Abstract
Patient: Male, 58-year-old
Final Diagnosis: Diabetes mellitus type 2
Symptoms: Confusion • diaphoresis • dizziness • dyspnea • palpitation • weakness
Medication: Metformin
Clinical Procedure: —
Specialty: Endocrinology and Metabolic • General and Internal Medicine • Psychiatry
Objective:
Unusual clinical course
Background:
Metformin has a longstanding reputation as the first-line treatment for glycemic control in the setting of diabetes mellitus type 2. A contributing factor to this reputation is metformin having a low risk of inducing hypoglycemia compared to other oral hypoglycemics or insulin. There are no case reports of hypoglycemia while on conventional or therapeutic doses of metformin. This case report is of a patient who developed symptomatic hypoglycemia while being treated with a therapeutic dose of metformin.
Case Report:
A 58-year-old man with history including diabetes mellitus type 2, hypertension, and schizoaffective disorder was dismissed early from work due to symptoms of severe weakness, confusion, diaphoresis, dizziness, shortness of breath, palpitations, and a sensation of feeling hot. Continuous glucose monitoring revealed hypoglycemic episodes up to 4% of the time. The hypoglycemic events appeared to occur primarily between midnight and 7 A.M., with the second likely time frame being between 7A.M. and noon. Within 2 weeks of discontinuing metformin, there were no further “attacks”, and the chronic daytime fatigue and somnolence significantly improved.
Conclusions:
This case report suggests that there is a risk of symptomatic hypoglycemia with therapeutic doses of metformin. Although advised to be taken with meals to avoid gastrointestinal upset, patients should also be educated to take metformin with meals to reduce the risk of metformin-associated hypoglycemia.
Keywords: Diabetes Mellitus, Hypoglycemia, Metformin
Background
Metformin has a longstanding reputation as the first-line treatment for glycemic control in the setting of diabetes mellitus type 2. A contributing factor to this reputation is metformin having a low risk of inducing hypoglycemia compared to other oral hypoglycemics or insulin. Metformin is a biguanide, which reduces blood glucose levels by decreasing glucose production by the liver, decreasing intestinal absorption of glucose, and improving insulin sensitivity [1–6]. The more common adverse effects of metformin include nausea, vomiting, flatulence, abdominal pain, and diarrhea, which is why it is advised to take metformin with food [6,7]. Metformin-associated lactic acidosis (MALA) is a rare adverse effect reported in postmarking cases, the majority of which were linked to metformin toxicity and/or predisposing conditions such as hypovolemia, infection or sepsis, acute or chronic kidney disease, or liver cirrhosis [1,4]. There are reports of metformin overdose or toxicity triggering hypoglycemia, but in only 2% of the cases [5]. There are no case reports of hypoglycemia while on conventional or therapeutic doses of metformin. This case report is of a patient who developed symptomatic hypoglycemia while being treated with a therapeutic dose of metformin. Given that the risk of hypoglycemia is rare but not zero, patients should be advised to take metformin with food, not only to minimize potential gastrointestinal adverse effects, but to also minimize the risk of hypoglycemia.
Case Report
A 58-year-old man presented to the clinic 1 day after experiencing what had historically been labelled a “schizophrenic attack”. The symptoms included severe weakness, confusion, diaphoresis, dizziness, shortness of breath, palpitations, and a sensation of feeling hot. This specific “attack” occurred in the morning while he was at work, and lasted approximately 3 hours. During that time, the patient was allowed to rest and was provided snacks. His medical history included diabetes mellitus type 2, hypertension, hyperlipidemia, gastroesophageal reflux disease, and schizoaffective disorder. His medications included aspirin 81 mg daily, lisinopril 10 mg daily, pantoprazole 40 mg daily, immediate-release metformin 500 mg twice daily, simvastatin 20 mg nightly, lurasidone 120 mg daily, sertraline 50 mg daily, and clozapine 250 mg daily.
On examination during the clinic visit, there were no physical abnormalities. The vital signs were within normal limits, with blood pressure of 118/70 mmHg, heart rate of 87 beats per minute, and oxygen saturation of 96%. He was mildly obese with a body mass index of 30.83. There were no subjective or objective findings indicative of active depression or mania. The patient was not internally stimulated, denied any visual hallucinations, and reported no change in his chronic auditory hallucinations that tell him that he is God. According to the patient’s wife, he had not voiced any recent paranoid or delusional statements, and continued to talk to himself as usual, with no change in behavior.
The complete blood count revealed normal cell counts, including white blood cell count of 6.30 K/uL with an absolute neutro-phil count of 3490 K/ul and red blood cell count of 5.02 M/uL. The clozapine level was within therapeutic range at 265 ng/mL with a normal combination clozapine + norclozapine level of 432 ng/mL. The comprehensive metabolic panel showed normal electrolytes, and normal liver and kidney function tests, without acidosis. The non-fasting blood glucose was 107 mg/dl. Inflammatory markers (CRP, sedimentation rate), thyroid-stimulating hormone (TSH), brain natriuretic peptide (BNP), and creatine phosphokinase (CPK) were all within normal limits. The serum C-peptide was elevated at 10.3 ng/mL, but the serum insulin and beta-hydroxybutyrate levels were normal at 11.4 mcIU/mL and 0.1 mmol/L, respectively. A CT scan of the abdomen and pelvis with contrast was normal, including no adrenal, pancreatic, or hepatic abnormalities. A transthoracic echocardiogram showed normal left ventricular ejection fraction of 55–60%, mild grade I diastolic dysfunction, and no wall motion or valvular abnormalities.
Given that the “attack” mimicked symptomatic hypoglycemia, continuous glucose monitor was performed as an out-patient. The monitor was worn for 15 days (from March 25 2020 through April 8 2020), with 100% active continuous glucose monitoring during that time. Most (96%) of the readings were within the target range (70–180 mg/dl), with 4% of the readings being in the hypoglycemic range (54–69 mg/dl). (Figure 1). The average reading was 92 mg/dl. The hypoglycemic events appeared to occur primarily between midnight and 7 A.M., with the second likely time frame being between 7A.M. and noon (Figure 2).
Figure 1.
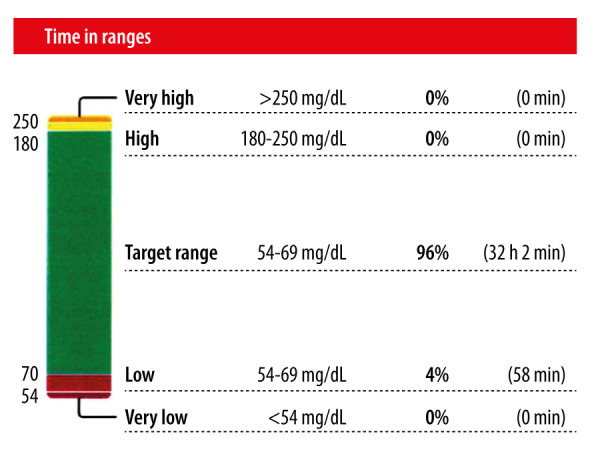
Composition of glucose readings during continuous glucose monitoring.
Figure 2.
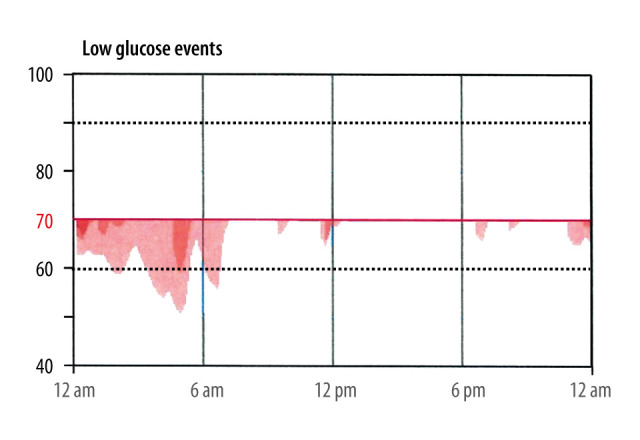
Frequency and timing of hypoglycemic events throughout a 24-hour period.
Given the documented hypoglycemia, metformin was subsequently discontinued. Approximately 1 month after the last “attack”, and approximately 2 weeks after discontinuing metformin, the patient and his wife reported noticing significant improvements. Both parties denied any further “attacks” of any severity. The patient’s wife stated that the patient was no longer “sleeping all the time”, a complaint for which previous sleep studies ruled out obstructive or central sleep apnea. The patient stated that he no longer had to consume “1 to 2 pots of coffee” to combat daytime fatigue. Unfortunately, his insurance would not cover repeat continuous glucose monitoring to reassess for ongoing hypoglycemia. However, repeat fasting blood tests were within normal limits, including glucose of 117 mg/dl, C-peptide 5.0 ng/mL, and creatinine of 0.8 mg/ dl with a GFR of greater than 60 mL/min/1.73 sq m.
Discussion
Medical and psychiatric evaluations did not support active schizoaffective disorder, orthostatic hypotension, hypoxia, myocarditis, cardiac valvular disorder, an infectious process, or thyroid dysfunction as contributing factors to this patient’s “attack”. Gastrointestinal pathology including insulinoma was also ruled out. Collateral information, including the metformin refill history through the pharmacy, as well as collateral information from his wife (who managed his medications) and from his social worker, verified that metformin use did not exceed the daily dose prescribed. There were no other residents in the home, and his wife was not on any diabetes medications. The initially elevated serum C-peptide level (with normal serum insulin level) were likely due to insulin insensitivity in the setting of type 2 diabetes mellitus and obesity. Due to the time of day of the majority of the hypoglycemic episodes (fasting state overnight), and an inability to perform a blood draw at or adjacent to his workplace, there was no way to capture his insulin level or his glucose level during a hypoglycemic episode.
The patient’s social worker estimated that he experienced 1 of these “attacks” at least once a month for several years and were attributed to his diagnosis of schizoaffective disorder, bipolar type. However, his wife estimated that he experienced an “attack” at least once or twice a week, at various levels of severity. His wife reported routinely bringing snacks to his work place in an effort to prevent these “attacks”. To her, the “attacks” were attributed to not eating before work, in combination with overexertion at work.
There are case reports estimating no more than 2% rate of hypoglycemia secondary to metformin overdose or toxicity [4,5], but hypoglycemia at a therapeutic metformin dose is thought to be rare. Several hypotheses have been proposed for metformin-induced hypoglycemia, but are currently controversial. Metformin-induced hypoglycemia hypotheses include a direct link to its mechanism of action – decreased hepatic glucose production and decreased glucose absorption [2]. However, it is thought that additional confounding circumstance(s) are required for hypoglycemia to occur. ACE-inhibitors are hypothesized to cause hypoglycemia by increasing insulin sensitivity, leading to vasodilation in the muscles with increased muscle uptake of glucose. The combination of ACE-inhibitor with 1 or more oral hypoglycemic agents, including metformin, is thought to increase the risk of hypoglycemia [8–10]. However, the risk does not appear to be significant, as ACE-inhibitors in combination with metformin is commonly prescribed in type 2 diabetes mellitus due the ACE-inhibitors’ protective benefits towards diabetic nephropathy. Also, a recent study of the ACE-inhibitors and ARBs with metformin and other oral hypoglycemics revealed no drug-drug interaction causing hypoglycemia (instead, it showed significant increase in total cholesterol and LDL) [11]. A pharmacy review update of medications commonly prescribed in the management of type 2 diabetes mellitus also did not report any hypoglycemic risk with ACE-inhibitors, nor of a significant drug-drug interaction between metformin and ACE-inhibitors [7,12].
Other hypotheses include concurrent presence of a fasting state, alcohol use, and/or strenuous activity [1,2]. Given that this patient has never consumed alcohol, in this case, the hypoglycemia appears to be multifactorial, including treatment with metformin combined with inadequate oral intake and overexertion at work. There were no drug-drug interactions identified between metformin and the following medications: aspirin, pantoprazole, simvastatin [6,13–15]. His psychotropic regimen, both individual medications and as a whole, (clozapine, lurasidone, sertraline), carries a risk of hyperglycemia (but not hypoglycemia) [6,16–18]. They each also carry the potential to minimize the glucose-reducing effects of metformin, while having no direct interaction with metformin. The initial diagnosis of diabetes mellitus type 2 and the initial treatment of immediate-release metformin was in March 2007 based on a fasting glucose 131 mg/dl. Our patient’s A1c had historically been between 5.4% and 6.0% while on metformin, and has ranged from 5.7% to 5.9% after the discontinuation of metformin.
The patient denied any other diabetic treatments or any previous adjustments in metformin since its initiation. Controlled studies would best determine risk factors for hypoglycemia regarding metformin. However, the rarity of such cases limits the ability to pursue such studies. Genetic studies on individuals who have experienced therapeutically-dosed metformin-induced hypoglycemia could be an alternate area of study.
Conclusions
This case report supports that there is a risk of symptomatic hypoglycemia with therapeutic doses of metformin. Although advised to be taken with meals to avoid gastrointestinal upset, patients should be educated to take metformin with meals to reduce the risk of metformin-associated hypoglycemia, especially in individuals who frequently engage in strenuous activities.
Footnotes
Department and Institution Where Work Was Done
Department of Internal Medicine and Psychiatry, Altru Health System, Grand Forks, ND, U.S.A.
Conflict of Interest
None.
References:
- 1.Bodmer M, Meier C, Krahenbuhl S, et al. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: A nested case-control analysis. Diabetes Care. 2008;31:2086–91. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasim GI. Hypoglycemia induced by therapeutic doses of metformin in the absence of other anti-diabetic drugs. FS J Pharm. 2013;4:1–2. [Google Scholar]
- 3.Nasri H, Rafieian-Kopaei M. Metformin: Current knowledge. J Res Med Sci. 2014;19(7):658–64. [PMC free article] [PubMed] [Google Scholar]
- 4.Jacob T, Garrick R, Goldberg M. Recurrent lactic acidosis and hypoglycemia with inadvertent metformin use: a case of look-alike pills. Endocrinol Diabetes Metab Case Rep. 2018;2018:17–0148. doi: 10.1530/EDM-17-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldobeaban S, Mzahim B, Ali Alshehri A. Recurrent hypoglycemia secondary to metformin toxicity in the absence of co-ingestions: A case report. J Med Case Rep. 2018;12:223. doi: 10.1186/s13256-018-1758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lexicomp. Wolters Kluwer Health, Inc; Riverwoods, IL: Metformin: Drug Information UpToDate. http://online.lexi.com. [Accessed March 5 2021] [Google Scholar]
- 7.Triplitt C. Drug interactions of medications commonly used in diabetes. Diabetes Spectrum. 2006;19(4):202–11. [Google Scholar]
- 8.Elshimy G, Techathaveewat P, Alysayed M, et al. Simple reason for hypoglycemia: ACE inhibitor-induced severe recurrent hypoglycemia in a non-diabetic patient. Cureus. 2019;11(8):e5449. doi: 10.7759/cureus.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May M, Schindler C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther Adv Endocrinol Metab. 2016;7(2):69–83. doi: 10.1177/2042018816638050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vue MH, Setter SM. Drug-induced glucose alterations part 1: Drug induced hypoglycemia. Diabetes Spectrum. 2011;24(3):171–77. [Google Scholar]
- 11.Daoud N, Qadi O, Al-talhi D, Al-sharabi H. Effect of anti-hypertensive agents on Biochemical Parameters in Diabetic Patients in Taif – KSA. International Journal of Diabetes and Clinical Research. 2019;6:113. [Google Scholar]
- 12.Lisinopril: Drug Information UpToDate. Wolters Kluwer Health, Inc; Riverwoods, IL: http://online.lexi.com [Accessed March 5 2021] [Google Scholar]
- 13.Simvastatin: Drug Information UpToDate. Wolters Kluwer Health, Inc; Riverwoods, IL: http://online.lexi.com [Accessed March 5 2021] [Google Scholar]
- 14.Pantoprazole: Drug Information UpToDate. Wolters Kluwer Health, Inc; Riverwoods, IL: http://online.lexi.com [Accessed March 5 2021] [Google Scholar]
- 15.Aspirin: Drug Information UpToDate. Wolters Kluwer Health, Inc; Riverwoods, IL: http://online.lexi.com [Accessed March 5 2021] [Google Scholar]
- 16.Clozapine: Drug Information UpToDate. Wolters Kluwer Health, Inc; Riverwoods, IL: http://online.lexi.com. [Accessed March 5 2021] [Google Scholar]
- 17.Lurasidone: Drug Information UpToDate. Wolters Kluwer Health, Inc; Riverwoods, IL: http://online.lexi.com. [Accessed March 5 2021] [Google Scholar]
- 18.Sertraline: Drug Information UpToDate. Wolters Kluwer Health, Inc; Riverwoods, IL: http://online.lexi.com [Accessed March 5 2021] [Google Scholar]


