Abstract
Background:
Levan or fructan, a polysaccharide of fructose, is widely used in various commercial industries. Levan could be produced by many organisms, including plants and bacteria. The cloning of the gene from Bacillus licheniformis, which expressed levansucrase in Escherichia coli host, was carried out successfully. In the present study, we performed the in vitro production of levan and analyzed its potential application as antibacterial and antioxidant agents.
Methods:
In vitro levan production catalyzed by heterologous-expressed levansucrase Lsbl-bk1 and Lsbl-bk2 was optimized with BW design. The antibacterial activity of the produced levan was carried out using agar well diffusion method, while its antioxidant activity was tested by free radical scavenging assays.
Results:
The optimum conditions for levan production were observed at 36 °C and pH 7 in 12% (w/v) sucrose for levansucrase Lsbl-bk1, while the optimum catalysis of levansucrase Lsbl-bk2 was obtained at 32 oC and pH 8 in the same sucrose concentration. The in vitro synthesized levan showed an antibacterial activity within a concentration range of 10-20% (w/v) against Staphylococcus aureus, E. coli, and Pseudomonas aeruginosa. The same levan was also able to inhibit the DPPH radical scavenging activity with the antioxidant strength of 75% compared to ascorbic acid inhibition.
Conclusion:
Our study, therefore, shows that the optimized heterologous expression of levansucrases encoded by Lsbl-bk1 and Lsbl-bk2 could open the way for industrial levan production as an antibacterial and antioxidant agent.
Key Words: Antioxidants, Fructans, In vitro technique, Levan
INTRODUCTION
Levansucrase, an enzyme belonging to the fructosyltransferase class, is secreted by several organisms, including plants and bacteria, and is widely distributed in both Gram-positive and Gram-negative bacteria[1]. Levansucrase from Gram-positive bacteria is secreted as a specific extracellular enzyme by two-step mechanisms as the enzyme possesses signal peptides cleaved after the carboxyl-terminal alanine during its export from the cytoplasmic to the outside of the cell[2-4]. In contrast, levansucrase from Gram-negative bacteria is fully secreted into its medium by a signal peptide-independent pathway involving the formation of an intermediate N-terminal protected amino acid in the periplasmic loop[5,6]. Levansucrase (EC 2.4.1.10) cleaves the β-1,2-glycosidic bond of sucrose and transfers the resulted fructosyl unit to numerous acceptor molecules, such as the short-chain acyl alcohols or mono- and di-saccharides[7], as well as catalyzes the conversion of sucrose to fructan polymer. Levansucrase structure is supposed to be cell-wall anchoring motifs in their C-terminal domains, which are involved in the proteolysis[8,9].
Levan is one of the fructose polymers (fructan) linked by the β-2,6-fructofuranosidic bond, synthesized in transfructosylation reaction, and catalyzed by levansucrase using sucrose as a substrate[10,11]. Fructan, as a product of fructosyltransferase activity, can be obtained from various sources with different chemical compositions, molecular weights, and structures[12,13]. Levan is naturally synthesized by several flowering plant species, some bacteria, and fungi[14,15]. Due to the unique characteristics, levans are being used in various industries, including food, cosmetic, and medicinal products. Levan is nontoxic and highly soluble in water and possesses low viscosity, strong adhesivity, and film-forming ability, as well as compatiblility with salts and surfactants, heatstability, acid and alkali adaptability, biodegradablility, and biocompatiblility to other substances[16-18]. In comparison to the levan product in plants, those synthesized by bacteria often have a higher degree of polymerization[19]. Bacterial levans contains around 103-104 fructosyl units, whereas plant levans consist of about 10-200 fructosyl units[20].
As the microbial levansucrase generates more branched levans, the levan products have larger molecular weight (~2-100 × 106 Da) and more extensively branched when compared to levans from plants (2000-3300 Da)[16,21,22]. Furthermore, plants make their levans in vacuoles, while bacteria produce them extracellularly[23]. Levan, commonly found as a microbial exopolysaccharide, is produced by hydrolysis and transfructosylating activities of levansucrase[24]. Production of microbial levansucrase is greatly affected by fermentation parameters, such as sucrose concentration, nutrients sources, pH, temperature, time of cultivation, agitation, and aerations rates[19,25,26]. Currently, optimization of levan productions is a very interesting field of research and is important in developing an efficient and cost-effective biopolymer production process[24]. Since levan is a non-toxic polymer and can easily be synthesized in vitro, many researchers have focused on levan production using bacterial native extracellular levansucrase[27]. In addition, the data on levansucrase gene and protein structures are available[19]. However, levan production using recombinant levansucrase is rarely been reported.
The wild types Bacillus licheniformis BK1 and BK2 are Gram-positive halophilic bacteria, which were isolated from Bledug Kuwu mud crater, Central Java, Indonesia. In our previous study, we successfully constructed the expression vectors pET-lsbl-bk1 and pET-lsbl-bk2 for high-level expression of levansucrase production[28]. In the present study, we aimed to investigate and optimize levan production catalyzed by recombinant levansucrase from B. licheniformis BK1 and BK2 expressed by pET-lsbl-bk1 and pET-lsbl-bk2 constructs in Escherichia coli host. Herein, we report the optimum condition of levan production by two recombinant enzymes. The potential uses of levan, as an antioxidant and antibacterial agent, were also studied.
MATERIALS AND METHODS
Strains, plasmids, and culture condition
E. coli BL21 (DE3) pLysS (our collection), as the expression host and plasmids pET-30a(+) (Invitrogen, USA), pET-lsbl-bk1 (GenBank: MF774877.1), and pET-lsbl-bk2 (GenBank: MF774878.1) as expression vectors were used in recombinant production of target enzyme Staphylococcus aureus, E. coli, and Pseudomonas aeruginosa (obtained from Microbiology Study Program, Universitas Hasanuddin, Indonesia) were used in antibacterial assays. The E. coli strains were grown in LB medium containing 1% (w/v) tryptone (Liofilchem, Italy), 0.5% (w/v) yeast extract (Liofilchem), and 1% (w/v) NaCl (Merck, Germany). Kanamycin (Bio Basic, USA) was u for selecting recombinant E. coli cells. Chemicals employed in this study were IPTG (Thermo Fisher Scientific, USA), 3,5-dinitrosalicyclic acid (Sigma Aldrich, USA), sucrose, Na2HPO4, NaH2PO4.H2O, ethanol, phenol, H2SO4 97%, DPPH, and ascorbic acid (Merck).
High level in vitro synthesis of levan
The in vitro levan synthesis was developed in two-stage processes. The first process was the heterologous overexpression of levansucrase gene cloned (pET-lsbl-bk1 and pET-lsbl-bk2) in E. coli BL21 (DE3) pLysS host cell to produce levansucrase, which its conditions had been optimized in our previous research[29]. The second process was the catalytic reaction in a sucrose medium employing the produced levansucrase to enhance levan production. The optimizations of in vitro levan were performed using the RSM approach[29,30].
Heterologous expression of B. licheniformis levansucrase
A single colony of E. coli BL21 (DE3) pLysS cells carrying pET-lsbl-bk1 was inoculated into a 5-mL LB medium containing 50 μg/mL of kanamycin and 0.6% (w/v) NaCl. The culture was grown at 37 °C overnight. Then 2 mL of this overnight culture was inoculated into 100 mL of a fresh LB medium containing kanamycin and NaCl, incubated at 37 °C with aeration until the OD600 reached 0.6-1. The expression of levansucrase was induced by IPTG (0.6-mM), and the culture was further incubated at 42 °C for 4 hours. Different conditions were performed for pET-lsbl-bk2, including 1.1% (w/v) NaCl, 0.7 mM of IPTG, and 40 °C incubation temperature. The expressed enzyme is known to be secreted into the medium[28]. Therefore, the supernatant of the culture, i.e. the levansucrase crude extract, was collected by centrifugation at 6,082 ×g at 4 °C for 15 min. The obtained crude extracts were used in experiments to optimize the in vitro levan production by RSM.
Experimental design
The RSM was applied to search for the optimum condition of levan production catalyzed by levansucrase Lsbl-bk2[29]. BW design was also used to optimize the in vitro levan production catalyzed by Lsbl-bk1. BW design and CCD are experimentally efficient technique for our study to develop a predictive model using experimental variables on in vitro levan production. In this design, the following three parameters were applied: the temperature of incubation (X1) between 20 °C to 54 °C, pH (X2) from 4 to 10, and sucrose concentration (X3) from 5% to 20% (w/v), as presented in Tables 1 and 2. The design was made up of BW full 23 factorial with eight cube points, augmented with five center points and six-star points. Accordingly, the total runs included 20 experiments. Levan concentration (Y) was considered as a dependent (response) variable. To estimate the optimal point and generate the contour plots, a second-order polynomial function was fitted to the experimental results. The predicted values of the model were validated by experiments performed in five replicates and compared with the model predicted values. The Minitab statistical software (version 18) was used for the experimental designs and regression analysis of the experimental data.
Table 1.
The CCD experimental design for in vitro levan production catalyzed by Lsbl-bk1
| Parameters |
Levels
|
||||
|---|---|---|---|---|---|
| -1,682 | -1 | 0 | +1 | +1,682 | |
| Temperature (°C) | 20.0 | 27.5 | 37.0 | 47.4 | 54.0 |
| Sucrose concentration (% w/v) | 5.0 | 8.0 | 12.5 | 16.9 | 20.0 |
| pH | 4.0 | 5.2 | 7.0 | 8.7 | 10.0 |
Table 2.
BW 23 factorial experimental results of in vitro levan production
| Run |
Temp
(°C) |
pH |
Sucrose concentration
(% w/v) |
Observed levan concentration
(mg/mL) |
|---|---|---|---|---|
| 1 | 54.0 | 7.0 | 12.5 | 58 |
| 2 | 37.0 | 7.0 | 12.5 | 96 |
| 3 | 37.0 | 4.0 | 12.5 | 63 |
| 4 | 47.1 | 8.8 | 17.0 | 61 |
| 5 | 37.0 | 7.0 | 12.5 | 96 |
| 6 | 37.0 | 7.0 | 5.0 | 76 |
| 7 | 26.9 | 5.2 | 17.0 | 61 |
| 8 | 37.0 | 7.0 | 12.5 | 97 |
| 9 | 47.1 | 5.2 | 8.0 | 64 |
| 10 | 26.9 | 5.2 | 8.0 | 64 |
| 11 | 26.9 | 8.8 | 8.0 | 64 |
| 12 | 37.0 | 10.0 | 12.5 | 51 |
| 13 | 47.1 | 8.8 | 8.0 | 61 |
| 14 | 37.0 | 7.0 | 12.5 | 96 |
| 15 | 20.0 | 7.0 | 12.5 | 64 |
| 16 | 47.1 | 5.2 | 17.0 | 63 |
| 17 | 37.0 | 7.0 | 20.0 | 60 |
| 18 | 37.0 | 7.0 | 12.5 | 96 |
| 19 | 26.9 | 8.8 | 17.0 | 63 |
| 20 | 37.0 | 7.0 | 12.5 | 96 |
Temp, temperature
In vitro levan production and determination of levan concentration
The in vitro levan synthesis was conducted with various conditions (Table 1) by mixing 8 mL of levansucrase fresh crude extract with 8 mL of phosphate buffer containing sucrose substrate, filtered through a 0.45-μm filter before loading with enzyme crude extract. The reaction mixtures were incubated at the desired temperature for 24 hours, stopped by heating at 80-100 °C for 5 min, and cooled down to room temperature. The suspensions were then centrifuged at 6,082 ×g at 25 °C for 10 min[30]. The concentration of the produced levan in the supernatants was determined by colorimetric phenolic-sulfuric method at 490 nm using fructose as a standard via determining the amount of sugar produced from sucrose hydrolysis by levansucrase[31,32]. Briefly, 450 µL of levan solution was transferred into a tube, and 50 µL of 5% (w/v) phenol solution was added. The mixture was then vortexed to homogenize. In the next step, 500 µL of concentrated sulfuric acid was added rapidly and carefully. The tube was allowed to stand on ice bath for 5 min shaken, and placed in a 90 °C water bath for 5 min to stop the reaction. The mixture was cooled down to room temperature before reading the absorbance of the final yellow-orange color product at 490 nm.
Levan extraction
The levan was extracted from the supernatant through the precipitation by adding three volumes of cold ethanol (3:1 v/v) at 4 °C overnight. The precipitate was recovered by centrifugation at 6,082 ×g at 4 °C for 20 min. To remove impurities, the precipitate was washed twice with ethanol 96% and once with deionized water, then freeze-dried for about 2 to 4 hours. The obtained levan was used for further analysis, such as morphological characterization, structure identification, thermal stability determination, and bioactivity.
Levan analysis
Characterization of levan
The morphology of the solid synthesized levan was analyzed by SEM (JEOL JSM 6510 LA, Japan), and its structure was characterized using FTIR (Shimadzu IR Prestige-21, Japan) and NMR spectroscopy (NMR spectrometer, Agilent 500 MHZ, USA) to obtain 1H NMR and 13C-NMR spectra. Besides, the characterization of levan thermal stability was determined under a nitrogen atmosphere using thermal gravimetric analysis (TG/DTG, Hitachi STA7300, USA).
Antioxidant activity of levan
Antioxidant activity of levan was tested by DPPH free radicals scavenging assays[33,34] using ascorbic acid as a standard. A fresh solution of DPPH was prepared by dissolving solid DPPH in ethanol absolute to obtain 0.5 mM of DPPH concentration. Ascorbic acid solutions were prepared in various concentrations (30, 120, 240, 420, 600, 960, and 1200 μg/mL), and levan solutions were prepared at the concentrations of 30, 250, 500, 1250, 2500, 3500, 4500, and 5000 μg/mL. Afterward, 250 μL of DPPH solution (0.5 mM) was added to 1.0 mL of the tested solution, which was then shaken vigorously to become homogeneous and incubated in the dark at room temperature for 30 minutes. The absorbance was read at 514 nm. Ethanol was utilized as a control solution. The percentage of inhibition was calculated using the following formula:
(Eq. 1)
The concentration of the sample with IC50 was calculated from the graph of % inhibition vs. sample concentration.
Antibacterial activity of levan
The antibacterial activity of levan was assessed against three species, namely E. coli, S. aureus, and P. aeruginosa. The test was carried out using agar well diffusion method utilizing a metal cup with a diameter of 6 mm and height of 10 mm. In this experiment, 0.5% (b/v) NA supplemented with 0.3% (b/v) beef extract and 0.5% (b/v) peptone was used. Also, 1 mL of the fresh liquid culture containing about 105-106 CFU was spread onto the NA solid medium, where the well was made using a metal cup, which was then filled with 0.2 mL levan solution (10% and 20% w/v). The system was incubated at 37 °C for 24 h, and the antimicrobial activity was detected by measuring the inhibitory clear zones around the well[35,36].
RESULTS
Optimization of high level in vitro levan synthesis
The in vitro synthesis of levan was carried out by levansucrase utilizing sucrose as a substrate. The conditions of in vitro levan production were optimized to achieve high efficiency and high yield of levan through RSM. Three parameters, the temperature of incubation (X1), pH of the medium (X2), and sucrose concentration (X3), influencing the in vitro levan production catalyzed by Lsbl-bk1 were treated as independent variables. The values of the obtained response under different experimental conditions of BW 23 factorial CCD are listed in Table 2. Optimization of in vitro levan production catalyzed by Lsbl-bk2 was not described as it had been reported in our previous study[29]. The results of CCD were fitted into second-order polynomial equation (equation 2) for the prediction of response on the basis of coded value.
(Eq. 2)
Equation 2 reveals the relationship between levan concentration, as a dependent variable, obtained from linear or quadratic of the three independent variables. Model significance and role of each variable were evaluated using ANOVA, and the results were presented in Table 3. The mathematical model obtained (Equation 2) resulting p < 0.05, indicated statistically significant of this model. The ANOVA of the regression model gave both R2 and adjusted R2 values close to 1.00, further confirming that the model was highly significant to predict the response. Besides, the quadratic model was statistically significant for the description of levan yield where the p value is 0.000, which is less than 0.05. It can be seen from Table 3 that the linear and quadratic terms of regression coefficients on sucrose concentration (X3) were significantly influenced by the in vitro levan production because the probability value is smaller than 0.05.
Table 3.
Analysis of variance for in vitro levan production catalyzed by Lsbl-bk1 (Y) as the function of temperature (X1), pH (X2), and sucrose concentration (X3)
| Source | Sum of squares | Degrees of freedom | Mean square | F-value | p value |
|---|---|---|---|---|---|
| Model | 5018.78 | 9 | 557.64 | 51.42 | 0.000 |
| Linear | 121.89 | 3 | 40.63 | 3.75 | 0.049 |
| X 1 | 9.33 | 1 | 9.33 | 0.86 | 0.375 |
| X 2 | 34.14 | 1 | 34.14 | 3.15 | 0.106 |
| X 3 | 78.43 | 1 | 78.43 | 7.23 | 0.023 |
| Square | 4889.84 | 3 | 1629.95 | 150.31 | 0.000 |
| X 1 X 1 | 2017.58 | 1 | 2017.58 | 186.06 | 0.000 |
| X 2 X 2 | 2558.31 | 1 | 2558.31 | 235.92 | 0.000 |
| X 3 X 3 | 1245.50 | 1 | 1245.50 | 114.86 | 0.000 |
| 2-way interaction | 7.05 | 3 | 2.35 | 0.22 | 0.883 |
| X1X2 | 4.16 | 1 | 4.16 | 0.38 | 0.550 |
| X1X3 | 1.09 | 1 | 1.09 | 0.10 | 0.758 |
| X2X3 | 1.80 | 1 | 1.80 | 0.17 | 0.692 |
| Error | 108.44 | 10 | 10.84 | ||
| Lack-of-fit | 107.82 | 5 | 21.56 | 174.01 | 0.000 |
| Pure error | 0.62 | 5 | 0.12 | ||
| Total | 5127.22 | 19 |
R 2 = 0.9789; R2 (adjusted) = 0.9598
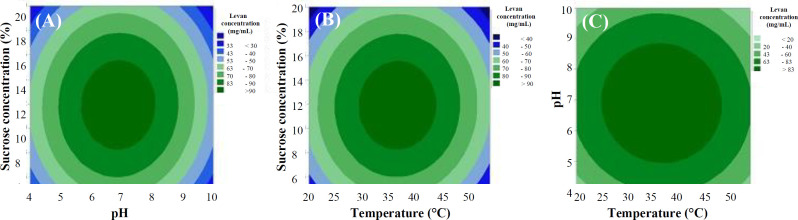
The three-dimensional response surface described by the contour plots model is presented in Figure 1. The highest levan concentration is predicted to be at 36 oC, 96.268 mg/mL levan. Five experiment replicates were then performed under this predicted optimum condition, and the obtained levan concentration was 96.117 mg/mL on average.
Fig. 1.
Contour plots predicting in vitro levan production as the function of pH and sucrose concentration (A), temperature and sucrose concentration (B), and temperature and pH (C).
Analysis of levan structure
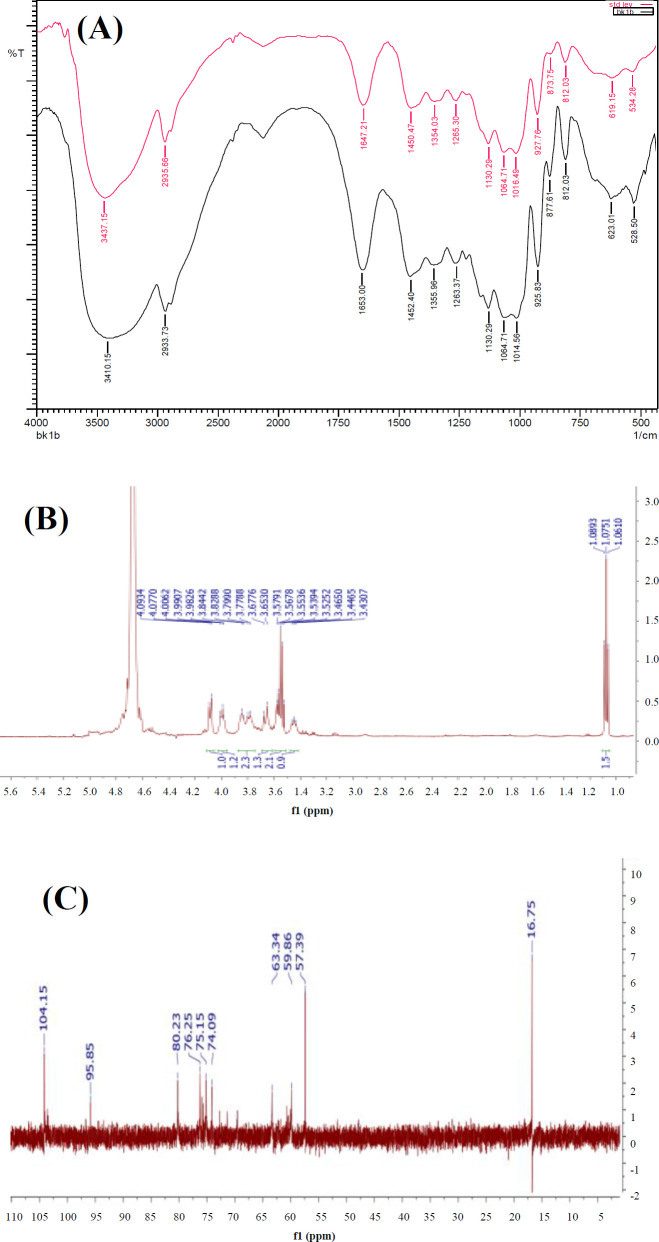
The structure of levan was verified by FTIR and NMR, as presented in Figure 2. In FTIR, absorption characteristic of levan (Fig. 2A) was attributed by the O-H stretching at around 3447-3414 cm-1, C-H stretching at around 2931.80 cm-1, and C=O stretching at 1658.78 cm-1. The fingerprint pH 7, and 12% (w/v) of sucrose, which produced spectrum between 1271.01 cm-1 and 925.83 cm-1 corresponded to carbohydrate molecules and related to overlapping signals of glycosidic (C-O-C) stretching. The levan spectra, 1H-NMR, and 13C-NMR can be seen in Figures 2B and 2C. The levan 1H-NMR spectrum exhibited the signal characteristic between 3.4 and 4.3 ppm, associated with sugar protons (Fig. 2B). The six broad resonance signals of levan 13C-NMR spectrum at 104.1 (C2), 80.2 (C5), 76.2 (C3), 75.1 (C4), 63.3 (C6), and 59.8 (C1) ppm corresponded to the carbon of β-fructofuranose, confirming that levan has successfully been synthesized.
Fig. 2.
Analysis of the produced levan catalyzed by Lsbl-bk1: (A) FTIR spectrum of levan (black line) compared with levan standard from E. herbicola (red line), (B) levan 1H-NMR spectrum, and (C) levan 13C-NMR spectrum
Morphology analysis
The in vitro levan product catalyzed by Lsbl-bk1 and Lsbl-bk2 were extracted with ethanol. The resulted levan showed different solid appearances, and then SEM was examined. The product of Lsbl-bk1 appeared as a white granule (Fig. 3A) with irregular circular pores, while the Lsbl-bk2 product showed yellow solid and a rather sticky texture (Fig. 3B), with a sheet-shaped solid without any pores. These morphology differences might be due to different synthesis conditions, particularly the pH, which would influence the morphological characteristic of the synthesis polymer[29].
Fig. 3.
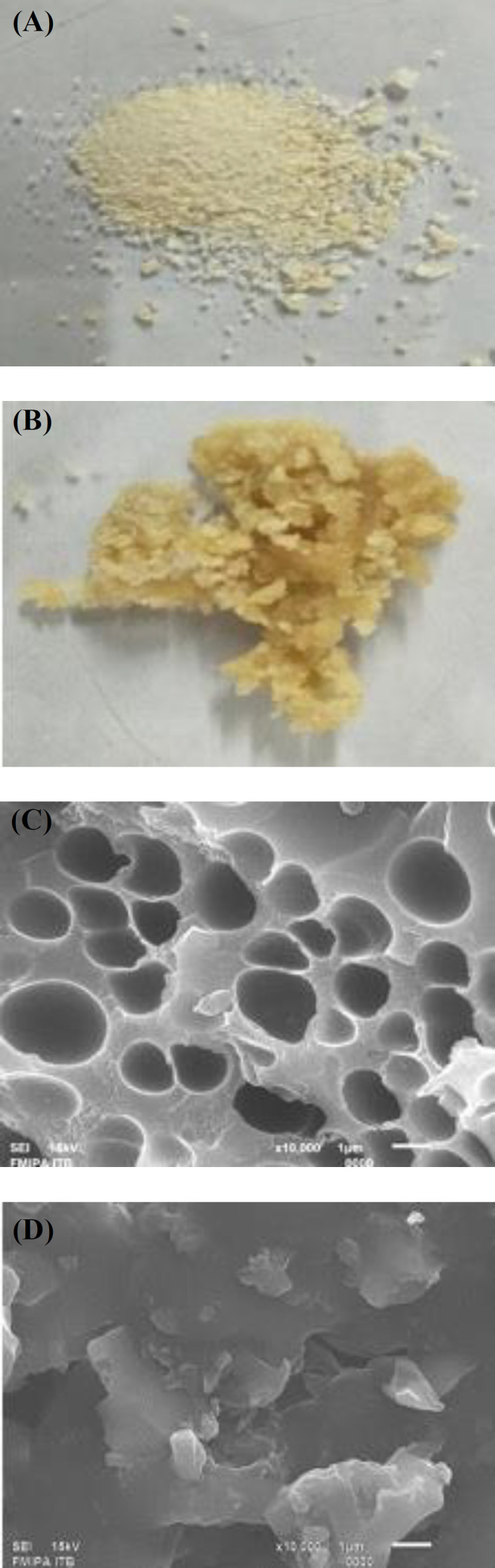
The morphological characteristic of solid produced levan catalyzed by (A) Lsbl-bk1 and (B) Lsbl-bk2 and the SEM image of levan catalyzed by (C) Lsbl-bk1 and (D) Lsbl-bk2
TG and DTG analysis
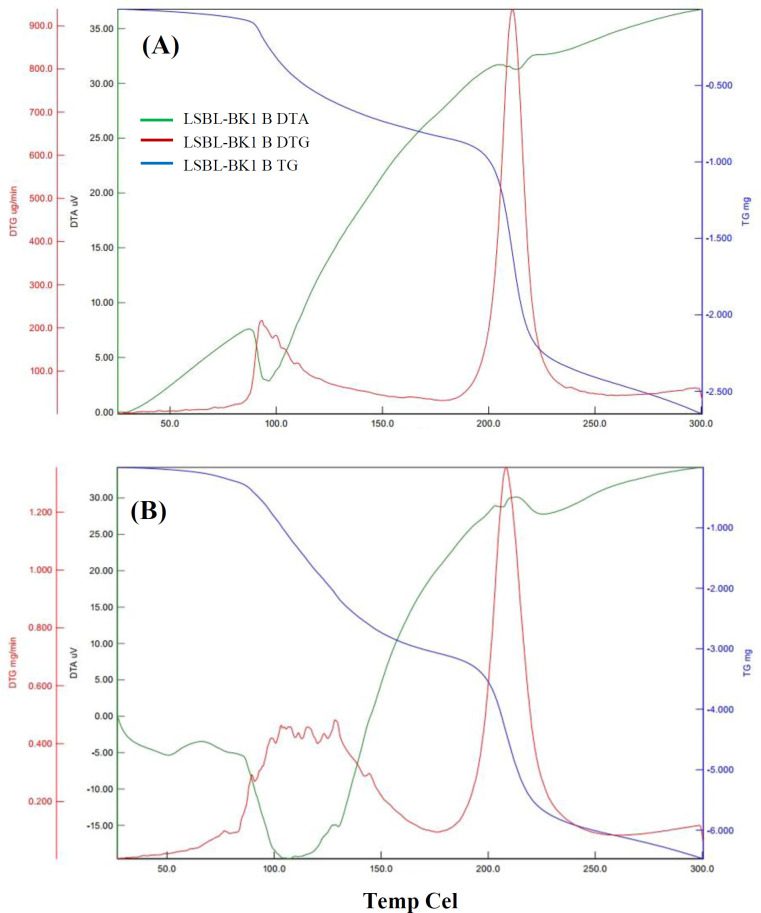
TG analysis and DTG profiles were used to investigate the thermal stability of the produced levan. The obtained thermograms of the in vitro produced levan catalyzed by Lsbl-bk1 and Lsbl-bk2 are presented in Figure 4, showing three stages of levan mass reduction at 0-100 °C, 100-200 °C, and 200-300 °C. The first region, 0-100 °C, is attributed to water and ethanol evaporation. Both levans attained their weight in the second region, 100-200 °C, indicating its thermal stability. The third region, 200-300 °C, which showed around 50% of mass reduction, might be corresponded to levan degradation. The DTG curves demonstrate that the main peak of levan degradation occurred at 211 °C and 208 °C for levan of Lsbl-bk1 and Lsbl-bk2, respectively.
Fig. 4.
TG and DTG curves of the produced levan catalyzed by (A) Lsbl-bk1 and (B) Lsbl-bk2. Blue, red, and green lines show TG, DTG, and DTA curves, respectively
Antioxidant activity
The antioxidant activity of the produced levan catalyzed by Lsbl-bk1 and Lsbl-bk2 was evaluated by DPPH free radical scavenging method. The results exhibited that the percentage activity of both levans is at the concentration of 30-1200 μg/mL, which could inhibit around 55% DPPH activity compared to ascorbic acid, which effectively inhibited up to 76% at the same concentration (Fig. 5). The scavenging activities of both levans directly increased to 75% if the concentration increased up to 5 mg/mL.
Fig. 5.
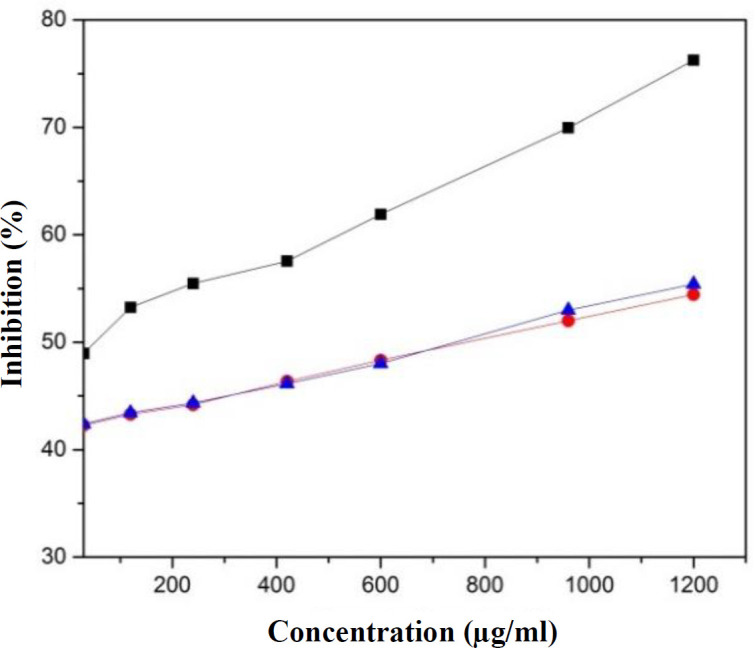
Antioxidant activities of ascorbic acid (black rectangle), levan Lsbl-bk1 (red circle) and levan Lsbl-bk2 (blue triangle)
Antibacterial activity
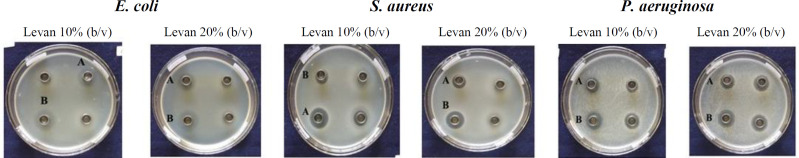
Antibacterial activity of the levan products was studied against E. coli, S. aureus, and P. aeruginosa. The results are depicted in Figure 6. The antibacterial activities were observed as a clear zone around the cup filled with levan. It can be seen in the Figure that both levans possessed antibacterial activity. Levan produced by Lsbl-bk1 catalysis showed the highest activity against S. aureus (12-16 mm), whereas those catalyzed by Lsbl-bk2 had the highest activity against E. coli (15-16 mm) and P. aeruginosa (14-16 mm). The appearance of the inhibition zone was not broad and not strong enough to necessarily kill the bacteria, indicating that levan owes a bacteriostatic effect on these tested bacteria.
Fig. 6.
Growth inhibitions of E. coli, S. aureus, and P. aeruginosa in levan containing nutrient agar. A and B show produced levan catalyzed by Lsbl-bk1 and Lsbl-bk2, respectively
DISCUSSION
BW design has been proved to be an efficient technique for optimization of the in vitro levan production catalyzed by levansucrase Lsbl-bk1, using three variables (temperature, pH, and sucrose concentration). The interaction between the three tested independent variables revealed no significant effect on in vitro levan production, as indicated by the p>0.05 (Table 3). The same result was also achieved from the in vitro levan production catalyzed by levansucrase Lsbl-bk2[29]. It is of advantageous that the three studied variables are non-interacting one to the other; hence, the changes of one variable would minimally affect the others.
Our study indicated that the optimum condition for the in vitro levan synthesis catalyzed by Lsbl-bk1 was at 36oC, pH 7, and 12% (w/v) sucrose, while this condition for Lsbl-bk2 was at 32oC, pH 8, and 12% (w/v) sucrose[29]. However, the in vitro levan synthesis by both levansucrases gave a similar high yield, which was about 95 mg/mL. Lu et al.[30] reported that the optimum condition for in vitro levan production catalyzed by recombinant levansucrase from B. licheniformis 8-37-0-1 occurred at 0.8 M sucrose concentration (equivalent to 14% w/v) in pH 6.5 at 40 °C for 24 hours with a yield of 7.1 mg/mL levan. These dissimilarities might be due to the different environmental origins of B. licheniformis 8-37-0-1, which was isolated from the hydrothermal vent of Panarea Island (Italy)[37], but our strain was obtained from Bledug Kuwu mud crater, Central Java, Indonesia. The in vitro production of levan in our research appeared to be more efficient, which resulted in higher levan yield at lower temperatures and lfor of in vitro levan production is more applicable and more feasible to industries.
The FTIR spectrum of levan catalyzed by Lsbl-bk1 was similar to that of levan from Erwinia herbicola[38] and consistent with the spectrum of levan obtained by the catalysis of Lsbl-bk2[29]. 1H-NMR and 13C-NMR spectra showed similar signals with the levan produced by Acetobacter xylinum NCIM2526 and Brenneria goodwinii[39,40]. The obtained FTIR and NMR patterns indicated that levan produced by the recombinant of B. licheniformis levansucrase represented β-(2,6)-levan polysaccharide.
The morphological properties of both synthetic levans were different due to variations in the synthesis of optimum conditions, especially pH. Accordingly, the synthesis of pH-responsive biopolymer was beneficial to develop preferred materials for drug delivery or other industrial desired materials[41]. Moreover, the thermal stability properties of levan produced were degraded at lower temperatures (208 oC and 211 oC) compared to other levans, such as levan produced by Zymomonas mobilis and Halomonas sp. AAD6 that degraded at 217oC and 253 °C, respectively[42,43].
The bioactivity of both levans has been tested in our study as antimicrobial and antioxidant agents. The antioxidant capacity of our levan was similar to that of Srikanth et al.’s[16] and Benattouche et al.’s[44] studies, in which the DPPH-scavenging effect increased with the elevation of levan concentration as exopoly-saccharides. The scavenging ability of levan on DPPH radical might be due to the ability of hydroxyl group to donate its electron as hydrogen radical[27,34,45]. These results suggest that the antioxidant activity of both levans is relatively lower compared to ascorbic acid. However, it would be possible and might be beneficial to be used in biomedicine industries. Aside from that, both Lsbl-bk1 and Lsbl-bk2 levan products, as exoploysaccharides, have beneficial potency as an antibacterial agent. Previous studies have shown that exopolysachharides with antimicrobial activity could inhibit the growth of S. aureus, E. coli, and P. aeruginosa with an inhibition zone diameter about 15-20 mm[44,46]. It has also been assumed that exopolysaccharide levans can disrupt the bacterial cell wall integrity by blocking nutrients input[36,46].
In summary, our preliminary research indicates that the produced levan possessed antioxidant and antibacterial activities; therefore, it is the potential to be used in food and pharmaceutical industries.
ACKNOWLEDGEMENTS
This research was supported by the Doctoral Research Grant from the Indonesian Ministry of Research and Technology with the contract number of 123/SP2H/PTNBH/DPRM 2018 and P3MI.
CONFLICT OF INTEREST.
None declared.
References
- 1.Malik A. Molecular cloning and in silico characterization of fructansucrase gene from Weissella confusa MBFCNC-2(1) isolated from local beverage. Asia-Pacific journal of molecular biology and biotechnology. 2012;20(1):33–42. [Google Scholar]
- 2.Tjalsma H, Bolhius A, Jongbloed DH, Bron S, van Dijil JM. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiology and molecular biology reviews. 2000;64(3):515–547. doi: 10.1128/mmbr.64.3.515-547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanigan-Gerdes S, Briceno G, Dooley AN, Faull KF, Lazazzera BA. Identification of residues important for cleavage of the extracellular signal peptide CSF of Bacillus subtilis from its precursor protein. Journal of bacteriology. 2008;190(20):6668–6675. doi: 10.1128/JB.00910-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonen M, Palva I. Protein secretion in Bacillus species. Microbiology reviews. 1993;57(1):109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakob F, Meißner D, Vogel RF. Comparison of novel GH 68 levansucrases of levan-overproducing Gluconobacter species. Acetic acid bacteria. 2012;1 [Google Scholar]
- 6.Van Hijum SA, Krajl S, Ozimek LK, Dijkhuizen L, van Geel-Schutten IG. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiology and molecular biology reviews. 2006;70(1):157–176. doi: 10.1128/MMBR.70.1.157-176.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versluys M, Kirtel O, Öner ET, den Ende WV. The fructant syndrome: evolutionary aspects and common themes among plants and microbes. Plant, cell and environment. 2018;41(1):16–38. doi: 10.1111/pce.13070. [DOI] [PubMed] [Google Scholar]
- 8.Desvaux M, Candela T, Serror P. Surfaceome and proteosurfaceome in parietal modern bacteria: focus on protein cell-surface display. Frontiers in microbiology. 2018;9 doi: 10.3389/fmicb.2018.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez L, Arrieta J, Menendez C, Vazquez R, Coego A, Suarez V, Selman G, Petit-Glatron MF, Chambert R. Isolation and enzymic properties of levansucrase secreted by Acetobacter diazotrophicus SRT4, a bacterium associated with sugar cane. Biochemical journal. 1995;309(Pt 1):113–118. doi: 10.1042/bj3090113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu FC, Chou SZ, Shih IL. Factors affecting the production and molecular weight of levan of Bacillus subtilis natto in batch and fed-batch culture in fermenter. Journal of the Taiwan institute chemical engineers. 2013;44(6):846–853. [Google Scholar]
- 11.Seo JW, Song KB, Jang KH, Kim CH, Jung BH, Rhee SK. Molecular cloning of a gene encoding the thermoactive levansucrase from Rahnella aquatilis and its growth phase-dependent expression in Escherichia coli. Journal of biotechnology. 2000;81(1):63–72. doi: 10.1016/s0168-1656(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 12.Zong A, Cao H, Wang F. Anticancer polysaccharides from natural resources : A review of recent research. Carbohydrate polymers. 2012;90(4):1395–1410. doi: 10.1016/j.carbpol.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Meyers MA, Chen PY, Lin AY, Seki Y. Biological materials: Structure and mechanical properties. Progress in materials science. 2008;53(1):1–206. doi: 10.1016/j.jmbbm.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Joaquim EO, Hayashi AH, Torres LMB, Figueiredo-Ribeiro RCL, Shiomi N, Sousa FS, Lago JHG, Carvalho MAM. Chemical structure and localization of levan, the predominant fructant type in underground systems of Gomphrenamarginata (Amaranthaceae) Frontiers in plant science. 2018;9 doi: 10.3389/fpls.2018.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Álvaro-benito M, Sainz-Polo MA, González-Pérez D, González B, Plou FJ, Fernández-Lobato M, Sanz-Aparicio J. Structural and kinetic insights reveal that the amino acid pair Gln-228/Asn-254 modulates the transfructosylating specificity of Schwanniomycesoccidentalis β-fructofuranosidase, an enzyme that produces prebiotics. Journal of biological chemistry. 2012;287(23):19674–19686. doi: 10.1074/jbc.M112.355503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srikanth R, Reddy CHSSS, Siddartha G, Ramaiah MJ, Uppuluri KB. Review on production, characterization and applications of microbial levan. Carbohydrate Polymers. 2015;120:102–114. doi: 10.1016/j.carbpol.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Divya JM, Sugumaran KR. Fermentation parameters and condition affecting levan production and its applications. Journal of chemical and pharmaceutical research. 2015;7(2):861–865. [Google Scholar]
- 18.Szwengiel A, Wiesner M. Effect of metal ions on levan synthesis ef fi ciency and its parameters by levansucrase from Bacillus subtilis. International journal of biological macromolecules. 2019;128:237–243. doi: 10.1016/j.ijbiomac.2019.01.155. [DOI] [PubMed] [Google Scholar]
- 19.Öner ET, Hernández L, Combie J. Review of levan polysaccharide: from a century experiences to future prospects. Biotecnology advances. 2016;34(5):827–844. doi: 10.1016/j.biotechadv.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Ritsema T, Smeekens S. Furctans: beneficial for plants and human. Current opinion in plant biology. 2003;6(3):223–230. doi: 10.1016/s1369-5266(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 21.Barone JR, Medynets M. Thermally processed levan polymers. Carbohydrate polymers. 2007;69(3):554–561. [Google Scholar]
- 22.Arvidson SA, Rinehart BT, Maria FG. Concentration regimes of solutions of levan polysaccharide from Bacillus sp. Carbohydr Polym. 2006;65:144–149. [Google Scholar]
- 23.Velázquez-Hernández ML, Cha MP. Microbial fructosyltransferases and the role of fructans. Journal of applied microbiology . 2009;106:1763–1778. doi: 10.1111/j.1365-2672.2008.04120.x. [DOI] [PubMed] [Google Scholar]
- 24.Belghith KS, Dahech I, Belghith H, Mejdoub H. Microbial production of levansucrase for synthesis of fructooligosaccharides and levan. International journal of biological macromolecules. 2012;50(2):451–458. doi: 10.1016/j.ijbiomac.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Maiorano AE, Piccolo RM, da Silva ES, de Andrade Rodrigues MF. Microbial production of fructosyltranferases for synthesis of prebiotics. Biotechnology letters. 2008;30(11):1867–1877. doi: 10.1007/s10529-008-9793-3. [DOI] [PubMed] [Google Scholar]
- 26.Yoo SH, Yoon EJ, Cha J, Lee HG. Antitumor activity of levan polysaccharides from selected microorganisms. International journal of biological macromolecules. 2004;34(1):37–41. doi: 10.1016/j.ijbiomac.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Luo J, Ye H, Zeng X. Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium Paenibacillus polymyxa EJS-3. Food and chemical toxicology. 2012;50(3-4):762–772. doi: 10.1016/j.fct.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Permatasari NU, Ratnaningsih E, Hertadi R. Molecular cloning and expression of levansucrase gene from Bacillus licheniformis BK1 isolated from Bledug Kuwu Mud Crater. EurAsian journal Bioscience. 2019;13:223–230. [Google Scholar]
- 29.Permatasari NU, Ratnaningsih E, Hertadi R. The use of response surface method in optimization of levan production by heterologous expressed levansucrase from halophilic bacteria Bacillus licheniformis BK2. IOP Conference series: earth and environmental science. 2018;209(1) [Google Scholar]
- 30.Lu L, Fu F, Zhao R, Jin L, He C, Xu L, Xiao M. A recombinant levansucrase from Bacillus licheniformis 8-37-0-1 catalyzes versatile transfructosylation reactions. Process biochemistry. 2014;49(9):1503–1510. [Google Scholar]
- 31.Xiao-Kui M, Qinqin R, Hong Z, Weidong Q. Developing a high-throughput microassay for large samples of fungal polysaccharides. Analytical methods. 2013;5(17):4310. [Google Scholar]
- 32.Zhang W, Zhang X, Cai L, Chen R, Zhang Q, Wang X. Determination of levan from Bacillus licheniformis by ultraviolet spectrophotometry. Tropical journal of pharmaceutical research. 2015;14(4):679–685. [Google Scholar]
- 33.Molyneux P. The use of the stable free radical diphenylpicryhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin journal of science and technology. 2004;26(2):211–219. [Google Scholar]
- 34.Noipa T, Srijaranai S, Tuntulani T, Ngeontae W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food research international. 2011;44(3):798–806. [Google Scholar]
- 35.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. Journal of pharmaceutical analysis. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wu Y, Zheng W, Han X, Jiang Y, Hu P, Tang Z, Shi L. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. Journal of functional foods. 2017;38(Part A):273–279. [Google Scholar]
- 37.Spanò A, Gugliandolo C, Lentini V, Teresa L, Maugeri , Anzelmo G, Poli A, Nicolaus B. A novel EPS-producing strain of Bacillus licheniformis isolated from a shallow vent off Panarea island (Italy) Current microbiology. 2013;67(1):21–29. doi: 10.1007/s00284-013-0327-4. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed KBA, Kalla D, Uppuluri KB, Anbazhagan V. Green synthesis of silver and gold nanoparticles employing levan, a biopolymer from Acetobacter xylinum NCIM 2526, as a reducing agent and capping agent. Carbohydrate polymers. 2014;112:539–545. doi: 10.1016/j.carbpol.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Srikanth R, Siddartha G, Sundhar Reddy CH, Harish BS, Janaki Ramaiah M, Uppuluri KB. Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM2526 and its statistical optimization. Carbohydrate polymers. 2015;123(5):8–16. doi: 10.1016/j.carbpol.2014.12.079. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Yu S, Zhang T, Jiang B, Mu W. Efficient biosynthesis of levan from sucrose by a novel levansucrase from Brenneria goodwinii. Carbohydrates polymers. 2017;157:1732–1740. doi: 10.1016/j.carbpol.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 41.Reyes-Ortega F, Aguilar MR. Smart Polymers and Their Applications: ph-Responsive Polymers: Properties, Synthesis and Applications. UK: Woodhead Publishing: 2014. pp. 45–92. [Google Scholar]
- 42.Cavalcanti OA, Petenuci B, Bedin AC, Pineda EAG, Hechenleitner AAW. Characterisation of ethylcellulose films containing natural polysaccharides by thermal analysis and FTIR spectroscopy. Acta Farmacéutica Bonaerense. 2004;23(1):53–57. [Google Scholar]
- 43.Poli A, Kazak H, Gürleyendağ B, Tommonaro G, Pieretti G, ToksoyÖner E, Nicolaus B. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydrate polymers. 2009;78(4):651–657. [Google Scholar]
- 44.Benattouche Z, Bouhadi D, Raho GB. Antioxidant and antibacterial of exopolysaccharides. International journal of food studies. 2018;7(2):30–37. [Google Scholar]
- 45.Zhang Z, Jin J, Shi L. Antioxidant activity of the derivatives of polysaccharide extract from a Chinese medical herb (Ramulus mori) Food science and technology research. 2008;14(2):160–168. [Google Scholar]
- 46.Li XL, Thakur K, Zhang YY, Tu XF, Zhang YS, Zhu DY, Zhang JG, Wei ZJ. Effects of different chemical modifications on the antibacterial activities of polysaccharides sequentially extracted from peony seed dreg. International journal of biological macromolecules. 2018;116:664–675. doi: 10.1016/j.ijbiomac.2018.05.082. [DOI] [PubMed] [Google Scholar]