Abstract
Inhibition of cytochrome P450 (CYP)-mediated retinoic acid (RA) metabolism by RA metabolism blocking agents (RAMBAs) increases endogenous retinoids and is an alternative to retinoid therapy. Currently available RAMBAs (i.e. liarozole and talarozole) tend to have fewer adverse effects than traditional retinoids but lack target specificity. Substrate-based inhibitor DX314 has enhanced selectivity for RA-metabolizing enzyme CYP26B1 and may offer an improved treatment option for keratinization disorders such as congenital ichthyosis and Darier disease. In this study we use RT-qPCR, RNA sequencing, pathway, upstream regulator, and histological analyses to demonstrate that DX314 can potentiate the effects of all-trans-RA (atRA) in healthy and diseased reconstructed human epidermis (RHE). We unexpectedly discovered that DX314, but not atRA or previous RAMBAs, appears to protect epidermal barrier integrity. Additionally, DX314-induced keratinization and epidermal proliferation effects are observed in a rhino mice model. Altogether, results indicate that DX314 inhibits atRA metabolism with minimal off-target activity and shows therapeutic similarity to topical retinoids in vitro and in vivo. Findings of a barrier-protecting effect require further mechanistic study but may lead to a unique strategy in barrier-reinforcing therapies. DX314 is a previously unreported promising candidate compound for further study and development in the context of keratinization disorders.
INTRODUCTION
Therapeutics targeting retinoid biopathways have been implemented in the clinical treatment of keratinization disorders such as the congenital ichthyoses (Vahlquist et al. 2008), Darier disease (DD) (Casals et al. 2009; Cooper and Burge 2003; Dicken et al. 1982; Steijlen et al. 1993), and other skin disorders (e.g. acne, psoriasis) (Dawson and Dellavalle 2013; Fisher and Voorhees 1996) to alleviate patient symptoms. Such therapies leverage the role of endogenous retinoids in regulating keratinocyte proliferation and differentiation. Retinoid bioactivity is primarily, although not solely, mediated by transcription factors such as retinoic acid receptors (RAR) and retinoid X receptors (RXR) (Fisher et al. 1994).
However, retinoid treatments also result in adverse effects including dry skin, irritation, redness, photosensitivity, teratogenicity and barrier impairment (Orfanos et al. 1997). Endogenously-occurring retinoids (e.g. tretinoin) autoinduce their own metabolism (Van Der Leede et al. 1997; Marikar et al. 1998), which in turn demands higher exogenous doses for effective treatment, resulting in increased systemic exposure. Synthetic retinoids, particularly tazarotenic acid, will both activate RARs and inhibit RA-metabolism (Foti et al. 2016), which may overstimulate retinoid biopathways and increase associated adverse effects. For these reasons, RA metabolism blocking agents (RAMBAs) (Verfaille et al. 2008), including liarozole and talarozole, were developed to target the primary RA-specific metabolizing enzymes of the cytochrome p450 family 26 (CYP26) (Ray et al. 1997).
RAMBAs, particularly when used topically, achieve therapeutic effects without high exposure to systemic levels of RA by utilizing endogenously available RA rather than high doses of exogenous retinoids, which theoretically reduces overexposure and adverse effects. Topical RAMBAs could be implemented either as standalone treatments or as adjunct therapies to reduce oral retinoid dosing without loss of therapeutic efficacy. Reports illustrate successful inhibition of RA metabolism using liarozole (Van Wauwe et al. 1992) and proven efficacy in treating several skin disorders (Berth-Jones et al. 2000; Bhushan et al. 2001; Kang et al. 1996; Kuijpers et al. 1998; Lucker et al. 2005; Lucker et al. 1997; Vahlquist et al. 2014), including in a comparative trial vs acitretin (an oral retinoid) for congenital ichthyosis, which showed a trend towards a better safety profile (Verfaille et al. 2007b). Problematically, liarozole also inhibits off-target CYPs such as aromatase (CYP19), an important enzyme in estradiol biosynthesis (Nelson et al. 2013). Previous RAMBAs have not progressed past clinical trials, suggesting the need for improved RAMBA candidates.
The promising results of early-generation RAMBAs led Diaz et al. to develop a series of compounds targeting RA metabolism via specific inhibition of two CYP26 isoforms (A1 and B1) (Diaz et al. 2016). Removal of the heme-interacting azole moiety, thought to contribute to the non-specific effects of previous azole-containing RAMBAs, may preserve the desired effects while minimizing off-target activity. One of these compounds, a CYP26B1-specific inhibitor, DX314 (IC50: CYP26A1=1752nM; CYP26B1=108nM), was described in US patent US009963439B2 as example 39 (Diaz et al. 2018).
Endogenous all-trans-RA (atRA) is a well-known regulator of epidermal proliferation and differentiation (Fisher and Voorhees 1996), in part by inducing heparin-binding EGF-like growth factor (HBEGF), which stimulates keratinocyte proliferation (Rittié et al. 2006; Stoll and Elder 1998; Xiao et al. 1999; Yoshimura et al. 2003), and involucrin (IVL), a late marker of epidermal differentiation (Eckert et al. 2004; Monzon et al. 1996; Poumay et al. 1999). Expression of both CYP26A1 and CYP26B1 is induced by atRA, but only the CYP26A1 promotor contains a RA response element (RARE) which directly binds RARs (Pavez Loriè et al. 2009a).
In this study, we investigate RAMBAs in vitro under tightly-controlled growth conditions that specifically do not contain RA or RA precursors (Giltaire et al. 2009; Minner et al. 2010; Pavez Loriè et al. 2009a; Poumay et al. 1999). In these conditions, a highly specific RAMBA will have a negligible effect in the absence of atRA, however when co-treated with a nanomolar dose of atRA, facilitate a relative increase in atRA concentration by inhibiting its metabolism, and therefore potentiate the expression of RA-responsive genes. Since in vivo concentrations of atRA in healthy human skin are typically 2-4nM (Mihály et al. 2011), we co-treated RAMBAs in vitro with 1nM atRA to provide a near-physiological basal level, without saturating nanomolar-sensitive atRA effects, which allowed us to observe if RAMBAs can potentiate those effects.
Skin acts as an effective barrier to the environment. Changes in epidermal barrier integrity can be investigated using transepithelial electrical resistance (TEER) and transepidermal water loss (TEWL). TEER is an in vitro assay assessing electrical properties to evaluate possible changes in trans-and paracellular (regulated by tight junctions) ion permeability across the epidermis. TEWL measures passive diffusion of water across the epidermis and can be performed in vivo or in vitro. Decreased TEER or increased TEWL typically indicates barrier integrity disruption.
Our data show that DX314 potentiates the effects of atRA on gene expression in healthy and diseased epidermis by inhibiting CYP26B1-mediated RA metabolism. Unexpectedly, DX314 mitigated the epidermal barrier dysregulation and irregular morphology displayed by other RAMBAs and high dose atRA. In addition, topical DX314 induced comedolytic/anti-keratinizing effects in the rhino mouse model, reflecting those observed in previous retinoid studies (Ashton et al. 1984; Fort-Lacoste et al. 1999; Kligman and Kligman 1979).
Together, this study suggests that DX314 exhibits potential as a keratinization disorder therapeutic that may address some shortcomings of previous retinoid-based treatments.
RESULTS
DX314 potentiates the effects of atRA in healthy and keratinization disorder keratinocytes
Based on RT-qPCR assays, a 4-day atRA treatment of RHE caused a dose-dependent increase in HBEGF, CYP26A1 and IVL gene expression (Figure 1a). DX314, together with near-physiological dosing of atRA (1nM; to provide basal RA without saturating sensitive RA pathways) mimics the consequences of high dose atRA on the expression of every gene analyzed, indicating potentiation of atRA. Liarozole with atRA significantly increases HBEGF and CYP26A1 expression relative to control, but only CYP26A1 expression is potentiated compared to 1nM atRA alone.
Figure 1: DX314 potentiates atRA gene expression effects in healthy RHE.

(a) Relative expression of HBEGF, IVL, and CYP26A1 mRNA by RT-qPCR. Symbol underneath indicates comparison group (n=3-4 times in duplicate; mean±95% CI; *p≤0.05, **p≤0.01, ***p≤0.001; one-way ANOVA with Tukey’s correction; *vs Control, †vs 1nM atRA, ¥vs 10nM atRA, ⨂vs DX314-alone, ⊕vs Liarozole-alone). (b) IVL localization in healthy RHE. (c) RNAseq: Relative mRNA expression of retinoid-responsive genes in healthy RHE. Non-grey cells differ from controls (FDR≤0.05; n=3-5). Adjacent green cell indicates likely RAR-mediated effect based on presence of RARE-promotor for respective gene. Predicted activation z-score of (d) upstream regulators or (e) canonical pathways determined by IPA software utilizing RNAseq data. Black dots indicate statistical insignificance (p≤0.05 and z-score ≥2 or ≤−2). Additional abbreviations: Table S4.
Immunostaining shows IVL (Figure 1b) primarily localized in the upper epidermis of control and DX314-alone RHE. Induction of early IVL expression is observed in basal and suprabasal layers of the epidermis with atRA-alone, and even more so, DX314 with 1nM atRA.
RNAseq confirmed that DX314 alone (atRA-free conditions) had no effect on HBEGF, IVL, CYP26A1 or CYP26B1 mRNA expression (Figure 1c), and that DX314 potentiated the effects of 1nM atRA, not only on these genes, but on numerous other known retinoid-responsive genes, among them several keratins (KRT) (Radoja et al. 1997), lecithin:retinol acyl-transferase (LRAT) (Kurlandsky et al. 1996), retinol binding protein 1 (RBP1) (Kang et al. 1995), and cellular retinoic acid binding protein 2 (CRABP2) (Aström et al. 1994). Changes in expression of other genes that involve both direct (RARE-containing promotor, indicated by adjacent green box) (Aström et al. 1992; Fisher et al. 1995; Lalevée et al. 2011; Laursen et al. 2015; Loudig et al. 2000; Radoja et al. 1997; de Thé et al. 1990; Tomic-Canic et al. 1992; Vasios et al. 1989) and indirect or unknown RA pathways supported the potentiation effect.
RNAseq data was further analyzed with Ingenuity Pathway Analysis (IPA) (Krämer et al. 2014) software for canonical pathway and upstream regulator prediction. The applied significance cutoffs (see Methods) resulted in 1360, 5480, 169, and 3015 differentially expressed genes in RHE treated with 1nM a/RA, 100nM atRA, 1000nM DX314, and 1000nM DX314 with 1nM atRA, respectively. Analysis results were sorted by overall |z-score|, indicating the strength and direction of each prediction (positive score = activation, negative score = inhibition). As expected, the upstream regulator with largest activation score was atRA (tretinoin), which displayed an activation pattern consistent with atRA potentiation (Figure 1d). Of the top 20 scoring regulators, only three weakly displayed any predicted activity by DX314 alone. Canonical pathway analysis (Figure 1e) found the overall most activated (“Integrin Signaling”) and inhibited (“RhoGDI Signaling”) pathways both display activation patterns suggesting a potentiation of atRA by DX314.
To test the potential of DX314 in certain keratinization disorders, we investigated CYP26A1 gene expression in keratinocytes from patients with Darier disease (DD), recessive x-linked ichthyosis (RXLI), and lamellar ichthyosis (LI). DX314 potentiates the effects of atRA in DD RHE (Figure 2a), RXLI full-thickness RHE (Figure 2b), LI RHE (Figure 2c), and RXLI monolayer cultures (Figure 2d). Talarozole potentiated atRA in DD RHE and liarozole potentiated atRA in RXLI full-thickness RHE.
Figure 2: DX314 potentiates the effects of atRA on CYP26A1 mRNA expression in keratinocytes from individuals with keratinization disorders.
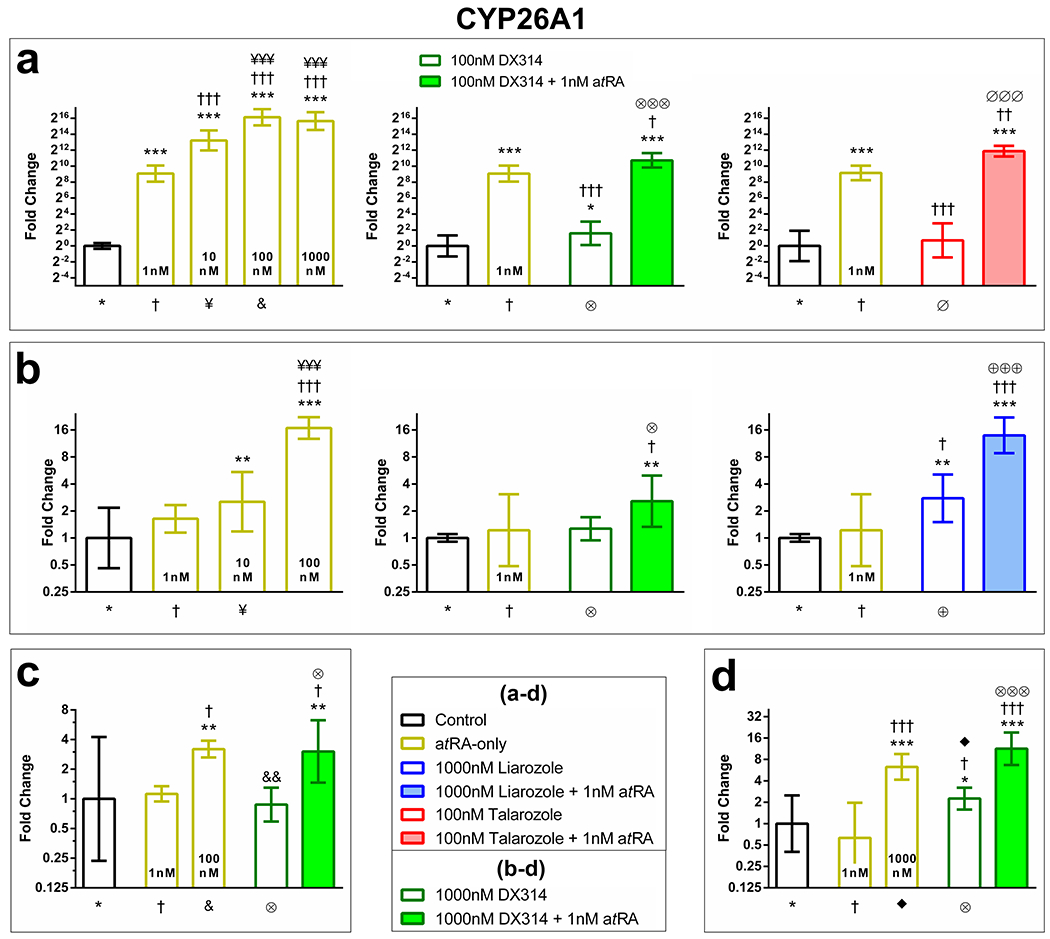
Relative CYP26A1 mRNA expression by RT-qPCR in; (a) Darier disease RHE, (b) recessive x-linked ichthyosis (RXLI) full-thickness RHE, (c) lamellar ichthyosis RHE, and (d) RXLI monolayer keratinocyte cultures. RHE were treated for 4 days and monolayer keratinocytes for 20hrs. Statistical significance was computed with (a) autoscaled or (b-d) raw dCt values. Symbol below each treatment indicates comparison group (n=3 independent replicates with technical duplicates; mean±95% CI; *p≤0.05; **p≤0.01; ***p≤0.001; one-way ANOVA with Tukey’s correction; *vs Control, †vs 1nM atRA, ¥vs 10nM atRA, &vs 100nM atRA, ◆vs 1000nM atRA, ⊗vs DX314-alone, ⊕vs Liarozole-alone, ∅vs Talarozole-alone).
Histological analysis shows that atRA induces robust morphological changes in DD RHE (Figure 3a) including a dramatic loss of SG (and their filaggrin-containing keratohyalin granules (KG)), denucleation and flattening of stratum spinosum (SS) keratinocytes, and an overall unhealthy appearance. When treated alone, DX314 and talarozole caused no major morphological changes. Co-treatment with talarozole and 1nM atRA shifted the cell morphology, most notably with loss of SG and flattening of epidermal keratinocytes, to more closely resemble the appearance of high dose atRA. DX314 with atRA also affected morphology relative to DX314 alone, but to a lesser extent, with the appearance not significantly different than control or only 1nM atRA-treated RHE.
Figure 3: DX314 potentiates the effects of atRA on the expression and localization of keratin 10 (KRT10) in Darier disease (DD) RHE. atRA, but not DX314, induces a loss of stratum granulosum.

(a) HE staining and (b) immunofluorecent staining of KRT10 (green) localization with nuclear stain (blue), in DD RHE treated for 4 days. Scale bars: black = 20μm, white =50 μm. (c) Relative KRT10 mRNA expression by qPCR. Symbol below each treatment indicates comparison group. (n=3 independent replicates with technical duplicates; mean±95% CI; *p≤0.05; **p≤0.01; ***p≤0.001; one-way ANOVA with Tukey’s correction on autoscaled values; *vs Control, †vs 1nM atRA, ¥vs 10nM atRA, &vs 100nM atRA, ⊗vs DX314-alone, ∅vs Talarozole-alone).
KRT10, a commonly used marker of epidermal differentiation that localizes to the suprabasal epidermis, showed reduced expression in atRA-treated RHE (Figure 3b). Treatment with DX314 or talarozole alone led to no change in KRT10 localization, but co-treatment with near-physiological levels of atRA reduced staining in the lower SS. KRT10 gene expression in DD RHE (Figure 3c) was decreased by atRA in a dose-dependent manner and was potentiated by DX314 and talarozole. This effect on KRT10 was also observed in healthy RHE (Figure 1c and S1).
DX314 induces barrier effects in healthy and diseased RHE
As a measure of barrier integrity, TEER of healthy RHE was assessed. Independent runs with RHE from several donors were pooled and normalized to their respective controls (Figure 4a). High dose atRA significantly decreased TEER. Liarozole and talarozole alone showed no effect on TEER, but co-treatment with atRA resulted in significant TEER decrease. Surprisingly, in both healthy and LI (Figure 4b) RHE, DX314 alone increased TEER and caused no decrease relative to control with atRA co-treatment. TEWL was similarly affected by atRA, but no significant change was seen with DX314 alone.
Figure 4: DX314 protects barrier function in RHE.

(a) Transepithelial electrical resistance (TEER) in healthy RHE. TEER was normalized to control RHEs for each run, then pooled for analysis. Graph shows Tukey’s boxplot with outliers. Sample sizes (n) are shown above x-axis. (b) LI RHE TEER (top), transepidermal water loss (middle), and the linear correlation between the two measures (bottom), (c) HE staining of lamellar ichthyosis (LI) RHE. Scale bar = 50μm. (d) Semi-quantitative analysis of relative stratum granulosum (SG) surface area in healthy RHE. (e) Relative expression of epidermal differentiation complex (EDC) genes and regulators by RNAseq. Colored (non-grey) cells indicate statistical significance from control (FDR≤0.05; n=3-5). All RHE received a 4-day treatment. (a,b,d) (*p≤0.05; **p≤0.01; ***p≤0.001; one-way ANOVA with Dunnett’s correction vs control).
Morphologically, atRA disrupted LI (Figure 4c) and healthy RHE (Figure S2 and S3) structure as described above. DX314 led to no major changes in morphology when dosed alone. However, unlike other RAMBAs (Figure S2), DX314 with atRA co-treatment reduced disruption in normal morphology (improved SG/KG, more columnar basal keratinocytes, and less disorganized upper epidermis). Semi-quantitative analysis of SG surface area (Figure 4d), measured in healthy RHE (Figure S3), confirmed a dramatic reduction of the SG by atRA, but no significant loss in DX314 treated groups.
The epidermal differentiation complex (EDC) is a cluster of genes on human locus 1q21 that are essential for epidermal differentiation (Kypriotou et al. 2012). As noted using RNAseq, expression of these genes (Figure 4e) is generally consistent with other retinoid-responsive genes. However, many cornified envelope (CE) precursor family genes, such as late cornified envelope (LCE) and small proline rich (SPRR) proteins, were dramatically downregulated by high dose atRA, but not affected by DX314 with atRA, which may play a role in the observed barrier effects. In addition, FLG expression was increased 2-fold with the DX314-atRA combination, but not with high dose atRA.
Nuclear receptor profiling revealed that DX314 acts as an inverse agonist for RAR-related orphan receptors (ROR) α and γ (Figure S4), while showing no activity on any other nuclear receptors studied.
DX314 reduces epidermal abnormalities in rhino mice
Rhino mice are commonly used as an in vivo model for screening comedolytic and anti-keratinizing compounds such as retinoids (Ashton et al. 1984; Fort-Lacoste et al. 1999; Griffiths et al. 1993; Seiberg et al. 1997). Overall, DX314 treatment improved skin morphology (Figure 5). DX314 decreased comedo density (Figure 6a and 6b), increased the mean comedo profile (ratio of comedo opening size to internal diameter) (Figure 6c), suggesting comedolysis, and induced epidermal thickening (Figure 6d), consistent with previous studies of topical retinoids. Again, DX314 treatment did not change TEWL relative to vehicle (Figure 6e). No abnormal behavior, adverse skin changes, changes in body weight or DRAIZE scoring (Table S5 and S6) were observed throughout the study.
Figure 5: DX314 reduces rhino mouse skin abnormalities.

Representative HE staining of skin biopsies from rhino mice topically treated for 11 days with vehicle (acetone) or 1% DX314. ImageJ software was used to quantify comedonal number, profile (d/D, ratio of opening to inner diameter), and epidermal thickness. Epidermal thickness was measured at multiple points across each sample by measuring the sum of epidermal areas (yellow), excluding the corneal layer, and dividing by the sum of the length of the basal layers (dotted blue line). Scale bar =200μm.
Figure 6: DX314 treatment reduces comedonal number, induces epidermal thickening, and increases comedonal profile, while having no effect on transepidermal water loss (TEWL) in treated rhino mice.

Semi-quantitative analysis of changes in (a) total (open + closed) and (b) open comedonal number, (c) comedonal profile, and (d) epidermal thickness in rhino mice topically treated with vehicle (acetone) or 1% DX314 over 11 days, (e) Daily TEWL measurements did not reveal any statistically significant differences between treatment groups. (n=5-6 mice per treatment; mean±SD; *p≤0.05; **p≤0.01; Student’s t-test vs vehicle control).
DISCUSSION
Retinoid-based drugs are well-accepted therapeutics for the treatment of many skin diseases (Dawson and Dellavalle 2013; Fisher and Voorhees 1996; Vahlquist et al. 2008). Despite their efficacy, use often leads to adverse reactions from their wide spectrum of non-therapeutically relevant endogenous roles, which are exacerbated by metabolic autoinduction and tolerance (Digiovanna et al. 2013; Orfanos et al. 1997). A strategy involving RAMBAs showed potential in preclinical and clinical studies (Berth-Jones et al. 2000; Bhushan et al. 2001; Bovenschen et al. 2007; Giltaire et al. 2009; Kang et al. 1996; Kuijpers et al. 1998; Lucker et al. 2005; Lucker et al. 1997; Pavez Lorie et al. 2009b; Stoppie et al. 2000; Vahlquist et al. 2014; Verfaille et al. 2007a; Van Wauwe et al. 1992), however, first-generation RAMBAs have not progressed to approved for clinical use. A highly selective RAMBA, with low risk of adverse events, could address the downsides of current treatment options. This study investigates the CYP26B1-selective compound, DX314, as a potential next-generation RAMBA.
Potentiation of the effects of a low, physiologically relevant dose of atRA by DX314 in healthy and keratinization disorder keratinocytes, but not DX314 in an atRA-free environment, confirms that DX314 acts by inhibiting atRA metabolism. These gene expression patterns were reproduced in keratinocyte cultures from individuals with DD and congenital ichthyosis, in addition to healthy skin, suggesting that the bioactivity of DX314 can be therapeutically relevant in skin disorders.
A broader investigation of gene expression changes using RNAseq also showed a strong pattern indicating potentiation of atRA by DX314 on both RARE-promoted, and indirectly regulated genes. Pathway analysis found compelling supporting evidence in predicted upstream regulator and canonical pathway activation patterns.
Immunostaining confirmed the atRA potentiating effects of DX314 on IVL localization in healthy RHE and KRT10 localization in DD RHE.
These experiments showed that DX314 alone had minimal effect on gene expression and therefor, minimal potential for off-target adverse effects, despite therapeutically relevant effects when paired with endogenous levels of at RA.
Keratinization disorders are associated with intrinsic epidermal barrier disruption and a therapy that improves barrier function would be highly desirable. Surprisingly, this study found a significant increase in TEER compared to controls in RHE treated with DX314 alone, and unlike with liarozole and talarozole, no decrease from control when co-treated with atRA. Although higher doses of atRA significantly decreased TEER and DX314 otherwise appeared to potentiate the effects of atRA, DX314 with atRA did not impair barrier function below that of the control. TEWL in LI RHE, which is expected to increase with retinoid treatment or ablation of CYP26B1 (Okano et al. 2012), was not increased by DX314 alone or beyond that of 1nM atRA when added as co-treatment. DX314 did not decrease TEWL in RHE, however, a lack of correlation between in vitro TEWL and barrier function has been previously documented (Chilcott et al. 2002). Despite previous studies in rhino mice showing retinoids inducing substantial increases in TEWL (Elias et al. 1981; Gendimenico et al. 1994), our study found a slight, albeit a non-significant, decrease in TEWL, which additionally suggests an in vivo barrier-protecting effect.
Morphologically, DX314-treated RHE show dramatically less disruption of the SG and KG, and display an overall healthy appearance compared to atRA, liarozole or talarozole-treated healthy, DD, and LI RHE. Semi-quantitative analysis of SG surface area also showed a significant decrease in atRA-treated RHE, but no significant change in RHE treated with DX314, with or without atRA, Nevertheless, DX314-treated RHE tissue sections had a more continuous SG/KG layer relative to controls, which may translate to an increase in TEER.
We observed minimal changes in the expression of many cornified envelope precursor proteins within the EDC, despite a large decrease in expression by high dose atRA and DX314 potentiating of the effects of atRA on other retinoid-responsive genes. We speculate that this preservation of CE protein expression may contribute to the ameliorative effect of DX314 on barrier integrity. In addition, DX314 displays unique inverse agonist activity on RORα and γ. Affinity for RORγ may be explained by DX314’s structural similarity to atRA, which has been previously shown to bind to, and inhibit, RORγ (Stehlin-Gaon et al. 2003). Conversely, atRA was not found to act on RORa, so the DX314 inverse agonism represents another unique property. Furthermore, a previously studied topical RORa/γ inverse agonist was found to inhibit inflammation in mouse models of atopic dermatitis (Dai et al. 2017), a skin disorder displaying pathological barrier disruption. Future investigations should explore the potential link between DX314’s RORa/γ activity and its barrier effects, as well as potential contribution to therapeutic effects of skin barrier protection.
Use of rhino mice to study the in vivo effects of dermatologically active compounds such as retinoids is well-established, and unlike in vitro, does not require co-treatment with atRA since adequate RA is generated in vivo through dietary sources. Reduction of the acne-like cysts (comedones), as well as the associated epidermal thickening (hyperplasia), are sensitive to retinoid treatment. In this preliminary study, DX314 led to significant improvement overall in comedo number (comedones per cm of skin), profile, and induced epidermal thickening. Optimization of the DX314 formulation (to eliminate the harsh acetone vehicle), dosing to improve bioavailability, and extending the treatment duration are likely to amplify DX314 efficacy in this model.
We conclude that our results provide strong evidence that DX314, which is known to specifically inhibit the RA-metabolizing enzyme CYP26B1, potentiates the effects of physiological levels of atRA in keratinocytes from healthy skin and keratinization disorders in vitro; may protect from epidermal skin barrier disruption by retinoids; and has a restorative effect on changes in vivo rhino mouse skin consistent with previous retinoid treatments. These observations merit further investigation as a unique keratinization disorder treatment with the ability to simultaneously correct abnormal keratinization while protecting critical skin barrier function. Together these findings present an exciting therapeutic candidate aimed at providing improved patient outcomes with minimal adverse effects, in contrast to currently available treatments.
MATERIALS AND METHODS
Primary keratinocytes
Healthy and DD primary keratinocytes, provided by Dr. Poumay’s lab (Namur, Belgium), were isolated as previously described (Poumay et al. 2004) from skin samples provided by Drs. B. Bienfait and J.S. Blairvacq (Clinique St Luc, Namur-Bouge, Belgium). Additional healthy keratinocytes were purchased from ThermoFisher (Cascade Biologics, Portland, OR). RXLI and LI keratinocytes were provided by Dr. Paller (Northwestern University, IL). Details in Table S1.
Monolayer culture and RHE
Monolayer cultures were prepared as previously described (Minner et al. 2010). Upon reaching confluence, keratinocytes were treated for 20hr to compounds solubilized in media (0.1% DMSO vehicle for all in vitro studies).
RHE were produced as previously described (Poumay et al. 2004; De Vuyst et al. 2014) in Epilife media with 1.5mM Ca2+ (Cascade Biologics, Portland, OR), 10ng/mL keratinocyte growth factor (Sigma, Saint Louis, MO) and 50μg/mL vitamin C (Sigma, Saint Louis, MO). Treatments were started day 7 of growth, refreshed day 9, and halted day 11.
Full-thickness RHE were prepared as previously described (Zheng et al. 2012). Briefly, keratinocytes were seeded atop a simulated dermis (collagen matrix containing J2-3T3 fibroblasts) and allowed to develop into stratified epidermis before receiving a 4-day treatment (refreshed day 2).
RHE histological analysis and immunostaining
RHE were processed as previously described (Frankart et al. 2012; De Vuyst et al. 2014) and stained with hematoxylin-eosin (HE) or prepared for immunostaining. Further described in Supplementary Methods.
Measures of epidermal barrier function
TEER was measured by a previously described method (Frankart et al. 2012) with a ERS-2 voltohmmeter (Millipore, Burlington, MA). TEWL measurement used an AquaFlux AF200 evaporimeter (Biox Systems, London, England). For RHE, a sterilized gasket was placed between the cell culture insert and TEWL probe to form airtight seal. TEWL was measured over 60-90s until reaching a steady state. Analysis of SG surface area was performed using Fiji/ImageJ (Schindelin et al. 2012). SG surface area, defined by the presence of KG, was manually outlined and the area divided by each tissue’s total area.
RNA isolation, RT-qPCR, RNAseq, and bioinformatics
Details on RT-qPCR, RNA-seq, and bioinformatics (Andrews 2010; Bolger et al. 2014; Durinck et al. 2009; Kim et al. 2017; Kim et al. 2016; Kim et al. 2015; Li et al. 2009; Love et al. 2013; Pertea et al. 2015; RCoreTeam 2018; Zhu et al. 2018) are provided in Supplementary Methods. Primer sequences (Giltaire et al. 2009) provided in Table S2.
When applicable, a method described by (Willems et al. 2008) was used to standardize the qPCR data to correct for interindividual variability before analysis.
Nuclear receptor profiling
Refer to Supplementary Methods.
Rhino mice
Eleven RHJ/LeJ rhino mice (2-3 males, 3 females per group) received daily topical application of 50μL vehicle (acetone), or 1% DX314, on a 2x2cm area of back skin for 11 days. All animal studies were approved by IACUC under NIH guidelines. Details in Supplementary Methods.
Statistical analysis
Statistics, apart from separately described RNAseq portion, were performed as described in respective figure legends using Prism 6 (Graphpad Software, La Jolla, CA).
Data availability statement
Data available upon request.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Drs. B. Bienfait and JS. Blairvacq (Clinique St. Luc, Namur-Bouge, Belgium) for providing skin samples used in this study. We would also like to thank Joanna Kreitinger and Larissa Walker of DermaXon™ and the Northwestern University Skin Biology and Diseases Resource-based Center (P30AR075049) for their assistance with various lab tasks.
Funding:
This work was supported by the by the National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health: R44AR069416 (PD), P20GM103546 (PD, JV). The content of this paper is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Abbreviations:
- RAMBA
retinoic acid metabolism blocking agents
- atRA
all-trans-retinoic acid
- RHE
reconstructed human epidermis
- TEER
transepithelial electrical resistance
- TEWL
transepidermal water loss
- CYP26
cytochrome p450 family 26
- DD
Darier disease
- LI
lamellar ichthyosis
- RXLI
Recessive x-linked ichthyosis
Footnotes
See supplementary materials for additional abbreviations (Table S4)
CONFLICTS OF INTEREST
PD is cofounder of DermaXon™ and inventor of the technology, he and The University of Montana are entitled to future royalty payments. JV was employed at DermaXon™ during a portion of this study.
REFERENCES
- Andrews S FastQC: A quality control tool for high throughput sequence data. [Internet], 2010. Available from: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Ashton RE, Connor MJ, Lowe NJ. Histologic changes in the skin of the rhino mouse (hrrhhrrh) induced by retinoids. J. Invest. Dermatol. Elsevier Masson SAS; 1984;82(6):632–5 [DOI] [PubMed] [Google Scholar]
- Aström A, Pettersson U, Chambon P, Voorhees JJ. Retinoic acid induction of human cellular retinoic acid-binding protein-II gene transcription is mediated by retinoic acid receptor-retinoid X receptor heterodimers bound to one far upstream retinoic acid-responsive element with 5-base pair spacing. J. Biol. Chem. 1994;269(35):22334–9 [PubMed] [Google Scholar]
- Aström A, Pettersson U, Voorhees JJ. Structure of the human cellular retinoic acid-binding protein II gene. Early transcriptional regulation by retinoic acid. J. Biol. Chem. 1992;267(35):25251–5 [PubMed] [Google Scholar]
- Berth-Jones J, Todd G, Hutchinson PE, Thestrup-Pedersen K, Vanhoutte FP. Treatment of psoriasis with oral liarozole: a dose-ranging study. Br. J. Dermatol. 2000; 143(6): 1170–6 [DOI] [PubMed] [Google Scholar]
- Bhushan M, Burden AD, McElhone K, James R, Vanhoutte FP, Griffiths CEM. Oral liarozole in the treatment of palmoplantar pustular psoriasis: A randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2001;145(4):546–53 [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovenschen HJ, Otero ME, Langewouters AMG, van Vlijmen-Willems IMJJ, van Rens DWA, Seyger MMB, et al. Oral retinoic acid metabolism blocking agent Rambazole for plaque psoriasis: an immunohistochemical study. Br. J. Dermatol. 2007;156(2):263–70 [DOI] [PubMed] [Google Scholar]
- Casals M, Campoy A, Aspiolea F, Carrasco MA, Camps A. Successful treatment of linear Darier’s disease with topical adapalene. J. Eur. Acad. Dermatol. Venereol. 2009;23(2):237–8 [DOI] [PubMed] [Google Scholar]
- Chilcott RP, Dalton CH, Emmanuel AJ, Allen CE, Bradley ST. Transepidermal water loss does not correlate with skin barrier function in vitro. J. Invest. Dermatol. Elsevier Masson SAS; 2002;118(5):871–5 Available from: 10.1046/j.1523-1747.2002.01760.x [DOI] [PubMed] [Google Scholar]
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am. J. Clin. Dermatol. 2003;4(2):97–105 [DOI] [PubMed] [Google Scholar]
- Dai J, Choo M-K, Park JM, Fisher DE. Topical ROR Inverse Agonists Suppress Inflammation in Mouse Models of Atopic Dermatitis and Acute Irritant Dermatitis. J. Invest. Dermatol. 2017; 137(12):2523—31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson AL, Dellavalle RP. Acne vulgaris. BMJ. 2013;346(6):f2634. [DOI] [PubMed] [Google Scholar]
- Diaz P, Huang W, Keyari CM, Buttrick B, Price L, Guilloteau N, et al. Development and Characterization of Novel and Selective Inhibitors of Cytochrome P450 CYP26A1, the Human Liver Retinoic Acid Hydroxylase. J. Med. Chem. 2016;59(6):2579–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P, Isoherranen N, Buttrick B, Guilloteau N. Specific Inhibitors of Cytochrome P450 26 Retinoic Acid Hydroxylase. United States: United States Patent Office; 2018. [Google Scholar]
- Dicken CH, Bauer EA, Hazen PG, Krueger GG, Marks JG, McGuire JS, et al. Isotretinoin treatment of Darier’s disease. J. Am. Acad. Dermatol. 1982;6(4):721–6 [DOI] [PubMed] [Google Scholar]
- Digiovanna JJ, Mauro T, Milstone LM, Schmuth M, Toro JR. Systemic retinoids in the management of ichthyoses and related skin types. Dermatol. Ther. 2013;26(l):26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009;4(8): 1184–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, Dashti SR, Deucher A, Bone F, et al. Regulation of involucrin gene expression. J. Invest. Dermatol. Elsevier Masson SAS; 2004; 123(1): 13–22 Available from: 10.1111/j.0022-202X.2004.22723.x [DOI] [PubMed] [Google Scholar]
- Elias PM, Fritsch PO, Lampe M, Williams ML, Brown BE, Nemanic M, et al. Retinoid effects on epidermal structure, differentiation, and permeability. Lab. Investig. 1981;44(6):531–40 [PubMed] [Google Scholar]
- Fisher GJ, Reddy AP, Datta SC, Kang S, Yi JY, Chambon P, et al. All-trans retinoic acid induces cellular retinol-binding protein in human skin in vivo. J. Invest. Dermatol. 1995; 105(1):80—6 [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Talwar HS, Xiao JH, Datta SC, Reddy AP, Gaub MP, et al. Immunological identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. J. Biol. Chem. 1994;269(32):20629–35 [PubMed] [Google Scholar]
- Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. Faseb J. 1996; 10(9): 1002–13 [DOI] [PubMed] [Google Scholar]
- Fort-Lacoste L, Verscheure Y, Tisne-Versailles J, Navarro R. Comedolytic effect of topical retinaldehyde in the rhino mouse model. Dermatology. 1999;199(SUPPL. 1):33—5 [DOI] [PubMed] [Google Scholar]
- Foti RS, Isoherranen N, Zelter A, Dickmann LJ, Buttrick BR, Diaz P, et al. Identification of Tazarotenic Acid as the First Xenobiotic Substrate of Human Retinoic Acid Hydroxylase CYP26A1 and CYP26B1. J. Pharmacol. Exp. Ther. 2016;357(2):281–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankart A, Malaisse J, De Vuyst E, Minner F, de Rouvroit CL, Poumay Y. Epidermal morphogenesis during progressive in vitro 3D reconstruction at the air-liquid interface. Exp. Dermatol. 2012;21(11):871–5 [DOI] [PubMed] [Google Scholar]
- Gendimenico GJ, Stim TB, Corbo M, Mezick JA, Janssen B. A Pleiotropic Response Is Induced in F9 Embryonal Carcinoma Cells and Rhino Mouse Skin by All-trans-Retinoic Acid, a RAR Agonist but Not by SR11237, a RXR-Selective Agonist. J. Invest. Dermatol. Elsevier Masson SAS; 1994; 102(5):676–80 [DOI] [PubMed] [Google Scholar]
- Giltaire S, Herphelin F, Frankart A, Hérin M, Stoppie P, Poumay Y. The CYP26 inhibitor R115866 potentiates the effects of all-trans retinoic acid on cultured human epidermal keratinocytes. Br. J. Dermatol. 2009;160(3):505–13 [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Elder JT, Bernard BA, Rossio P, Cromie MA, Finkel LJ, et al. Comparison of CD271 (adapalene) and all-trans retinoic acid in human skin: dissociation of epidermal effects and CRABP-II mRNA expression. J. Invest. Dermatol. 1993. p. 325–8 [DOI] [PubMed] [Google Scholar]
- Kang S, Duell EA, Fisher GJ, Datta SC, Wang ZQ, Reddy AP, et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J. Invest. Dermatol. Elsevier Masson SAS; 1995;105(4):549–56 [DOI] [PubMed] [Google Scholar]
- Kang S, Duell EA, Kim KJ, Voorhees JJ. Liarozole inhibits human epidermal retinoic acid 4-hydroxylase activity and differentially augments human skin responses to retinoic acid and retinol in vivo. J. Invest. Dermatol. 1996; 107(2): 183–7 [DOI] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12(4):357–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Langmead B, Salzberg S. genome_tran [Internet], 2017. [cited 2018 Jun 25], Available from: http://ftp.ccb.jhu.edu/pub/infphilo/hisat2/data/grch38_tran.tar.gz [Google Scholar]
- Kim D, Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA- seq experiments with HISAT , StringTie and Transcript-level expression analysis of RNA-seq experiments with HISAT , StringTie and Ballgown. Nat. Protoc. Nature Publishing Group; 2016; 11(9): 1650–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman LH, Kligman AM. The effect on rhino mouse skin of agents which influence keratinization and exfoliation. J. Invest. Dermatol. Elsevier Masson SAS; 1979;73(5 I):354–8 [DOI] [PubMed] [Google Scholar]
- Krämer A, Green J, Pollard J, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30(4):523–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers ALA, Van Pelt JPA, Bergers M, Boegheim PJ, Den Bakker JEN, Siegenthaler G, et al. The effects of oral liarozole on epidermal proliferation and differentiation in severe plaque psoriasis are comparable with those of acitretin. Br. J. Dermatol. 1998;139(3):380–9 [DOI] [PubMed] [Google Scholar]
- Kurlandsky SB, Duell EA, Kang S, Voorhees JJ, Fisher GJ. Auto-regulation of retinoic acid biosynthesis through regulation of retinol esterification in human keratinocytes. J. Biol. Chem. 1996;271(26): 15346–52 [DOI] [PubMed] [Google Scholar]
- Kypriotou M, Huber M, Hohl D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the “fused genes” family. Exp. Dermatol. 2012;21(9):643–9 [DOI] [PubMed] [Google Scholar]
- Lalevée S, Anno YN, Chatagnon A, Samarut E, Poch O, Laudet V, et al. Genome-wide in silico identification of new conserved and functional retinoic acid receptor response elements (direct repeats separated by 5 bp). J. Biol. Chem. 2011;286(38):33322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen KB, Kashyap V, Scandura J, Gudas LJ. An alternative retinoic acid-responsive Stra6 promoter regulated in response to retinol deficiency. J. Biol. Chem. 2015;290(7):4356–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Leede BJM, Van Den Brink CE, Pijnappel WWM, Sonneveld E, Van Der Saag PT, Van Der Burg B. Autoinduction of retinoic acid metabolism to polar derivatives with decreased biological activity in retinoic acid-sensitive, but not in retinoic acid-resistant human breast cancer cells. J. Biol. Chem. 1997;272(29): 17921–8 [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudig O, Babichuk C, White J, Abu-Abed S, Mueller C, Petkovich M. Cytochrome P450RAI(CYP26) promoter: a distinct composite retinoic acid response element underlies the complex regulation of retinoic acid metabolism. Mol. Endocrinol. 2000; 14(9): 1483–97 [DOI] [PubMed] [Google Scholar]
- Love M, Anders S, Huber W. Differential analysis of RNA-Seq data at the gene level using the DESeq package. 2013; 1–32 [Google Scholar]
- Lucker GPH, Heremans AMC, Boegheim PJ, Van De Kerkhof PCM, Steijlen PM. Oral treatment of ichthyosis by the cytochrome P-450 inhibitor liarozole. Br. J. Dermatol. 1997; 136(1):71—5 [PubMed] [Google Scholar]
- Lucker GPH, Verfaille CJ, Heremans AMC, Vanhoutte FP, Boegheim JPJ, Steijlen PPM. Topical liarozole in ichthyosis: A double-blind, left-right comparative study followed by a long-term open maintenance study [2]. Br. J. Dermatol. 2005;152(3):566–9 [DOI] [PubMed] [Google Scholar]
- Marikar Y, Wang Z, Petkovich M, Voorhees JJ, Fisher GJ, Duell EA. Retinoic Acid Receptors Regulate Expression of Retinoic Acid 4-Hydroxylase that Specifically Inactivates All-Trans Retinoic Acid in Human Keratinocyte HaCaT Cells. J. Invest. Dermatol. Elsevier Masson SAS; 1998; 111 (3):434—9 [DOI] [PubMed] [Google Scholar]
- Mihaly J, Gamlieli A, Worm M, Rühl R. Decreased retinoid concentration and retinoid signalling pathways in human atopic dermatitis. Exp. Dermatol. 2011;20(4):326–30 [DOI] [PubMed] [Google Scholar]
- Minner F, Herphelin F, Poumay Y. Study of Epidermal Differentiation in Human Keratinocytes Cultured in Autocrine Conditions. Biol. Integument. 2010. p. 71–82 [DOI] [PubMed] [Google Scholar]
- Monzon RI, LaPres JJ, Hudson LG. Regulation of involucrin gene expression by retinoic acid and glucocorticoids. Cell Growth Differ. 1996;7(12): 1751—9 [PubMed] [Google Scholar]
- Nelson CH, Buttrick BR, Isoherranen N. Therapeutic potential of the inhibition of the retinoic acid hydroxylases CYP26A1 and CYP26B1 by xenobiotics. Curr. Top. Med. Chem. NIH Public Access; 2013; 13(12): 1402–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano J, Lichti U, Mamiya S, Aronova M, Zhang G, Yuspa S, et al. Increased retinoic acid levels through ablation of Cyp26b1 determine the processes of embryonic skin barrier formation and peridermal development. J. Cell Sci. 2012;125(Pt 7):1827–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanos CE, Zouboulis CC, Almond-Roesler B, Geilen CC. Current use and future potential role of retinoids in dermatology. Drugs. 1997;53(3):358–88 [DOI] [PubMed] [Google Scholar]
- Pavez Loriè E, Chamcheu JC, Vahlquist A, Törmä H. Both all-trans retinoic acid and cytochrome P450 (CYP26) inhibitors affect the expression of vitamin A metabolizing enzymes and retinoid biomarkers in organotypic epidermis. Arch. Dermatol. Res. Springer-Verlag; 2009a;301(7):475–85 [DOI] [PubMed] [Google Scholar]
- Pavez Loriè E, Cools M, Borgers M, Wouters L, Shroot B, Hagforsen E, et al. Topical treatment with CYP26 inhibitor talarozole (R115866) dose dependently alters the expression of retinoid-regulated genes in normal human epidermis. Br. J. Dermatol. Blackwell Publishing Ltd; 2009b; 160(1):26–36 [DOI] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33(3):290–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poumay Y, Dupont F, Marcoux S, Leclercq-Smekens M, Hérin M, Coquette A. A simple reconstructed human epidermis: Preparation of the culture model and utilization in in vitro studies. Arch. Dermatol. Res. 2004;296(5):203–11 [DOI] [PubMed] [Google Scholar]
- Poumay Y, Herphelin F, Smits P, De Potter IY, Pittelkow MR. High-cell-density phorbol Ester and retinoic acid upregulate involucrin and downregulate Suprabasal Keratin 10 in autocrine cultures of human epidermal keratinocytes. Mol. Cell Biol. Res. Commun. 1999;2(2): 138–44 [DOI] [PubMed] [Google Scholar]
- Radoja N, Diaz DV, Minars TJ, Freedberg IM, Blumenberg M, Tomic- Canic M. Specific organization of the negative response elements for retinoic acid and thyroid hormone receptors in keratin gene family. J. Invest. Dermatol. 1997;109(4):566–72 [DOI] [PubMed] [Google Scholar]
- Ray WJ, Bain G, Yao M, Gottlieb DI. CYP26, a novel mammalian cytochrome P450, is induced by retinoic acid and defines a new family. J. Biol. Chem. 1997;272(30): 18702–8 [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet], Vienna, Austria; 2018. Available from: https://www.r-project.org [Google Scholar]
- Rittié L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J. Invest. Dermatol. 2006; 126(4):732–9 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9(7):676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiberg M, Siock P, Wisniewski S, Cauwenbergh G, Shapiro SS. The effects of trypsin on apoptosis, utriculi size, and skin elasticity in the Rhino mouse. J. Invest. Dermatol. 1997;109(3):370–6 [DOI] [PubMed] [Google Scholar]
- Stehlin-Gaon C, Willmann D, Zeyer D, Sanglier S, Van Dorsselaer A, Renaud JP, et al. All-trans retinoic acid is a ligand for the orphan nuclear receptor RORβ. Nat. Struct. Biol. 2003;10(10):820–5 [DOI] [PubMed] [Google Scholar]
- Steijlen PM, Reifenschweiler DOH, Ramaekers FCS, van Muijen GNP, Happle R, Link M, et al. Topical treatment of ichthyoses and Darier’s disease with 13-cis-retinoic acid-A clinical and immunohistochemical study. Arch. Dermatol. Res. 1993;285(4):221–6 [DOI] [PubMed] [Google Scholar]
- Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Exp. Dermatol. 1998;7(6):391—7 [DOI] [PubMed] [Google Scholar]
- Stoppie P, Borgers M, Borghgraef P, Dillen L, Goossens J, Sanz G, et al. R115866 inhibits all-trans-retinoic acid metabolism and exerts retinoidal effects in rodents. J. Pharmacol. Exp. Ther. 2000;293(1):304–12 [PubMed] [Google Scholar]
- de Thé H, Vivanco-Ruiz MM, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343(6254): 177–80 [DOI] [PubMed] [Google Scholar]
- Tomic-Canic M, Sunjevaric I, Freedberg IM, Blumenberg M. Identification of the retinoic acid and thyroid hormone receptor-responsive element in the human K14 keratin gene. J. Invest. Dermatol. 1992;99(6): 842–7 [DOI] [PubMed] [Google Scholar]
- Vahlquist A, Blockhuys S, Steijlen P, Van Rossem K, Didona B, Blanco D, et al. Oral liarozole in the treatment of patients with moderate/severe lamellar ichthyosis: Results of a randomized, double-blind, multinational, placebo-controlled phase II/III trial. Br. J. Dermatol. 2014; 170(1): 173—81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlquist A, Gånemo A, Virtanen M. Congenital ichthyosis: An overview of current and emerging therapies. Acta Derm. Venereol. 2008. p. 4–14 [DOI] [PubMed] [Google Scholar]
- Vasios GW, Gold JD, Petkovich M, Chambon P, Gudas LJ. A retinoic acid-responsive element is present in the 5’ flanking region of the laminin B1 gene. Proc Natl Acad Sci USA. 1989;86(23):9099–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaille CJ, Borgers M, van Steensel MAM. Retinoic acid metabolism blocking agents (RAMBAs): a new paradigm in the treatment of hyperkeratotic disorders. J. der Dtsch. Dermatologischen Gesellschaft = J. Ger. Soc. Dermatology JDDG. 2008;6(5):355–64 [DOI] [PubMed] [Google Scholar]
- Verfaille CJ, Thissen CACB, Bovenschen HJ, Mertens J, Steijlen PM, Van De Kerkhof PCM. Oral R115866 in the treatment of moderate to severe plaque-type psoriasis. J. Eur. Acad. Dermatology Venereol. 2007a;21(8): 1038–46 [DOI] [PubMed] [Google Scholar]
- Verfaille CJ, Vanhoutte FP, Blanchet-Bardon C, Van Steensel MA, Steijlen PM. Oral liarozole vs. acitretin in the treatment of ichthyosis: A phase II/III multicentre, double-blind, randomized, active-controlled study. Br. J. Dermatol. 2007b; 156(5):965—73 [DOI] [PubMed] [Google Scholar]
- De Vuyst E, Charlier C, Giltaire S, De Glas V, de Rouvroit CL, Poumay Y. Reconstruction of normal and pathological human epidermis on polycarbonate filter. Methods Mol. Biol. 2014; 1195(1202): 191–201 [DOI] [PubMed] [Google Scholar]
- Van Wauwe J, Van Nyen G, Coene MC, Stoppie P, Cools W, Goossens J, et al. Liarozole, an inhibitor of retinoic acid metabolism, exerts retinoid-mimetic effects in vivo. J. Pharmacol. Exp. Ther. 1992;261(2):773–9 [PubMed] [Google Scholar]
- Willems E, Leyns L, Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008;379(1): 127–9 [DOI] [PubMed] [Google Scholar]
- Xiao JH, Feng X, Di W, Peng ZH, Li L a, Chambon P, et al. Identification of heparin-binding EGF-like growth factor as a target in intercellular regulation of epidermal basal cell growth by suprabasal retinoic acid receptors. EMBO J. 1999;18(6):1539–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Uchida G, Okazaki M, Kitano Y, Harii K. Differential expression of heparin-binding EGF-like growth factor (HB-EGF) mRNA in normal human keratinocytes induced by a variety of natural and synthetic retinoids. Exp. Dermatol. Munksgaard International Publishers; 2003; 12 Suppl 2(s2):28–34 [DOI] [PubMed] [Google Scholar]
- Zheng D, Giljohann DA, Chen DL, Massich MD, Wang X-Q, Iordanov H, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc. Natl. Acad. Sci. 2012; 109(30): 11975–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Ibrahim JG, Love MI. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2018;(November):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request.


