Supplemental Digital Content is available in the text
Keywords: adolescence, HIV, longitudinal, MRI, neuroimaging
Abstract
Objective:
Cross-sectional studies, including one from our NOVICE cohort [Neurological Visual and Cognitive performance in children with treated perinatally acquired HIV (PHIV) compared with matched HIV-negative controls], have revealed that the brains of children with PHIV have lower white matter and grey matter volumes, more white matter hyperintensities (WMH) and poorer white matter integrity. This longitudinal study investigates whether these differences change over time.
Methods:
We approached all NOVICE participants to repeat MRI after 4.6 ± 0.3 years, measuring total white matter and grey matter volume, WMH volume and white matter integrity, obtained by T1-weighted, fluid-attenuated inversion recovery (FLAIR) and diffusion tensor imaging (DTI), respectively. We compared rates of change between groups using multivariable linear mixed effects models, adjusted for sex and age at enrolment. We investigated determinants of developmental deviation, and explored associations with cognitive development.
Results:
Twenty out of 31 (65%) PHIV-positive, and 20 out of 37 (54%) HIV-negative participants underwent follow-up MRI. Groups did not significantly differ in terms of age and sex. Over time, we found no statistically different changes between groups for white matter and WMH volumes, and for white matter integrity (P > 0.1). Total grey matter volume decreased significantly less in PHIV [group∗time 10 ml, 95% confidence interval −1 to 20, P = 0.078], but this difference in rate of change lost statistical significance after additional adjustment for height (group∗time 9 ml, 95% confidence interval −2 to 20, P = 0.112). We found no HIV-associated determinants for potential reduced grey matter pruning, nor associations with cognitive development.
Conclusion:
While using long-term antiretroviral treatment, structural brain development of adolescents growing up with perinatally acquired HIV appears largely normal.
Introduction
HIV enters the brain shortly after infection, thereby potentially posing a threat to normal brain development. Although severe neurological complications are rare due to combination antiretroviral therapy (cART) [1], children with perinatally acquired HIV (PHIV) continue to show cognitive impairment, cerebral abnormalities and persistent immune inflammation and immune activation [2–4].
Normal brain development starts prior to birth and continues throughout early adulthood [5,6]. It is a highly dynamic process in which white matter volume increases, whereas grey matter volume – after initially increasing similarly – declines from puberty onward.
Up to now, we do not know whether brain development in cART-treated PHIV-positive children is similar to that of healthy peers. The few studies that have investigated this were mostly cross-sectional [7]. Those studies, including a prior analysis by our group, have reported that PHIV-positive children have lower brain volumes, poorer white matter integrity and more white matter hyperintensities (WMH) [4]. MRI studies in adults on cART have shown similar changes over 2 years to be similar compared with HIV-negative comparable controls, suggesting that most damage in HIV-positive adults likely occurred during the preceding period of untreated HIV infection [8–10]. However, this may not be generalizable to children, as HIV-positive adults generally acquire HIV long after the critical stages of early brain development.
This analysis from the prospective NOVICE (Neurological Visual and Cognitive performance in perinatally HIV-positive children) observational cohort study [3,4] compared structural brain development between children with PHIV and controls over 4–5 years of follow-up.
Materials and methods
Study design and participants
Between December 2012 and January 2014, 35 PHIV children 8–18 years of age were enrolled into the NOVICE observational cohort study from the outpatient department of the Amsterdam University Medical Centers (Amsterdam UMC), location Academic Medical Center, Amsterdam, the Netherlands, and 37 HIV-negative controls were recruited from the same communities as the PHIV children and frequency matched regarding age, sex, ethnicity and socioeconomic status (SES). The outcomes of the first neuroimaging assessment have previously been published in this journal [11]. Exclusion criteria were (non-HIV associated) chronic neurological diseases such as seizure disorders, (history of) intracerebral neoplasms and severe infections, a history of severe traumatic brain injury (with loss of consciousness longer than 30 min) and severe psychiatric disorders. Between February 2017 and July 2018, we approached all original NOVICE participants for follow-up assessment and included those who provided consent.
Standard protocol approvals, registrations and patient consents
We obtained written informed consent from participants older than 12 years and from all parents or legal guardians of participants younger than 18 years. The Ethics Committee of the Amsterdam UMC approved the study. The NOVICE study is registered with the Netherlands Trial Register (ID NL6813).
MRI data acquisition and processing
We repeated advanced brain imaging on the same 3 Tesla MRI (Ingenia, Philips Healthcare, Best, the Netherlands) using the same 16-channel phased array head coil (Supplementary data for details on acquisition). At both time-points, we assessed two types of outcomes. First, we assessed brain volumes, and second, we assessed white matter microstructure. For brain volumes, we assessed total grey matter and white matter volume by T1-weighted structural MRI, and total WMH volume, by T2-weighted fluid-attenuated inversion recovery (FLAIR). We assessed the microstructure of white matter by diffusion tensor imaging (DTI), a technique generally used for this purpose. We assessed the following DTI measures: fractional anisotropy, axial diffusivity, radial diffusivity and mean diffusivity (Supplementary data for details on MRI data processing).
Neuropsychological assessment
All participants underwent neurocognitive assessment by a well trained neuropsychologist (AMtH) on the same day the MRI was performed. Neurocognitive assessment resulted in the following outcomes: intelligence quotient (IQ), working memory, processing speed, learning abilities, executive functioning and visual-motor functioning. The details and results of both baseline and follow-up neurocognitive assessment have been previously published elsewhere [3,12].
Disease and treatment related characteristics
The Dutch HIV Monitoring Foundation provided data on historical HIV and cART-related characteristics, such as age at HIV diagnosis, age at cART initiation, HIV viral load and CD4+ T-cell counts.
Statistical analysis
We investigated selective dropout in the NOVICE cohort by comparing MRI outcomes at first assessment between PHIV-positive and HIV-negative controls who did or did not consent to the second MRI assessment. We generated descriptive statistics for those PHIV and HIV-negative controls who underwent both MRI examinations and we compared demographic characteristics using the unpaired t-test or Mann–Whitney U-test for normally and nonnormally distributed variables respectively, and Fischer's exact test for categorical data.
We performed linear mixed effects model analysis to assess the association between HIV infection and the rate of change in total grey matter volume, white matter volume, WMH volume, fractional anisotropy, axial diffusivity, radial diffusivity and mean diffusivity over time. We handled all outcomes as continuous variables. For each MRI outcome, we used the model to assess cross-sectional differences between groups at both first and follow-up assessment. Subsequently, we assessed the group-by-visit interaction term for each outcome to evaluate differences in the rates of change over time between PHIV-positive and HIV-negative controls. We adjusted all models for the predefined determinants sex and age at time of first MRI as confounders, as both are known to be highly associated with brain developmental trajectories [5]. For interaction terms, we used a predefined P value cut-off of 0.1. We used a higher Type I error rate of 10% instead of the traditional 5% to gain statistical power. When significant, we concluded that development differs across groups.
We presented least square means and pairwise contrasts between groups with 95% confidence intervals (95% CIs). We performed all analyses using R (version 1.1.383) [13]. With respect to white matter integrity, we additionally performed voxelwise analyses to investigate differences between groups in specific regions of the brain.
For each neuroimaging outcome that developed significantly different between groups, we explored the association with cognitive development among all participants. Again, we adjusted for sex and age at first MRI. Moreover, we analysed possible associations with HIV and cART-related factors among the PHIV participants by univariate analysis, given the limited sample size.
Finally, we performed a sensitivity analysis to determine whether rates of change over time in those with detectable HIV viral load were different compared with the entire PHIV group. We excluded PHIV participants with episodes of detectable HIV viral load (HIV viral load), defined as HIV viral load above 200 copies/ml during follow-up time.
Data availability statement
Data are available from the corresponding author (M.vdH.) on request, in anonymized form.
Results
Participants’ characteristics
Of the 31 PHIV-positive and 37 HIV-negative controls who underwent first MRI examination, 20 PHIV (65%) and 23 HIV-negative controls (62%) provided consent for follow-up MRI. For the 11 PHIV and 14 HIV-negative controls, reasons to not participate were unwillingness to participate (nine PHIV and nine HIV-negative), inability to contact (five HIV-negative) or relocation (two PHIV). PHIV-positive and HIV-negative controls who consented to follow-up MRI were not statistically significantly different at baseline compared with those who did not provide consent with regards to brain volumetric measures and fractional anisotropy at the time of first MRI assessment (all P > 0.05). Table 1 summarizes the characteristics of PHIV-positive and HIV-negative controls who consented to follow-up. Three HIV-negative controls were not eligible for MRI, due to dental braces (n = 2), and misfit of the MRI headcoil (n = 1). We performed follow-up MRI after a mean of 4.6 years (0.3 SD), which was not significantly different between groups (P = 0.694). Participants’ mean age at time of follow-up, was 17.0 years (SD 3.1). PHIV were more often born in sub-Saharan Africa and had more often been adopted (P = 0.008), while the majority of controls was born in the Netherlands (P < 0.001) to immigrant parents. The PHIV-positive group had been diagnosed with HIV at a median age of 1.5 years (IQR 0.8–4.1). Of the 20 PHIV-positive participants, 19 used cART and had been on treatment for a median of 15.0 years (IQR 12.3–19.6). At study entry, children predominantly used an NNRTI-based regimen (efavirenz). Among the PHIV, 65% had HIV viral load below 200 copies/ml during the entire period of follow-up.
Table 1.
Characteristics of perinatally HIV-positive and HIV-negative participants who underwent two MRI examinations as part of the NOVICE cohort.
| Characteristics | n | PHIV-positive | n | HIV-negative | P |
| No. and proportion consenting to second MRI | 20/31 (65%) | 23/37 (62%) | |||
| No and proportion that were eligible for MRI | 20 | 20 (100%) | 23 | 20 (87%) | |
| Interval (y) between MRIs, mean (SD) | 20 | 4.6 (0.3) | 20 | 4.6 (0.3) | 0.694 |
| Age at first MRI (years), mean (SD) | 20 | 13.3 (3.4) | 20 | 12.6 (2.7) | 0.443 |
| Age at second MRI (years), mean (SD) | 20 | 18.0 (3.4) | 20 | 17.2 (2.7) | 0.425 |
| Male sex, No. (%) | 20 | 11 (55%) | 20 | 8 (40%) | 0.527 |
| Internationally adopted, No. (%) | 20 | 3 (15%) | 20 | 0 (0%) | 0.008 |
| Region of birth, No (%) | 20 | 20 | <0.001 | ||
| The Netherlands | 5 (25%) | 19 (95%) | |||
| Sub-Sahara Africa | 12 (60%) | 1 (5%) | |||
| Othera | 3 (15%) | 0 (0%) | |||
| Ethnicity, No (%) | 20 | 20 | |||
| Black | 16 (80%) | 15 (75%) | 0.533 | ||
| Caucasian | 0 (0%) | 2 (10%) | |||
| Other | 4 (20%) | 3 (15%) | |||
| ISCED level of most educated parentb, median (IQR) | 20 | 5.5 (4.8–6) | 20 | 5 (4–6) | 0.359 |
| Number of parents with a jobb, No. (%) | 18 | 20 | 0.228 | ||
| 0 | 7 (39%) | 6 (30%) | |||
| 1 | 9 (50%) | 7 (35%) | |||
| 2 | 2 (11%) | 7 (35%) | |||
| Height at first MRI (m) | 20 | 1.51 (0.2) | 20 | 1.57 (0.1) | 0.204 |
| Height at second MRI (m) | 18 | 1.65 (0.2) | 20 | 1.69 (0.1) | 0.303 |
| HIV and cART-related variables | |||||
| Age at HIV diagnosis (years), median (IQR) | 20 | 1.5 (0.7–4.1) | |||
| Nadir CD4+ T-cell z-score, median (IQR) | 20 | −0.9 (−1.2 to −0.8) | |||
| Zenith HIV viral load (log copies/ml), median (IQR) | 20 | 5.5 (4.7–5.8) | |||
| CDC category, No. (%) | 20 | ||||
| N/A | 12 (60%) | ||||
| B | 2 (10%) | ||||
| C | 6 (30%) | ||||
| Age at cART initiation (years), median (IQR) | 17 | 2.5 (1.2–4.3) | |||
| Time between HIV diagnosis and start cART (months), median (IQR) | 17 | 2.5 (1.4–8.3) | |||
| Years on cART (years), median (IQR) | 17 | 15.0 (12.3–19.6) | |||
| cART at first assessment, No (%) | 20 | 18 (90%) | |||
| cART at second assessment, No. (%) | 20 | 19 (95%) | |||
| Number of children with VL<200 copies/ml during entire follow-up, No. (%) | 20 | 13 (65%) | |||
| MRIs of good quality for assessment | |||||
| T1-weighted | 20 | 18 (90%) | 20 | 17 (85%) | |
| FLAIR | 20 | 19 (95%) | 20 | 18 (90%) | |
| DTI | 20 | 17 (85%) | 20 | 17 (85%) | |
ISCED, International Standard Classification of Education.
Other regions of birth include Latin America and Asia.
At first assessment.
Cross-sectional comparisons of brain volumes and brain white matter integrity at both baseline and follow-up
Brain volumetric assessment
Total white matter volume was significantly lower among PHIV than controls both at first MRI (adjusted beta coefficient −34 ml, 95% CI −63 to −5, P = 0.031), and at follow-up (−32 ml, 95% CI −59 to −5, P = 0.032) (Figs. 1 and 2 and supplementary Tables 1 and 2). Grey matter and WMH volume were not significantly different between groups at both first MRI (adjusted beta coefficient grey matter volume −34 ml, 95% CI −74 to 5, P = 0.104; WMH volume 0.090 ml, 95% CI −0.004 to 0.185, P = 0.078) and at follow-up (grey matter volume −15 ml, 95% CI −51 to 22, P = 0.447; WMH volume 0.084 ml, 95% CI −0.010 to 0.178, P = 0.098).
Fig. 1.
Longitudinal changes over time in brain volumetric and white matter integrity measures.
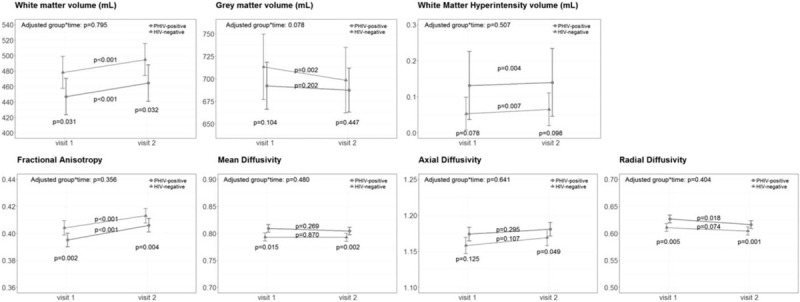
Changes between first and follow-up MRI measures for perinatally HIV-positive (PHIV) adolescents (in red) and HIV-negative controls (in green). Graphs show the least-square means with the 95% confidence intervals, adjusted for sex and age at enrolment, and P values for the adjusted group∗time interaction term, cross-sectional comparison between groups at both time-points and within group over time.
Fig. 2.
Group averages of total white matter, grey matter and white matter hyperintensity volume of perinatally HIV-positive adolescents and HIV-negative controls at first and second MRI.
Brain white matter integrity assessment
Regarding whole brain white matter integrity at first assessment, we found significantly lower fractional anisotropy (adjusted beta coefficient −0.015, 95% CI −0.023 to −0.006, P = 0.002), higher mean diffusivity (0.025, 95% CI 0.006–0.043, P = 0.015) and higher radial diffusivity (0.026, 95% CI 0.009–0.042, P = 0.005) among PHIV-positive compared with HIV-negative controls, indicating poorer white matter diffusion in PHIV. AD was not statistically different between groups at first MRI (0.023, 95% CI −0.003 to 0.050, P = 0.125). At time of follow-up, fractional anisotropy, radial diffusivity and mean diffusivity remained significantly poorer in PHIV compared to controls (adjusted beta coefficient FA −0.011, 95% CI −0.017 to −0.004, P = 0.004; mean diffusivity 0.016, 95% CI 0.007–0.025, P = 0.002; radial diffusivity 0.017, 95% CI 0.008–0.013, P = 0.001). At follow-up, axial diffusivity was significantly higher in PHIV (0.014, 95% CI 0.001–0.028, P = 0.049).
Voxel-wise analysis found no significant differences between groups regarding fractional anisotropy at first MRI (P =>0.05), yet we found significant higher adjusted measures of axial diffusivity, radial diffusivity and mean diffusivity in PHIV-positive compared with HIV-negative participants (all P < 0.05) (data not shown). At follow-up, voxel-wise analysis showed significant lower fractional anisotropy, and significant higher axial diffusivity, radial diffusivity and mean diffusivity in PHIV-positive adolescents compared with controls (all P < 0.05).
Longitudinal analysis
Figure 1 and Table 2 show the outcomes for PHIV-positive adolescents and HIV-negative controls over time. We found a significant group-by-time interaction for grey matter volume (adjusted beta coefficient 10 ml, 95% CI −1 to 20, P = 0.078), indicating that grey matter volume decreased significantly less in PHIV. After we additionally adjusted for height – as PHIV were consistently, yet not significantly shorter compared to HIV-negative controls – we found that the adjusted interaction term lost statistical significance (group∗time 9 ml, 95% CI −2 to 20, P = 0.112). We found no statistically different changes over time in white matter volume (group∗time 1 ml, 95% CI −6 to 8, P = 0.795), nor in WMH volume (group∗time −0.003 ml, 95%CI −0.013 to 0.006, P = 0.507), and in white matter integrity measures (FA group∗time 0.002, 95% CI −0.002 to 0.006, P = 0.356; MD group∗time −0.004, 95% CI −0.013 to 0.006, P = 0.480; RD group∗time −0.004, 95% CI −0.020 to 0.012, P = 0.641; AD group∗time −0.004, 95% CI −0.014 to 0.006, P = 0.404).
Table 2.
Longitudinal changes over time in brain volumetric and white matter integrity measures in perinatally HIV-positive and HIV-negative participants who underwent two MRI examinations as part of the NOVICE cohort.
| Group ∗ Time unadjusted | Group ∗ Time adjusted | ||||
| T1-weighted MRI | n | Coefficient (95% CI) | P | Coefficient (95% CI) | P |
| Total GM volume | 35 | 10 (–1 to 20) | 0.078 | 10 (–1 to 20) | 0.078 |
| Total WM volume | 35 | 1 (–6 to 8) | 0.795 | 1 (–6 to 8) | 0.795 |
| T2-weighted FLAIR | |||||
| WMH volume | 37 | –0.003 (–0.013 to 0.006) | 0.507 | –0.003 (–0.013 to 0.006) | 0.507 |
| Diffusion MRI | |||||
| Whole brain FA | 34 | 0.002 (–0.002 to 0.006) | 0.356 | 0.002 (–0.002 to 0.006) | 0.356 |
| Whole brain MD | 34 | –0.004 (–0.016 to 0.007) | 0.480 | –0.004 (–0.013 to 0.006) | 0.480 |
| Whole brain RD | 34 | –0.004 (–0.014 to 0.006) | 0.404 | –0.004 (–0.020 to 0.012) | 0.641 |
| Whole brain AD | 34 | –0.004 (–0.021 to 0.013) | 0.641 | –0.004 (–0.014 to 0.006) | 0.404 |
We present unadjusted outcomes, and outcomes adjusted for sex and age at first MRI. We report total white matter (WM), grey matter (GM) and white matter hyperintensity (WMH) volume in ml. Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) represent diffusivity measures. MD, AD and RD values are x 1000.
Associations with cognitive outcomes and HIV and cART-related factors
Among all participants, the rate of grey matter decline was not associated with any of the cognitive outcomes (P > 0.149). Likewise, the rate of grey matter decline among PHIV-positive participants was not associated with any historical HIV and cART-related factors (P > 0.186).
Sensitivity analyses
We repeated the longitudinal model excluding six PHIV participants with detectable HIV viral load at or between the two MRI assessments. This did not result in major changes in the significance of the association between HIV and any of the brain outcome measures (data not shown). Initially, we did not adjust for intracranial volume (ICV), that is cranial size, as we expected ICV volume differences across groups as part of the effect of HIV. As a sensitivity analysis, we repeated all models including adjustment for ICV, which did not result in major changes in our findings (data not shown).
Discussion
In this unique longitudinal study, we investigated structural brain development within a cohort of long-term treated PHIV-positive adolescents and an extensively matched HIV-negative control group over approximately five years. We aimed to understand whether previously found brain differences are subject to change. This study suggests that structural brain development, that is the rate of change over time, in individuals growing up with PHIV is normal during adolescence. Although this study may indicate a lower rate of normal grey matter decline in adolescents living with PHIV, we found no HIV or cART-related associations, and all other outcomes of structural brain development appear to develop similarly.
As expected for normal brain development [14], we observed an increase in total white matter volume and a decrease in total grey matter volume, both in PHIV-positive and HIV-negative study participants who, over a period of approximately 5 years time, had transitioned into adolescence and young adulthood. Although the rate of change in white matter volume did not differ between groups, white matter volume remained significantly lower in PHIV-positive adolescents and young adults, as had been the case during their childhood.
Although our initial analyses suggested that PHIV might have a slower age-appropriate pruning in total grey matter, the observed difference between PHIV and controls appeared largely attributable to variation in height. In addition, we found no HIV or cART-related factors determinants of grey matter decline, and no association with cognitive development. In particular, we did not find an association with executive functioning development, which we found to be delayed in a previous analysis in the same cohort [12]. Altogether, this further supports our previously proposed hypothesis that the developmental delay in executive functioning might be explained by brain damage induced during the time these children were exposed to uncontrolled HIV replication [12].
Up to now, only two other longitudinal studies have investigated brain development in PHIV-positive children transitioning into adulthood [15,16]. Both studies were limited by a follow-up time of only one year, as well as by the fact that they investigated brain development from a limited perspective, primarily focusing on either subcortical structures using 1.5T MRI [15] or solely performing region of interest analysis, studying grey matter and cortical thickness development [16]. Nonetheless, in line with the results of our findings, the effect of HIV on brain morphology was suggested to be subtle.
This study suggests an increase in WMH volume over 5 years of follow-up in both PHIV-positive and HIV-negative participants. In general, it is important to realize that absolute WMH volumes are low. WMHs are common findings in elderly, positively associated with age [17], presumed to result from chronic ischaemia associated with cerebral small vessel disease [18], and associated with an increased risk of cognitive decline, stroke, dementia and death [19]. In a young and healthy population, however, WMHs are not commonly observed, with a reported prevalence of 5.3% between the age of 16 and 65 years [20]. Research on WMH in the paediatric population is scarce and clinical implications are understudied [11,21–23]. Only a few cross-sectional studies mention WMHs in PHIV children [11,24], yet this is the first study that has investigated the development of WMHs over time.
Although this study makes an important contribution to research in the field of paediatric HIV due to its longitudinal design with long-term follow-up of treated PHIV participants and well matched controls, some limitations need to be acknowledged. The software of the scanner was updated between the first and follow-up MRI. We observed no major differences between time-points, but this does not fully rule out improved image quality at follow-up MRI. As this likely would have occurred for both HIV-positive and HIV-negative participants, we consider this less of a limitation. Although the number of participants is considered substantial in the field of neuroimaging research, we acknowledge that this may still have been too small to detect some potentially meaningful differences and also precluded us from performing extensive multivariable analyses. Although we used a higher Type I error rate for interaction terms, this cut-off remains arbitrary and accordingly results need to be interpreted cautiously [25]. Future studies may benefit from the inclusion of HIV-exposed but uninfected controls to further elucidate the impact of prenatal HIV/cART exposure. Moreover, we encourage future studies to validate our findings by undertaking longitudinal studies that start at birth.
In conclusion, this longitudinal study paints a fairly optimistic picture on structural brain development in adolescents with perinatally acquired HIV on sustained antiretroviral treatment. Although cross-sectional differences persist, this longitudinal study suggests that the development of brain structure during adolescence appears to be normal.
Acknowledgements
The authors thank all study participants and their parents, Sandra van den Berg-Faaij for technical assistance; Annouschka Weijsenfeld and Claudia de Boer for help in participant recruitment.
For author's contributions, See Appendix.
Conflicts of interest
M.vdH., P.E.J.J., A.M.tH., H.J.S., A.S., A.K., D.P. and H.-J.M.M.M. report no disclosures.
M.C. is a shareholder of Nico-Lab.
C,B,L.M.M. reports grants from CVON/Dutch Heart Foundation, grants from European Commission, grants from TWIN Foundation, grants from Stryker, outside the submitted work (all paid to institution); and is Shareholder of Nico-lab, a company that focuses on the use of artificial intelligence for medical image analysis.
P.R. through his institution has received independent scientific grant support, not related to the current work, from Gilead Sciences, Janssen Pharmaceuticals Inc, Merck & Co and ViiV Healthcare, and has served on scientific advisory boards for Gilead Sciences, ViiV Healthcare, Merck & Co, and Teva pharmaceutical industries, for which honoraria were all paid to his institution.
F.W.N.M.W. has received personal fees, not related to the current work, for advisory board membership for ViiV Healthcare and Gilead Sciences.
Statistical analysis conducted by M.vdH. and P.E.J.J. Amsterdam University Medical Centers, University of Amsterdam, Emma Children's Hospital, Pediatric Infectious Diseases, Amsterdam, the Netherlands.
Study funded by AIDSfonds (grant number 2015009).
Supplementary Material
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (http://www.AIDSonline.com).
References
- 1.Patel K, Ming X, Williams PL, Robertson KR, Oleske JM, Seage GR, 3rd. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS 2009; 23:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blokhuis C, Peeters CFW, Cohen S, Scherpbier HJ, Kuijpers TW, Reiss P, et al. Systemic and intrathecal immune activation in association with cerebral and cognitive outcomes in paediatric HIV. Sci Rep 2019; 9:8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S, Ter Stege JA, Geurtsen GJ, Scherpbier HJ, Kuijpers TW, Reiss P, et al. Poorer cognitive performance in perinatally HIV-infected children versus healthy socioeconomically matched controls. Clin Infect Dis 2015; 60:1111–1119. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Caan MW, Mutsaerts H-J, Scherpbier HJ, Kuijpers TW, Reiss P, et al. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology 2016; 86:19–27. [DOI] [PubMed] [Google Scholar]
- 5.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going?. Neuron 2010; 67:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 2011; 31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Hof M, ter Haar AM, Caan MWA, Spijker R, van der Lee JH, Pajkrt D. Brain structure of perinatally HIV-infected patients on long-term treatment: a systematic review. Neurol Clin Pract 2019; 9:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole JH, Caan MWA, Underwood J, De Francesco D, van Zoest RA, Wit F, et al. No evidence for accelerated ageing-related brain pathology in treated HIV: longitudinal neuroimaging results from the Comorbidity in Relation to AIDS (COBRA) project. Clin Infect Dis 2018; 66:1899–1909. [DOI] [PubMed] [Google Scholar]
- 9.Correa DG, Zimmermann N, Tukamoto G, Doring T, Ventura N, Leite SC, et al. Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. J Magn Reson Imaging 2016; 44:1262–1269. [DOI] [PubMed] [Google Scholar]
- 10.van Zoest RA, Underwood J, De Francesco D, Sabin CA, Cole JH, Wit FW, et al. Structural brain abnormalities in successfully treated HIV infection: associations with disease and cerebrospinal fluid biomarkers. J Infect Dis 2017; 217:69–81. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Caan MW, Mutsaerts HJ, Scherpbier HJ, Kuijpers TW, Reiss P, et al. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology 2016; 86:19–27. [DOI] [PubMed] [Google Scholar]
- 12.Van den Hof M, Ter Haar AM, Scherpbier HJ, van der Lee JH, Reiss P, Wit F, et al. Neurocognitive development in perinatally HIV-infected adolescents on long-term treatment compared to healthy matched controls: a longitudinal study. Clin Infect Dis 2020; 70:1364–1371. [DOI] [PubMed] [Google Scholar]
- 13. R Core Team (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, Available online at https://www.R-project.org/ [Google Scholar]
- 14.Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn 2010; 72:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wade BSC, Valcour VG, Puthanakit T, et al. Mapping abnormal subcortical neurodevelopment in a cohort of Thai children with HIV. Neuroimage Clin 2019; 23:101810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X, Gao L, Wang H, Yin Z, Fang J, Chen J, et al. Neuroanatomical changes underlying vertical HIV infection in adolescents. Front Immunol 2019; 10:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015; 11:157–165. [DOI] [PubMed] [Google Scholar]
- 19.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging 2006; 16:243–251. [DOI] [PubMed] [Google Scholar]
- 21.van der Land V, Hijmans CT, de Ruiter M, Mutsaerts HJ, Cnossen MH, Engelen M, et al. Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br J Haematol 2015; 168:553–556. [DOI] [PubMed] [Google Scholar]
- 22.Blackmon K, Ben-Avi E, Wang X, Pardoe HR, Di Martino A, Halgren E, et al. Periventricular white matter abnormalities and restricted repetitive behavior in autism spectrum disorder. Neuroimage Clin 2016; 10:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyoo IK, Lee HK, Jung JH, Noam GG, Renshaw PF. White matter hyperintensities on magnetic resonance imaging of the brain in children with psychiatric disorders. Compr Psychiatry 2002; 43:361–368. [DOI] [PubMed] [Google Scholar]
- 24.Ackermann C, Andronikou S, Laughton B, Kidd M, Dobbels E, Innes S, et al. White matter signal abnormalities in children with suspected HIV-related neurologic disease on early combination antiretroviral therapy. Pediatr Infect Dis J 2014; 33:e207–e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov 2007; 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author (M.vdH.) on request, in anonymized form.


