Abstract
Context
Huoxiangzhengqi oral liquid (HXZQ-OL), a traditional Chinese medicine formula, has antibacterial, anti-inflammation and gastrointestinal motility regulation effects.
Objective
The study investigates the anti-allergic activity and underlying mechanism of HXZQ-OL.
Materials and methods
IgE/Ag-mediated RBL-2H3 cells were used to evaluate the anti-allergic activity of HXZQ-OL (43.97, 439.7 and 4397 μg/mL) in vitro. The release of cytokines and eicosanoids were quantified using ELISA. RT-qPCR was used to measure the gene expression of cytokines. The level of intracellular Ca2+ was measured with Fluo 3/AM. Immunoblotting analysis was performed to investigate the mechanism of HXZQ-OL. In the passive cutaneous anaphylaxis (PCA), BALB/c mice (5 mice/group) were orally administrated with HXZQ-OL (263.8, 527.6 and 1055 mg/kg/d) or dexamethasone (5 mg/kg/d, positive control) for seven consecutive days.
Results
HXZQ-OL not only inhibited degranulation of mast cells (IC50, 123 μg/mL), but also inhibited the generation and secretion of IL-4 (IC50, 171.4 μg/mL), TNF-α (IC50, 88.4 μg/mL), LTC4 (IC50, 52.9 μg/mL) and PGD2 (IC50, 195.8 μg/mL). Moreover, HXZQ-OL suppressed the expression of IL-4 and TNF-α mRNA, as well as the phosphorylation of Fyn, Lyn and multiple downstream signalling proteins including MAPK and PI3K/NF-κB pathways. In addition, HXZQ-OL (527.5 mg/kg) attenuated the IgE-mediated PCA with 55% suppression of Evans blue exudation in mice.
Conclusions
HXZQ-OL attenuated the activation of mast cell and PCA. Therefore, HXZQ-OL might be used as an alternative treatment for allergic diseases.
Keywords: IgE-mediated mast cell activation, high-affinity IgE receptor, inflammatory mediators, passive cutaneous anaphylaxis
Introduction
Allergic diseases, such as asthma, allergic rhinitis, atopic dermatitis, food allergy and drug hypersensitivity, have a high prevalence in all age groups and have become a worldwide clinical health problem because of their serious consequences (Asher et al. 2006; Ring et al. 2014). High-affinity IgE receptor (FcεRI) expressed on the surface of mast cells that are the major effector cells of allergic diseases is a key point in IgE-mediated allergic reactions (Kraft and Kinet 2007). After mast cells are challenged with antigen, the aggregation of FcεRI liberates preformed and de novo synthesized mediators such as LTC4, PGD2, IL-4 and TNF-α that can induce an allergic inflammatory response in a wide variety of tissues and organs (Theoharides and Kalogeromitros 2006; Mukai et al. 2018; Yoo et al. 2019). Therefore, inhibiting the activation of IgE/Ag-stimulated mast cells is an important target for the development of novel anti-allergic drugs.
Signalling pathways relating to the degranulation of mast cells have been well studied (Kraft and Kinet 2007; Roth et al. 2008; Gilfillan and Rivera 2009). Aggregation of FcεRI activates receptor-proximal tyrosine kinases that initiate the downstream cascade and promote the phosphorylation of various key proteins such as PLCγ leading to the influx of Ca2+ eventually that is a key event in the degranulation of mast cells (Beaven et al. 1984). FcεRI crosslinking also induces activation of the MAPK, PI3K/NF-κB signalling pathways that in turn active arachidonic acid-associated enzymes which are responsible for the production of multiple pro-inflammatory mediators (Gilfillan and Tkaczyk 2006).
Thus far, there are no therapies that can cure allergic diseases completely. The treatment of allergic diseases includes clinically-prescribed mast cell stabilizers (e.g., disodium cromoglycate, tranilast and ketotifen fumarate), H1 receptors antagonists (e.g., cetirizine, diphenhydramine and loratadine) and immune suppressors (e.g., adrenal cortical hormones, dexamethasone and hydrocortisone) which have side effects such as drowsiness, dizziness, dry mouth and skin atrophy (Oppenheimer and Casale 2002; Schoepe et al. 2006). As such, there is inspiring research using herbal medicines that have multi-component, multi-target and multi-mechanism anti-allergic characteristics with few side effects (Wang et al. 2015). In addition, since herbal medicines are widely available and inexpensive, they could be valuable approaches for the treatment of allergic diseases (Man et al. 2018).
Huoxiangzhengqi oral liquid (HXZQ-OL) is a Chinese traditional patent medicine derived from huoxiangzhengqi formula, a famous traditional Chinese medicine recipe that has been used for more than a thousand years and is recorded in Pharmacopoeia of the People's Republic of China (Commission CP 2020). HXZQ-OL has been shown to possess a wide variety of pharmacological effects, including antibacterial, anti-inflammation and gastrointestinal motility regulation activities and has played a positive role in the treatment of heat wet cold and gastrointestinal disorder (He et al. 2006; Zhao et al. 2018). Interestingly, HXZQ-OL has also been used as an alternative medicine for the clinical management of allergic diseases, such as asthma, eczema and urticaria (Tan 1995; Tang 1998; Wan et al. 2000; Yu et al. 2005). In addition, the Chinese medicinal materials that make up HXZQ-OL exert anti-allergic effects. Orally administered Angelicae dahuricae (Fish. ex Hoffm.) Benth. et Hook. f. (Apiaceae) radix (200 mg/kg) suppresses the progression of AD induced by DNCB in BALB/c mice (Ku et al. 2017). Pinellia ternate (Thunb.) Makino (Araceae) and Citrus reticulata Blanco (Rutaceae) inhibit eosinophil infiltration and airway hyperresponsiveness by suppressing CCR3+ and Th2 cytokines production in the OVA-induced asthma model (Ok et al. 2009). Poria cocos F.A.Wolf (Polyporaceae) bark extract has the potential as an oral immune suppressor for the treatment of AD and FA through the generation and maintenance of regulatory T cells in an AhR-dependent manner (Bae et al. 2016). Aqueous extract of Magnolia officinalis Rehder & E. H. Wilson (Magnoliaceae) bark (0.1 and 1 g/kg) inhibits compound 48/80 induced systemic anaphylaxis and IgE/Ag-mediated passive cutaneous anaphylaxis (PCA) reaction, as well as the histamine release from rat peritoneal mast cells (RPMC) activated by compound 48/80 or IgE/Ag complex (Shin et al. 2001). However, the effect of HXZQ-OL on allergic reaction is still poorly understood and the underlying mechanism has not been investigated yet. Therefore, we designed this study to investigate the anti-allergic activity of HXZQ-OL and further explore its underlying mechanism using IgE/Ag-mediated RBL-2H3 cells and PCA in mice.
Materials and methods
Materials
Eagle’s minimal essential medium (EMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (GIBCO™, New York, NY, USA). Penicillin, streptomycin, p-nitrophenyl-N-acetyl-β-d-glucosaminide (PNAG), anti-DNP-IgE and pluronic F127 were obtained from Sigma-Aldrich (St. Louis, MO, USA). DNP-BSA was purchased from Biosearch Technologies (Petaluma, CA, USA). Fluo 3/AM was obtained from Solarbio (Beijing, China). ELISA kits for TNF-α and IL-4 were purchased from R&D Systems (Minneapolis, MN, USA). ELISA kits for LTC4 and PGD2 were obtained from SenBeiJia Biological Technology (Nanjing, China). Advanced cDNA synthesis kit for RT-qPCR was purchased from Bio-Rad Laboratories (Hercules, CA, USA). Nuclear and cytoplasmic protein extraction kit and BCA protein assay kit were purchased from Beyotime Biotechnology (Shanghai, China). Specific antibodies against Syk (ab40781), Fyn (ab125016), GAB2 (ab32365), AKT(ab28422), extracellular signal-regulated kinase 1/2 (ERK1/2, ab17942), p38 (ab170099), cytosolic phospholipase A2 (cPLA2, ab58375), 5-lipoxygenase (5-LO, ab169755), cyclooxygenase 2 (COX-2, ab15191), IKKα/β (ab178870), NF-κB p65 (ab16502), β-actin (ab115777), phospho-Fyn (ab182661), phospho-AKT (ab81283), phospho-p38 (ab4822), phospho-cPLA2 (ab53105), phospho-5-LO (ab30573) and phospho-IKKα/β (ab194528) were obtained from Abcam (Cambridge, UK). A specific antibody against PI3K (sc-1637) was procured from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Specific antibodies against Lyn (2796), PLCγ1 (2822), PKCδ (9616), c-Jun N-terminal kinase (JNK,9252), IκBα (4814), phospho-PLCγ1 (2821), phospho-PKCδ (2055), phospho-GAB2 (3881), phospho-PI3K p85/p55 (17366), phospho-ERK1/2 (4370), phospho-JNK (9255) and phospho-IκBα (2859) were obtained from Cell Signalling Technology (Boston, MA, USA). Specific antibodies against PLCγ2 (AF7738), phospho-Lyn (AF3119), phospho-Syk (AF8404) and phospho-PLCγ2 (AF3192) were purchased from Affinity Biosciences (Cincinnati, OH, USA). Liquiritin, hesperidin, ammonium glycyrrhizinate, 5-hydroxymethyl-2-furaldehyde (5-HMF), honokiol and magnolol were purchased from National Institutes for Food and Drug Control (Beijing, China). Narirutin and isoliquiritin were purchased from Pufei De Biotech Co., Ltd (Chengdu, China). All other chemicals were of analytical grade.
Fingerprint analysis of HXZQ-OL by HPLC
HXZQ-OL (Chinese Food and Drug Administration approval number: Z50020409; Lot Number: 19050190), containing 10 traditional Chinese medicinal medicines summarized in Table 1, were manufactured by Taiji Group Chongqingfuling Pharmaceutical Co., Ltd (Chongqing, China) according to Good Manufacturing Practices. HXZQ-OL, as well as the standards of ammonium glycyrrhizinate, honokiol, magnolol, liquiritin, narirutin, hesperidin, 5-HMF and isoliquiritin, were dissolved and diluted with methanol. The major components of HXZQ-OL were quantified using an HPLC system (SHIMADZU, Kyoto, Japan) equipped with LC-20AT liquid chromatograph, SPD-M20A diode array detector, SIL-20A autosampler, CTO-20A column oven, DGU-20A5R degasser and LC solution software. The constituents of HXZQ-OL were separated using Gemini® NX-C18 column (4.6 mm × 250 mm, 5 µm; Phenomenex, CA, USA). The mobile phase was 0.4% formic acid-acetonitrile by gradient elution (0.01 min, 98:2; 8 min, 98:2; 20 min, 92:8; 30 min, 91:9; 35 min, 91:9; 45 min, 85:15; 60 min, 85:15; 75 min, 78:22; 85 min, 78:22; 95 min, 73:27; 120 min, 58:42; 145 min, 35:65; 160 min, 10:90). The column temperature was 30 °C, the flow rate was 1.0 mL/min, and the detection wavelength was set at 250 nm (glycyrrhizin, honokiol, magnolol), 276 nm (liquiritin), 284 nm (narirutin, hesperidin, 5-HMF) and 360 nm (isoliquiritin). To determine the solid content of HXZQ-OL, 10 mL sample was placed in a 25 mL evaporating dish, followed by evaporating with water bath, and then was dried at 105 C for 3 h. The solid content of HXZQ-OL was 87.9 mg/mL.
Table 1.
Constituents of HXZQ-OL.
| Chinese medicinal materials | Botanical family | Species | Portion used | Amount |
|---|---|---|---|---|
| Atractylodis Rhizoma | Compositae | Atractylodes lancea (Thunb.) DC. | Rhizome | 80 g |
| Citri Reticulatae Pericarpium | Rutaceae | Citrus reticulata Blanco | Pericarp | 80 g |
| Magnoliae Officinalis Cortex | Magnoliaceae | Magnolia officinalis Rehder & E.H.Wilson | Bark | 80 g |
| Angelicae Dahuricae Radix | Apiaceae | Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. | Radix | 120 g |
| Poria | Polyporaceae | Poria cocos (Schw.) Wolf | Sclerotium | 120 g |
| Arecae pericarpium | Arecaceae | Areca catechu L. | Pericarp | 120 g |
| Pinelliae rhizome | Araceae | Pinellia ternata (Thunb.) Ten. ex Breitenb. | Tuber | 80 g |
| Licorice extract | Leguminosae | Glycyrrhiza uralensis Fisch. | Radix and rhizome extract | 10 g |
| Patchouli oil | Lamiaceae | Pogostemon cablin (Blanco) Benth. | Overground extract | 0.8 mL |
| Perilla leaves oil | Lamiaceae | Perilla frutescens (L.) Britton | Leaf extract | 0.4 mL |
Animals
BALB/c mice (female, 18–22 g, and 6 weeks), purchased from SPF Biotechnology Co., Ltd. (Beijing, China) were housed in polypropylene plastic cages (5 mice per cage) and bred at the Experimental Animal Centre of Chongqing Academy of Chinese Materia Medica under standard husbandry conditions. All animal experiments were approved by the Experimental Animal Centre of Chongqing Academy of Chinese Materia Medica and followed the National Act on Use of Experimental Animals of China.
PCA
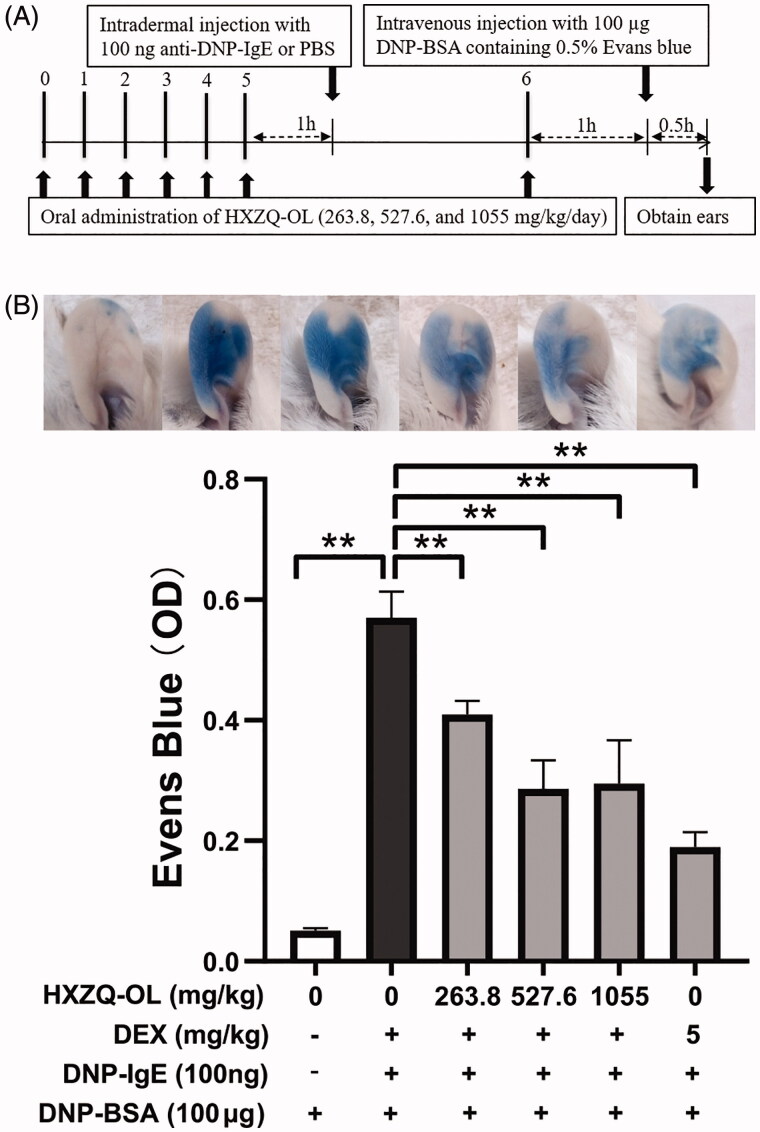
PCA was conducted following the previous method with modification (Hada et al. 2019). HXZQ-OL at dosages of 263.8, 527.6 and 1055 mg/kg that was equivalent to 1/2, 1, 2 times the adult clinical dosage were orally administrated for seven consecutive days. One hour after the administration with HXZQ-OL, the ears of mice were intradermally injected with 100 ng anti-DNP-IgE on the sixth day. Twenty-four hour after ears sensitisation with IgE, mice were intravenously injected with 100 µg DNP-BSA containing 0.5% Evans blue for 30 min. Finally, the mice were sacrificed, ears were cut with scissors, and Evans blue was extracted using 500 µL formamide at 65 °C for 12 h. The absorbance at 630 nm was measured using a microplate reader (KHB ST-360, Shanghai Kehua Experimental System Co., Ltd, China).
Cell culture
RBL-2H3 cells were purchased from the American Tissue Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in EMEM containing 10% FBS, with penicillin and streptomycin at 37 °C, 5% CO2.
Cell viability
The cytotoxic effect of the HXZQ-OL on RBL-2H3 cells was evaluated by methyl thiazolyl tetrazolium (MTT) assay. After incubation with indicated concentrations of HXZQ-OL (0–4397 µg/mL) for 24 h, RBL-2H3 cells were further incubated with 20 μL MTT (5 mg/mL) for 4 h in dark condition. Finally, 150 μL DMSO was added to each well to dissolve the formazan crystals after removing the supernatants. The absorbance was determined at 570 nm using a microplate reader.
β-Hexosaminidase release assay
β-Hexosaminidase (β-HEX) release assay was conducted as previously described (Tewtrakul et al. 2008). Briefly, RBL-2H3 cells were sensitized with IgE (0.45 µg/mL) for 24 h and then preincubated with HXZQ-OL (0–4397 µg/mL) for 30 min, followed by addition of 10 µg/mL DNP-BSA at 37 °C for 10 min to stimulate the cells to degranulate. The supernatant (50 µL) was mixed with 50 µL PNAG in 0.1 M citrate buffer (pH4.5) and then incubated for 1h at 37 °C. At the end of the reaction terminated by stop solution, the absorbance was measured at 405 nm using a microplate reader.
Measurement of intracellular Ca2+
RBL-2H3 cells were sensitized with IgE (0.45 µg/mL) for 24 h and then incubated with 4 µM Fluo 3/AM working solution for 30 min. Then, the cells were treated with HXZQ-OL (43.97, 439.7 and 4397 μg/mL) for 30 min after washing with HBSS to remove free Fluo 3/AM. Finally, the cells were challenged with 10 µg/mL DNP-BSA for 10 min. The fluorescence intensity was measured using Flow Cytometer (BD LSRFortessa™, Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
ELISA for IL-4, TNF-α, LTC4 and PGD2
RBL-2H3 cells were sensitized with IgE (0.45 µg/mL) for 24 h and then treated with HXZQ-OL (43.97, 439.7 and 4397 μg/mL) for 30 min. After the cells were challenged with 10 µg/mL of DNP-BSA for 4 h, the supernatants were collected and the amounts of IL-4, TNF-α, LTC4 and PGD2 were determined using ELISA kits following the manufacturers’ instructions.
RT-qPCR assay for IL-4 and TNF-α mRNA
RBL-2H3 cells were treated as above. After the treated cells were collected, total RNA was extracted using TRIzol® reagent (Thermo Fisher, NY, USA), and reverse transcription was performed following the manufacturer’s instructions. RT-qPCR was conducted under the following conditions that were identical for all primers (Table 2): an initial incubation at 95 °C for 30 s, followed by 45 cycles of 95, 55 and 72 °C for 10 s, respectively. The levels of gene expression were calculated using the 2−ΔΔCt method and normalized to the level of GAPDH.
Table 2.
Forward and reverse primers sequences of genes.
| Gene | Forward primers sequences (5′-3′) | Reverse primers sequences (5′-3′) |
|---|---|---|
| IL-4 | TCCACGGATGTAACGACAGC | TCATTCACGGTGCAGCTTCT |
| TNF-α | CATCCGTTCTCTACCCAGCC | AATTCTGAGCCCGGAGTTGG |
| GAPDH | TGAGATCAACGTGTTCCAGTG | ACCAGATGAAATGTGCCCC |
Immunoblotting analysis
IgE-sensitized RBL-2H3 cells were challenged with DNP-BSA for 10 min to measure the expression levels of phospho-Lyn (1:500), phospho-Syk (1:1000), phospho-Fyn (1:500), phospho-PLCγ1/2 (1:500), phospho-PKCδ (1:1000), phospho-Gab2 (1:1000), phospho-PI3K (1:1000), phospho-Akt (1:5000), phospho-p38 (1:1000), phospho-JNK (1:2000) and phospho-ERK1/2 (1:2000) or 4 h to measure the expression levels of phospho-cPLA2 (1:500), 5-LO (1:1000), COX-2 (1:1000), NF-κB p65 (1:2000), phospho-IKKα/β (1:5000) and phospho-IκBα (1:1000). The cells were then lysed in RIPA lysis buffer with PMSF and centrifuged to obtain total protein extracts. The nuclear and cytoplasmic protein extracts were obtained using the nuclear and cytoplasmic protein extraction kit following the manufacturer’s instructions. The concentrations of the protein extracts were determined using a BCA kit. The protein extracts (60 μg) were separated by 12% (w/v) SDS-PAGE and transferred to PVDF membranes. After transference, the membranes were blocked in TBST solution containing 5% nonfat-dried milk, subsequently incubated with primary antibodies and then with HRP-conjugated secondary antibody. Finally, Bands were developed with enhanced chemiluminescence reagents and visualized using Tanon 6600 luminous imaging workstation (Shanghai Tianneng Technology Co., Ltd., Shanghai, China).
Statistical analyses
All experimental data were presented as the mean ± SD. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Tukey test using GraphPad Prism version 8.0.1 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was set at the *p < 0.05 and **p < 0.01.
Results
HXZQ-OL inhibited the degranulation of IgE/Ag-mediated RBL-2H3 cells
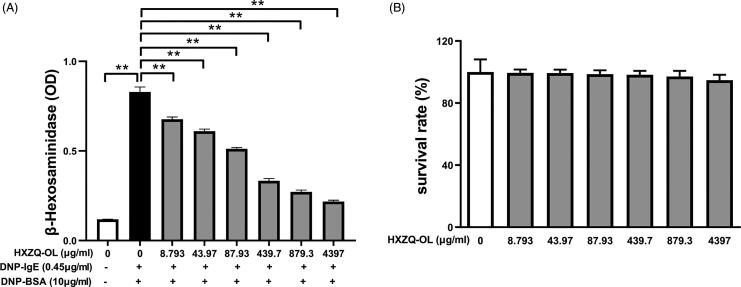
The effect of HXZQ-OL on the release of preformed β-HEX in IgE/Ag-mediated RBL-2H3 cells was investigated. The release of β-HEX was increased up to six times by DNP-BSA challenge compared to normal cells. While the release of β-HEX was inhibited in IgE/Ag-activated RBL-2H3 cells in a concentration-dependent manner when cells were pre-treated with various concentrations of HXZQ-OL (Figure 1(A); IC50, 123 μg/mL). In addition, we also examined the effect of HXZQ-OL on the viability of RBL-2H3 cells. As shown in Figure 1(B), HXZQ-OL possessed no significant cytotoxicity indicating that the inhibitory effect of HXZQ-OL on the degranulation was not caused by cytotoxicity.
Figure 1.
Effects of HXZQ-OL on degranulation (A) and cell viability (B) in IgE/Ag-mediated RBL-2H3 cells. The data were expressed as the mean ± SD values of five independent experiments. *p < 0.05 and **p < 0.01.
HXZQ-OL inhibited the release of proinflammatory mediators in IgE/Ag-mediated RBL-2H3 cells
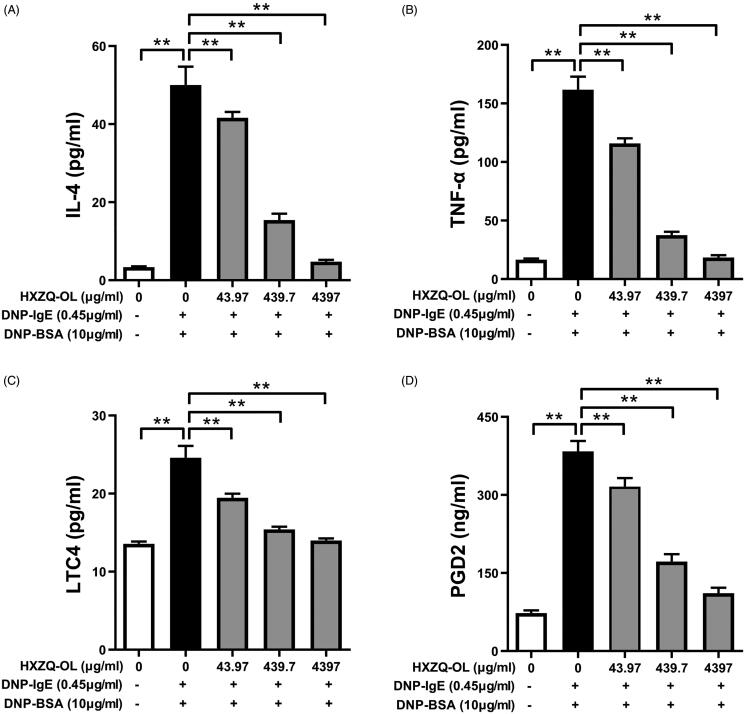
Because HXZQ-OL showed potent anti-degranulation action, the effects of HXZQ-OL on the release of de novo synthesized proinflammatory mediators were investigated in IgE/Ag-activated mast cells. As shown in Figure 2, the release of IL-4, TNF-α, PGD2 and LTC4 was increased up to 14, 8, 4 and 1 times, respectively, by DNP-BSA challenge compared to normal cells. Pre-treatment of HXZQ-OL (43.97, 439.7 and 4397 μg/mL) significantly reduced the production of IL-4 (IC50, 171.4 μg/mL), TNF-α (IC50, 88.4 μg/mL), PGD2 (IC50, 195.8 μg/mL) and LTC4 (IC50, 52.9 μg/mL). Interestingly, HXZQ-OL at concentration of 4397 μg/mL reduced the increased release of IL-4, TNF-α and LTC4 from IgE/Ag-activated mast cells to normal levels (p > 0.05).
Figure 2.
HXZQ-OL inhibited the release of proinflammatory mediators: IL-4 (A), TNF-α (B), LTC4 (C) and PGD2 (D) in IgE/Ag-mediated RBL-2H3 cells. IgE-sensitized RBL-2H3 cells were incubated with HXZQ-OL (43.97, 439.7 and 4397 μg/mL) for 30 min, followed by DNP-BSA challenge for 4 h. The amounts of IL-4, TNF-α, LTC4 and PGD2 were determined using ELISA kits following the manufacturers’ instructions. The data were expressed as the mean ± SD values of three independent experiments. *p < 0.05 and **p < 0.01.
HXZQ-OL inhibited the gene expression of IL-4 and TNF-α in IgE/Ag-mediated RBL-2H3 cells
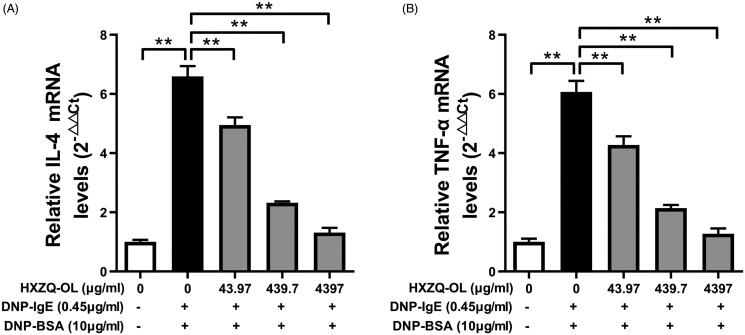
As HXZQ-OL showed potent inhibitory effects on the release of de novo synthesized IL-4 and TNF-α, we further investigated the effects of HXZQ-OL on IL-4 and TNF-α mRNA expression using RT-qPCR. As shown in Figure 3(A,B), the mRNA levels of IL-4 and TNF-α were significantly increased by DNP-BSA challenge in IgE/Ag-stimulated cells. However, pre-treatment of HXZQ-OL (43.97, 439.7 and 4397 μg/mL) significantly reduced the mRNA levels of IL-4 and TNF-α up to 95% in comparison to the IgE/Ag-stimulated cells.
Figure 3.
HXZQ-OL reduced the levels of IL-4 mRNA (A) and TNF-α mRNA (B) in IgE/Ag-mediated RBL-2H3 cells. IgE-sensitized RBL-2H3 cells were incubated with HXZQ-OL (43.97, 439.7 and 4397 μg/mL) for 30 min, followed by DNP-BSA challenge for 4 h. The gene expression of IL-4 and TNF-α were measured by RT-qPCR. The data were expressed as the mean ± SD values of three independent experiments. *p < 0.05 and **p < 0.01.
HXZQ-OL reduced the intracellular Ca2+ concentration in IgE/Ag-mediated RBL-2H3 cells
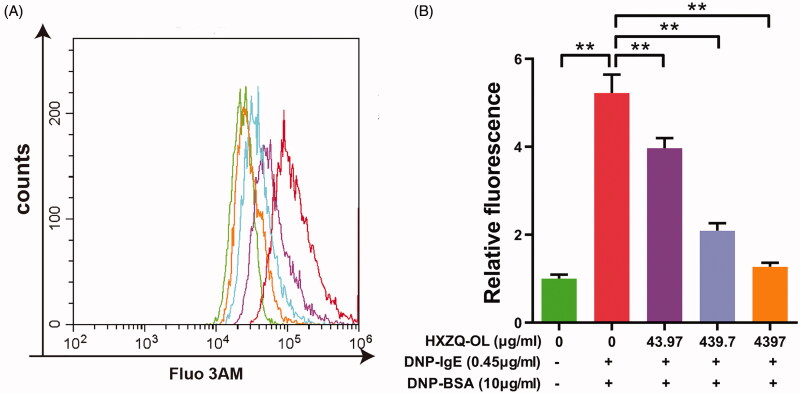
Because the increase of the intracellular Ca2+ concentration is one of the critical events for mast cell degranulation, the effect of HXZQ-OL on the intracellular Ca2+ concentration in IgE/Ag-stimulated RBL-2H3 cells was examined. The level of intracellular Ca2+ was increased up to five times by DNP-BSA challenge in the IgE/Ag-stimulated cells compared to normal cells, whereas the elevation was significantly suppressed by HXZQ-OL (Figure 4). The inhibitory rates of HXZQ-OL (43.97, 439.7 and 4397 μg/mL) on the elevation of intracellular Ca2+ were 30%, 74% and 94%, respectively, indicating that the inhibitory effect of HXZQ-OL on the degranulation of IgE/Ag-mediated RBL-2H3 cells might be through reducing the elevation of intracellular Ca2+.
Figure 4.
HXZQ-OL inhibited the intracellular Ca2+ concentration in IgE/Ag-mediated RBL-2H3 cells. IgE-sensitized RBL-2H3 cells were incubated with HXZQ-OL for 30 min, followed by DNP-BSA challenge for 10 min. The intracellular Ca2+ concentration was determined using flow cytometry with Fluo 3/AM probe. The data were expressed as the mean ± SD values of three independent experiments. *p < 0.05 and **p < 0.01.
HXZQ-OL inhibited the phosphorylation of FcεRI-induced degranulation signalling Cascades in IgE/Ag-mediated RBL-2H3 cells
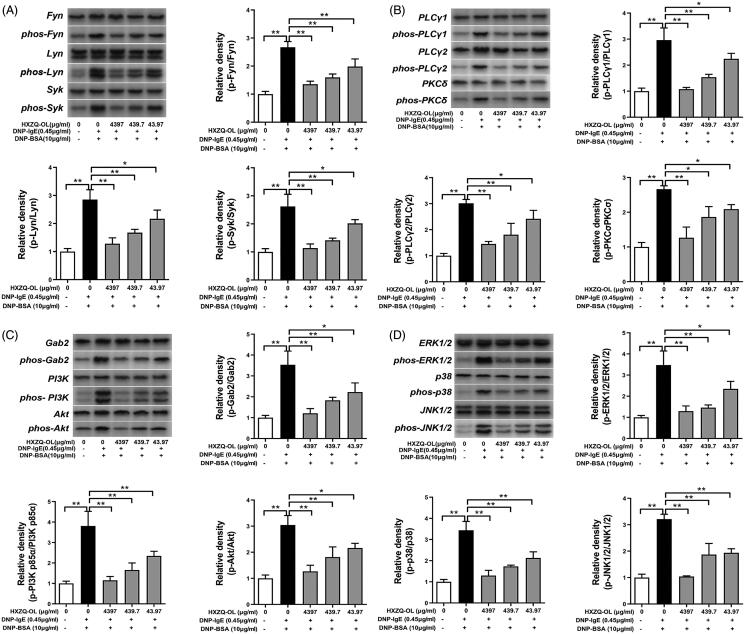
The effects of HXZQ-OL on phosphorylation of FcεRI signalling cascades that are important for mast cell activation were investigated to further understand the mechanism of HXZQ-OL on IgE/Ag-stimulated RBL-2H3 cells. As shown in Figure 5(A–C), HXZQ-OL significantly inhibited the phosphorylation of Fyn, Lyn, Syk, PLCγ1/2, PKCδ, Gab2 and PI3K that were significantly up-regulated by DNP-BSA challenge in the early phase (10 min). An aliquot of 4397 μg/mL HXZQ-OL reduced the increased phosphorylation of Fyn, Lyn, Syk, PLCγ1/2, PKCδ, Gab2 and PI3K in IgE/Ag-activated mast cells to normal levels (p > 0.05). About 43.97 and 439.7 μg/mL HXZQ-OL significantly reduced the phosphorylation of Fyn, Lyn, Syk, PLCγ1/2, PKCδ, Gab2 and PI3K by 30–52% and 48–77%, respectively. These findings suggested that HXZQ-OL can inhibit mast cells degranulation by suppressing the phosphorylation of Fyn, Lyn, Syk, PLCγ1/2, PKCδ, Gab2 and PI3K.
Figure 5.
Inhibitory effects of HXZQ-OL on the phosphorylation of FcεRI-induced degranulation signalling cascades (A, B, C) and MAPKs (D) in IgE/Ag-mediated RBL-2H3 cells. IgE-sensitized RBL-2H3 cells were incubated with HXZQ-OL for 30 min, followed by DNP-BSA challenge for 10 min. The expression levels of p-Fyn, p-Lyn, p-Syk, p-PLCγ1, p-PLCγ2, p-PKCδ, p-Gabs, p-PI3K, p-Akt, p-ERK1/2, p-p38 and p-JNK1/2 were normalized to total proteins, respectively. The data were expressed as the mean ± SD values of three independent experiments. *p < 0.05 and **p < 0.01.
HXZQ-OL inhibited the activation of MAPK proteins and PI3K/NF-κB pathway in IgE/Ag-mediated RBL-2H3 cells
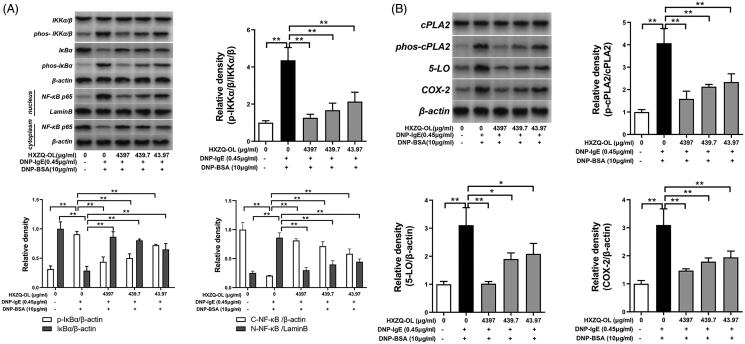
Since MAPK proteins and PI3K/NF-κB pathway collectively led to the synthesis of lipid mediators and cytokines in IgE/Ag-stimulated mast cells (Hirasawa et al. 1995; Lu et al. 2011), we further examined the effects of HXZQ-OL on the activation of MAPK proteins and PI3K/NF-κB pathway in IgE/Ag-mediated RBL-2H3 cells. Figure 5(D) showed that the phosphorylation levels of ERK1/2, p38 and JNK were significantly increased by DNP-BSA challenge in IgE/Ag-stimulated cells. However, pre-treatment of HXZQ-OL (43.97, 439.7 and 4397 μg/mL) significantly reduced the phosphorylation levels of ERK1/2, p38 and JNK compared to the IgE/Ag-stimulated cells. 43.97 and 439.7 μg/mL HXZQ-OL significantly reduced the phosphorylation of all MAPKs by 46–58% and 61–81%, respectively. Moreover, 4397 μg/mL HXZQ-OL almost reduced the increased phosphorylation of all MAPKs in IgE/Ag-activated mast cells to normal levels (p > 0.05). In addition, HXZQ-OL also significantly inhibited the phosphorylation of PI3K, Akt, IKKα/β, and IκBα, as well as degradation of IκBα and nuclear translocation of NF-κB p65 induced by DNP-BSA challenge (Figures 5(C) and 6(A)). Taken together, these results indicated that HXZQ-OL would inhibit the lipid mediator generation and cytokines production via suppressing the activation of MAPK proteins and PI3K-NFκB pathway in IgE/Ag-mediated RBL-2H3 cells.
Figure 6.
Inhibitory effects of HXZQ-OL on the activations of PI3K/NF-κB (A) and eicosanoid cascades (B) in IgE/Ag-mediated RBL-2H3 cells. IgE-sensitized RBL-2H3 cells were preincubated with HXZQ-OL for 30 min, followed by DNP-BSA challenge for 4 h. P-IKKα/β and p-cPLA2 were normalized to total proteins, respectively. The endogenous reference protein used for p-IκB, IκB, 5-LO, COX-2 and cytosolic NF-κB p65 was β-actin whereas for nuclear NF-κB p65 was Lamin B. The data were expressed as the mean ± SD values of three independent experiments. *p < 0.05 and **p < 0.01.
HXZQ-OL inhibited the activation of eicosanoid cascades in IgE/Ag-mediated RBL-2H3 cells
It is well known that the production of LTC4 and PGD2 is regulated by the eicosanoid cascades (Tan et al. 2017). As HXZQ-OL reduced the release of LTC4 and PGD2, we further investigated the effects of HXZQ-OL on the activation of eicosanoid cascades-related key proteins: cPLA2, 5-LO and COX-2 in IgE/Ag-activated mast cells. As shown in Figure 6(B), HXZQ-OL (43.97, 439.7 and 4397 μg/mL) significantly inhibited the phosphorylation level of cPLA2 as well as the expression levels of the downstream proteins: both 5-LO and COX-2 by 49–99%. 4397 μg/mL HXZQ-OL almost reduced the up-regulated expression levels of p-cPLA2, 5-LO and COX-2 in IgE/Ag-activated mast cells to normal levels (p > 0.05).
HXZQ-OL inhibited the IgE/Ag-mediated PCA in mice
We had investigated the underlying anti-allergic mechanisms of HXZQ-OL using IgE receptor-bearing RBL-2H3 in vitro. We then examined the anti-allergic effect of HXZQ-OL in vivo using mouse PCA model (Inagaki et al. 1984). Figure 7 shows that Evans blue exudation increased more than ten times by DNP-BSA challenge in IgE/Ag-stimulated mice. However, pre-treatment of HXZQ-OL (263.8, 527.5 and 1055 mg/kg/d) for seven consecutive days followed by antigen challenge significantly reduced the IgE/Ag-mediated mast cell-dependent PCA in mice. HXZQ-OL at 263.8 and 527.6 mg/kg exhibited 31% and 55% suppression of Evans blue exudation, respectively, indicating that HXZQ-OL has anti-allergic effect in vivo. Therefore, it suggested that HXZQ-OL has the potential for the treatment of allergic diseases.
Figure 7.
HXZQ-OL inhibited IgE/Ag-mediated PCA in mice. (A) Experimental schedule. (B) Evans blue exudation. BALB/c mice were orally administrated with HXZQ-OL (263.8, 527.6 and 1055 mg/kg/d) or dexamethasone (DEX, 5 mg/kg/d) for seven consecutive days. One hour after the administration with HXZQ-OL, the ears of mice were intradermally injected with 100 ng anti-DNP-IgE on the sixth day. Twenty-four hour after ears sensitisation with IgE, mice were intravenously injected with 100 µg DNP-BSA containing 0.5% Evans blue for 30 min. After 30 min, Evans blue was extracted, and the absorbance was measured at 630 nm. The data were expressed as the mean ± SD values of five independent experiments. *p < 0.05 and **p < 0.01.
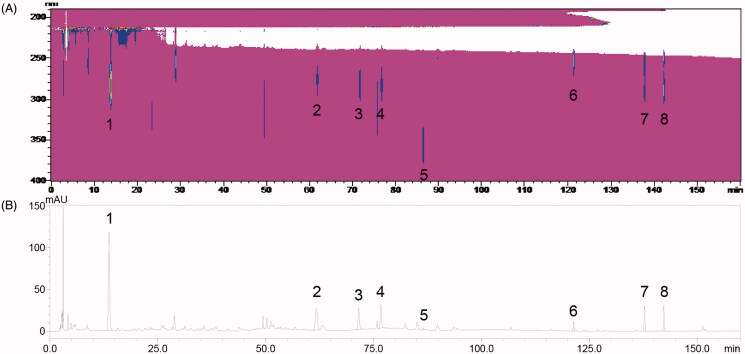
Identification of active compounds in HXZQ-OL
To confirm the active ingredients that are related to the anti-allergic actions, the phytochemical composition of HXZQ-OL was analysed using an HPLC system. The peaks of major compounds were identified as 5-HMF, liquiritin, narirutin, hesperidin, isoliquiritin, glycyrrhizin, honokiol and magnolol by comparing with the reference standard whose retention times were 13.70, 61.75, 71.58, 76.71, 86.41, 121.40, 137.82 and 142.28 min on the HPLC chromatogram (Figure 8). Thus, we further quantified the amounts of 5-HMF, liquiritin, narirutin, hesperidin, isoliquiritin, glycyrrhizin, honokiol and magnolol in HXZQ-OL whose amounts were 0.173, 0.262, 0.204, 0.194,0.057, 0.545, 0.151 and 0.208 mg/mL, respectively. These results suggested that 5-HMF, liquiritin, narirutin, hesperidin, isoliquiritin, glycyrrhizin, honokiol and magnolol might be responsible for the anti-allergic actions of HXZQ-OL.
Figure 8.
HPLC chromatograms of HXZQ-OL (A) Contour plot (190–400 nm) (B) 276 nm. 5-HMF (1), liquiritin (2), narirutin (3), hesperidin (4), isoliquiritin (5), glycyrrhizin (6), honokiol (7) and magnolol (8) were identified. HPLC analysis was described in the materials and methods.
Discussion
HXZQ-OL is one of the acclaimed Traditional Chinese Medicine preparations which has been used for thousands of years and has a variety of pharmacological activities, such as antibacterial, anti-inflammation, antithrombotic, antioxidative activity, immune protection and gastrointestinal motility regulation effects (He et al. 2006; Zhao et al. 2018). Chinese medicinal materials contained in HXZQ-OL exerted anti-allergic effects including anti-asthma, anti-food allergy and anti-atopic dermatitis. Nonetheless, the effect of HXZQ-OL on allergic reactions is still poorly understood and the underlying mechanism has not been investigated yet.
In this research, HXZQ-OL possessed inhibitory effects on IgE-mediated allergic actions both in vitro and in vivo. Mast cell mediator release that follows antigen-mediated aggregation of FcεRI represents a pivotal event in the initiation and progression of allergic diseases (Gilfillan and Rivera 2009). After FcεRI crosslinking, intracellular signalling cascades are initiated, namely the primary Lyn pathway and the complementary Fyn pathway, to regulate FcεRI-induced degranulation (Parravicini et al. 2002; Kraft and Kinet 2007). Our study showed that HXZQ-OL markedly inhibited IgE/Ag-mediated degranulation of RBL-2H3 cells. HXZQ-OL also decreased the phosphorylation of Lyn, Syk, PLCγ1/2 and Fyn, Gab2, PKCδ and influx of Ca2+, suggesting that HXZQ-OL influences degranulation through attenuation of both Lyn pathway and Fyn pathway.
The proinflammatory mediators related to the onset and development of allergic diseases are generated and released by IgE/Ag-activated mast cells. We observed that HXZQ-OL significantly reduced the generation and release of de novo synthesized proinflammatory cytokines and lipid mediators including IL-4, TNF-α, PGD2 and LTC4 in the late phase of allergic reaction. In line with the ELISA results, the results from RT-qPCR showed that HXZQ-OL also inhibited the expression of IL-4 and TNF-α mRNA. These findings have shown that HXZQ-OL decreases the production of IL-4 and TNF-α through both the protein level and the transcription level. Taken together, HXZQ-OL not only attenuates the release of preformed mediators by inhibiting Lyn and Fyn pathways, but also decreases subsequent production and release of late-phase proinflammatory mediators.
The activation of MAPKs and PI3K/NF-κB signalling pathways can induce the expression of proinflammatory mediators through the activation of transcription factors and enzymes related to arachidonic acids, such as cPLA2, 5-LO and COX-2 (Hirasawa et al. 1995; Lu et al. 2011). As expected, HXZQ-OL decreased the phosphorylation level of cPLA2 and expression levels of 5-LO and COX-2 that are associated with the production of lipid mediators. Our data also showed that HXZQ-OL not only attenuated the phosphorylation levels of all MAPKs including ERK1/2, JNK and p38, but also inhibited both the phosphorylation of PI3K, Akt, IKKα/β and IκBα, as well as degradation of IκBα and nuclear translocation of NF-κB p65. Therefore, the inhibitory effect of HXZQ-OL on the production and release of proinflammatory mediators, as well as eicosanoid cascades would be likely attributed to the inhibition on the activation of MAPK and PI3K/NF-κB signalling pathways.
PCA is one of the most important local allergic reaction models in vivo (Inagaki et al. 1984). In our study, oral administration of HXZQ-OL for consecutive 7 d inhibited the PCA in BALB/c mice, whereas the single administration did not (data not shown). Hence, we also tested the inhibitory effect of HXZQ-OL on the IgE/Ag-mediated PCA by topical application. Interestingly, single topical application of HXZQ-OL at 0.88 and 1.76 mg/ear exhibited 51% and 62% suppression of Evans blue exudation, respectively (data not shown). It is worth noting that several main compounds in HXZQ-OL have relatively low hydrophilicity, like magnolol and honokiol which are more likely to penetrate through the skin and thus absorbed by topical application. Based on this, the effective blood concentration would be reached only by continuous oral administration of HXZQ-OL to earn the inhibitory effect on PCA. Taken together, these results suggested that the bioactive components in HXZQ-OL might be lipophilic molecules.
To confirm the active ingredients, HPLC fingerprint of HXZQ-OL was performed, and the major peaks were confirmed as 5-HMF, liquiritin, narirutin, hesperidin, isoliquiritin, glycyrrhizin, honokiol and magnolol. 5-HMF, liquiritin, narirutin, hesperidin, isoliquiritin, glycyrrhizin, honokiol and magnolol have already been reported to have anti-allergic effects (Hamasaki et al. 1999; Funaguchi et al. 2007; Shin et al. 2007; Kuo et al. 2010; Munroe et al. 2010; Jeong et al. 2013; Yu et al. 2015; Han et al. 2017; Uchida et al. 2020). It has been reported that narirutin significantly reduces OVA-induced airway inflammation, as well as the levels of eosinophil, IL-4, and IgE (Funaguchi et al. 2007). Hesperidin inhibits compound 48/80-induced systemic anaphylactic reaction and IgE-induced PCA reaction, as well as intracellular Ca2+ levels and the release of histamine and tryptase from RPMC (Han et al. 2012). Orally or intraperitoneally administration of isoliquiritin can inhibit PCA reaction induced by the IgE/Ag complex, while liquiritigenin, the aglycone of liquiritin, potently inhibits the degranulation of RBL-2H3 cells induced by IgE/Ag complex and RPMC induced by compound 48/80 (Shin et al. 2007). Glycyrrhizin significantly attenuates the mast cell-dependent PCA reaction and β-HEX release from RBL-2H3 cells through inhibiting IgE/Ag-stimulated Ca2+ influx (Han et al. 2017). About 800 µg/mL 5-HMF reduces IgE/Ag-induced bone marrow-derived mast cells degranulation and IL-6 production through inhibiting phosphorylation of PLCγ1 and ERK1/2 (Uchida et al. 2020). Magnolol (IC50, 1.04 µg/mL) and honokiol (IC50, 2.77 µg/mL) inhibit C48/80-induced histamine release from RPMC. Magnolol also inhibits anti-IgE- and A23187-stimulated synthesis of LTC4 and LTB4 in RBL-2H3 cells through reducing the increase of intracellular Ca2+ concentration, resulting in the inhibition of cPLA2 and 5-LO (Hamasaki et al. 1999; Ikarashi et al. 2001). Therefore, 5-HMF, liquiritin, narirutin, hesperidin, isoliquiritin, glycyrrhizin, honokiol and magnolol as the major components may contribute to the anti-allergic effect of HXZQ-OL. In addition, the lipid-water partition coefficient of 5-HMF, honokiol and magnolol are larger than that of five glycosides: liquiritin, narirutin, hesperidin, isoliquiritin, and glycyrrhizin. Taken together, 5-HMF, honokiol and magnolol would serve as the major active components in HXZQ-OL. Further study is needed to verify the relationship between these compounds and HXZQ-OL in anti-allergy actions.
Conclusions
HXZQ-OL has anti-allergic effects in IgE/Ag-mediated allergic responses through strongly inhibiting the degranulation of mast cells and the production and release of allergic response elicitors in vitro and in vivo. The molecular mechanism underlying anti-allergic effects of HXZQ-OL is through negative regulation of Lyn and Fyn pathways, as well as the downstream MAPK, PI3K/NF-κB and eicosanoid signalling pathways in IgE/Ag-stimulated mast cells. The anti-allergic actions of HXZQ-OL may be concern with active phytochemicals such as 5-HMF, liquiritin, narirutin, hesperidin, isoliquiritin, glycyrrhizin, honokiol and magnolol in HXZQ-OL that would be used for the development of novel medicine for the treatment of allergic diseases.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This work was supported by the Ministry of Science and Technology of the People’s Republic of China [grant numbers 2018YFC1707407].
Disclosure statement
The authors declare that they have no conflict of interests.
References
- Asher MI, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK, Williams H.. 2006. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 368(9537):733–743. [DOI] [PubMed] [Google Scholar]
- Bae MJ, See HJ, Choi G, Kang CY, Shon DH, Shin HS.. 2016. Regulatory T cell induced by Poria cocos bark exert therapeutic effects in murine models of atopic dermatitis and food allergy. Mediat Inflamm. 2016:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven MA, Rogers J, Moore JP, Hesketh TR, Smith GA, Metcalfe JC.. 1984. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J Biol Chem. 259(11):7129–7136. [PubMed] [Google Scholar]
- Commission CP. 2020. Pharmacopoeia of the people’s republic of China. 2020 ed. Beijing, China: China Medical Science Press. [Google Scholar]
- Funaguchi N, Ohno Y, La BL, Asai T, Yuhgetsu H, Sawada M, Takemura G, Minatoguchi S, Fujiwara T, Fujiwara H.. 2007. Narirutin inhibits airway inflammation in an allergic mouse model. Clin Exp Pharmacol Physiol. 34(8):766–770. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Rivera J.. 2009. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 228(1):149–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C.. 2006. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 6:218–230. [DOI] [PubMed] [Google Scholar]
- Hada M, Nishi K, Ishida M, Onda H, Nishimoto S, Sugahara T.. 2019. Inhibitory effect of aqueous extract of Cuminum cyminum L. seed on degranulation of RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Cytotechnology. 71:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki Y, Kobayashi I, Zaitu M, Tsuji K, Kita M, Hayasaki R, Muro E, Yamamoto S, Matsuo M, Ichimaru T, et al. . 1999. Magnolol inhibits leukotriene synthesis in rat basophilic leukemia-2H3 cells. Planta Med. 65:222–226. [DOI] [PubMed] [Google Scholar]
- Han NR, Kim HM, Jeong HJ.. 2012. Pyeongwee-San extract (KMP6): a new anti-allergic effect. J Pharm Pharmacol. 64:308–316. [DOI] [PubMed] [Google Scholar]
- Han S, Sun L, He F, Che H.. 2017. Anti-allergic activity of glycyrrhizic acid on IgE-mediated allergic reaction by regulation of allergy-related immune cells. Sci Rep. 7(1):7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YH, Zhao HY, Liu ZL, Lu C, Luo XJ, Lin SQ, Qian XW, Chen SL, Lu AP.. 2006. Effects of huoxiangzhengqi liquid on enteric mucosal immune responses in mice with Bacillus dysenteriae and Salmonella typhimurium induced diarrhea. World J Gastroenterol. 12:7346–7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa N, Santini F, Beaven MA.. 1995. Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. Indications of different pathways for release of arachidonic acid and secretory granules. J Immunol. 154:5391–5402. [PubMed] [Google Scholar]
- Ikarashi Y, Yuzurihara M, Sakakibara I, Nakai Y, Hattori N, Maruyama Y.. 2001. Effects of the extract of the bark of Magnolia obovata and its biphenolic constituents magnolol and honokiol on histamine release from peritoneal mast cells in rats. Planta Med. 67:709–713. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Goto S, Nagai H, Koda A.. 1984. Mouse ear PCA as a model for evaluating antianaphylactic agents. Int Arch Allergy Immunol. 74(1):91–92. [DOI] [PubMed] [Google Scholar]
- Jeong HH, Kim SR, Pyo MY.. 2013. Suppressive effects of hesperidin on Th2-associated cytokines expression in RBL-2H3 cells. Korean J Pharmacog. 44(2):104–109. [Google Scholar]
- Kraft S, Kinet JP.. 2007. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 7(5):365–378. [DOI] [PubMed] [Google Scholar]
- Ku JM, Hong SH, Kim HI, Seo HS, Shin YC, Ko SG.. 2017. Effects of Angelicae dahuricae radix on 2, 4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice model. BMC Complement Altern Med. 17(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DH, Lai YS, Lo CY, Cheng AC, Wu H, Pan MH.. 2010. Inhibitory effect of magnolol on TPA-induced skin inflammation and tumor promotion in mice. J Agric Food Chem. 58:5777–5783. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yang JH, Li X, Hwangbo K, Hwang SL, Taketomi Y, Murakami M, Chang YC, Kim CH, Son JK, et al. . 2011. Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation. Biochem Pharmacol. 82:1700–1708. [DOI] [PubMed] [Google Scholar]
- Man G, Hu LZ, Elias PM, Man MQ.. 2018. Therapeutic benefits of natural ingredients for atopic dermatitis. Chin J Integr Med. 24:308–314. [DOI] [PubMed] [Google Scholar]
- Mukai K, Tsai M, Saito H, Galli SJ.. 2018. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 282(1):121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe ME, Businga TR, Kline JN, Bishop GA.. 2010. Anti-inflammatory effects of the neurotransmitter agonist honokiol in a mouse model of allergic asthma. J Immunol. 185:5586–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ok IS, Kim SH, Kim BK, Lee JC, Lee YC.. 2009. Pinellia ternata, Citrus reticulata, and their combinational prescription inhibit eosinophil infiltration and airway hyperresponsiveness by suppressing CCR3+ and Th2 cytokines production in the ovalbumin-induced asthma model. Mediat Inflamm. 2009:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer JJ, Casale TB.. 2002. Next generation antihistamines: therapeutic rationale, accomplishments and advances. Expert Opin Investig Drugs. 11:807–817. [DOI] [PubMed] [Google Scholar]
- Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J.. 2002. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 3(8):741–748. [DOI] [PubMed] [Google Scholar]
- Ring J, Akdis C, Lauener R, Schäppi G, Traidl-Hoffmann C, Akdis M, Ammann W, Behrendt H, Bieber T, Biedermann T, et al. . 2014. Global allergy forum and second Davos declaration 2013 allergy: barriers to cure–challenges and actions to be taken. Allergy. 69:978–982. [DOI] [PubMed] [Google Scholar]
- Roth K, Chen WM, Lin TJ.. 2008. Positive and negative regulatory mechanisms in high-affinity IgE receptor-mediated mast cell activation. Arch Immunol Ther Exp. 56:385–399. [DOI] [PubMed] [Google Scholar]
- Schoepe S, Schäcke H, May E, Asadullah K.. 2006. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 15:406–420. [DOI] [PubMed] [Google Scholar]
- Shin TY, Kim DK, Chae BS, Lee EJ.. 2001. Antiallergic action of Magnolia officinalis on immediate hypersensitivity reaction. Arch Pharm Res. 24(3):249–255. [DOI] [PubMed] [Google Scholar]
- Shin YW, Bae EA, Lee B, Lee SH, Kim JA, Kim YS, Kim DH.. 2007. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta Med. 73:257–261. [DOI] [PubMed] [Google Scholar]
- Tan FY. 1995. Clinical observation of 45 cases of type I allergic disease treated with huoxiangzhengqi liquid. Chin J Integrat Med Cardio-Cerebro Dis. (2):16–16. [Google Scholar]
- Tan JW, Israf DA, Md Hashim NF, Cheah YK, Harith HH, Shaari K, Tham CL.. 2017. LAT is essential for the mast cell stabilising effect of tHGA in IgE-mediated mast cell activation. Biochem Pharmacol. 144:132–148. [DOI] [PubMed] [Google Scholar]
- Tang GH. 1998. Treatment of 21 cases of eczema with huoxiangzhengqi liquid. Shaanxi J Trad Chin Med. 19(9):414–415. [Google Scholar]
- Tewtrakul S, Subhadhirasakul S, Kummee S.. 2008. Anti-allergic activity of compounds from Kaempferia parviflora. J Ethnopharmacol. 116:191–193. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kalogeromitros D.. 2006. The critical role of mast cells in allergy and inflammation. Ann NY Acad Sci. 1088(1):78–99. [DOI] [PubMed] [Google Scholar]
- Uchida R, Kato M, Hattori Y, Kikuchi H, Watanabe E, Kobayashi K, Nishida K.. 2020. Identification of 5-hydroxymethylfurfural (5-HMF) as an active component Citrus jabara that suppresses FcεRI-mediated mast cell activation. Int J Mol Sci. 21(7):2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan P, Li Q, Ouyang XY, Liu HH, Wang ZW, Cao DH.. 2000. Observation on curative effect of huoxiangzhengqi liquid in treating chronic urticaria. Chin Trad Herbal Drugs. 31(3):47–48. [Google Scholar]
- Wang HL, Li ZH, Xu YT.. 2015. Advances in pharmacologic studies on treatments of allergic diseases by Chinese materia medica. Chin Trad Herbal Drugs. 46:1542–1555. [Google Scholar]
- Yoo JM, Park KI, Yang JH, Cho WK, Lee B, Ma JY.. 2019. Anti-allergic actions of F-PASA, a novel herbal cocktail, in IgE/antigen-mediated allergic responses in RBL-2H3 cells and passive cutaneous anaphylaxis in mice. Phytomedicine. 55:229–237. [DOI] [PubMed] [Google Scholar]
- Yu CX, Yan GZ, Lin J.. 2005. Clinical observation on the asthma in outbreak period with spray inhalation of ultrasonic oxygen mixed huoxiangzhengqi oral liquid. J Fujian Coll TCM. 15(5):5–7. [Google Scholar]
- Yu JY, Ha JY, Kim KM, Jung YS, Jung JC, Oh S.. 2015. Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules. 20:13041–13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Chen Y, Wang C, Xiao W, Chen S, Zhang S, Yang L, Li Y.. 2018. Systems pharmacology dissection of multi-scale mechanisms of action of Huo-Xiang-Zheng-Qi formula for the treatment of gastrointestinal diseases. Front Pharmacol. 9(:1448):1448. [DOI] [PMC free article] [PubMed] [Google Scholar]