine-needle biopsy of the pancreas using a 19G needle, bile duct biopsy by endoscopic retrograde cholangiopancreatography and liver biopsy were performed.
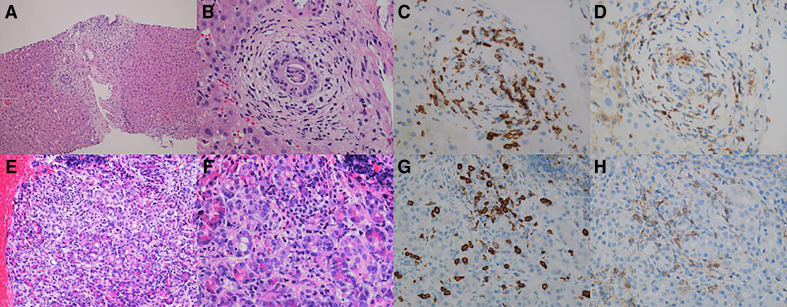
Liver needle biopsy showed small-duct cholangitis (figure 2A, B) and mild panlobular hepatitis. We found periductal fibrosis of the intrahepatic small bile ducts due to lymphocytic infiltration. In the parenchyma, we observed foci of spotty necrosis with lymphocytic infiltration and steatohepatitis (figure 2A). The infiltrating cells were mainly lymphocytes and a few neutrophils, with inconspicuous plasma cell infiltration. Immunostaining revealed the presence of CD8+ and CD4+ T lymphocytes, with the CD8+ cells showing greater infiltration than the CD4+ cells, particularly in the intraepithelial infiltrate of the bile ducts (figure 2C, D). IgG4+ plasma cells did not infiltrate.
Figure 2.
Findings from liver tissues obtained by liver needle biopsy and pancreatic tissues obtained by endoscopic ultrasound-guided fine needle biopsy (EUS-FNB). Liver needle biopsy: (A) hepatic irAE with small-duct cholangitis, mild panlobular hepatitis and steatohepatitis; (B) small duct sclerosing cholangitis; (C, D) immunostaining for CD8 (C) and CD4 (D) in hepatic irAEs showing periductal fibrosis and infiltration of CD8+intraepithelial T-lymphocytes. EUS-FNB: (E, F) infiltration of lymphocytes and sparse eosinophils; (G, H) immunostaining for CD8+ (G) and CD4+ (H) lymphocytes, showing that the infiltrating lymphocytes were predominantly CD8+T-lymphocytes.
Subsequently, EUS-guided fine-needle biopsy of the pancreas revealed lymphocyte predominance and mild inflammatory infiltration (figure 2E, F), whereas immunostaining revealed an increased number of CD8+ cell infiltrates, which were more abundant than the CD4+ cell infiltrates (figure 2G, H). We also observed the focal infiltration of a few eosinophils and plasma cells. We found no evidence of IgG4+ plasma cell infiltration, storiform fibrosis or obstructive phlebitis. Biopsy of the common bile duct, stomach and rectum also revealed CD8+ and CD4+ lymphocytic infiltration. The dominance of CD8+ cells was confirmed by immunostaining of the intraepithelial lymphocyte infiltrate.
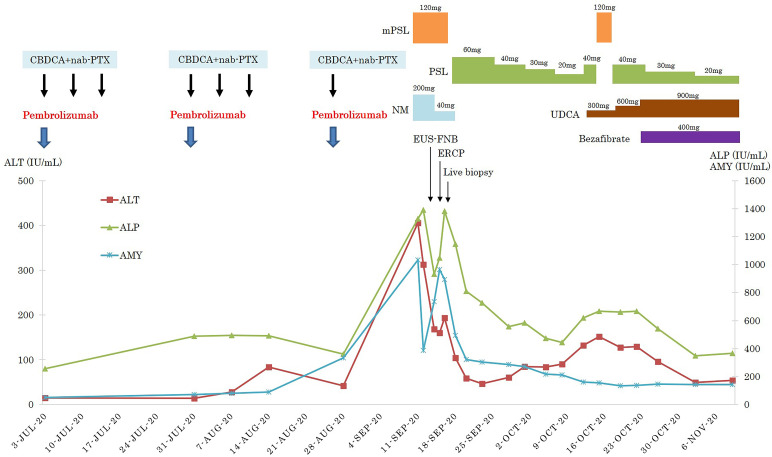
The treatment course administered to the patient is presented in figure 3. Briefly, methylprednisolone (120 mg/day) was intravenously administered for 5 days. Subsequently, 60 mg/day prednisolone (PSL) was orally administered for 1 week; the PSL dose was subsequently reduced to 40 mg/day and then decreased by 10 mg/day weekly thereafter. When the PSL dose was reduced to 20 mg/day, signs of liver injury were noted in blood tests. Hence, we increased the PSL dosage to 40 mg/day and further administered methylprednisolone (120 mg/day) for 3 days. Furthermore, 300 mg/day ursodeoxycholic acid and 400 mg/day bezafibrate were administered in combination. The ursodeoxycholic acid dose was finally increased to 900 mg/day.
Figure 3.
The clinical course of a 57-year-old man with the simultaneous occurrence of autoimmune pancreatitis and sclerosing cholangitis as immune-related adverse events. CBDCA, carboplatin; nab-PTX, nanoparticle albumin-bound-paclitaxel; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AMY, amylase; NM, nafamostat mesylate; PSL, prednisolone; mPSL, methylprednisolone; UDCA, ursodeoxycholic acid.
Outcome
The patient’s liver injury and pancreatitis appeared to be manageable with this treatment protocol. The patient was discharged after the PSL dose was decreased to 20 mg/day.
Discussion
Although pancreatitis may occur as an irAE due to pembrolizumab, only a few such cases have been reported.5 One report revealed that the incidence of pancreatitis in patients treated with PD-1/PD-L1 inhibitors, including pembrolizumab, was 1%.6 If PD-1/PD-L1 inhibitor use is limited to pembrolizumab, pancreatitis has been shown to occur in 0.4%–0.6% of patients with PD-L1-positive unresectable advanced/recurrent nonsmall cell lung cancer.7 8
Although there was no sign of a capsule-like rim, the CT results showed diffuse sausage-like swelling of the pancreas in the present case. The observed radiological features were similar to those of AIP. There have been two previous cases in which nivolumab, another PD-1 inhibitor, caused pancreatitis, with the CT images mimicking those of AIP.9 10 However, similar CT observations related to an adverse event caused by pembrolizumab have not yet been reported.
AIP is considered to be an IgG4-related sclerosis systemic disease that also affects the pancreas11; currently, it is recognised as a pancreatic lesion associated with IgG4-related disease. Histopathological findings of IgG4-related disease include IgG4-positive plasma cells, storiform fibrosis and obstructive phlebitis.
Our patient showed no elevation in the serum levels of IgG4, and no infiltration of IgG4-positive plasma cells, storiform fibrosis or obstructive phlebitis in histopathological examination. Hence, the occurrence of AIP as an IgG4-related disease was excluded.
Our patient showed CD8+ and CD4+ lymphocyte infiltration in the pancreatic glands, with CD8+ lymphocytes infiltrating the pancreatic acinus as determined histopathologically; CD8+ lymphocytes were slightly predominant in the pancreatic glands and pancreatic acinus.
As previous reports indicated that infiltration of CD8+ lymphocytes in the tissue is beneficial for diagnosing irAEs,12–14 we noted that AIP-like features, as an irAE, are characterised by CD8+ cells, the dominant type of infiltrating lymphocytes in the pancreas. There have been no previous histopathological reports of irAE-related AIP. Although we previously presented cases of AIP caused by nivolumab, histopathological studies regarding this condition are lacking.9 10
The features of early chronic pancreatitis, such as lobularity with honeycombing, hyperechoic foci without shadowing and a hyperechoic main pancreatic duct margin were observed by EUS. One previously described case of nivolumab-induced AIP showed a similar feature in the EUS.9 However, it is unknown whether these features are typical. As our patient had no history of alcohol abuse, we did not consider alcoholic chronic pancreatitis. Further investigations are needed to interpret the EUS findings in the pancreas.
Moreover, our patient was simultaneously affected by AIP and SC. AIP is most frequently associated with IgG4-SC in IgG4-related disease.15 However, to the best of our knowledge, the simultaneous occurrence of SC and AIP has not been reported as an irAE.
In our patient, AIP and SC occurred simultaneously as irAEs, although the pancreatitis-like features as an irAE pathologically differed from AIP as an IgG4-related disease. Specifically, CD8+ lymphocytes were the dominant infiltrating lymphocytes in the AIP as an irAE.
Learning points.
Pembrolizumab could cause autoimmune pancreatitis as an immune-related adverse event.
Simultaneous occurrence of autoimmune pancreatitis and sclerosing cholangitis appeared as immune-related adverse events.
Pathologically, CD8+ lymphocytes dominantly infiltrated the pancreatitis-like and sclerosing cholangitis tissues.
Steroid therapy was effective in treating the autoimmune pancreatitis and sclerosing cholangitis as immune-related adverse events.
Acknowledgments
We are grateful to Prof Kenichi Harada (Kanazawa University, Department of Human Pathology) for his assistance in making a pathological diagnosis and Dr Tokio Wakabayashi (Saiseikai Kanazawa Hospital, Department of Gastroenterology) for valuable inputs on the principles of autoimmune pancreatitis and sclerosing cholangitis.
Footnotes
Contributors: TS cared for the patient, edited the manuscript, prepared the figures and obtained the written informed consent from the patient. MK made the pathological diagnosis, edited the manuscript and prepared the figures. KK cared for the patient, edited the manuscript and gave the expert opinion on immune checkpoint inhibitors treatment and immune-related adverse events. EM edited the manuscript and gave expert opinions on gastroenterology and hepatology.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sharon E, Streicher H, Goncalves P, et al. Immune checkpoint inhibitors in clinical trials. Chin J Cancer 2014;33:434–44. 10.5732/cjc.014.10122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magee DE, Hird AE, Klaassen Z, et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Oncol 2020;31:50–60. 10.1016/j.annonc.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 3.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2:1346–53. 10.1001/jamaoncol.2016.1051 [DOI] [PubMed] [Google Scholar]
- 4.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med 2017;377:1409–12. 10.1056/NEJMp1709968 [DOI] [PubMed] [Google Scholar]
- 5.Kakuwa T, Hashimoto M, Izumi A, et al. Pembrolizumab-related pancreatitis with elevation of pancreatic tumour markers. Respirol Case Rep 2020;8:e00525. 10.1002/rcr2.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y-B, Zhang Q, Li H-J, et al. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2017;6:S8–20. 10.21037/tlcr.2017.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Sakai A, Kobayashi T, et al. Nivolumab-related pancreatitis with autoimmune pancreatitis-like imaging features. J Gastroenterol Hepatol 2019;34:1274. 10.1111/jgh.14620 [DOI] [PubMed] [Google Scholar]
- 10.Saito H, Ono K. Nivolumab-induced pancreatitis: an immune-related adverse event. Radiology 2019;293:521. 10.1148/radiol.2019191603 [DOI] [PubMed] [Google Scholar]
- 11.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 2003;38:982–4. 10.1007/s00535-003-1175-y [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci 2012;57:2233–40. 10.1007/s10620-012-2140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imoto K, Kohjima M, Hioki T, et al. Clinical features of liver injury induced by immune checkpoint inhibitors in Japanese patients. Can J Gastroenterol Hepatol 2019;2019:1–12. 10.1155/2019/6391712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka R, Ichimura Y, Kubota N, et al. Activation of CD8 T cells accelerates anti-PD-1 antibody-induced psoriasis-like dermatitis through IL-6. Commun Biol 2020;3:571. 10.1038/s42003-020-01308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamisawa T, Takuma K, Egawa N, et al. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol 2010;7:401–9. 10.1038/nrgastro.2010.81 [DOI] [PubMed] [Google Scholar]