Abstract
Background:
LINC-ROR, as a cancer-related lncRNA, has vital roles in stem cell survival, pluripotency, differentiation, and self-renewal in hESCs. However, cancer-related molecular mechanisms, its functional roles, and clinical value of LINC-ROR in GC remain unclear. In this study, we aimed to investigate probable interplay between LINC-ROR with SALL4 stemness regulator and their role with the development of the disease.
Methods:
The mRNA expression profile of LINC-ROR and SALL4 was assessed in tumoral and adjacent non-cancerous tissues of GC patients, using quantitative real-time PCR.
Results:
Significant LINC-ROR underexpression and SALL4 overexpression were observed in 55.81% and 75.58% (p < 0.0001) of samples, respectively. The expression of LINC-ROR and SALL4 were significantly correlated with each other (p = 0.044). There was an association between the underexpression of LINC-ROR and sex, stage of tumor progression, tumor type, and location of tumor (p < 0.05), and H. pylori infection with SALL4 expression (p = 0.036). There were also significant correlations between concomitant mRNA expression of SALL4 and LINC-ROR in tumors located at distal noncardiac, positive for H. pylori infection, tumors with invasion into the muscle layer of the stomach, and grade II tumor (p < 0.05).
Conclusion:
The clinical results of the SALL4-LINC-ROR association propose a probable functional interaction between these markers in tumor maintenance and aggressiveness. Our study can help to understand one of the mechanisms involved in the progression of GC through the function of these regulators.
Key Words: LINC-ROR, Non-coding RNA, SALL4
INTRODUCTION
Gastric cancer results from interactions between the molecular pathways, genetic/epigenetics alterations, and environmental factors[1,2]. A series of genetic susceptibilities have been supposed to stem cdebe involved in GC development. These include various polymorphisms as well as alteration in gene expression profile of such as tumor suppressors, inflammation related genes, cellular metabolism genes, EMT markers, transmembrane proteins, and matrix metalloproteinases[3]. Moreover, epigenetic changes can cause the dysregulation of tumor-related markers, leading to GC initiation and progression[4]. Among fundamental epigenetic changes in GC, methylation of CpG islands within the promoter region of certain genes, hydroxymethylation, chromatin remodeling, histone modification, and dysregulation of ncRNAs have been reported[5].
Evidence has shown a complicated association between ncRNAs and coding genes in the development of human cancers through the regulation of cellular and molecular processes[6]. These ncRNAs are mainly classified into several types of snRNAs, miRNAs, siRNAs, snRNAs, lncRNAs, and lincRNAs[6]. lncRNAs, as transcripts longer than 200 nucleotides, are involved in various biological processes, such as maintenance and modification of chromatin, genome imprinting, DNA methylation, dosage compensation, transcription, splicing, and translation[5]. LncRNAs regulate the expression of their target genes at three prominent levels of transcriptional, post-transcriptional, and epigenetic[7]. Besides, the defective function of lncRNAs can lead to apoptosis, invasion, progression, and metastasis in different human cancers[8]. As a result, lncRNAs are applied as diagnostic, prognostic and therapeutic markers in numerous types of solid tumors such as gastrointestinal cancers[9-11]. ANRIL, FENDRR, AF147447, CAT1, GAS5, HULC, MEG3, HOTAIR, H19, GHET1, GAPLINC, and MALAT1 are among dysregulated lncRNAs recognized in GC[11]. LincRNAs as a member of lncRNAs family demonstrate a highly specific expression pattern in cell or tissue levels [12]. Moreover, they can often be expressed with their neighboring coding genes and regulate gene expression via affecting nuclear bodies and chromatin complexes[13,14]. LincRNAs have crucial roles in diverse cellular and molecular processes, e.g. functioning as competing endogenous RNA in modulation of miRNAs expression with the transcriptional network in hESCs, reprogramming somatic cells toward iPSCs, early development in ESCs, and response to cellular stress by coordinating with p53[14].
LINC-ROR, a type of lincRNAs with 2.6 kb in length, has broad roles in stress response regulation, coordination in cell-cell and cell-environment interactions, inhibition of miRNAs function, tumor development, and prevention of p53 translation in DNA damage response, to boost pluripotency and stem cell survival[15,16]. Additionally, LINC-ROR has functional roles in differentiation and self-renewal of hESCs, iPSCs, and ESCs via the regulation of OCT4, SOX2, and NANOG expression[14,17]. Of note, SALL4, a C2H2 zinc finger TF, correlates with ESC markers of SOX2, OCT4, NANOG, and signaling pathways of BMI-1 and Wnt/β-catenin, leading to control the tumor cell renewal and preserve the pluripotency of ESCs and iPSCs[18,19]. SALL4 acts as a stemness state regulator in various cancers via ESCC, GC, lung, breast, colon, and hematopoietic cancers[20-25]. High expression of SALL4 is correlated with the early steps of tumor development, metastasis to lymph nodes, poor prognosis, and invasion in various malignancies[21]. Studies have investigated the dysregulation of LINC-ROR in tumorigenesis by evaluating the increased LINC-ROR expression in numerous malignancies, including pancreatic, colon, lung, bladder, endometrial, breast, hepatocellular, nasopharyngeal cancers, and ESCC, as well as its heterogenic function in glioblastoma[15,26-28]. Overexpression of LINC-ROR was significantly related to the advanced stages of malignancies, metastasis to lymph nodes, and vascular invasion, suggesting that LINC-ROR could serve as a prognostic marker in some human cancers[16].
Considering the prognostic role of LINC-ROR, its function in tumor progression, and its correlation with stemness markers, a probable correlation may be existed between the expression of LINC-ROR and SALL4 in tumorigenesis. Therefore, we aimed in the current study to evaluate the expression of LINC-ROR in tumor and adjacent non-cancerous tissues of GC patients and to investigate its probable linkage with SALL4 stemness regulator, as well as their correlation with clinicopathological features of patients.
MATERIALS AND METHODS
Patients and tissue samples
The tumor and adjacent non-cancerous tissues were collected from 86 GC patients who underwent gastrectomy at two affiliated Hospitals of Mashhad University of Medical Sciences (MUMS), Mashhad, Iran, Imam Reza and Omid, from 2008 to 2012. The fresh specimens were quickly transferred to the RNAlater solution (Qiagen, Hilden, Germany) and stored at -20 °C until further use. The information of tumor tissues and patients were characterized according to Union International Cancer TNM classification by a pathologist[29]. Inclusion criteria included patients who had not been received preoperative chemo-radio treatment before the surgery. Based on hematoxylin and eosin analysis, histopathologic examination was performed for all the samples to verify that all cancer cells have at least 70% tumor cells.
RNA isolation and cDNA synthesis
Total RNA was extracted from adjacent non-cancerous and tumoral tissue samples using the RNeasy Mini Kit (Qiagen). Five to seven tissue sections (100 mg) were first lysed by adding 1 mL of lysis buffer and then centrifuged at 50,000 ×g for three minutes. All steps were performed as per the manufacturer's instructions. The quantity and purity of total RNAs were measured by a NanoDrop spectrophotometer (WPA, Biowave II+, Germany); pure RNA had an A260/A280 absorbance ratio of 1.8 to 2.0. The integrity of RNA preparation was evaluated by the electrophoresis on 1% agarose gel and viewed as the 28S and 18S rRNA bands. Then total RNA was treated with DNase I (Thermo Fisher Scientific, Waltham, MA) in accordance with the manufacturer’s procedures for DNA contamination prevention. The first strand cDNA synthesis was carried out by the primeScriptTM RT Reagent kit (Takara, Japan) with 1 µg of treated RNA, oligo(dT), and random primers according to the manufacturer’s instructions (37 °C for 15 min and 85 °C for 5 s). Afterward, the quality of cDNA was verified by the amplification of GAPDH as the control, and cDNA was stored at -20 °C until the real-time PCR.
Comparative real-time PCR analysis
Comparative real-time PCR experiment was performed based on the Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines[30]. We applied the adjacent tissue as a reference sample and compared mRNA expression in tumor with that of adjacent non-cancerous tissues through comparative relative real-time PCR. GAPDH was employed as an internal control to normalize the data in a comparative relative real-time PCR (SYBR Green, AMPLIQON, Denmark) on a LightCycler® 96 Real-Time PCR System thermocycler (Roche, Germany) by gaining the relative Ct values and calculating LINC-ROR and SALL4 mRNA expression through the 2-ΔΔCt method[26,31]. Tumor mRNA expression higher or less than onefold relative to corresponding gene expression in adjacent non-cancerous tissues was considered as overexpression or underexpression, respectively, whereas the fold changes between -1 and +1 were regarded as no change in the gene expression. All RT-PCRs were performed in duplicates. The thermal cycling conditions, and the sequences of the specific primers set for LINC-ROR and SALL4 are illustrated in Table 1[21,26].
Table 1.
Primer sequences and the thermal cycling used in real-time PCR
| Transcripts | Sequence | Thermal profile |
|---|---|---|
| LINC-ROR | F: ACAAGGAGGAAAGGGCTGAC R: TTCTGGAAGCTAAGTGCACATG |
95 °C (15 min) [95 °C (15 s)/63 °C (20 s)/72 °C (20 s)] 40 |
| SALL4 | F: CCAAAGGCAACTTAAAGGTTCAC R: GAGATCTCATTGGTCTTCACGG |
95 °C (10 min) [95 °C (30 s)/58 °C (15 s)/72 °C (30 s)] 40 |
| 16S rRNA | F:GCTATGACGGGTATCC R:GATTTTACCCCTACACCA |
95 °C (5 min) [92 °C (30 s)/55 °C (40 s)/72 °C (40 s)] 40 |
| UreC (glmM) | F:AGCTTTTAGGGGTGTTAGGGGTTT R:AAGCTTACTTTCTAACACTAACGC |
95 °C (5 min) [92 °C (30 s)/55 °C (40 s)/72 °C (40 s)] 40 |
| CagA | F:GATAACAGGCAAGCTTTTGAGG F:CTGCAAAAGATTGTTTGGCAGA |
95 °C (5 min) [92 °C (30 s)/55 °C (40 s)/72 °C (40 s)] 40 |
| GAPDH | F: GGAAGGTGAAGGTCGGAGTCA R: GTCATTGATGGCAACAATATCCACT |
95 °C (10 min) [95 °C (30 s)/58 °C (30 s)/72 °C (30 s)] 40 |
DNA extraction and detection of H . Pylori infection
Total genomic DNA was extracted from normal and tumoral tissue samples by enzymatic method (digestion buffer, proteinase K, and RNase) as previously described[32]. Briefly, tissues were lysed in 200 μl of digestion buffer, 20 μl of proteinase K, and 5 μl of RNase at 55 °C for 3 h incubation. Thereafter, 200 μl of phenol/chloroform solution was added to the mixture, and samples were centrifuged at room temperature for one minutes. After the addition of two volumes of absolute ethanol and 0.1 volume of 3 M sodium acetate to the supernatant, the mixture was incubated at -20 °C overnight. The mixture was then centrifuged for 10 minutes, and the DNA pellet was washed with 75% ethanol and resuspended in Tris-EDTA buffer. PCR was accomplished on extracted DNA using specific primers set for H. pylori genes of UreC (glmM), 16S rRNA, and virulence factor of CagA. The primer sequences and the thermalprofile for PCR amplification are demonstrated in Table 1.
Statistical analysis
Statistical analyses were performed using SPSS 19.9 software (SPSS, Chicago, IL) and GraphPad Prism 5.0 (La Jolla, CA, USA). The Kolmogorov–Smirnov (K-S) test was applied for normal or non-normal distribution of the data. The correlation between expression of LINC-ROR and SALL4 with different clinicopathological characteristics of GC patients was evaluated by the χ2 or Fisher exact test, paired-samples T-test, independent-samples T-test, and ANOVA. The probable correlation between the LINC-ROR and SALL4 expression was assessed by Pearson correlation. A p < 0.05 was considered a statistically significant level.
Ethical statement
The above-mentioned sampling protocol was approved by the Ethics Committee of MUMS, Mashhad, Iran (ethical code: 921706). Prior to participation, the informed constant was obtained from all patients participating in this study.
RESULTS
Patients’ histopathological characteristics
The clinicopathological characteristics of 86 GC patients involving 63 males and 23 females enrolled in the present study are summarized in Table 2. The general mean age ± SD of patients at the time of diagnosis was 63.03 ± 10.98 years, and the mean size ± SD of tumor samples was 6.46 ± 3.11 cm. Most cases (64/86, 74.5%) had T3/T4 tumor depth of invasion, 65 out of 86 (75.5%) samples were in stages II/III of tumor, and 73/86 (84.9%) of tumor samples had lymph node metastasis. Based on the histopathological analysis, 60 (69.8%) tumor samples were categorized as intestinal type, whereas 21 (24.4%) and 5 (5.8%) tumors were diffused and mixed types, respectively. Tumors located in the gastric cardia were 37/86 (43%), while 49/86 (57%) was found in noncardia regions of the stomach. The gastric tissue samples were investigated for H. pylori infection using PCR for three genes of 16s rRNA, UreC, and CagA. Also, 43 (50%) tumor tissue samples were positive for 16s rRNA/UreC, while 44.2% was positive for CagA.
Table 2.
Clinicopathological features of the 86 GC patients under study
| Factor | Patients (%) |
|---|---|
| Age (mean ± SD) | 63.03 ± 10.98 years |
| Sex | |
| Male Female |
63 (73.3) 23 (26.7) |
| Tumor size (mean ± SD) | 6.46 ± 3.11 cm |
| Differentiation | |
| PD MD WD |
20 (23.3) 55 (64) 11 (12.8) |
| Lymph node metastasis (N) | |
| N0 N1 N2 N3 |
13 (15.1) 40 (46.5) 26 (30.2) 7 (8.1) |
| Grade | |
| I II III |
13 (15.1) 53 (61.6) 20 (23.3) |
| Stage of tumor progression | |
| I II III IV |
7 (8.1) 15 (17.4) 50 (58.1) 14 (16.3) |
| Depth of tumor invasion (T) | |
| T2 T3 T4 |
22 (25.6) 47 (54.7) 17 (19.8) |
| Tumor type | |
| Intestinal Diffused Mixed |
60 (69.8) 21 (24.4) 5 (5.8) |
| Location | |
| Cardiac Noncardiac |
37 (43) 49 (57) |
| H. pylori (16s rRNA/UreC) | |
| Positive Negative |
43 (50) 43 (50) |
| H. pylori (CagA) | |
| Positive Negative |
38 (44.2) 48 (55.8) |
PD, poorly differentiated; MD, moderately differentiated; WD, well differentiated; N0, no. of regional lymph node metastasis; N1, metastasis in one to two regional lymph nodes; N2, metastasis in three to six regional lymph nodes; N3, metastasis in seven or more regional lymph nodes
Expression analysis of LINC-ROR and SALL4
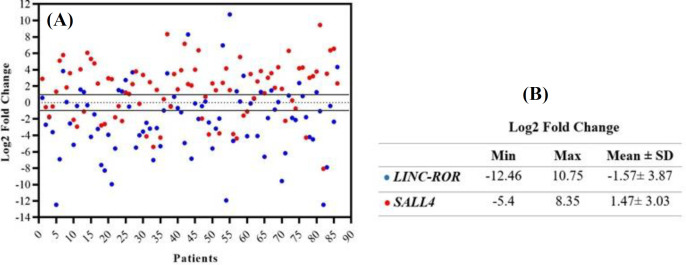
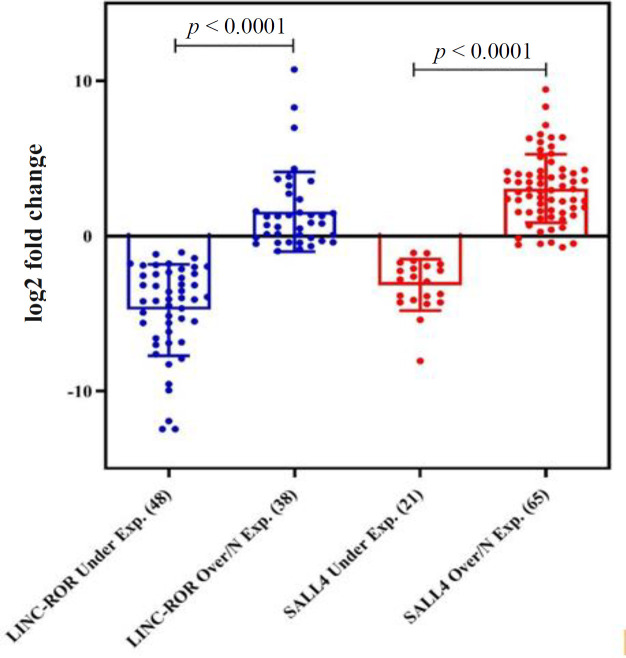
Relative comparative qRT-PCR indicated that the log2 fold change of LINC-ROR and SALL4 decreased in 55.81% (48/86) and 24.41% (21/86) of tumor samples compared to the adjacent non-cancerous tissues (p < 0.0001); however, 38 (44.18%) and 65 (75.58%) samples showed the normal or overexpression of LINC-ROR and SALL4 (P < 0.0001), respectively (Table 4). The profile of mRNA expression in all patients is displayed in Figure 1A as a scatter plot. The minimum and maximum log2 fold changes ranged from -12.46 to 10.57 for LINC-ROR and -5.4 to 8.35 for SALL4, respectively (Fig. 1B). Expression levels of LINC-ROR and SALL4 in underexpressed vs. overexpressed or normal expression of tumor specimens are represented in Figure 2 as a dot plot. The mean log2 fold change of LINC-ROR and SALL4 expression level were -1.57 ± 3.87 and 1.47 ± 3.03 (Fig. 1B), respectively. We identified that the LINC-ROR level was significantly lower in cancerous tissues compared with the adjacent non-cancerous tissues (normal; Fig. 3A; p < 0.0001). Moreover, the SALL4 level was significantly higher in cancerous tissues compared with the paired non-neoplastic gastric tissues (normal; Fig. 3B; p < 0.0005). In addition, the average LINC-ROR and SALL4 expression levels of GC tissues were 1.53 ± 4.23 and -0.38 ± 3.34, as well as that of matched adjacent non-cancerous tissues were 3.36 ± 4.81 and -1.74 ± 3.06, respectively, as demonstrated in Figure 3C. We identified that the LINC-ROR level was significantly lower in cancerous tissues compared with the adjacent non-cancerous tissues (normal; Fig. 3A; p < 0.0001). Moreover, the SALL4 level was significantly higher in cancerous tissues compared with the paired non-neoplastic gastric tissues (normal; Fig. 3B; p < 0.0005).
Table 4.
Expression profile of LINC-ROR and SALL4 in different clinicopathological features of the enrolled GC patients
| Factor |
LINC-ROR
|
p
value |
SALL4
|
p value |
Coexpression of markers
( p value ) |
||
|---|---|---|---|---|---|---|---|
| ─ / ↑ (%) | ↓ (%) | ─ / ↑ (%) | ↓ (%) | ||||
| Sex Male Female |
26 (30.2) 13 (15.1) |
37 (43) 10 (11.6) |
0.05 * | 25 (29) 12 (13.9) |
38 (44.1) 11 (12.7) | NS | 0.246 0.031* |
| Differentiation | |||||||
| PD MD WD |
12 (13.9) 30 (34.8) 5 (5.8) |
8 (9.3) 25 (29) 6 (6.9) |
NS | 10 (11.6) 23 (26.7) 4 (4.6) |
10 (11.6) 32 (37.2) 7 (8.1) |
NS |
0.07
0.02* 0.48 |
| Lymph node metastasis (N) | |||||||
| N0 N1 N2 N3 |
7 (8.1) 17 (19.7) 11 (12.7) 4 (4.6) |
6 (6.9) 23 (26.7) 15 (17.4) 3 (3.4) |
NS | 7 (8.1) 14 (16.2) 14 (16.2) 2 (2.3) |
6 (6.9) 26 (30.2) 12 (13.9) 5 (5.8) |
NS |
0.61
0.19 0.13 0.7 |
| Grade | |||||||
| I II III |
7 (8.1) 28 (32.5) 12 (13.9) |
6 (6.9) 25 (29) 8 (9.3) |
NS | 6 (6.9) 21 (24.4) 10 (11.6) |
7 (8.1) 32 (37.2) 10 (11.6) |
NS |
0.62
0.023* 0.081 |
| Stage of tumor progression | |||||||
| I II III IV |
4 (4.6) 5 (5.8) 33 (38.3) 5 (5.8) |
3 (3.4) 10 (11.6) 17 (19.7) 9 (10.4) |
0.029 * | 5 (5.8) 5 (5.8) 22 (25.5) 5 (5.8) |
2 (2.3) 10 (11.6) 28 (32.55) 9 (10.4) |
NS |
0.16
0.23 0.34 0.59 |
| Depth of tumor invasion (T) | |||||||
| T2 T3 T4 |
11 (12.7) 26 (30.2) 10 (11.6) |
11(12.7) 10 (11.6) 2 (2.3) |
NS | 9 (10.4) 20 (23.25) 8 (9.3) |
13 (15.11) 27 (31.39) 9 (10.4) |
NS |
0.002
*
0.96 0.76 |
| Tumor type | |||||||
| Intestinal Diffuse Mixed |
33 (38.3) 11 (12.7) 3 (3.4) |
27 (31.3) 11 (12.7) 3 (3.4) |
0.05 * | 25 (29) 8 (9.3) 4 (4.6) |
35 (40.6) 13 (15.11) 1 (1.1) |
NS | 0.19 0.14 0.75 |
| Location | |||||||
| Cardiac Noncardiac |
23 (26.7) 24 (27.9) |
14 (16.2) 25 (29) |
0.048 * | 15 (17.4) 22 (25.5) |
22 (25.5) 27 (31.39) |
NS | 0.77 0.021* |
| H. pylori (16s rRNA/UreC) | |||||||
| Positive Negative |
23 (26.7) 24 (27.9) |
19 (22) 20 (23.2) |
NS | 24 (27.9) 13 (15.1) |
19 (22) 30 (34.8) |
0.047 * |
0.03
*
0.96 |
| H. pylori (CagA) | |||||||
| Positive Negative |
23 (26.7) 24 (27.9) |
25 (29) 14 (16.2) |
NS | 12 (13.9) 25 (29) |
23 (26.7) 26 (30.2) |
0.036 * |
0.041
*
0.87 |
PD, poorly differentiated; MD, moderately differentiated; WD, well differentiated; N0, no. of regional lymph node metastasis; N1, metastasis in one to two regional lymph nodes; N2, metastasis in three to six regional lymph nodes; N3, metastasis in seven or more regional lymph nodes; NS, non-significant correlation
Fig. 1.
(A) Scatter plot representing descriptive analysis of relative gene expression of LINC-ROR and SALL4 in GC patients. The black lines indicate the thresholds for the over- and under-expression. The range between over- and under-expression shows the cases with normal LINC-ROR and SALL4 mRNA expression; (B) Minimum, maximum, and mean of log2 fold change for the LINC-ROR and SALL4 mRNA expression
Fig. 2.
Dot plot representative of relative mRNA expression of LINC-ROR and SALL4 in GC patients. Dot plots represent the lowest, lower quartile, median, upper quartile, and highest observations of fold changes in patients with normal/ over- or under-expressed LINC-ROR and SALL4
Fig. 3.
(A and B) The expression alteration of LINC-ROR and SALL4 in GC tissues and adjacent noncancerous tissues. LINC-ROR and SALL4 expression were assessed by qRT-PCR in tissue. Data was evaluated statistically using the two-way ANOVA. (C) Minimum, maximum, and mean of level relative expression for the LINC-ROR and SALL4 in GC tissues and adjacent noncancerous tissues
Association between concomitant expression of LINC-ROR and SALL4 in GC
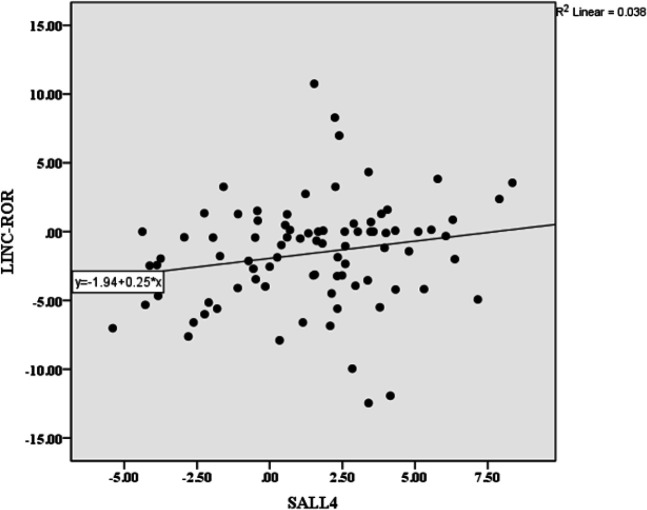
The mRNA levels of LINC-ROR and SALL4 were significantly correlated with each other (p = 0.044; Table 3). Interestingly, the expression level of LINC-ROR decreased in tumor specimens with a low level of SALL4 expression (p < 0.05) compared to SALL4 overexpression samples (p = 0.92). Correlation between LINC-ROR and SALL4 expression levels is represented as a regression plot in Figure 4. There was also no significant association between LINC-ROR and SALL4 under- or overexpression (Table 4).
Table 3.
Association between LINC-ROR and SALL4 expression in GC samples
| SALL4 | SALL4 underexpression |
SALL4
overexpression |
|
|---|---|---|---|
|
LINC-ROR
Pearson correlation Sig. (two‐tailed) |
0.218* 0.044 |
||
|
LINC-ROR underexpression (N) Pearson correlation Sig. (two‐tailed) |
11 0.043 0.900 |
16 0.143 0.597 |
|
|
LINC-ROR overexpression (N) Pearson correlation Sig. (two‐tailed) |
0 | 7 -0.578 0.174 |
*Correlation is significant at the 0.05 level
Fig. 4.
Regression plot illustrating a correlation between the level of LINC-ROR and SALL4 expression (p = 0.044)
Clinicopathological relevance of LINC-ROR and SALL4 expression in GC
To indicate LINC-ROR expression on GC progression, we investigated the correlation of LINC-ROR expression with patients’ clinicopathological traits (Table 4). Based on the Table, there were some significant correlations between the data, including the stage of tumor progression (p = 0.029), the tumor type (p = 0.05), and the location of tumor (p = 0.048) with
LINC-ROR mRNA expression. Among samples with LINC-ROR underexpression, 16.2% (14/86) and 29% (25/86) samples were located in cardiac and noncardiac regions, and 31.3% (27/86) indicated intestinal tumor type compared with diffused or mixed type 16.1% (14/86) GC. Moreover, there was a correlation between sex and LINC-ROR expression (p = 0.05), but correlation between LINC-ROR and other clinicopathological factors was insignificant. However, GC patients with underexpression of LINC-ROR were prone to N1/N2 steps of lymph node metastasis (47.1%), indicating that metastasis of tumor cells to the lymph node was declined via decreasing in LINC-ROR expression. Most specimens with LINC-ROR underexpression had grade II (29%) and 13.9% was invaded to the adventitia (T3). There was not any significant correlation between the level of SALL4 mRNA expression with patients’ clinicopathological traits (Table 4).
Correlation between LINC-ROR and SALL4 expression and H. pylori infection
A significant correlation was found between SALL4 mRNA expression and H. pylori infection in which 50% (43/86) and 40.7% (35/86) gastric tumor samples were positive for 16s rRNA/UreC (p = 0.047) and CagA (p = 0.036), respectively (Table 4). Furthermore, our results indicated that no significant correlation between LINC-ROR expression and H. pylori infection in GC. The majority of LINC-ROR underexpression samples were positive for 16s rRNA/UreC (48.7%) and CagA (64.1%).
Correlation between the concomitant expression of LINC-ROR and SALL4 with different pathological variables
There was significant correlation of the concomitant expression level of LINC-ROR with SALL4 in specimens positive for H. pylori infection (p = 0.03). The co-expression of LINC-ROR and SALL4 was significantly associated with the depth of tumor invasion (T2; p = 0.002) and tumor grade of II (p = 0.05). Moreover, co-expression of LINC-ROR and SALL4 in tumor samples was significantly correlated with moderately differentiated (p = 0.05). Furthermore, co-expression of both LINC-ROR and SALL4 in the tumors located at distal noncardiac region (p = 0.05). We found no significant correlation between co-expression of LINC-ROR and SALL4 with metastasis of tumor cells into lymph nodes, stage of tumor progression, and tumor type. The correlations between the co-expression of LINC-ROR and SALL4 with different pathological states of the patients are summarized in Table 4.
DISCUSSION
Recent studies in the last decade have displayed high incidence and poor diagnosis with a survival rate of 31% in GC patients[33,34]. Identification of new biomarkers is critical in early detection, diagnosis, and treatment that would help to improve the quality of life in cancer patients[35]. Recent data have illustrated that lncRNAs have widely participated in biological procedures, and their misexpression has an impact on the pivotal function of molecules involved in signaling pathways and tumor pathogenesis[28].
LncRNAs play key roles in tumorigenesis and many aspects of cancer development; therefore, evaluating the probable correlation between LINC-ROR and SALL4 in GC development is needed. We reported for the first time the significantly declined expression of LINC-ROR at the transcript level in GC tissues. Moreover, we indicated a significant correlation between concomitant expression of LINC-ROR and stemness transcriptional factor SALL4 with clinicopathological features, including H. pylori infection, depth of tumor invasion, tumor location, differentiation, tumor grade, and sex in gastric tissues. These data display that co-expression of LINC-ROR and SALL4 may contribute to the development and progression of GC.
LincRNA function as TSG or oncogene in pathogenesis and development of different cancers suggests that they take a part in various cellular and molecular processes, from ESC commitment to tumorigenesis-associated gene expression system as vital regulators[36]. Interaction of lincRNAs with other coding genes and ncRNAs can change various mechanisms, e.g. chromatin modifications, transcription, and post-transcription processes, acting as protein and RNA decoys in leukemia and solid malignancies[37]. Some lncRNAs are identified as biomarkers in the diagnosis and prognosis of malignancies, including H19, HOTAIR, and MALAT1[38]. Moreover, aberrant expression of LINC-ROR, located at 18q21, has been indicated in the progression of some human malignancies[36]. The expression level of LINC-ROR was significantly associated with metastasis to lymph nodes, vascular invasion, and advanced stages of tumor progression, where its expression activated during tumor progression[16]. It has been shown that dysregulation of LINC-ROR is correlated with poor prognosis and overall shorter survival in GC patients, implying that LINC-ROR may be identified as an independent prognostic marker and a vital modulator for GC[31]. Moreover, increased LINC-ROR expression in CD133+ GC stem cells was correlated with proliferation, invasion, and apoptosis inhibition, as well as with the overexpression of OCT4, SOX2, and NANOG[39]. Interestingly, we found LINC-ROR downregulation in GC tissues, and this observation is not in accordance with those reported in a previous study[40]. Therefore, we can conclude that LINC-ROR demonstrates a heterogeneous expression level in different populations of GC patients. In this regard, a former study has examined the expression of LINC-ROR in various cell lines and human tumor tissue samples and reported that the expression of LINC-ROR increases in cervical, esophageal, and ovarian cancers, but decreases in breast, colon, sarcoma and melanoma cancers. In addition, underexpression of LINC-ROR has been observed in somatic cancer cell lines, except for undifferentiated ESCs and embryonic carcinoma cell lines[40]. It is noteworthy that LINC-ROR has also been indicated heterogeneous expression levels in GC. Our outcomes propel the hypothesis that the underexpression of LINC-ROR in GC tissues may be due to more prevalent of monosomy chromosome 18 (location of LINC-ROR) compared with trisomy 18 (based on the Mitelman database of chromosome aberrations and gene fusions in cancer[39,41]. Another hypothesis about the diversity of LINC-ROR expression in GC is that different small subpopulations from the cancerous mass of GC stem cells may lead to the heterogeneous expression of LINC-ROR in different populations[40]. Our results indicated that the underexpression of LINC-ROR is associated with tumor type and location, stage of tumor progression, and gender of patients. In line with our reports, the heterogeneous expression level of LINC-ROR has been illustrated in glioblastoma, as the up-regulation of LINC-ROR was related to overall survival and poor progression[42]. On the other hand, the underexpression of LINC-ROR is correlated with the mRNA expression levels of SOX11 and KLF4 in glioma[36]. Herein, we have sought to examine the significant changes in the expression level of LINC-ROR in some malignancies. It has been illustrated that the upregulation of LINC-ROR is associated with cell proliferation, EMT, migration, invasion, metastasis, poor prognosis, inhibition of NANOG expression, and alteration of cancer stem-like cells properties via the regulation of miRNAs in pancreatic cancer[43,44]. Recent studies have found that LINC-ROR overexpression can trigger proliferation, EMT, progression, and metastasis via interaction with miRNAs and the TGF-β signaling pathway in breast cancer[45,46]. Moreover, LINC-ROR silencing results in the inhibition of cell proliferation, invasion, and EMT through reducing CDH1 expression and increasing the expression of ZEB1/2 and Vimentin in ESCC tumor tissues[26,47]. Increased LINC-ROR expression was found in bladder cancer tissues and cell lines, leading to the induction of EMT, promotion of cell proliferation, migration, invasion, and tumor progression[28]. It has been divulged that LINC-ROR is overexpressed and linked to the hypoxia network via modulating miR-145-HIF-1α expression in hepatocellular cancer[48]. LINC-ROR is upregulated in lung cancer stem cell and non-small cell lung cancer and associated with poor prognosis[27]. Evidence has shown that LINC-ROR, as an epigenetic regulator implicated in lineage commitment, sorely associates with tumorigenesis and stemness[49].
The stemness state is involved in EMT and tumor growth in different steps of tumorigenesis through a set of TFs[21]. It has been demonstrated that LINC-ROR regulates the pluripotency levels of TFs, including SALL4, LIN28, OCT4, SOX2, and NANOG via acting as a miRNA-145 sponge, leading to the preservation of hESCs and cancer stem cells undifferentiated status[14,50,51]. Moreover, the dysregulation of LINC-ROR is correlated with the upregulation of stemness TFs of OCT4, SOX2, and NANOG and the pluripotent factor of CD133 in GC stem cells[39]. The expression of LINC-ROR represses ESC differentiation, resulting in boosting the survival of ESCs and iPSCs through suppressing cellular stress pathways[52]. LINC-ROR stimulates cancer stem cell phenotype and ESCC progression through the deregulation of SOX9, as a TF involved in embryogenesis and maintenance of stem/progenitor cells, to coordinately promote cell proliferation, motility, self-renewal capacity, and cancer stemness state[53]. Expression of LINC-ROR has been reported to be upregulated in undifferentiated oral squamous cell carcinoma and correlated with the overexpression of KLF4, C-MYC, OCT4, and SOX2[49]. According to the role of SALL4 in stemness state of GC cells as well as the function of LINC-ROR as a cell stemness regulator, we assessed whether there is a correlation between the expression of SALL4 and LINC-ROR in GC[49,54]. Accordingly, we indicated a significant co-expression of markers in the GC tissues patients. These tumor tissues are located in noncardiac regions with invasion into the muscle layer of the stomach (T2). In addition, these samples were in grade II tumor and moderate differentiation status, introducing this correlation as an effective axis of markers in GC patients. Our findings revealed that the co-expression of LINC-ROR and SALL4 was significantly correlated with each other in the early steps of tumor development in patients. Remarkably, the association between LINC-ROR and SALL4 expression may enhance the stemness characteristics and promote the progression of GC. Induction of EMT via the deregulation of LINC-ROR may activate the expression of stemness marker, SALL4, as a TF with an oncogenic role in GC, to promote cancer phenotype. It has been illustrated that lncRNA differentiation antagonizing non-protein coding RNA boosts the proliferation, metastasis, and invasion in GC cells through activating SALL4, to adjust the expression of EMT regulators, such as TWIST, SLUG, and E-cadherin[19]. However, no reports are available on the clinical importance of LINC-ROR and SALL4 in GC aggressiveness. Our results may provide evidence for the possible linkage between LINC-ROR and SALL4 stemness markers in GC tumorigenesis.
There is a direct interaction between H. pylori infection and GC as change in the host cell microenvironment can activate oncogenic pathways[32]. Identification of epigenetic alterations can assist to predict and diagnose cancer patients’ prognosis[55]. Accordingly, the deregulation of lncRNAs in combination with H. pylori infection can be worthwhile in predicting markers involved in GC development[56]. The LINC-ROR expression pattern in gastric cells infected by H. pylori has never been reported until now. Considering the widespread functions of lncRNAs in molecular processes and their role in pathogenesis and tumorigenesis, the aberrant expression of LINC-ROR may relate to H. pylori infection in GC patients. Thus, in the present study, we have shown that the significant co-expression of LINC-ROR and SALL4 is correlated with H. pylori-positivity. Moreover, the LINC-ROR expression declined in specimens with H. pylori infection, and dysregulation of LINC-ROR may be correlated with the H. pylori-related carcinogenesis. Previous studies have indicated that H. pylori infection could deregulate the downstream target genes of the Wnt signaling pathway, such as CDX1. Dysregulation of CDX1 can induce the expression level of stemness markers such as SALL4 to transform gastric epithelial cells into dedifferentiated stem/progenitor-like cells, which then converts to intestinal metaplasia[57,58]. Moreover, it has been indicated that the infection of H. pylori alters the expression level of lncRNAs, leading to H. pylori-related cancer[56]. Our results may consider a signaling axis of LINC-ROR-SALL4 through its correlation with H. pylori infection, to display the possible crosstalk between LINC-ROR and SALL4 in GC tumorigenesis.
Overall, our findings reveal the downregulation of LINC-ROR in GC tissues. The decreased expression of LINC-ROR may be affected by mechanism underlying SALL4 deregulation and H. pylori infection, which may provide a new viewpoint to understand the dual function of LINC-ROR as oncogene or TSG in GC. Our data also suggest a linkage between tumorigenesis and dedifferentiation and stemness status. However, our results propose that concomitant dysregulation of LINC-ROR and SALL4 in transcript level may have a potential role in tumor initiation and invasion of GC. This conclusion requires further functional analysis for better understanding of the mechanisms involved in the pathogenesis and progression of GC.
ACKNOWLEDGEMNTS
This work has been supported by the Mashhad University of Medical Sciences (grant 921706). We gratefully acknowledge the Human Genetics Division, Immunology Research Center, Avicenna Research Institute (Mashhad University of Medical Sciences) for their help and cooperation.
CONFLICT OF INTEREST.
None declared.
References
- 1.FU DG. Epigenetic alterations in gastric cancer. Molecular medicine reports. 2015;12(3):3223–3230. doi: 10.3892/mmr.2015.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamashita S, Kishino T, Takahashi T, Shimazu T, Charvat H, Kakuqawa R, Nakajima T, Lee YC, Lida N, Maeda M, Hattori N, Takeshima H, Nagano R, Oda Ichiro Tsugane S, Shiang M, Ushijirna T. Genetic and epigenetic alterations in normal tissues have differential impacts on cancer risk among tissues. Proceedings of the national academy of sciences. 2018;115(6):1328–1333. doi: 10.1073/pnas.1717340115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura G. Genetic and epigenetic alterations of tumor suppressor and tumor-related genes in gastric cancer. Histology and histopathology. 2002;17(1):323–329. doi: 10.14670/HH-17.323. [DOI] [PubMed] [Google Scholar]
- 4.Patel TN, S Roy, R Ravi. Gastric cancer and related epigenetic alterations. Ecancermedicalscience . 2017;11:714. doi: 10.3332/ecancer.2017.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Z, Lin Z, Pang X, Tariq MA, Ao X, Li P, Wang J. Epigenetic regulation of long non-coding RNAs in gastric cancer. Oncotarget . 2018;9(27):19443–19458. doi: 10.18632/oncotarget.23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao-Po L, He L. Noncoding RNAs in cancer development. Annual review of cancer biology. 2017;1:163–184. [Google Scholar]
- 7.Chen J, Wang R, Zhang K, Chen LB. Long non‐coding RNAs in non‐small cell lung cancer as biomarkers and therapeutic targets. Journal of cellular and molecular medicine. 2014;18(12):2425–2436. doi: 10.1111/jcmm.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheetham S, Gruhi F, Matick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. British journal of cancer. 2013;108(12):2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han W, Xiao R, Zhang C, Suyila Q, Li X, Su X. Selecting lncRNAs in gastric cancer cells for directed therapy with bioactive peptides and chemotherapy drugs. Oncotarget. 2017;8(49):86082–86097. doi: 10.18632/oncotarget.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin MT, Song HJ, Ding XY. Long non-coding RNAs involved in metastasis of gastric cancer. World journal of gastroenterology. 2018;24(33):3724–3737. doi: 10.3748/wjg.v24.i33.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Mo X, Fu L, Xiao B, Guo J. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget. 2016;7(8):8601–8612. doi: 10.18632/oncotarget.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: New links in cancer progression. Cancer research. 2011;71(1):3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabili MN, Trapnell C, Goff L, Koziol M, Vega BT, Regev A, Rinn J. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes and development. 2011;25(18):1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Xu z, Jiang J, Jiuhon C, Leixiao GK, Wu M, Xiong J, Guo X, Liu H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Developmental cell. 2013;25(1):69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Pan Y, Li Chen, Zhang K, Chu X, Wang R, Chen L. The emerging roles of long noncoding RNA ROR (lincRNA-ROR) and its possible mechanisms in human cancers. Cellular physiology and biochemistry. 2016;40(1-2):219–229. doi: 10.1159/000452539. [DOI] [PubMed] [Google Scholar]
- 16.Lu R, Chen J, Kong L, Zhu H. Prognostic value of lncRNA ROR expression in various cancers: A meta-analysis. Bioscience reports. 2018;38(5):20181095. doi: 10.1042/BSR20181095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fico A, Fiorenzano A, Pascale E, Patriarca EJ, Minchiotti G. Long non-coding RNA in stem cell pluripotency and lineage commitment: functions and evolutionary conservation. Cellular and molecular life sciences. 2019;76(8):1459–1471. doi: 10.1007/s00018-018-3000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Tam WL, Tong GQ, Wu Q, Yun chan H, SEnsoh B, LOu Y, Yang J, Ma Y, Chai Li, Hui Ng H, Lufkin T, Robson P, Lim B. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nature cell biology. 2006;8(10):1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 19.Pan L, Liang W, Gu J, Zang X, Huang Z, Shi H, Chen J, Fu M, Zhang P, Xiao X, Qian H, Xu W, Jiang P, Zhang X. Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget. 2018;9(2):1915–1930. doi: 10.18632/oncotarget.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viiri LE, Rantapero T, Kiamehr M, Alexanova A, Oittinen M, Viiri K, Niskanen H, Nykter M, Kaikkonen MU, Aalto-Setala K. Extensive reprogramming of the nascent transcriptome during iPSC to hepatocyte differentiation. Scientific reports. 2019;9(1) doi: 10.1038/s41598-019-39215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forghanifard MM, Khales SA, Mallak A, Farshchian M, Abbaszadegan MR. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Medical oncology. 2014;31(4):922. doi: 10.1007/s12032-014-0922-7. [DOI] [PubMed] [Google Scholar]
- 22.Yuan X, Zhang X, Zhang W, Liang W, Zhang P, Shi H, Zhang B, Shao M, Yam Y, Qian H, Xu W. SALL4 promotes gastric cancer progression through activating CD44 expression. Oncogenesis . 2016;5(11) doi: 10.1038/oncsis.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dirican E, Akkiprik M. Functional and clinical significance of SALL4 in breast cancer. Tumor biology. 2016;37(9):11701–11709. doi: 10.1007/s13277-016-5150-7. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi D, Kuribayashi K, Tanaka M. Watanabe N. Overexpression of SALL4 in lung cancer and its importance in cell proliferation. Oncology reports. 2011;26(4):965–970. doi: 10.3892/or.2011.1374. [DOI] [PubMed] [Google Scholar]
- 25.Khales SA, Abbaszadegan MR, Abdollahi A, Raeisossadati R, Tousi F, Forghanifard MM. SALL4 as a new biomarker for early colorectal cancers. Journal of cancer research and clinical oncology. 2015;141(2):229–235. doi: 10.1007/s00432-014-1808-y. [DOI] [PubMed] [Google Scholar]
- 26.Sahebi R, Malakootian M, Balalaee B, Shahrvari A, Khoshnia M, Abbaszadehgan MR, Moradi A, Mowta SJ. Linc-ROR and its spliced variants 2 and 4 are significantly up-regulated in esophageal squamous cell carcinoma. Iranian journal of basic medical sciences. 2016;19(10):1131–1135. [PMC free article] [PubMed] [Google Scholar]
- 27.Xia F, Xiong Y, Li Q. Interaction of lincRNA ROR and p53/miR-145 correlates with lung cancer stem cell signatures. Journal of cellular biochemistry. 2017 doi: 10.1002/jcb.25960. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Peng Ya, Xu Z, Ge Bo, Xiang X, Zhang T, Gao Li, shi H, Wang C, Huang J. LncROR promotes bladder cancer cell proliferation, migration, and epithelial-mesenchymal transition. Cellular physiology and biochemistry. 2017;41(6):2399–2410. doi: 10.1159/000475910. [DOI] [PubMed] [Google Scholar]
- 29.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Yumours. 7th edition. UK:: John Wiley & Sons; 2011. [Google Scholar]
- 30.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Noian T, Wpfaffi MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clinical chemistry. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 31.Zou Z, Ding Q, Li P, Cao R, Shi L, Feng Y, Peng G. Overexpression of lincRNA-ROR predicts poor prognosis in patients with gastric cancer. International journal of clinical and experimental pathology. 2016;9(9):9467–9472. [Google Scholar]
- 32.Barooei R, Mahmoudian RA, Abbaszadegan MR, Mansouri A, Gholamin M. Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Medical oncology. 2015;32(8):217. doi: 10.1007/s12032-015-0653-4. [DOI] [PubMed] [Google Scholar]
- 33.Rivas-Ortiz CI, Vidal YL, Arrendondo Hernandez LR, Rojas GC. Genetic alterations in gastric cancer associated with Helicobacter pylori infection. Frontiers in medicine. 2017;4:47. doi: 10.3389/fmed.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veisani Y, Delpisheh A. Survival rate of gastric cancer in Iran; a systematic review and meta-analysis. Gastroenterology and hepatology from bed to bench. 2016;9(2):78–86. [PMC free article] [PubMed] [Google Scholar]
- 35.Raju KL, Augustine D, Rao RS, SVS , Haragannavar VC, Nambiar S, Prasad K, Awan KH, Patil S. Biomarkers in tumorigenesis using cancer cell lines: A systematic review. Asian Pacific journal of cancer prevention. 2017;18(9):2329–2337. doi: 10.22034/APJCP.2017.18.9.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng S, Yao J, Chen Y, Geng P, Zhang H, Ma X, Zhao J, Yu X. Expression and functional role of reprogramming-related long noncoding RNA (lincRNA-ROR) in glioma. Journal of molecular neuroscience . 2015;56(3):623–630. doi: 10.1007/s12031-014-0488-z. [DOI] [PubMed] [Google Scholar]
- 37.Gyvyte U, Kupcinskas J, Juzenas S, Inciuraite R, Poskiene L, Salteniene V, Link A, Fassan M, Franke A, Kupcinskas L, Skieceviciene J. Identification of long intergenic non-coding RNAs (lincRNAs) deregulated in gastrointestinal stromal tumors (GISTs) PloS one . 2018;13(12) doi: 10.1371/journal.pone.0209342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo C, Cao J, Peng R, Guo Q, Ye H, Wang P, Wang K, Song C. Functional variants in linc-ROR are associated with mRNA expression of LINC-ROR and breast cancer susceptibility. Scientific reports . 2018;8(1):4680. doi: 10.1038/s41598-018-22881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Liu F, Deng J, Cai X, Han J, Liu Qi. Long noncoding RNA ROR regulates proliferation, invasion, and stemness of gastric cancer stem cell. Cellular reprogramming . 2016;18(5):319–326. doi: 10.1089/cell.2016.0001. [DOI] [PubMed] [Google Scholar]
- 40.Rezaei M, Emadi-Baygi M, Hoffmann MJ, Schulz WA, Nikpour P. Altered expression of LINC-ROR in cancer cell lines and tissues. Tumor biology. 2016;37(2):1763–1769. doi: 10.1007/s13277-015-3933-x. [DOI] [PubMed] [Google Scholar]
- 41.Hiyama T, Tanaka S, Yoshihara M, Sasao S, Kose K, Shima H, Tuncel H, Ueno Y, Ito M, Kitadai Y, Yasui W, Haruma K, Chayama K. Chromosomal and microsatellite instability in sporadic gastric cancer. Journal of gastroenterology and hepatology. 2004;19(7):756–760. doi: 10.1111/j.1440-1746.2004.03369.x. [DOI] [PubMed] [Google Scholar]
- 42.Toraih EA, El-Wazir A, Hussein MH, Khashana MS, Matter A, Fawzy MS, Hosny S. Expression of long intergenic non-coding RNA, regulator of reprogramming, and its prognostic value in patients with glioblastoma. The International journal of biological markers. 2019;34(1):69–79. doi: 10.1177/1724600818814459. [DOI] [PubMed] [Google Scholar]
- 43.Fu Z, Li Guolin, Li Zhihua, Wang Y, Zhao Y, Zheng S, Ye H, Luo Y, Zhao x, Wei L, Liu Y, Lin Q, Zhou Q, Chen R. Endogenous miRNA Sponge LincRNA-ROR promotes proliferation, invasion and stem cell-like phenotype of pancreatic cancer cells. Cell death discovery . 2017 doi: 10.1038/cddiscovery.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao S, Wang P, Hua Y, Xi H, Meng Z, Liu T, Chen Z, Liu L. ROR functions as a ceRNA to regulate Nanog expression by sponging miR-145 and predicts poor prognosis in pancreatic cancer. Oncotarget . 2016;7(2):1608–1616. doi: 10.18632/oncotarget.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou P, Zhao y, Li z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell death and disease . 2014;5(6) doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou L, Tu J, Cheng F, Yang H, Yu F, Wang M, Lu J, Fan J, Zhou G. Long noncoding RNA ROR promotes breast cancer by regulating the TGF-β pathway. Cancer cell international . 2018;18(1) doi: 10.1186/s12935-018-0638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, L Cui, J Liu. Increased lincRNA ROR is association with poor prognosis for esophageal squamous cell carcinoma patients. Intertional journal of clinical experimental pathology. 2017;10:4654–4660. [Google Scholar]
- 48.Takahashi K, Irene K, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. Journal of cell science. 2014;127(Pt7):1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arunkumar G, Arunagiri KD, Magendhra Rao, Mayakannan M, Kanagaraj A, Vilvanathan V, Sundaramoorthy R, Kottayasamy SR, Ramamurthy R, Arasambattu KM. Expression profiling of long non-coding RNA identifies linc-RoR as a prognostic biomarker in oral cancer. Tumor biology . 2017;39(4):1010428317698366. doi: 10.1177/1010428317698366. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, Gao Q, Wang J, Zhang X, Liu K, Duan Z. Linc-RNA-RoR acts as a “sponge” against mediation of the differentiation of endometrial cancer stem cells by microRNA-145. Gynecologic oncology. 2014;133(2):333–339. doi: 10.1016/j.ygyno.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 51.Cheng , EC , Lin H. Repressing the repressor: a lincRNA as a MicroRNA sponge in embryonic stem cell self-renewal. Developmental cell. 2013;25(1):1–2. doi: 10.1016/j.devcel.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, Manos PD, Datta S, Lander ES, Schlaeger TM, Daley G, Rinn JL. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nature genetics. 2010;42(12):1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, YU L, Zhang Z, Pang L, Xu J, Jiang J, Liang W, Chai Y, Hou J, Li F. LINC-ROR promotes esophageal squamous cell carcinoma progression through the derepression of SOX9. Journal of experimental and clinical cancer research. 2017;36(1) doi: 10.1186/s13046-017-0658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan X, Zhang X, Zhang W, Liang W, Zhang P, Shi H, Zhang B, Shao M, Yan Y, Qian H, Xu W. SALL4 promotes gastric cancer progression through activating CD44 expression. Oncogenesis. 2016;5(11) doi: 10.1038/oncsis.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osman MA, Bloom GS, Tagoe EA. Helicobacter pylori‐induced alteration of epithelial cell signaling and polarity: A possible mechanism of gastric carcinoma etiology and disparity. Cytoskeleton. 2013;70(7):349–359. doi: 10.1002/cm.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou X, Chen H, Zhu L, Hao B, Zhang W, Hua J, Gu H, Jin W, Zhang G. Helicobacter pylori infection related long noncoding RNA (lncRNA) AF147447 inhibits gastric cancer proliferation and invasion by targeting MUC2 and up-regulating miR-34c. Oncotarget. 2016;7(50):82770–82782. doi: 10.18632/oncotarget.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amieva M. and RM Peek Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150(1):64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sue S, Shibata W, Maeda S. Helicobacter pylori-induced signaling pathways contribute to intestinal metaplasia and gastric carcinogenesis. Biomed research international. 2015;2015:737621. doi: 10.1155/2015/737621. [DOI] [PMC free article] [PubMed] [Google Scholar]