Abstract
Bacteria utilize type VI secretion system (T6SS) to deliver antibacterial toxins to target co‐habiting bacteria. Here, we report that Burkholderia gladioli strain NGJ1 deploys certain T6SS effectors (TseTBg), having both DNase and RNase activities to kill target bacteria. RNase activity is prominent on NGJ1 as well as other bacterial RNA while DNase activity is pertinent to only other bacteria. The associated immunity (TsiTBg) proteins harbor non‐canonical helix–turn–helix motifs and demonstrate transcriptional repression activity, similar to the antitoxins of type II toxin–antitoxin (TA) systems. Genome analysis reveals that homologs of TseTBg are either encoded as TA or T6SS effectors in diverse bacteria. Our results indicate that a new ORF (encoding a hypothetical protein) has evolved as a result of operonic fusion of TA type TseTBg homolog with certain T6SS‐related genes by the action of IS3 transposable elements. This has potentially led to the conversion of a TA into T6SS effector in Burkholderia. Our study exemplifies that bacteria can recruit toxins of TA systems as T6SS weapons to diversify its arsenal to dominate during inter‐bacterial competitions.
Keywords: DNA adenine methylase, effector neutralization, LysR proteins, protein‐DNA interaction, restriction modification system
Subject Categories: Microbiology, Virology & Host Pathogen Interaction
Tox‐REase‐5 domain containing T6SS effectors of Burkholderia exhibit dual nuclease activity and cognate immunity proteins exert transcription repression activity, reminiscent of type II antitoxins. T6SS recruit intracellular toxins as extracellular weapons to target prey bacteria.

Introduction
Under natural conditions, bacteria have to compete with co‐habiting bacteria for available resources. This imparts severe evolutionary pressure on bacteria to adopt strategies to limit the growth of other co‐habiting microbes (Hibbing et al, 2010). Several bacterial species use a specialized protein secretion system called the type VI secretion system (T6SS) to target co‐habiting bacteria in a contact‐dependent manner (Ho et al, 2014; Sana et al, 2017; Anderson et al, 2017; Bernal et al, 2018; Chassaing & Cascales, 2018; Coulthurst, 2019). The T6SS is a syringe‐like apparatus composed of a membrane complex (spanning the inner and outer membrane), a base plate, and an inner tube, being wrapped in a sheath‐like structure (Silverman et al, 2012; Wang et al, 2017; Cherrak et al, 2018; Park et al, 2018). Hexamers of the Hemolysin‐coregulated protein (Hcp) form the inner tube of the T6SS apparatus (Ruiz et al, 2015; Wang et al, 2017) while a trimer of the valine–glycine repeat protein G (VgrG protein) forms a spike‐like structure on the top of the inner tube (Bondage et al, 2016). The PAAR (proline–alanine–alanine–arginine) repeat‐containing protein binds to the distal end of the spike and forms a sharp pointed tip (Shneider et al, 2013; Wood et al, 2019). Contraction of the sheath enables the Hcp‐VgrG‐PAAR protein complex to puncture the bacterial membrane and deliver various T6SS effectors into the extracellular environment or directly into the target bacterial cells (Silverman et al, 2012; Cianfanelli et al, 2016a; Cianfanelli et al, 2016b). The effectors are either encoded in the T6SS apparatus‐encoding gene cluster or elsewhere in the bacterial genome (commonly referred to as orphan effectors). Notably, Hcp/VgrG/PAAR is required as a carrier protein for their T6SS‐mediated delivery (Silverman et al, 2013; Hachani et al, 2014; Whitney et al, 2014; Bondage et al, 2016; Cianfanelli et al, 2016b; Lien & Lai, 2017). Recent studies have suggested that some of the effectors require certain additional proteins such as chaperones (DUF4123, DUF1795, and DUF2169) for T6SS‐mediated delivery (Liang et al, 2015; Unterweger et al, 2015; Lien & Lai, 2017; Burkinshaw et al, 2018). Diverse kinds of proteins including phospholipases (Tle), amidases (Tae), glucosaminidase (Tge), nucleases, peptidases, and pore‐forming toxins have been identified as T6SS effectors from different bacteria (Russell et al, 2011, 2013, 2014; Das et al, 2021). These effectors demonstrate potent bactericidal activity by targeting different components (DNA/cell membrane/cell wall) of the prey bacteria (Russell et al, 2011, 2014; Burkinshaw et al, 2018). However to protect self as well as sister cells from intoxication, bacteria have evolved cognate immunity proteins against each of the T6SS effectors (Russell et al, 2011; Benz & Meinhart, 2014; Yang et al, 2018). The effector–immunity pairs are encoded together in an operonic fashion and the immunity protein neutralizes the toxic effect of the cognate effector via direct binding/interaction (Coulthurst, 2013; Ho et al, 2014; Alcoforado Diniz et al, 2015; Yang et al, 2018).
Besides T6SS effectors, bacteria encode various toxins as part of toxin–antitoxin (TA) systems (Unterholzner et al, 2013; Van Acker et al, 2014; Wen et al, 2014; Page & Peti, 2016; Harms et al, 2018; Kang et al, 2018; Fraikin et al, 2020). Similar to that of immunity proteins, the antitoxin of type II TA systems binds to the cognate toxin and neutralizes it to keep the cells protected (Goormaghtigh et al, 2018; Kang et al, 2018; Fraikin et al, 2020). Moreover, besides directly neutralizing the toxin, the antitoxin alone or in complex with cognate toxin binds to the promoter of the type II TA operon and represses the expression of TA genes (Unterholzner et al, 2013; Harms et al, 2018; Fraikin et al, 2020; LeRoux et al, 2020). The degradation of antitoxin causes de‐repression of toxin gene expression and the ratio of toxin and antitoxin proteins decides the fate of the cells. The plasmid‐encoded TA system has been predominantly involved in post‐segregational killing while the role of chromosomal TA system has been debatable (Page & Peti, 2016; Yang & Walsh, 2017; Fraikin et al, 2020). Various studies have proposed their role in regulating important cellular processes, genome stability, biofilm, persister cell formation, programmed cell death, growth arrest, and stress tolerance (Wen et al, 2014; Yang & Walsh, 2017; Kang et al, 2018; Fraikin et al, 2020; LeRoux et al, 2020). It is noteworthy that some of the recent studies have suggested that certain TA modules serve as a common evolutionary gene pool which can be adopted by bacteria for different functions (Triplett et al, 2016; Harms et al, 2017).
In the present study, we demonstrate that a rice‐associated bacterium, Burkholderia gladioli strain NGJ1, utilizes two different T6SSs (here named as T6SS‐1 and T6SS‐2) to target co‐habiting bacteria. Bioinformatics analysis indicates that the NGJ1 bacterium encodes fourteen different T6SS effectors and immunity proteins. The restriction endonuclease (Tox‐REase‐5) domain‐containing nuclease effector (here onward referred as TseTBg; Type VI secreted effector Tox‐REase proteins of B. gladioli strain NGJ1) proteins demonstrated dual nuclease (DNase as well as RNase) activity. The immunity proteins (TsiTBg) associated with these effectors showed transcriptional repression activity. Functional similarity and genome organization suggest that the TseTBg effectors have potentially evolved from a bacterial TA system. The transposition of IS3 family elements appears to have played a major role in this evolutionary process which has enabled the bacteria to adapt the function of these intracellular toxins as extracellular weapons to kill other bacteria.
Results
Burkholderia gladioli strain NGJ1 utilizes two different type VI secretion systems for antibacterial activity
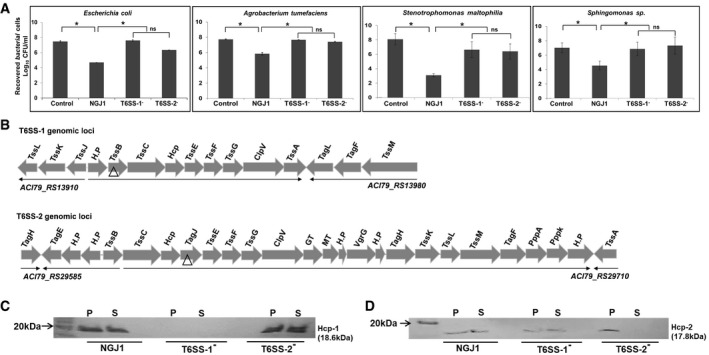
We observed that B. gladioli strain NGJ1 demonstrates strong antibacterial activity against Escherichia coli, Agrobacterium tumefaciens as well as some of the rice endophytic bacteria (Stenotrophomonas maltophilia and Sphingomonas sp.) in a contact‐dependent manner (Fig 1A). We anticipated that such antibacterial activity of NGJ1 may be mediated by a type VI secretion system (T6SS). Genome analysis revealed that NGJ1 contains two different T6SS apparatus‐encoding gene clusters, here onward named as T6SS‐1 (Burkholderia genome database locus id: ACI79_RS13910–ACI79_RS13980) and T6SS‐2 (Burkholderia genome database locus id: ACI79_RS29585–ACI79_RS29710; Fig 1B). We disrupted one important gene from each of these clusters through plasmid integration to obtain T6SS‐1 defective (referred as T6SS‐1−; TssB gene being disrupted) and T6SS‐2 defective (referred as T6SS‐2−; TagJ gene being disrupted) strains. Western blot analysis revealed that wild‐type NGJ1 bacterium is capable of secreting Hcp‐1 (that is associated with T6SS‐1 cluster) and Hcp‐2 (that is associated with T6SS‐2 cluster) proteins into extracellular milieu (Fig 1C and D). However, T6SS‐1− strain was defective in Hcp‐1 secretion but proficient in Hcp‐2 secretion. On the other hand, T6SS‐2− strain was defective in secretion of Hcp‐2 but proficient in secretion of Hcp‐1.
Figure 1. Burkholderia gladioli strain NGJ1 utilizes two different type VI secretion system (T6SS) apparatus for antibacterial activity.
-
AAntibacterial activity of NGJ1, T6SS‐1−, and T6SS‐2− strains against target bacteria.
-
BSchematic representation of two different T6SS apparatus‐encoding gene clusters (named as T6SS‐1 and T6SS‐2) of NGJ1. The locus id (as per Burkholderia genome database) of upstream and downstream genes of the T6SS locus is provided. The gene disrupted in the respective T6SS mutants is marked by triangle (△).
-
C, DImmunoblots showing secretion profile of Hcp‐1 (associated with T6SS‐1) and Hcp‐2 (associated with T6SS‐2) proteins, using protein‐specific peptide antibody in different NGJ1 strains. The T6SS‐1− mutant was defective in Hcp‐1 secretion (due to polar effect of plasmid integration, synthesis was also prevented) but proficient in Hcp‐2 secretion. On the other hand, the T6SS‐2− mutant was defective in secretion of Hcp‐2 (although the protein was synthesized) but proficient in secretion of Hcp‐1.
Data information: In (A), graphs show mean values ± SD of three biological replicates. Asterisk * indicates significant difference at P < 0.05 and “ns” indicates non‐significant difference (estimated using one‐way ANOVA). In (C, D), P: total protein from bacterial pellet and S: total protein from cell‐free supernatant. Similar results were obtained in at least three independent experiments.
Source data are available online for this figure.
Further, we investigated the ability of T6SS‐1− and T6SS‐2− mutants to kill the prey bacteria in a contact‐dependent manner. The data revealed that both the mutants are compromised in killing the prey bacteria (Fig 1A). This suggested that certain antibacterial effectors are secreted by NGJ1 through T6SS‐1 while others are secreted through T6SS‐2 and disruption of either of T6SS apparatus compromises the antibacterial activity of NGJ1.
Burkholderia gladioli strain NGJ1 harbors diverse antibacterial T6SS effectors
Using computational analysis, we identified fourteen different T6SS effector operons in NGJ1 genome (Appendix Fig S1). Besides effector proteins, each of these operons also encoded a cognate immunity protein and certain carrier (VgrG and/or PAAR) as well as chaperone (DUF4123/DUF1795) proteins that potentially assist in T6SS‐mediated delivery of effectors. The putative functions of various T6SS effectors of NGJ1 are summarized in Appendix Table S1. We ectopically expressed some of these effectors and observed them to be lethal for the recombinant E. coli (BL21 [DE3]) cells. In most of the cases, co‐expression of cognate immunity proteins using separate plasmids (pET23b:effector + pET28a:immunity) protected the cells from effector‐mediated killing (Appendix Fig S2). However, co‐expression of certain effectors (TseTBg; TseTBg1–3) and their cognate immunity (TsiTBg; TsiTBg1–3) proteins failed to protect the E. coli cells (Fig 2A and Appendix Fig S3A–C). On the other hand, when transcriptionally fused effector–immunity pair (TseTBg:TsiTBg) was co‐expressed using a single plasmid (pET28a), the recombinant cells got protected (Fig 2A and Appendix Fig S3A–C).
Figure 2. The TseTBg effectors of NGJ1 exhibit potent antibacterial activity.
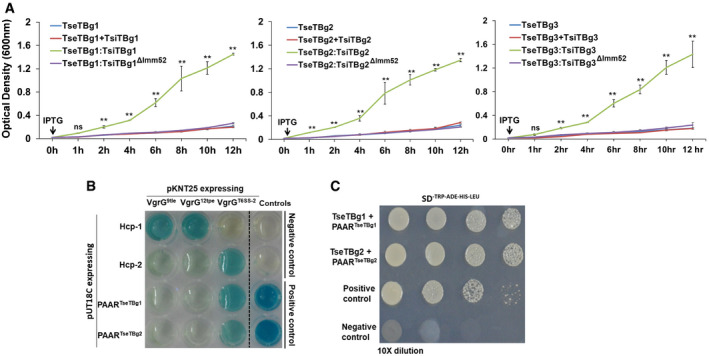
- Growth curve of recombinant BL21 (DE3) cells upon IPTG‐mediated induction of proteins, represented as colored line graphs.
- Bacterial two‐hybrid assay reflecting interaction between proteins of T6SS apparatus and effector operons. Interaction between T25‐zip and T18‐zip was used as positive control while pKNT25 and pUT18C (empty vectors) were used as negative control. Appearance of blue color suggests positive interaction while the absence of color suggests lack of interaction.
- Yeast two‐hybrid assay reflecting interaction of TseTBg1 and TseTBg2 effector proteins with the corresponding PAAR (PAARTseTBg) proteins encoded in their operon. Interaction between P53 and SV40 large T‐antigen (T) proteins was used as positive control. The pGBKT7 and pGADT7 (empty vectors) were used as negative control.
Data information: In (A), graphs represent mean ± SD of three biological replicates. Asterisk ** indicates significant difference at P < 0.01, and “ns” indicates non‐significant difference (estimated using one‐way ANOVA) at the given time point. In (B, C), similar results were obtained in at least three independent experiments.
Bioinformatics analysis revealed that TsiTBg immunity proteins contain a conserved Imm52 domain (Appendix Fig S4A and B) that is potentially involved in neutralization of cognate effector proteins in other bacterial systems (Zhang et al, 2012; Burkinshaw et al, 2018). We generated Imm52 domain‐deleted variant of each of these immunity proteins and observed that the co‐expression of transcriptionally fused effector and Imm52 domain‐deleted immunity protein (TseTBg:TsiTBgΔImm52) failed to protect the recombinant E. coli cells from effector‐mediated killing (Fig 2A and Appendix Fig S3A–C). Notably, a similar observation was observed during microscopic analysis of the recombinant E. coli cells. Lack of fluorescence signal upon DAPI staining was prominent in the cells that express either effector (TseTBg) or co‐express effector and immunity proteins using two different plasmids (TseTBg + TsiTBg; Fig EV1). On the other hand, an intense signal was observed in the cells that express transcriptionally fused effector–immunity pairs (TseTBg:TsiTBg). However, the signal was very minimal in cells that express transcriptionally fused effector and Imm52 domain‐deleted immunity proteins (TseTBg:TsiTBgΔImm52).
Figure EV1. TseTBg effector proteins elicit nuclear degradation/ cell death in Escherichia coli .
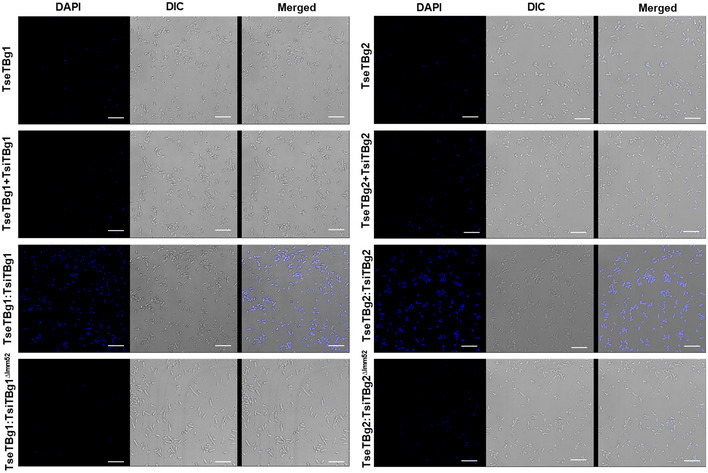
Fluorescence microscopic images of DAPI (nuclear staining dye)‐stained E. coli cells that express TseTBg, TseTBg + TsiTBg, TseTBg:TsiTBg, and TseTBg:TsiTBgΔImm52 proteins upon IPTG (1 mM) induction. Lack of DAPI staining suggested nuclear degradation/ cell death while proper staining reflected intact nuclei. DIC: differential interference contrast microscopy image.
Data information: scale bar = 10µm. Similar results were obtained in at least three independent experiments.
Source data are available online for this figure.
The TseTBg effector proteins are secreted through T6SS‐2 of NGJ1
We observed that TseTBg operons have significant sequence similarity with a previously reported T6SS effector Tox‐REase (TseT) operon of Pseudomonas aeruginosa strain PAO1 (Burkinshaw et al, 2018). The gene organization is also identical between TseT and TseTBg operons and in both the cases PAAR is encoded as upstream ORF (referred as PAARTseTBg). RT–PCR analysis revealed that various genes of TseTBg operon are expressed as a single transcriptional unit (Fig EV2A–C). Previously, interactions of TseT with PAARTseT and PAARTseT with VgrG of T6SS apparatus had been shown to be required for T6SS‐mediated delivery of TseT effector (Burkinshaw et al, 2018). We observed that PAARTseTBg exhibits physical interaction with the VgrG of T6SS‐2 apparatus (VgrGT6SS‐2) of NGJ1, as revealed in bacterial two‐hybrid assay (Fig 2B). However, PAARTseTBg did not interact with VgrG of other effector (9tle/12tpe) operons (VgrG9tle/VgrG12tpe). Similarly, the Hcp‐2 interacted with the VgrGT6SS‐2 but not with the VgrG9tle/VgrG12tpe. We further investigated the interaction of TseTBg with the cognate PAARTseTBg using yeast two‐hybrid assays (due to antibacterial nature of TseTBg, bacterial two‐hybrid assay was not used). The analysis revealed the interaction of TseTBg proteins with the cognate PAAR proteins (Fig 2C). Based upon this, we speculate that the interaction of PAARTseTBg with VgrGT6SS‐2 would enable T6SS‐2 apparatus‐mediated secretion of TseTBg proteins (Appendix Fig S5). The Western blot analysis using specific peptide antibody revealed the presence of TseTBg2 protein in the supernatant of wild‐type as well as T6SS‐1− strains but not in the T6SS‐2− strain of NGJ1 (Fig EV3A). However, the protein was detected in the pellet of each of these strains. This reinforced that the TseTBg2 protein is secreted by NGJ1 in a T6SS‐2‐dependent manner.
Figure EV2. RT–PCR analysis reflects genes of TseTBg operon of NGJ1 being expressed as single transcriptional unit.
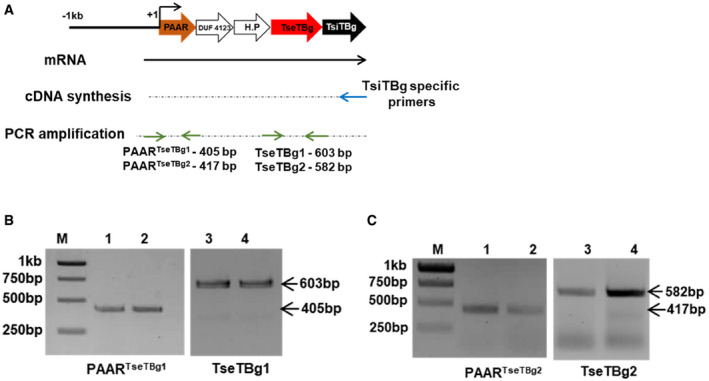
- Strategy used for validating the expression of different genes of TseTBg operon as single transcriptional unit.
- PCR amplification of PAARTseTBg1 and TseTBg1 genes from cDNA synthesized using TsiTBg1‐specific primer.
- PCR amplification of PAARTseTBg2 and TseTBg2 genes from cDNA synthesized using TsiTBg2‐specific primer.
Data information: In (B, C), lanes 1 and 3 represent gene expression during NGJ1 interaction with Escherichia coli, while lanes 2 and 4 represent gene expression during NGJ1 interaction with Agrobacterium tumefaciens. Similar results were obtained in at least three independent experiments. M: DNA size marker.
Source data are available online for this figure.
Figure EV3. NGJ1 utilizes TseTBg2 effector to kill prey bacteria in a T6SS dependent manner.
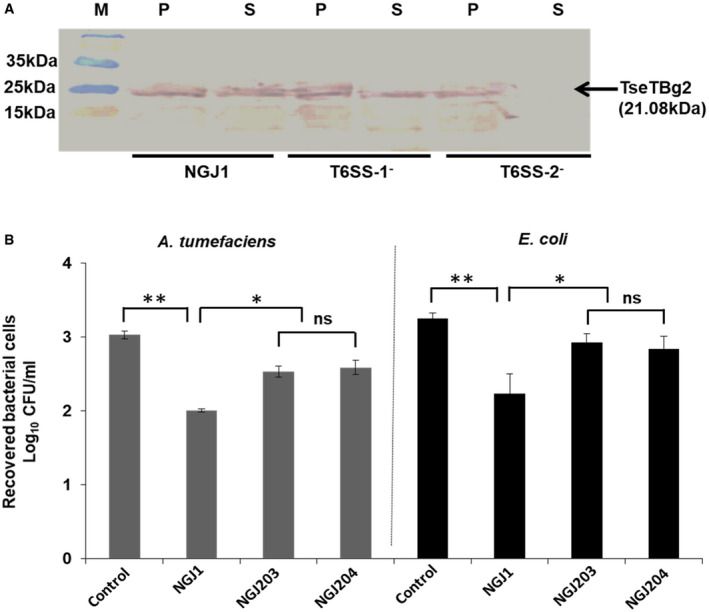
- Western blot analysis reflecting the T6SS‐2‐dependent secretion of TseTBg2 protein in NGJ1. The total proteins from cell‐free supernatant (S) as well as pellet (P) of different strains were immunoblotted using TseTBg2‐specific peptide antibody.
- Antibacterial activity of the TseTBg2 mutants (NGJ203 and NGJ204; two independent mutants) and wild‐type NGJ1 bacteria against Agrobacterium tumefaciens and Escherichia coli.
Data information: In (A), M: Protein size marker. Similar results were obtained in at least three independent experiments. In (B), asterisks ** and * indicate significant difference at P < 0.01 and P < 0.05, respectively, while “ns” indicates non‐significant difference (estimated using paired t‐test). Graphs show mean values ± SD of three biological replicates.
Source data are available online for this figure.
To investigate the role of TseTBg2 during inter‐bacterial competition, we created TseTBg2− mutant strains of NGJ1 (NGJ203 and NGJ204; independently isolated). We observed that both the mutant strains are compromised in contact‐dependent antibacterial activity against E. coli and A. tumefaciens (Fig EV3B). This indicates that at least some of the TseTBg proteins are involved in contact‐dependent killing of target bacteria by NGJ1.
The TseTBg effectors of NGJ1 have dual nuclease activity
NCBI Conserved Domain Database (CDD) analysis revealed the presence of a conserved restriction endonuclease‐5 (Tox‐REase‐5) domain at the C terminus of TseTBg proteins (Appendix Fig S4C and D). The Tox‐REase‐5 domain‐containing proteins, including TseT of P. aeruginosa strain PAO1, belong to PD‐DxK superfamily of restriction endonucleases (Burkinshaw et al, 2018). To investigate further, we ectopically overexpressed and purified two of the TseTBg (TseTBg1/TseTBg2) proteins from E. coli (BL21 (DE3) pLysS). Notably, both the proteins were able to degrade the genomic DNA of E. coli (Fig 3A) as well as lambda (Appendix Fig S6A) and plasmid (pET28a) DNA (Appendix Fig S6B). However, treatment with either of TseTBg proteins failed to degrade the genomic DNA of NGJ1 (Fig 3B). Moreover, we observed that the purified TseTBg1/TseTBg2 proteins also exhibit RNase activity, being capable of degrading the RNA of E. coli and NGJ1, under in vitro conditions (Fig 3C and D, and Appendix Fig S6C and D). RNase activity of these proteins was retained, even in the presence of RNaseOUTTm (a recombinant Ribonuclease inhibitor; Appendix Fig S6E and F). On the other hand, we observed that supplementation of TsiTBg1 immunity protein prevents RNase activity of TseTBg1 effector protein but not of TseTBg2 protein (Fig 3C and D). This highlights that RNase activity of TseTBg proteins is neutralized by the cognate TsiTBg proteins.
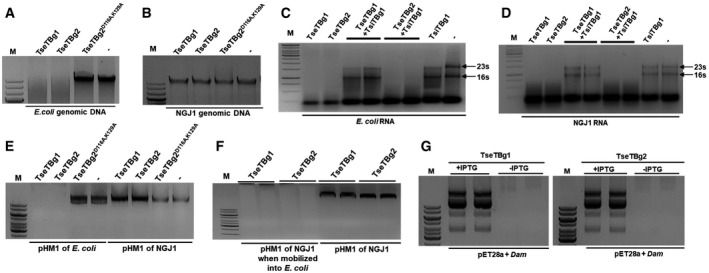
Figure 3. The TseTBg effector proteins of NGJ1 harbor potent DNase and RNase activity.
-
A, BEffect of purified TseTBg (TseTBg1/TseTBg2) and one of the variants (TseTBg2D116A,K129A) proteins on degradation of bacterial genomic DNA.
-
C, DRNase activity of TseTBg effector proteins, in the presence or absence of cognate TsiTBg immunity proteins.
-
EEffect of TseTBg1, TseTBg2, TseTBg2D116A,K129A proteins on degradation of pHM1, a broad host‐range plasmid isolated from Escherichia coli and NGJ1.
-
FEffect of TseTBg1 and TseTBg2 proteins on degradation of pHM1, reintroduced into E. coli from NGJ1.
-
GEffect of ectopic expression of DNA adenine methylase (Dam) protein on TseTBg proteins mediated degradation of plasmid DNA.
Data information: In (A–G), similar results were obtained in at least three independent experiments. M; DNA marker (1 kb); −; negative control.
We observed that similar to TseT, TseTBg proteins also harbor certain aspartate (D) and lysine (K) amino acids (Appendix Fig S7A) that are conserved in the active site of PD‐DxK superfamily of restriction endonucleases in different bacteria (Burkinshaw et al, 2018). We obtained a variant of TseTBg2 (TseTBg2D116A,K129A) protein wherein conserved D116 and K129 residues had been replaced with alanine (A) (Appendix Fig S7B). Notably, the TseTBg2D116A,K129A protein was defective in DNase activity but still proficient in RNase activity (Fig 3A and B and Appendix Fig S6A–F). We observed that IPTG‐mediated induction of TseTBg2D116A,K129A (DNase−RNase+) protein causes delay in growth in the recombinant E. coli cells (Fig EV4A and B). On the other hand, induction of native TseTBg2 (DNase+RNase+) strongly inhibited the growth of the cells.
Figure EV4. Effect of ectopic expression of TseTBg2 variant protein (TseTBg2D116A,K129A) on the growth of recombinant Escherichia coli cells.
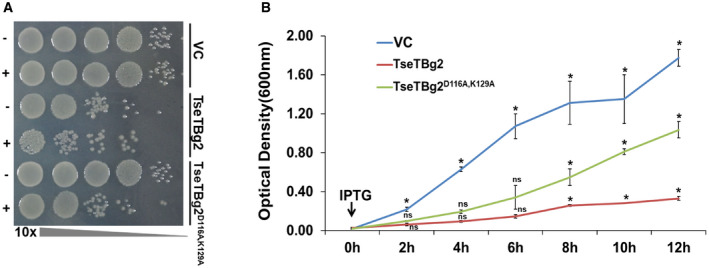
- Growth of recombinant BL21 (DE3) cells with or without induction of TseTBg2 and its variant (TseTBg2D116A,K129A, DNase−RNase+).
- Growth curve of recombinant BL21 (DE3) cells with or without IPTG‐mediated induction of TseTBg2 or TseTBg2D116A,K129A proteins.
Data information: In (A, B), VC, vector control. In (A), similar results were obtained in at least three independent experiments. +/− indicates presence/absence of 1 mM IPTG. In (B), graph represents mean ± SD of three biological replicates. Asterisk * indicates significant difference at P < 0.05 and “ns” indicates non‐significant difference (estimated using one‐way ANOVA) at the given time point.
TseTBg effector proteins exhibit host methylation‐sensitive DNase activity
It was intriguing why TseTBg proteins are unable to degrade the genomic DNA of NGJ1. For this, we tested the DNase activity of TseTBg proteins on pHM1, a broad host‐range plasmid that can replicate in both NGJ1 and E. coli. We observed that treatment of TseTBg1/TseTBg2 proteins completely degraded the pHM1 plasmid (isolated from E. coli), but failed to degrade it when isolated from NGJ1 (Fig 3E). Moreover, when the pHM1 from NGJ1 was mobilized into E. coli, the isolated plasmid was completely degraded by TseTBg proteins (Fig 3F). This suggests that DNase activity of TseTBg is host methylation sensitive. To investigate further, we identified a DNA adenine methylase (Dam) (AC179_RS12830), being encoded in the proximity of TseTBg1 operon. We ectopically expressed the Dam protein in E. coli using pET28a plasmid and isolated the plasmid with and without IPTG‐mediated induction of the protein. Notably, plasmid (pET28a) isolated from Dam‐expressing E. coli cells was not degraded by either of TseTBg1/TseTBg2 proteins (Fig 3G). However, plasmid isolated from cells that do not express Dam protein was completely degraded by both of them.
Immunity proteins associated with TseTBg effectors demonstrate transcriptional repressor activity
The TsiTBg proteins of NGJ1 as well as several of their homologs have been annotated as putative LysR family transcriptional regulators in NCBI as well as Burkholderia genome database. The CDD domain analysis and HMM‐based searches did not reveal the presence of a canonical DNA‐binding helix–turn–helix (HTH) motif that is a characteristic of bacterial LysR family transcriptional regulator. However, by using an online HTH prediction tool GYM2 (Narasimhan et al, 2002), we detected potential HTH motifs in some of the TsiTBg homologs (n = 33; but not in TsiTBg proteins of NGJ1; Appendix Fig S8 and Appendix Table S2). Homology searches using the consensus sequence of these HTH motifs (compiled using FIMO tool (Grant et al, 2011)) revealed the presence of non‐canonical HTH motif at N terminus of TsiTBg immunity proteins of NGJ1 (Fig 4A and B) and many of their homologs in other bacteria (Dataset EV1). To investigate further, we obtained HTH motif‐deleted variants of two of the TsiTBg proteins and analyzed transcriptional regulation ability of native (TsiTBg1/TsiTBg2) as well as variant (TsiTBg1ΔHTH/TsiTBg2ΔHTH) proteins.
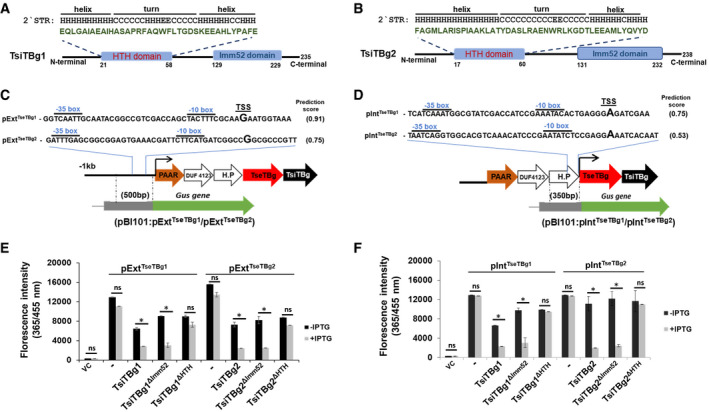
Figure 4. The TsiTBg immunity proteins of NGJ1 demonstrate transcriptional repressor activity.
-
A, BSchematics reflecting the presence of a non‐canonical HTH domain and an Imm52 domain in TsiTBg1 and TsiTBg2 proteins.
-
C, DRepresentation of predicted external (pExtTseTBg1/pExtTseTBg2) and internal (pIntTseTBg1/pIntTseTBg2) promoter regions in TseTBg1 and TseTBg2 operons. Strategy for obtaining promoter:Gus reporter constructs in pBI101 is also provided.
-
E, FGraph showing the effect of native TsiTBg and HTH or Imm52 domain‐deleted variant of TsiTBg proteins on Gus reporter gene expression (quantified using 4‐methylumbelliferyl ß‐d‐glucuronide, MUG, as substrate) under the external (pExtTseTBg1/pExtTseTBg2) and internal (pIntTseTBg1/pIntTseTBg2) promoters in Escherichia coli.
Data information: In (C, D), TSS represents putative transcription start site. In (E, F), VC: vector control; ‐: absence of TsiTBg protein. Graphs show mean values ± SD of three independent biological replicates. Asterisk * indicates significant difference at P < 0.05 and “ns” indicates non‐significant difference (estimated using paired t‐test).
For this, we first predicted promoter region in the TseTBg1/TseTBg2 operon using online prokaryotic promoter prediction tool; Neural Network Promoter Prediction (Reese, 2001). The analysis predicted an external promoter upstream to the PAAR gene (pExtTseTBg1/pExtTseTBg2) that can drive the expression of entire operon (Fig 4C) and an internal promoter within the gene‐encoding hypothetical protein (pIntTseTBg1/pIntTseTBg2) that can only transcribe TseTBg and TsiTBg genes (Fig 4D). Gene expression analysis using a β‐glucuronidase reporter gene indicated that both external and internal promoters are active in A. tumefaciens and in E. coli (Appendix Fig S9A). However, co‐expression of the TsiTBg immunity proteins (TsiTBg1/TsiTBg2) significantly inhibited expression of β‐glucuronidase from the external (pExtTseTBg1/pExtTseTBg2) promoter (Fig 4E and Appendix Fig S9B) as well as the internal (pIntTseTBg1/pIntTseTBg2) promoter (Fig 4F and Appendix Fig S9C). Further we tested the transcription repression ability of HTH domain (TsiTBg1ΔHTH/TsiTBg2ΔHTH) and Imm52 domain (TsiTBg1ΔImm52/TsiTBg2ΔImm52) deleted variants of immunity proteins. The deletion of HTH domain compromised the transcription repression activity of TsiTBg proteins on both the promoters while the Imm52 domain‐deleted variants had retained the activity (Fig 4E and F and Appendix Fig S9B and C).
To verify the binding of immunity protein to the promoter region, we performed Electromobility shift assay (EMSA) using one of the purified TsiTBg (TsiTBg1) protein. Shifts in the mobility of pExtTseTBg1 and pIntTseTBg1 promoter DNA fragments were observed in the presence of TsiTBg1 protein, and position of the shifted DNA was found to be coinciding with the protein (Fig 5A). However, the HTH motif‐deleted variant (TsiTBg1ΔHTH) protein failed to produce a shift in the mobility of the promoter DNA (Fig 5B). It is to be noted that TsiTBg1 protein was unable to interact and cause shift in the mobility of promoter DNA of TseTBg2 operon (pExtTseTBg2/pIntTseTBg2; Fig 5C) or control (random) DNA (Fig 5D). These results suggest a novel mode of regulation of TseTBg operon in NGJ1, wherein immunity protein represses the expression of the operon via binding to both external and internal promoters.
Figure 5. Electromobility shift assay (EMSA) reflecting promoter DNA‐binding ability of TseTBg1 protein† .
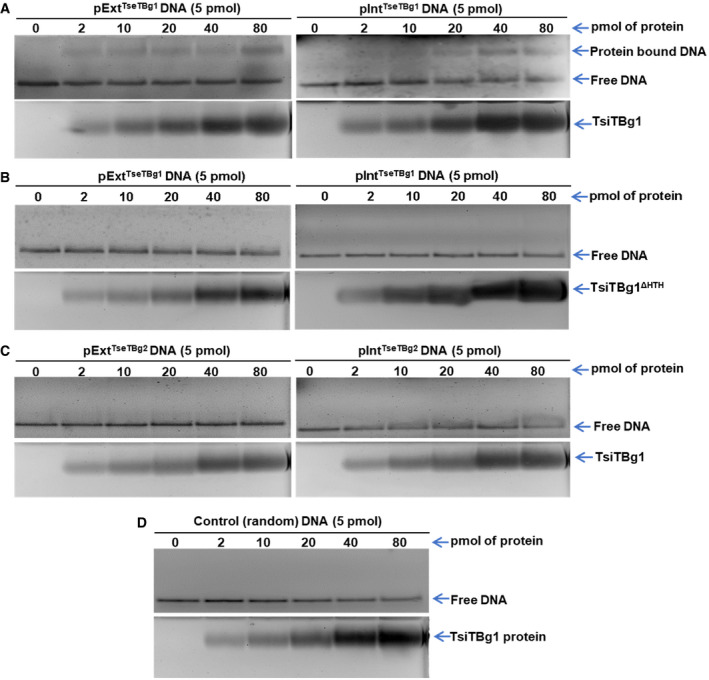
- Interaction of external (pExtTseTBg1) and internal (pIntTseTBg1) promoter DNA fragments of TseTBg1 operon with the TsiTBg1 protein.
- Interaction of pExtTseTBg1 and pIntTseTBg1 promoter DNA fragments of TseTBg1 operon with the HTH domain‐deleted variant of TseTBg1 protein (TsiTBg1ΔHTH).
- Interaction of pExtTseTBg2 and pIntTseTBg2 promoter DNA of TseTBg2 operon with the TsiTBg1 protein.
- Interaction of control (random) DNA with the TsiTBg1 protein.
Data information: In (A–D), lower panel represents visualization of the protein on the gel. Equimolar concentration of promoter DNA probe (5 pmol) was titrated with increasing concentration of protein (2–80 pmol). Similar results were obtained in at least three independent experiments.
Source data are available online for this figure.
The homologs of TseTBg proteins may function either as T6SS effectors or part of TA systems in different bacteria
Searches against Pfam database revealed that homologs of TseTBg proteins are encoded in diverse bacterial genomes (Appendix Fig S10). To understand the evolutionary relationship among TseTBg homologs, we carried out phylogenetic and gene neighborhood analysis, using Randomized Axelerated Maximum Likelihood (RAxML) (Stamatakis et al, 2012) and webFlaGs (Saha et al, 2020) tools, respectively. The analysis reflected that similar to NGJ1, several Beta‐proteobacteria (including Burkholderia sp.) and Gamma‐proteobacteria (including Pseudomonas sp.) encode homologs of TseTBg and TsiTBg proteins along with certain T6SS‐related proteins (PAAR, DUF4123, and hypothetical protein) in the same operon (Fig 6 and Dataset EV2). We anticipate that such TseTBg homologs may serve as T6SS effectors. Indeed one of such homologs, TseT had already been demonstrated to be a T6SS effector in P. aeruginosa strain PAO1 (Burkinshaw et al, 2018).
Figure 6. Phylogenetic analysis indicates homologs of TseTBg proteins to be encoded as TA or T6SS effectors in different bacteria.
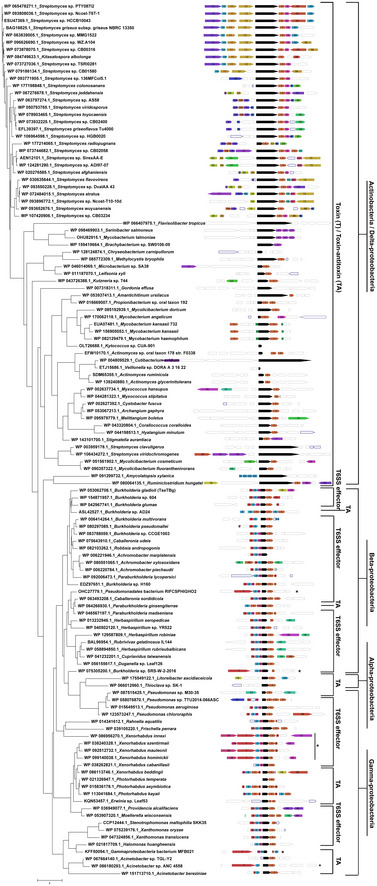
Phylogenetic tree of different TseTBg homologs (n = 121) constructed by RAxML tool using Maximum Likelihood algorithm. The tree is drawn to scale wherein branch lengths indicate number of substitutions per site. For each of the TseTBg homologs, gene neighborhood conservation (depicted by color‐coded box) by webFlaGs tool is also presented. The full output of webFlaGs analysis is provided in Dataset EV2. Bacterial classification is denoted in the outermost bar line.
Data information: The TseTBg homologs when encoded together with TsiTBg homologs are considered as TA whereas, when TseTBg and TsiTBg homologs are encoded along with certain T6SS related (PAAR, DUF4123, and hypothetical protein [H.P]) proteins, they are considered as T6SS effectors. *VgrG is encoded (instead of PAAR) as upstream ORF in these operons.
On the other hand, some closely related strains of these bacteria as well as several Actinobacteria, Alpha‐proteobacteria, and Delta‐proteobacteria encode only TseTBg and TsiTBg homologs in a particular operon (Fig 6). The absence of T6SS‐related proteins (PAAR, DUF4123, and hypothetical protein) in the operons suggests that such TseTBg homologs may not be functioning as T6SS effectors. Considering that the TseTBg proteins act as potent antibacterial toxins and the TsiTBg proteins act as antitoxins, either by neutralizing the effector protein via direct binding or by repressing the expression of effector through transcriptional repression, we anticipate that these TseTBg and TsiTBg homologs may function as TA systems.
The TseTBg homologs may have evolved as T6SS effectors from ancestral bacterial TA systems
An examination of the genomes of approximately 1,800 strains of different Burkholderia sp. that are available in the Burkholderia genome database (Winsor et al, 2008) revealed intraspecific variation wherein some of the strains appear to have TseTBg homologs as TA systems (Appendix Fig S11A) while others appear to encode them as T6SS effectors (Appendix Fig S11B). For example, the Burkholderia pseudomallei strain D7 3230‐3018 harbors a particular TseTBg homolog as part of a TA system while in the B. pseudomallei strain 2002721100 (Fig 7A) and several other strains (Fig EV5), it is encoded as a T6SS effector. The presence of several IS3 family transposable elements suggests their possible role in the evolution of this locus (Fig 7B). As seen in B. pseudomallei strain 3000015237 (isolate MX2014), two IS3 elements are present immediately upstream of TA genes and two T6SS‐related genes (PAAR and DUF4123) are sandwiched between the IS elements. However, in several other B. pseudomallei strains (Nau24B‐3, Vgh16R, 2002721100, and 2011756189 isolate swiss2010), one of the IS3 elements appears to have excised bringing the PAAR and DUF4123 genes upstream of the TA genes (Fig 7B and Appendix Fig S12). The precise excision of this IS element has also created a new ORF of 474 bp which encodes a hypothetical protein (Fig 7C and Appendix Fig S12). Notably, promoter of the TA operon is embedded within the ORF that encodes a hypothetical protein and could function as an internal promoter (pIntTseTBg) to drive expression of downstream genes (Fig 4D). The sequence variations in different B. pseudomallei strains suggest some intermediary evolutionary steps mediated at least in part by IS element action, wherein a TA pair could have been converted into a T6SS effector–immunity proteins. Apparently, a similar evolutionary progression that can lead to conversion of TA systems into T6SS effector operon could have also occurred in other Burkholderia sp. (Appendix Fig S13) as well as Pseudomonas sp. (Appendix Fig S14). This suggests that such a conversion may not be a one‐off event and might have occurred several times during evolution of Gamma‐proteobacteria and Beta‐proteobacteria.
Figure 7. Potential conversion of TseTBg homologs of TA system into T6SS effectors in Burkholderia pseudomallei .
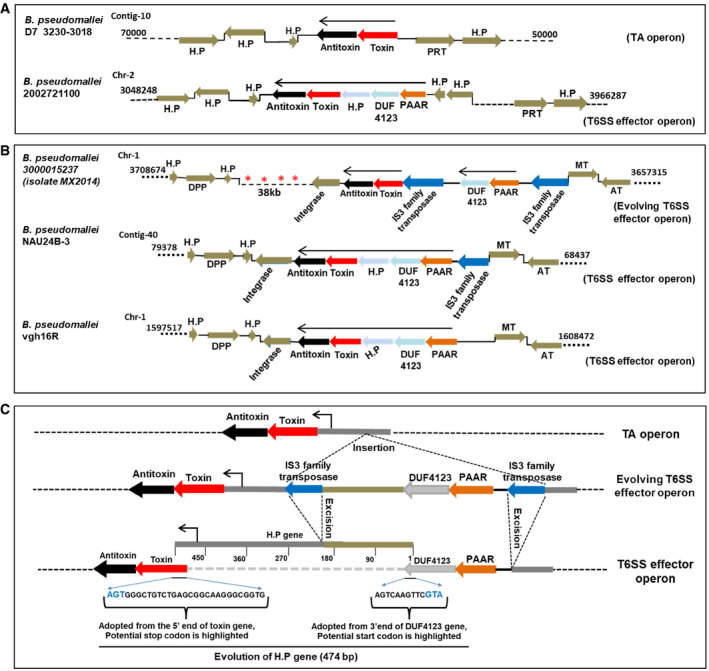
- The genomic loci containing TseTBg (toxin) and TsiTBg (antitoxin) homologs in different B. pseudomallei strains.
- In B. pseudomallei 3000015237 isolate MX2014, two IS3 family transposable elements that carry two of the T6SS‐related genes (PAAR and DUF4123) are present upstream to the TA genes. However, in B. pseudomallei Nau24B‐3 and vgh16R strains, the IS3 element which flank the TA and T6SS‐related genes have been excised and a new ORF encoding hypothetical protein (H.P) has evolved at its place.
- The excision of IS3 family transposase has created operonic fusion of TA and T6SS related genes. Moreover, a new ORF of 474 bp that encode a H.P has evolved at the locus, through adoption of 3′ end of the DUF4123 and 5′ end of the toxin gene.
Data information: In (A–C), H.P, hypothetical protein, PRT, Phosphoribosyl transferase, DPP, Decaprenyl diphosphatase, MT, MerR transcriptional regulator, AT, Aspartate aminotransferase. In (A, B), genomic location of each of the locus on the contig/chromosome is provided. In (B), asterisks indicate presence of multiple IS3 family transposase.
Figure EV5. Representation of genomic loci harboring TseTBg homologs as TA or T6SS effectors in different Burkholderia pseudomallei strains.
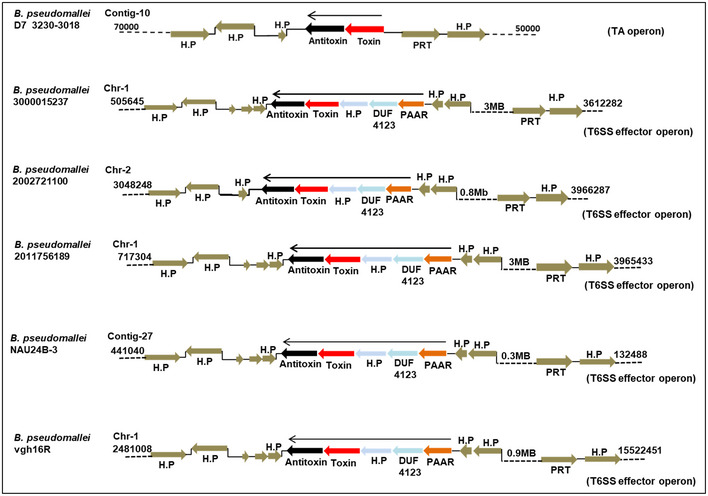
The TseTBg homologs (toxins) and TsiTBg homologs (antitoxins) were either encoded alone (considered as TA) or encoded along with certain T6SS‐related genes (PAAR, DUF4123, and H.P; considered as T6SS effector). The conservation of flanking genes suggests that TA or T6SS effector type TseTBg homologs are encoded at the same genomic locus in different B. pseudomallei strains.
Data information: H.P—hypothetical protein, PRT—Phosphoribosyltransferase. Genomic location of each of the locus on the contig/chromosome is provided.
Discussion
The genus Burkholderia constitutes a large group of bacterial species, being present as soil dwellers or living in association with plants, animals, fungi (endosymbiont), and insects (as symbiont) (Compant et al, 2008; Eberl & Vandamme, 2016; Flórez et al, 2018). Recently, we had demonstrated that the rice endophytic B. gladioli strain NGJ1 utilizes a type III secretion system (T3SS) to feed on fungi (phenomenon known as mycophagy) (Swain et al, 2017). In this study, we report that the NGJ1 bacterium also exhibits antibacterial activity and utilizes two different type VI secretion systems (T6SS‐1 and T6SS‐2) to kill co‐habiting bacteria in a contact‐dependent manner.
The presence of a diverse arsenal of T6SS effectors highlights the potency of antibacterial repertoire of NGJ1. However, to protect itself as well as sister cells from intoxication, NGJ1 encodes immunity proteins against each of the effector proteins. Among various effectors, the Tox‐REase‐5 domain‐containing effectors (TseTBg; TseTBg1/TseTBg2/TseTBg3) demonstrate strong homology with a previously reported T6SS effector (TseT) of P. aeruginosa strain PAO1 (Burkinshaw et al, 2018). Our observation that the TseTBg2 mutant of NGJ1 is compromised in contact‐dependent killing of E. coli as well as A. tumefaciens indicates important role of this effector protein in killing the prey bacteria. Further, dual nuclease activity of TseTBg proteins emphasizes that they can target prey bacteria through both DNase and RNase activities, when delivered as T6SS effectors. However, they exhibit only RNase but not DNase activity against NGJ1. This is potentially due to host DNA methylation‐sensitive nature of the proteins, as ectopic expression of a DNA adenosine methylase (Dam) gene of NGJ1 prevents TseTBg‐mediated degradation of plasmid DNA. This highlights that DNase activity of TseTBg proteins has functional similarity with endonucleases of Restriction–modification (R–M) systems (Vasu & Nagaraja, 2013). This may provide protection to NGJ1 against invasion of foreign DNA or assist in repair of replication error, through degradation of a newly synthesized strand of hemi‐methylated DNA (Oliveira & Fang, 2021). On the other hand, RNase activity of TseTBg proteins may enable NGJ1 to modulate its growth through degradation of cellular RNA, as postulated earlier in case of toxins of TA systems (Page & Peti, 2016; Thomet et al, 2019; Agarwal et al, 2020; Sierra et al, 2020). Indeed, heterologous expression of a variant of TseTBg2D116A,K129A (DNase−RNase+) imparts growth arrest but does not kill the recombinant E. coli cells. This suggests that the DNase/RNase activity of TseTBg proteins can be uncoupled. Similarly, previous reports had suggested that alteration in the active site of certain nucleases compromise the DNase but not the RNase activity (Lesniewicz et al, 2013; Dixit et al, 2016).
The expression of cognate immunity (TsiTBg) proteins protects from TseTBg effector‐mediated killing. This protection requires the presence of an Imm52 domain in the immunity protein, which has been previously postulated to be involved in effector neutralization (Zhang et al, 2012; Burkinshaw et al, 2018). Interestingly, the TsiTBg proteins also exhibit transcriptional repressor activity, dependent on the non‐canonical HTH motif. Bioinformatics analysis has revealed two potential promoters (pExtTseTBg and pIntTseTBg) in the TseTBg operon of NGJ1 and both of them can be repressed by the cognate TsiTBg proteins, suggesting the possibility of autoregulation.
Apparently, activation of pExtTseTBg can lead to co‐expression of various T6SS‐related proteins (PAAR, DUF4123, and hypothetical protein) along with TseTBg and TsiTBg proteins. As homologs of these proteins are important for T6SS‐mediated delivery of TseT in P. aeruginosa strain PAO1 (Burkinshaw et al, 2018), we anticipate that their co‐expression will enable T6SS‐mediated secretion of TseTBg proteins. Indeed, we observed that TseTBg effectors of NGJ1 are secreted in a T6SS‐2‐dependent manner. On the other hand, when the pIntTseTBg is active, only TseTBg and TsiTBg proteins can be expressed. Generally, certain carrier proteins that are encoded either as upstream ORFs in the operon or as additional domains of effectors are required for T6SS‐mediated delivery (Hachani et al, 2014; Liang et al, 2015; Lien & Lai, 2017; Wettstadt & Filloux, 2020). In the absence of the carrier and chaperone proteins, T6SS‐mediated secretion of TseTBg proteins will be compromised. We speculate that such configurations of proteins may enable intracellular function as TA systems. Similar to the toxins, TseTBg proteins exert antibacterial activity and can impart growth arrest while similar to the antitoxins of type II TA systems (Unterholzner et al, 2013; Goormaghtigh et al, 2018; Kang et al, 2018; Fraikin et al, 2020), the TsiTBg proteins protect the cells by transcriptional repression as well as neutralization of cognate effectors. Overall, this suggests that the TseTBg‐TsiTBg proteins of NGJ1 can potentially serve as effector–immunity pairs of T6SS or as TA proteins of a TA system.
In support of this hypothesis, we observed that TseTBg‐TsiTBg homologs are either encoded as T6SS effector–immunity pairs (when encoded along with T6SS‐related proteins; PAAR, DUF4123, and hypothetical protein) or as part of a TA system (when encoded without T6SS‐related proteins) in different Burkholderia as well as several other bacterial species. The presence of TseTBg homolog as TA in some strains of Burkholderia sp. and as T6SS effectors in other strains of the same species suggests that the TseTBg proteins might have evolved as T6SS effectors from an ancestral TA system. Further, sequence analysis of this locus in different B. pseudomallei strains has enabled us to identify some notable genetic events which might lead to the conversion of a TA into a T6SS effector. The insertion and excision of IS3 family transposable elements has created genomic rearrangements at a potential TA locus which led to the operonic fusion of T6SS‐related functions (PAAR, DUF4123, and hypothetical protein) with the TA encoding genes. The hypothetical protein encompasses the junction region and this ORF seems to be a result of operon fusion. We speculate that the evolution of hypothetical protein is a significant step toward conversion of TseTBg homolog into T6SS effector, as one of the homolog of hypothetical protein, i.e., co‐TecT plays an important role as co‐chaperone to assist the chaperone (TecT; a DUF4123 domain‐containing protein) in T6SS‐mediated delivery of TseT effector in P. aeruginosa strain PAO1 (Burkinshaw et al, 2018).
Several previously conducted studies have indicated that bacterial toxins can be co‐opted into intracellular functions (TA systems) or as extracellular polymorphic toxins (Zhang et al, 2012; Jamet & Nassif, 2015; Triplett et al, 2016; Harms et al, 2017; Stårsta et al, 2020). The results presented here indicate that TA systems can also be converted into extracellular T6SS effectors through action of insertion elements (and possibly other kinds of genetic changes), providing novel weapons in the bacterial armory to dominate during intense competition with co‐habiting bacteria. Other examples of toxins serving as extracellular weapons of bacteria include the VbhT, an inter‐bacterial type IV secretion system (T4SS) effector in Bartonella that has been potentially co‐opted from the FIC (filamentation induced by cAMP) domain‐containing FicT toxin of FicTA module (Harms et al, 2017), and the AvrRxo1, a type III secretion system (T3SS) effector of Xanthomonas oryzae pv. oryzicola (that modulate plant immune response) is related to the Zeta toxin of Streptococcus pyogenes (Han et al, 2015; Triplett et al, 2016). The data presented in this study not only describes the genetic co‐occurrence of TA‐ and T6SS‐related functions, but also represents real synthesis of findings that demonstrate the concept of T6SS stealing intracellular TA systems and converting them into extracellular T6SS effectors. By doing this, certain Burkholderia strains may have gained the ability to inhibit the growth of co‐habiting bacteria. In NGJ1, we have demonstrated that the co‐opted TseTBg effector enables the bacterium to restrict the growth of other bacteria. By doing this, NGJ1 may be reducing competition for resources, including those that are released upon degradation of fungal biomass.
Materials and Methods
Bacterial strains and growth conditions
The bacterium B. gladioli strain NGJ1 (rifR derivative of NGJ1) and its derivatives were grown on PDA (Potato Dextrose Agar; HiMedia, India) plates at 28°C. Escherichia coli and its derivatives were grown on LBA (Luria‐Bertani Agar; HiMedia, India) plates at 37°C. Agrobacterium tumefaciens strain EHA105 and the endophytic bacteria, S. maltophilia and Sphingomonas sp. (Isolated from surface‐sterilized leaves of ~ 45‐day‐old Pusa Basmati‐1 rice) were grown on PDA at 28°C. The Saccharomyces cerevisiae (yeast) strains were grown on Yeast Extract Peptone Dextrose (YPD; HiMedia, India) at 28°C. Whenever required, the media was supplemented with antibiotics: kanamycin, 50 µg/ml; rifampicin, 20 µg/ml; ampicillin, 50 µg/ml; and spectinomycin, 50 µg/ml. The list of bacterial/fungal strains and plasmids used in this study are summarized in Appendix Table S3.
Inter‐bacterial competition assay
The inter‐bacterial competition of NGJ1 with selected prey bacteria was set up as described previously (Jana et al, 2019). Briefly, the NGJ1 (or its derivatives) and the prey bacteria cells were mixed in 5:1 ratio and spotted on a LBA plate. Upon 4 h of incubation at 28°C, the spotted cells were re‐suspended in 1 ml LB broth and dilution plating was performed on antibiotic‐containing selection plates (PDA/LBA, depending upon the prey bacteria). The colony forming unit (cfu) of the surviving prey bacterial cells were counted. The cfu of the prey bacteria grown in the absence of NGJ1 (or its derivatives) was used as control. The experiment was independently repeated at least three times, and similar results were observed in each replicate.
In silico mining of T6SS apparatus and effector encoding gene clusters
The draft genome sequence of NGJ1 (Jha et al, 2015) was used for the identification of putative T6SS apparatus‐encoding gene clusters using a web‐based online tool; T346Hunter (Martínez‐García et al, 2015). The presence of two different T6SS apparatus in the NGJ1 genome was further verified using Burkholderia Genome Database (Winsor et al, 2008). Considering that T6SS effectors are predominantly encoded along with certain carrier proteins (Hcp, VgrG or PAAR) (Salomon et al, 2014; Shyntum et al, 2014; Liang et al, 2015; Lien & Lai, 2017), we carried out BlastN searches against Burkholderia Genome Database to identify paralogs of Hcp (AC179_RS13930; AC179_RS29610), VgrG (AC179_RS29655), and PAAR (AC179_RS13885) encoding genes in NGJ1 genome. The identified paralogs were found to be encoded along with potential effector, immunity, and certain chaperonic protein (DUF4123, DUF1795, and DUF2169)‐encoding genes in operonic fashion. A similar approach has been used in previous studies to identify potential T6SS effectors in different bacterial systems (Shyntum et al, 2014; Bondage et al, 2016; Barbosa & Lery, 2019; Repizo et al, 2019). The NCBI conserved domain database (Marchler‐Bauer et al, 2015) and Pfam database (Finn et al, 2014) were used to identify the conserved domains in proteins that are encoded in the effector operons.
Construction of T6SS mutants
Partial fragments (~ 300 bp) of one of the core T6SS apparatus genes of T6SS‐1 (TssB) and T6SS‐2 (TagJ) were PCR amplified from the genomic DNA of NGJ1 using gene‐specific primers (Appendix Table S4) and cloned into pK18mob vector. The recombinant plasmid was electroporated (Gene pulsar XcellTm; Bio‐Rad) into NGJ1, as per the method described in Swain et al (2017). The insertion mutants were selected on kanamycin‐ and rifampicin‐containing KBA (King's medium B Base; HiMedia, India) plates. The T6SS‐1− (insertion in TssB) and T6SS‐2− (insertion in TagJ) mutant strains were confirmed by PCR using gene‐specific flanking forward and vector‐specific reverse (M13) primers (Appendix Table S4).
Western blot analysis
Burkholderia gladioli strain NGJ1 and its various mutant strains were grown overnight in the presence of prey bacteria (E. coli DH5α) in 5:1 ratio. The cell‐free supernatant of overnight grown culture (100 ml) was precipitated using TCA (12% w/v) and dissolved in 2 ml PBS (10 mM) to obtain the supernatant (S) protein. Further to isolate total protein, bacterial pellet (obtained from 10 ml culture) was crushed in liquid N2 and dissolved in 2 ml of buffer (10 mM PBS: phosphate buffer saline; pH 7.4, 1 mM lysozyme, and 1 mM PMSF: phenyl methane sulfonyl fluoride). The soluble fraction collected upon centrifugation was used as crude protein extract of the pellet (P).
10 μg of protein samples were resolved on SDS–PAGE gel (12%) and electro‐blotted onto PVDF membrane, as described in (Swain et al, 2017). The membrane was individually probed with Hcp‐1 (T6SS‐1), Hcp‐2 (T6SS‐2), or TseTBg2‐specific polyclonal peptide antibodies (raised in rabbit by ABGENEX Pvt. Ltd., India, a service provider) at 1:25,000 dilutions. The alkaline phosphatase‐conjugated anti‐rabbit IgG (Sigma‐Aldrich) was used as secondary antibody (1:10,000 dilutions), and the blot was developed as per the manufacturer's instruction.
Effector–immunity functionality assay
The functionality of effector and immunity proteins was tested using the previously described approach (Ding et al, 2012; Brooks et al, 2013; Fitzsimons et al, 2018). The full‐length coding sequences of selected effector and immunity genes were PCR amplified from NGJ1 genomic DNA using gene‐specific primers (Appendix Table S4). The effector genes were cloned in pET23b (pET23b:effector), and the cognate immunity genes were cloned in pET28a (pET28a:immunity). Escherichia coli BL21 (DE3) cells were transformed with the pET23b:effector or co‐transformed with the cognate pET28a:immunity plasmid. The positive transformed bacterial cells were selected by colony PCR using gene‐specific primers (Appendix Table S4).
In order to test toxicity of T6SS effectors, the recombinant E. coli BL21 (DE3) cells were grown in 10 ml liquid media to mid‐log phase (O.D600 ~ 0.5) and protein expression was induced using 1 mM IPTG (Isopropyl β‐d‐1‐thiogalactopyranoside; Sigma‐Aldrich) for 3 h at 37°C. In control (‐IPTG), an equivalent amount of sterile distilled H2O was added instead of IPTG. Subsequently, the cells were serially diluted and spotted on appropriate antibiotics containing LBA plates and their growth was monitored. Similarly, survival of E. coli cells that co‐express effector and cognate immunity proteins was analyzed.
Expression of transcriptionally fused effector–immunity proteins
The complete coding sequences of TseTBg (TseTBg1, TseTBg2, and TseTBg3) effectors along with their cognate immunity (TsiTBg) genes were PCR amplified from NGJ1 using effector‐specific forward and immunity‐specific reverse primers (Appendix Table S4). The PCR products were cloned into pET28a to express transcriptionally fused effector–immunity proteins (TseTBg:TsiTBg). Similarly, the coding sequences of TseTBg effectors along with their cognate immunity genes without the Imm52 domain (putatively involved in cognate effector neutralization through physical binding) encoding sequence were PCR amplified and cloned into pET28a to obtain TseTBg:TsiTBgΔImm52 constructs, respectively. Subsequently, these constructs were individually transformed into BL21 (DE3) cells and positive transformants were selected by colony PCR. Upon 3h of IPTG induction, survival rate of the recombinant bacteria was analyzed.
Bacterial growth curve
1% of overnight grown cultures of recombinant BL21 (DE3) E. coli cells were inoculated in 100 ml of LB broth (with or without 1 mM IPTG supplementation) and incubated at 37°C with constant shaking. Optical density (OD600) of the bacterial cultures was measured at 2‐h time intervals and growth curve was plotted (time interval on x‐axis and OD600 on y‐axis).
DAPI staining and microscopic analysis
4′,6‐diamidino‐2‐phenylindole (DAPI) staining and microscopic analysis were performed to visualize cell death and nuclear degradation in the recombinant E. coli cells, as described previously (Pissaridou et al, 2018; Song et al, 2021). Briefly, 10 ml of recombinant E. coli (BL21) cells were grown in LB media containing appropriate antibiotics. 2 ml of culture was pelleted down upon 3h of 1 mM IPTG induction and washed thrice with PBS buffer (pH‐7.0). Subsequently, the cells were stained with DAPI staining solution (GeneTex, Catalog number: GTX16206) for 10 min in dark at 37°C. Upon washing with the buffer, cells were analyzed under fluorescence microscope (AOBS TCS‐SP8; LEICA, GERMANY) under DAPI filter (447 nm). The experiment was independently repeated three times, with a minimum of three technical repeats, and similar results were obtained.
Mapping of TseTBg operon
To investigate whether genes of TseTBg operon are expressed as a single transcriptional unit, reverse transcription‐PCR (RT–PCR) was performed. For this, the NGJ1 and the prey bacterial (E. coli or A. tumefaciens) culture were mixed in 5:1 ratio and grown for 12 h in PDB. The total RNA was isolated using TRIzol reagent (Invitrogen) and upon DNase I (Thermo Scientific) treatment for 30 min at 37°C, and 2 µg RNA of each sample was reverse transcribed into cDNA by using TsiTBg‐specific reverse primer (Appendix Table S4) and Superscript III RT enzyme (Invitrogen) as per the supplier’s instructions. Subsequently, the cDNA was used as a template for PCR amplification of PAAR and TseTBg genes of the operon, using gene‐specific primers (Appendix Table S4). PCR products were resolved on 1% agarose gel.
Bacterial two‐hybrid analysis
In order to understand the potential T6SS‐1/T6SS‐2‐mediated secretion of TseTBg effectors, bacterial two‐hybrid analysis was performed following an established protocol (Giesecke & Joung, 2007). Genes encoding the proteins of interest (X and Y) were PCR amplified from the NGJ1 genomic DNA using appropriate primers (Appendix Table S4) and cloned into pKNT25 and pUT18C vectors, respectively. The recombinant plasmids encoding the T25‐X and T18‐Y hybrid proteins were transformed into the competent BTH101 reporter cells. The interaction was visually monitored by appearance of blue color due to activity of β‐galactosidase on X‐gal (20 mg/ml; 5‐bromo‐4‐chloro‐3‐indolyl‐β‐D‐galactopyranoside). The bacterial cells transformed with plasmids pKT25‐zip and pUT18C‐zip served as positive controls and those with pKNT25 and pUT18C served as negative controls.
Yeast two‐hybrid analysis
Yeast two‐hybrid (Y2H) assay was performed using Matchmaker Gold Yeast Two‐Hybrid library screening system (Clontech, USA). The full‐length copy of PAAR and TseTBg effector protein‐encoding genes were PCR amplified from the NGJ1 genomic DNA using gene‐specific primer pairs (Appendix Table S4) and cloned into pGBKT7 and pGADT7 vectors, respectively. Both the recombinant plasmids were co‐transformed into Y2H Gold strain of yeast, following the manufacturer's instructions. Positive transformants were selected on synthetically defined media lacking leucine and tryptophan amino acids (SD‐Leu‐Trp; double dropout). The interaction assay was performed by growing positive transformed colonies at 30°C on synthetically defined media lacking leucine, tryptophan, histidine, and adenine (SD‐Leu‐Trp‐His‐Ade; quadruple dropout) but supplemented with 30 mM concentration of 3‐Amino‐1, 2, 4‐triazole (3AT). The yeast cells co‐transformed with pGBKT7‐P53 and pGADT7‐T (SV40 large T‐antigen) were used as positive control while the cells co‐transformed with pGBKT7 and pGADT7 (empty vectors) were used as negative control.
Construction of TseTBg2 mutant strain of NGJ1
Using a pK18mobsacB‐Km suicide plasmid, having kanamycin as selection and sucrose as counterselection marker, we knocked out one of the TseTBg (TseTBg2) gene in NGJ1 (Schäfer et al, 1994). For this, 5′ and 3′ sequences (each 250 bp) flanking to the TseTBg2 gene (fragment 1 and fragment 2) were PCR amplified from NGJ1 using appropriate primers mentioned in Appendix Table S4 and cloned into two different sites of the MCS of pK18mobsacB. The deletion construct thus obtained was electroporated (Gene pulsar XcellTm; Bio‐Rad) into NGJ1, as per the method described in Swain et al (2017). The single recombinants were selected on kanamycin‐ and rifampicin‐containing King's medium B Base (KBA; HiMedia) plates and were found to be sucrose sensitive. To obtain inframe deletion, the single recombinants were passaged for five generations in KB broth and dilution plating was performed to select the sucroseR and kanamycinS colonies, as per the methodology described in Kumar Verma et al (2018). The deletion of gene fragment was confirmed by colony PCR using flanking primers (Appendix Table S4).
Ectopic expression and purification of effector and immunity proteins
The gene‐encoding TseTBg, TsiTBg proteins, and their variants were cloned into pET28a and mobilized into E. coli BL21 (DE3) pLysS strain, which is generally used to isolate proteins that are toxic to bacterial cells (Rosano & Ceccarelli, 2014). 250 ml culture of the recombinant pLysS cells was grown up to O.D600 ~ 0.5 at 37°C and following 3 h of 1 mM IPTG induction, the bacterial pellet (obtained through centrifugation) was sonicated in extraction buffer (10 mM PBS; pH 7.4, 1 mg/ml lysozyme, 0.1% N‐Lauroylsarcosine sodium salt and 1 mM PMSF). The protein was purified using Ni2+‐NTA beads as per previously described method (Swain et al, 2017).
Gene synthesis of a variant of TseTBg2 protein (TseTBg2D116A,K129A)
The protein sequence of TseTBg effectors of NGJ1 was aligned with a previously reported Tox‐REase‐5 domain‐containing TseT effector protein of P. aeruginosa strain PAO1 using ClustalW algorithm (Thompson et al, 2003). Conservation of various amino acid residues including aspartate and lysine in the potential active site was noticed. We designed a variant TseTBg2 gene wherein nucleotide sequence corresponding to the conserved aspartate (D116) and lysine (K129) amino acid residues present in the native TseTBg2 protein were replaced by alanine (A) encoding nucleotides. The modified gene was synthesized and cloned into pET28a vector by a service provider (Gene Universal Inc.; Newark, USA). Upon sequence verification, the pET28a:TseTBg2D116AK129A construct was transformed into E. coli BL21 (DE3) as well as pLysS cells for further study.
Nuclease (DNase/RNase) assay
DNase assay was performed using 1 μg of lambda DNA (Thermo Scientific™), E. coli (DH5α) as well as NGJ1 genomic DNA (isolated using QIAamp genomic DNA kit; Qiagen), and plasmid (pET28a) DNA. Each of these DNA samples was treated with 20 pmol of the purified TseTBg1/TseTBg2 proteins for 30 min at 28°C. The buffer‐treated DNA was used as control.
Similarly, the DNase activity of TseTBg proteins was tested on 1 μg of pHM1 plasmid isolated from E. coli (DH5α). Also, the pHM1 was mobilized into NGJ1 cells through electroporation and the isolated plasmid was treated with TseTBg proteins. Further, the pHM1 plasmid isolated from NGJ1 was transformed into E. coli (DH5α) cells through heat‐shock method and isolated plasmid was treated with TseTBg proteins.
For RNase assay, 1 μg of NGJ1 as well as E. coli RNA (isolated using TRIzol reagent, Invitrogen) was either treated with 20 pmol of TseTBg effector proteins or along with equimolar concentration (20 pmol) of TsiTBg immunity proteins. The treatment with TsiTBg alone (20 pmol), buffer, or heat‐inactivated TseTBg protein was used as control. The RNase activity was also tested in the presence of RNaseOUTTm (a recombinant Ribonuclease inhibitor from Invitrogen that inhibits RNase (RNase A, RNase B, and RNase C) contaminations during sample preparations).
The treated DNA as well as RNA samples were resolved on 0.8% agarose gel and visualized under UV Trans‐illuminator (ChemiDoc MP System, Bio‐Rad).
Identification and characterization of DNA adenine methylase encoded in NGJ1
An ORF (AC179_RS12830) encoding DNA adenine methylase (Dam) was found to be encoded in the proximity of TseTBg1 operon in NGJ1. Also, BlastX search in the Burkholderia genome database revealed the presence of another paralog of the Dam protein (AC179_RS17275) at a different genomic locus in NGJ1 genome (having 98.4% identity with AC179_RS12830). The full‐length nucleotide sequence of one of the Dam gene (AC179_RS12830) was PCR amplified from NGJ1 genomic DNA using gene‐specific primers (Appendix Table S4), cloned in pET28a (pET28a:Dam), and transformed into E. coli BL21 (DE3) cells, using heat‐shock method. The plasmid was isolated from the recombinant cells, with or without 3 h of IPTG‐mediated induction of the Dam protein. Subsequently, the plasmid was treated with 20 pmol of TseTBg proteins for 30 min at 28°C and resolved on 0.8% agarose gel. The gel was visualized under UV Tran‐illuminator (ChemiDoc MP System, Bio‐Rad).
Promoter identification and characterization
The putative promoters of TseTBg (TseTBg1/TseTBg2) effector operon were identified using online prokaryotic promoter identification tool (Neural network promoter prediction (Reese, 2001)). The nucleotide sequence of the complete operon along with 1 kb upstream sequence was scanned. In this process, two promoter regions; namely the external promoter (pExtTseTBg1/pExtTseTBg2) upstream to the PAAR encoding gene and the internal promoter (pIntTseTBg1/pIntTseTBg2) present upstream to the effector (TseTBg) gene were predicted.
Further, the complete nucleotide sequence of both the promoter regions were PCR amplified (500 bp for pExtTseTBg1/pExtTseTBg2 and 350 bp for pIntTseTBg1/pIntTseTBg2) from NGJ1 genomic DNA using promoter‐specific primers mentioned in Appendix Table S4. The PCR‐amplified DNA fragments were cloned in pBI101 (pBI101:pExtTseTBg1, pBI101:pExtTseTBg2, pBI101:pIntTseTBg1, and pBI101:pIntTseTBg2) upstream to the Gus (β‐glucuronidase) gene. Each of these constructs were transformed into A. tumefaciens strain EHA105 as well as E. coli BL21 cells and the Gus expression was visualized by the appearance of blue color in the presence of 5‐bromo‐4‐chloro‐3‐indolyl glucuronide (X‐Gluc) (Platteeuw et al, 1994). Empty vector (pBI101)‐transformed cells were used as control.
Helix–turn–helix motif (DNA‐binding region) prediction
BlastX analysis was carried out using default parameters to identify homologs of TsiTBg proteins in NCBI database, and the presence of potential HTH domain was predicted using GYM2.0 tool (Narasimhan et al, 2002). The consensus HTH motif of the positive hits was compiled and used for HTH motif scanning (e‐value > 10−5) in TsiTBg proteins of NGJ1 as well as their homologs in other bacteria, using FIMO tool (Grant et al, 2011). The secondary structures of sequences were predicted using PSIPRED (McGuffin et al, 2000), and β‐turn regions were predicted using NetTurnP 1.0 tool (Petersen et al, 2010). The HTH motif predicted by FIMO tool was found to be overlapping with the predicted secondary structure of TsiTBg proteins.
Construction of HTH and Imm52 domain‐deleted variants of TsiTBg
The coding sequences of TsiTBg (TsiTBg1/TsiTBg2) proteins of NGJ1 were PCR amplified without HTH domain/Imm52 domain encoding regions using specific primers (Appendix Table S4). The amplified PCR products were further cloned into pET23b to obtain pET23b:TsiTBg1ΔHTH/pET23b:TsiTBg2ΔHTH and pET23b:TsiTBg1ΔImm52/pET23b:TsiTBg2ΔImm52 constructs.
Trans‐repression assay
The expression of Gus reporter gene (β‐glucuronidase) under pExtTseTBg/pIntTseTBg promoter (pBI101:promoter:Gus) was analyzed in recombinant E. coli BL21 (DE3) cells that express TsiTBg immunity proteins (pET28a:TsiTBg) or their variants (pET23a:TsiTBgΔImm52/pET23a:TsiTBgΔHTH). The expression of β‐glucuronidase protein was quantified spectrophotometrically using a fluorescent substrate; 4‐methylumbelliferyl ß‐d‐glucuronide (4‐MUG) (Ramsay, 2013). Furthermore, the expression of β‐glucuronidase was visualized as appearance of blue color using 5‐bromo‐4‐chloro‐3‐indolyl glucuronide (X‐Gluc) as substrate (Platteeuw et al, 1994).
Electromobility shift assay
Electromobility shift assay was performed to validate binding of TsiTBg1/TsiTBg1ΔHTH proteins with the promoter DNA of TseTBg1/TseTBg2 operons, using previously described method (Stead et al, 2006; Ream et al, 2016). The DNA fragment containing promoter region (500 bp for pExtTseTBg1/pExtTseTBg2 and 350 bp for pIntTseTBg1/pIntTseTBg2) was PCR amplified using appropriate primers (Appendix Table S4) and used as DNA probe. The DNA–protein‐binding reaction was performed in a 100 μl reaction volume containing 10 mM Tris–HCl; pH 7.5, 10 mM NaCl, 1 mM DTT, 100 μg/ml BSA, DNA probe (5 pmol), and different concentrations (2, 10, 20, 40, and 80 pmol) of the purified protein. The reaction was incubated for 1 h at 4°C followed by electrophoresis on 0.8% agarose gel. The DNA was visualized upon staining with SYBR‐green EMSA dye (Invitrogen, Catalog no. E33075) under ChemiDoc XRS+ gel imaging system (Bio‐Rad). The same gel was subsequently stained with SYPRO Ruby EMSA dye (Invitrogen, Catalog no. E33075) for visualization of protein.
Phylogenetic analysis
The homologs of TseTBg proteins were identified and downloaded from Pfam database (Finn et al, 2014) using Tox‐REase 5 domain (PF15648) as query keyword. After removal of duplicate entries corresponding to the same bacterial strain, a total of 121 protein sequences were aligned using ClustalW algorithm (Thompson et al, 2003). The sequences were trimmed using trimal tool (Capella‐Gutiérrez et al, 2009) to generate a uniform aligned sequence containing 713 positions in the final dataset (Dataset EV3). A comprehensive multi‐furcating tree was constructed using Maximum Likelihood (ML) and JTT matrix‐based model using MEGAX (Kumar et al, 2018). To increase the robustness and reproducibility of the phylogenetic tree, RAxML tool was used to construct a scalable ML tree (Stamatakis et al, 2012).
Further, gene neighborhood analysis was carried out using webFlaGs tool (Saha et al, 2020). The NCBI id of the TseTBg homologs was used as input and the flanking gene‐encoded proteins were clustered using HMM‐based JackHMMER method, as per default parameters. The to‐scale color‐coded representation as well as description of flanking genes was obtained as output (Fig 6 and Dataset EV2).
Evolutionary analysis
The genomic organization of TseTBg and TsiTBg homologs were studied in different Burkholderia sp. using Burkholderia Genome Database (Winsor et al, 2008). In most of the strains, these proteins were found to be encoded along with certain T6SS related proteins (PAAR, DUF4123: a chaperon and a hypothetical protein) and we considered them as candidate T6SS effectors. However, in some strains, only TseTBg and TsiTBg homologs were encoded in the operon and the T6SS‐related proteins were absent. These homologs were considered as part of TA system. Further, the presence of IS3 family transposases was detected at these loci. The conservation of flanking genes was analyzed in Burkholderia Genome Database (Winsor et al, 2008).
Statistical analysis
One‐way ANOVA and paired t‐test analysis were performed using Sigma Plot 12.0 (SPSS, Inc. Chicago, IL, USA). Statistical significance was calculated at P < 0.01 or P < 0.05 and mentioned in the figure legend, wherever required.
Author contributions
Overall planning and study supervision: GJ. Initiation of the project and execution of most of the experiments: SKY. Assistance in characterization of T6SS effectors and their antibacterial property: AM and AK. Phylogenetic analysis and assistance in computational analysis: SG. Assistance in proteins isolation: AP. Assistance in molecular cloning: RK, JD, and MS. Manuscript writing: SKY, SG, and GJ. Manuscript approval: All authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 5
Source Data for Expanded View
Acknowledgements
SKY and JD acknowledge fellowship from DBT, Govt. of India. AP acknowledges fellowship from UGC, Govt. of India. SG and RK acknowledge SPM and SRA fellowship from CSIR, Govt. of India, respectively. GJ acknowledges Swarna Jayanti Fellowship from DST, Govt. of India. We sincerely thank RV Sonti (NIPGR) for providing E. coli strains, Manjula Reddy (CSIR‐CCMB) for sharing different strains/plasmids for bacterial two‐hybrid assay. We also acknowledge Prabhu Patil and Kanika Bansal (IMTECH, Chandigarh) for valuable discussion and suggestions on genome analysis. Deepti Jain (RCB, Faridabad) is acknowledged for suggestion on HTH domain prediction. RV Sonti is greatly acknowledged for valuable suggestions and comments on the manuscript. This work was supported by core research grant from National Institute of Plant Genome Research, India. Also, funding from DBT, Govt. of India, to support research in GJ laboratory is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
EMBO reports (2021) 22: e51857.
Footnotes
Correction added on 4 June 2021, after first online publication: Figure 5B has been replaced; see the associated Corrigendum at https://doi.org/10.15252/embr.202153112
Data availability
No primary datasets have been generated and deposited. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- Agarwal S, Sharma A, Bouzeyen R, Deep A, Sharma H, Mangalaparthi KK, Datta KK, Kidwai S, Gowda H, Gowda H et al (2020) VapBC22 toxin‐antitoxin system from Mycobacterium tuberculosis is required for pathogenesis and modulation of host immune response. Sci Adv 6: eaba6944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcoforado Diniz J, Liu YC, Coulthurst SJ (2015) Molecular weaponry: diverse effectors delivered by the Type VI secretion system. Cell Microbiol 17: 1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Vonaesch P, Saffarian A, Marteyn BS, Sansonetti PJ (2017) Shigella sonnei encodes a functional T6SS used for interbacterial competition and niche occupancy. Cell Host Microbe 21: 769–776.e3 [DOI] [PubMed] [Google Scholar]
- Barbosa VAA & Lery LMS (2019) Insights into Klebsiella pneumoniae type VI secretion system transcriptional regulation. BMC Genom 20: 2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz J, Meinhart A (2014) Antibacterial effector/immunity systems: it's just the tip of the iceberg. Curr Opin Microbiol 17: 1–10 [DOI] [PubMed] [Google Scholar]
- Bernal P, Llamas MA, Filloux A (2018) Type VI secretion systems in plant‐associated bacteria. Environ Microbiol 20: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondage DD, Lin JS, Ma LS, Kuo CH, Lai EM (2016) VgrG C terminus confers the type VI effector transport specificity and is required for binding with PAAR and adaptor‐effector complex. Proc Natl Acad Sci USA 113: E3931–E3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks TM, Unterweger D, Bachmann V, Kostiuk B, Pukatzki S (2013) Lytic activity of the Vibrio cholerae type VI secretion toxin VgrG‐3 is inhibited by the antitoxin TsaB. J Biol Chem 288: 7618–7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkinshaw BJ, Liang X, Wong M, Le ANH, Lam L, Dong TG (2018) A type VI secretion system effector delivery mechanism dependent on PAAR and a chaperone‐co‐chaperone complex. Nat Microbiol 3: 632–640 [DOI] [PubMed] [Google Scholar]
- Capella‐Gutiérrez S, Silla‐Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large‐scale phylogenetic analyses. Bioinformatics 25: 1972–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Cascales E (2018) Antibacterial weapons: targeted destruction in the microbiota. Trends Microbiol 26: 329–338 [DOI] [PubMed] [Google Scholar]
- Cherrak Y, Rapisarda C, Pellarin R, Bouvier G, Bardiaux B, Allain F, Malosse C, Rey M, Chamot‐Rooke J, Cascales E et al (2018) Biogenesis and structure of a type VI secretion baseplate. Nat Microbiol 3: 1404–1416 [DOI] [PubMed] [Google Scholar]
- Cianfanelli FR, Alcoforado Diniz J, Guo M, De Cesare V, Trost M, Coulthurst SJ (2016a) VgrG and PAAR proteins define distinct versions of a functional type VI secretion system. PLoS Pathog 12: e1005735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfanelli FR, Monlezun L, Coulthurst SJ (2016b) Aim, load, fire: the type VI secretion system, a bacterial nanoweapon. Trends Microbiol 24: 51–62 [DOI] [PubMed] [Google Scholar]
- Compant S, Nowak J, Coenye T, Clément C, Ait Barka E (2008) Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol Rev 32: 607–626 [DOI] [PubMed] [Google Scholar]
- Coulthurst SJ (2013) The type VI secretion system ‐ a widespread and versatile cell targeting system. Res Microbiol 164: 640–654 [DOI] [PubMed] [Google Scholar]
- Coulthurst S (2019) The type VI secretion system: a versatile bacterial weapon. Microbiology (United Kingdom) 165: 503–515 [DOI] [PubMed] [Google Scholar]
- Das J, Yadav SK, Ghosh S, Tyagi K, Magotra A, Krishnan A, Jha G (2021) Enzymatic and non‐enzymatic functional attributes of plant microbiome. Curr Opin Biotechnol 69: 162–171 [DOI] [PubMed] [Google Scholar]
- Ding J, Wang W, Feng H, Zhang Y, Wang DC (2012) Structural insights into the Pseudomonas aeruginosa type VI virulence effector tse1 bacteriolysis and self‐protection mechanisms. J Biol Chem 287: 26911–26920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit B, Ghosh KK, Fernandes G, Kumar P, Gogoi P, Kumar M (2016) Dual nuclease activity of a Cas2 protein in CRISPR‐Cas subtype I‐B of Leptospira interrogans. FEBS Lett 590: 1002–1016 [DOI] [PubMed] [Google Scholar]
- Eberl L, Vandamme P (2016) Members of the genus Burkholderia: good and bad guys [version 1; referees: 3 approved]. F1000Research 5: 1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J et al (2014) Pfam: the protein families database. Nucleic Acids Res 42: D222–D230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons TC, Lewis JM, Wright A, Kleifeld O, Schittenhelm RB, Powell D, Harper M, Boyce JD (2018) Identification of novel Acinetobacter baumannii type VI secretion system antibacterial effector and immunity pairs. Infect Immun 86: e00297‐18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flórez LV, Scherlach K, Miller IJ, Rodrigues A, Kwan JC, Hertweck C, Kaltenpoth M (2018) An antifungal polyketide associated with horizontally acquired genes supports symbiont‐mediated defense in Lagria villosa beetles. Nat Commun 9: 2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraikin N, Goormaghtigh F, Van Melderen L (2020) Type II toxin‐antitoxin systems: evolution and revolutions. J Bacteriol 202: e00763–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke AV, Joung JK (2007) The bacterial two‐hybrid system as a reporter system for analyzing protein‐protein interactions. Cold Spring Harb Protoc 2007: pdb.prot4672 [DOI] [PubMed] [Google Scholar]
- Goormaghtigh F, Fraikin N, Putrinš M, Hallaert T, Hauryliuk V, Garcia‐Pino A, Sjödin A, Kasvandik S, Udekwu K, Tenson T et al (2018) Reassessing the role of type II toxin‐antitoxin systems in formation of Escherichia coli type II persister cells. MBio 9: e00640–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CE, Bailey TL, Noble WS (2011) FIMO: scanning for occurrences of a given motif. Bioinformatics 27: 1017–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachani A, Allsopp LP, Oduko Y, Filloux A (2014) The VgrG proteins are ‘à la carte’ delivery systems for bacterial type VI effectors. J Biol Chem 289: 17872–17884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Zhou C, Wu S, Liu Y, Triplett L, Miao J, Tokuhisa J, Deblais L, Robinson H, Leach JE et al (2015) Crystal structure of Xanthomonas AvrRxo1‐ORF1, a type III effector with a polynucleotide kinase domain, and its interactor AvrRxo1‐ORF2. Structure 23: 1900–1909 [DOI] [PubMed] [Google Scholar]
- Harms A, Liesch M, Körner J, Québatte M, Engel P, Dehio C (2017) A bacterial toxin‐antitoxin module is the origin of inter‐bacterial and inter‐kingdom effectors of Bartonella. PLoS Genet 13: e1007077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms A, Brodersen DE, Mitarai N, Gerdes K (2018) Toxins, targets, and triggers: an overview of toxin‐antitoxin biology. Mol Cell 70: 768–784 [DOI] [PubMed] [Google Scholar]
- Hibbing ME, Fuqua C, Parsek MR, Peterson SB (2010) Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 8: 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BT, Dong TG, Mekalanos JJ (2014) A view to a kill: the bacterial type VI secretion system. Cell Host Microbe 15: 9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet A, Nassif X (2015) New players in the toxin field: Polymorphic toxin systems in bacteria. MBio 6: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana B, Fridman CM, Bosis E, Salomon D (2019) A modular effector with a DNase domain and a marker for T6SS substrates. Nat Commun 10: 3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha G, Tyagi I, Kumar R, Ghosh S (2015) Draft genome sequence of broad‐spectrum antifungal bacterium Burkholderia gladioli strain NGJ1, isolated from healthy rice seeds. Genome Announc 3: e00803–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Kim DH, Jin C, Lee BJ (2018) A systematic overview of type II and III toxin‐antitoxin systems with a focus on druggability. Toxins (Basel) 10: 515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Verma R, Samal B, Chatterjee S (2018) Xanthomonas oryzae pv. oryzae chemotaxis components and chemoreceptor Mcp2 are involved in the sensing of constituents of xylem sap and contribute to the regulation of virulence‐associated functions and entry into rice. Mol Plant Pathol 19: 2397–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoux M, Culviner PH, Liu YJ, Littlehale ML, Laub MT (2020) Stress can induce transcription of toxin‐antitoxin systems without activating toxin. Mol Cell 79: 280–292.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewicz K, Karlowski WM, Pienkowska JR, Krzywkowski P, Poreba E (2013) The plant s1‐like nuclease family has evolved a highly diverse range of catalytic capabilities. Plant Cell Physiol 54: 1064–1078 [DOI] [PubMed] [Google Scholar]
- Liang X, Moore R, Wilton M, Wong MJQ, Lam L, Dong TG (2015) Identification of divergent type VI secretion effectors using a conserved chaperone domain. Proc Natl Acad Sci USA 112: 9106–9111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YW, Lai EM (2017) Type VI secretion effectors: methodologies and biology. Front Cell Infect Microbiol 7: 2235–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler‐Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI et al (2015) CDD: NCBI’s conserved domain database. Nucleic Acids Res 43: D222–D226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐García PM, Ramos C, Rodríguez‐Palenzuela P (2015) T346Hunter: a novel web‐based tool for the prediction of type III, type IV and type VI secretion systems in bacterial genomes. PLoS One 10: e0119317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT (2000) The PSIPRED protein structure prediction server. Bioinformatics 16: 404–405 [DOI] [PubMed] [Google Scholar]
- Narasimhan G, Bu C, Gao Y, Wang X, Xu N, Mathee K (2002) Mining protein sequences for motifs. J Comput Biol 9: 707–720 [DOI] [PubMed] [Google Scholar]
- Oliveira PH, Fang G (2021) Conserved DNA Methyltransferases: a window into fundamental mechanisms of epigenetic regulation in bacteria. Trends Microbiol 29: 28–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R, Peti W (2016) Toxin‐antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol 12: 208–214 [DOI] [PubMed] [Google Scholar]
- Park YJ, Lacourse KD, Cambillau C, DiMaio F, Mougous JD, Veesler D (2018) Structure of the type VI secretion system TssK–TssF–TssG baseplate subcomplex revealed by cryo‐electron microscopy. Nat Commun 9: 5385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B, Lundegaard C, Petersen TN (2010) NetTurnP ‐ neural network prediction of beta‐turns by use of evolutionary information and predicted protein sequence features. PLoS One 5: e15079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissaridou P, Allsopp LP, Wettstadt S, Howard SA, Mavridou DAI, Filloux A (2018) The Pseudomonas aeruginosa T6SS‐VgrG1b spike is topped by a PAAR protein eliciting DNA damage to bacterial competitors. Proc Natl Acad Sci USA 115: 12519–12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platteeuw C, Simons G, De Vos WM (1994) Use of the Escherichia coli β‐glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl Environ Microbiol 60: 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay J (2013) High‐throughput β‐galactosidase and β‐glucuronidase assays using fluorogenic substrates. Bio‐Protocol 3: e827 [Google Scholar]
- Ream JA, Lewis LK, Lewis KA (2016) Rapid agarose gel electrophoretic mobility shift assay for quantitating protein: RNA interactions. Anal Biochem 511: 36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese MG (2001) Application of a time‐delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput Chem 26: 51–56 [DOI] [PubMed] [Google Scholar]
- Repizo GD, Espariz M, Seravalle JL, Salcedo SP (2019) Bioinformatic analysis of the type VI secretion system and its potential toxins in the Acinetobacter genus. Front Microbiol 10: 2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5: 172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz FM, Santillana E, Spínola‐Amilibia M, Torreira E, Culebras E, Romero A (2015) Crystal structure of hcp from Acinetobacter baumannii: a component of the type VI secretion system. PLoS One 10: e013697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Hood RD, Bui NK, Leroux M, Vollmer W, Mougous JD (2011) Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475: 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Leroux M, Hathazi K, Agnello DM, Ishikawa T, Wiggins PA, Wai SN, Mougous JD (2013) Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496: 508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AB, Peterson SB, Mougous JD (2014) Type VI secretion system effectors: poisons with a purpose. Nat Rev Microbiol 12: 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha CK, Sanches Pires R, Brolin H, Delannoy M, Atkinson GC (2020) FlaGs and webFlaGs: discovering novel biology through the analysis of gene neighbourhood conservation. Bioinformatics bta9788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D, Kinch LN, Trudgian DC, Guo X, Klimko JA, Grishin NV, Mirzaei H, Orth K (2014) Marker for type VI secretion system effectors. Proc Natl Acad Sci USA 111: 9271–9276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana TG, Lugo KA, Monack DM (2017) T6SS: the bacterial ‘fight club’ in the host gut. PLoS Pathog 13: e1006325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A, Tauch A, Jsger W, Kalinowski J, Thierbachb G, Piihler A (1994) pK18mobsacB. Gene 145: 69–73 [DOI] [PubMed] [Google Scholar]
- Shneider MM, Buth SA, Ho BT, Basler M, Mekalanos JJ, Leiman PG (2013) PAAR‐repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500: 350–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyntum DY, Venter SN, Moleleki LN, Toth I, Coutinho TA (2014) Comparative genomics of type VI secretion systems in strains of Pantoea ananatis from different environments. BMC Genom 15: 163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra R, Prados J, Panasenko OO, Andrey DO, Fleuchot B, Redder P, Kelley WL, Viollier PH, Renzoni A (2020) Insights into the global effect on Staphylococcus aureus growth arrest by induction of the endoribonuclease MazF toxin. Nucleic Acids Res 48: 8545–8561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Brunet YR, Cascales E, Mougous JD (2012) Structure and regulation of the type VI secretion system. Annu Rev Microbiol 66: 453–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Agnello DM, Zheng H, Andrews BT, Li M, Catalano CE, Gonen T, Mougous JD (2013) Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol Cell 51: 584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Pan J, Yang Y, Zhang Z, Cui R, Jia S, Wang Z, Yang C, Xu L, Dong TG et al (2021) Contact‐independent killing mediated by a T6SS effector with intrinsic cell‐entry properties. Nat Commun 12: 423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Aberer AJ, Goll C, Smith SA, Berger SA, Izquierdo‐Carrasco F (2012) RAxML‐Light: A tool for computing terabyte phylogenies. Bioinformatics 28: 2064–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stårsta M, Hammarlof DL, Waneskog M, Schlegel S, Xu F, Gynnå AH, Borg M, Herschend S, Koskiniemi S (2020) RHS‐elements function as type II toxinantitoxin modules that regulate intramacrophage replication of Salmonella Typhimurium. PLoS Genet 16: e1008607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead JA, Keen JN, McDowall KJ (2006) The identification of nucleic acid‐interacting proteins using a simple proteomics‐based approach that directly incorporates the electrophoretic mobility shift essay. Mol Cell Proteomics 5: 1697–1702 [DOI] [PubMed] [Google Scholar]
- Swain DM, Yadav SK, Tyagi I, Kumar R, Kumar R, Ghosh S, Das J, Jha G (2017) A prophage tail‐like protein is deployed by Burkholderia bacteria to feed on fungi. Nat Commun 8: 404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomet M, Trautwetter A, Ermel G, Blanco C (2019) Characterization of HicAB toxin‐antitoxin module of Sinorhizobium meliloti . BMC Microbiol 19: 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG (2003) Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinforma 2.3.1–2.3.22 [DOI] [PubMed] [Google Scholar]
- Triplett LR, Shidore T, Long J, Miao J, Wu S, Han Q, Zhou C, Ishihara H, Li J, Zhao B et al (2016) AvrRxo1 Is a Bifunctional type III Secreted effector and toxin‐antitoxin system component with homologs in diverse environmental contexts. PLoS One 11: e0158856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner SJ, Poppenberger B, Rozhon W (2013) Toxin–antitoxin systems. Mob Genet Elements 3: e26219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterweger D, Kostiuk B, Ötjengerdes R, Wilton A, Diaz‐Satizabal L, Pukatzki S (2015) Chimeric adaptor proteins translocate diverse type VI secretion system effectors in Vibrio cholerae . EMBO J 34: 2198–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker H, Sass A, Dhondt I, Nelis HJ, Coenye T (2014) Involvement of toxin‐antitoxin modules in Burkholderia cenocepacia biofilm persistence. Pathog Dis 71: 326–335 [DOI] [PubMed] [Google Scholar]
- Vasu K, Nagaraja V (2013) Diverse functions of restriction‐modification systems in addition to cellular defense. Microbiol Mol Biol Rev 77: 53–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Brackmann M, Castaño‐Díez D, Kudryashev M, Goldie KN, Maier T, Stahlberg H, Basler M (2017) Cryo‐EM structure of the extended type VI secretion system sheath‐tube complex. Nat Microbiol 2: 1507–1512 [DOI] [PubMed] [Google Scholar]
- Wen Y, Behiels E, Devreese B (2014) Toxin‐Antitoxin systems: their role in persistence, biofilm formation, and pathogenicity. Pathog Dis 70: 240–249 [DOI] [PubMed] [Google Scholar]
- Wettstadt S, Filloux A (2020) Manipulating the type VI secretion system spike to shuttle passenger proteins. PLoS One 15: e0228941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JC, Beck CM, Goo YA, Russell AB, Harding BN, De Leon JA, Cunningham DA, Tran BQ, Low DA, Goodlett DR et al (2014) Genetically distinct pathways guide effector export through the type VI secretion system. Mol Microbiol 92: 529–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor GL, Khaira B, Van Rossum T, Lo R, Whiteside MD, Brinkman FSL (2008) The Burkholderia genome database: facilitating flexible queries and comparative analyses. Bioinformatics 24: 2803–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TE, Howard SA, Wettstadt S, Filloux A (2019) PAAR proteins act as the ‘sorting hat’ of the type VI vsecretion system. Microbiology (United Kingdom) 165: 1208–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QE, Walsh TR (2017) Toxin‐antitoxin systems and their role in disseminating and maintaining antimicrobial resistance. FEMS Microbiol Rev 41: 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Long M, Shen X (2018) Effector–immunity pairs provide the T6SS nanomachine its offensive and defensive capabilities. Molecules 23: 1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L (2012) Polymorphic toxin systems: comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct 7: 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Review Process File
Source Data for Figure 1
Source Data for Figure 3
Source Data for Figure 5
Source Data for Expanded View
Data Availability Statement
No primary datasets have been generated and deposited. The data supporting the findings of this study are available from the corresponding author upon reasonable request.


