Objective:
This meta-analysis aims to combine the latest research evidence to assess the effect of probiotics on preventing antibiotic-associated diarrhea (AAD) in adults.
Methods:
PubMed, Cochrane Library, EMBASE, and Web of Science were searched for randomized placebo-controlled trials on probiotics preventing AAD. A random or fixed effect model was used to combine the incidence of AAD (primary outcome) and the adverse event rates. The authors performed subgroup analyses to explore the effects of different participants population, probiotics species, and dosage.
Results:
Thirty-six studies were included with 9312 participants. Probiotics reduced the incidence of AAD by 38% (pooled relative risk, 0.62; 95% confidence interval, 0.51-0.74). The protective effect of probiotics was still significant when grouped by reasons for antibiotics treatment, probiotic duration, probiotic dosage, and time from antibiotic to probiotic. However, there were no statistically significant increased adverse events in the probiotics group (relative risk, 1.00; 95% confidence interval, 0.87-1.14).
Conclusions:
This updated meta-analysis suggested that using probiotics as early as possible during antibiotic therapy has a positive and safe effect on preventing AAD in adults. Further studies should focus on the optimal dosage and duration of probiotics to develop a specific recommendation.
Key Words: probiotics, prevention, antibiotic-associated diarrhea, diarrhea, adults
Antibiotic-associated diarrhea (AAD) is defined as diarrhea developing from the beginning of antibiotic treatment to 6 to 8 weeks after discontinuation, which may contribute to antimicrobial prescription noncompliance and the overconsumption of second-line antibiotics.1 The prevalence of AAD varies between 5% and 39% in adults. It largely depends on the antibacterial spectrum and pharmacokinetic characteristics including the absorption rate of oral administration and enterohepatic circulation of parenteral administration.2 The pathogenesis of AAD includes the following 2 aspects: (1) the direct effect of antibacterial agents on the intestinal mucosa; (2) the interference of antibacterial agents on the intestinal flora ecosystem, which leads to normal metabolic dysfunction and overgrowth of pathogens (especially Clostridioides difficile).3
As a live microorganism, probiotic with adequate amounts can bring health benefits to the host.4 The mechanisms by which probiotics work on AAD may associate with the following: (1) altering the gut microbiota composition and metabolism; (2) modulating the solute secretion and absorption; and (3) improving the intestinal barrier function and intestinal immune responses.5 Although several randomized controlled trials (RCTs) and meta-analyses have shown its efficacy in preventing AAD, there are currently no clear clinical practice guidelines for probiotics use in preventing AAD.6 A review comparing the effectiveness of multiple probiotics suggested that positive or negative generalization about probiotics was inadequate. Strain specificity, the designated patient population, and various treatment conditions would change the effect of probiotics.7 Therefore, our meta-analysis aims to combine the latest research evidence and compare the effects of probiotic products under different conditions through the most comprehensive subgroup analyses.
METHODS
This meta-analysis was conducted strictly following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.8
Selection Criteria
Inclusion criteria: (1) patients limited to the adults both inpatients and outpatients who were prescribed antibiotics for various reasons with probiotics (experimental groups) or placebo (control group); (2) providing the occurrence of AAD; and (3) the study designed as an RCT.
Exclusion criteria: (1) duplicate studies, animal researches, preclinical studies, and case reports; (2) not-blinded trials; (3) unknown probiotics composition; and (4) existing diarrhea in baseline or containing laxative-related diarrhea.
Literature Search
The databases involving the PubMed, EMBASE, Web of Science, and Cochrane Library were searched for the RCTs on probiotics to prevent AAD. Publications in any language from the databases inception to February 2020 were included. The search terms were the combinations of the following Mesh terms and key words: “probiotic(s),” “diarrhea,” “anti-bacterial agents,” “antibiotic(s),” “antibiotic-associated diarrhea,” “placebo,” “randomized,” and “randomized controlled trial.”
Data Extraction and Quality Assessment
The data extraction was conducted using the standardized form by 2 independent researchers (W.L. and Q.Z.). The primary outcome was the occurrence of AAD during the follow-up period. The secondary outcome was the incidence of adverse events. Other data extracted included demographics, participant setting, indications for antibiotics, probiotics species and dosage, probiotics duration, time from antibiotics to probiotics, follow-up period, and diarrhea definition.
The Cochrane Handbook for Systematic Reviews of Interventions9 was applied to assess the quality of the selected studies. Two researchers assessed the eligibility and quality of each article independently. Any discrepancies were resolved through consensus, adjudicated with the support of a third investigator.
Statistical Analyses
We used the RevMan V.5.210 and Stata Release V.15.1 (StataCorp, College Station, TX) to perform the data analyses. The pooled relative risk (RR) and the 95% confidence interval (CI) were determined by a random-effects model (DerSimonian-Laird method11) or a fixed-effects model (Mantel-Haenszel method12). The χ2 test and I 2 statistic were used to evaluate the heterogeneity of included studies.13,14 P<0.1 or I 2>50% indicated substantial heterogeneity and a random effect would be adopted. Otherwise, a fixed-effects model would be applied. Sensitivity analysis and subgroup analyses were carried out to explore the sources of heterogeneity. In addition, we assessed the publication bias by the funnel plot, Begg test, and Egger tests.15–17
RESULTS
Eligible Studies
A systematic search conducted in February 2020 identified 1789 citations (PubMed 204, Cochrane Library 439, EMBASE 533, and Web of Science 613). Of these studies, 36 RCTs18–53 with 9312 subjects met the inclusion criteria (35 published in English and one in Spanish). Details of the search flow are depicted in Figure 1. The probiotics species studied in the trials primarily included Lactobacillus, Saccharomyces, Bifidobacterium, and Streptococcus. Probiotics were used at the same time as antibiotics or were prolonged by 2 to 28 days after the therapy. Diarrhea was defined by the World Health Organization (WHO) criterion in 8 studies (≥3 loose stools within a 24-h period).54 Six studies applied an adjusted WHO criterion (≥3 loose or liquid stools/day for at least 2 d). Other RCTs defined diarrhea based on the number of bowel movements per day and the consistency of the stool. Table 1 summarizes the details of participants and intervention.
FIGURE 1.
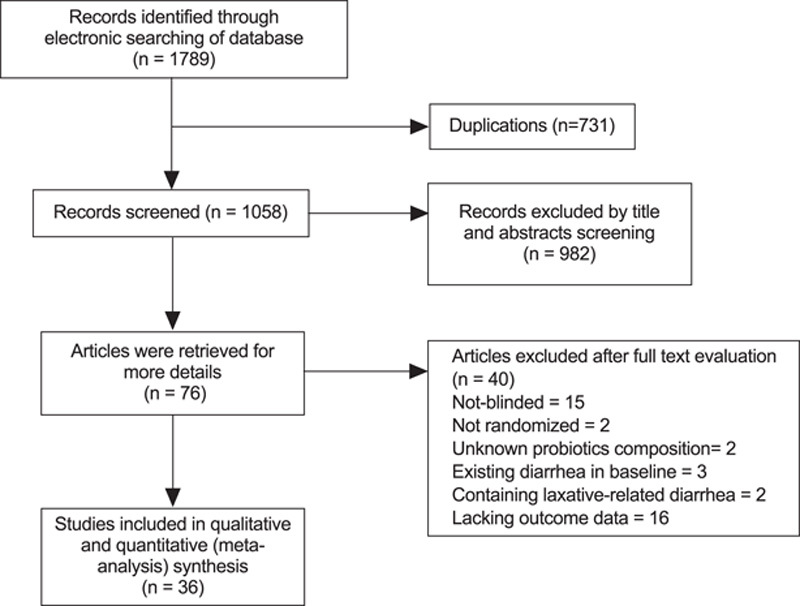
Selection process of meta-analysis.
TABLE 1.
Characteristics of Enrolled Studies
| References | Risk of Bias (Based on Cochrane Handbook) | Setting | Sample Size (Treatment Group; Placebo Group) | Mean Age/Range (Treatment Group; Placebo Group) | Diarrhea Definition | Antibiotic (s) | Time From Antibiotic to Probiotic, d | Probiotic Species | Dosage Per Day | Probiotic Duration (d) | Follow-up Period (From the Cessation of Antibiotics Treatment) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Armuzzi et al18 | Low | Adults, asymptomatic | 30/30 | 40 | NR | H. pylori eradication | 0 | Lactobacillus GG | 1.2×1010 CFU | 14 d, AC†+7 | 3 wk |
| Thomas et al19 | Low | Adults, in-patient | 133/134 | 57.2/54.4 | Other definition | Various | 1 | Lactobacillus GG | 1×1010 CFU | 14 d | 1 wk |
| Cremonini et al20 | Low | Adults, asymptomatic | 63/20 | 18-61 | NR | H. pylori eradication | 0 | Lactobacillus GG, Saccharomyces boulardii, or the combination of L. acidophilus and Bifidobacterium lactis | 6×109, 5×109, or 5×109 CFU | 14 d, AC+7 | 3 wk |
| Nista et al21 | Unclear | Adults, asymptomatic | 54/52 | 46.0/43.0 | NR | H. pylori eradication | 0 | Bacillus clausii | 6×109 CFU | 14 d, AC+7 | 3 wk |
| Can et al22 | Unclear | Adults, in-patient | 73/78 | 25-50 | NR | Various | 2 | S. boulardii | 1×1010 CFU | Various, AC | 4 wk |
| Beausoleil et al23 | High | Adults, in-patient | 44/45 | 68.8/72.9 | WHO* | Various | 2 | A combination of L. acidophilus and L. casei | 2.5×1010 CFU for the first 2 days, 5×1010vCFU for the remaining days | Various, AC | 3 wk |
| Cindoruk et al24 | Unclear | adults | 62/62 | 45.82/47.56 | NR | H. pylori eradication | 0 | S. boulardii | 1000 mg | 14 d, AC | 6 wk |
| Hickson et al25 | Unclear | Adults, in-patient | 57/56 | 73.7/73.9 | Other definition | Various | 2 | A combination of L. casei, S. thermophilus and L. bulgaricus | 1.94×1010, 1.94×1010, and 1.94×109 CFU, respectively | Various, AC+7 | 4 wk |
| Bravo et al26 | High | Adults, out-patient | 41/45 | 49.78/50.98 | WHO* | Amoxicillin | 1 | S. boulardii | 1×1010 CFU | 12 d, AC+ at least 2 d | At least 11 d |
| Koning et al27 | Unclear | Adults, healthy volunteers | 19/19 | 25.5/28.2 | Other definition | Amoxycillin | 0 | A combination of B. bifidum, B. lactis, B. Longum, E. faecium, L. acidophilus, L. paracasei, L. plantarum, L. rhamnosus L. salivarius | 1×1010 CFU | 14 d, AC+7 | 8 wk |
| Wenus et al28 | Unclear | Adults, in-patient | 34/29 | 58.8/56.2 | Adjusted WHO† | Various | 3 | A combination of Lactobacillus GG L. acidophilus and Bifidobacterium | 2.50×1010, 2.50×109, and 2.50×1010 CFU, respectively | 14 d | 0 |
| Gao et al29 | Unclear | Adults, in-patient | 171/84 | 60/60 | WHO* | One of penicillin, cephalosporin, or clindamycin | 1.5 | A combination of L. acidophilus and L. casei | 5×1010 or 1×1011 CFU | Various, AC+5 | 26 d |
| Lonnermark et al30 | Unclear | Adults, in-patient, and out-patient | 80/83 | 47/43 | Adjusted WHO† | Various | 2 | L. plantarum | 1×1010 CFU | Various, AC+7 | 2 wk |
| Song et al31 | High | Adults, in-patient | 103/111 | 61/60 | Adjusted WHO† | Various | 2 | A combination of L. rhamnosus and L. acidophilus | 4×109 CFU | 14 d | 0 |
| Bekar et al32 | Unclear | Adults | 46/36 | 46/43 | NR | H. pylori eradication | 0 | A combination of Lactobacilli, lactic streptococci, yeasts, and acetic acid bacteria | 500 mL | 14 d, AC | 0 |
| Cimperman et al33 | High | Adults, in-patient | 13/10 | 42.8/63.6 | Adjusted WHO† | Various | 4 | L. reuteri | 2×108 CFU | 28 d | 2 wk |
| Manfredi et al34 | Low | Adults | 73/76 | 46.4/50.6 | NR | H. pylori eradication | 0 | A combination of L. acidophilus, L. bulgaricus, B. bifidum, and Streptococcus thermophilus | 2×109, 2×109, 1×109, and 2×109 CFU, respectively | 10 d, AC | 0 |
| Pozzoni et al35 | Low | Adults, in-patient | 106/98 | 79.9/78.5 | Other definition | Various | 2 | S. boulardii | 1×1010 CFU | Various, AC+7 | 12 wk |
| Allen et al36 | Low | Adults, in-patient | 1470/1471 | 77.2/77.0 | WHO* | Various | 7 | A combination of L. acidophilus, B. bifidum and B. lactis | 6×1010 CFU | 21 d | 5 wk |
| Chatterjee et al37 | Low | Adults, out-patient | 176/167 | 18-70 | Adjusted WHO† | One of cefadroxil or amoxycillin | 0 | A combination of L. acidophilus and Bifidobacterium | 4×109 CFU | 14 d, AC+7 | 1 wk |
| Padilla et al38 | Unclear | Adults | 29/30 | 56.6 | NR | H. pylori eradication | 0 | L. rhamnosus | 1.2×1010 CFU | 7 d, AC | 0 |
| Selinger et al39 | Unclear | Adults, in-patient | 117/112 | 57.9/57.0 | Other definition | Various | 2 | A combination of B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. Bulgaricus and Streptococcus thermophilus | 9×1011 CFU | Various, AC+7 | 4 wk |
| Shavakhi et al40 | Low | Adults | 90/90 | 42.3/42.2 | NR | H. pylori eradication | 0 | A combination of L. casei, L. rhamnosus, L. acidophilus, and L. bulgaricus, B. breve and B. longum, and Streptococcus thermophiles | 2×108 CFU | 14 d, AC | 4 wk |
| Francavilla et al41 | Low | Adults, dyspepsia | 44/43 | 49/44 | NR | H. pylori eradication | 0 | A combination of 2 strains of L. reuteri | 2×108 CFU | 7 d, AC | 61 d |
| Ouwehand et al42 | Low | Adults, in-patient | 336/167 | 49.9/50.0 | WHO* | One of broad-spectrum penicillin, cephalosporin, or clindamycin | 1.5 | A combination of L. acidophilus, L. paracasei and B. lactis | 4.17×109 or 1.70×1010 CFU | 10-21 d, AC+7 | 4 wk |
| Helps et al43 | Low | Adults, in-patient | 44/41 | 62.27/62.49 | WHO* | Various | 2 | L. casei, Shirota | 1.3×1010 CFU | Various, AC+7 | 12 wk after recruitment |
| Wright et al44 | Low | Adults, in-patient | 41/46 | 85.4/86.1 | Adjusted WHO† | Various | NA | L. casei, Shirota | 130 mL | Various, AC | 4 wk after recruitment |
| Ehrhardt et al45 | Unclear | Adults, in-patient | 246/231 | 60.1/56.5 | WHO* | Various | 2 | S. boulardii | 3.6×1010 CFU | Various but <8 wk, AC+7 | 7 wk |
| Evans et al46 | Low | Adults, healthy volunteers | 80/80 | 34.6/33.9 | Other definition | Amoxicillin-clavulanic acid | 0 | A combination of L. helveticus and L. rhamnosus | 0·4×109 and 7.6×109 CFU, respectively | 14 d, AC+7 | 8 wk |
| Shafaghi et al47 | High | Adults | 38/38 | 43.75/43.35 | NR | H. pylori eradication | 3 | A combination of L. casei, L. rhamnosus, Streptococcus thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus | 4×108 CFU | 17 d, 3 days earlier+AC | 1 wk |
| Chotivitayatarakorn et al48 | Unclear | Adults, dyspepsia | 54/54 | 54.15 | NR | H. pylori eradication | 0 | S. boulardii | 565 mg | 7 or 14 d, AC | 2-3 wk |
| Haghdoost et al49 | Unclear | Adults, dyspepsia | 88/88 | 28.34 | NR | H. pylori eradication | 0 | A combination of L. actobacillus and Bifidobacterium | 3×109 CFU | 38 d, AC+28 | 10 wk |
| Jiang and Zhu50 | Unclear | Adults | 111/111 | 35.2/34.8 | NR | H. pylori eradication | 0 | Bifidobacterium | 6 capsules | 14 d, AC | 4 wk |
| Trallero et al51 | Unclear | Adults | 18/18 | 38.5 | Other definition | Amoxicillin-clavulanic acid | 0 | A combination of L. acidophilus, L. rhamnosus, B. breve, B. longum, B. lactis and B. bifidum | 1×109 CFU | 30 d, AC+22 | 22 d |
| Romeo et al52 | Unclear | Adults | 74/73 | 18-65 | WHO* | Amoxicillin/clavulanic acid | 0 | Combination including Lactobacillus GG | Unclear | 7 d, AC | 0 |
| Rajkumar et al53 | Unclear | Adults, in-patient | 549/577 | 73.7/73.5 | Other definition | Various | 2 | A combination of L. casei, L. delbrueckii subspecies bulgaricus and S. thermophilus | 2×1010, 2×108, and 2×108 CFU, respectively | Various, AC+7 | 3 wk |
WHO, diarrhea was defined as ≥3 loose stools within a 24-hour period.
Adjusted WHO, diarrhea was defined as ≥3 loose stools/day for at least 2 days.
AC indicates antibiotic course; NR, not reported; WHO, World Health Organization.
Quality Assessment
The quality assessment results are shown in Figure 2, whereas Figure 3 displays the risk of bias of individual study. Among the eligible studies, 13 RCTs were triple-blinded, and the reminders were not clearly reported about the detection bias. Attrition bias and other biases were assessed to be higher for lacking an intention-to-treat analysis (11/36), excessive or unbalanced loss of follow-up (6/36), funding bias (4/36), small sample size (3/36), unbalanced baseline (3/36), or short follow-up period (1/36).
FIGURE 2.
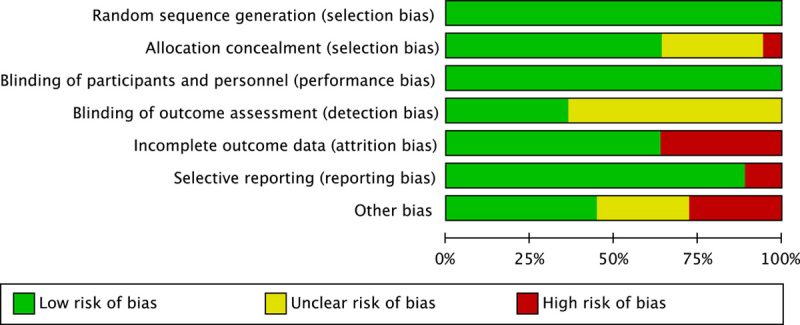
Risk of bias.
FIGURE 3.
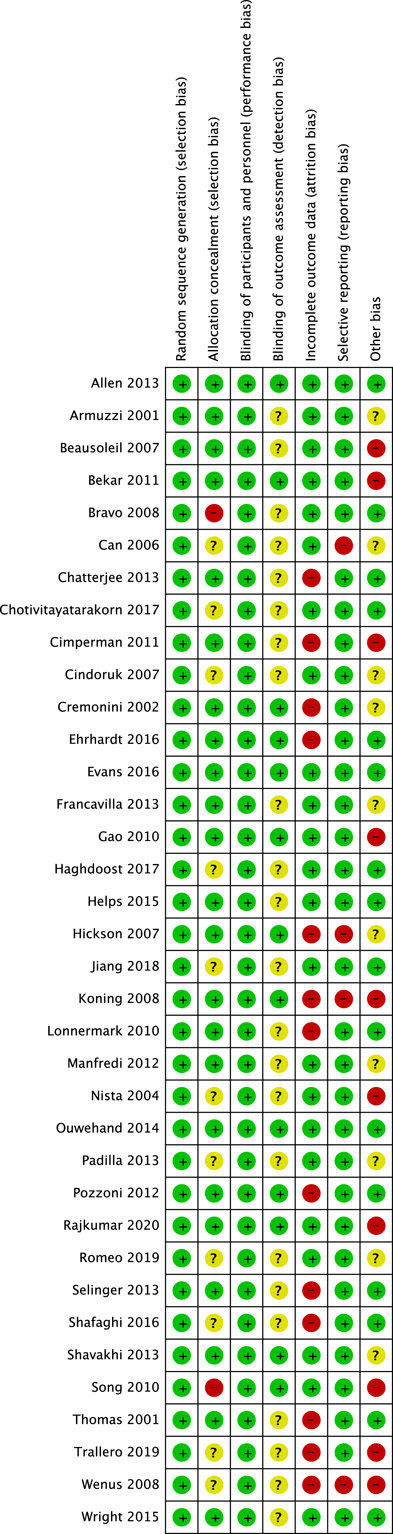
Risk of bias summary: green, low risk; yellow, unclear risk; red, high risk.
Overall Effect of Probiotics
As substantial heterogeneity was observed among the included studies (P<0.1, I 2=58%>50%), we calculated the overall AAD rate using a random effect model. Probiotics reduced the incidence of AAD by 38% (RR, 0.62; 95% CI, 0.51-0.74) in comparison with placebo (Fig. 4).
FIGURE 4.
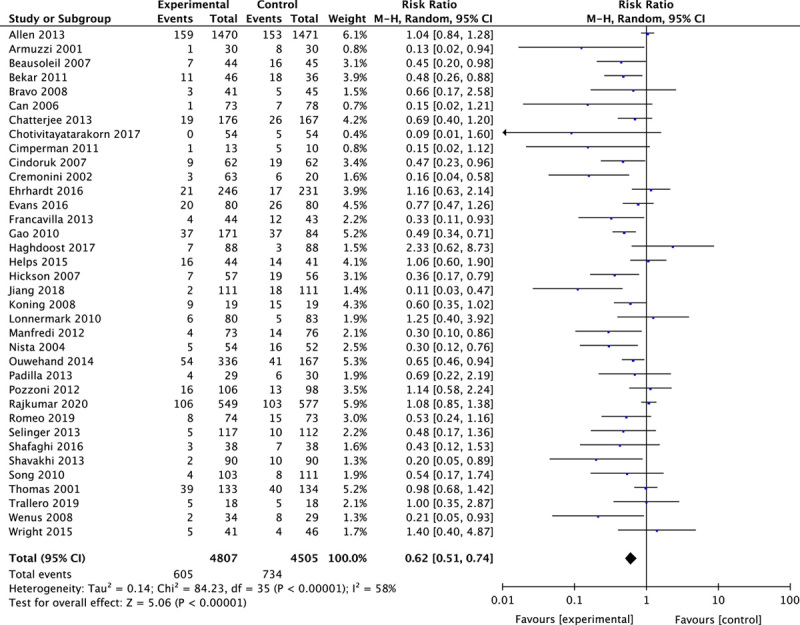
Forest plot for the overall effect of probiotics.
Sensitivity Analyses and Subgroup Analyses
Sensitivity analysis revealed that the pooled RR of probiotic effectiveness was robust. No single study significantly affected the overall effect.
Based on the characteristics of the studies, such as the quality of publications, age, participant setting, dosage, and intervention duration, we carried out a series of subgroup analyses. There were significant differences (P<0.1) among the 4 subgroups including reasons for antibiotics treatment (P=0.0007), probiotic duration (P=0.006), probiotic dosage (P=0.05), and time from antibiotic to probiotic (P=0.03).
Thirteen studies during Helicobacter pylori eradication had a higher efficacy than those used antibiotics for other reasons (RR, 0.36; 95% CI, 0.25-0.53; I 2=31% vs. RR, 0.75; 95% CI, 0.63-0.90; I 2=49%).
Probiotic duration equal to the antibiotics course is more effective than prolonging at least 7 days after the end of antibacterial treatment (RR, 0.42; 95% CI, 0.31-0.58; I 2=10% vs. RR, 0.74; 95% CI, 0.58-0.95; I 2=55%).
The daily dose of probiotics <1010 CFU is more effective for preventing AAD (RR, 0.49; 95% CI, 0.33-0.72; I 2=43% vs. RR, 0.77; 95% CI, 0.60-0.98; I 2=52%).
Using probiotics within the first 2 days of antibiotic treatment is more beneficial to prevent diarrhea (RR, 0.54; 95% CI, 0.43-0.67; I 2=43% vs. RR, 0.79; 95% CI, 0.60-1.03; I 2=52%).
Other subgroups, as shown in Table 2, were also evaluated but were not statistically different.
TABLE 2.
The Results of Subgroup Analyses
| Effect Estimate | Heterogeneity Test | ||||
|---|---|---|---|---|---|
| Subgroup | No. Trials | Risk Ratio | 95% CI | I², P | P for Interaction |
| Overall effect | 36 | 0.62 | 0.51-0.74 | 58%, <0.1 | — |
| Risk of bias | |||||
| Low risk | 13 | 0.72 | 0.55-0.93 | 59%, 0.003 | 0.25 |
| Unclear risk | 18 | 0.57 | 0.42-0.77 | 63%, 0.0002 | |
| High risk | 5 | 0.45 | 0.27-0.76 | 0%, 0.82 | |
| Diarrhea definition | |||||
| WHO definition | 8 | 0.74 | 0.55-0.99 | 64%, 0.007 | 0.27 |
| Adjusted WHO definition | 6 | 0.64 | 0.37-1.11 | 30%, 0.21 | |
| Others | 22 | 0.53 | 0.40-0.70 | 63%, <0.01 | |
| Reasons for antibiotics treatment | |||||
| For H. pylori eradication | 13 | 0.36 | 0.25-0.53 | 31%, 0.13 | 0.0007 |
| For other reasons | 23 | 0.75 | 0.63-0.90 | 49%, 0.005 | |
| Participant setting | |||||
| Hospital | 16 | 0.75 | 0.60-0.94 | 61%, 0.0007 | 0.64 |
| Community | 4 | 0.69 | 0.51-0.92 | 0%, 0.92 | |
| No. antibiotics | |||||
| One | 8 | 0.62 | 0.52-0.75 | 0%, 0.84 | 0.68 |
| Others | 28 | 0.58 | 0.45-0.75 | 64%, <0.01 | |
| Probiotic duration | |||||
| During antibiotics treatment | 12 | 0.42 | 0.31-0.58 | 10%, 0.34 | 0.006 |
| At least 1 week after antibiotics | 16 | 0.74 | 0.58-0.95 | 55%, 0.004 | |
| No. probiotics species | |||||
| One | 15 | 0.64 | 0.44-0.93 | 56%, 0.004 | 0.86 |
| Mixture | 20 | 0.61 | 0.49-0.76 | 60%, 0.0003 | |
| Probiotic dosage (CFU/d) | |||||
| ≥1010 | 14 | 0.77 | 0.60-0.98 | 52%, 0.01 | 0.05 |
| <1010 | 12 | 0.49 | 0.33-0.72 | 43%, 0.06 | |
| Follow-up duration (from the cessation of antibiotics treatment) (wk) | |||||
| ≥4 | 14 | 0.64 | 0.47-0.86 | 64%, 0.0006 | 0.45 |
| <4 | 20 | 0.54 | 0.41-0.72 | 57%, 0.0008 | |
| Probiotic species | |||||
| Lactobacillus | 12 | 0.67 | 0.50-0.91 | 44%, 0.05 | 0.10 |
| S. boulardii | 6 | 0.69 | 0.39-1.22 | 47%, 0.09 | |
| Lactobacillus+Bifidobacterium | 6 | 0.82 | 0.57-1.17 | 56%, 0.04 | |
| Other (mixed) species | 12 | 0.41 | 0.27-0.63 | 71%, <0.01 | |
| Time from antibiotic to probiotic (d) | |||||
| <2 | 22 | 0.54 | 0.43-0.67 | 43%, 0.02 | 0.03 |
| 2-7 | 13 | 0.79 | 0.60-1.03 | 52%, 0.01 | |
H. pylori indicates Helicobacter pylori; S. boulardii, Saccharomyces boulardii.
Adverse Events
A total of 15 studies described adverse events, mainly involving nausea, bloating, and dyspepsia. Four of them reported no adverse events either in the probiotics group or in the placebo, and 2 registered serious adverse events but not attributable to probiotics. There were no statistically significant increased adverse events in the probiotics group (RR, 1.00; 95% CI, 0.87-1.14; P=0.97 ) (Fig. 5).
FIGURE 5.
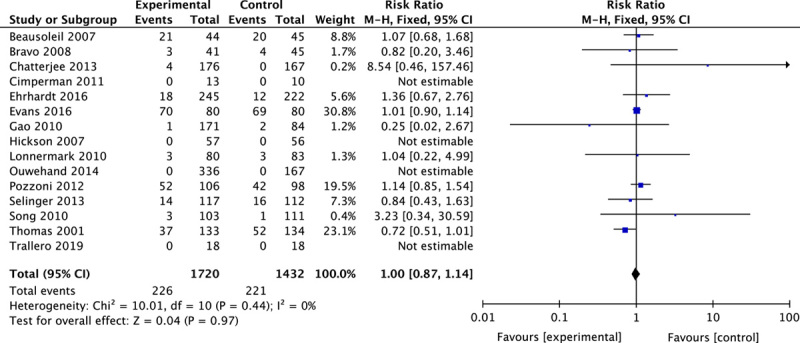
Forest plot of adverse events.
Publication Bias
The funnel plot, Begg test, and Egger test were applied to assess the publication bias of the enrolled studies. These results provided evidence of publication bias (Begg test: z=2.36, Pr > |z|=0.018<0.05; and Egger test: t=−4.77; 95% CI, −2.40 to −0.97; P<0.05). We use the trim and fill method to correct the publication bias and yielded the same pooled RR of 0.62 as initial outcomes, which suggested that results of the overall effect were stable, and publication bias had few effects on the results. Therefore, our asymmetric funnel plot may be caused by other reasons such as studies with low quality or small sample size (Fig. 6).
FIGURE 6.
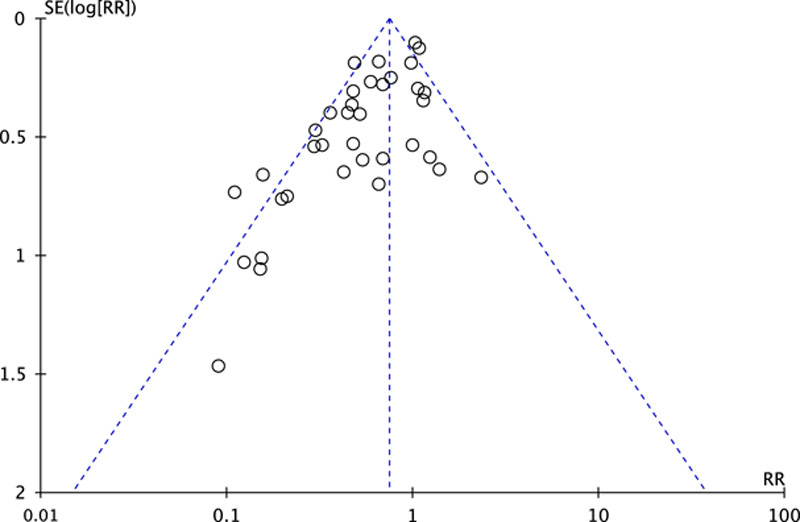
Funnel plot of publication bias. RR indicates relative risk; SE, standard error.
DISCUSSION
Our meta-analysis indicated a reduction of AAD from 16% in placebo to 13% in probiotic-treated groups (RR, 0.62; 95% CI, 0.51-0.74; random-effects). Further subgroup analyses suggested that the protective effect was still significant when grouped by reasons for antibiotics treatment, probiotic duration, probiotic dosage, and time from antibiotic to probiotic.
Compared with antibiotics treatments for other reasons, probiotics showed more effective protection during H. pylori treatment. Certain probiotics, when used as an auxiliary in H. pylori eradication, can increase the eradication rate and reduce side effects.55 Meta-analyses for Saccharomyces boulardii and Lactobacillus both showed statistically promising results. S. boulardii significantly improved the eradication rates (RR, 1.11; 95% CI, 1.06-1.17) and reduced the incidence of diarrhea (RR, 0.51; 95% CI, 0.42-0.62).56 So was the Lactobacillus (improving eradication rates: OR, 1.78; 95% CI, 1.21-2.62; reducing incidence of diarrhea: OR, 0.23; 95% CI, 0.11-0.48).57 In terms of the mechanism of probiotics in H. pylori eradication, animal investigations have indicated that probiotics may regulate immune activity by controlling cytokine and inflammatory/anti-inflammatory chemokine balance, such as interleukin-8 and secretory immunoglobulin A, thereby reducing gastric activity and inflammation. Also, probiotics assisted in promoting the H. pylori eradication through a physiological or nonspecific mechanism. Certain probiotics directly or in combination with their products stimulated gastric epithelium to produce antibacterial peptides, inhibited the growth of H. pylori by secreting short-chain fatty acids, competitively inhibited the adhesion of pathogens to the gastric mucosal layer, improved the epithelial barrier function, and increased mucin production.58
We also explored the dose effect of probiotics in our meta-analysis. Our results showed that high-dose probiotics (≥1010 CFU/d) were statistically less effective than low-dose probiotics (P=0.05<0.10). However, a previous meta-analysis conducted by Johnston et al (involving adults and children) demonstrated that higher dosage (>1010 CFU/d) had a more effective trend than lower dosage but not significantly (RR, 0.34; 95% CI, 0.23-0.49 vs. RR, 0.61; 95% CI, 0.08-4.60; P=0.57>0.10).59 This may be because we excluded children and the difference in sample size between subgroups. Hence, more RCTs on dose-response were needed to determine whether probiotics in higher doses were more effective and safe.
Our results are almost consistent with the previous meta-analysis in terms of the duration and starting time of probiotics.60,61 It is beneficial to use probiotics as early as possible to maintain the gut flora’s stability and prevent the overgrowth of pathogens. Concerning the optimal duration of probiotics, we suggested that probiotics use during antibiotic therapy can effectively prevent AAD. However, whether it is necessary to prolong the use of probiotics after the end of antibiotic treatment still needs more clinical evidence and theoretical support.
Twelve studies applied Lactobacillus as intervention indicated a more protective trend among all the probiotics species (RR, 0.67; 95% CI, 0.50-0.91). Among them, L. rhamnosus GG (LGG) is the most studied. A meta-analysis proposed that LGG significantly reduced the risk of diarrhea (RR, 0.49; 95% CI, 0.29-0.83).62 This effect may be related to the colonization of LGG in the intestine. It not only enhances the survival rate of the intestinal epithelium survival and preserves cytoskeletal integrity, but also secretes lectin-like proteins 1 and 2 to resist biofilms produced by various pathogens.63 Unfortunately, because of the insufficient sample size, some probiotics strains cannot be analyzed separately. In addition, we did not find significant differences in the efficacy of single species and multiple species (RR, 0.64; 95% CI, 0.44-0.93 vs. RR, 0.61; 95% CI, 0.49-0.76; P=0.86>0.1).
The type of antibiotic was reported as the strongest predictor for AAD. Although ampicillin/amoxicillin, cephalosporins, and clindamycin used alone were most frequently associated with AAD, other antibiotics, when used in combination, also increased the risk of AAD.64 Unfortunately, many RCTs did not register specific antibiotics, which prevented us from performing subgroup analysis.
We extracted the data related to adverse events from 15 studies and thus calculated the pooled RR of 1.00 with no statistical significance (95% CI, 0.87-1.14; P=0.97). A comprehensive systematic review on probiotics safety based on 622 studies displayed a pooled RR of 1.00 (95% CI, 0.93-1.07; P=0.999), which was close to our finding.65 These pieces of evidence were sufficient to show that short-term use of probiotics would not bring about serious side effects on a population without severe systemic disease or immunodeficiency. However, specific patients, including critical illness, using a central venous catheter, immunosuppression, should be sensitive to the adverse effects.66 Some case reports and clinical studies have reported probiotics-related adverse events involving systemic infections, gastrointestinal side effects, deleterious metabolic activities, and gene transfer.67 In short, probiotics are safe to use in preventing AAD.
There were some limitations. First, some heterogeneity was observed in our results. Both the subgroup analyses and sensitivity analysis failed to explain the source of heterogeneity. Second, some included studies failed to mention all specific characteristics. Thus, several subgroup analyses could not enroll all the 36 RCTs.
Nevertheless, our research also had some advantages. We adopted rigorous inclusion criteria to collect more representative data. During the citations identified, we excluded 2 publications with unknown probiotics composition. To avoid interference with baseline conditions, RCTs that included existing diarrhea or containing laxative-related diarrhea were also excluded. In addition, we conducted subgroup analyses as comprehensive as possible, and the trend of probiotics in some specific situations had been explored.
Our study suggests that using probiotics within 2 days during antibiotic treatment significantly reduces the incidence of AAD in adults and is safe. Besides, the existing evidence showed that S. boulardii supplementation or Lactobacillus supplementation in H. pylori eradication therapy significantly increased the eradication rate and reduced the incidence of diarrhea. But the role of other probiotics in H. pylori eradication had not yet been fully clarified. Of course, to match the population included in this meta-analysis, these findings are restricted to adults without immunodeficiency and the history of intensive care unit.
CONCLUSIONS
Our meta-analysis suggested that during antibiotic treatment, taking probiotics as early as possible has a positive and safe effect on preventing antibiotic-related diarrhea in adults. However, further studies should focus on the optimal dosage and duration of probiotics and pay attention to the strain specificity to develop a specific recommendation.
Footnotes
The authors declare that they have no conflict of interest.
REFERENCES
- 1. Beaugerie L, Petit JC. Microbial-gut interactions in health and disease. Antibiotic-associated diarrhoea. Best Practice & Res Clin Gastroenterol. 2004;18:337–352. [DOI] [PubMed] [Google Scholar]
- 2. McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563–578. [DOI] [PubMed] [Google Scholar]
- 3. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. [DOI] [PubMed] [Google Scholar]
- 4. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol Hepatol. 2014;11:506–514. [DOI] [PubMed] [Google Scholar]
- 5. Mekonnen SA, Merenstein D, Fraser CM, et al. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr Opin Biotechnol. 2020;61:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guarino A, Guandalini S, Lo Vecchio A. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2015;49(suppl 1):S37–S45. [DOI] [PubMed] [Google Scholar]
- 7. Brussow H. Probiotics and prebiotics in clinical tests: an update. F1000Res. 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JPT, Green S. Cochrane Collaboration Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England/Hoboken NJ: Wiley-Blackwell; 2008. [Google Scholar]
- 10. Review Manager (RevMan) [Computer Program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- 11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 12. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 13. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available at: www.cochrane-handbook.org. Accessed March 18, 2014.
- 14. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 15. Langan D, Higgins JP, Gregory W, et al. Graphical augmentations to the funnel plot assess the impact of additional evidence on a meta-analysis. J Clin Epidemiol. 2012;65:511–519. [DOI] [PubMed] [Google Scholar]
- 16. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armuzzi A, Cremonini F, Bartolozzi F, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163–169. [DOI] [PubMed] [Google Scholar]
- 19. Thomas MR, Litin SC, Osmon DR, et al. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76:883–889. [DOI] [PubMed] [Google Scholar]
- 20. Cremonini F, Di Caro S, Covino M, et al. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97:2744–2749. [DOI] [PubMed] [Google Scholar]
- 21. Nista EC, Candelli M, Cremonini F, et al. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther. 2004;20:1181–1188. [DOI] [PubMed] [Google Scholar]
- 22. Can M, Beşirbellioglu BA, Avci IY, et al. Prophylactic Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea: a prospective study. Med Sci Monit. 2006;12:PI19–PI22. [PubMed] [Google Scholar]
- 23. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus CL1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cindoruk M, Erkan G, Karakan T, et al. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. 2007;12:309–316. [DOI] [PubMed] [Google Scholar]
- 25. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bravo MV, Bunout D, Leiva L, et al. Effect of probiotic Saccharomyces boulardii on prevention of antibiotic associated diarrhea in adult outpatients with amoxicillin treatment. Rev Med Chile. 2008;136:981–988. [PubMed] [Google Scholar]
- 27. Koning CJM, Jonkers D, Stobberingh EE, et al. The effect of a multispecies probiotic on the intestinal microbiota and bowel movements in healthy volunteers taking the antibiotic amoxycillin. Am J Gastroenterol. 2008;103:178–189. [DOI] [PubMed] [Google Scholar]
- 28. Wenus C, Goll R, Loken EB, et al. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr. 2008;62:299–301. [DOI] [PubMed] [Google Scholar]
- 29. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636–1641. [DOI] [PubMed] [Google Scholar]
- 30. Lönnermark E, Friman V, Lappas G, et al. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J Clin Gastroenterol. 2010;44:106–112. [DOI] [PubMed] [Google Scholar]
- 31. Song HJ, Kim JY, Jung SA, et al. Effect of probiotic Lactobacillus (Lacidofil® Cap) for the prevention of antibiotic-associated diarrhea: a prospective, randomized, double-blind, multicenter study. J Korean Med Sci. 2010;25:1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bekar O, Yilmaz Y, Gulten M. Kefir improves the efficacy and tolerability of triple therapy in eradicating Helicobacter pylori . J Med Food. 2011;14:344–347. [DOI] [PubMed] [Google Scholar]
- 33. Cimperman L, Bayless G, Best K, et al. A randomized, double-blind, placebo-controlled pilot study of Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic-associated diarrhea in hospitalized adults. J Clin Gastroenterol. 2011;45:785–789. [DOI] [PubMed] [Google Scholar]
- 34. Manfredi M, Bizzarri B, Sacchero RI, et al. Helicobacter pylori infection in clinical practice: probiotics and a combination of probiotics+lactoferrin improve compliance, but not eradication, in sequential therapy. Helicobacter. 2012;17:254–263. [DOI] [PubMed] [Google Scholar]
- 35. Pozzoni P, Riva A, Bellatorre AG, et al. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: a single-center, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2012;107:922–931. [DOI] [PubMed] [Google Scholar]
- 36. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet (London, England). 2013;382:1249–1257. [DOI] [PubMed] [Google Scholar]
- 37. Chatterjee S, Kar P, Das T, et al. Randomised placebo-controlled double blind multicentric trial on efficacy and safety of Lactobacillus acidophilus LA-5 and Bifidobacterium BB-12 for prevention of antibiotic-associated diarrhoea. J Assoc Phys India. 2013;61:708–712. [PubMed] [Google Scholar]
- 38. Padilla Ruiz M, Fernández Aguiar ME, Arce Nuñez M, et al. Lactobacillus rhamnosus GG supplementation to reduce side-effects of anti-Helicobacter pylori treatment. Rev Gastroenterol Peru. 2013;33:121–130. [PubMed] [Google Scholar]
- 39. Selinger CP, Bell A, Cairns A, et al. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J Hosp Infect. 2013;84:159–165. [DOI] [PubMed] [Google Scholar]
- 40. Shavakhi A, Tabesh E, Yaghoutkar A, et al. The effects of multistrain probiotic compound on bismuth-containing quadruple therapy for Helicobacter pylori infection: a randomized placebo-controlled triple-blind study. Helicobacter. 2013;18:280–284. [DOI] [PubMed] [Google Scholar]
- 41. Francavilla R, Polimeno L, Demichina A, et al. Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol. 2014;48:407–413. [DOI] [PubMed] [Google Scholar]
- 42. Ouwehand AC, DongLian C, Weijian X, et al. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32:458–463. [DOI] [PubMed] [Google Scholar]
- 43. Helps A, Bell E, Mactier R. Prospective randomized double-blind study of efficacy of probiotic milk drink in reducing the incidence of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea. Int J Probiotics Prebiotics. 2015;11:145–152. [Google Scholar]
- 44. Wright K, Wright H, Murray M. Probiotic treatment for the prevention of antibiotic-associated diarrhoea in geriatric patients: a multicentre randomised controlled pilot study. Aust J Ageing. 2015;34:38–42. [DOI] [PubMed] [Google Scholar]
- 45. Ehrhardt S, Guo N, Hinz R, et al. Saccharomyces boulardii to prevent antibiotic-associated diarrhea: a randomized, double-masked, placebo-controlled trial. Open Forum Infect Dis. 2016;3:ofw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Evans M, Salewski RP, Christman MC, et al. Effectiveness of Lactobacillus helveticus and Lactobacillus rhamnosus for the management of antibiotic-associated diarrhoea in healthy adults: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2016;116:94–103. [DOI] [PubMed] [Google Scholar]
- 47. Shafaghi A, Pourkazemi A, Khosravani M, et al. The effect of probiotic plus prebiotic supplementation on the tolerance and efficacy of Helicobacter pylori eradication quadruple therapy: a randomized prospective double blind controlled trial. Middle East J Digest Dis. 2016;8:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chotivitayatarakorn P, Mahachai V, Vilaichone RK. Effectiveness of 7-day and 14-day moxifloxacin-dexlansoprazole based triple therapy and probiotic supplement for Helicobacter pylori eradication in thai patients with non-ulcer dyspepsia: a double-blind randomized placebo-controlled study. Asian Pac J Cancer Prev. 2017;18:2839–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haghdoost M, Taghizadeh S, Montazer M, et al. Double strain probiotic effect on Helicobacter pylori infection treatment: a double-blinded randomized controlled trial. Casp J Intern Med. 2017;8:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang L, Zhu W. Probiotics improved the effectiveness and safety of the quadruple Helicobacter pylori eradication therapy. Biomed Res (India). 2018;29:2053–2056. [Google Scholar]
- 51. García Trallero O, Herrera Serrano L, Bibián Inglés M, et al. Effect of the administration of a probiotic with a combination of Lactobacillus and Bifidobacterium strains on antibiotic-associated diarrhea. Rev Esp Quimioter. 2019;32:268–272. [PMC free article] [PubMed] [Google Scholar]
- 52. Romeo A, Barreiro C, Miegimolle M, et al. Efficacy of a 7-strain synbiotic mixture in combination with oral antibiotic treatment in preventing antibiotic-associated diarrhea (AAD): prodeggio study. Turk J Gastroenterol. 2019;30:S298. [Google Scholar]
- 53. Rajkumar C, Wilks M, Islam J, et al. Do probiotics prevent antibiotic associated diarrhoea? Results of a multicentre randomised placebo-controlled trial. J Hosp Infect. 2020;105:280–288.32035998 [Google Scholar]
- 54. WHO. Diarrhoeal disease. Available at: http://www.who.int/mediacentre/factsheets/fs330/en/. Accessed June 1, 2017.
- 55. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. [DOI] [PubMed] [Google Scholar]
- 56. Szajewska H, Horvath A, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2015;41:1237–1245. [DOI] [PubMed] [Google Scholar]
- 57. Zou J, Dong J, Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97–107. [DOI] [PubMed] [Google Scholar]
- 58. Eslami M, Yousefi B, Kokhaei P, et al. Are probiotics useful for therapy of Helicobacter pylori diseases? Comp Immunol Microbiol Infect Dis. 2019;64:99–108. [DOI] [PubMed] [Google Scholar]
- 59. Johnston BC, Ma SS, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878–888. [DOI] [PubMed] [Google Scholar]
- 60. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355–1369. [DOI] [PubMed] [Google Scholar]
- 61. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889–1900. [DOI] [PubMed] [Google Scholar]
- 62. Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149–1157. [DOI] [PubMed] [Google Scholar]
- 63. Mantegazza C, Molinari P, D’Auria E, et al. Probiotics and antibiotic-associated diarrhea in children: a review and new evidence on Lactobacillus rhamnosus GG during and after antibiotic treatment. Pharmacol Res. 2018;128:63–72. [DOI] [PubMed] [Google Scholar]
- 64. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(suppl 2):S129–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hempel S, Newberry S, Ruelaz A, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep/Technol Assess. 2011:1–645. [PMC free article] [PubMed] [Google Scholar]
- 66. Szajewska H, Konarska Z, Kolodziej M. Probiotic bacterial and fungal strains: claims with evidence. Digest Dis (Basel, Switzerland). 2016;34:251–259. [DOI] [PubMed] [Google Scholar]
- 67. McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Digest Dis (Basel, Switzerland). 1998;16:292–307. [DOI] [PubMed] [Google Scholar]


