ABSTRACT
Objective:
Increased mortality risk because of severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) infection in adults with native liver disease (LD) and liver transplant (LT) is associated with advanced age and comorbid conditions. We aim to report outcomes for children with LD and LT enrolled in the NASPGHAN/SPLIT SARS-CoV2 registry.
Methods:
In this multicenter observational cohort study, we collected data from 91 patients <21 years (LD 44, LT 47) with laboratory-confirmed SARS-CoV2 infection between April 21 and September 17, 2020.
Results:
Patients with LD were more likely to require admission (70% vs 43% LT, P = 0.007) and pediatric intensive care unit (PICU) management (32% vs 4% LT, P = 0.001). Seven LD patients required mechanical ventilation (MV) and 2 patients died; no patients in the LT cohort died or required MV. Four LD patients presented in pediatric acute liver failure (PALF), 2 with concurrent multisystem inflammatory syndrome in children (MIS-C); all recovered without LT. Two LD patients had MIS-C alone and 1 patient died. Bivariable logistic-regression analysis found that patients with nonalcoholic fatty LD (NAFLD) (odds ratio [OR] 5.6, P = 0.02) and LD (OR 6.1, P = 0.01, vs LT) had higher odds of severe disease (PICU, vasopressor support, MV, renal replacement therapy or death).
Conclusions:
Although not directly comparable, LT recipients had lower odds of severe SARS-CoV2 infection (vs LD), despite immunosuppression burden. NAFLD patients reported to the registry had higher odds of severe SARS-CoV2 disease. Future controlled studies are needed to evaluate effective treatments and further stratify LD and LT patients with SARS-CoV2 infection.
Keywords: acute liver failure, chronic liver disease, multisystem inflammatory syndrome in children, pediatric liver transplant, severe acute respiratory syndrome coronavirus-2 infection, viral hepatitis
What Is Known/What Is New
What Is Known
Older age and obesity are associated with increased mortality due to severe acute respiratory syndrome coronavirus-2 in adult liver transplant recipients.
Multisystem inflammatory syndrome in children is a unique pediatric disease phenotype, with the potential for significant hepatic injury.
What Is New
Liver transplant recipients do not appear to be at increased risk of worse outcomes than children with liver disease despite immunosuppression exposure.
Obesity associated with nonalcoholic fatty liver disease is a potential risk factor for severe severe acute respiratory syndrome coronavirus-2 infection in children.
Patients with severe acute respiratory syndrome coronavirus-2-related pediatric acute liver failure may recover without liver transplantation.
The health effects of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) pandemic have generally been mild in children, although hepatic manifestations can be prominent (1–8). Although the SARS-CoV2 pandemic continues to produce staggering disease incidence, children account for 2% to 7.6% of reported SARS-CoV2 cases, with death rarely reported (<0.1%) (1,9). A subset of children requires hospitalization for severe illness, of which 50% have an underlying medical condition, most commonly, obesity (9–13). Multisystem inflammatory syndrome in children (MIS-C) is also a unique pediatric disease phenotype, with a potential for significant hepatic injury (14–17).
Amongst infected adults with chronic liver disease (LD), 90% were hospitalized and 32% died (18). In a multicenter cohort study of adult solid organ transplant recipients, age >65 years and obesity were associated with mortality but immunosuppression intensity was not (19). Webb et al (20) similarly reported that liver transplant (LT) status in adults was not independently associated with death. Available data on the impact of SARS-CoV2 in children with native LD or with a LT are limited to case reports and small series (21–24).
To address this knowledge gap in children, the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) Hepatology Committee and the Society of Pediatric Liver Transplantation (SPLIT) Quality Improvement and Clinical Care Committee partnered to create a SARS-CoV2 registry dedicated to pediatric patients with LD and LT. We aimed to evaluate the prevalence, clinical course, and outcomes of SARS-CoV2 infection in this population and highlight the differences between children with LD and LT. We additionally summarize treatment practices and evaluate risk factors for morbidity and mortality.
METHODS
Study Design and Setting
This multicenter retrospective observational cohort study analyzed de-identified data collected using the secure Research Electronic Data Capture (REDCap) software program (25,26) hosted by Queens University, Canada from patients under 21 years of age with acute liver failure (ALF), chronic LD (CLD), and recipients of LT with laboratory confirmed SARS-CoV2 infection. Data in this report was amassed from the time of registry launch, September 17, 2020 through August 21, 2020 with data collection ongoing. The study was approved by the Columbia University Institutional Review Board (IRB) and the Queen's University Research Ethics Board. Contributing study sites underwent review by local IRB as required to access data outside of routine care. All research was conducted following the Declaration of Helsinki guidelines of good practice.
Participants
We asked clinicians to submit patients <21 years of age with LD or LT and laboratory-confirmed SARS-CoV2 infection by reverse transcriptase-polymerase chain reaction of nasopharyngeal swabs or SARS-CoV2 IgM/IgG antibodies and clinical symptoms with or without known exposure. Testing for SARS-CoV2 infection was at the discretion of the individual center based on their institutional protocols and availability of testing. Recipients of multivisceral (n = 3), isolated intestine (n = 3), and multiorgan (n = 2) transplantation were excluded to improve the generalizability of our findings to a distinct single organ recipient population.
Variables
Data requested from the registry included demographics, pertaining details regarding LD (etiology of LD, LT and immunosuppression, laboratory variables, liver-related complications). SARS-CoV2 infection data included the method of diagnosis, symptoms, imaging, and additional co-infections. Other important variables collected included details about treatment, clinical course, and outcome. Table 1 (Supplemental Digital Content) shows the data elements collected.
Definitions
Pediatric acute liver failure (PALF) has been previously defined (27). MIS-C was diagnosed by Center for Disease Control criteria (17). Severe SARS-CoV2 infection was defined by the presence of at least one of the following: death, pediatric intensive care unit (PICU) level of care, mechanical ventilation, vasopressor support, and/or renal replacement therapy (RRT).
Statistical Methods and Analytical Software
Descriptive statistics were applied to collected data. We calculated median and interquartile range (IQR) for continuous variables, categorical variables were reported as count and numeric proportions. Continuous variables were compared with rank-sum analyses and proportions with chi-square analyses as appropriate. The association between baseline covariates and disease severity was assessed using logistic regression among the full cohort. Given the small cohort size, the model was limited to 2 variables based on univariable analysis significance: LD and non-alcoholic fatty LD (NAFLD). Statistical analyses were conducted using STATA 13. A P value <0.05 was considered significant.
RESULTS
Participants
Between April 21 and September 17, 2020, 91 patients (53% males, median age 10 years, LD 44 [48%], LT 47 [52%]) were reported to the registry with laboratory-confirmed SARS-CoV2 infection (Table 1). Twenty-eight centers from 6 countries (United States, Canada, China, Pakistan, Argentina, and Colombia) contributed data, with 77 (85%) patients from North America.
TABLE 1.
Baseline characteristics and clinical data for patients with native liver disease and liver transplant recipients infected with severe acute respiratory syndrome coronavirus-2
| Native liver disease, N = 44 | Liver transplant recipient, N = 47 | |
| Baseline characteristics | ||
| Age, years; median (IQR) | 11 (0.9–17) | 10 (4–16) |
| Male gender, % | 26 (59) | 22 (46.8) |
| Race and ethnicity, % | ||
| White | 20 (45.5) | 20 (42.6) |
| Hispanic | 13 (29.5) | 18 (38.2) |
| Black | 5 (11.4) | 2 (4.3) |
| Asian | 4 (9.1) | 3 (6.4) |
| Other | 2 (4.5) | 3 (6.4) |
| Missing | 0 | 1 (2.1) |
| Primary liver condition, % | ||
| Biliary atresia | 10 (22.7) | 20 (42.6) |
| Autoimmune hepatitis | 8 (18.2) | 3 (6.4) |
| Acute liver failure | 4 (9.2) | 7 (14.9) |
| NAFLD | 10 (22.7) | 0 |
| Metabolic∗ | 3 (6.8) | 6 (12.8) |
| Malignancy | 2 (4.5) | 5 (10.6) |
| Other cholestatic liver disease | 5 (11.4) | 1 (2.1) |
| Other† | 2 (4.5) | 5 (10.6) |
| Comorbid conditions, %‡ | ||
| None | 15 (34) | 28 (59.6) |
| Obesity | 14 (31.8) | 3 (6.4) |
| Cardiac | 6 (13.6) | 5 (10.6) |
| Pulmonary | 5 (11.4) | 3 (6.4) |
| Gastrointestinal | 5 (11.4) | 1 (2.1) |
| Endocrine | 2 (4.5) | 2 (4.3) |
| Renal | 1 (2.3) | 3 (6.4) |
| Other autoimmune conditions | 1 (2.3) | 3 (6.4) |
| Clinical data | ||
| Presenting symptoms, %‡ | ||
| Respiratory symptoms | 22 (50) | 17 (36.2) |
| Fever | 21 (47.8) | 16 (34) |
| Gastrointestinal symptoms | 12 (27.3) | 12 (25.5) |
| Constitutional symptoms§ | 4 (9.1) | 3 (6.4) |
| Asymptomatic | 8 (18.2) | 13 (27.7) |
| Method of SARS-CoV2 diagnosis, % | ||
| NP swab | 35 (79.5) | 39 (83) |
| Serum antibody | 6 (13.6) | 8 (17) |
| NP swab + serum antibody | 3 (6.9) | 0 |
| Imaging findings‡ | ||
| CXR: normal | 11 (25) | 8 (10.6) |
| CXR: diffuse patchy opacities | 9 (20.5) | 2 (4.3) |
| CXR: interstitial pneumonia | 2 (4.5) | 2 (2.1) |
| CXR: lobar pneumonia | 2 (4.5) | 0 |
| Chest CT: ground-glass opacities | 1 (2.3) | 0 |
| Imaging data not available | 20 (45.5) | 35 (74.4) |
| Liver biopsy performed | 1 (2.3) | 3 (6.4) |
CT = computed tomography; CXR = chest X-ray; IQR = interquartile range; n = number; NAFLD = nonalcoholic fatty liver disease; NP = nasopharyngeal; SARS-CoV2 = severe acute respiratory syndrome coronavirus-2.
Cystic fibrosis, alpha-1 antitrypsin deficiency, citrullinemia; liver transplant: ornithine transcarbamylase deficiency, maple syrup urine disease, urea cycle disorder, glycogen storage disease.
GVHD with cirrhosis, idiopathic portal vein thrombosis; liver transplant: cryptogenic (n = 2), factor V deficiency, Ellis Van Creveld syndrome, hereditary hemochromatosis.
Category is not mutually exclusive.
Fatigue, sore throat, myalgia, loss of smell or taste.
Descriptive Data
Twenty-three percent of patients were asymptomatic. Respiratory symptoms were the most common presentation (50% of LD and 36% of LT patients), followed by fever. Co-infections occurred in 4.5% of LD patients and 15% of LT recipients (Table 2, Supplemental Digital Content). The most common causes of LD were NAFLD, biliary atresia (BA), and autoimmune hepatitis (AIH) (Table 1). PALF was reported in 4 LD patients attributed to SARS-CoV2 infection, with concurrent MIS-C in 2 (50%); 2 patients had MIS-C alone, of which 1 patient died (Table 3, Supplemental Digital Content). No patients presenting with PALF or MIS-C had a prior history of LT, AIH, or exposure to immunosuppressive agents. No patients with PALF required LT. Obesity was more common in patients with LD (32%) compared with LT (6%) and all patients with NAFLD were obese.
Most LT recipients were transplanted for BA. Median time from LT to SARS-CoV2 infection was 3.4 years. Table 4 (Supplemental Digital Content) shows baseline immunosuppression and changes for both LD and LT patients. Amongst LD patients with AIH on immunosuppression, 2 of 7 underwent dose reduction. Amongst LT recipients, immunosuppression changes included reduction (28%) and/or discontinuation (15%) of a second agent, with no adjustments in 68%. Higher baseline immunosuppression in LT recipients did not predict a need for higher level care (Fig. 1A).
FIGURE 1.
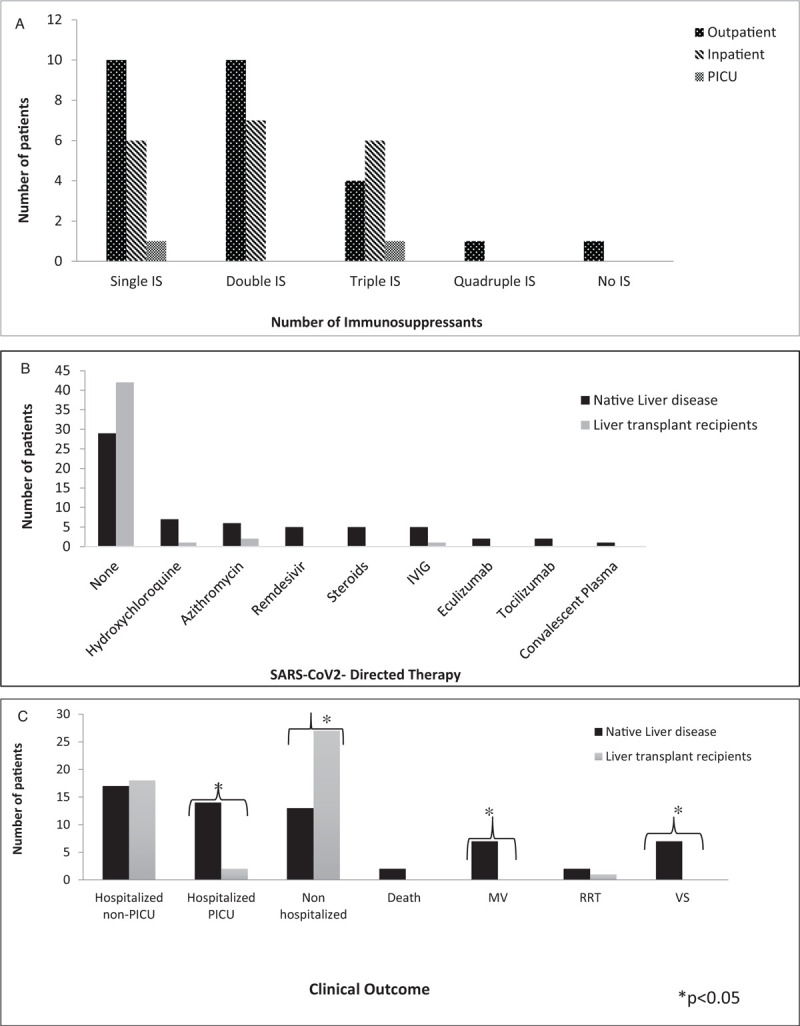
(A) Highest level of care by baseline number of immunosuppressive medications for liver transplant recipients. (B) Severe acute respiratory syndrome coronavirus-2 directed medical therapies in patients with native liver disease and liver transplant recipients. (C) Clinical outcomes for patients with native liver disease and liver transplant recipients. IS = immunosuppression; IVIG = intravenous immunoglobulin; MV = mechanical ventilation; PICU = pediatric intensive care unit; RRT = renal replacement therapy; VS = vasopressor support.
Twenty LT recipients had reported increased alanine aminotransferase (ALT) values with 30% having an elevated ALT (>100 IU/mL) during SARS-CoV2 infection, representing a significant increase from baseline. Of LD patients with reported ALT (n = 36), 52% had an elevated ALT (>100 IU/L) (additional labs, Table 5, Supplemental Digital Content). Treatments varied significantly with 75% of the total cohort having received no SARS-CoV2-directed treatments (Fig. 1B).
Clinical Outcomes
Patients with LD were less likely to be managed as outpatients (30% LD vs 57% LT) and more likely to require PICU management (32% LD vs 4% LT) (Fig. 1C). Patients in the LD cohort were more likely to have respiratory compromise requiring mechanical ventilation (16% LD vs 0% LT) and to need vasopressor support (16% LD vs 0% LT). Three LT patients developed severe SARS-CoV2 infection; immunosuppression intensity is shown in Table 4 (Supplemental Digital Content). Only 3% of the total cohort required RRT. Patients with LD had longer hospitalizations (median 9.5 vs 2 days). Complications occurred more often in the LD cohort, including new-onset ascites (n = 8) and bacterial infection (n = 1). Two BA patients presented with their first episode of variceal bleeding: 1 remains listed for LT, the other was subsequently transplanted. Of the 6 LT patients with elevated transaminases, 2 (1 in the immediate post-LT period, 1 >10 years post-LT) developed biopsy-proven acute cellular rejection in the setting of immunosuppression reduction. Both resolved with immunosuppression modulation with no reported graft loss.
The only 2 reported deaths were from the LD cohort (Table 6, Supplemental Digital Content). One morbidly obese adolescent required mechanical ventilation and died of multiorgan failure. The second adolescent who met diagnostic criteria for MIS-C had a remote history of bone marrow transplant for Fanconi anemia complicated by cirrhosis from graft-versus-host disease and died from complications of acute on-chronic liver failure and respiratory failure.
Predictors of Severe Acute Respiratory Syndrome Coronavirus-2 Infection
Severe illness from SARS-CoV2 infection occurred in 20 (22%) total patients and was significantly more likely in children reported to the registry with LD than LT (85% vs 15%). Patients with severe disease were also significantly more likely to have obesity, NAFLD, no history of immunosuppression use, higher peak ALT, higher international normalized ratio, higher ferritin, and lower nadir albumin levels (Table 2). Both univariable and bivariable analyses showed that patients with NAFLD (odds ratio [OR] 5.6; confidence interval (CI): 1.19–26.3] vs non-NAFLD) and LD (OR 6.1; CI: 1.5–24 vs LT) were significantly more likely to develop severe SARS-CoV2 infection (Table 3).
TABLE 2.
Comparison between severe∗ and nonsevere clinical presentation in patients with severe acute respiratory syndrome coronavirus-2 infection
| Severe∗ SARS-CoV2 infection, N = 20 | Nonsevere SARS-CoV2 infection, N = 71 | P values | |
| Baseline characteristics | |||
| Native liver disease, % | 17 (85) | 27 (38) | <0.001 |
| Liver transplant, % | 3 (15) | 44 (62) | <0.001 |
| Age, years; median (IQR) | 13.5 (1–17) | 10 (4–16) | 0.8 |
| Male gender, % | 13 (65) | 35 (49) | 0.3 |
| Obesity, % | 8 (40) | 9 (13) | 0.006 |
| NAFLD diagnosis, % | 7 (35) | 3 (4) | 0.001 |
| Biliary atresia diagnosis, % | 4 (20) | 26 (37) | 0.12 |
| Clinical data | |||
| Peak INR, median (IQR) | 1.5 (1.2–2.4) | 1.1 (1–1.2) | <0.001 |
| Nadir albumin, g/dL; median (IQR) | 2.7 (2.2–3.2) | 3.9 (3.2–4.2) | <0.001 |
| Peak ALT, IU/L; median (IQR) | 227 (75–2722) | 84 (43–168) | 0.001 |
| Peak ferritin, ng/mL, median (IQR) | 1,495 (312–4,321) | 352 (97–611) | 0.03 |
| Peak total bilirubin, mg/dL, median (IQR) | 3.1 (0.7–6.3) | 1.05 (0.4–2.2) | 0.06 |
| CRP, median (IQR) | 10.9 (3.3–34) | 2.5 (0.5–10.6) | 0.08 |
| Baseline platelet count, ×109/L, median (IQR) | 197 (134–278) | 245 (166–300) | 0.2 |
| Baseline platelet count <150 × 109/L, n (%) | 6 (30) | 11 (15) | 0.1 |
| Absolute lymphocyte count, cell/mm3, median (IQR) | 1,300 (760–2,470) | 2,000 (920–4920) | 0.2 |
ALT = alanine aminotransferase; CRP = C-reactive protein; INR = international normalized ratio; IQR = interquartile range; IU = international units; n = number; NAFLD = nonalcoholic fatty liver disease; ng = nanograms; SARS-CoV2 = severe acute respiratory syndrome coronavirus-2.
Severe SARS-CoV2 infection defined as pediatric intensive care unit level of care, respiratory failure requiring mechanical ventilation, use of vasoactive medications, use of renal replacement therapy, and/or death
TABLE 3.
Univariate and bivariate logistic regression to evaluate risk factors for severe∗ clinical presentation of the severe acute respiratory syndrome coronavirus-2 infection, n = 20
| Univariate analysis | Bivariate analysis | |||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Native liver disease LT† | 9.2 (2.4–34) | 0.001 | 6.1 (1.5–24) | 0.01 |
| NAFLD | 12 (2.7–53) | 0.001 | 5.6 (1.19–26.3) | 0.02 |
| Non-NAFLD† | ||||
| Obese | 4.5 (1.4–14) | 0.008 | ||
| Nonobese† | ||||
| Biliary atresia | 0.4 (0.11–1.4) | 0.1 | ||
| Nonbiliary atresia† | ||||
| Female | 0.52 (0.18–1.4) | 0.2 | ||
| Male† | ||||
| Age | 1.01 (0.94–1.06) | 0.7 | ||
| Immunosuppression use | 0.1 (0.03–0.37) | <0.001 | ||
| No immunosuppression use† | ||||
CI = confidence interval; IS = immunosuppressive medication; LT = liver transplant; NAFLD = nonalcoholic fatty liver disease.
Severe SARS-CoV2 infection defined as pediatric intensive care unit level of care, respiratory failure requiring mechanical ventilation, use of vasoactive medications, use of renal replacement therapy and/or death.
Reference group.
DISCUSSION
This international multicenter observational cohort study is the largest reported experience of laboratory-confirmed SARS-CoV2 infection in children with LD and LT. Notably, despite long-term immunosuppression use, pediatric LT recipients in this registry were significantly less likely to develop severe SARS-CoV2 disease than patients with LD. No LT recipients who tested positive for SARS-CoV2 required mechanical ventilation or died. Similar to adult studies, we observed that NAFLD patients had an increased risk of severe SARS-CoV2 infection. Finally, we report that despite severe morbidity from SARS-CoV2 in children manifesting as PALF, all recovered without LT.
In adults, obesity and NAFLD are predictors of worse outcomes and rapid disease progression following SARS-CoV2 infection (28,29). Zachariah et al previously identified obesity as the most significant predictor of mechanical ventilation in children >2 years of age (9). In our pediatric cohort, NAFLD predicted a nearly 6-fold increased odds of severe illness from SARS-CoV2 infection. NAFLD may increase liver injury risk during infection as it reportedly exacerbates viral oxidative stress in hepatocytes and promotes release of pro-inflammatory cytokines by adipose cells and Kupffer cells (30). In adults, obesity is a notable predictor of worse outcomes in SARS-CoV2 infection, and patients with NAFLD appear to have rapid disease progression and prolonged viral shedding (31). One of the 2 deaths reported, one occurred in a morbidly obese teenager with NAFLD. Obesity in children remains a public health crisis, magnified in the setting of a global pandemic. It is imperative that clinicians aggressively target weight loss in obese patients to potentially mitigate their risk for poor outcomes secondary to SARS-CoV2 infection.
Our target population's overall clinical presentation and low mortality align with previous reports that children have a milder course than adults (32). In our cohort, LT recipients were less likely to require PICU care, mechanical ventilation, or die despite significant immunosuppression exposures; this is illustrated in a previously described case of a 6-month-old infected immediately post-transplant amid full steroid induction who completely recovered (22,23). These findings mirror prior reports of pediatric (24) and adult (19,20) solid organ transplant recipients in whom immunosuppression intensity was not associated with inferior outcomes or increased mortality. Furthermore, recent report suggests that tacrolimus may favorable mitigate disease severity (33). There is not enough evidence to support reduction or withholding immunosuppression in LT recipients during SARS-CoV2 infection unless indicated for other clinical reasons.
In adult series, SARS-CoV2-associated hepatitis (5,34), acute on-chronic liver failure (35–37) and ALF without need for LT (38) have been reported. We report 4 cases of SARS-CoV2-associated PALF that resolved spontaneously without LT. LD patients more commonly developed acute hepatitis compared with LT recipients. In our cohort, disease severity was associated with more severe transient hepatocellular injury in alignment with previous reports in children (39) and adults (35,36,38,40–42). The interpretation and management of elevated transaminases in patients with LD or LT are complex, considering the potential for drug-induced liver injury with SARS-CoV2-directed therapies, underlying conditions, and direct cytopathic effects. Long-term follow-up is essential to characterize patients with LD at risk for hepatic decompensation and LT recipients at risk for graft rejection because of immunosuppression modulation during SARS-CoV2 infection. Given the lack of proven direct SARS-CoV2 therapy, treatment approaches reported to the registry varied widely over time, precluding meaningful assessment of the reported agents’ safety or efficacy. Overall, supportive care is essential and prevention is critical.
In our study, 8% of registry enrollees were black and 34% were Hispanic. Both SARS-CoV2-related deaths occurred in Hispanic children. Racial disparities in SARS-CoV2 infection are widely reported, including a higher disease prevalence and mortality among black and Hispanic people, compared with white people (43). As advocates for children, our mandate is to divest from racial health inequities by implementing meaningful changes to address structural racism and ensure equitable outcomes (44).
The strengths of this study remain our international collaborative focus on SARS-CoV2 infection in children with LD or LT and the opportunity to share real-time aggregate data on the largest cohort available to date, world-wide. Study limitations include retrospective data collection and incomplete data because of lack of standardized SARS-CoV2 diagnostics. Reporting bias is inherent to all registry designs and providers may have been more likely to report both mild and severe cases of SARS-CoV2 infection to the registry in LT and pre-LT patients, but only severe cases in patients with other types of native LD. Our findings must be interpreted with this potential limitation in mind especially when directly comparing cohorts. The lack of data to distinguish cirrhotic from noncirrhotic patients in our LD group further limits our ability to better predict the impact of the stage of LD on outcomes. The majority of cases originated in the United States, restricting geographic diversity as SARS-CoV2 has various mutations (45). Given a lack of guidelines, therapeutic approaches varied widely, precluding any assessment of the safety or efficacy of treatments employed.
CONCLUSIONS
We identified NAFLD and LD as potential factors of severe SARS-CoV2 infection. LT recipients do not appear to be at increased risk of worse outcomes than children with LD despite daily immunosuppression exposure. We report a broad spectrum of liver injury from mild to severe with a reasonable probability of spontaneous recovery in children. Although limited by reporting bias, our data supports that SARS-CoV2 infection in children with LD or LT is different than adults with less morbidity and associated mortality. Continued meticulous attention to primary preventive measures, counseling and family support, particularly with return to school challenges, is, however, paramount (46). Further data collection is required to permit the large-scale analyses essential to inform optimal immunosuppression management and disease-specific treatments for pediatric LD and LT patients.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of the NASPGHAN Hepatology Committee, the SPLIT Quality Improvement and Clinical Care Committee, the SPLIT Executive, and The Transplantation Society. We additionally thank Dr Elizabeth Rand, Dr Rohit Kohli, Julie Economides, Trang Nguyen, Angela Hui, Anna Banc Husu, Ryan Cunningham, Anna Rundle, and the Center for Advanced Computing at Queens University for their scientific or administrative support of the registry.
Footnotes
Supplemental digital content is available for this article. www.jpgn.org).
Drs Mohit Kehar and Noelle H. Ebel are equal first authors.
Drs Steven Lobritto and Mercedes Martinez contributed equally to this study.
The majority of the authors have no financial relationship except for V.L.N.: consulting, Albireo; D.H.L.: consulting, Gilead, Merck and grants and contract, Abbvie, Gilead, and Mirum; T.M.: consulting, Genflix, Alexion. Speakers Bureau: Alexion. S.L., grants and contracts: Gilead, Abbvie. Advisory board: Gilead. Consulting: various law firms; M.M., Advisory board: Gilead.
REFERENCES
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.Patel KP, Patel PA, Vunnam RR, et al. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol 2020; 128:104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology 2020; 159:765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020; 40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020; 5:428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry BM, Benoit SW, de Oliveira MHS, et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin Biochem 2020; 81:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou YH, Zheng KI, Targher G, et al. Abnormal liver enzymes in children and infants with COVID-19: a narrative review of case-series studies. Pediatr Obes 2020; 15:e12723. [DOI] [PubMed] [Google Scholar]
- 8.Dona D, Torres Canizales J, Benetti E, et al. ERN TransplantChild. Pediatric transplantation in Europe during the COVID-19 pandemic: early impact on activity and healthcare. Clin Transplant 2020; 34:e14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr 2020; 174:e202430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://www.cdc.gov/covid-data-tracker/index.html#demographics. Demographic trends of COVID-19 cases and deaths in the U.S. reported to CDC; c2020. Accessed September 6, 2020. [Google Scholar]
- 12.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to U.S. and Canadian pediatric intensive care units. JAMA Pediatr 2020; 174:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotzinger F, Santiago-Garcia B, Noguera-Julian A, et al. ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020; 4:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfred-Cato S, Bryant B, Leung J, et al. California MIS-C Response Team. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantor A, Miller J, Zachariah P, et al. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: a single-center report. Hepatology 2020; 72:1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.cdc.gov/mis-c/hcp/. Information for healthcare providers about Multisystem Inflammatory Syndrome in Children (MIS-C); c2020. Accessed September 6, 2020. [Google Scholar]
- 18.https://covidcirrhosis.web.unc.edu/. SECURE-Cirrhosis registry; c2020. Accessed September 6, 2020. [Google Scholar]
- 19.Kates OS, Haydel BM, Florman SS, et al. UW COVID-19 SOT Study Team. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis 2020; Aug 7:ciaa1097. doi: 10.1093/cid/ciaa1097. Online ahead of print. [Google Scholar]
- 20.Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol 2020; 5:1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morand A, Roquelaure B, Colson P, et al. Child with liver transplant recovers from COVID-19 infection. A case report. Arch Pediatr 2020; 27:275–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagana SM, De Michele S, Lee MJ, et al. COVID-19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med 2020; doi: 10.5858/arpa.2020-0186-SA. [DOI] [PubMed] [Google Scholar]
- 23.Heinz N, Griesemer A, Kinney J, et al. A case of an Infant with SARS-CoV-2 hepatitis early after liver transplantation. Pediatr Transplant 2020; 24:e13778.doi: 10.1111/petr.13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl 2020; 26:832–834. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Minor BL, et al. REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso EM, Horslen SP, Behrens EM, et al. Pediatric acute liver failure of undetermined cause: a research workshop. Hepatology 2017; 65:1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020; 43:1392–1398. [DOI] [PubMed] [Google Scholar]
- 29.Gao F, Zheng KI, Wang XB, et al. Obesity Is a risk factor for greater COVID-19 severity. Diabetes Care 2020; 43:e72–e74. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Fan C, Chen Y, et al. Effect of hepatic steatosis on the progression of chronic hepatitis B: a prospective cohort and in vitro study. Oncotarget 2017; 8:58601–58610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji D, Cheng G, Lau G. Reply to: “NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression – The debate continues”. J Hepatol 2021; 74:484–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020; 109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belli LS, Fondevila C, Cortesi PA, et al. ELITA-ELTR COVID-19 Registry. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology 2020; S0016-5085(20)35514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phipps MM, Barraza LH, LaSota ED, et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology 2020 May 30. Hepatology 2020; 72:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji D, Zhang D, Yang T, et al. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int 2020; 14:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu H, Wander P, Bernstein D, et al. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Liver Int 2020; 40:1590–1593. [DOI] [PubMed] [Google Scholar]
- 37.Rhee Y, Chan EL, Eswaran SL, et al. Fatal COVID-19 in a patient with end-stage liver disease wait-listed for liver transplantation: an evidence-based review of COVID-19 screening modalities prior to transplant. Clin Liver Dis 2020; 15:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber S, Mayerle J, Irlbeck M, et al. Severe liver failure during SARS-CoV-2 infection. Gut 2020; 69:1365–1367. [DOI] [PubMed] [Google Scholar]
- 39.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int 2020; 14:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhoori S, Rossi RE, Citterio D, et al. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol 2020; 5:532–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anastasiou OE, Korth J, Herbstreit F, et al. Mild vs severe liver injury in SARS-CoV-2 infection. Dig Dis 2021; 39:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooper MW, Napoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020; 323:2466–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardeman RR, Medina EM, Boyd RW. Stolen breaths. N Engl J Med 2020; 383:197–199. [DOI] [PubMed] [Google Scholar]
- 45.Mercatelli D, Giorgi FM. Geographic and genomic distribution of SARS-CoV-2 mutations. Front Microbiol 2020; 11:1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Downes KJ, Danziger-Isakov LA, Cousino MK, et al. Return to school for pediatric solid organ transplant recipients in the United States during the COVID-19 pandemic: expert opinion on key considerations and best practices. J Pediatric Infect Dis Soc 2020; 9:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


