Abstract
Timely removal of dying or pathogenic cells by phagocytes is essential to maintaining host homeostasis. Phagocytes execute the clearance process with high fidelity while sparing healthy neighboring cells, and this process is at least partially regulated by the balance of “eat‐me” and “don't‐eat‐me” signals expressed on the surface of host cells. Upon contact, eat‐me signals activate “pro‐phagocytic” receptors expressed on the phagocyte membrane and signal to promote phagocytosis. Conversely, don't‐eat‐me signals engage “anti‐phagocytic” receptors to suppress phagocytosis. We review the current knowledge of don't‐eat‐me signaling in normal physiology and disease contexts where aberrant don't‐eat‐me signaling contributes to pathology.
Keywords: ‘don't‐eat‐me’, anti‐phagocytic receptor, efferocytosis, ITIM, phagocytosis
Subject Categories: Immunology, Membrane & Intracellular Transport, Molecular Biology of Disease
Timely removal of dying or pathogenic cells is essential for host homeostasis. This review summarizes the current knowledge of don't‐eat me signaling in normal physiology and highlights how aberrant don't eat me signaling contributes to pathology.
Glossary
- 18F‐FDG
fluorodeoxyglucose 18F
- Aβ
amyloid beta
- AD
Alzheimer's disease
- ADCP
antibody‐dependent cellular phagocytosis
- AIHA
autoimmune hemolytic anemia
- B2M
beta‐2 microglobulin
- C3
complement component 3
- cDC2
classical dendritic cell 2
- dLGN
dorsal lateral geniculate nucleus
- F4/80
EGF‐like module‐containing mucin‐like hormone receptor‐like 1 (EMR1)
- FAK
focal adhesion kinase
- Fc
fragment crystallizable
- Gas6
growth arrest‐specific protein 6
- Grb2
growth factor receptor‐bound protein 2
- ICAM‐3
intracellular adhesion molecule‐3
- IFNγ
interferon gamma
- Ig
immunoglobulin
- IL‐10
interleukin 10
- IL‐6
interleukin 6
- ITIM
immunoreceptor tyrosine‐based inhibitory motif
- ITSM
immunoreceptor tyrosine‐based switch motif
- LILRB1
leukocyte immunoglobulin‐like receptor B1
- LRP1
low‐density lipoprotein receptor‐related protein 1
- mAb
monoclonal antibody
- MerTK
mer proto‐oncogene tyrosine kinase
- mev
viable motheaten
- MFG‐E8
milk fat globule EGF factor VIII
- MHC class I
major histocompatibility complex class I
- MI
myocardial infarction
- MIAT
myocardial infarction‐associated transcript
- MyD88
myeloid differentiation primary response 88
- NF‐κB
nuclear factor‐kappa B
- NK
natural Killer
- NOD
non‐obese diabetic
- NSG
NOD scid gamma
- PD‐1
programmed cell death protein 1
- PD‐L1
programmed death ligand 1
- PD‐L2
programmed death ligand 2
- PECAM‐1
platelet endothelial cell adhesion molecule‐1
- PET/CT
positron emission tomography/computed tomography
- PI3K
phosphoinositide 3‐kinase
- PLCγ
phospholipase C gamma
- PS
phosphatidylserine
- Pyk2
proline‐rich tyrosine kinase 2
- RGC
retinal ganglion cells
- RhoA
Ras homolog family member A
- RIG‐I
retinoic acid‐inducible gene I
- SFK
Src family kinase
- SH2
Src homology 2
- SH3
Src homology 3
- SHIP
Src homology 2 domain‐containing inositol polyphosphate 5‐phosphatase
- SHP‐1
Src homology region 2 domain‐containing phosphatase‐1
- SHP‐2
Src homology region 2 domain‐containing phosphatase‐2
- Siglec
sialic acid‐binding immunoglobulin‐type lectin
- SIRPα
signal regulatory protein α
- SMCs
smooth muscle cells
- SP‐A
surfactant protein‐A
- SP‐D
surfactant protein‐D
- Syk
spleen tyrosine kinase
- TAM
tumor‐associated macrophage
- TGFβ
transforming growth factor β
- TIDC
tumor‐infiltrating dendritic cell
- TLR
Toll‐like receptor
- TNFα
tumor necrosis factor α
- TSP
thrombospondin
Introduction
Removal of unwanted or noxious cells is important for proper development, tissue integrity, and protection against pathogenic and immunogenic damage to the host (Arandjelovic & Ravichandran, 2015). Phagocytosis is a highly efficient process, and dying cells are rarely observed in vivo during homeostasis despite routine cellular turnover in several tissues (Elliott & Ravichandran, 2016). The “clearance crew” mediating phagocytosis can be divided into two broad categories: “professional phagocytes” and “non‐professional phagocytes” (Arandjelovic & Ravichandran, 2015). Innate immune cells (e.g., immature dendritic cells, monocyte‐derived macrophages, tissue‐resident macrophages, and microglia) are considered professional phagocytes as they can sense and migrate toward target cells, as well as rapidly and successively internalize multiple targets (Elliott et al, 2009; Ariel & Ravichandran, 2016; Medina et al, 2020). Other tissue‐resident cells (e.g., epithelial cells, fibroblasts, and endothelial cells) also play important roles in phagocytosis, albeit less efficiently, and are considered non‐professional phagocytes (Wood et al, 2000; Monks et al, 2005; Juncadella et al, 2013; Arandjelovic & Ravichandran, 2015; Lee et al, 2016; Davies et al, 2018). The phagocytic response can be described in four phases: “smell”, “taste”, “ingestion”, and “digestion/response”; and the molecular details promoting the clearance of dying cells have been excellently reviewed (Arandjelovic & Ravichandran, 2015; Morioka et al, 2019).
The phagocytic process must be tightly controlled, however, to prevent unwarranted removal of healthy cells. Regulation during the taste phase involves “integrated” recognition of eat‐me signals and don't‐eat‐me signals expressed on the target cell. Eat‐me signals include antibody and complement opsonins, exposed phosphatidylserine (PS), calreticulin, oxidized low‐density lipoprotein, cell‐bound thrombospondin (TSP), modified intracellular adhesion molecule ICAM‐3, annexin I, and other modifications to surface proteins (Arandjelovic & Ravichandran, 2015). While living cells generally do not express eat‐me signals, there are specific physiologic contexts when some living cells transiently express markers that partially mimic a dying cell such as during lymphocyte activation, skeletal muscle formation, and sperm–egg fusion (Elliott et al, 2005; Gardai et al, 2005; Hochreiter‐Hufford et al, 2013; Hochreiter‐Hufford et al, 2015; Rival et al, 2019). Thus, the expression of don't‐eat‐me signals provides an additional regulatory mechanism to prevent unwarranted clearance of “healthy” cells.
In this review, we discuss the current knowledge of don't‐eat‐me signaling in phagocytosis with an emphasis on anti‐phagocytic receptors that mediate the recognition of don't‐eat‐me signals. Most known anti‐phagocytic receptors are single‐pass, type I transmembrane proteins belonging to the immunoglobulin (Ig) superfamily that contain one or more immunoreceptor tyrosine‐based inhibitory motifs (ITIM) in their cytoplasmic tail (Daeron et al, 2008). The canonical ITIM consensus sequence is (I/V/L)xYxx(L/V), where x can be any amino acid. Many anti‐phagocytic receptors also have non‐canonical “ITIM‐like” motifs with a more divergent sequence (I/V/L/SxYxxL/V/I), as well as immunoreceptor tyrosine‐based switch motifs (ITSM) and other protein‐binding domains (Ravetch & Lanier, 2000; Daeron et al, 2008). In this review, we refer to all inhibitory motifs as ITIMs in an effort to reduce complexity. Anti‐phagocytic receptor activation induces tyrosine phosphorylation of the cytoplasmic ITIMs and binding of cytosolic SH2 domain‐containing phosphatases, such as the SHP tyrosine phosphatases and the SHIP1/2 inositol phosphatases (Neel et al, 2003; Daeron et al, 2008). These phosphatases act as downstream effectors to mediate the inhibitory function of anti‐phagocytic receptors, although their downstream substrates have been challenging to identify. In antibody‐dependent cellular phagocytosis (ADCP) of opsonized targets, the FcγRIIb inhibitory receptor plays an important regulatory role via activation of SHIP1/2 (Aman et al, 2000; Getahun & Cambier, 2015). Most other anti‐phagocytic receptors dependent on SHP‐1 and/or SHP‐2 tyrosine phosphatases for their inhibitory function, and we will focus our discussion on anti‐phagocytic receptor signaling mediated by these tyrosine phosphatases (Getahun & Cambier, 2015). Structurally, SHP‐1 and SHP‐2 contain two N‐terminal SH2 domains, a catalytic phosphatase domain and a C‐terminal tail (Lorenz, 2009). Phosphorylation of two tandem ITIMs is thought to be required for SHP binding and activation downstream of anti‐phagocytic receptors, but the exact molecular details are not entirely clear (Lorenz, 2009; Getahun & Cambier, 2015).
We will first discuss the CD47‐SIRPα axis, as it represents one of the better characterized don't‐eat‐me checkpoints. Next, we will highlight what is known about other don't‐eat‐me checkpoints, and discuss several diseases with potential links to dysregulated phagocytosis as a result of aberrant anti‐phagocytic signaling.
SIRPα as a prototypic anti‐phagocytic receptor
Molecular characterization of SIRPα
SIRP⍺ (also known as SIRP⍺1, PTPNS1, SHPS‐1, BIT, p84, MFR, MyD‐1, and CD172a) is a 115–120 kDa glycoprotein of the SIRP paired receptor family (Fujioka et al, 1996; Comu et al, 1997; Kharitonenkov et al, 1997; Yamao et al, 1997; Matozaki et al, 2009). It is expressed in most tissues and enriched on monocytes, macrophages, CD8α‐ classical type II dendritic cells (cDC2), neutrophils, and osteoclasts, as well as microglia and neurons. It is also moderately expressed on fibroblasts, endothelial cells, and some epithelial cells (Adams et al, 1998; Veillette et al, 1998; Johansen & Brown, 2007). The extracellular region of SIRPα contains an N‐terminal IgV domain followed by two IgC domains (Fig 1) (Fujioka et al, 1996; Kharitonenkov et al, 1997; Yamao et al, 1997). Species and tissue‐specific differences in the molecular weight and ligand affinity of SIRPα are attributed to the highly polymorphic IgV domain and the varying number of N‐linked glycosylation sites in the extracellular region (Yamao et al, 1997; Takenaka et al, 2007). Conversely, the cytoplasmic tail of SIRPα is highly conserved with four ITIMs and a proline‐rich region predicted to bind SH3 domain‐containing proteins (Fig 1) (Fujioka et al, 1996; Kharitonenkov et al, 1997; Yamao et al, 1997; Veillette et al, 1998). Although originally identified in 1990 as an adhesion protein on neurons, SIRPα was independently characterized as an ITIM‐containing receptor and a substrate of activated receptor tyrosine kinases in response to various growth factors and mitogens (Chuang & Lagenaur, 1990; Fujioka et al, 1996; Noguchi et al, 1996; Comu et al, 1997; Kharitonenkov et al, 1997; Yamao et al, 1997; Veillette et al, 1998). Additionally, SIRPα is tyrosine phosphorylated in response to integrin‐mediated adhesion to specific extracellular matrix components (Tsuda et al, 1998; Oh et al, 1999). The tyrosine kinases responsible for direct phosphorylation of SIRPα have remained unclear and may be context‐specific; however, Src family kinases (SFK), as well as focal adhesion kinase (FAK) and proline‐rich tyrosine kinase 2 (Pyk2), have been implicated in SIRPα signaling (Takeda et al, 1998; Timms et al, 1999). Nonetheless, tyrosine phosphorylation of SIRPα at specific ITIMs permits the binding of SHP‐1 or SHP‐2 via their SH2 domains, which relieves repression on the catalytic phosphatase domain and allows for dephosphorylation of their respective substrates (Neel et al, 2003; Lorenz, 2009). It remains unclear whether these phosphatases compete for the same binding site(s), or bind distinct sites on SIRPα; however, current evidence supports the latter model (Takada et al, 1998; Myers et al, 2020). In addition to being a binding partner, SIRPα is also a substrate of these phosphatases (Timms et al, 1998). In professional phagocytes such as macrophages, SHP‐1 predominantly mediates inhibitory signaling downstream of SIRPα, consistent with the abundant expression of SHP‐1 in hematopoietic cells (Veillette et al, 1998; Lorenz, 2009; Abram & Lowell, 2017). Conversely, SHP‐2 is ubiquitously expressed and predominately binds SIRPα in non‐hematopoietic cells in response to stimulation with growth factors, mitogens, and cellular adhesion (Kharitonenkov et al, 1997; Tsuda et al, 1998; Barclay & Van den Berg, 2014). While SHP‐2 can modulate cytokine receptor signaling and macrophage activation states, whether it has a direct role in regulating phagocytosis is unclear (Neel et al, 2003; Tao et al, 2014; Niogret et al, 2019).
Figure 1. Inhibition of phagocytosis by SIRP⍺.
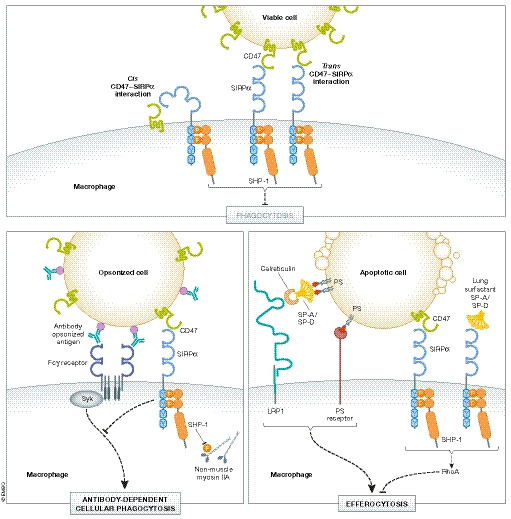
Engagement of the CD47‐SIRP⍺ axis occurs in cis and in trans, which induces tyrosine phosphorylation of the ITIMs in the cytoplasmic tail of SIRP⍺. Phosphorylation at Y429 and Y453 of human SIRPα mediate binding of tyrosine phosphatase SHP‐1 (Myers et al, 2020). Dephosphorylation of non‐muscle myosin IIA is one proposed substrate of SHP‐1, resulting in disassembly of the actomyosin cytoskeleton. (top) Viable cells express don't‐eat‐me signals such as CD47 and lack expression of eat‐me signals, thus limiting phagocytic clearance. (bottom‐left) Antibody‐dependent cellular phagocytosis (ADCP) is also negatively regulated by the CD47‐SIRPα axis (Zent & Elliott, 2017). (bottom‐right) Efferocytosis by alveolar macrophages in the lung can be negatively regulated by SIRPα activation in response to binding CD47 and surfactant proteins, SP‐A and SP‐D. The mechanism of this suppression is thought to involve activation of the GTPase RhoA (Janssen et al, 2008).
The best‐known ligand of SIRPα is CD47 (also known as integrin‐associated protein, OV‐3, and Rh‐related protein), which is a conserved, ubiquitously expressed 45–55‐kDa transmembrane glycoprotein belonging to the Ig superfamily (Brown et al, 1990; Lindberg et al, 1993; Lindberg et al, 1994; Reinhold et al, 1995; Jiang et al, 1999). CD47 has a single N‐terminal extracellular IgV domain followed by five hydrophobic membrane‐spanning segments and a short C‐terminal cytoplasmic tail that is alternately spliced to form four isoforms (Fig 1) (Brown et al, 1990; Lindberg et al, 1993; Reinhold et al, 1995). CD47‐SIRPα binding via their IgV domains regulates many cellular processes, including leukocyte transmigration, lymphocyte homeostasis, dendritic cell maturation and activation, and bone resorption (Latour et al, 2001; de Vries et al, 2002; Liu et al, 2002; Hagnerud et al, 2006; Van et al, 2006; Lundberg et al, 2007; Saito et al, 2010; Legrand et al, 2011; Maile et al, 2011; Sato‐Hashimoto et al, 2011). As we will discuss in the following sections, it is also one of the most studied don't‐eat‐me checkpoints in suppressing phagocytosis.
CD47 is a marker of “self” for phagocytosis
Seminal work by Oldenborg et al (2000) established CD47 as a marker of “self” on erythrocytes to prevent their premature clearance from circulation. The average circulating lifespan of wild‐type murine erythrocytes is between 40 and 60 days (Goodman & Smith, 1961; Ishikawa‐Sekigami et al, 2006; Oldenborg, 2013). In stark contrast, CD47‐negative erythrocytes were shown to be rapidly cleared from circulation within 24 h of transfusion into syngeneic wild‐type recipient mice in a manner independent of complement and adaptive immunity. Rather, splenic red pulp macrophages were found to facilitate the clearance of CD47‐negative erythrocytes, as splenectomy and depletion of macrophages using liposomal clodronate permitted their circulation in wild‐type mice. Moreover, blockade of SIRPα on wild‐type splenic macrophages enhanced the clearance of wild‐type erythrocytes ex vivo. Since this work, several studies over the past two decades have shown that other cell types use CD47 to avoid phagocytosis, including platelets, lymphocytes, and hematopoietic stem cells (Blazar et al, 2001; Yamao et al, 2002; Olsson et al, 2005; Ahrens et al, 2006; Ishikawa‐Sekigami et al, 2006; Jaiswal et al, 2009; Catani et al, 2011; Kuriyama et al, 2012). These data are consistent with the mild thrombocytopenia and lymphopenia observed in mice globally deficient in either CD47 or SIRPα expression (Lindberg et al, 1996; Yamao et al, 2002; Ishikawa‐Sekigami et al, 2006; Li et al, 2012). Recent evidence suggests that the CD47‐SIRPα axis also regulates neuronal pruning by microglia during postnatal development (Lehrman et al, 2018). Retinal ganglion cells (RGCs) extend axons in the dorsal lateral geniculate nucleus (dLGN) of the thalamus to synapse with relay neurons (Guido, 2008). RGC inputs need to be refined for proper development of eye‐specific territories, and this process involves phagocytosis of RGC inputs by microglia in the dLGN (Guido, 2008; Schafer et al, 2012). CD47 was found enriched on active RGC inputs and its expression protected against microglial phagocytosis in a manner dependent on SIRPα (Lehrman et al, 2018). An observed reduction in CD47 on less active RGC inputs suggests that microglia use this marker, at least in part, to decide which inputs to remove (Lehrman et al, 2018). Together, these data support a role for the CD47‐SIRP⍺ axis as a brake on the phagocytosis of “self”.
Given the importance of the CD47‐SIRPα axis in protecting host cells from phagocytosis, it is confounding that mice deficient in either CD47 or SIRPα expression do not have severe developmental or homeostatic abnormalities (Lindberg et al, 1996; Li et al, 2012). Moreover, CD47‐deficient mice are tolerant to transfused syngeneic CD47‐negative bone marrow cells, suggesting a more complex role for this signaling axis than previously thought (Blazar et al, 2001). A phagocytic “licensing” role for CD47 has been hypothesized, analogous to licensing of natural killer (NK) cells for functional cytotoxicity. Inhibitory receptors expressed on the surface of NK cells must interact with MHC class I molecules on host cells in order for NK cells to acquire proper cytotoxic function (Jonsson & Yokoyama, 2009). Elegant bone marrow reconstitution studies suggest that CD47 expression on non‐hematopoietic cells is important for regulating CD47‐dependent phagocytosis (Wang et al, 2007). Endogenous and donor CD47‐negative leukocytes were tolerated in CD47‐deficient mice that were either partially or fully reconstituted with wild‐type bone marrow (Wang et al, 2007). Conversely, wild‐type recipient mice reconstituted with bone marrow lacking CD47 expression rapidly eliminated donor CD47‐negative cells.
Senescent erythrocytes are known to be cleared by F4/80high splenic red pulp macrophages of yolk‐sac and fetal liver origin; however, bone marrow‐derived monocytes can contribute to the F4/80high splenic macrophage population to a certain degree (Schulz et al, 2012; Guilliams & van de Laar, 2015; Gonzalez & Castrillo, 2018). Interestingly, both wild‐type mice and CD47‐deficient mice reconstituted with wild‐type bone marrow rapidly eliminated donor CD47‐negative erythrocytes 24 weeks post‐bone marrow transplantation (Wang et al, 2007). Perhaps, in this situation the donor monocyte‐derived splenic macrophages contributed to the clearance of CD47‐deficient erythrocytes. Alternatively, erythrocytes may lack expression of other don't‐eat‐me signals expressed on nucleated cells, thus making CD47‐deficient erythrocytes more vulnerable to clearance. Mechanism notwithstanding, these findings suggest an important role for CD47 in phagocyte development and/or effector function, with relevance for CD47‐based immunotherapies currently attempted.
CD47‐SIRPα axis in antibody‐dependent cellular phagocytosis
After identifying CD47 as a marker of “self” on erythrocytes, Oldenborg et al (2001) showed that the CD47‐SIRPα axis suppresses pro‐phagocytic signaling downstream of activated Fcγ receptors and complement receptors. In this study, IgG‐opsonized CD47‐negative erythrocytes were completely absent from circulation within 8 h post‐transfusion into wild‐type syngeneic recipient mice, whereas unopsonized CD47‐negative erythrocytes and IgG‐opsonized wild‐type erythrocytes remained for > 24 h. These data are consistent with the earlier onset and more severe autoimmune hemolytic anemia (AIHA) observed in CD47‐deficient non‐obese diabetic (NOD) mice, which are prone to autoimmune diseases (Oldenborg et al, 2002; Wong et al, 2014). Moreover, IgG‐opsonized wild‐type and CD47‐negative erythrocytes were also eliminated from circulation within 8 h post‐transfusion into viable motheaten (mev/mev) recipient mice, which have markedly reduced SHP‐1 phosphatase activity (Shultz et al, 1984; Kozlowski et al, 1993; Shultz et al, 1993; Oldenborg et al, 2001). While target cell binding and Fcγ receptor activation appear mostly unaffected by CD47‐induced inhibitory signaling, the mechanism of suppression at least partially involves regulation of cytoskeletal elements important for target cell internalization (Lowry et al, 1998; May & Machesky, 2001; Diakonova et al, 2002; Kant et al, 2002; Tsai & Discher, 2008; Gomez & Descoteaux, 2018). Global tyrosine phosphorylation is reduced in phagocytes following activation of CD47‐SIRPα signaling, including reduced tyrosine phosphorylation of the non‐muscle myosin IIA motor protein, which was previously shown to be a direct substrate of SHP‐1 following B‐cell activation (Fig 1) (Baba et al, 2003; Tsai & Discher, 2008). CD47‐SIRPα‐mediated inhibition of inside‐out integrin activation may also reduce macrophage spreading around the bound target cell (Tsai & Discher, 2008; Morrissey et al, 2020). Phagocytic receptors are suggested to be restricted in lateral movement on the plasma membrane due to interactions with the underlying cytoskeleton (Freeman et al, 2018). SIRPα has been shown to form clusters near FcγRI receptors in resting macrophages, and engagement of the CD47‐SIRPα axis promotes receptor clustering, whereas unligated SIRPα is suggested to be excluded from the phagocytic cup following FcγRI stimulation with IgG or disruption of filamentous actin (Lopes et al, 2017). Thus, co‐ligation of pro‐phagocytic receptors (e.g., IgG activation of Fcγ receptors) and anti‐phagocytic receptors (e.g., CD47‐SIRPα axis) may function to fine‐tune phagocytosis in some contexts, perhaps to allow for proper antigen digestion and presentation. In the absence of anti‐phagocytic receptor co‐ligation, such as during the clearance of apoptotic cells as we will discuss in the next section, these phagocytic “brakes” are mostly excluded from the phagocytic synapse.
Studies investigating the role of the CD47‐SIRPα axis in phagocytosis have largely focused on trans engagement of CD47 expressed on the target cell with SIRPα expressed on the phagocyte (Fig 1). It is important to note that many professional and non‐professional phagocytes express both CD47 and SIRPα on their surface. Recent work investigating the potential for cis engagement of the CD47‐SIRPα axis showed that loss of CD47 on macrophages also enhanced phagocytosis of unopsonized and IgG‐opsonized erythrocytes in vitro (Hayes et al, 2020). Additionally, compared to wild‐type macrophages, CD47‐deficient macrophages bound more soluble CD47, presumably due to the lack of cis CD47‐SIRPα interactions. Basal tyrosine phosphorylation of macrophage SIRPα was also reduced following deletion of macrophage CD47, and the loss of phosphorylation signal correlated with the loss of CD47 expression (Johansen & Brown, 2007; Hayes et al, 2020). Together, these data support a model whereby the SIRPα extracellular domains bend over to interact with the CD47 IgV domain in cis to influence the dynamics of cell clearance (Fig 1). Interestingly, other immune receptor‐ligand pairs have been shown to engage in cis, suggesting that this may be a more general regulatory mechanism for immune cell function such as phagocytosis (Doucey et al, 2004). Whether the signaling strength downstream of SIRPα differs by binding CD47 in cis versus in trans remains unclear (Hayes et al, 2020). Localization and clustering of CD47‐SIRPα signaling complexes near the phagocytic cup is clearly an important determining factor as to the potency of inhibitory signaling and the overall impact on phagocytosis. The conformational state of these molecules may also influence differences between cis and trans signaling downstream of SIRPα (Hayes et al, 2020).
Together, these data support an inhibitory role for the CD47‐SIRPα axis in ADCP via activation of SHP‐1 and subsequent inhibition of contractile forces necessary for internalization of the target cell.
CD47‐SIRPα axis in apoptotic cell clearance
Over 200–300 billion cells die in the human body every day as a part of routine cellular turnover, and most of these cells are thought to die by apoptosis (Arandjelovic & Ravichandran, 2015). The clearance of apoptotic cells, also referred to as efferocytosis, is not only immunologically silent, but also actively immunosuppressive via the secretion of anti‐inflammatory mediators such as lactate, IL‐10, and TGFβ (Gardai et al, 2003; Morioka et al, 2018; Perry et al, 2019). An early event following induction of apoptosis is loss of phospholipid asymmetry and exposure of phosphatidylserine (PS), a potent eat‐me signal for efferocytosis, as well as membrane blebbing (Fig 1) (Fadok et al, 2001). Given the importance of the CD47‐SIRPα axis in suppressing other forms of phagocytosis, its potential role in regulating efferocytosis has been explored, albeit to a lesser extent. Reduced CD47 expression is observed on some cell types (e.g., neutrophils, fibroblasts) following induction of apoptosis in vitro, as well as on senescent erythrocytes (Gardai et al, 2005; Khandelwal et al, 2007; Lv et al, 2015). However, CD47 expression changes are not evident on other cell types such as Jurkat T cells and thymocytes during early stages of apoptotic cell death. Changes in the localization of CD47 on the plasma membrane of dying cells have also been observed such that fewer molecules of CD47 engage SIRPα within the phagocytic synapse, thus reducing the binding avidity and inhibitory signaling strength downstream of SIRPα (Gardai et al, 2005; Lv et al, 2015). Further, tyrosine phosphorylation of SIRPα has been shown to be reduced in macrophages co‐cultured with apoptotic cells, suggesting less involvement of the CD47‐SIRPα axis in regulating efferocytosis. Interestingly, the activation of SIRPα signaling by cross‐linking or recombinant CD47 has been shown to reduce efferocytosis in vitro (Gardai et al, 2005; Janssen et al, 2008; Lv et al, 2015). Thus, the CD47‐SIRPα axis is capable of inhibiting efferocytosis, at least in some conditions. “Brakes” for phagocytosis such as the CD47‐SIRPα axis may largely be excluded from the phagocytic synapse during efferocytosis to prevent delayed clearance of apoptotic cells, which can result in secondary necrosis and chronic inflammatory pathologies (Morioka et al, 2019).
Paradoxically, a pro‐phagocytic role for CD47 has also been suggested in efferocytosis. In the presence of serum, CD47‐deficient apoptotic cells were shown to be engulfed less efficiently than their wild‐type counterparts in vitro (Tada et al, 2003; Nilsson & Oldenborg, 2009). CD47 is known to bind the C‐terminal RFYVVM domain of thrombospondins (TSP), which are platelet‐derived soluble coagulation factors, and TSP‐1 promotes efferocytosis by acting as a molecular bridge between apoptotic cells and specific pro‐phagocytic receptors (e.g., CD36 and αvβ3 integrin) (Savill et al, 1992; Arandjelovic & Ravichandran, 2015). Moreover, erythrocyte aging studies suggest that oxidative stress induces a conformational change in CD47 that permits TSP‐1 binding via its C‐terminal domain, suggesting that under specific conditions CD47 may be converted to an eat‐me signal (Gao et al, 1996a; Gao et al, 1996b; Burger et al, 2012). In some contexts, ligation of CD47 has also been reported to induce a form of cell death that is phenotypically similar to apoptosis, including PS exposure, but often lacks nuclear changes and is caspase‐independent (Oldenborg, 2013). It is not clear to what extent, if any, CD47‐induced cell death contributes to cellular turnover or the clearance process.
Other don't‐eat‐me ligands for SIRPα: lung surfactant proteins
Surfactant proteins A and D (SP‐A and SP‐D, respectively) are oligomeric structures of the collectin family that are present in lung surfactant and participate in innate host defense (LeVine & Whitsett, 2001; Wright, 2005). In addition to modulating inflammation, SP‐A and SP‐D can facilitate phagocytosis of microorganisms and apoptotic cells by alveolar macrophages (Schagat et al, 2001; Vandivier et al, 2002; Reidy & Wright, 2003). The globular lectin domain of SP‐A and SP‐D binds the target cell, and the collagenous domain binds to pro‐phagocytic calreticulin/low‐density lipoprotein receptor 1 (LRP1) complexes on the phagocyte membrane. Paradoxically, the globular lectin domain of SP‐A and SP‐D has also been shown to interact with SIRPα on alveolar macrophages to suppress efferocytosis and pro‐inflammatory cytokine production via tyrosine phosphorylation of SHP‐1 and activation of the GTPase, RhoA (Fig 1) (Gardai et al, 2003; Tosello‐Trampont et al, 2003; Janssen et al, 2008). Alveolar macrophages deficient in SHP‐1 demonstrated enhanced efferocytosis in vitro, presumably due to loss of inhibitory signaling downstream of the SP‐A/SP‐D:SIRPα axis (Janssen et al, 2008). However, peritoneal macrophages lacking SHP‐1 were not significantly affected in efferocytosis, suggesting tissue context differences (Janssen et al, 2008). In response to lung infection and trauma, SIRPα was recently shown to suppress alveolar macrophage phagocytosis and promote an immunosuppressive microenvironment conducive to secondary infection (Roquilly et al, 2020). Collectively, these observations support a role for SIRPα in suppressing alveolar macrophage function. The mechanisms regulating the dichotomous functions of SP‐A and SP‐D are not clear and may depend on other factors present in the lung environment.
Additional anti‐phagocytic receptors
In addition to SIRPα, several other ITIM‐containing receptors have also been suggested to inhibit phagocytosis via activation of SHP‐1 and/or SHP‐2 tyrosine phosphatases. Many of these receptors have been studied in the context of disease, and further studies are needed to assess the roles of these inhibitory receptors in modulating phagocytosis in normal physiology. Of note, CD31 (also known as PECAM‐1) is an ITIM‐containing receptor that has been shown to promote de‐attachment of viable cells from phagocytes; however, its proposed mechanism differs from the anti‐phagocytic receptors discussed in this review (Brown et al, 2002).
CD300a and CD300f
Inhibitory receptors of the CD300 family include CD300a and CD300f, and both contain a single extracellular IgV domain (Borrego, 2013). In humans and mice, these inhibitory receptors are primarily expressed on myeloid cells, and to varying levels on some lymphoid subsets (Borrego, 2013). Phosphatidylserine (PS) is thought to be a ligand for CD300a and CD300f as both receptors have been shown to bind apoptotic cells and PS‐containing liposomes in a calcium‐dependent manner (Choi et al, 2011; Nakahashi‐Oda et al, 2012a; Nakahashi‐Oda et al, 2012b; Simhadri et al, 2012). Additionally, the PS‐binding proteins annexin V and milk‐fat globule EGF factor VIII (MFG‐E8) can block apoptotic cell binding to CD300a and CD300f. Other lipids have been suggested as ligands for these receptors, but further studies are warranted to clarify conflicting studies (Borrego, 2013).
CD300a
The cytoplasmic tail of human CD300a contains four ITIMs, whereas mouse CD300a contains two ITIMs and a third tyrosine associated with a tyrosine‐based sorting motif (Fig 2) (Borrego, 2013). Activation of CD300a by PS induces SHP‐1 binding and inhibits efferocytosis (Nakahashi‐Oda et al, 2012a; Nakahashi‐Oda et al, 2012b; Simhadri et al, 2012). The PS‐dependent inhibitory function of this receptor is confounding since PS is a potent eat‐me signal for efferocytosis. A potential explanation is that CD300a may be important to inhibit inflammatory responses to apoptotic cell uptake, albeit potentially at the expense of reduced efferocytosis (Simhadri et al, 2012). In support of this hypothesis, CD300a expression is induced on several types of immune cells in response to inflammatory stimuli and has been shown to inhibit MyD88 inflammatory cytokine production (Kim et al, 2012; Borrego, 2013). Additionally, activation of CD300a on human neutrophils was shown to suppress production of reactive oxygen species in response to FcγRIIa stimulation (Alvarez et al, 2008). Thus, CD300a expression is modulated on several immune cell types in response to infection and downregulates inflammatory responses to prevent excessive tissue damage. Whether CD300a plays a role in regulating efferocytosis in the absence of inflammatory stimuli such as during homeostatic cell clearance warrants further investigation.
Figure 2. The structure of human and mouse anti‐phagocytic receptors.
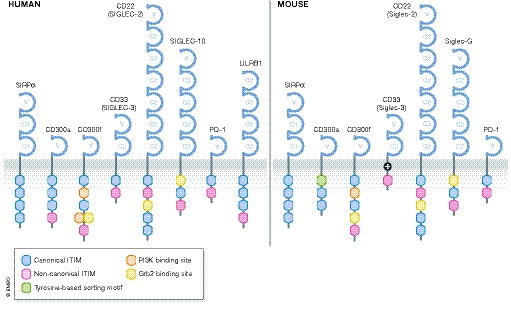
Several anti‐phagocytic receptors have been identified in humans and mice. These are single‐pass type I transmembrane receptors belonging to the immunoglobulin (Ig) superfamily and contain one or more immunoreceptor tyrosine‐based inhibitory motifs (ITIM) in their cytoplasmic tails. Many of these receptors also have additional binding sites for signaling molecules that can endow these receptors with activation functions.
CD300f
Both human and mouse CD300f have three cytoplasmic ITIMs, as well as binding motifs for PI3K and Grb2 signaling molecules (Fig 2) (Borrego, 2013). The role of CD300f in regulating phagocytosis is more complex. Studies suggest both anti‐phagocytic and pro‐phagocytic functions, depending on the cell type and activation context, perhaps due to its ability to bind SHP‐1 and SHP‐2 (Borrego, 2013). Activation of CD300f was shown to promote apoptotic cell binding and internalization in a manner dependent on Y276 phosphorylation and p85α‐PI3K signaling for phagocytic cup formation (Choi et al, 2011; Tian et al, 2014; Tian et al, 2016). Conversely, the other four tyrosines and expression of SHP‐1 had an inhibitory effect on efferocytosis in vitro, and loss of CD300f enhanced efferocytosis and T‐cell priming by dendritic cells, suggesting that it also functions as an anti‐phagocytic receptor (Tian et al, 2014; Tian et al, 2016). Thus, the role of CD300f in modulating efferocytosis is complex and likely depends on interactions with other receptors, as well as available signaling molecules within different types of phagocytes.
Siglecs
Siglecs, or sialic acid‐binding Ig‐like lectins, are a large family of receptors primarily expressed on immune cells and have important roles in attenuating inflammatory responses to pathogen and damage‐associated molecular patterns, mediating adhesion and phagocytosis, and regulating immune cell activation (Pillai et al, 2012). Many of these lectins bind specific sialic acid‐containing glycoconjugates in cis, as well as in trans, to fine‐tune responses following immune cell activation (Pillai et al, 2012). Below, we will focus our discussion on three Siglecs suggested to function as anti‐phagocytic receptors.
CD33/Siglec‐3
Human CD33 (also called Siglec‐3) has an extracellular region consisting of one IgV domain and one IgC domain, and a cytoplasmic tail containing two ITIMs (Fig 2) (Ulyanova et al, 1999; Pillai et al, 2012). It is expressed on cells of the myeloid lineage and microglia (Pillai et al, 2012; Griciuc et al, 2013). Human CD33 binds α2,3‐ and α2,6‐linked sialic acid‐containing glycans (Brinkman‐Vand der Linden et al, 2003). Activation of CD33 induces SHP‐1 binding, and the membrane proximal ITIM is necessary and sufficient for SHP‐1 activation (Ulyanova et al, 1999). Genome‐wide association studies of genetic variants associated with Alzheimer's disease (AD) suggested a potential link to the CD33 loci (Hollingworth et al, 2011; Naj et al, 2011). Further analysis revealed a positive correlation in CD33 expression on microglia and the presence of insoluble Aβ42 levels and amyloid plaque burden in AD patients (Griciuc et al, 2013). Moreover, loss of CD33 in mouse models of AD was sufficient to reduce insoluble Aβ42 levels and amyloid plaque burden via enhanced uptake by microglia (Griciuc et al, 2013; Griciuc et al, 2019). These studies suggest CD33 as an anti‐phagocytic receptor on microglia and its potential involvement in AD pathogenesis.
Unlike human CD33, the mouse ortholog has one ITIM and a positively charged lysine residue in the transmembrane region that forms a putative binding site for signaling molecules associated with immune cell activation (Fig 2) (Brinkman‐Vand der Linden et al, 2003; Pillai et al, 2012). Given these structural differences, divergent functions between human and mouse CD33 orthologs may exist and one study suggests that mouse CD33 may not regulate Aβ42 phagocytosis (Bhattacherjee et al, 2019). The reasons for these conflicting data are not clear.
CD22/Siglec‐2
CD22 (also called Siglec‐2) has an extracellular region that contains an N‐terminal IgV domain followed by six IgC domains, and the cytoplasmic tail contains four ITIMs and a Grb2 binding motif (Fig 2) (Pillai et al, 2012). It is highly expressed in B cells, as well as some myeloid‐derived cells and microglia (Pillai et al, 2012). Mouse CD22 binds Neu5Gc glycans, and human CD22 binds both Neu5Ac and Neu5Gc glycans, although the specific glycoconjugates are not fully clear (Pillai et al, 2012). Activation of CD22 has been shown to bind and activate SHP‐1, as well as a variety of other signaling molecules (Doody et al, 1995; Law et al, 1996; Blasioli et al, 1999; Poe et al, 2000; Otipoby et al, 2001). A recent study investigating age‐related genes involved in microglial phagocytosis found CD22 enriched on microglia from aged mice and its expression negatively correlated with cognitive function (Pluvinage et al, 2019). CD22 was shown to inhibit microglial phagocytosis, presumably via activation of SHP‐1, and loss of CD22 enhanced the clearance of several neurodegenerative factors, including myelin debris, Aβ oligomers, and α‐synuclein fibrils in vitro and in vivo (Pluvinage et al, 2019). Thus, in addition to CD33, this study suggests that microglia also utilize CD22 as an anti‐phagocytic receptor to restrict phagocytosis in the central nervous system, perhaps in early development as well as pathological contexts when there is an overabundance of cellular debris. Additional studies are needed to assess whether blockade of CD22 is of benefit in treating age‐related cognitive decline and neurodegenerative diseases.
Siglec‐10
Human Siglec‐10, or mouse Siglec‐G, has an extracellular region consisting of an N‐terminal IgV domain followed by four IgC domains, and a cytoplasmic tail containing two ITIMs as well as a Grb2‐binding motif (Fig 2) (Munday et al, 2001; Pillai et al, 2012). It is expressed on myeloid cells and some lymphocytes, and binds both SHP‐1 and SHP‐2 (Munday et al, 2001; Whitney et al, 2001; Pillai et al, 2012). CD24 is a sialylated glycosyl phosphoinositol‐anchored protein and a cognate ligand of human Siglec‐10, and mouse Siglec‐G (Chen et al, 2009). Signaling through this axis suppresses NF‐κB‐mediated inflammation in response to damage‐associated molecular patterns via SHP‐1 activation (Chen et al, 2009; Chen et al, 2011). Additionally, NF‐κB activation has been shown to increase Siglec‐G expression in innate immune cells in response to viral infection, leading to retinoic acid‐inducible gene I (RIG‐I) degradation and attenuation of anti‐viral responses in a manner involving SHP‐2 (Chen et al, 2013). Mouse Siglec‐G has also been shown to negatively regulate MHC class I‐peptide cross‐presentation by CD8α+ dendritic cells (Ding et al, 2016). Mechanistically, the activation of SHP‐1 downstream of Siglec‐G led to an increase in phagolysosomal pH such that MHC class I‐peptide complex formation was impaired (Ding et al, 2016). Recently, CD24 was also identified as a don't‐eat‐me signal that is highly expressed on several cancers (Barkal et al, 2019). This study showed that tumor‐associated macrophages (TAMs) from ovarian and breast tumors also expressed Siglec‐10 (Barkal et al, 2019). Blockade or genetic depletion of either CD24 on tumor cells, or Siglec‐10 on macrophages, enhanced tumor cell clearance in vitro and in vivo, presumably by disrupting trans engagement of this inhibitory axis (Barkal et al, 2019). Thus, in addition to dampening inflammatory responses, the CD24‐Siglec‐10 axis also regulates phagocytosis processes in professional phagocytes. Additional studies are needed to investigate the mechanism(s) driving increased expressed of CD24 on tumor cells, and Siglec‐10 expression on TAMs, and if blockade of this signaling axis would provide enhanced anti‐tumor immunity in cancers resistant to clinically available therapies. Future studies should also evaluate dysregulation of this don't‐eat‐me signaling axis in other contexts where impaired phagocytosis is evident such as in cardiovascular diseases.
PD‐1
PD‐1 (also known as programmed cell death protein 1 and CD279) is a well‐known immune inhibitory receptor of the B7/CD28 family and has been extensively studied in suppressing T‐cell activation, at least in part via SHP‐2 (Okazaki et al, 2013; Rota et al, 2018; Marasco et al, 2020; Patsoukis et al, 2020). Structurally, it has one extracellular IgV domain and two cytoplasmic ITIMs (Fig 2) (Patsoukis et al, 2020). Its two cognate ligands are PD‐L1 (also known as CD274 and B7‐H1) expressed on non‐lymphoid cells, and PD‐L2 (also known as CD273 and B7‐DC) expressed on antigen‐presenting cells (Okazaki et al, 2013). In addition to being expressed on lymphocytes, PD‐1 expression is induced in macrophages in response to infection, which is negatively associated with an inflammatory macrophage phenotype and pathogen clearance (Huang et al, 2009; Shen et al, 2016; Gordon et al, 2017). Upon microbial infection, Toll‐like receptor/NF‐κB activation induces PD‐1 expression in macrophages, and PD‐1 activation in turn suppresses NF‐κB/p65‐mediated inflammatory responses (Bally et al, 2015; Chen et al, 2016). In several cancers, a subset of TAMs and tumor‐infiltrating dendritic cells (TIDCs) highly express PD‐1, which negatively correlates with anti‐tumor immune responses (Karyampudi et al, 2016; Gordon et al, 2017). Specifically, PD‐1 expression on TAMs correlated with reduced phagocytosis of tumor cells, and inhibition of the PD‐L1:PD‐1 axis in lymphocyte‐deficient tumor‐bearing mice reduced tumor burden and extended survival, suggesting its potential role in don't‐eat‐me signaling (Gordon et al, 2017). Together, these studies establish a model whereby PD‐1 is induced in innate immune cells to modulate their inflammatory state and act as a rheostat to prevent excessive inflammation and tissue damage, which can indirectly influence phagocytic activity to specific targets (Yurdagul et al, 2020). Additional studies are needed to assess whether PD‐1 plays a more direct role in modulating phagocytic processes such as target cell internalization.
LILRB1
Human LILRB1 (leukocyte immunoglobulin‐like receptor B1; also known as CD85J, ILT2, LIR‐1) is an inhibitory receptor expressed on myeloid cells and subsets of lymphoid cells (Borges et al, 1997; Colonna et al, 1997; Fanger et al, 1998). Structurally, it has an extracellular region consisting of four IgC domains and a cytoplasmic tail containing four ITIMs (Fig 2) (Borges et al, 1997; Samaridis & Colonna, 1997). Tyrosine phosphorylation of the two membrane‐distal ITIMs permits SHP‐1 binding and activation (Fanger et al, 1998; Bellon et al, 2002). LILRB1 binds MHC class I molecules expressed on all nucleated cells, and the invariant β2‐microglobulin subunit of MHC class I has been shown to be important for this interaction (Borges et al, 1997; Colonna et al, 1997; Fanger et al, 1998; Barkal et al, 2018). In a recent study, MHC class I expression was shown to protect some cancer cells from phagocytosis via engagement of LILRB1 (Barkal et al, 2018). Loss of either MHC class I or LILRB1 was shown to enhance tumor cell phagocytosis in vitro when combined with antibody opsonization to provide eat‐me signaling through Fc receptor activation (Barkal et al, 2018). Additionally, loss of both MHC class I and CD47 don't‐eat‐me signals on tumor cells provided better anti‐tumor immune responses in a manner dependent on macrophages, as well as contribution from adaptive immune cells in vivo (Barkal et al, 2018). This study highlights MHC class I as an additional don't‐eat‐me signal utilized by some cancers to avoid clearance. Activation of LILRB1‐SHP1 inhibitory signaling has previously been shown to suppress early tyrosine phosphorylation events downstream of FcγRI signaling (Fanger et al, 1998). Thus, it is likely that engagement of the MHC class I‐LILRB1 axis suppresses ADCP, and loss of this inhibitory axis allows for better uptake of opsonized cells.
Regulation gone awry: don't‐eat‐me signaling in disease
The expression of don't‐eat‐me signals by host cells is thought to prevent their unwarranted removal by neighboring phagocytes, and dysregulation of this innate immune checkpoint has pathological consequences. In addition to the studies discussed in the previous sections, below we highlight two disease categories where enhanced CD47 expression contributes to pathology.
Cancer
CD47 expression is upregulated in many cancers, including both hematologic and solid tumor malignancies, and its expression correlates with poor prognosis (Campbell et al, 1992; Jaiswal et al, 2009; Majeti et al, 2009; Willingham et al, 2012; Weiskopf et al, 2016; Michaels et al, 2018). The mechanisms contributing to enhanced CD47 expression differ among cancer subtypes, but aberrant transcriptional regulation is a commonality (Zhang et al, 2015; Case y et al, 2016; Betancur et al, 2017; Liu et al, 2017). Blocking the CD47‐SIRPα axis was shown to promote phagocytosis of tumor cells in vitro, and tumor regression in animal models, when an eat‐me signal was available to activate pro‐phagocytic signaling (Fig 3). A first‐in‐human phase I trial evaluated the efficacy of a humanized anti‐CD47 monoclonal antibody (called magrolimab or Hu5F9‐G4) as monotherapy for advanced solid tumors and lymphomas, but limited anti‐tumor responses were observed (Sikic et al, 2019; Veillette & Tang, 2019). Conversely, Advanti et al reported partial or complete responses in 50% of patients with relapsed/refractory non‐Hodgkin's lymphoma enrolled in a small phase 1b trial where patients received co‐administration of magrolimab and rituximab (an anti‐CD20 monoclonal antibody). Additionally, 95% of responding patients were previously refractory to single agent rituximab treatment (Advani et al, 2018; Veillette & Tang, 2019). These studies provided the first clinical evidence that loss of CD47 don't‐eat‐me signaling promotes anti‐tumor immunity at least partially via ADCP. Mild anemia and lymphopenia were the only low‐grade adverse events observed with magrolimab treatment, suggesting promising tolerability to CD47 blockade, albeit larger trials with longer follow‐ups are needed to fully assess treatment‐related toxicities, including potential cognitive‐related issues (Advani et al, 2018; Sikic et al, 2019; Veillette & Tang, 2019). Additional clinical trials investigating the efficacy and safety of immunotherapies targeting the CD47‐SIRPα axis are currently underway for both hematologic and solid tumors, including their use as single agents and in combination with other checkpoint blockade therapies, as well as azacytidine—a hypomethylating agent (Feng et al, 2019). Blocking strategies include the use of several different anti‐CD47 monoclonal antibodies, as well as a recombinant SIRPαFc fusion protein (TTI‐621) consisting of the Fc region of human IgG1 and the extracellular IgV domain of human SIRPα (Feng et al, 2019). Altogether, these initial observations suggest that immunotherapies targeting the CD47‐SIRPα axis to enhanced tumor cell ADCP are well‐tolerated and demonstrate promising anti‐tumor effects. Future studies investigating the efficacy of targeting other don't‐eat‐me signaling molecules are needed for cancers showing resistance to CD47 blockade (Barkal et al, 2018).
Figure 3. Therapeutically blocking the CD47‐SIRP⍺ axis to enhance cancer cell phagocytosis.
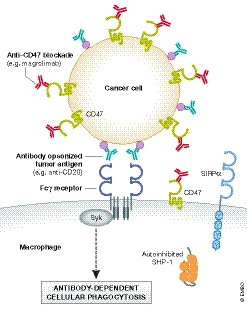
Many cancer cells have increased expression of CD47. Preclinical and clinical studies have shown promise in enhancing anti‐tumor immunity when anti‐CD47 blockade is used in combination with other immunotherapies such as the anti‐CD20 monoclonal antibody Rituximab. This strategy provides an eat‐me signal via activation of antibody‐dependent cellular phagocytosis (ADCP) while concurrently blocking the CD47‐SIRPα don't‐eat‐me signaling axis.
Cardiovascular disease
Atherosclerosis
Atherosclerosis is a major contributing factor to cardiovascular and cerebrovascular diseases, and mortality worldwide (Benjamin et al, 2019). In the early stages of atherosclerosis, modified circulating lipoproteins become trapped in the subendothelial space of arteries and stimulate inflammatory leukocyte recruitment into the vessel wall (Doran et al, 2019). A portion of macrophages that phagocytose entrapped lipoproteins become cholesterol‐laden and undergo apoptosis (Yurdagul et al, 2018; Morioka et al, 2019). These dying cells are removed by other plaque‐associated macrophages, but over time efferocytosis becomes impaired in diseased vessels and advanced plaques form. The accumulation of uncleared dying cells forms the “necrotic core” of advanced plaques, which are prone to rupture.
A combination of aberrations contributes to impaired efferocytosis in atherosclerotic lesions. These include reduced surface expression of pro‐phagocytic receptors such as LRP1 and the Mer proto‐oncogene tyrosine kinase (MerTK) via proteolytic cleavage and receptor degradation (Fig 4) (Doran et al, 2019). Additionally, enhanced CD47 expression was observed in human lesions, and blockade of CD47 in murine atherosclerotic models showed fewer plaque‐associated dead cells and smaller necrotic cores (Kojima et al, 2016; Gerlach et al, 2020). One mechanism contributing to enhanced CD47 expression on plaque cells involved NF‐κB activation downstream of tumor necrosis factor (TNF) signaling (Kojima et al, 2016). In agreement with this finding, other reports in a variety of disease contexts have also linked pro‐inflammatory signaling to increased CD47 expression, suggesting that CD47 is induced in response to some inflammatory conditions to fine‐tune inflammatory responses (Betancur et al, 2017; Cui et al, 2020; Tal et al, 2020; Wang et al, 2020). It is possible that increased CD47 expression on plaque‐associated phagocytes may also contribute to impaired efferocytosis via cis engagement of the CD47‐SIRPα axis (Fig 4) (Hayes et al, 2020; Wang et al, 2020). Increased expression of the long non‐coding RNA called “myocardial infarction‐associated transcript” (MIAT) was also observed in advanced lesions (Ye et al, 2019). MIAT was shown to negatively regulate the microRNA miR‐149‐5p, thereby relieving repression of CD47 expression (Fig 4) (Ye et al, 2019). Knockdown of MIAT in vitro and in murine atherosclerosis promoted efferocytosis, reduced plaque necrosis, and improved plaque stability, presumably via reduced CD47 expression (Ye et al, 2019).
Figure 4. Impaired efferocytosis in cardiovascular disease.
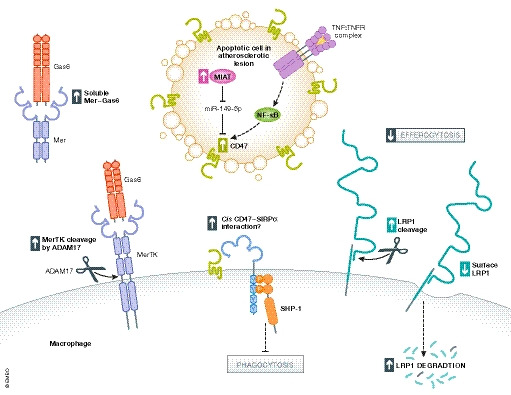
Several mechanisms contribute to impaired efferocytosis in cardiovascular diseases, such as atherosclerosis (Doran et al, 2019). Tumor necrosis factor (TNF) signaling through the TNF receptor activates the nuclear factor‐κB pathway leading to enhanced CD47 expression on apoptotic cells in atherosclerotic lesions. Enhanced expression of the “myocardial infarct‐associated transcript” (MIAT) long non‐coding RNA inhibits processing of the microRNA, miR‐149‐5p, and in turn, results in increased CD47 expression. CD47 can bind SIRPα in cis on the phagocyte surface to inhibit phagocytosis. Loss of pro‐phagocytic receptors on the phagocyte surface including LRP1 and MerTK contributes to impaired efferocytosis. Metalloproteinase ADAM17 cleaves MerTK, resulting in soluble Mer that may act to sequester growth arrest‐specific protein 6 (Gas6) needed for recognition of phosphatidylserine (PS) on the surface of apoptotic cells. Parts of this figure were adapted from Doran et al (2019).
Potential therapeutic benefit in blocking CD47 for atherosclerosis was suggested based on a retrospective analysis of 9 patients enrolled on the Advanti trial (Jarr et al, 2021). Reduced inflammation in the carotid arteries was observed by 18F‐FDG PET/CT scans following combination treatment with magrolimab and rituximab. Future trials directly evaluating the efficacy of blocking CD47 for treating atherosclerosis are needed. It should also be noted that one study using CD47‐deficient mice with hypercholesterolemia showed larger atherosclerotic plaques compared with wild‐type mice, and this phenotype was associated with enhanced lymphocyte activation, particularly INFγ‐producing CD90+ NK cells (Engelbertsen et al, 2019). Thus, CD47 appears to play a more general role in controlling both innate and adaptive immune responses, and detailed studies are needed to evaluate both on‐target and off‐target effects of CD47 blockade.
Myocardial infarction
Heart failure after myocardial infarction (MI) is another major source of cardiac‐related mortality (Frangogiannis, 2012). Efficient clearance of dying cardiomyocytes and timely resolution of acute inflammation following myocardial infarction is critical to cardiac repair (Frangogiannis, 2012; Doran et al, 2019). Cleavage of the MerTK receptor is one mechanism of impaired cardiomyocyte efferocytosis (Wan et al, 2013; DeBerge et al, 2017). Cardiomyocytes associated with infarcted tissue also express more CD47, and acute blockade of CD47 or SIRPα during reperfusion injury could enhance cardiomyocyte clearance and reduce infarct size and collagen content (Zhang et al, 2017). The mechanisms underlying enhanced CD47 expression on cardiomyocytes in infarcted tissue remain unclear, and additional studies are needed to assess the benefit of blocking the CD47‐SIRPα axis following ischemic injury.
While professional phagocytes are important for reparative responses post‐myocardial infarct, non‐professional phagocytes such as cardiac myofibroblasts can assist in the clearance process (Nakaya et al, 2017). The physiological response to myofibroblast efferocytosis in the damaged myocardium remains unclear. It is also not known if efferocytosis by non‐professional phagocytes such as cardiac myofibroblasts is regulated by don't‐eat‐me signaling, and future studies are needed to explore this area of phagocytosis research in more depth.
Conclusions
Efficient clearance of dying and pathogenic cells is critical to preventing unnecessary inflammation and maintaining immune quiescence. However, this process must be tightly controlled to avoid aberrant removal of healthy cells. Regulation during the taste phase of phagocytosis involves phagocyte recognition of eat‐me and don't‐eat‐me signals expressed on host cells. In general, it is thought that don't‐eat‐me signaling raises the activation threshold needed for phagocytosis to occur.
In ADCP, evidence supports a role for the CD47‐SIRP⍺ axis, and other don't‐eat‐me checkpoints, in fine‐tuning the clearance response. Many anti‐phagocytic receptors play important roles in controlling inflammatory responses by suppressing cytokine and TLR signaling, and in some cases, antigen presentation for the activation of adaptive immune responses. Additional studies are needed to decipher how don't‐eat‐me signaling directly regulates phagocytosis, and this includes identifying the cellular substrates of SHP‐1 and SHP‐2 following anti‐phagocytic receptor activation. Specifically, whether regulation beyond suppression of integrin activation and the actomyosin cytoskeleton occurs needs to be defined (see also Box 1).
Box 1: In need of answers.
What is (are) the mechanism(s) by which anti‐phagocytic receptors regulate phagocytosis? Specifically, what are the substrates of SHP‐1 and SHP‐2 regulating phagocytosis?
Is there specificity in which phagocytes are regulated by particular anti‐phagocytic receptors?
Do anti‐phagocytic receptors cooperate or compensate, and in which tissue contexts?
Which types of anti‐phagocytic receptors are important for regulating ADCP versus efferocytosis?
Does dysregulation of phagocytosis via aberrant don't‐eat‐me signaling contribute to fibrosis?
What are the long‐term consequences of therapeutically blocking anti‐phagocytic receptors?
During efferocytosis, exclusion of some anti‐phagocytic receptors from the phagocytic synapse may reduce the activation threshold to allow for efficient removal of dying cells. Additionally, efferocytosis is immunologically silent and can actively promote anti‐inflammatory responses. Engagement of anti‐phagocytic receptors may contribute to suppressing inflammation in response to the uptake of apoptotic cells. The degree of regulation on efferocytosis by anti‐phagocytic receptors is less understood and requires further investigation.
Several new don't‐eat‐me checkpoints have been identified in recent years, specifically in cancers. Similarly, several pro‐phagocytic receptors have been identified for efferocytosis, and while initially thought to be a matter of redundancy, evidence suggests that pro‐phagocytic receptor expression is likely tissue and phagocyte‐specific (Penberthy et al, 2017). Is there similar specificity for anti‐phagocytic receptors? Do anti‐phagocytic receptors regulate phagocytosis by non‐professional phagocytes? Studies aimed at genetically deleting individual anti‐phagocytic receptors in specific phagocyte populations will be useful to address these questions (see also Box 1).
Lastly, we and others have reviewed several examples of how impaired phagocytosis contributes to pathology (Doran et al, 2019; Feng et al, 2019; Morioka et al, 2019). Currently available studies evaluating the efficacy and safety of blocking the CD47‐SIRPα axis have shown promising results. Additional studies are needed to evaluate the long‐term safety and efficacy of these therapies, as well as therapeutically targeting other don't‐eat‐me checkpoints (see also Box 1). Finally, are there other diseases where aberrant don't‐eat‐me signaling contributes to pathology? Recent studies suggest that CD47 and PD‐L1 are upregulated in pulmonary fibrosis, but it is unclear from these studies if dysregulated phagocytosis contributes to fibrotic pathologies (Wernig et al, 2017; Cui et al, 2020).
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors thank members of the Ravichandran Laboratory for useful discussions. This work is supported by AI59551, GM122542, HL120840 (to KSR), and a fellowship from the Carter Immunology Center at the University of Virginia.
EMBO reports (2021) 22: e52564.
See the Glossary for abbreviations used in this article.
References
- Abram CL, Lowell CA (2017) Shp1 function in myeloid cells. J Leukoc Biol 102: 657–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams S, van der Laan LJW, Vernon‐Wilson E, de Lavalette CR, Dopp EA, Dijkstra CD, Simmons DL, van den Berg TK (1998) Signal‐regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol 161: 1853–1859 [PubMed] [Google Scholar]
- Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP et al (2018) CD47 blockade by Hu5F9‐G4 and rituximab in non‐Hodgkin's lymphoma. N Engl J Med 379: 1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens N, Pagenkopf C, Kiesewetter H, Salama A (2006) CD47 is expressed at normal levels in patients with autoimmune haemolytic anaemia and/or immune thrombocytopenia. Transfus Med 16: 397–402 [DOI] [PubMed] [Google Scholar]
- Alvarez Y, Tang X, Coligan JE, Borrego F (2008) The CD300a (IRp60) inhibitory receptor is rapidly up‐regulated on human neutrophils in response to inflammatory stimuli and modulates CD32a (Fc gamma IIa) mediated signaling. Mol Immunol 45: 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MJ, Walk SF, March ME, Su HP, Carver DJ, Ravichandran KS (2000) Essential role for the C‐terminal noncatalytic region of SHIP in Fc gamma RIIB1‐mediated inhibitory signaling. Mol Cell Biol 20: 3576–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arandjelovic S, Ravichandran KS (2015) Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16: 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel A, Ravichandran KS (2016) 'This way please': apoptotic cells regulate phagocyte migration before and after engulfment. Eur J Immunol 46: 1583–1586 [DOI] [PubMed] [Google Scholar]
- Baba T, Fusaki N, Shinya N, Iwamatsu A, Hozumi N (2003) Myosin is an in vivo substrate of the protein tyrosine phosphatase (SHP‐1) after mIgM cross‐linking. Biochem Biophs Res Commun 304: 67–72 [DOI] [PubMed] [Google Scholar]
- Bally APR, Lu PY, Tang Y, Austin JW, Scharer CD, Ahmed R, Boss JM (2015) NF‐kappa B regulates PD‐1 expression in macrophages. J Immunol 194: 4545–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AN, Van den Berg TK (2014) The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 32: 25–50 [DOI] [PubMed] [Google Scholar]
- Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM et al (2018) Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol 19: 76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal LJ et al (2019) CD24 signalling through macrophage Siglec‐10 is a target for cancer immunotherapy. Nature 572: 392–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon T, Kitzig F, Sayos J, Lopez‐Botet M (2002) Mutational analysis of immunoreceptor tyrosine‐based inhibition motifs of the Ig‐like transcript 2 (CD85j) leukocyte receptor. J Immunol 168: 3351–3359 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR et al (2019) Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation 139: e56–e528 [DOI] [PubMed] [Google Scholar]
- Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M, Kuo AH, McKenna K, Kojima Y, Leeper NJ et al (2017) A CD47‐associated super‐enhancer links pro‐inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun 8: 14802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacherjee A, Rodrigues E, Jung J, Luzentales‐Simpson M, Enterina JR, Galleguillos D, St. Laurent CD, Nakhaei‐Nejad M, Fuchsberger FF, Streith L et al (2019) Repression of phagocytosis by human CD33 is not conserved with mouse CD33. Commun Biol 2: 450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasioli J, Paust S, Thomas ML (1999) Definition of the sites of interaction between the protein tyrosine phosphatase SHP‐1 and CD22. J Biol Chem 274: 2303–2307 [DOI] [PubMed] [Google Scholar]
- Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis‐Mortari A, Oldenborg PA, Iizuka K, Yokoyama WM, Taylor PA (2001) CD47 (integrin‐associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med 194: 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges L, Hsu ML, Fanger N, Kubin M, Cosman D (1997) A family of human lymphoid and myeloid Ig‐like receptors, some of which bind to MHC class I molecules. J Immunol 159: 5192–5196 [PubMed] [Google Scholar]
- Borrego F (2013) The CD300 molecules: an emerging family of regulators of the immune system. Blood 121: 1951–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman‐Vand der Linden ECM, Angata T, Reynolds SA, Powell LD, Hedrick SM, Varki A (2003) CD33/Siglec‐3 binding specificity, expression pattern, and consequences of gene deletion in mice. Mol Cell Biol 23: 4199–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, Hooper L, Ho T, Gresham H (1990) Integrin‐associated protein: a 50‐kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol 111: 2785–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J (2002) Apoptosis disables CD31‐mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418: 200–203 [DOI] [PubMed] [Google Scholar]
- Burger P, Hilarius‐Stokman P, de Korte D, van den Berg TK, van Bruggen R (2012) CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 119: 5512–5521 [DOI] [PubMed] [Google Scholar]
- Campbell IG, Freemont PS, Foulkes W, Trowsdale J (1992) An ovarian tumor‐marker with homology to vaccinia virus contains an Igv‐like region and multiple transmembrane domains. Cancer Res 52: 5416–5420 [PubMed] [Google Scholar]
- Casey Sc, Tong L, Li Y, Do R, Walz S, Fitzgerald Kn, Gouw Am, Baylot V, Gutgemann I, Eilers M et al (2016) MYC regulates the antitumor immune response through CD47 and PD‐L1. Science 352: 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani L, Sollazzo D, Ricci F, Polverelli N, Palandri F, Baccarani M, Vianelli N, Lemoli RM (2011) The CD47 pathway is deregulated in human immune thrombocytopenia. Exp Hematol 39: 486–494 [DOI] [PubMed] [Google Scholar]
- Chen GY, Tang J, Zheng P, Liu Y (2009) CD24 and Siglec‐10 selectively repress tissue damage‐induced immune responses. Science 323: 1722–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G‐Y, Chen Xi, King S, Cavassani KA, Cheng J, Zheng X, Cao H, Yu H, Qu J, Fang D et al (2011) Amelioration of sepsis by inhibiting sialidase‐mediated disruption of the CD24‐SiglecG interaction. Nat Biotechnol 29: 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Han C, Xie B, Hu X, Yu Q, Shi L, Wang Q, Li D, Wang J, Zheng P et al (2013) Induction of Siglec‐G by RNA viruses inhibits the innate immune response by promoting RIG‐I degradation. Cell 152: 467–478 [DOI] [PubMed] [Google Scholar]
- Chen W, Wang J, Jia L, Liu J, Tian Y (2016) Attenuation of the programmed cell death‐1 pathway increases the M1 polarization of macrophages induced by zymosan. Cell Death Dis 7: e2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SC, Simhadri VR, Tian LJ, Gil‐Krzewska A, Krzewski K, Borrego F, Coligan JE (2011) Cutting edge: mouse CD300f (CMRF‐35‐Like Molecule‐1) recognizes outer membrane‐exposed phosphatidylserine and can promote phagocytosis. J Immunol 187: 3483–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang W, Lagenaur CF (1990) Central‐nervous‐system antigen‐P84 can serve as a substrate for neurite outgrowth. Dev Biol 137: 219–232 [DOI] [PubMed] [Google Scholar]
- Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, LopezBotet M (1997) A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 186: 1809–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comu S, Weng W, Olinsky S, Ishwad P, Mi Z, Hempel J, Watkins S, Lagenaur CF, Narayanan V (1997) The murine P84 neural adhesion molecule is SHPS‐1, a member of the phosphatase‐binding protein family. J Neurosci 17: 8702–8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Chen SY, Lerbs T, Lee JW, Domizi P, Gordon S, Kim YH, Nolan G, Betancur P, Wernig G (2020) Activation of JUN in fibroblasts promotes pro‐fibrotic programme and modulates protective immunity. Nat Commun 11: 2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeron M, Jaeger S, Du Pasquier L, Vivier E (2008) Immunoreceptor tyrosine‐based inhibition motifs: a quest in the past and future. Immunol Rev 224: 11–43 [DOI] [PubMed] [Google Scholar]
- Davies SP, Reynolds GM, Stamataki Z (2018) Clearance of apoptotic cells by tissue epithelia: a putative role for hepatocytes in liver efferocytosis. Front Immunol 9: 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerge M, Yeap XY, Dehn S, Zhang S, Grigoryeva L, Misener S, Procissi D, Zhou X, Lee DC, Muller WA et al (2017) MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ Res 121: 930–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakonova M, Bokoch G, Swanson JA (2002) Dynamics of cytoskeletal proteins during Fcgamma receptor‐mediated phagocytosis in macrophages. Mol Biol Cell 13: 402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Guo Z, Liu Y, Li X, Zhang Q, Xu X, Gu Y, Zhang Y, Zhao D, Cao X (2016) The lectin Siglec‐G inhibits dendritic cell cross‐presentation by impairing MHC class I‐peptide complex formation. Nat Immunol 17: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin JJ, Thomas ML, Fearon DT (1995) A role in B‐cell activation for Cd22 and the protein‐tyrosine‐phosphatase Shp. Science 269: 242–244 [DOI] [PubMed] [Google Scholar]
- Doran AC, Yurdagul A Jr, Tabas I (2019) Efferocytosis in health and disease. Nat Rev Immunol 20: 254–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucey MA, Scarpellino L, Zimmer J, Guillaume P, Luescher IF, Bron C, Held W (2004) Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat Immunol 5: 328–336 [DOI] [PubMed] [Google Scholar]
- Elliott JI, Surprenant A, Marelli‐Berg FM, Cooper JC, Cassady‐Cain RL, Wooding C, Linton K, Alexander DR, Higgins CF (2005) Membrane phosphatidylserine distribution as a non‐apoptotic signalling mechanism in lymphocytes. Nat Cell Biol 7: 808–816 [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P et al (2009) Nucleotides released by apoptotic cells act as a find‐me signal to promote phagocytic clearance. Nature 461: 282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Ravichandran KS (2016) The dynamics of apoptotic cell clearance. Dev Cell 38: 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbertsen D, Autio A, Verwilligen RAF, Depuydt MAC, Newton G, Rattik S, Levinsohn E, Saggu G, Jarolim P, Wang H et al (2019) Increased lymphocyte activation and atherosclerosis in CD47‐deficient mice. Sci Rep 9: 10608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL (2001) Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem 276: 1071–1077 [DOI] [PubMed] [Google Scholar]
- Fanger NA, Cosman D, Peterson L, Braddy SC, Maliszewski CR, Borges L (1998) The MHC class I binding proteins LIR‐1 and LIR‐2 inhibit Fc receptor‐mediated signaling in monocytes. Eur J Immunol 28: 3423–3434 [DOI] [PubMed] [Google Scholar]
- Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL (2019) Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer 19: 568–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG (2012) Regulation of the inflammatory response in cardiac repair. Circ Res 110: 159–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SA, Vega A, Riedl M, Collins RF, Ostrowski PP, Woods EC, Bertozzi CR, Tammi MI, Lidke DS, Johnson P et al (2018) Transmembrane pickets connect Cyto‐ and pericellular skeletons forming barriers to receptor engagement. Cell 172: 305–317.e310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M (1996) A novel membrane glycoprotein, SHPS‐1, that binds the SH2‐domain‐containing protein tyrosine phosphatase SHP‐2 in response to mitogens and cell adhesion. Mol Cell Biol 16: 6887–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA (1996a) Thrombospondin modulates alpha(v)beta(3) function through integrin‐associated protein. J Cell Biol 135: 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA (1996b) Integrin‐associated protein is a receptor for the C‐terminal domain of thrombospondin. J Biol Chem 271: 21–24 [DOI] [PubMed] [Google Scholar]
- Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM (2003) By binding SIRP alpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115: 13–23 [DOI] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy‐Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM (2005) Cell‐surface calreticulin initiates clearance of viable or apoptotic cells through trans‐activation of LRP on the phagocyte. Cell 123: 321–334 [DOI] [PubMed] [Google Scholar]
- Gerlach BD, Marinello M, Heinz J, Rymut N, Sansbury BE, Riley CO, Sadhu S, Hosseini Z, Kojima Y, Tang DD et al (2020) Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ 27: 525–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun A, Cambier JC (2015) Of ITIMs, ITAMs, and ITAMis: revisiting immunoglobulin Fc receptor signaling. Immunol Rev 268: 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CP, Descoteaux A (2018) Moesin and myosin IIA modulate phagolysosomal biogenesis in macrophages. Biochem Biophys Res Commun 495: 1964–1971 [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Castrillo A (2018) Origin and specialization of splenic macrophages. Cell Immunol 330: 151–158 [DOI] [PubMed] [Google Scholar]
- Goodman JW, Smith LH (1961) Erythrocyte life span in normal mice and in radiation bone marrow chimeras. Am J Physiol 200: 764–770 [DOI] [PubMed] [Google Scholar]
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D et al (2017) PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature 545: 495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, Serrano‐Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE (2013) Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78: 631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, Patel S, Federico AN, Choi SH, Innes BJ, Oram MK, Cereghetti G, McGinty D, Anselmo A, Sadreyev RI et al (2019) TREM2 acts downstream of CD33 in modulating microglial pathology in Alzheimer's disease. Neuron 103: 820–835.e827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W (2008) Refinement of the retinogeniculate pathway. J Physiol 586: 4357–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, van de Laar L (2015) A Hitchhiker's guide to myeloid cell subsets: practical implementation of a novel mononuclear phagocyte classification system. Front Immunol 6: 406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagnerud S, Manna PP, Cella M, Stenberg A, Frazier WA, Colonna M, Oldenborg PA (2006) Deficit of CD47 results in a defect of marginal zone dendritic cells, blunted immune response to particulate antigen and impairment of skin dendritic cell migration. J Immunol 176: 5772–5778 [DOI] [PubMed] [Google Scholar]
- Hayes BH, Tsai RK, Dooling LJ, Kadu S, Lee JY, Pantano D, Rodriguez PL, Subramanian S, Shin JW, Discher DE (2020) Macrophages show higher levels of engulfment after disruption of cis interactions between CD47 and the checkpoint receptor SIRPα. J Cell Sci 133: jcs237800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter‐Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA, Klibanov AL, Yan Z, Mandell JW, Ravichandran KS (2013) Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 497: 263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter‐Hufford AE, Arandjelovic S, Ravichandran KS (2015) Using phosphatidylserine exposure on apoptotic cells to stimulate myoblast fusion. Methods Mol Biol 1313: 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J‐C, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V et al (2011) Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet 43: 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Venet F, Wang Yl, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung C‐s et al (2009) PD‐1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA 106: 6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa‐Sekigami T, Kaneko Y, Okazawa H, Tomizawa T, Okajo J, Saito Y, Okuzawa C, Sugawara‐Yokoo M, Nishiyama U, Ohnishi H et al (2006) SHPS‐1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood 107: 341–348 [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL (2009) CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138: 271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen WJ, McPhillips KA, Dickinson MG, Linderman DJ, Morimoto K, Xiao YQ, Oldham KM, Vandivier RW, Henson PM, Gardai SJ (2008) Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP alpha. Am J Respir Crit Care Med 178: 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarr KU, Nakamoto R, Doan BH, Kojima Y, Weissman IL, Advani RH, Iagaru A, Leeper NJ (2021) Effect of CD47 blockade on vascular inflammation. N Engl J Med 384: 382–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Lagenaur CF, Narayanan V (1999) Integrin‐associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 274: 559–562 [DOI] [PubMed] [Google Scholar]
- Johansen ML, Brown EJ (2007) Dual regulation of SIRP alpha phosphorylation by integrins and CD47. J Biol Chem 282: 24219–24230 [DOI] [PubMed] [Google Scholar]
- Jonsson AH, Yokoyama WM (2009) Natural killer cell tolerance: licensing and other mechanisms. Adv Immunol 101: 27–79 [DOI] [PubMed] [Google Scholar]
- Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter‐Hufford A, Borish L, Ravichandran KS (2013) Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493: 547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant AM, De P, Peng XD, Yi TL, Rawlings DJ, Kim JS, Durden DL (2002) SHP‐1 regulates Fc gamma receptor‐mediated phagocytosis and the activation of RAC. Blood 100: 1852–1859 [PubMed] [Google Scholar]
- Karyampudi L, Lamichhane P, Krempski J, Kalli KR, Behrens MD, Vargas DM, Hartmann LC, Janco JM, Dong H, Hedin KE et al (2016) PD‐1 blunts the function of ovarian tumor‐infiltrating dendritic cells by inactivating NF‐kappaB. Cancer Res 76: 239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal S, van Rooijen N, Saxena RK (2007) Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion 47: 1725–1732 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Chen ZJ, Sures I, Wang HY, Schilling J, Ullrich A (1997) A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386: 181–186 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Lee SM, Suk K, Lee WH (2012) CD300a and CD300f differentially regulate the MyD88 and TRIF‐mediated TLR signalling pathways through activation of SHP‐1 and/or SHP‐2 in human monocytic cell lines. Immunology 135: 226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye JQ, Connolly AJ et al (2016) CD47‐blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536: 86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski M, Mlinaric‐Rascan I, Feng GS, Shen R, Pawson T, Siminovitch KA (1993) Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med 178: 2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama T, Takenaka K, Kohno K, Yamauchi T, Daitoku S, Yoshimoto G, Kikushige Y, Kishimoto J, Abe Y, Harada N et al (2012) Engulfment of hematopoietic stem cells caused by down‐regulation of CD47 is critical in the pathogenesis of hemophagocytic lymphohistiocytosis. Blood 120: 4058–4067 [DOI] [PubMed] [Google Scholar]
- Latour S, Tanaka H, Demeure C, Mateo V, Rubio M, Brown EJ, Maliszewski C, Lindberg FP, Oldenborg A, Ullrich A et al (2001) Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal‐regulator protein‐alpha: down‐regulation of IL‐12 responsiveness and inhibition of dendritic cell activation. J Immunol 167: 2547–2554 [DOI] [PubMed] [Google Scholar]
- Law CL, Sidorenko SP, Chandran KA, Zhao ZH, Shen SH, Fischer EH, Clark EA (1996) CD22 associates with protein tyrosine phosphatase 1C, Syk, and phospholipase C‐gamma 1 upon B cell activation. J Exp Med 183: 547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Penberthy KK, Wheeler KM, Juncadella IJ, Vandenabeele P, Lysiak JJ, Ravichandran KS (2016) Boosting apoptotic cell clearance by colonic epithelial cells attenuates inflammation in vivo . Immunity 44: 807–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand N, Huntington ND, Nagasawa M, Bakker AQ, Schotte R, Strick‐Marchand H, de Geus SJ, Pouw SM, Bohne M, Voordouw A et al (2011) Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T‐ and natural killer‐ (NK) cell homeostasis in vivo . Proc Natl Acad Sci USA 108: 13224–13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, Walker AJ, Heller MD, Umemori H, Chen CF et al (2018) CD47 protects synapses from excess microglia‐mediated pruning during development. Neuron 100: 120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine AM, Whitsett JA (2001) Pulmonary collectins and innate host defense of the lung. Microbes Infect 3: 161–166 [DOI] [PubMed] [Google Scholar]
- Li LX, Atif SM, Schmiel SE, Lee SJ, McSorley SJ (2012) Increased susceptibility to Salmonella infection in signal regulatory protein alpha‐deficient mice. J Immunol 189: 2537–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg FP, Gresham HD, Schwarz E, Brown EJ (1993) Molecular cloning of integrin‐associated protein: an immunoglobulin family member with multiple membrane‐spanning domains implicated in alpha v beta 3‐dependent ligand binding. J Cell Biol 123: 485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg FP, Lublin DM, Telen MJ, Veile RA, Miller YE, Doniskeller H, Brown EJ (1994) Rh‐related antigen Cd47 is the signal‐transducer integrin‐associated protein. J Biol Chem 269: 1567–1570 [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ (1996) Decreased resistance to bacterial infection and granulocyte defects in IAP‐deficient mice. Science 274: 795–798 [DOI] [PubMed] [Google Scholar]
- Liu Y, Buhring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA (2002) Signal regulatory protein (SIRP alpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem 277: 10028–10036 [DOI] [PubMed] [Google Scholar]
- Liu F, Jiang CC, Yan XG, Tseng H‐Y, Wang CY, Zhang YY, Yari H, La T, Farrelly M, Guo ST et al (2017) BRAF/MEK inhibitors promote CD47 expression that is reversible by ERK inhibition in melanoma. Oncotarget 8: 69477–69492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes FB, Balint S, Valvo S, Felce JH, Hessel EM, Dustin ML, Davis DM (2017) Membrane nanoclusters of FcgammaRI segregate from inhibitory SIRPalpha upon activation of human macrophages. J Cell Biol 216: 1123–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz U (2009) SHP‐1 and SHP‐2 in T cells: two phosphatases functioning at many levels. Immunol Rev 228: 342–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry MB, Duchemin AM, Robinson JM, Anderson CL (1998) Functional separation of pseudopod extension and particle internalization during Fc gamma receptor‐mediated phagocytosis. J Exp Med 187: 161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P, Koskinen C, Baldock PA, Lothgren H, Stenberg A, Lerner UH, Oldenborg PA (2007) Osteoclast formation is strongly reduced both in vivo and in vitro in the absence of CD47/SIRPalpha‐interaction. Biochem Biophys Res Commun 352: 444–448 [DOI] [PubMed] [Google Scholar]
- Lv Z, Bian Z, Shi L, Niu S, Ha B, Tremblay A, Li L, Zhang X, Paluszynski J, Liu M et al (2015) Loss of cell surface CD47 clustering formation and binding avidity to SIRPalpha facilitate apoptotic cell clearance by macrophages. J Immunol 195: 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile LA, DeMambro VE, Wai C, Lotinun S, Aday AW, Capps BE, Beamer WG, Rosen CJ, Clemmons DR (2011) An essential role for the association of CD47 to SHPS‐1 in skeletal remodeling. J Bone Miner Res 26: 2068–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, van Rooijen N, Weissman IL (2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138: 286–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco M, Berteotti A, Weyershaeuser J, Thorausch N, Sikorska J, Krausze J, Brandt Hj, Kirkpatrick J, Rios P, Schamel Ww et al (2020) Molecular mechanism of SHP2 activation by PD‐1 stimulation. Sci Adv 6: eaay4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T, Murata Y, Okazawa H, Ohnish H (2009) Functions and molecular mechanisms of the CD47‐SIRP alpha signalling pathway. Trends Cell Biol 19: 72–80 [DOI] [PubMed] [Google Scholar]
- May RC, Machesky LM (2001) Phagocytosis and the actin cytoskeleton. J Cell Sci 114: 1061–1077 [DOI] [PubMed] [Google Scholar]
- Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, Barron B, Walk SF, Ghesquière B, Krupnick AS et al (2020) Metabolites released from apoptotic cells act as tissue messengers. Nature 580: 130–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels AD, Newhook TE, Adair SJ, Morioka S, Goudreau BJ, Nagdas S, Mullen MG, Persily JB, Bullock TNJ, Slingluff CL Jr et al (2018) CD47 blockade as an adjuvant immunotherapy for resectable pancreatic cancer. Clin Cancer Res 24: 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, Fadok VA (2005) Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ 12: 107–114 [DOI] [PubMed] [Google Scholar]
- Morioka S, Perry JSA, Raymond MH, Medina CB, Zhu Y, Zhao L, Serbulea V, Onengut‐Gumuscu S, Leitinger N, Kucenas S et al (2018) Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563: 714–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka S, Maueroder C, Ravichandran KS (2019) Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50: 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey MA, Kern N, Vale RD (2020) CD47 Ligation repositions the inhibitory receptor SIRPA to suppress integrin activation and phagocytosis. Immunity 53: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday J, Kerr S, Ni J, Cornish AL, Zhang JQ, Nicoll G, Floyd H, Mattei MG, Moore P, Liu D et al (2001) Identification, characterization and leucocyte expression of Siglec‐10, a novel human sialic acid‐binding receptor. Biochem J 355: 489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers DR, Abram CL, Wildes D, Belwafa A, Welsh AMN, Schulze CJ, Choy TJ, Nguyen T, Omaque N, Hu Y et al (2020) Shp1 loss enhances macrophage effector function and promotes anti‐tumor immunity. Front Immunol 11: 576310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang L‐S, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK et al (2011) Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late‐onset Alzheimer's disease. Nat Genet 43: 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahashi‐Oda C, Tahara‐Hanaoka S, Honda S, Shibuya K, Shibuya A (2012a) Identification of phosphatidylserine as a ligand for the CD300a immunoreceptor. Biochem Biophs Res Commun 417: 646–650 [DOI] [PubMed] [Google Scholar]
- Nakahashi‐Oda C, Tahara‐Hanaoka S, Shoji M, Okoshi Y, Nakano‐Yokomizo T, Ohkohchi N, Yasui T, Kikutani H, Honda S‐I, Shibuya K et al (2012b) Apoptotic cells suppress mast cell inflammatory responses via the CD300a immunoreceptor. J Exp Med 209: 1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya M, Watari K, Tajima M, Nakaya T, Matsuda S, Ohara H, Nishihara H, Yamaguchi H, Hashimoto A, Nishida M et al (2017) Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J Clin Invest 127: 383–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel BG, Gu HH, Pao L (2003) The 'Shp'ing news: SH2 domain‐containing tyrosine phosphatases in cell signaling. Trends Biochem Sci 28: 284–293 [DOI] [PubMed] [Google Scholar]
- Nilsson A, Oldenborg PA (2009) CD47 promotes both phosphatidylserine‐independent and phosphatidylserine‐dependent phagocytosis of apoptotic murine thymocytes by non‐activated macrophages. Biochem Biophs Res Commun 387: 58–63 [DOI] [PubMed] [Google Scholar]
- Niogret C, Birchmeier W, Guarda G (2019) SHP‐2 in lymphocytes' cytokine and inhibitory receptor signaling. Front Immunol 10: 2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Matozaki T, Fujioka Y, Yamao T, Tsuda M, Takada T, Kasuga M (1996) Characterization of a 115‐kDa protein that binds to SH‐PTP2, a protein‐tyrosine phosphatase with Src homology 2 domains, in Chinese hamster ovary cells. J Biol Chem 271: 27652–27658 [DOI] [PubMed] [Google Scholar]
- Oh ES, Gu HH, Saxton TM, Timms JF, Hausdorff S, Frevert EU, Kahn BB, Pawson T, Neel BG, Thomas SM (1999) Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP‐2. Mol Cell Biol 19: 3205–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T (2013) A rheostat for immune responses: the unique properties of PD‐1 and their advantages for clinical application. Nat Immunol 14: 1212–1218 [DOI] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP (2000) Role of CD47 as a marker of self on red blood cells. Science 288: 2051–2054 [DOI] [PubMed] [Google Scholar]
- Oldenborg PA, Gresham HD, Lindberg FP (2001) CD47‐signal regulatory protein alpha (SIRP alpha) regulates Fc gamma and complement receptor‐mediated phagocytosis. J Exp Med 193: 855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg PA, Gresham HD, Chen Y, Izui S, Lindberg FP (2002) Lethal autoimmune hemolytic anemia in CD47‐deficient nonobese diabetic (NOD) mice. Blood 99: 3500–3504 [DOI] [PubMed] [Google Scholar]
- Oldenborg PA (2013) CD47: a cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol 2013: 614619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA (2005) Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood 105: 3577–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otipoby KL, Draves KE, Clark EA (2001) CD22 regulates B cell receptor‐mediated signals via two domains that independently recruit Grb2 and SHP‐1. J Biol Chem 276: 44315–44322 [DOI] [PubMed] [Google Scholar]
- Patsoukis N, Wang Q, Strauss L, Boussiotis VA (2020) Revisiting the PD‐1 pathway. Sci Adv 6: eabd2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy KK, Rival C, Shankman LS, Raymond MH, Zhang J, Perry JSA, Lee CS, Han CZ, Onengut‐Gumuscu S, Palczewski K et al (2017) Context‐dependent compensation among phosphatidylserine‐recognition receptors. Sci Rep 7: 14623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JSA, Morioka S, Medina CB, Iker Etchegaray J, Barron B, Raymond MH, Lucas CD, Onengut‐Gumuscu S, Delpire E, Ravichandran KS (2019) Interpreting an apoptotic corpse as anti‐inflammatory involves a chloride sensing pathway. Nat Cell Biol 21: 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Netravali IA, Cariappa A, Mattoo H (2012) Siglecs and immune regulation. Annu Rev Immunol 30: 357–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluvinage JV, Haney MS, Smith BAH, Sun J, Iram T, Bonanno L, Li L, Lee DP, Morgens DW, Yang AC et al (2019) CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 568: 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe JC, Fujimoto M, Jansen PJ, Miller AS, Tedder TF (2000) CD22 forms a quaternary complex with SHIP, Grb2, and Shc ‐ A pathway for regulation of B lymphocyte antigen receptor‐induced calcium flux. J Biol Chem 275: 17420–17427 [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Lanier LL (2000) Immune inhibitory receptors. Science 290: 84–89 [DOI] [PubMed] [Google Scholar]
- Reidy MF, Wright JR (2003) Surfactant protein A enhances apoptotic cell uptake and TGF‐beta 1 release by inflammatory alveolar macrophages. Am J Physiol‐Lung C 285: L854–L861 [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ (1995) In vivo expression of alternatively spliced forms of integrin‐associated protein (CD47). J Cell Sci 108(Pt 11): 3419–3425 [DOI] [PubMed] [Google Scholar]
- Rival CM, Xu W, Shankman LS, Morioka S, Arandjelovic S, Lee CS, Wheeler KM, Smith RP, Haney LB, Isakson BE et al (2019) Phosphatidylserine on viable sperm and phagocytic machinery in oocytes regulate mammalian fertilization. Nat Commun 10: 4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquilly A, Jacqueline C, Davieau M, Molle A, Sadek A, Fourgeux C, Rooze P, Broquet A, Misme‐Aucouturier B, Chaumette T et al (2020) Alveolar macrophages are epigenetically altered after inflammation, leading to long‐term lung immunoparalysis. Nat Immunol 21: 636–648 [DOI] [PubMed] [Google Scholar]
- Rota G, Niogret C, Dang AT, Barros CR, Fonta NP, Alfei F, Morgado L, Zehn D, Birchmeier W, Vivier E et al (2018) Shp‐2 Is dispensable for establishing T cell exhaustion and for PD‐1 signaling in vivo . Cell Rep 23: 39–49 [DOI] [PubMed] [Google Scholar]
- Saito Y, Iwamura H, Kaneko T, Ohnishi H, Murata Y, Okazawa H, Kanazawa Y, Sato‐Hashimoto M, Kobayashi H, Oldenborg PA et al (2010) Regulation by SIRPalpha of dendritic cell homeostasis in lymphoid tissues. Blood 116: 3517–3525 [DOI] [PubMed] [Google Scholar]
- Samaridis J, Colonna M (1997) Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur J Immunol 27: 660–665 [DOI] [PubMed] [Google Scholar]
- Sato‐Hashimoto M, Saito Y, Ohnishi H, Iwamura H, Kanazawa Y, Kaneko T, Kusakari S, Kotani T, Mori M, Murata Y et al (2011) Signal regulatory protein alpha regulates the homeostasis of T lymphocytes in the spleen. J Immunol 187: 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Hogg N, Ren Y, Haslett C (1992) Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest 90: 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron 74: 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagat TL, Wofford JA, Wright JR (2001) Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol 166: 2727–2733 [DOI] [PubMed] [Google Scholar]
- Schulz C, Perdiguero EG, Chorro L, Szabo‐Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SEW, Pollard JW et al (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90 [DOI] [PubMed] [Google Scholar]
- Shen L, Gao Y, Liu YY, Zhang BY, Liu QQ, Wu J, Fan L, Ou QF, Zhang WH, Shao LY (2016) PD‐1/PD‐L pathway inhibits M.tb‐specific CD4(+) T‐cell functions and phagocytosis of macrophages in active tuberculosis. Sci Rep 6: 38362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL (1984) Viable Motheaten, a new allele at the Motheaten locus. 1. PATHOLOGY. Am J Pathol 116: 179–192 [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Rajan TV, Yi TL, Ihle JN, Matthews RJ, Thomas ML, Beier DR (1993) Mutations at the Murine Moth‐Eaten Locus are within the hematopoietic‐cell protein‐tyrosine‐phosphatase (Hcph) gene. Cell 73: 1445–1454 [DOI] [PubMed] [Google Scholar]
- Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, Colevas AD, O'Rourke T, Narayanan S, Papadopoulos K et al (2019) First‐in‐human, first‐in‐class phase I trial of the anti‐CD47 antibody Hu5F9‐G4 in patients with advanced cancers. J Clin Oncol 37: 946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhadri VR, Andersen JF, Calvo E, Choi SC, Coligan JE, Borrego F (2012) Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood 119: 2799–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada K, Tanaka M, Hanayama R, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2003) Tethering of apoptotic cells to phagocytes through binding of CD47 to Src homology 2 domain‐bearing protein tyrosine phosphatase substrate‐1. J Immunol 171: 5718–5726 [DOI] [PubMed] [Google Scholar]
- Takada T, Matozaki T, Takeda H, Fukunaga K, Noguchi T, Fujioka Y, Okazaki I, Tsuda M, Yamao T, Ochi F et al (1998) Roles of the complex formation of SHPS‐1 with SHP‐2 in insulin‐stimulated mitogen‐activated protein kinase activation. J Biol Chem 273: 9234–9242 [DOI] [PubMed] [Google Scholar]
- Takeda H, Matozaki T, Fujioka Y, Takada T, Noguchi T, Yamao T, Tsuda M, Ochi F, Fukunaga K, Narumiya S et al (1998) Lysophosphatidic acid‐induced association of SHP‐2 with SHPS‐1: roles of RHO, FAK, and a SRC family kinase. Oncogene 16: 3019–3027 [DOI] [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JCY, Mortin‐Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS (2007) Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 8: 1313–1323 [DOI] [PubMed] [Google Scholar]
- Tal MC, Torrez Dulgeroff LB, Myers L, Cham LB, Mayer‐Barber KD, Bohrer AC, Castro E, Yiu YY, Lopez Angel C, Pham E et al (2020) Upregulation of CD47 is a host checkpoint response to pathogen recognition. MBio 11: e01293–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao B, Jin W, Xu J, Liang Z, Yao J, Zhang Y, Wang K, Cheng H, Zhang X, Ke Y (2014) Myeloid‐specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J Immunol 193: 2801–2811 [DOI] [PubMed] [Google Scholar]
- Tian LJ, Choi SC, Murakami Y, Allen J, Morse HC, Qi CF, Krzewski K, Coligan JE (2014) p85 alpha recruitment by the CD300f phosphatidylserine receptor mediates apoptotic cell clearance required for autoimmunity suppression. Nat Commun 5: 3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Choi SC, Lee HN, Murakami Y, Qi CF, Sengottuvelu M, Voss O, Krzewski K, Coligan JE (2016) Enhanced efferocytosis by dendritic cells underlies memory T‐cell expansion and susceptibility to autoimmune disease in CD300f‐deficient mice. Cell Death Differ 23: 1086–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms JF, Carlberg K, Gu HH, Chen HY, Kamatkar S, Nadler MJS, Rohrschneider LR, Neel BG (1998) Identification of major binding proteins and substrates for the SH2‐containing protein tyrosine phosphatase SHP‐1 in macrophages. Mol Cell Biol 18: 3838–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms JF, Swanson KD, Marie‐Cardine A, Raab M, Rudd CE, Schraven B, Neel BG (1999) SHPS‐1 is a scaffold for assembling distinct adhesion‐regulated multi‐protein complexes in macrophages. Curr Biol 9: 927–930 [DOI] [PubMed] [Google Scholar]
- Tosello‐Trampont A‐C, Nakada‐Tsukui K, Ravichandran KS (2003) Engulfment of apoptotic cells is negatively regulated by Rho‐mediated signaling. J Biol Chem 278: 49911–49919 [DOI] [PubMed] [Google Scholar]
- Tsai RK, Discher DE (2008) Inhibition of "self" engulfment through deactivation of myosin‐II at the phagocytic synapse between human cells. J Cell Biol 180: 989–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Matozaki T, Fukunaga K, Fujioka Y, Imamoto A, Noguchi T, Takada T, Yamao T, Takeda H, Ochi F et al (1998) Integrin‐mediated tyrosine phosphorylation of SHPS‐1 and its association with SHP‐2. Roles of Fak and Src family kinases. J Biol Chem 273: 13223–13229 [DOI] [PubMed] [Google Scholar]
- Ulyanova T, Blasioli J, Woodford‐Thomas TA, Thomas ML (1999) The sialoadhesin CD33 is a myeloid‐specific inhibitory receptor. Eur J Immunol 29: 3440–3449 [DOI] [PubMed] [Google Scholar]
- Van VQ, Lesage S, Bouguermouh S, Gautier P, Rubio M, Levesque M, Nguyen S, Galibert L, Sarfati M (2006) Expression of the self‐marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs. EMBO J 25: 5560–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE (2002) Role of surfactant proteins D, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 169: 3978–3986 [DOI] [PubMed] [Google Scholar]
- Veillette A, Thibaudeau E, Latour S (1998) High expression of inhibitory receptor SHPS‐1 and its association with protein‐tyrosine phosphatase SHP‐1 in macrophages. J Biol Chem 273: 22719–22728 [DOI] [PubMed] [Google Scholar]
- Veillette A, Tang Z (2019) Signaling regulatory protein (SIRP)α‐CD47 blockade joins the ranks of immune checkpoint inhibition. J Clin Oncol 37: 1012–1014 [DOI] [PubMed] [Google Scholar]
- de Vries HE, Hendriks JJA, Honing H, de Lavalette CR, van der Pol SMA, Hooijberg E, Dijkstra CD, van den Berg TK (2002) Signal‐regulatory protein alpha‐CD47 interactions are required for the transmigration of monocytes across cerebral endothelium. J Immunol 168: 5832–5839 [DOI] [PubMed] [Google Scholar]
- Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K et al (2013) Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid‐epithelial‐reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113: 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Madariaga ML, Wang S, Van Rooijen N, Oldenborg PA, Yang YG (2007) Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci USA 104: 13744–13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nanda V, Direnzo D, Ye J, Xiao S, Kojima Y, Howe KL, Jarr KU, Flores AM, Tsantilas P et al (2020) Clonally expanding smooth muscle cells promote atherosclerosis by escaping efferocytosis and activating the complement cascade. Proc Natl Acad Sci USA 117: 15818–15826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer JP, Liu J, Lim JS et al (2016) CD47‐blocking immunotherapies stimulate macrophage‐mediated destruction of small‐cell lung cancer. J Clin Invest 126: 2610–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig G, Chen SY, Cui L, Van Neste C, Tsai JM, Kambham N, Vogel H, Natkunam Y, Gilliland DG, Nolan G et al (2017) Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci USA 114: 4757–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney G, Wang SL, Chang H, Cheng KY, Lu P, Zhou XD, Yang WP, McKinnon M, Longphre M (2001) A new siglec family member, siglec‐10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur J Biochem 268: 6083–6096 [DOI] [PubMed] [Google Scholar]
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras‐Trujillo H, Martin R, Cohen JD et al (2012) The CD47‐signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA 109: 6662–6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AS, Mortin‐Toth S, Sung M, Canty AJ, Gulban O, Greaves DR, Danska JS (2014) Polymorphism in the innate immune receptor SIRPalpha controls CD47 binding and autoimmunity in the nonobese diabetic mouse. J Immunol 193: 4833–4844 [DOI] [PubMed] [Google Scholar]
- Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, Martin P (2000) Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development 127: 5245–5252 [DOI] [PubMed] [Google Scholar]
- Wright JR (2005) Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5: 58–68 [DOI] [PubMed] [Google Scholar]
- Yamao T, Matozaki T, Amano K, Matsuda Y, Takahashi N, Ochi F, Fujioka Y, Kasuga M (1997) Mouse and human SHPS‐1: molecular cloning of cDNAs and chromosomal localization of genes. Biochem Biophys Res Commun 231: 61–67 [DOI] [PubMed] [Google Scholar]
- Yamao T, Noguchi T, Takeuchi O, Nishiyama U, Morita H, Hagiwara T, Akahori H, Kato T, Inagaki K, Okazawa H et al (2002) Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS‐1. J Biol Chem 277: 39833–39839 [DOI] [PubMed] [Google Scholar]
- Ye ZM, Yang S, Xia YP, Hu RT, Chen S, Li BW, Chen SL, Luo XY, Mao L, Li Y et al (2019) LncRNA MIAT sponges miR‐149‐5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis 10: 138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurdagul A, Doran AC, Cai BS, Fredman G, Tabas IA (2018) Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front Cardiovasc Med 4: 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurdagul A Jr, Subramanian M, Wang X, Crown SB, Ilkayeva OR, Darville L, Kolluru GK, Rymond CC, Gerlach BD, Zheng Z et al (2020) Macrophage metabolism of apoptotic cell‐derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab 31: 518–533.e510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zent CS, Elliott MR (2017) Maxed out macs: physiologic cell clearance as a function of macrophage phagocytic capacity. FEBS J 284: 1021–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, Gilkes DM, He J, Semenza GL (2015) HIF‐1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA 112: E6215–E6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yeap XY, DeBerge M, Naresh NK, Wang K, Jiang Z, Wilcox JE, White SM, Morrow JP, Burridge PW et al (2017) Acute CD47 blockade during ischemic myocardial reperfusion enhances phagocytosis‐associated cardiac repair. JACC Basic Transl Sci 2: 386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]


