Abstract
Purpose of review
The last decade has witnessed tremendous advances in revealing an important role for the interleukin (IL)-17 cytokine family in the pathogenesis of spondyloarthritis (SpA). Although most attention has been focused on IL-17A, a potential role of other IL-17 family members in inflammation and tissue remodelling is emerging. Herein, I review recent studies covering the role of IL-17B-F cytokines in the pathogenesis of SpA.
Recent findings
Several recent studies provided new insights into the cellular source, regulation and function of IL-17F. IL-17F/IL-17A expression ratio is higher in psoriatic skin compared to SpA synovitis. IL-17F-expressing T cells produce different proinflammatory mediators than IL-17A-expressing cells, and IL-17F and IL-17A signal through different receptor complex. Dual IL-17A and IL-17F neutralization resulted in greater suppression of downstream inflammatory and tissue remodelling responses. Furthermore, there is additional evidence of IL-23-independent IL-17 production. In contrast to IL-17A, IL-17F and IL-17C, which play proinflammatory roles in skin and joint inflammation, an anti-inflammatory function is proposed for IL-17D. An increase in IL-17E is associated with subclinical gut microbiome alterations after anti-IL-17A therapy in SpA patients.
Summary
IL-17 family cytokines may act as agonists or antagonists to IL-17A contributing in concert to local inflammatory responses. Understanding their function and identifying their cellular sources, and molecular mechanisms driving their expression will be the key to designing rational therapies in SpA.
Keywords: interleukin-17C, interleukin-17D, interleukin-17E, interleukin-17F, interleukin-17 family cytokines, spondyloarthritis
INTRODUCTION
Strong evidence from clinical trials firmly placed interleukin 17A (IL-17A) in the centre of the pathogenesis of the spondyloarthritides (SpA), the group of related but phenotypically heterogeneous conditions that share common genetic and pathogenetic features [1–6]. Responders to anti-IL-17A therapy included naïve patients and those who did not respond to previous treatments [7–10]. Importantly, emerging evidence indicates that targeting IL-17A slows down structural damage (including bone erosions and pathological new bone formation) as IL-17A blockade inhibits radiographic disease progression in both, psoriatic arthritis (PsA) [6,11] and ankylosing spondylitis (AS) [12]. In addition, recent data suggest that IL-17A inhibition improves enthesitis in patients with PsA [13] and AS [14].
As to the related extra-articular manifestations, anti-IL-17A therapy demonstrated impressive clinical efficacy in treating skin psoriasis (PsO) [3,15,16], but was not effective in treating colitis [17] or uveitis [18]. In contrast, unexpectedly, blocking IL-23, the cytokine upstream of IL-17A was not effective in AS [19,20] though anti-IL-23 therapy did improve colitis [21,22]. Overall, the IL-17 axis holds great promise for the development of further disease-modifying therapeutic opportunities in SpA. However, the inability of IL-17A blockers to cover the entire disease spectrum and to achieve a major clinical response and sustained remission underscores the importance of the identification of additional drivers of the pathologic immune responses, tissue-specific pathways, and hierarchies. The list of attractive candidates comprises other IL-17 family members: IL-17B, IL-17C, IL-17D, IL-17E and IL-17F [23–25]. These structurally related to IL-17A yet less well-characterized cytokines could play complementary or antagonistic roles, hence may affect IL-17A-driven tissue inflammation and/or remodelling, contributing to the pathology of SpA. This review highlights the most recent studies featuring the role of IL-17B-F cytokines in SpA.
Box 1.

no caption available
INTERLEUKIN-17F
Among the IL-17 family members, IL-17F shares the highest homology (55%) with IL-17A. Both cytokines can exist as disulphide-linked homodimers or as IL-17A/IL-17F heterodimers [26]. It was postulated that IL-17F is co-produced with IL-17A by Th17 cells under the control of STAT3 and RORgt transcription factors [27] and signals via the same heterodimeric receptor consisting of IL-17RC and IL-17RA. Similar to IL-17A, although to a lesser extent, IL-17F can synergize with other pro-inflammatory molecules, particularly with tumor necrosis factor alpha, but also with IL-1β, interferon (IFN)-γ and lipopolysaccharide, amplifying its inflammatory potential [28]. Therefore, a similar, albeit less potent pro-inflammatory function has been proposed for IL-17F in driving pathogenic responses. Recent studies have provided new insights into the cellular source, regulation and function of IL-17F.
Interleukin-23-independent production of interleukin-17A and interleukin-17F
Cole and colleagues [29▪▪] present important novel insight into the biology of IL-17A-producing and IL-17F-producing innate cells. They demonstrated that IL-17F is the dominant isoform produced by in vitro-stimulated mucosal-associated invariant T (MAIT) cells, a unique population of innate-like T cells with restricted T cell receptor (TCR) diversity that can function through both TCR-dependent and -independent pathways [30,31]. IL-17A-producing MAIT cells were identified in PsO skin [32] and PsA and AS joint [33–35], and their potential role in SpA pathogenesis is emerging [36,37]. Importantly, Cole et al. showed that MAIT cells can produce IL-17F (and IL-17A) in an IL-23-independent fashion, in response to TCR triggering combined with IL-12 and IL-18 cytokines stimulation in vitro[29▪▪]. In addition, ILC3s and γδ T cells were also capable of an IL-23-independent IL-17A and IL-17F production [29▪▪], supporting recent evidence that human entheseal γδ T cells can produce IL-17A without IL-23 receptor expression [38]. These data prompt the notion that IL-23-independent IL-17A and IL-17F production is a feature shared among innate lymphocyte family members [29▪▪]. Remarkably, the cytokine milieu that tunes the IL-17A and IL-17F production seems to be cell-type dependent. In contrast to MAIT and γδ T cells, which were dependent on IL-12 for IL-23-independent IL-17A and IL-17F production, ILC3s did not require IL-12 or IL-23 and produced IL-17A and IL-17F upon stimulation with IL-1β, IL-2 and IL-7 [29▪▪]. The ability of T cells and innate(-like) lymphocytes to produce IL-17A in response to cytokines other than canonical IL-23, in particular to IL-7 and IL-9 [34,39,40], has been demonstrated before [41–43]. Such IL-12-IL-23-independent IL-17A and IL-17F production by these, presumably (but not yet proven) pathogenic cellular subsets could explain why targeting p19 subunit that is unique to IL-23, or p40 subunit common to both, IL-12 and IL-23, were not efficacious in AS [19,20]. As to the peripheral disease, our recent study investigating cellular and molecular changes in the PsA joint in response to IL-12/IL-23 blockade with ustekinumab revealed that although ustekinumab suppressed synovial inflammation through modulation of key pathogenic pathways, expression of IL-17A and IL-17F remained unaffected [44], supporting IL-23-independent IL-17A and IL-17F production in PsA joint. Whether it has a pathogenetic significance has to be assessed in head-to-head clinical trials of IL-17A or IL-17A-IL-17F versus IL-23 antagonists. Yet, a recent retrospective study in PsA demonstrated that treatment with secukinumab has a greater persistence rate than the treatment with ustekinumab [45]. Taken together, the emergence of distinct pathways culminating in the secretion of IL-17A and IL-17F cytokines, in addition to the canonical IL-23/IL-17A pathway, underscores the importance of the IL-17A/IL-17F axis in the pathogenesis of SpA, provides insights into understanding results of clinical trials and urges to identify pathogenic cell populations in target tissues.
Distinct regulation and function of interleukin-17F
Recent findings challenged the notion that IL-17F has a redundant role in SpA pathogenesis. In the study of Cole et al., only a minor population of MAIT cells produced IL-17A upon in vitro stimulation despite uniform expression of RORgt. Instead, MAIT cells as well as ILC3s and γδ T cells produced predominantly IL-17F [29▪▪], supporting the concept that IL-17A and IL-17F are differentially regulated [46,47]. High expression of IL-17F can be also induced in canonical CD4+ T cells [48▪▪], but in contrast to innate lymphocytes, this process is dependent on IL-23. In this study, Burns et al. identified and characterized three CD4+ T cell subsets: IL-17A+IL-17F-, IL-17A+IL-17F+, and IL-17A-IL-17F+. Interestingly, these populations displayed different cytokine profiles: while all subsets contained similarly high frequencies of cells expressing TNF, IL-17A-IL-17F+ cells expressed less IL-10 and GM-CSF and more IFN-γ compared to IL-17A+IL-17F- CD4+ T cells [48▪▪]. Based on previous molecular characterization of IL-10-expressing Th17 subsets [49], the authors proposed that IL-17F-expressing CD4+ T cells might represent the ‘pathogenic’ subtype, although in-depth molecular and functional characterization of these cells is required to conclude about their pathogenicity. Notably, IL-17F and IL-17A-expressing T cells differ not only in their molecular profiles but also are differentially regulated. Comparing the induction of CD4+ T cells by LPS-activated monocytes versus soluble anti-CD28 mAb and L-1β and IL-23 stimulation, Burns and colleagues observed that while both stimuli induced IL-17A+IL-17F+ CD4+ T cells, only the latter resulted in IL-17F+IL-17A- CD4+ T cells [48▪▪]. Further analysis revealed that IL-17F expression in CD4+ T cells is driven by high-strength TCR stimulation in the presence of IL-23 and IL-1β. IL-17F induction is partially mediated via IL-2-dependent mechanism, as IL-2 blockade significantly reduced the CD28-mediated increase in frequencies of IL-17F+ CD4+ T cells [48▪▪], in line with previous findings showing that high levels of IL-2 shift the balance between IL-17A and IL-17F towards IL-17F production by murine T cells in vitro[50]. Interestingly, another study in mice demonstrated that the activation of transmembrane TNF (tmTNF)-TNF Receptor 2 signalling stimulates IL-2 expression and regulates IL-2 mRNA stability [51]. Given a marked increase of tmTNF in SpA synovitis and its impact on key pathological features of SpA [52] along with the observation that IL-17F levels are strikingly higher than IL-17A in the blood of patients with SpA [53] it might be revealing to examine tmTNF-IL-17F axis in SpA. Importantly, reports by Cole et al. and Burns et al. demonstrate that IL-17F is not only differentially regulated but also significantly contributes to inflammation, as dual IL-17A and IL-17F blockade were more effective at reducing IL-17-driven pro-inflammatory responses by human dermal fibroblasts [29▪▪] and synovial fibroblasts [48▪▪] compared to blockade of IL-17A alone, according to previous findings [54] (Fig. 1). Attempting to detect IL-17F-expressing cells ex vivo, Cole et al. confirmed the presence of single-positive for IL-17A or IL-17F, as well as double-positive MAIT cells in psoriatic lesional skin [29▪▪] (Fig. 1). In contrast, Burns et al. failed to detect the presence of IL-17F-expressing cells in PsA synovial fluid directly ex vivo, although confirmed the potential of synovial fluid mononuclear cells to produce IL-17F upon in vitro stimulation [48▪▪]. Could be these discrepancies explained by tissue-specific expression of IL-17F? Previous findings demonstrated that IL-17F levels are approximately 30-fold higher than IL-17A levels in PsO skin [53]. Our recent study using paired biopsies of skin and synovium collected from PsA patients with active PsO confirmed a higher IL-17F to IL-17A ratio in the inflamed skin and revealed that the relative expression of IL-17A versus IL-17F is inversed in inflamed joint and skin compartments with IL-17A being more than 30-fold higher than IL-17F in the joint [55▪] (Fig. 1). Taken together these in vitro and ex vivo data point towards a nonredundant role for IL-17F and provide new pathobiological insights in joint versus skin inflammation, suggesting that (1) the contribution of IL-17F to chronic tissue inflammation may be more prominent in the skin than in joint; (2) IL-17F has the potential to contribute to pathology, therefore dual blockade of IL-17A and IL-17F can further reduce inflammation.
FIGURE 1.
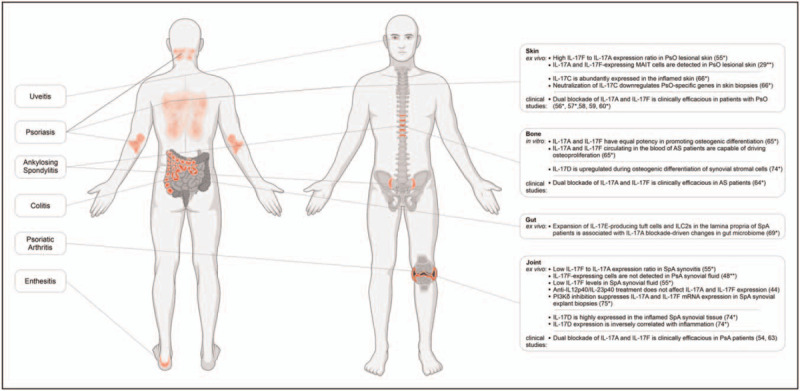
Recent ex vivo, in vitro and in vivo evidence supporting the role of IL-17 family cytokines in the pathogenesis of SpA. Created with BioRender.com. SpA, spondyloarthritis.
The preclinical data supporting the efficacy of the dual IL-17A and IL-17F blockade is further underpinned by recent clinical-trials evidence for bimekizumab, a humanized monoclonal IgG1 antagonist neutralizing both cytokines [54]. Two recent Phase 3 studies reported the safety and efficacy of bimekizumab for the treatment of moderate to severe plaque PsO [56▪,57▪] confirming phase 2 findings [58,59] and revealing the superiority of dual IL-17A and IL-17F targeting to the targeting of IL-12/IL-23 in achieving complete skin clearance. Similarly, simultaneous inhibition of IL-17A and IL-17F in patients with PsO was more effective than inhibition of TNF in terms of the speed, depth and durability of skin clearance [60▪]. Superior efficacy of IL-17A blockade relative to inhibition of IL-12/IL-23[61] and TNF [62] in clearing skin PsO has been demonstrated previously. Ongoing head-to-head comparator study of bimekizumab and anti-IL-17A treatment (BE RADIANT, http://clinicaltrials.gov/ct/show/NCT03536884) will provide important knowledge on whether targeting of both cytokines is clinically more beneficial than inhibition of IL-17A alone. Bimekizumab is also effective in treating peripheral and axial SpA. It has been first assessed in the proof-of-concept study [54] and strengthened in followed up phase 2b study that patients with PsA, who were administered bimekizumab, showed marked and sustained improvements in their condition compared with placebo [63]. Also, for an axial disease, a phase 2b study revealed a rapid onset and greater ASAS40 response rates as well as sustained improvements across secondary outcomes of disease activity for bimekizumab versus placebo [64▪].
Role in bone pathology
Another recent study employing an in vitro model of osteogenic differentiation of human periosteal cells puts forward the argument that IL-17F does not only contribute to IL-17A but has equal potency in promoting osteogenic differentiation, in contrast to its less potent role in driving inflammatory responses [65▪] (Fig. 1). IL-17A and IL-17F cytokines, circulating in the blood of AS patients, are also functionally active as they were capable of driving osteoproliferation in vitro[65▪] (Fig. 1). Accordingly, neutralization of both cytokines by bimekizumab resulted in greater suppression of γδ or Th17 T-cell supernatants-mediated, or AS patient's serum-mediated in vitro bone formation than the blockade of IL-17A or IL-17F individually [65▪]. These results provide further scientific evidence to validate the clinical relevance of the dual IL-17A and IL-17F blockade in patients with AS for preventing or suppressing pathological periosteal bone formation.
OTHER MEMBERS OF THE INTERLEUKIN-17 FAMILY
A very limited number of recent studies address the role of other IL-17 family members in SpA. Lauffer et al. demonstrated that IL-17C, a member of the IL-17 family that, in contrast to IL-17A and IL-17F, is mainly produced by epithelial cells and keratinocytes, is broadly expressed in the inflamed skin of patients with various inflammatory skin diseases including but not limited to PsO [66▪]. The study revealed that IL-17C establishes a self-amplifying circuit in synergy with TNF, leading to the secretion of pro-inflammatory cytokines by keratinocytes and the recruitment of immune cells to the site of inflammation (Fig. 1). Using human disease models, Lauffer et al. demonstrated significant downregulation of PsO-specific genes after neutralization of IL-17C, considering IL-17C as a promising drug target for the treatment of inflammatory skin diseases [66▪]. However, since IL-17C is regulated by IL-17A and TNF, as both therapies rapidly reduce IL-17C expression in PsO skin [67,68], the added-value of the developing of IL-17C-specific therapy in SpA needs to be further established.
Another recent study suggests an association between IL-17A blockade-driven changes in the gut microbiome of SpA patients and the expansion of IL-17E-producing tuft cells and ILC2s in the lamina propria [69▪]. Whether IL-17E drives gut inflammation after IL-17A inhibition remains to be assessed. IL-17E has been shown to promote PsO [70], however, its role in gut inflammation is confusing as it has been demonstrated to induce colitis [71,72] or to protect against colitis [73].
IL-17D is the least investigated member of the IL-17 family. Our recent data on the cellular source and function of IL-17D suggest its unique position among other IL-17 family cytokines [74▪]. First, IL-17D is abundantly expressed in inflamed SpA joint, higher than other IL-17 cytokines. Second, IL-17D is expressed by stromal cells, in particular, by cells similar to multipotent mesenchymal stromal cells. Third, IL-17D expression inversely correlates with inflammation (Fig. 1). Furthermore, IL-17D is upregulated during osteogenic differentiation of synovial stromal cells in vitro. However, in vitro functional assays in bone precursor cells and in vivo experiments in IL-17d–/– mice failed to demonstrate a critical role for IL-17D in bone homeostasis. Instead, IL-17d–/– mice were more prone to arthritis development than littermate controls and presented with enhanced systemic inflammation at the peak of serum-transfer arthritis [74▪]. Based on these data it is tempting to propose that IL-17D exerts an anti-inflammatory effect on synovial cells, yet further research is required to address its role in the pathogenesis of SpA.
DIRECTIONS FOR FUTURE RESEARCH
Further investigations of the exact mechanisms of production and function of IL-17 family members will provide novel insights into their roles in SpA pathogenesis and may have direct relevance for the targeted therapy. Could we imagine other ways to target IL-17A and IL-17F production? Recently we demonstrated that PI3Kδ inhibition dampens both IL-17A and IL-17F expression in innate-like lymphocytes and Th17 cells in IL-23-independent and the dependent manner in vitro as well as in primary cells derived from blood and synovial fluid of SpA patients [75▪]. This inhibition has functional anti-inflammatory and anti-remodelling effects on target cells, such as synovial fibroblasts. Furthermore, we demonstrated that the PI3K-Akt-mTOR pathway is active in the SpA joint and PI3Kδ inhibition suppresses IL-17A and IL-17F expression in SpA synovial explant biopsies ex vivo[75▪]. In light of the results from in vitro models, simultaneous suppression of IL-17A and IL-17F is a promising direction in IL-17-mediated diseases, however, more data is needed to conclude about its added value on clinical response over IL-17A inhibition. Moreover, accumulating evidence suggests that IL-17A and IL-17F may exert distinct, even opposite downstream activities, which may impact the clinical outcome. For instance, IL-17A-blockade is ineffective for Crohn's disease [17]. It was concluded, that IL-17A is important for maintaining barrier integrity and has a protective role in colitis [76]. However recent data may suggest an alternative explanation. First, the IL-17F pathway has been demonstrated to promote inflammation in the intestines through its effect on the intestinal microbiome. Consequently, IL-17F neutralization suppressed the development of colitis whereas blocking of IL-17A did not [77]. Second, a recent mechanistic study revealed that IL-17A inhibits the expression of IL17-lineage cytokines through a negative feedback loop. Accordingly, the loss of IL-17A in Th17 cells did not reduce their pathogenicity, resulting in the elevated expression of GM-CSF and IL-17F cytokines [78]. Third, recent findings demonstrated that in contrast to IL-17A homodimers or IL-17A/IL-17F heterodimers that signal via heterodimeric IL-17RA/IL-17RC receptor, IL-17F preferentially associates with IL-17RC homodimers, leading to IL-17RA-independent signalling [79▪]. Given that it is plausible to propose that aggravation of Crohn's pathology by IL-17A neutralization could be not due to a decrease in IL-17A but rather due to upregulation of IL-17F and increased signalling via IL-17RC/IL-17F axis. In this context, it is perhaps not surprising that anti-IL-17RA treatment with brodalumab resulted in worsening Crohn's disease [80].
CONCLUSION
Accumulating evidence suggests that IL-17 family members have tissue-specific functions in inflammation. Their differential cellular sources, expression levels and function in different target tissues could contribute to tissue-discrete results for IL-17 axis inhibition across the SpA spectrum. Additionally, there is evidence for interaction between IL-17 cytokines, including self-reinforcing, feed-forward as well as negative feedback mechanisms leading to agonistic or antagonistic effects on tissue inflammation and/or remodelling. Therefore understanding the function of IL-17 family cytokines, as well as detailed characterization of cellular subsets and molecular mechanisms culminating in their expression, will be the key to designing rational therapies in SpA.
Acknowledgements
I am grateful to Dr Marleen van de Sande (AMC, Amsterdam University Medical Centers) and Dr Troy Noordenbos (Leiden University Medical Center) for the critical review of the manuscript.
Financial support and sponsorship
The author acknowledges support from the FOREUM Foundation for Research in Rheumatology.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015; 373:2534–2548. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human antiinterleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015; 386:1137–1146. [DOI] [PubMed] [Google Scholar]
- 3.Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017; 76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014; 370:2295–2306. [DOI] [PubMed] [Google Scholar]
- 5.Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in Patients with psoriatic arthritis. N Engl J Med 2015; 373:1329–1339. [DOI] [PubMed] [Google Scholar]
- 6.Mease P, van der Heijde D, Landewe R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis 2018; 77:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sieper J, Deodhar A, Marzo-Ortega H, et al. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 Study. Ann Rheum Dis 2017; 76:571–592. [DOI] [PubMed] [Google Scholar]
- 8.Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a Phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol 2019; 71:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with nonradiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020; 395:53–64. [DOI] [PubMed] [Google Scholar]
- 10.Dougados M, Wei JC, Landewe R, et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann Rheum Dis 2020; 79:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Heijde D, Mease PJ, Landewe RBM, et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology 2020; 59:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun J, Baraliakos X, Deodhar A, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology 2019; 58:859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladman DD, Orbai AM, Klitz U, et al. Ixekizumab and complete resolution of enthesitis and dactylitis: integrated analysis of two phase 3 randomized trials in psoriatic arthritis. Arthritis Res Ther 2019; 21:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schett G, Baraliakos X, Van den Bosch F, et al. Secukinumab efficacy on enthesitis in patients with ankylosing spondylitis: pooled analysis of four pivotal Phase 3 studies. J Rheumatol 2021; Mar 15:jrheum.201111. doi: 10.3899/jrheum.201111. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015; 73:400–409. [DOI] [PubMed] [Google Scholar]
- 16.Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016; 375. [DOI] [PubMed] [Google Scholar]
- 17.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61:1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dick AD, Tugal-Tutkun I, Foster S, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology 2013; 120:777–787. [DOI] [PubMed] [Google Scholar]
- 19.Baeten D, Ostergaard M, Wei JC, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis 2018; 77:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 2019; 71:258–270. [DOI] [PubMed] [Google Scholar]
- 21.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019; 381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 22.Hanzel J, D’Haens GR. Antiinterleukin-23 agents for the treatment of ulcerative colitis. Expert Opin Biol Ther 2020; 20:399–406. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Chen J, Huang A, et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA 2000; 97:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Ho WH, Maruoka M, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem 2001; 276:1660–1664. [DOI] [PubMed] [Google Scholar]
- 25.Starnes T, Robertson MJ, Sledge G, et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol 2001; 167:4137–4140. [DOI] [PubMed] [Google Scholar]
- 26.Dubin PJ, Kolls JK. Interleukin-17A and interleukin-17F: a tale of two cytokines. Immunity 2009; 30:9–11. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol 2009; 21:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 2010; 129:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪▪.Cole S, Murray J, Simpson C, et al. Interleukin (IL)-12 and IL-18 synergize to promote MAIT cell IL-17A and IL-17F production independently of IL-23 signaling. Front Immunol 2020; 11:585134. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrated that innate lymphocytes, including MAIT cells, gd T cells and ILC3s produce predominantly IL-17F cytokine and identified IL-17F-producing cells in psoriatic lesional skin. The authors extended a previous finding that dual blockade of IL-17A and IL-17F is required for optimal inhibition of downstream inflammatory responses. Furthermore, this study provides further evidence towards the contribution of IL-17A and IL-17F to tissue inflammation independently of IL-23, advancing our understanding of divergent results of therapeutic targeting IL-17A and IL-23 in AS.
- 30.Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012; 491:717–723. [DOI] [PubMed] [Google Scholar]
- 31.Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003; 422:164–169. [DOI] [PubMed] [Google Scholar]
- 32.Teunissen MBM, Yeremenko NG, Baeten DLP, et al. The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J Investig Dermatol 2014; 134:2898–2907. [DOI] [PubMed] [Google Scholar]
- 33.Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014; 66:1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gracey E, Qaiyum Z, Almaghlouth I, et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis 2016; 75:2124–2132. [DOI] [PubMed] [Google Scholar]
- 35.Raychaudhuri SK, Abria C, Mitra A, Raychaudhuri SP. Functional significance of MAIT cells in psoriatic arthritis. Cytokine 2020; 125:154855. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi E, Chiba A, Tada K, et al. Involvement of mucosal-associated invariant T cells in ankylosing spondylitis. J Rheumatol 2016; 43:1695–1703. [DOI] [PubMed] [Google Scholar]
- 37.Toussirot E, Saas P. MAIT cells: potent major cellular players in the IL-17 pathway of spondyloarthritis? RMD Open 2018; 4:e000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuthbert RJ, Watad A, Fragkakis EM, et al. Evidence that tissue resident human enthesis gammadeltaT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis 2019; 78:1559–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webster KE, Kim HO, Kyparissoudis K, et al. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol 2014; 7:1058–1067. [DOI] [PubMed] [Google Scholar]
- 40.Hassane M, Jouan Y, Creusat F, et al. Interleukin-7 protects against bacterial respiratory infection by promoting IL-17A-producing innate T-cell response. Mucosal Immunol 2020; 13:128–139. [DOI] [PubMed] [Google Scholar]
- 41.Yoshiga Y, Goto D, Segawa S, et al. Invariant NKT cells produce IL-17 through IL-23-dependent and -independent pathways with potential modulation of Th17 response in collagen-induced arthritis. Int J Mol Med 2008; 22:369–374. [PubMed] [Google Scholar]
- 42.Hasegawa E, Sonoda KH, Shichita T, et al. IL-23-independent induction of IL-17 from gammadeltaT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J Immunol 2013; 190:1778–1787. [DOI] [PubMed] [Google Scholar]
- 43.Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015; 43:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiechter RH, de Jong HM, van Mens LJJ, et al. IL-12p40/IL-23p40 blockade with ustekinumab decreases the synovial inflammatory infiltrate through modulation of multiple signaling pathways including MAPK-ERK and Wnt. Front Immunol 2021; 12:611656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letarouilly JG, Flachaire B, Labadie C, et al. Secukinumab and ustekinumab treatment in psoriatic arthritis: results of a direct comparison. Rheumatology (Oxford) 2020; Nov 24:keaa710. doi: 10.1093/rheumatology/keaa710. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Melton AC, Melrose J, Alajoki L, et al. Regulation of IL-17A production is distinct from IL-17F in a primary human cell co-culture model of T cell-mediated B cell activation. PLoS One 2013; 8:e58966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Rodriguez J, Sahu N, Handon R, et al. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity 2009; 31:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪▪.Burns LA, Maroof A, Marshall D, et al. Presence, function, and regulation of IL-17F-expressing human CD4(+) T cells. Eur J Immunol 2020; 50:568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence that IL-17A and IL-17F are differentially regulated upon T cell stimulation and that IL-17F-producing and IL-17A-producing CD4+ T cells display different cytokine profiles. Findings demonstrate that IL-17F contributes to inflammation since dual IL-17A and IL-17F blockade is more effective at reducing inflammatory cytokine production than blockade of IL-17A alone.
- 49.Aschenbrenner D, Foglierini M, Jarrossay D, et al. An immunoregulatory and tissue-residency program modulated by c-MAF in human TH17 cells. Nat Immunol 2018; 19:1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol 2011; 12:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller PG, Bonn MB, McKarns SC. Transmembrane TNF-TNFR2 impairs Th17 differentiation by promoting Il2 expression. J Immunol 2015; 195:2633–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaaij MH, van Tok MN, Blijdorp IC, et al. Transmembrane TNF drives osteoproliferative joint inflammation reminiscent of human spondyloarthritis. J Exp Med 2020; 217:e20200288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol 2017; 139:923–932. e8. [DOI] [PubMed] [Google Scholar]
- 54.Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018; 77:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Chen S, Blijdorp IC, van Mens LJJ, et al. Interleukin 17A and IL-17F expression and functional responses in rheumatoid arthritis and peripheral spondyloarthritis. J Rheumatol 2020; 47:1606–1613. [DOI] [PubMed] [Google Scholar]; This study demonstrates that IL-17A and IL-17F expression are higher in psoriatic skin compared to the inflamed SpA joint, with a striking inverse IL-17A/IL-17F expression ratio in the joint compared to the skin compartment in SpA. These data suggest that the relative contribution of IL-17F in chronic tissue inflammation may be more prominent in the skin than in joints.
- 56▪.Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 2021; 397:475–486. [DOI] [PubMed] [Google Scholar]; This phase 3 randomised controlled trial study showed a rapid and substantial response in patients with moderate to severe plaque psoriasis, which was durable over 56 weeks. These data further support the therapeutic value of dual inhibition of IL-17A and IL-17F in PsO.
- 57▪.Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021; 397:487–498. [DOI] [PubMed] [Google Scholar]; The head-to-head trial evaluated dual IL-17A and IL-17F versus IL-12/IL-23 inhibition in psoriasis over 52 weeks, with results suggesting that bimekizumab is superior to ustekinumab and placebo as shown by significantly higher response rates for all primary and secondary endpoints.
- 58.Blauvelt A, Papp KA, Merola JF, et al. Bimekizumab for patients with moderate to severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled, phase 2b extension study. J Am Acad Dermatol 2020; 83:1367–1374. [DOI] [PubMed] [Google Scholar]
- 59.Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: Results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol 2018; 79:277–286. e10. [DOI] [PubMed] [Google Scholar]
- 60▪.Warren RBA, Bagel J, Papp KA, et al. Bimekizumab efficacy and safety versus adalimumab in patients with moderate to severe plaque psoriasis: results from a multicenter, randomized, double-blinded active comparator-controlled phase 3 trial (Be Sure). SKIN J Cutan Med 2021; 5:s15. [Google Scholar]; The head-to-head trial in patients with moderate to severe plaque psoriasis that demonstrated uperiority of bimekizumab to adalimumab over 16 weeks of treatment in terms of the speed, depth and durability of skin clearance.
- 61.Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J Am Acad Dermatol 2017; 76:60–69. e9. [DOI] [PubMed] [Google Scholar]
- 62.Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015; 386:541–551. [DOI] [PubMed] [Google Scholar]
- 63.Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet 2020; 395:427–440. [DOI] [PubMed] [Google Scholar]
- 64▪.van der Heijde D, Gensler LS, Deodhar A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis 2020; 79:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study of bimekizumab in patients with active AS, demonstrating that bimekizumab-treated patients had rapid and significant ASAS40 responses versus placebo at week 12, supported by all secondary efficacy endpoints. These results validate a dual neutralisation of IL-17A and IL-17F with bimekizumab as a novel therapeutic option for the treatment of AS.
- 65▪.Shah M, Maroof A, Gikas P, et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open 2020; 6:e001306. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows a direct equal effect of IL-17A and IL-17F on progenitor cells from the periosteum. T helper 17 or γδ-T cell supernatants or serum obtained from patients with AS potently stimulated in vitro bone formation, which was blocked deeper by dual inhibition of IL-17A and IL-17F than by neutralisation of IL-17A or IL-17F alone.
- 66▪.Lauffer F, Jargosch M, Baghin V, et al. IL-17C amplifies epithelial inflammation in human psoriasis and atopic eczema. J Eur Acad Dermatol Venereol 2020; 34:800–809. [DOI] [PubMed] [Google Scholar]; This study reported elevated IL-17C in various inflammatory skin diseases and demonstrated that IL-17C amplifies epithelial inflammation and immune cell influx to the skin. IL-17C depletion significantly reduced inflammatory mediators in human skin biopsies of psoriasis ex vivo.
- 67.Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Investig Dermatol 2011; 131:677–687. [DOI] [PubMed] [Google Scholar]
- 68.Krueger JG, Wharton KA, Jr, Schlitt T, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol 2019; 144:750–763. [DOI] [PubMed] [Google Scholar]
- 69▪.Manasson J, Wallach DS, Guggino G, et al. Interleukin-17 inhibition in spondyloarthritis is associated with subclinical gut microbiome perturbations and a distinctive interleukin-25-driven intestinal inflammation. Arthritis Rheumatol 2020; 72:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that IL-17 inhibition-induced subclinical gut inflammation and intestinal dysbiosis in SpA patients is associated with overexpression of IL-25/IL-17E–producing cells, suggesting that IL-17i-induced Crohn's disease might be driven by a specific IL-17 pathway.
- 70.Senra L, Stalder R, Alvarez Martinez D, et al. Keratinocyte-derived IL-17E contributes to inflammation in psoriasis. J Investig Dermatol 2016; 136:1970–1980. [DOI] [PubMed] [Google Scholar]
- 71.Reynolds JM, Lee YH, Shi Y, et al. Interleukin-17B antagonizes interleukin-25-mediated mucosal inflammation. Immunity 2015; 42:692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Camelo A, Barlow JL, Drynan LF, et al. Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J Gastroenterol 2012; 47:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McHenga SS, Wang D, Janneh FM, et al. Differential dose effects of recombinant IL-25 on the development of dextran sulfate sodium-induced colitis. Inflamm Res 2010; 59:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪.Chen SMC, van Tok, Melissa, et al. Interleukin-17D, a cytokine derived from stromal cells, attenuates joint inflammation. Arthritis Rheumatol 2020; 72: suppl 10: https://acrabstracts.org/abstract/interleukin-17d-a-cytokine-derived-from-stromal-cells-attenuates-joint-inflammation/https://acrabstracts.org/abstract/interleukin-17d-a-cytokine-derived-from-stromal-cells-attenuates-joint-inflammation/. [Accessed 26 April 2021]. [Google Scholar]; This study demonstrates that expression of IL-17D is localized to synovial stromal cells and inversely correlates with synovial inflammation in SpA. Mice lacking IL-17D. This suggests that IL-17D may exert an anti-inflammatory effect on joint inflammation in SpA and requires further investigation.
- 75▪.Chen S, Paveley R, Kraal L, et al. Selective targeting of PI3Kdelta suppresses human IL-17-producing T cells and innate-like lymphocytes and may be therapeutic for IL-17-mediated diseases. J Autoimmun 2020; 111:102435. [DOI] [PubMed] [Google Scholar]; This publication reports that selective inhibition of PI3Kδ suppresses IL-23-dependent and IL-23-independent production IL-17A and IL-17F by Th17-like cells and innate T cells from a healthy donor and SpA primary cells. This inhibition has a functional impact on the inflammatory and tissue remodelling responses in target cells in psoriasis and SpA. This supports a rationale to target PI3Kδ in IL-17-driven diseases.
- 76.O’Connor W, Jr, Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol 2009; 10:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol 2018; 19:755–765. [DOI] [PubMed] [Google Scholar]
- 78.Chong WP, Mattapallil MJ, Raychaudhuri K, et al. The cytokine IL-17A limits Th17 pathogenicity via a negative feedback loop driven by autocrine induction of IL-24. Immunity 2020; 53:384–397. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79▪.Goepfert A, Lehmann S, Blank J, et al. Structural analysis reveals that the cytokine IL-17F forms a homodimeric complex with receptor IL-17RC to drive IL-17RA-independent signaling. Immunity 2020; 52:499–512. e5. [DOI] [PubMed] [Google Scholar]; This publication reports the crystal structure of the extracellular domain of human IL-17RC in complex with IL-17F, providing a structural basis for IL-17F signalling through IL-17RC and suggesting the possibility of IL-17RA-independent IL-17 signalling pathways.
- 80.Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn's disease. Am J Gastroenterol 2016; 111:1599–1607. [DOI] [PubMed] [Google Scholar]


