Abstract
Targeted oncogenesis is the process of driving tumor formation by engineering transgenic mice that express an oncogene under the control of a cell-type specific promoter. Such tumors can be adapted to cell culture, providing immortalized cell lines. To make it feasible to follow the process of tumorigenesis and increase the opportunity for generating cell lines, we developed a mouse strain that expresses SV40 T antigens in response to Cre-recombinase. Using CRISPR/Cas9 we inserted a cassette with coding sequences for SV40 T antigens and an internal ribosome entry site with green fluorescent protein cassette (IRES-GFP) into the Rosa26 locus, downstream from a stop sequence flanked by loxP sites: Rosa26LSL-SV40-GFP. These mice were mated with previously established Prop1-cre and Tshb-cre transgenic lines. Both the Rosa26LSL-SV40-GFP/+; Prop1-cre and Rosa26LSL-SV40-GFP/+; Tshb-cre mice developed fully penetrant dwarfism and large tumors by 4 weeks. Tumors from both of these mouse lines were adapted to growth in cell culture. We have established a progenitor-like cell line (PIT-P1) that expresses Sox2 and Pitx1, and a thyrotrope-like cell line (PIT-T1) that expresses Pou1f1 and Cga. These studies demonstrate the utility of the novel, Rosa26 LSL-SV40-GFP mouse line for reliable targeted oncogenesis and development of unique cell lines.
Keywords: PROP1, PITX1, SOX2, thyrotrope, progenitor, adenoma
Immortalized cell lines have been invaluable tools for understanding the molecular mechanisms that underlie the pituitary gland’s response to hypothalamic regulation, feedback from end organs, and intracellular signaling. They have also been useful in understanding the formation of pituitary adenomas (1). Cell lines offer the ease of manipulation and obviate the need to rely on primary tissues. This is particularly true for small organs like the pituitary gland. Additional pituitary cell lines would be useful for dissecting the changes associated with stem cells transitioning to differentiation and commitment to the thyrotrope fate.
Pituitary and hypothalamic cell lines have been developed by targeted oncogenesis. This involved using cell-specific transcriptional regulatory sequences to drive expression of large and small simian virus 40 (SV40) T antigens in transgenic mice. Invariably, tumors develop in some of the mice, and the cells in these tumors can sometimes be adapted to grow in culture into stable, immortalized cell lines that maintain some of the features of differentiated cells. Tumors often develop early and cause infertility or death, making it difficult to generate cell lines from a single founder mouse. For this reason, we generated a mouse line that can express SV40 T antigen in response to Cre-recombinase excision of a stop sequence flanked by loxP sites.
Both the SV40 large and small T antigens contribute to immortalization. The large T antigen causes transformation by binding to and disrupting the function of HSC70, Rb, p300, and p53, whereas the small t antigen binds to phosphatase 2A (PP2A) to contribute to the transformation, characterized by uncontrolled proliferation (2). The ability of SV40 to inactivate p300-related activity is critical to its capacity to immortalize cells (3). SV40-mediated immortalization has been used to create cell lines that represent pre-gonadotropes (αT3-1), gonadotropes (LβT2), precursors of the POU1F1 lineage (GHFT1, Pit1-zero), differentiated cells of the POU1F1 lineage (Pit1-triple, TαT1, and Pit1-PRL), and GnRH neurons (GT1-1) (4-8). αT3-1 cells were developed by driving SV40 T antigen expression with 1.8 kb of the CGA promoter. CGA encodes a common alpha subunit of 3 heterodimeric pituitary hormones: follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are expressed in the gonadotropes, and thyrotropin (TSH; thyroid-stimulating hormone), which is expressed in thyrotropes. The αT3-1 cells do not express any beta subunits, but they do express Cga and the gonadotropin-releasing hormone (GnRH) receptor, indicating commitment to the gonadotrope fate. These cells have been invaluable for studying GnRH-mediated cell signaling and regulation of gene expression (9-12). A more differentiated gonadotrope-like cell line, the LβT2 cell line, was generated by driving SV40 T antigen expression from the rat LHβ gene regulatory elements. LβT2 cells express GnRH receptor, CGA, LH, and FSH. They secrete LH in response to GnRH stimulation and respond appropriately to steroid hormone feedback (13). These cells have been used widely to study regulation of gene expression in response to various stimuli (14-18). The TαT-1 cell line was generated from a tumor produced by driving SV40 T antigen expression with an expanded, human CGA regulatory region. These thyrotrope-like cells respond to thyrotropin-releasing hormone and retinoids, and they secrete TSH in response to diurnal cues (19-21). Several cell lines were generated using the Pou1f1 regulatory elements to drive T antigen. While each of these cell lines express Pou1f1, they vary in hormone expression from none (GHFT1 and Pit1-zero) to 3 hormones (Pit1-triple), including growth hormone (GH), TSH, and prolactin. These cell lines were valuable for studying regulation of the human GH gene cluster, which was introduced as a transgene, and for studying interactions between POU1F1 and the CCAAT enhancer binding protein, CEBPα (22). A GnRH neuronal-like cell line, GT1-7, was generated by driving SV40 T antigen from GnRH promoter elements, and these cells were used to study GnRH expression and responsiveness to external stimulation (23-25). Despite these success stories, pituitary tumors often lead to dwarfism, infertility, and sudden death, and it can be difficult to adapt tumors to culture.
Additional pituitary cell lines would be valuable for understanding the process of differentiation from progenitors to hormone-producing cells. Pituitary stem cells express the common pluripotency factor Sox2. SOX2-positive cells are capable of giving rise to all hormone-producing cells within the pituitary (26-28). The commitment to pituitary fate is associated with expression of the transcription factors PITX1, PITX2, LHX3, and LHX4 (27, 29). Prop1 is initially co-expressed with Sox2. Prop1 is a pituitary-specific transcription factor necessary for the POU1F1 lineage, which comprises thyrotropes, somatotropes and lactotropes (30). Lineage tracing suggests that all cells of the anterior and intermediate lobes of the pituitary gland pass through a Prop1-expressing progenitor (31). Pituitary stem cells can be grown as organoids and stimulated to differentiate into all hormone-producing cell types (32-34). This process is inefficient, making it difficult to get enough material to analyze chromatin accessibility and epigenetic marks. There are currently no Sox2-expressing pituitary progenitor cell lines.
To expand the repertoire of cell lines available for study, we generated a mouse line that conditionally expresses SV40 T antigen from the Rosa26 locus: Rosa26LSL-SV40-GFP. Well-characterized cre strains can be used to initiate cell-type specific oncogenesis, and the development of tumors can be followed because targeted oncogenesis is initiated with 2 different transgenes. This approach also provides multiple opportunities to adapt tumors to culture. As rare, novel pituitary cell populations have been discovered with the use of single-cell RNA-seq, we have created a reagent for the generation of limitless immortalized cell lines that permits characterization of these elusive populations.
Methods
Donor Plasmid Construction
The donor plasmid was constructed using pR26 GFP Dest (gift from Ralf Kuehn, Addgene plasmid # 74283), and SV40 small and Large T in pENTR1A (w611-7, gift from Eric Campeau, Addgene plasmid # 22297). The pR26 GFP Dest plasmid is 14.7 kb and contains 2 arms of homology to the Rosa26 locus: 1 kb 5′ and 4 kb 3′. The pR26 GFP Dest was recombined with the SV40 small and Large T plasmid with a Gateway LR clonase II reaction (ThermoFisher, 11791100). The resulting plasmid was amplified and purified with the Qiagen Maxi Prep Plasmid (Qiagen, 12163) kit. Endotoxins were removed from the plasmid using the Endotoxin Removal Solution (Sigma, E4274).
Mice and Genotyping
All mice were housed in a 12-hour light, 12-hour dark cycle in ventilated cages with unlimited access to tap water and Purina 5020 chow. All procedures were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guidelines on the Care and Use of Experimental Animals and approved by our Institutional Animal Care and Use Committee.
The Rosa26LSL-SV40-GFP/+ allele was generated by microinjecting enhanced specificity Cas9 protein (50 ng/μL from Sigma), circular DNA donor plasmid (20 ng/μL, adapted from Addgene), chemically modified sgRosa26-1 (30 ng/μL from Synthego.com) into fertilized eggs obtained by mating (C57BL/6 X SJL)F1 or C57BL/6 female mice with (C57BL/6 X SJL)F1 male mice purchased from the Jackson Laboratory. The guide RNA, sgROSA26-1, was previously shown to be highly efficient and has the sequence: 5′-TCT GCA ACT CCA GTC TTT CTA GAA GAT GGG CGG GAC TCT TCT-3′ (35). Pronuclear microinjection was performed as describe (36).
Founders were confirmed to have the correct insertion by 3 separate polymerase chain reaction (PCR) reactions. The first set was between the first Rosa26 intron and the splice acceptor of the knock-In allele (forward 5′-GCCTCCTGGCTTCTGAGGACCG-3′ and reverse 5′-CCTGGACTACTGCGCCCTACAGA-3′). The second set of primers was designed to detect the presence of the presence of SV40 T antigen (forward 5′-AAAGTGGCATTGCTTTGCTT-3′ and reverse 5′-AAATGAGCCTTGGGACTGTG-3′). The third set of primers were designed to distinguish correct insertion into the Rosa26 locus from random insertion of the donor plasmid. The forward primer was specific for a segment of Rosa26 genomic DNA not included in the donor plasmid and the reverse primer was specific to the donor plasmid: (forward 5′-CTGCCCGAGCGGAAACGCCACTGAC-3′ and reverse 5′-CCTGGACTACTGCGCCCTACAGA-3′). Sixteen out of 79 potential founders were positive for all 3 PCRs. Subsequent genotyping of mice for the Rosa26LSL-SV40-GFP/+ allele was done using the T antigen specific primer pair described above.
Tg(Prop1-cre) 432Sac , referred to here as Prop1-cre, were generated at University of Michigan (31). They were genotyped with the following primers: forward 5′-GGTCTCCCTCCGTTTTTCTC-3′ and reverse 5′-CTGCACACAGACAGGAGCAT-3′.
Tg(Tshb-cre) Sac , referred to here as Tshb-cre, were generated at University of Michigan (37). They were genotyped with these primers: forward 5′-GGACATGTTCAGGGATCGCCAGGCG-3′ and reverse 5′-GCATAACCAGTGAAACAGCATTGCTG-3′.
Gt(ROSA)26Sor tm1(EYFP)Cos , referred to here as Rosa26YFP, were generated at Columbia University and were purchased from Jackson Laboratories, stock number 006148, and they were genotyped according to Jackson Laboratory recommended primers and conditions (38).
Detection of a copulation plug was designated as embryonic day 0.5 (e0.5), and the day of birth was designated postnatal day 1 (P1).
Histology and Immunohistochemistry
Heads from mice younger than 2 weeks of age were fixed in PFA (5 mL formaldehyde in 35 mL of phosphate-buffered saline [PBS]) overnight, followed by 3 washes in PBS. They were put in 10% EDTA for 3 days, then dehydrated for a minimum of 4 hours in each step of a gradient of ethanol solutions: 25%, 50%, and 75%. Pituitaries from mice 2 weeks or older were fixed overnight in PFA, followed by 3 PBS washes. They were put in 10% EDTA for 3 hours, then dehydrated for a minimum of 1 hour in each step of ethanol solutions described above. Pituitaries and heads were embedded in paraffin with 4-hour cycles in a Tissue Tek VIP paraffin tissue processing machine. Sections at 6 μm thickness from each at least 3 different controls and experimental mice were analyzed by hematoxylin and eosin (H&E) and immunohistochemical markers as previously described (39). Immunostaining for pituitary hormone markers was performed using anti-TSH and anti-GH (1:1000, National Hormone and Peptide Program, UCLA Medical Center, Torrance, CA, USA). Immunostaining for proteins was performed using rabbit anti-POU1F1 (kindly provided by Dr. Simon Rhodes, University of North Florida, Jacksonville) rabbit anti-Ki67 (1:250, Novocastra, Newcastle, United Kingdom), rabbit anti-NR5A1 (1:100, kindly provided by Dr. Gary Hammer, University of Michigan), guinea pig anti-PROP1 (1:100, kindly provided by Aimee Ryan, Montreal, Quebec), mouse anti-SV40 (1:1000, kindly provided by Michael J. Imperiale, University of Michigan), and rabbit anti-PECAM (1:100, Thermo Scientific). The following secondary antibodies were used: biotinylated anti-rabbit IgG (1:100, Jackson Immunoresearch) for anti-POU1F1, anti-PECAM, anti-Ki67, anti-NR5A1; anti-human biotin (1:200, ab97223, Abcam) for anti-GH, and biotinylated anti-guinea pig IgG (Jackson Immunoresearch) for anti-TSH and anti-PROP1. For anti-SV40 the M.O.M. (Mouse on Mouse) Immunodetection Kit, Basic (Vector Laboratories, Burlingame, CBMK-2202) was used.
Antibodies were detected using the tyramide signal amplification (TSA) (33002 CFF488A Streptavidin HRP, Biotium, Fremont, CA), streptavidin-conjugated Alexa-fluor 488 (1:200, S11223, Invitrogen), and streptavidin-conjugated Cy3 (Thermo Fisher 434315). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (1:200) for 5 minutes. Slides were mounted with permount mounting medium containing DABCO. Images were captured with a Leica DMRB fluorescent microscope and a Leica MZ10F dissecting scope.
Cell Dispersion and Fluorescence-Activated Cell Sorting
Pituitaries were removed from mice 2 weeks or older and placed in PBS. They were transferred to an enzyme mix consisting of collagenase, DNAse, Fungizone, and trypsin in HBSS. The pituitaries were incubated at 37 °C for 2 hours, followed by trituration with a siliconized P1000 tip. The pituitaries were incubated at 37 °C for 2 additional hours, followed by trituration with a siliconized P200 tip. The dispersed cells were collected by a 5-minute centrifugation at 600 relative centrifugal force and resuspended in 500 µL of PBS and 2 µL DAPI. For fluorescence-activated cell sorting (FACS), the cells were sorted on a Synergy cell sorter with a 488 nm laser. Samples were either taken immediately for RNA or placed in 200 μL RNA Later (Invitrogen AM 7020).
RNA Extraction, cDNA Conversion and Quantitative PCR
RNA was extracted from cells or cell lines using either the RNAqueous Micro Kit (Invitrogen AM1931) or the Qiagen RNeasy Micro Kit (74004), and cDNA was made from the RNA using SuperScript First Strand (Invitrogen, 11904-018). Transcripts were quantified using the TaqMan Universal PCR Master Mix (Applied Biosystems 4304437), and TaqMan probes for Cga (probe Mm00438189_m1), Gata2 (probe Mm00492300_m), Hprt (probe Mm03024075_m1), Lhx3, (probe Mm01333633_m1), Pitx1 (probe Mm00440824_m1), Pou1f1 (probe Mm00476852_m1), Prop1 (probe Mm00839471_m1), Sox2 (probe Mm03053810_s1), and Tshb (probe Mm03990915_g1). The quantitative PCR (qPCR) reaction conditions were 2 initial hold stages of 50 °C for 2 minutes and 95 °C for 10 minutes followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute.
Tissue Culture
GHFT1 cells were provided by Pamela Mellon, University of California San Diego (5).
Tumorous pituitaries were dispersed by mincing and placing in a mix of HBSS (Gibco, 14175-095) with 10 mg/mL collagenase (Gibco, 17104-019) at 37 °C for 30 minutes. Cells were collected by centrifugation at 600g for 5 minutes, followed by resuspension in cell culture media. All subsequent cell culture was done in Dulbecco’s Modified Eagle Medium (Gibco, 11995-065) with 10% Fetal Bovine Serum (Corning 35016CV), 1% Penicillin Streptomycin (Sigma-Aldrich P4333), 10 μg/mL hEGF (Gibco, PHG0314), and 1X ITS (Corning MT25800CR).
Panning was done by collecting the media (without trypsin) every 2 days, collecting the cells by centrifugation, resuspending in fresh media, and plating on a new, non-Matrigel-coated plate. After 2 weeks of panning, the cells were plated on Matrigel until 50% to 80% confluence was achieved. A sample of the heterogenous population of cells was collected for RNA analysis. Clonal cell lines were developed via limited dilution.
We passaged clonal cell lines when they achieved ~80% confluence by a wash of the cells, trypsinization (Invitrogen 25300054) for 3 minutes at 37 °C, collection with media, centrifugation, resuspension, and plating on Matrigel-covered plates at approximately a 1:10 dilution.
Results
Constructing Mice With an Inducible Knock-In of SV40 at the Rosa26 Locus
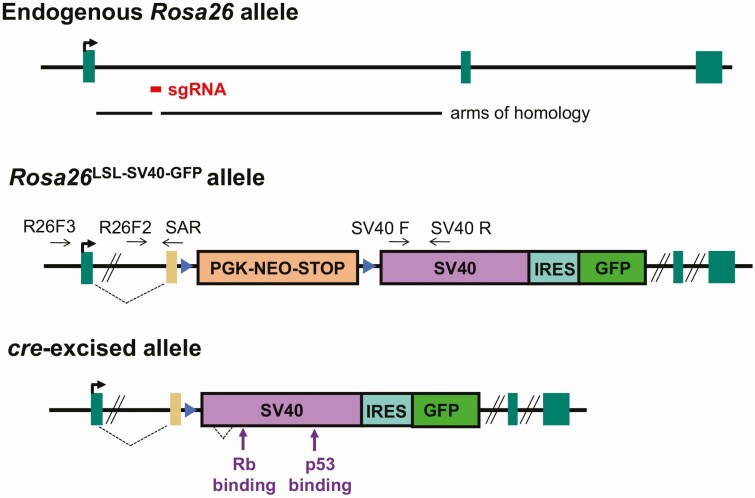
Our objective was to create a mouse line with inducible oncogene expression and a fluorescent marker for tracking induction and generating immortalized cell lines. We chose to use the SV40 large and small T antigens (SV40 TAg) as the oncogene, given their ability to immortalize cells with high penetrance and their track record of successful transformation (40). We selected the Rosa26 locus for targeting because it has nearly ubiquitous expression (including in the pituitary), is a nonessential gene, and is optimized for targeting. We used a targeting vector (pR26 GFP Dest) that had arms of homology with the first intron of the Rosa26 locus (35). It contains a splice acceptor sequence, a floxed stop sequence, an internal ribosome entry site (IRES), and a green fluorescent protein (GFP) coding region. We used the gateway recombination method to add the large and small SV40 T antigen coding sequences to this vector (Fig. 1). This vector was injected into fertilized eggs together with CRISPR-Cas9 and the previously described short guide RNA, sgRosa26-1, to produce transgenic mice (35). Genomic DNA of potential founder mice was genotyped by PCR. The knock-in was highly efficient, as 16 of 79 (20%) potential founders had the correct allele, verified by PCR amplification and DNA sequencing.
Figure 1.
Development of Rosa26LSL-SV40-GFP mice. The endogenous Rosa26 allele is shown with the arms of homology in the targeting vector and the location targeted by the small guide RNA (sgRNA). Rosa26LSL-SV40-GFP knock-in allele contains the Pgk-neo stop sequence flanked by loxP sites and the downstream SV40 T antigen coding region, internal ribosome entry site (IRES), and green fluorescent protein (GFP) coding region. The new allele was characterized by PCR with a primer outside of the targeting vector (R26F3) and a primer complimentary to the gene trap splice acceptor sequence (SAR). Subsequent genotyping utilized the primer pairs R26F2 and SAR and SV40 forward and reverse (SV40F, SV40R). After cre-mediated excision of the stop sequence, SV40 and GFP will be expressed from the Rosa26 regulatory sequences.
Cre-Recombination Results in Dwarfed Mice
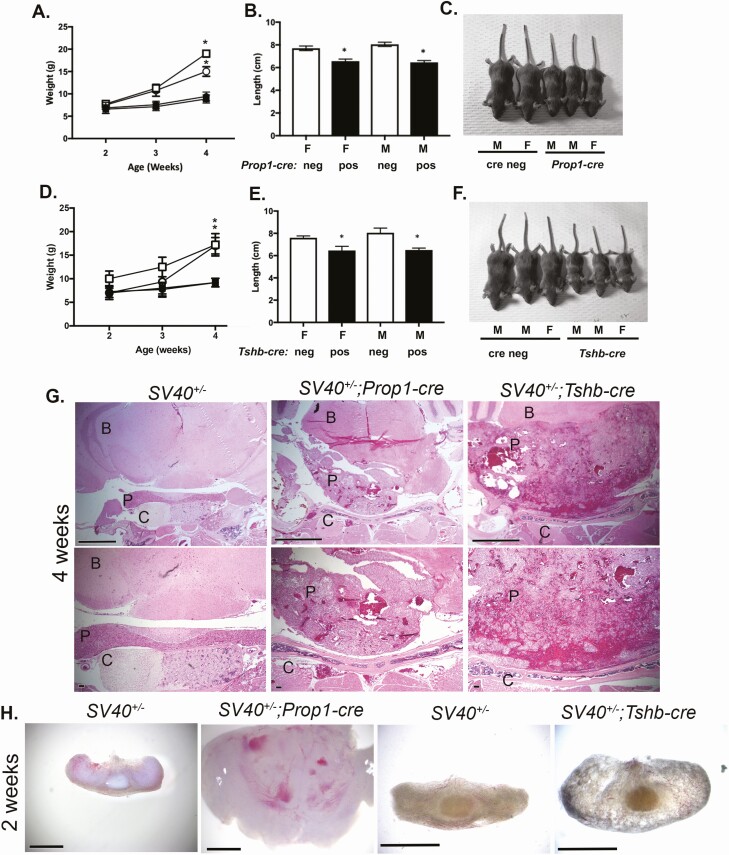
We intercrossed the new Rosa26LSL-SV40-GFP/+ strain to produce homozygotes, which appeared normal and fertile. We selected 2 cre transgenic lines, Prop1-cre and Tshb-cre, to cross with the Rosa26LSL-SV40-GFP homozygotes. Prop1-cre genetically labels a few pituitary cells in Rathke’s pouch at e11.5 and completely labels cells in the anterior and intermediate lobes by e12.5 (31). Tshb-cre labels thyrotropes beginning at e14.5 (37). Both Rosa26LSL-SV40-GFP/+; Prop1-cre (hereafter referred to as SV40; Prop1-cre) and Rosa26LSL-SV40-GFP/+; Tshb-cre (hereafter referred to as SV40; Tshb-cre) mice and their littermates were weighed at 2, 3, and 4 weeks (Fig. 2A-F). There were no significant weight differences among the genotypes at 2 weeks, but both male and female SV40; Prop1-cre mice had immature faces typical of hypopituitarism and head enlargement not observed with other genotypes. By 4 weeks all of the SV40; Prop1-cre mice (N = 13) and all of the SV40; Tshb-cre (N = 12) exhibited dwarfism relative to their SV40, cre-negative littermates (N = 12 and N = 7, respectively). The body lengths of SV40; Prop1-cre and SV40; Tshb-cre mice were significantly less than their SV40, cre-negative littermates. Males and females were equally affected with reduced weight and length. Most of the SV40; Prop1-cre and SV40; Tshb-cre mice had enlarged heads at 4 weeks.
Figure 2.
Induction of SV40 TAg expression with Prop1-cre and Tshb-cre causes dwarfism and large pituitary tumors by 4 weeks. A, Weights of SV40; Prop1-cre (N = 7 female, N = 6 male, black symbols) and SV40 positive, cre negative littermates (N = 8 female, N = 4 male, white symbols) at 2, 3, and 4 weeks. Males and females are indicated with the square and circle symbols, respectively. B, Lengths of female (F) and male (M) mice (nose to base of tail) were measured at 4 weeks. White bars are SV40 positive, cre negative and black bars are SV40; Prop1-cre animals. C, Sample images of male (M) and female (F) SV40 positive, cre negative and SV40; Prop1-cre littermates at 4 weeks. D, Weights of SV40; Tshb-cre mice (N = 6 female, N = 6 male black symbols) and SV40 positive, cre negative littermates (N = 3 female, N = 4 male, white symbols) at 2, 3, and 4 weeks. Males and females are indicated with the square and circle symbols, respectively. E, Lengths of female (F) and male (M) mice (nose to base of tail) measured at 4 weeks. White bars are SV40 positive, cre negative and black bars are SV40; Tshb-cre animals. F, Sample images of male (M) and female (F) SV40 positive, cre negative and SV40; Tshb-cre littermates at 4 weeks. G, H&E staining revealed abnormal pituitary histology of 4-week-old SV40; Prop1-cre and SV40; Tshb-cre mice compared with SV40 positive, cre negative littermates (N = 3/genotype). The brain (B), pituitary (P), and cartilage (C) are labeled. Top panel magnification is 50× and scale bar is 1000 μm. Bottom panel magnification is 100× and scale bar is 100 μm. H, Brightfield images of whole pituitaries from 2-week-old SV40 positive, cre negative, SV40; Prop1-cre, and SV40; Tshb-cre mice (N = 3/genotype). SV40; Prop1-cre and control pituitary magnification is 32× with 1000 μm and SV40; Tshb-cre and control pituitary magnification is 50× with a 1000 μm scale bar.
Mice Expressing SV40 T Antigen in the Pituitary Gland Have Large Tumors at 4 Weeks
Mice of both the SV40; Prop1-cre and the SV40; Tshb-cre genotypes had large pituitary masses with abnormal blood accumulation and vascularization at 4 weeks of age (Fig. 2G). At 2 weeks, the pituitaries of SV40; Prop1-cre mice were large (n = 3) (Fig. 2H). There was some variability in the degree of enlargement and vascularization of SV40; Tshb-cre pituitaries at 2 weeks, but they were all significantly enlarged relative to littermate controls (N = 3). No sex differences were noted for either cross.
SV40; Prop1-cre Mice Have Early Onset Hyperplasia
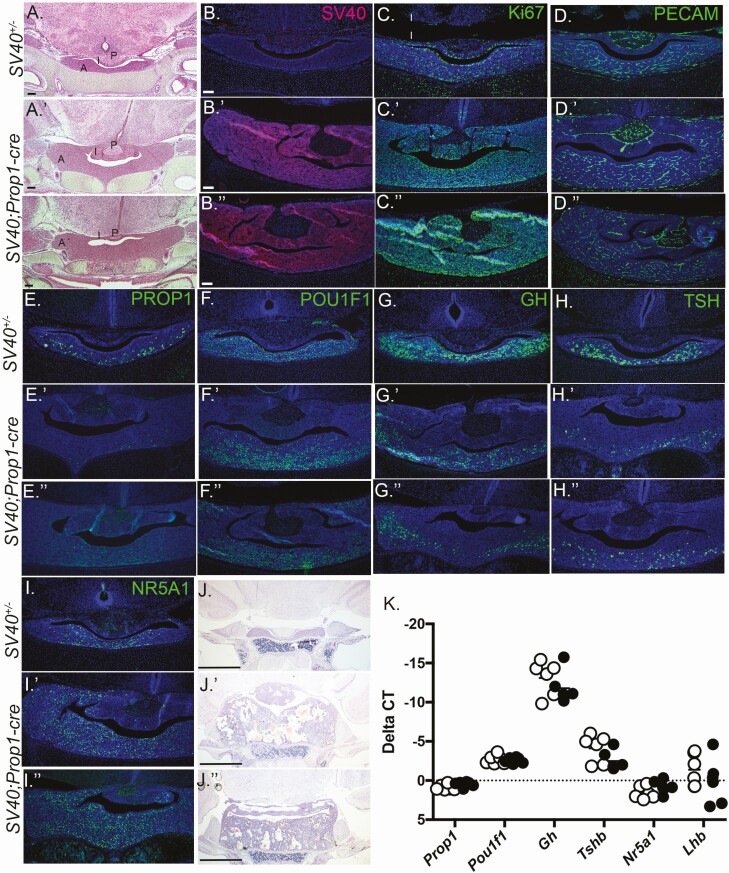
We examined pituitaries at birth by histology and immunostaining (N = 3/genotype). Newborn SV40; Prop1-cre mice had hyperplastic pituitaries (Fig. 3A-3D). Both the intermediate and anterior lobes were enlarged and stained broadly for SV40 T antigen. No SV40 staining was detectable in SV40; cre-negative littermate controls. In control pituitaries, immunostaining for the proliferation marker, Ki67, was enriched in the cells in the marginal zone and in cells scattered throughout the anterior lobe, as expected. In contrast, nearly every cell in the SV40; Prop1-cre pituitaries was positive for Ki67, consistent with the pituitary enlargement. CD31 or platelet endothelial cell adhesion molecule (PECAM) immunostaining did not reveal significant changes in vascularization between the controls and the SV40; Prop1-cre mutants at this age.
Figure 3.
SV40, Prop1-cre mice exhibit pituitary hyperplasia at birth. Coronal sections of P1 pituitaries from SV40 positive, cre negative littermates (A-I) and SV40; Prop1-cre mice (A′-I′′) were stained with H&E and various antibodies (N = 3/genotype). (A, A′, A′′) H&E staining at 50× magnification with a 100 μm scale bar. Immunostaining for SV40 (B, B′, B′′), Ki67 (C, C′, C′′), PROP1 (D, D′, D′′), POU1F1 (E, E′, E′′), GH (F, F′, F′′), TSH (G, G′, G′′), and NR5A1 (I, I′, I′′). DAPI staining (blue) of cell nuclei. B-I′′ magnification is 100× with 100 μm scale bar. Coronal sections of P10 pituitaries from SV40 positive, cre negative (J) and SV40; Prop1-cre (J′, J′′) stained with H&E, (N = 3 individuals/genotype), magnification is 50× with 100 μm scale bars (J-J′′). RNA prepared from P1 pituitary glands was used to synthesize cDNA and PCR amplified with probes specific for Prop1, Pou1f1, Gh, Tshb, Nr5a1, and Lhb. Open circles indicate SV40 positive, cre negative animals and black filled circles indicate SV40; Prop1-cre mice (N = 6 individuals/genotype).
To assess effects of early hyperplasia on gene expression we carried out immunostaining and qRT-PCR for lineage-specific transcription factors and hormones (Fig. 3E-K). PROP1 immunostaining is detectable in the cytoplasm of cells scattered throughout the parenchyma of the anterior lobe of control newborn mice, but very little to no PROP1 immunostaining was visible in the SV40; Prop1-cre mice. POU1F1 immunostaining was similar in control and SV40; Prop1-cre mice. GH and TSH immunostaining were reduced in the SV40; Prop1-cre mice relative to controls. Normally, NR5A1 staining is enriched in the midline and ventral aspect of the pituitary gland, but NR5A1 immunostaining was increased and laterally expanded in the SV40; Prop1-cre anterior pituitary gland. Hematoxylin and eosin staining revealed that the hyperplasia of the anterior and intermediate lobes became even more severe by P10. At birth, we saw no significant differences in mRNA levels of Prop1, Pou1f1, Nr5a1, Gh, or Lhb.
Onset of Hyperplasia and Effects on Gene Expression in SV40; Tshb-cre Mice
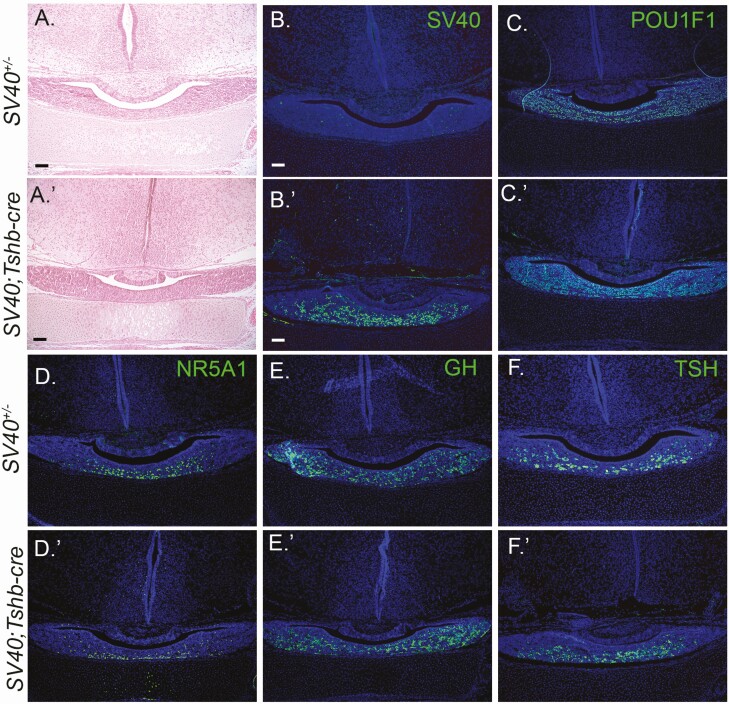
The pituitaries of SV40; Tshb-cre mice were indistinguishable from their SV40; cre-negative littermates at birth by H&E staining (N = 3/genotype, Fig. 4). SV40 immunostaining was present in the ventral parenchyma of SV40; Tshb-cre mice, as expected, because this is where most thyrotropes are found. No SV40 staining was detected in SV40; cre-negative littermate pituitaries. Immunostaining for POU1F1, GH, TSH, and NR5A1 was indistinguishable between genotypes.
Figure 4.
SV40; Tshb-cre mice have normal pituitary morphology and cell specification at birth. Coronal sections of P1 pituitaries from SV40 positive, cre negative (A-F) and SV40; Tshb-cre mice (A′-F′) were stained with H&E and various antibodies (N = 3 individuals/genotype). The magnification is 100× with 100 μm scale bar. H&E staining (A, A′) and immunostaining for SV40 (B, B′), POU1F1 (C, C′), NR5A1 (D, D′), GH, (E, E′), and TSH (F, F′). DAPI staining (blue) of cell nuclei B-F′.
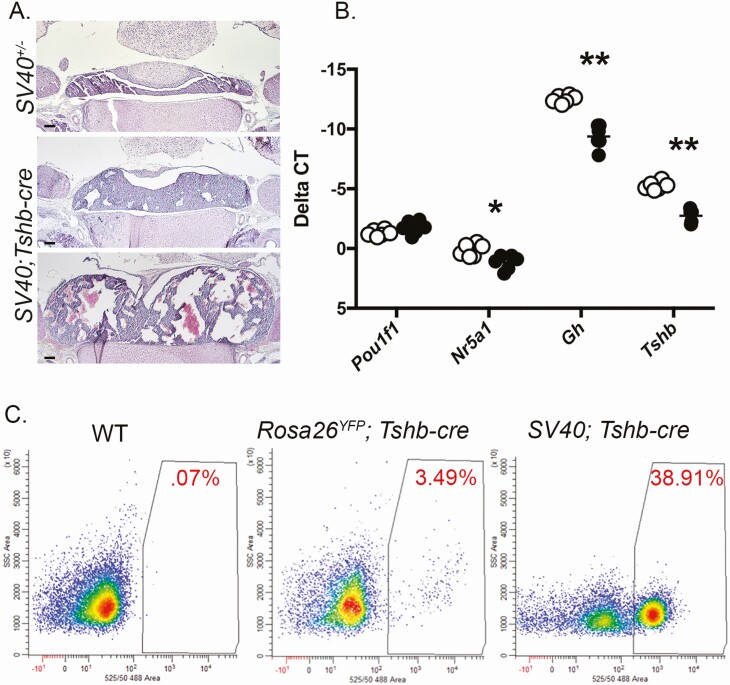
At P10, the SV40; Tshb-cre mice had consistently larger pituitaries with varying abnormalities associated with oncogenesis, including evidence for increased vascularization and large, acellular spaces (Fig. 5A). Thus, hyperplasia and oncogenesis occurred between P1 and P10. We carried out qRT-PCR at P7 to assess the effect of hyperplasia on differentiation and hormone production (N = 6/genotype) (Fig. 5B). There was no difference in Pou1f1 transcripts, but both Gh and Tshb transcripts were reduced in SV40; Tshb-cre mice relative to SV40; cre-negative animals (P < 0.01). Nr5a1 transcript levels were also reduced (P < 0.05).
Figure 5.
SV40; Tshb-cre pituitaries have altered gene expression postnatally. A, Coronal sections of P10 pituitaries from SV40 positive, cre negative and SV40; Tshb-cre littermates stained with H&E, (N = 3 individuals/genotype) magnification is 50× with 100 μm scale bars. B, Transcripts for Pou1f1, Nr5a1, Gh, and Tshb were measured by qRT-PCR in pituitaries from P7 SV40 positive, cre negative mice (open circles) and SV40; Tshb-cre littermates (black circles), N = 6 individuals/genotype. The statistical significance is indicated as * for P < 0.05 and ** for P < 0.01. C, Representative fluorescent-activated cell sorting of pituitary cell dispersions from P14 wild-type (WT), Gt(ROSA)26Sortm1(EYFPCos); Tshb-cre (RosaYFP; Tshb-cre), and SV40; Tshb-cre mice. The percentage of fluorescent cells is indicated in red.
FACS Sorting of Genetically Marked Cells
The IRES-GFP expression cassette in the Rosa26LSL-SV40-GFP/+ mice made it feasible to flow sort cells derived from cre-mediated excision of the stop sequence. Wild-type mice contain very few auto-fluorescent pituitary cells (Fig. 5C). Mice with genetically labeled thyrotropes were generated by crossing Tshb-cre mice with the cre-reporter strain, Gt(ROSA)26Sortm1(EYFP)Cos (Rosa26YFP). Pituitaries of 2-week-old mice of both Rosa26YFP; Tshb-cre and SV40; Tshb-cre mice were dispersed and subjected to FACS to serve as a control for the normal number of genetically labeled thyrotropes. Two different tumors of SV40; Tshb-cre mice were collected at 2 weeks of age and subjected to FACS. Approximately 3.5% of the Rosa26YFP; Tshb-cre cells had high levels of fluorescence, similar to the expected value of ~5% (41). The SV40; Tshb-cre pituitaries had a significantly higher number of fluorescent cells, ~ 40%. The fluorescent cells had Tshb transcripts, but the non-fluorescent cells did not (data not shown). Nearly all of the cells from a 2-week-old SV40; Prop1-cre pituitary had strong green fluorescence (data not shown), consistent with the expectation that all hormone-producing cells in the pituitary gland are derived from Prop1-expressing cells (31).
Developing Immortalized Cell Lines
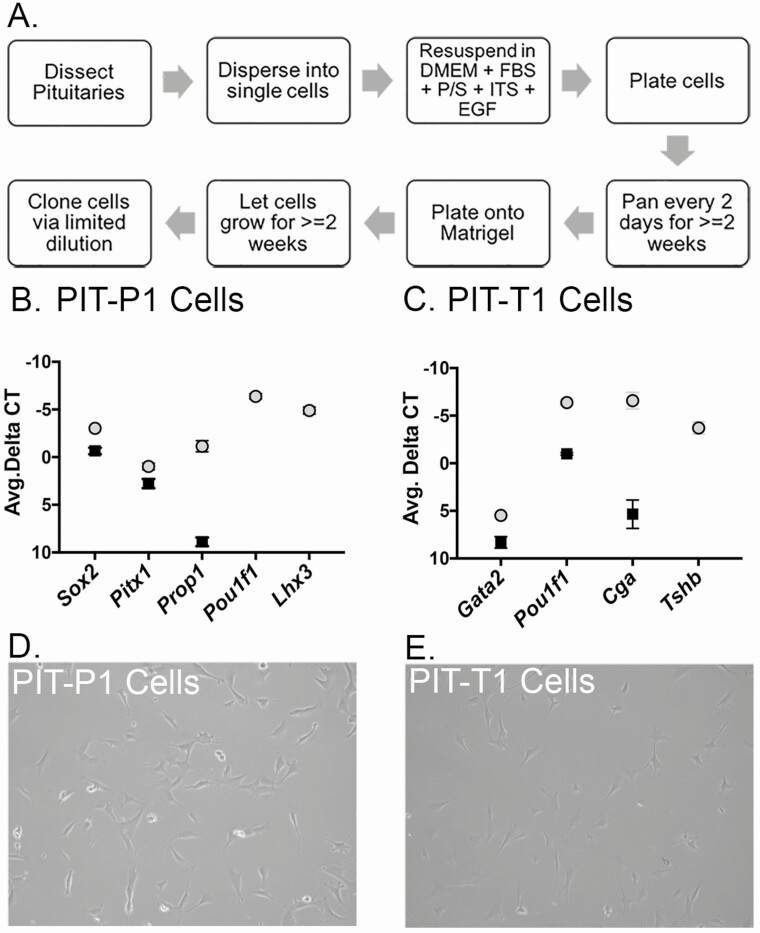
Single pituitary tumors were dissected from a 4-week-old SV40; Prop1-cre and a SV40; Tshb-cre mouse and dispersed separately into single cells in cell culture media (Fig. 6A). These heterogeneous cell populations were panned separately for 2 weeks to reduce fibroblast contamination. After 2 weeks, the cells from each tumor were collected and plated onto Matrigel. Once the cells reached 50% confluence, the cells were collected and cloned via limited dilution into a 96-well plate. The cells were left for 2 weeks in the plate and then transferred to 24-well and 6-well plates, respectively, as the cells grew confluent. Several clonal cell lines were derived from a tumor from a SV40; Prop1-cre mouse and a tumor from a SV40; Tshb-cre mouse.
Figure 6.
Characterization of immortalized cell lines by gene expression profiling. A, Protocol for generating clonal cell lines from SV40-induced tumors. B, RNA from SV40 immortalized Pit-P1 cells (black squares) and P4 pituitary glands (light gray circles) was analyzed for expression of Prop1, Sox2, Pitx1, Pou1f1, Lhx3, and Hprt using qRT-PCR. Values were normalized to Hprt. Assays were performed in triplicate on 6 independent batches of RNA. Pou1f1 and Lhx3 were not detectable in the Pit-P1 cells. C, RNA from SV40 immortalized Pit-T1 cells (black squares) and P4 pituitary glands (light gray circles) was analyzed for expression of Gata2, Pou1f1, Cga, Tshb, and Hprt using qRT-PCR. Values were normalized to Hprt. Assays were performed in triplicate on 6 independent batches of RNA. Tshb was not detectable in the Pit-T1 cells. D, Brightfield image of the PIT-P1 cell line. E, Brightfield image of the PIT-T1 cell line.
We tested the cell lines for expression of several key genes using qRT-PCR (N = 6), using Hprt as an expression control, and wild-type P4 pituitary gland RNA as a positive control (N = 6). The precursor cell line (PIT-P1) expressed detectable levels of Sox2, Pitx1, and Prop1. No expression of Pou1f1, or Lhx3 was detected in PIT-P1 cells (Fig. 6B). The thyrotrope-like cell line (PIT-T1) had detectable expression of Gata2, Pou1f1, and Cga, but no expression of Tshb was detected (Fig. 6C). The morphology of these 2 cell types is subtly different, with the PIT-P1 line having less pronounced projections (Fig. 6D) in comparison with the PIT-T1 line (Fig. 6E).
Discussion
We have developed a mouse line that conditionally expresses the powerful SV40 T antigen oncogene from the Rosa26 safe harbor locus. We demonstrate the power of this novel mouse line by mating it to 2 distinct pituitary-specific cre strains. Both Prop1-cre and Tshb-cre induced reproducible, high penetrance, cell-type specific induction of SV40 T antigen expression and tumor formation. We successfully adapted tumors from each of these crosses to cell culture, and they retained expression of some markers specific to pituitary progenitors and thyrotrope cells. This demonstrates the utility of this new line as a tool for immortalizing stable cell lines that represent discrete stages of development.
We observed high penetrance and rapid onset of pituitary hyperplasia and tumors in the SV40; Prop1-cre and SV40; Tshb-cre mice. Pituitary hyperplasia was consistently evident in newborn SV40; Prop1-cre mice, and variable degrees of oncogenesis were evident at P10. The onset of hyperplasia was slightly later in SV40; Tshb-cre mice. Pituitaries appeared normal at birth, hyperplasia was obvious at 2 weeks, and extensive oncogenesis was obvious in all of the mice at 4 weeks. Founder mice containing fusions of the regulatory elements of Pou1f1, Cga, Lhb, or Gh and the coding region of SV40 were infertile or died before they could be bred (4, 5, 7). The surviving founders developed tumors at between 4 weeks and 5 months, and the penetrance varied from ~ 6% to 20%. The binary transgene system we developed circumvents the problems of infertility and sudden death, and it makes it possible to study the path from hyperplasia to oncogenesis in progeny of the same strain. We hypothesize that the high penetrance and rapid latency of oncogenesis we observed in the binary system is the result of 2 factors: small amounts of Cre-recombinase are sufficient for elimination of the floxed stop sequence, and the Rosa26 locus drives consistent and ample expression of SV40 to drive hyperplasia and oncogenesis. This contrasts with random integration of SV40 transgenes into the genome, which is subject to position effects that commonly diminish expression.
An added benefit of having inserted the SV40 T antigen into the Rosa26 locus is the ability to dissect the effects of SV40 expression under different induction conditions. The SV40; Prop1-cre mice consistently had earlier hyperplasia and oncogenesis than the SV40; Tshb-cre mice. The Prop1-cre allele induces cre-mediated expression by e12.5 in undifferentiated, proliferating progenitor cells, while Tshb-cre does not become active until e14.5 in cells that have normally exited the cell cycle and committed to the thyrotrope lineage (31, 37). The earlier onset of hyperplasia in Prop1-cre mice may be attributable to the activation of oncogene expression prior to cell cycle exit.
SV40 expression prevents cells from leaving the cell cycle. This was made clear by the continued, broad expression of Ki67 in the SV40; Prop1-cre mice, and the pituitary enlargement noted in both of the mouse lines. While it is generally believed that cells must exit the cell cycle to differentiate, SV40; Prop1-cre mouse pituitaries have significant expression of lineage-specific transcription factors (including POU1F1 and NR5A1) and hormones. This phenotype of persistent proliferation and differentiation is reminiscent of p27 and p57 double knockout mice. Embryonic pituitaries from p27, p57 knockout mice reveal cycling cells positive for both POMC and TBX19 (TPIT), suggesting that cell cycle exit is not absolutely critical for differentiation (42).
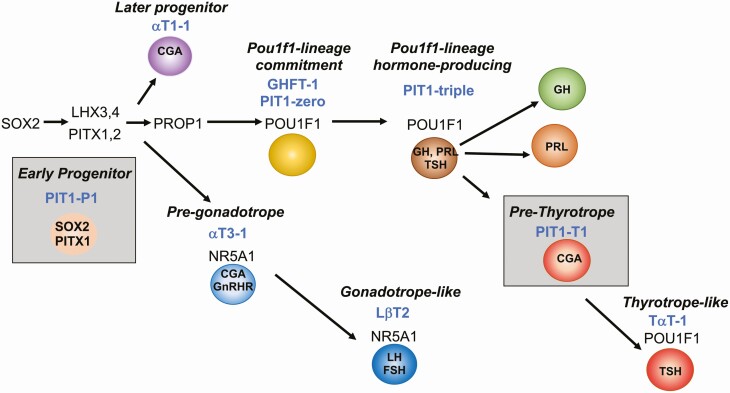
Existing immortalized pituitary cell lines represent distinct time points in development (Fig. 7) (4). We were successful in developing stable, clonal cell lines from SV40; Prop1-cre mice and SV40; Tshb-cre mice that represent pituitary precursors (PIT-P1 cells), and pre-thyrotropes (PIT-T1 cells) respectively. The PIT-P1 cells we report here are the first immortalized, SOX2-positive pituitary precursor cell line. The expression of PITX1 indicates commitment to the pituitary fate, and these cells will be useful for understanding the first steps in that process. The PIT-T1 cells are a pre-thyrotrope-like cell line that is characterized by the expression of POU1F1, and CGA. Although Tshb expression was not detected, reduction in SV40 expression might permit these progenitors to differentiate (43). Nevertheless, they provide an excellent companion cell line for comparison with the more differentiated TαT-1 cells, just as comparison of αT3-1 and LβT-2 cells have revealed important genetic changes as gonadotrope differentiation progresses (Fig. 7). It is possible that the binary system we developed could give rise to additional cell lines that represent different times in development if tumors were adapted to culture from younger mice. In support of this idea, the Mellon and Liebhaber groups have generated multiple cell lines with different features from the tumors of different transgene founders (4, 7).
Figure 7.
PIT-P1 and PIT-T1 cells represent 2 new niches within the pituitary differentiation cascade. Representation of transcription factors regulating pituitary growth and differentiation and the stages represented by commonly used immortalized cell lines. The PIT-P1 cell line represents a Sox2- and Pitx1-expressing pituitary precursor, whereas PIT-T1 cells represents a pre-thyrotrope expressing Pou1f1 and Cga.
These mice represent a significant advance for two reasons: (1) they are a powerful tool for the rapid immortalization of cell lines; and (2) they allow for careful dissection of the effect of SV40 T antigen-mediated immortalization on proper differentiation and organ function during the process of oncogenesis. The binary system is universally applicable because Rosa26LSL-SV40-GFP/+ mice can be mated with any cre strain, including drug-inducible cre strains. A strain with universal potential was previously developed that expresses a heat-labile copy of SV40 oncogene driven by the H-2Kb promoter, and tissues incubated at 33 °C in the presence of interferon-γ are immortalized at high frequency (44). A binary system with heat-labile SV40 oncogene is an intriguing future direction. Similarly, it is valuable to have a universal system in which the oncogene can be extinguished after immortalization has taken place. Such a system was developed in which flp recombinase can extinguish SV40 T antigen expression (45). In the meantime, these mice will help to characterize rare cell types being discovered at increasing frequency with single-cell technology. Our ability to generate immortalized cell lines is now only limited by the availability of cre alleles.
Acknowledgments
We thank Thom Saunders, Wanda Filipiak, and Galina Gavrilina of the Transgenic Animal Model Core for assistance with the gene targeting (NIH P30CA046592). We thank Pamela Mellon for advice on adaptation of tumors to cell culture. We thank Michelle Brinkmeier for the Rosa26YFP; Tshb-cre mice. We thank Owen Funk, Ken Kwan, Rachna Sridhar, and Lindsey A. Dudley for their contributions to the work.
Financial Support: NIH R01 HD097096, HD034283 (to S.A.C.), NIH T32GM007544 and T32HG000040 (to A.Z.D.), and the Japan Society for the Promotion of Science Overseas Fellowship (to H.B.).
Glossary
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- e#
embryonic day #
- FACS
fluorescence-activated cell sorting
- FSH
follicle-stimulating hormone
- GFP
green fluorescent protein
- GH
growth hormone
- GnRH
gonadotropin-releasing hormone
- H&E
hematoxylin and eosin
- IRES
internal ribosome entry site
- LH
luteinizing hormone
- P#
postnatal day #
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PECAM
platelet endothelial cell adhesion molecule
- qPCR
quantitative polymerase chain reaction
- TSH
thyrotropin (thyroid-stimulating hormone)
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Zhu Z, Cui W, Zhu D, Gao N, Zhu Y. Common tools for pituitary adenomas research: cell lines and primary cells. Pituitary. 2020;23(2):182-188. [DOI] [PubMed] [Google Scholar]
- 2. Ahuja D, Sáenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24(52):7729-7745. [DOI] [PubMed] [Google Scholar]
- 3. Manfredi JJ, Prives C. The transforming activity of simian virus 40 large tumor antigen. Biochim Biophys Acta. 1994;1198(1):65-83. [DOI] [PubMed] [Google Scholar]
- 4. Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122(10):3319-3329. [DOI] [PubMed] [Google Scholar]
- 5. Lew D, Brady H, Klausing K, et al. GHF-1-promoter-targeted immortalization of a somatotropic progenitor cell results in dwarfism in transgenic mice. Genes Dev. 1993;7(4):683-693. [DOI] [PubMed] [Google Scholar]
- 6. Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5(1):1-10. [DOI] [PubMed] [Google Scholar]
- 7. Sizova D, Ho Y, Cooke NE, Liebhaber SA. Research resource: T-antigen transformation of pituitary cells captures three novel cell lines in the Pit-1 lineage. Mol Endocrinol. 2010;24(11):2232-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Windle JJ, Weiner RI, Mellon PL. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4(4):597-603. [DOI] [PubMed] [Google Scholar]
- 9. Bilezikjian LM, Corrigan AZ, Blount AL, Chen Y, Vale WW. Regulation and actions of Smad7 in the modulation of activin, inhibin, and transforming growth factor-beta signaling in anterior pituitary cells. Endocrinology. 2001;142(3):1065-1072. [DOI] [PubMed] [Google Scholar]
- 10. Fowkes RC, Burch J, Burrin JM. Stimulation of extracellular signal-regulated kinase by pituitary adenylate cyclase-activating polypeptide in alpha T3-1 gonadotrophs. J Endocrinol. 2001;171(3):R5-10. [DOI] [PubMed] [Google Scholar]
- 11. McGillivray SM, Bailey JS, Ramezani R, Kirkwood BJ, Mellon PL. Mouse GnRH receptor gene expression is mediated by the LHX3 homeodomain protein. Endocrinology. 2005;146(5):2180-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navratil AM, Bliss SP, Berghorn KA, et al. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J Biol Chem. 2003;278(34):31593-31602. [DOI] [PubMed] [Google Scholar]
- 13. Xie H, Hoffmann HM, Iyer AK, et al. Chromatin status and transcription factor binding to gonadotropin promoters in gonadotrope cell lines. Reprod Biol Endocrinol. 2017;15(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breen KM, Thackray VG, Coss D, Mellon PL. Runt-related transcription factors impair activin induction of the follicle-stimulating hormone {beta}-subunit gene. Endocrinology. 2010;151(6):2669-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cherrington BD, Bailey JS, Diaz AL, Mellon PL. NeuroD1 and Mash1 temporally regulate GnRH receptor gene expression in immortalized mouse gonadotrope cells. Mol Cell Endocrinol. 2008;295(1-2):106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21(5):1175-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasson R, Luu SH, Thackray VG, Mellon PL. Glucocorticoids induce human glycoprotein hormone alpha-subunit gene expression in the gonadotrope. Endocrinology. 2008;149(7):3643-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thackray VG, Mellon PL. Synergistic induction of follicle-stimulating hormone beta-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology. 2008;149(3):1091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakajima Y, Yamada M, Taguchi R, et al. NR4A1 (Nur77) mediates thyrotropin-releasing hormone-induced stimulation of transcription of the thyrotropin β gene: analysis of TRH knockout mice. PLoS One. 2012;7(7):e40437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janssen JS, Sharma V, Pugazhenthi U, Sladek C, Wood WM, Haugen BR. A rexinoid antagonist increases the hypothalamic-pituitary-thyroid set point in mice and thyrotrope cells. Mol Cell Endocrinol. 2011;339(1-2):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aninye IO, Matsumoto S, Sidhaye AR, Wondisford FE. Circadian regulation of Tshb gene expression by Rev-Erbα (NR1D1) and nuclear corepressor 1 (NCOR1). J Biol Chem. 2014;289(24):17070-17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Day RN, Voss TC, Enwright JF III, Booker CF, Periasamy A, Schaufele F. Imaging the localized protein interactions between Pit-1 and the CCAAT/enhancer binding protein alpha in the living pituitary cell nucleus. Mol Endocrinol. 2003;17(3):333-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann HM, Gong P, Tamrazian A, Mellon PL. Transcriptional interaction between cFOS and the homeodomain-binding transcription factor VAX1 on the GnRH promoter controls Gnrh1 expression levels in a GnRH neuron maturation specific manner. Mol Cell Endocrinol. 2018;461:143-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Longo KM, Sun Y, Gore AC. Insulin-like growth factor-I effects on gonadotropin-releasing hormone biosynthesis in GT1-7 cells. Endocrinology. 1998;139(3):1125-1132. [DOI] [PubMed] [Google Scholar]
- 25. Nelson SB, Lawson MA, Kelley CG, Mellon PL. Neuron-specific expression of the rat gonadotropin-releasing hormone gene is conferred by interactions of a defined promoter element with the enhancer in GT1-7 cells. Mol Endocrinol. 2000;14(9):1509-1522. [DOI] [PubMed] [Google Scholar]
- 26. Andoniadou CL, Matsushima D, Mousavy Gharavy SN, et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13(4):433-445. [DOI] [PubMed] [Google Scholar]
- 27. Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105(8):2907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizzoti K, Akiyama H, Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell. 2013;13(4):419-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis SW, Castinetti F, Carvalho LR, et al. Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Mol Cell Endocrinol. 2010;323(1):4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perez Millan MI, Brinkmeier ML, Mortensen AH, Camper SA. PROP1 triggers epithelial-mesenchymal transition-like process in pituitary stem cells. Elife. 2016;5:e14470. Published online June 28, 2016. doi:10.7554/eLife.14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davis SW, Keisler JL, Pérez-Millán MI, Schade V, Camper SA. All hormone-producing cell types of the pituitary intermediate and anterior lobes derive from prop1-expressing progenitors. Endocrinology. 2016;157(4):1385-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ozone C, Suga H, Eiraku M, et al. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun. 2016;7:10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suga H, Kadoshima T, Minaguchi M, et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480(7375):57-62. [DOI] [PubMed] [Google Scholar]
- 34. Zimmer B, Piao J, Ramnarine K, Tomishima MJ, Tabar V, Studer L. Derivation of diverse hormone-releasing pituitary cells from human pluripotent stem cells. Stem Cell Reports. 2016;6(6):858-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chu VT, Weber T, Graf R, et al. Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes. BMC Biotechnol. 2016;16:4. Published online January 16, 2016. doi:10.1186/s12896-016-0234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pease S, Saunders TL, International Society for Transgenic Technologies. Advanced protocols for animal transgenesis: an ISTT manual. Heidelberg; New York: Springer; 2011. [Google Scholar]
- 37. Castinetti F, Brinkmeier ML, Gordon DF, et al. PITX2 AND PITX1 regulate thyrotroph function and response to hypothyroidism. Mol Endocrinol. 2011;25(11):1950-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. Published online March 27, 2001. doi:10.1186/1471-213x-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mortensen AH, Schade V, Lamonerie T, Camper SA. Deletion of OTX2 in neural ectoderm delays anterior pituitary development. Hum Mol Genet. 2015;24(4):939-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mellon PL, Windle JJ, Weiner RI. Immortalization of neuroendocrine cells by targeted oncogenesis. Recent Prog Horm Res. 1991;47:69-93; discussion 93. [DOI] [PubMed] [Google Scholar]
- 41. Kulig E, Camper SA, Kuecker S, Jin L, Lloyd RV. Remodeling of hyperplastic pituitaries in hypothyroid us-subunit knockout mice after thyroxine and 1713-estradiol treatment: role of apoptosis. Endocr Pathol. 1998;9(3):261-274. [DOI] [PubMed] [Google Scholar]
- 42. Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol. 2009;29(7):1895-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chou JY. Differentiated mammalian cell lines immortalized by temperature-sensitive tumor viruses. Mol Endocrinol. 1989;3(10):1511-1514. [DOI] [PubMed] [Google Scholar]
- 44. Jat PS, Noble MD, Ataliotis P, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991;88(12):5096-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu X, Li L, Yu X, et al. CRISPR/Cas9-mediated reversibly immortalized mouse bone marrow stromal stem cells (BMSCs) retain multipotent features of mesenchymal stem cells (MSCs). Oncotarget. 2017;8(67):111847-111865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.