Abstract
Background:
The objective of our study was to investigate the location, extension and type of novel coronavirus-induced disease 2019 (COVID-19) infection involvement and hepatic steatosis on initial chest computed tomography (CT). The relationship between fatty liver and severity of the disease was also investigated by measuring the liver attenuation index (LAI).
Methods:
This study evaluated the chest CT images of 343 patients (201 male, mean age 48.43 years) who were confirmed to have COVID-19, using nasopharyngeal swab. The chest CTs were analyzed for laterality, number of involved lobes, diffuseness, number of lesions, and lesion types. The CT attenuation values of liver and spleen were measured, and LAI was calculated for the detection of hepatic steatosis. Univariate and multivariate logistic regression analysis were used to identify the independent early predictors for severe COVID-19.
Results:
There was no significant difference between genders in terms of clinical course. Liver density and LAI were significantly lower in the intensive care unit (ICU) patients. The prevalence of severe disease was higher in the patients with hepatic steatosis than in the non-steatotic group (odds ratio [OR] 3.815, 95% confidence interval [CI] 1.97–7.37, P < 0.001). After adjusting for age and comorbidities including hypertension, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, and chronic kidney disease, multivariate logistic regression analysis showed that non-alcoholic fatty liver disease (NAFLD) was an independent risk factor for COVID-19 severity (OR 3.935, 95% CI 1.77-8.70, P = 0.001). The optimal cut-off value for LAI was calculated as 0.5 for predicting patients who required ICU treatment.
Conclusions:
On the initial chest CT images of COVID-19 patients, presence of fatty liver is a strong predictor for severe disease.
Keywords: Computed tomography, COVID-19, hepatic steatosis, liver
INTRODUCTION
Severe acute respiratory syndrome, coronavirus 2 (SARS-CoV-2)-induced Coronavirus disease 2019 (COVID-19) first appeared in Wuhan, China in December 2019, and later spread to the whole of China and the world.[1,2,3] The ongoing outbreak was declared as a global public health emergency on January 30, 2020 by the World Health Organization (WHO), which raised the assessment of the spread risk and impact of COVID-19 to a very high level on February 28, 2020.[4] On March 11, 2020, the disease was declared as a global pandemic by WHO.[3]
The virus is transmitted through large droplets scattered in the environment during coughing and sneezing. Fever, cough, sore throat, headache, fatigue, and dyspnea are common symptoms.[2,3,5] COVID-19 may be asymptomatic in mild cases, while severe cases can progress to pneumonia, acute respiratory distress syndrome, and multi-organ dysfunction.[3,5]
Real-time reverse transcription polymerase chain reaction (RT-PCR) is accepted as the reference standard in the diagnosis of COVID-19.[1,3,6] However, the sensitivity of RT-PCR is reported to be not very high in the literature, and the rate of false negativity varies between 30 and 70%. Continuation of negative results in the repeated tests causes difficulties in the diagnosis and delays treatment.[3,7,8,9] In COVID-19 patients with false negative PCR, the computed tomography (CT) of the chest is a valuable diagnostic tool with high sensitivity.[1,2,6,7,8]
Although COVID-19 is a disease that primarily affects the lungs, the involvement of different organ systems has also been described in recent studies in the literature. Liver injury related to COVID-19 is a frequently mentioned consequence of such involvement.[9,10,11,12] The incidence of liver injuries has been reported to vary between 14% and 53%, and abnormal liver function test results are the main indicator. It may be accompanied by a slight elevation in bilirubin levels.[9,10] Liver injury develops significantly, more frequently in severe disease than in mild disease.[10,13] The relationship between non-alcoholic fatty liver disease (NAFLD) and COVID-19 has also been investigated in a limited number of studies. Pre-existing NAFLD is associated with a severe course of COVID-19.[9,11] Comorbidities such as hypertension (HT), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), and cardiovascular diseases are also more common among patients with severe COVID-19.[9] Patients with NAFLD may be vulnerable to COVID-19; thus, identifying those with pre-existing liver disease is important in the early stage of the disease.[14]
The aim of the study was to retrospectively evaluate the chest CT of PCR-confirmed COVID-19 cases and classify lung involvement by location, extension, and type, and to investigate the relationship between this classification and whether the patient had steatosis or not. Since the liver is visualized in the lower sections of CT, steatosis was evaluated by density measurements, and its association with the severity of the disease was investigated.
METHODS
The retrospective study was approved by the ethics committee of our hospital (Approval Number: E1-20-498). From March 15 to April 30, 2020, the chest CT examinations and medical records of COVID-19-suspected patients were evaluated on the day of admission to the emergency department. Patients under the age of 18 years, those with image artifacts, those that received an intravenous contrast agent for examinations, such as CT angiography, and those with chronic liver disease were excluded from the study. RT-PCR was accepted as the reference standard in the diagnosis of COVID-19. Any positive result was considered as a confirmed COVID-19 infection. In all patients, PCR and chest CT were performed on the same day. High-resolution chest CT was performed using two 128-slice multidetector scanners (GE Revolution EVO 128 Slice CT Scanner, GE Medical Systems, Milwaukee, WI, USA) reserved only for COVID-19-suspected cases. All scans were performed without intravenous contrast media with the patient in the supine position during end-inspiration. The following technical parameters were used: tube voltage 100 kV, tube current 90-300 mAs, spiral pitch factor 0.98, collimation width, 0.625, and slice thickness 1.3 mm with a sharp reconstruction kernel.
The following CT features were recorded by a radiologist: (a) laterality, (b) involved lobes, (c) peripheral vs central involvement, (d) number of lesions (single or multiple), (e) lesion types (ground glass opacity [GGO], consolidation, interlobular septal thickening, crazy paving, air bronchogram, linear opacity, adjacent pleural thickening, pleural effusion, halo sign, pericardial effusion, bronchial dilatation, vascular enlargement, atelectasis, and lymphadenopathy). The mean CT attenuation values of the liver and spleen were obtained in Hounsfield unit (HU) for the detection of hepatic steatosis. Density measurements were made by placing four regions of interest (ROI) of approximately 150 mm2 in the right liver lobe and two ROI in the spleen Figure 1. While measurements were made from homogeneous areas, vascular structures and bile ducts were not included in the ROI. The liver attenuation index (LAI) was defined as the difference between the mean hepatic and splenic attenuations.[15] Fatty liver was considered if the attenuation of the liver was at least 10 HU less than that of the spleen or if the attenuation of the liver was less than 40 HU.[16]
Figure 1.
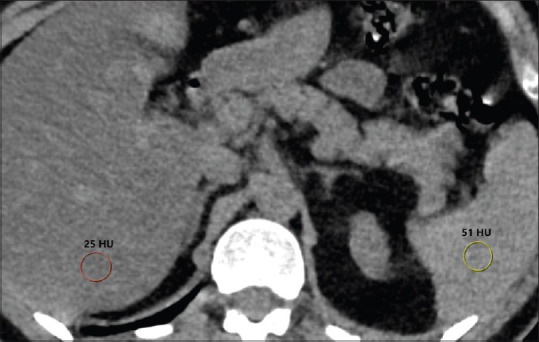
Hounsfield attenuation values of liver and spleen with round shaped region of interests (ROI) which were placed to the right hepatic lobe and spleen on non-contrast CT. Liver attenuation index is -26 (25-51) and mean liver attenuation is 25 HU (<40 HU) indicating hepatic steatosis
The disease severity ranges from mild to critical according to clinical status. While hospitalization may not be required in mild/moderate groups, hospitalization and intensive care unit (ICU) may be required in severe and critical disease.[17] We divided the study population into two subgroups according to ICU admissions; ICU (treatment in ICU) and non-ICU (hospitalization or management at home). In addition, patients were compared in two subgroups as mortality and surviving. The preference of clinical physicians referring to CT examination can be considered as a patient selection bias. We obtained the patients' laboratory parameters from medical records, including the alanine aminotransferase (ALT) (normal range, <50 U/L), aspartate aminotransferase (AST) (normal range, <35 U/L), alkaline phosphatase (ALP) (normal range 53-128 U/L), gamma glutamyl transferase (GGT) (normal range <73 U/L), albumin (normal range 32-48 g/L), total bilirubin (normal range 0.3-1.2 mg/dL). The pre-existing comorbidities such as DM, HT, coronary artery disease (CAD), COPD, and chronic kidney disease (CKD) were also noted from medical records.
Statistical analysis
Statistical analysis was performed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). In descriptive statistics, normal distribution was determined by one-sample Kolmogorov-Smirnov test, and continuous variables that were not normally distributed were expressed as median (min-max), while categorical variables were presented in numbers and percentages. The Mann-Whitney U test was used to compare the continuous variables between the two groups. The difference between the categorical variables was calculated by the Chi-square test. The independent effect of the parameters were calculated by logistic regression analyses. Variables having P < 0.10 in univariate analysis were inserted into a forward multivariate logistic model. A receiver operating characteristic (ROC) curve was applied to obtain the optimum cut-off value of LAI in predicting severe disease. A P value of <0.05 was considered statistically significant.
RESULTS
In this retrospective study, we reviewed the CT scans of 343 adult patients (201 male, 142 female, mean age 48.43 ± 16.85 years). There was no significant difference between genders in terms of clinical course (P = 0.478) [Table 1]. Older ages, bilateral involvement, right upper lobe, right middle lobe, left upper lobe and lingula involvement, diffuse distribution in the lobe, and multiple lesions were significantly higher in the ICU patients (P < 0.05). CT appearances, such as consolidation, air bronchogram, pleural effusion, crazy paving pattern, pleural thickening adjacent to GGO, and pericardial effusion were also observed to be significantly higher in this group. Serum AST, ALT, GGT and total bilirubin levels were higher and albumin levels lower in the ICU patients (P < 0.05) [Table 1].
Table 1.
Demographic and clinical features, chest CT and laboratory findings of PCR-confirmed COVID-19 patients. Comparison between the ICU and non-ICU patients™ and between the mortality and surviving groups
| Total (n=343) | ICU (n=54) | Non-ICU (n=289) | P | Mortality (n=20) | Surviving (n=323) | P | |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Female, n (%) | 142 (41.3%) | 20 (37%) | 122 (42.2%) | 0.478 | 8 (40%) | 134 (41.4%) | 0.896 |
| Male, n (%) | 201 (58.6%) | 34 (62.9%) | 167 (57.7%) | 12 (60%) | 189 (58.5%) | ||
| DM, n (%) | 38 (11.1%) | 11 (20.4%) | 27 (9.3%) | 0.018 | 5 (25%) | 33 (10.2%) | 0.041 |
| HT, n (%) | 63 (18.4%) | 19 (35.2%) | 44 (15.2%) | 0.001 | 7 (35%) | 56 (17.3%) | 0.048 |
| CAD, n (%) | 9 (2.6%) | 4 (7.4%) | 5 (1.7%) | 0.017 | 1 (5%) | 8 (2.5%) | 0.494 |
| COPD, n (%) | 20 (5.8%) | 7 (13%) | 13 (4.5%) | 0.015 | 3 (15%) | 17 (5.3%) | 0.071 |
| CKD, n (%) | 8 (2.3%) | 4 (7.4%) | 4 (1.4%) | 0.007 | 4 (20%) | 4 (1.2%) | <0.001 |
| Age, mean±SD, years | 48.43±16.85 | 65.87±14.62 | 45.11±15.14 | <0.001 | 69.05±13.53 | 47.13±16.2 | <0.001 |
| Liver density (HU), mean±SD | 53.06±10.54 | 47.55±7.81 | 54.1±10.67 | <0.001 | 46.85±5.59 | 53.45±10.66 | <0.001 |
| Fatty liver, n (%) | 55 (16%) | 19 (35.1%) | 36 (12.4%) | <0.001 | 8 (40%) | 47 (14.5%) | 0.007 |
| LAI, mean±SD | 2.28±10.69 | -4.57±8.36 | 3.56±10.6 | <0.001 | -6.25±5.08 | 2.85±10.72 | <0.001 |
| AST (U/L), mean±SD | 32.15±33.77 | 57.61±70.6 | 27.39±17.12 | <0.001 | 57.35±59.46 | 30.59±31 | <0.001 |
| ALT (U/L), mean±SD | 36.73±38.73 | 51.5±82 | 33.97±22.25 | 0.002 | 56±89.26 | 35.54±33.14 | 0.021 |
| ALP (U/L), mean±SD | 72.2±29.3 | 76.3±36.9 | 71.4±27.7 | 0.265 | 79.7±33.7 | 71.7±29.0 | 0.240 |
| GGT (U/L), mean±SD | 38.5±37.5 | 57.85±54.63 | 34.86±32.23 | <0.001 | 56.85±32.11 | 37.34±37.57 | <0.001 |
| Albumin (g/L), mean±SD | 43.0±6.1 | 38.02±10.05 | 43.98±4.49 | <0.001 | 35.33±7.25 | 43.52±5.71 | 0.024 |
| Total bilirubin (mg/dL), mean±SD | 0.58±0.29 | 0.70±0.45 | 0.55±0.24 | 0.001 | 0.89±0.65 | 0.56±0.23 | 0.031 |
CT: Computed tomography; PCR: Polymerase chain reaction; ICU: Intensive care unit; DM: Diabetes mellitus; HT: Hypertension; CAD: Coronary artery disease; COPD: Chronic obstructive pulmonary disease; CKD: Chronic kidney disease; LAI: Liver attenuation index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; GGT: Gamma glutamyl transferase; HU: Hounsfield unit
Increased number of affected lobes, bilateral involvement, upper-middle lobe involvement, diffuse distribution of lesions, and consolidation, air bronchogram and paving patterns were observed at a significantly higher rate in patients with NAFLD compared to the non-steatotic patients.
Liver density and LAI were significantly lower in the ICU patients [Figure 2]. The optimum cut-off value for LAI was calculated as 0.5 for predicting patients who required ICU treatment [Figure 3]. The disease resulted in mortality in 20 patients who were older than surviving patients (P < 0.001) and fatty liver was higher in the mortality group (P = 0.007). The univariate logistic regression analysis showed that fatty liver, age, HT, DM, CAD, COPD and CKD were associated with the disease severity. In the multivariate model, fatty liver (odds ratio [OR] 3.935, 95% confidence interval [CI] 1.77-8.70, P = 0.001) and age (OR 1.090, 95% CI 1.06-1.12, P < 0.001) were the independent early predictors for COVID-19 progression. Multivariate analysis also showed that fatty liver (OR 4.522, 95% CI 1.44-14.17, P = 0.010), age (OR 1.094, 95% CI 1.05-1.14, P < 0.001), and CKD (OR 34.842, 95% CI 4.10-295.59, P = 0.001) were independent risk factors for mortality [Table 2].
Figure 2.
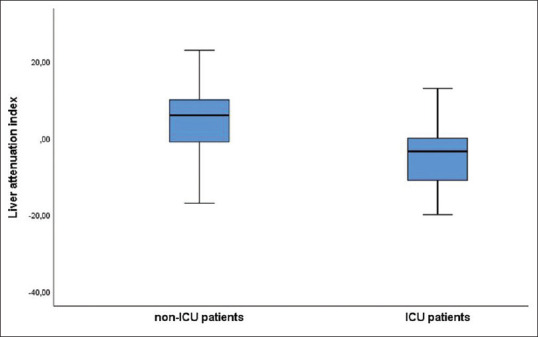
Liver attenuation index of the ICU and non-ICU patients
Figure 3.
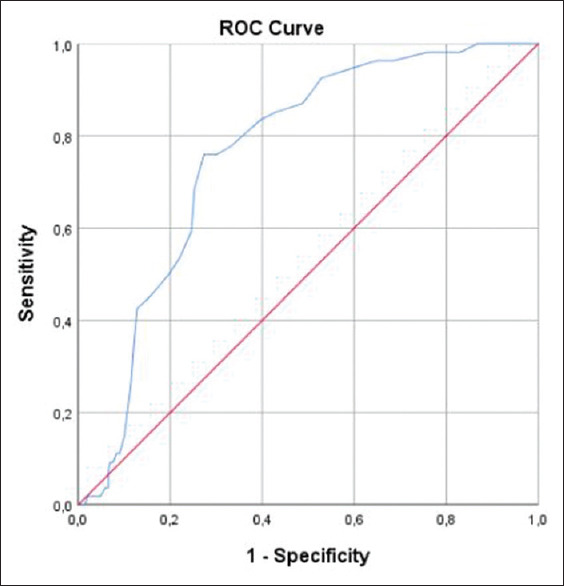
The ROC curve of the liver attenuation index for the ICU and non-ICU patients
Table 2.
Univariate and multivariate logistic regression analysis of risk factors associated with COVID-19 progression
| COVID-19 Severity | COVID-19 Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age, years | 1.092 (1.066-1.119) | <0.001 | 1.090 (1.060-1.119) | <0.001 | 1.091 (1.053-1.129) | <0.001 | 1.094 (1.051-1.138) | <0.001 |
| Gender, male | 1.242 (0.682-2.262) | 0.479 | 1.063 (0.423-2.673) | 0.896 | ||||
| Fatty liver | 3.815 (1.974-7.371) | <0.001 | 3.935 (1.778-8.707) | 0.001 | 3.915 (1.519-10.088) | 0.005 | 4.522 (1.443-14.173) | 0.010 |
| DM | 2.482 (1.147-5.370) | 0.021 | 0.534 (0.188-1.517) | 0.239 | 2.929 (1.000-8.576) | 0.050 | 0.430 (0.082-2.254) | 0.318 |
| HT | 3.023 (1.587-5.756) | 0.001 | 1.052 (0.453-2.444) | 0.906 | 2.567 (0.980-6.725) | 0.055 | 0.826 (0.229-2.988) | 0.771 |
| CAD | 4.544 (1.180-17.506) | 0.028 | 1.796 (0.392-8.233) | 0.451 | 2.072 (0.246-17.435) | 0.502 | ||
| COPD | 3.162 (1.199-8.337) | 0.020 | 1.774 (0.512-6.148) | 0.366 | 3.176 (0.848-11.902) | 0.086 | 1.177 (0.225-6.168) | 0.847 |
| CKD | 5.700 (1.380-23.537) | 0.016 | 5.399 (0.877-33.225) | 0.069 | 19.938 (4.566-87.066) | <0.001 | 34.842 (4.107-295.586) | 0.001 |
Data are presented as odds ratios (ORs) and 95% confidence intervals (CIs) measured by univariate and multivariate logistic regression analyses. HT: Hypertension; DM: Diabetes mellitus; CAD: Coronary artery disease; COPD: Chronic obstructive pulmonary disease; CKD: Chronic kidney disease
DISCUSSION
A radiological examination is an easy-to-apply diagnostic method that provides rapid diagnosis. With these advantages, it is of great importance for the early detection and treatment of patients affected by COVID-19. Chest CT with high sensitivity plays an important role in the diagnosis of COVID-19.[2,6,18] Of the 343 COVID-19 patients, 289 (84.2%) were treated on an outpatient basis or hospitalized, all discharged with full recovery. Fifty four (15.7%) patients were treated in ICU, and 20 (5.8%) of these died during treatment. Our center is the largest hospital in our country, and is the main COVID-19 treating facility. Patients with severe clinical findings and those that require ambulance transport due to their comorbidities are accepted in our emergency department. This may be the reason for the high number of ICU patients.
The incidence of liver injury ranges from 14% to 53% in COVID-19 patients. Abnormal ALT/AST ratios and accompanying slightly elevated bilirubin levels are the main indicators in the liver injury.[10] The cause of liver injury has not yet been proven. Systemic inflammatory response, drug toxicity, and progression of pre-existing liver diseases are considered as probable underlying factors.[12] In a postmortem study, hepatomegaly with hepatocyte degeneration accompanied by focal necrosis, neutrophil, lymphocyte and monocyte infiltration in the portal region, and hepatic sinus congestion with microthrombosis were detected.[12] Angiotensin-converting enzyme 2 (ACE2) expression is very low in the hepatocytes of a normal liver, but the upregulation of ACE2 expression has been shown in a mouse model of acute liver injury.[19] In severe COVID-19 patients, liver injury occurs at a significantly higher rate than in mild cases.[10] Xie et al. found that the patients with liver injury had severe imaging findings which might be the predictor of liver injury in COVID-19. They concluded that the patients with a high CT score should be followed up closely with liver function tests in order to assess liver injury.[19] Huang et al. found that liver injury was significantly higher in the ICU patients than in the non-ICU patients.[20] It was also reported that progression to severe disease and worse outcomes in COVID-19 was higher in patients with NAFLD.[9,11,13] Ji et al. investigated NAFLD in 202 patients with COVID-19 by calculating the hepatic steatosis index based on AST, ALT, body mass index, presence of diabetes, and gender and/or by an ultrasound examination and showed that pre-existing comorbidities (OR: 6.3) and NAFLD (OR: 6.4) were associated with COVID-19 progression, in univariate and multivariate logistic regression analysis.[9] In a study by Zhou et al., the risk of severe COVID-19 increases fourfold (adjusted OR 4.07, 95% CI 1.20-13.79, P = 0.02) by the coexistence of metabolic associated fatty liver disease.[21] Similar to the mentioned studies, univariate and multivariate analysis indicated that patients with NAFLD were at increased risk of disease progression. Pre-existing comorbidities such as DM, HT, CAD, and COPD are described as additional risks to progression of COVID-19.[9,22] In the current study, pre-existing comorbidities contributed to disease severity including ICU admissions and death.
The current study showed a relationship between pulmonary findings and fatty liver. An increased number of affected lobes, especially the involvement of the upper lobes and diffuse infiltrations in the form of consolidation, air bronchogram, and paving pattern were detected more frequently in patients with NAFLD, and the incidence of these findings was significantly higher among the ICU patients than in the non-ICU group. Patients with fatty liver showed higher odds of severe disease, even after including age and medical comorbidities in the multivariate model. In light of these results, we concluded that hepatic steatosis was a poor prognostic factor in COVID-19. Patients with hepatic steatosis detected on COVID-19-positive chest CT can be added to the report to alert the clinician to the risk of progressive disease, especially when LAI is lower than 0.5.
There are several limitations to this study. The considerable false negativity of the PCR test continues to be an important problem worldwide. We did not include patients with typical CT findings but with a negative PCR for COVID-19. Due to our hospital being the main COVID-19 center, a great number of patients had been referred from other hospitals, but these cases were not included in the study since it was not possible to evaluate their initial CT findings, which constituted the primary subject of this study. Another limitation is that some of the clinical findings were missing due to the overloaded nature of the emergency department during the pandemic. Lastly, the CT scans of all patients could not be performed on the same symptomatic day because they presented to the emergency department on different days after the onset of their symptoms.
In conclusion, chest CT which plays a central role in the diagnosis of COVID-19, can provide information about the prognosis of the disease. Fatty liver is an important sign for a poor prognosis and can easily be detected on chest CT taken for the diagnosis of the COVID-19 disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 2020;30:4381–9. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT Findings: A systematic review and meta-analysis. J Am Coll Radiol. 2020;17:701–9. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W, Sirajuddin A, Zhang X, Liu G, Teng Z, Zhao S, et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19) Eur Radiol. 2020;30:4874–82. doi: 10.1007/s00330-020-06827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Situation reports. [Last accessed on 2020 Feb 22]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 5.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–6. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;296:E32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruso D, Zerunian M, Polici M, Pucciarelli F, Polidori T, Rucci C, et al. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020;296:E79–85. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): A systematic review of ımaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 9.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451–3. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–52. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Fan JG. Characteristics and mechanism of liver ınjury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-What we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267–75. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prins GH, Olinga P. Potential implications of COVID-19 in non-alcoholic fatty liver disease. Liver Int. 2020;40:2568. doi: 10.1111/liv.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: Comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–85. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 16.Boyce CJ, Pickhardt PJ, Kim DH, Taylor AJ, Winter TC, Bruce RJ, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol. 2010;194:623–8. doi: 10.2214/AJR.09.2590. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19) [Last accessed on 2020 Nov 29]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html .
- 18.Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): Analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321–6. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160–3. doi: 10.1111/liv.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. doi: 10.1111/eci.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]


