ABSTRACT
Cancer stem cells (CSCs) represent a small subpopulation of cells found within tumors that exhibit properties of self-renewal, like normal stem cells. CSCs have been defined as a crucial factor involved in driving cancer relapse, chemoresistance and metastasis. Prominin-1 (CD133) is one of the most well-characterized markers of CSCs in various tumor types, including hepatocellular carcinoma (HCC). CD133+ cells have been demonstrated to be involved in metastasis, tumorigenesis, tumor recurrence, and resistance to treatment in HCC. CD133-related clinical prognosis prediction, and targeted therapy have highlighted the clinical significance of CD133 in HCC. However, there remains controversy over the role of CD133 in experimental and clinical research involving HCC. In this article, we summarize the fundamental cell biology of CD133 in HCC cells and discuss the important characteristics of CD133+ in HCC cells. Furthermore, the prognostic value of CD133, and therapeutic strategies for its targeting in HCC, is also reviewed.
KEYWORDS: CD133, cancer stem cells, hepatocellular carcinoma, prognosis, therapeutic
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent types of liver cancers, and one of the leading causes of cancer-related deaths and is usually a consequence of chronic hepatitis infection and cirrhosis.1 At present, the effective treatment for HCC remains liver transplantation and surgical resection. However, most patients with HCC are found to be at an advanced stage and therefore, inoperable. Even after surgical resection or transplantation, the long-term prognosis for HCC remains unsatisfactory due to its high recurrence and rate of metastasis. Cancer stem cells (CSCs) or tumor-initiating cells (TICs) are a subpopulation of cells within tumors that exhibit self-renewal properties similar to those of normal stem cells.2 CSCs have been implicated in relapse, metastasis, and chemo-resistance in different cancer types; thus, these cells represent promising targets for cancer therapy and prevention.3
CSCs have been identified in various solid tumor types, and this identification mainly involves the use of surface markers.4 In HCC, accumulating evidence has demonstrated the existence of CSCs and several CSC markers have been identified, including CD133, CD90, CD44, OV6, EpCAM, CD13, CD24, DLK1, α2δ1, ALDH and K19.5,6 The identification of subpopulations of CSCs using specific markers will help to give new insights into further understanding HCC development, which may bring hope for the development of future treatments. To achieve this, we must further investigate markers of stemness and the cell properties associated with prognosis, metastasis, and resistance.
CD133/prominin 1 (PROM1) is one of the most frequently used cell surface markers used for the detection and isolation of CSCs from various solid tumors, including HCC.7 Moreover, over a decade ago, several studies found that CD133‐positive HCC cells have a potential for tumor initiation.8–10 Since then, CD133 has become the focus of research in liver cancer stem cells (LCSCs), and it has been demonstrated to be involved in metastasis, tumorigenesis, tumor recurrence, as well as treatment resistance in HCC. Furthermore, CD133 expression in HCC tissue has been found to be an independent prognostic factor for survival and tumor recurrence in HCC patients. However, currently, there remain some controversial points concerning the accuracy associated with using CD133 as a specific marker for LCSC detection and isolation.
The aim of this review, therefore, is to discuss the recent progress in CD133+ LCSC research with regard to identification, regulation and clinical relevance of CD133 in HCC.
Function of the CD133 molecule in liver cancer cells
CD133 is a single-chain transmembrane glycoprotein with five transmembrane domains separating two large glycosylated extracellular loops and two small intracellular loops, which localize into the protrusions of the cellular plasma membrane.11,12 To date, most studies have focused on the use of CD133 as a marker for CSCs, with little knowledge of its biological role in these cells. In addition to being widely used as a marker in LCSC identification and isolation, CD133 has been postulated to be involved in signal transduction. Recent evidence suggests that CD133 facilitates CSC-like properties by stabilizing EGFR-AKT signaling in HCC.13 It can also promote TM4SF5 expression, and this CD133-induced TM4SF5 expression and function, are important for liver cancer spherical cell growth.14 Knockdown of CD133 in CD133+ cells results in the downregulation of the stemness-related gene c-Myc.15 In addition to regulating stemness in LCSCs, CD133 can also confer a malignancy potential by regulating the expressions of matrix metalloproteinase (MMP)-2 and a disintegrin, as well as the metalloproteinase (ADAM)9 in human HCC.16 CD133 has also been shown to be preferentially localized to plasma membrane protrusions and microvilli, suggesting that it may be involved in membrane organization.7 CD133-containing membrane particles, such as microvesicles (MV), have been reported to be released into various human body fluids.17,18 One study has found that CD133 released from the plasma membrane into the cytoplasm is involved in autophagy and promotes glucose uptake and also may function in HCC cell survival.19
Therefore, in summary, although CD133 is widely used as a marker for LCSC, its role in LCSCs remains controversial, and more research is needed to elucidate its exact function. Only when this is achieved, can an effective means of clearing CD133+ cells be obtained.
Characteristics of CD133+ HCC cells
Since CD133+ cells are thought to represent a subpopulation that exhibits stem cell properties in several solid tumors, the identification of these cells in HCC has been investigated, based on the analysis of tumorigenesis, or other stemness functional characteristics.
Tumorigenesis, self-renewal and aggressiveness
In HCC, the role of CD133+ cells as a representative subset of LCSCs was first demonstrated by Suetsugu et al. in 2006. They isolated CD133+ cells from Huh7 cells and found these possessed a high proliferative and tumorigenic potential.8 The tumorigenic potential of CD133+ cells was further confirmed by Ma et al.9 In addition to their proliferative and tumorigenic properties, CD133+ cells were demonstrated to possess unique characteristics such as the ability to self-renew, and the ability to differentiate.9 These researchers also found that CD133+ HCC cells could promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling.20 CD133+ cells also demonstrated great invasiveness and lymphatic metastatic capacity in HCC.21,22
Chemoresistance
CSCs have been identified as the reason for resistance to chemotherapeutic agents and radiotherapy. Several studies have found that the CD133+ populations of HCC cells are responsible for multiple drug resistance. A recent study has shown that CD133+ HCC cells had significant capacity for cisplatin-resistance when compared to CD133– HCC cells, and that overexpression of miR-124 caused sensitization of cisplatin-induced cytotoxicity against CD133+ HCC cells by targeting the SIRT1/ROS/JNK pathway.23 CD133+ HCC cells can also confer chemoresistance to doxorubicin (DOX) and 5-fluorouracil (5-FU), through activation of the Akt/PKB pathway.24 Furthermore, a recent study has found that CD133+ cells in HCC were resistant to vincristine and 5-FU through the NOTCH signaling pathway activation.25 Sorafenib and regorafenib are multikinase inhibitors used for the treatment of advanced HCC; however, CD133+ HCC cells frequently confer resistance to Sorafenib and Regorafenib.26 Although CD133+ cells have been reported to be resistant to a variety of anti-tumor drugs, the mechanism of drug resistance remains to be further elucidated.
Radioresistance
CD133+ HCC cells have been suggested to contribute to radioresistance in several studies. Piao et al. demonstrated that the CD133+ cell subpopulation was significantly enriched after radiation exposure due to resistance in radiation-induced apoptosis. Furthermore, an in vivo study found that there was an increased tumor formation in irradiated CD133+ cells when compared to a CD133– cell group.27 They also showed that CD133+ HCC cells were involved in radioresistance through the activation of the MAPK/ERK survival pathway. Lee et al. found that a knockdown of 14-3-3ζ can inhibit radioresistance and enhance apoptosis induced by γ-irradiation in CD133+ liver CSCs.28 Furthermore, CD133+ HCC cells also showed greater resistance to sublethal irradiation and had enhanced metastatic capability after irradiation.29
Metabolic reprogramming and plasticity
Due to the high rate of proliferation and a lack of vasculature, the nutrient and oxygen supply to solid tumors is generally lower than that seen in normal tissues. Therefore, cancer cells must adjust their metabolic phenotypes to adapt to these more unfavorable microenvironmental conditions. One important common feature of this altered metabolism is an increase in glucose uptake.30 It has been reported that in comparison to CD133– cells, CD133+ HCC cells preferentially survived when glucose was restricted by the IL-6/STAT3 pathway mediated glucose uptake.31 Similarly, a different study found that overexpression of CD133 enhanced glucose uptake and autophagy in low glucose media, and may help cells to survive in the harsh tumor microenvironments.19 Autophagy is important as it allows tumor cells to survive under conditions of nutrient stress, by recycling the by‐products of autophagic degradation. Autophagy can also influence CSCs phenotype through autophagy mediated regulation of metabolism.32 Significantly, CD133+ HCC cells showed higher survival rates during times of oxygen and nutrient deprivation which was found to be dependent upon higher rates of autophagy.33
The identification of LCSCs using a combination of CD133 and other surface markers
Although CD133+ cells are regarded as mainstream LCSC markers because of their strong tumor initiating capability, there remains some controversy whether CD133 represents a single marker for CSCs. Salnikov et al. found that CD133+ and CD133– HCC cells have no significant difference in migratory properties, and that the expression of CD33 had no correlation with prognosis in HCC patients.34 It has been observed that there are differential expression patterns of CD133 in several HCC cell lines, which ranged from 48% to 60% in PLC8024s to 49% to 65% in Huh7s. Therefore, as a single marker, CD133 may not be sufficient to determine CSCs in HCC.35 A recent study has provided evidence that LCSCs at the single‐cell level are phenotypically, functionally, and transcriptionally heterogeneous.36 Thus, identification of other markers, expressed along with CD133, is needed to better characterize the CSC population in HCC. Other cell surface markers that are expressed along with CD133 have been found to be capable of identifying CSCs in HCC. Ma et al. found that CD133+ ALDH+ cells confer significantly more tumorigenic capacity than their CD133-ALDH+ or CD133-ALDH− counterparts, which may help to characterize the liver CSCs more specifically.35 Wu et al. identified a subset of CD13+ CD133+ cells as liver CSCs through the detection of sphere formation, stemness analysis and tumor formation in xenograft models.37 A different study by Wang et al. isolated the CD13+ CD133+ cells from both HCC cell lines and HCC primary cells and further identified them as liver CSCs.38 When compared to CD133+ CD44– cells, these CD133+ CD44+ cells had greater tumorigenic and chemoresistance capabilities, and also exhibited preferential expression of stem cell-related genes.39 CD133+ CD44+ cells have also been reported to be associated with metastasis in liver cancer40,41 and CD133+/CD44+ in HCC cells have been found to have stem cell properties.42 A study by Chen et al. revealed that CD133+ EpCAM+ cells represent precisely CSCs in Huh7 cells.43 As compared to CD133+ EpCAM–, CD133–EpCAM+, CD133-EpCAM- cells, they found that CD133+ EpCAM+ cells have higher differentiation, colony-formation, drug-resistant, spheroid formation and tumorigenic capacity.43 A number of subsequent studies found that EpCAM+/CD133+ cells from the Huh7 cell line further verified their stem cell properties.44–46 However, this subgroup has not been found in other HCC cells. A recent clinical study found that patients with CD24+ CD133+ cells present in their tumors, had a worse clinical outcome than those with CD24+ CD133−, CD24− CD133+, or CD24− CD133−, present. Furthermore, CD24+ CD133+ cells showed significantly greater tumorigenicity and chemotherapy resistance to sorafenib than CD24− CD133− cells.47
Regulation of CD133+ LCSCs self-renewal
Signaling pathways
Among all the stem cell-related signaling pathways (Figure 1), the Wnt/β-catenin pathway has been found to be one of the most important in CSCs, including CD133+ LCSCs. Wnt/β-catenin signaling has been found to be hyperactivated in CD133+ LCSCs and plays a crucial role in the maintenance of stemness in CD133+ LCSCs.48,49 Accumulating evidence has revealed that aberrant NF-κB signaling is present in many types of CSCs.50 In CD133+ LCSCs, increased activation of the NF-κB signaling pathway was seen, and blockade of NF-κB activation significantly inhibited the self-renewal of CD133+ LCSCs.51,52 Furthermore, several studies have shown that activation of the STAT3 signaling pathway promotes the expression of CD133 through induction of transcription and the maintenance of CD133+ CSC stemness.53,54 Maehara et al. found that AMPK-CEBPβ signaling plays an important role in regulating the expression of CD133 and self-renewal of CD133+ liver CSCs.55 Many studies have reported a correlation between the JNK signaling pathway and CSCs, however, the role of JNK signaling in CSCs remains unclear.56 Tong et al. found that p-JNK was preferentially expressed in CD133+ HCC cells, and that activation of the JNK pathway promoted characteristics associated with CD133+ CSCs.57 In contrast, Jin et al. demonstrated that the expression of JNK-associated signaling molecules was inversely correlated to CD133 expression, and that CD133+ CSCs have high metastatic capacity due to inhibition of the JNK signaling transduction pathway.21 Thus, further studies are needed to elucidate these disputed functions related to JNK signaling in CSCs. Furthermore, the role of TGF-β signaling in CSCs remains controversial due to its complex functions in the development of cancer. It has been demonstrated that TGF-β can enhance the expression of CD133 and impart an aggressive EMT phenotype.58–61 Interestingly, a study by Chen et al. revealed that the inhibition of the TGF-β pathway through the activation of TLR4/NANOG exhibited enhanced tumorigenesis and chemoresistance in CD133+ CSCs.62
Figure 1.
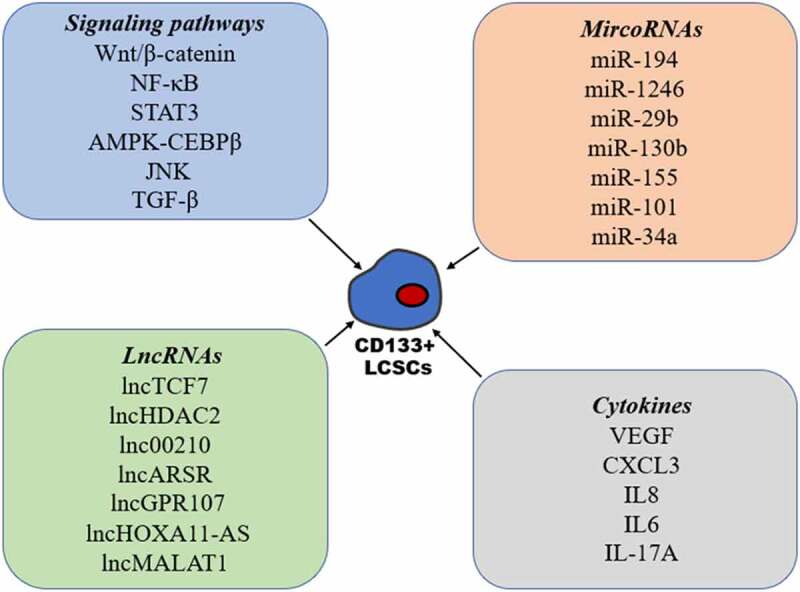
The molecular mechanism that regulates the stemness of CD133+ liver cancer stem cells
MircoRNAs
There is accumulating evidence linking microRNAs (miRNAs) to the regulation of CSC stemness, including CD133+ LCSCs (Figure 1). Ran et al. found that miR-194 decreased remarkably in CD133+ LCSCs, and that interference of miR-194 facilitated the self-renewal of CD133+ LCSCs by targeting Ras-related C3 botulinum toxin substrate 1 (RAC1).63 MiR-1246 has been found to be overexpressed in CD133+ LCSCs, which may play an important role in CSCs stemness via suppression of AXIN2 and GSK3β expression, and the constitutive activation of the Wnt signaling pathway.48 A study by Bai et al. revealed that miR‐29b could directly bind with CD133 to regulate the expression of CD133, and that miR‐29b is responsible for CD133+ cell stemness both in vitro and in vivo.64 Using miRNA expression profiling of CD133+ and CD133– cells, Ma et al. found that miR-130b was overexpressed in CD133+ spheres; they also demonstrated that miR-130b was involved in self renewal, tumorigenicity, and chemoresistance in CD133+ cells and this was achieved by targeting TP53INP1.65 MiR-155 was also demonstrated to regulate the expression of CD133 by targeting TP53INP1 in HCC cells.66 Using miRNA microarray analysis, Ma et al. found that miR-101 expression was downregulated in CD133+ liver CSCs. Furthermore, increasing the miR-101 expression markedly inhibited CD133+ CSC proliferation, and their tumorigenesis by targeting AXNA2,67 and a recent study showed that miR-34a plays an important role in the maintenance of CD133+ LCSC stemness via FOXM1.68
LncRNAs
Currently, there is a tremendous amount of work exploring the involvement of long noncoding RNAs (lncRNAs) in CD133+ LCSC self-renewal (Figure 1). Using transcriptome microarray analysis, Wang et al. identified lncTCF7 to be highly expressed in HCC tumors and CD133+ CD13+ cells and was also involved in CD133+ CD13+ LCSC self-renewal.38 A separate study by the same group identified a further lncRNA termed lncHDAC2 by conducting a transcriptome microarray analysis of CD13+ CD133+ cells and CD13− CD133− cells, and found that this lncRNA was able to promote the self-renewal of liver CD13+ CD133+ CSCs.37 A further transcriptome microarray analysis, by Fu et al., identified lnc00210 as being overexpressed in CD133+ cells and found that it played an important role in CD133+ LCSC self-renewal.69 Yang et al. showed that lncARSR expression was increased in CD133+ LCSCs, and that this lncRNA when silenced caused inhibition of the self-renewing capacity of CD133+ LCSCs.70 LncGPR107 was also highly expressed in HCC and CD133+ LCSCs, where it regulates the self-renewal of CD133+ CSCs through GPR107.71 A study by Guo et al. demonstrated that CD133+ cells showed high expression of lncHOXA11-AS, and that its silencing suppressed the self-renewal and tumorigenicity of CD133+ cells in vivo.72 A recent study further indicated that silencing lncMALAT1 downregulates β-catenin expression and attenuates CD133+ HCC cell populations.73
Cytokines
It is generally accepted that CSCs reside in a particular niche microenvironment, which has a complex architecture consisting of endothelial cells, fibroblastic cells, immune cells, an extracellular matrix, and a variety of cytokines.74 Cytokines have an important role in determining the characteristics of CSCs in both an autocrine or paracrine manner (Figure 1). Liu et al. demonstrated that vascular endothelial growth factor (VEGF) increased the proportion of CD133+ CSCs and enhanced their capacity for self-renewal via activation of the VEGF receptor (VEGFR2).75 Furthermore, the expression of CXCL3 in HCC cells is positively correlated with CD133, and CXCL3 plays an important role in CD133+ LCSCs maintenance via MAPK signaling.76 Kahraman et al. found that IL8 expression was increased significantly upon Sorafenib treatment in CD133+/EpCAM+ LCSCs and that IL8 inhibition with the inhibitor reparixin or siRNA, repressed the characteristics of LCSCs, and increased their sensitivity to sorafenib.26 A recent study also indicated that IL6-mediated inflammatory programs and enhanced the metastatic capacity of CD133+ LCSCs via constitutive activation of TAK1-NF-κB signaling, and that this program reacts to deficient TGFβ signaling.77 Finally, it has been found that IL-17A secreted from lymphatic endothelial cells promotes self-renewal and the immune escape of CD133+ LCSCs through upregulation of PD-L1.78
Clinical significance of CD133 in HCC
According to our current thinking, the characteristics of CSCs should be closely correlated with patient prognosis. Thus, detection of liver CSCs might contribute to a more accurate prediction of prognosis in HCC patients. Several studies have shown that high CD133 expression in HCC was an independent risk factor for the overall survival (OS) and relapse-free survival (RFS) rates in these patients.79–82 A separate study has indicated that CD133 expression was an independent prognostic factor for OS, and a highly accurate prognostic factor in patients with stage I disease in HCC.83 CSC biomarkers are expressed heterogeneously, and the positive rate of a single biomarker in cancers is always low. Thus, it is reasonable to assume that applying a set of CSC biomarkers may be more sensitive and specific than employing a single marker to predict prognoses in cancer patients. Based on Cox regression, Yang et al. used a simplified model including the CSC markers CD133, CD44, nestin and angiogenesis microvessel density (MVD) markers and found that these were a good independent predictor of OS and RFS, regardless of the α-fetoprotein (AFP) level, tumor stage, or recurrence time in HCC.84 Based on CD133 immunostaining and serum AFP levels, Dai et al. subclassified HCC cases into four subtypes and found that when compared to CD133+ AFP− HCC, CD133− AFP+ HCC and CD133− AFP− HCC, only CD133+ AFP+ HCC was associated with a relatively poor prognosis.85 Several studies have found that the PTEN−/CD133+ group had shorter OS and RFS times when compared to the PTEN−/CD133−, PTEN+/CD133+ and PTEN+/CD133− groups in HCC.86,87
The clinical significance of CD133 in the response to various treatments for HCC was also explored. It has been reported that high expression of CD133 was linked to a poor response to sorafenib, suggesting that CD133 may be a predictive biomarker for the nature of the response to sorafenib treatment in HCC.88 A further study showed that CD133 expression was related to the response to sorafenib; however, the association was not statistically significant for CD133 alone. Furthermore, they also found that the levels of CD133 and CD90 expression were associated with the clinical response to sorafenib.89 By studying resected livers from HCC patients during transplantation, CD133 was found to be an independent risk factor associated with increased recurrence and a worsening OS.90,91 Further studies with greater sample sizes and a longer follow-up period are required to elucidate the real clinical associations between CD133 and HCC.
Therapeutic strategies targeting CD133 in HCC
As mentioned above, CD133 is functionally important for LCSCs, making it an attractive therapeutic target. There have been therapeutic advances in the field of CD133+ LCSCs (Figure 2).
Figure 2.
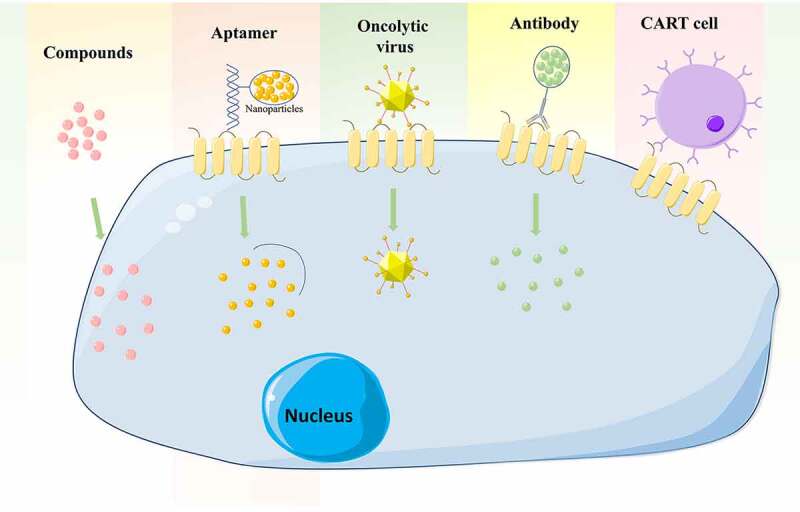
Therapeutic strategies targeting CD133 in HCC, including antibodies, aptamers, CAR T cells, oncolytic viruses and some drugs or compounds
Antibody
Monoclonal antibody targeting CSC markers in neoplasia is an attractive option for a novel therapeutic approach. A study by Chen et al. determined the effect of a monoclonal antibody directed against CD133 (CD133mAb) LCSCs in HCC cells, and found that treatment with CD133mAb inhibited spheroid and colony formation, suppressed xenografted tumor growth and promoted chemotherapy efficacy.92 Antibody-drug conjugates (ADC) have developed to recognize tumor-associated antigens and to release cytotoxic payloads to specific subsets of tumor cells.93 One such study used a murine anti-human CD133 antibody (AC133) conjugated to a cytotoxic drug, monomethyl auristatin F (MMAF), to inhibit the growth of Hep3B tumors in SCID mice.94 They also found AC133-vcMMAF treatment caused elimination of most CD133+ cells within the tumors investigated.94
Aptamers
Aptamers are small single-stranded DNA or RNA oligomers which form unique tertiary structures that bind to target molecules. Compared to antibodies, aptamers have low immunogenicity, low toxicity, and are chemically stable.95 One study used a CD133 aptamer A15 conjugated with salinomycin-loaded nanoparticles. Once internalized, into CD133+ HCC cells, the salinomycin was released to the cytoplasm and cells were destroyed.96 Zhou et al. developed a specific aptamer against CD133 (CD133-apt) that was loaded with the anticancer drug, doxorubicin (CD133-apt-Dox), which inhibited the self-renewal capacity of liver CSCs and attenuated their stemness phenotypes in vitro and in vivo.97
T‑cell therapy
Chimeric antigen receptor (CAR) T cells have been engineered to express synthetic antigen receptors that are specific to tumor surface antigens.98 CART cells directed toward CD133,
(CART-133) were tested in a clinical trial by Wang et al. They found encouraging antitumor activity from CART-133 for the treatment of HCC patients with advanced and CD133+ tumors.99 Results from a recent clinical trial have shown an important therapeutic potential for CART-133 cell therapy in patients with advanced HCC.100
CD133-targeted viruses
A growing body of evidence has demonstrated that oncolytic viruses can efficiently eliminate CSCs in many types of cancer, including HCC CSCs. Bach et al. generated CD133-targeted viruses using oncolytic measles virus, which selectively eliminate CD133+ HCC cells and exerted strong antitumoral effects on tumor growth subcutaneously or in a multifocal cancer model in NOD/SCID mice.101 Another study, by Terai et al., developed a mutated version of Herpes simplex virus‐1 (HSV‐1), which was transcriptionally targeted against CD133+ HCC cells, and the virus showed a robust inhibitory activity against tumor growth and invasiveness.102
Drugs and compounds
A study by Song et al. revealed that treatment with sulfasalazine (SASP) sensitizes CD133+ HCC to chemotherapies by decreasing the levels of glutathione (GSH), and suggested that a combination of SASP and conventional chemotherapy may effectively overcome resistance in HCC.103 Using highthroughput screening, Song et al. identified oxytetracycline as exhibiting a significant inhibition of the CD133+ population. They also demonstrated that oxytetracycline suppresses stemness and malignancies in HCC cells through destabilization of CD133 in CD133+ HCC cells.104 Ye et al. found that osthole reversed the resistance of CD133+ HCC cells to cisplatin through inhibited AKT/Bad/Bcl-2 pathway via the increase of PTEN expression.105 Zhang et al. indicated that As2O3 induced CD133+ HCC CSC differentiation and inhibited recurrence after radical resection and prolonged survival in a mouse model.106 Research by Song et al. demonstrated that chromenopyrimidinone (CPO) effectively suppressed stemness and malignancies caused by CD133+ cells in vitro and in vivo, by inducing degradation of CD133.107
Conclusion
CD133 is one of the most frequently used cell surface markers for the detection and isolation of CSCs from HCC. In the past two decades, there have been numerous studies relating to CD133+ LCSCs, and many novel and controversial views have emerged. The confirmation that CSC heterogeneity has led to an increasing number of researchers combining CD133 with other markers for the identification of LCSCs. CD133 is not just a surface marker of stem cells in HCC, its expression also plays an important role in signal transduction involved in the maintenance of stemness in liver CSCs.
Here, we have summarized the relevant mechanisms that regulate the stemness of CD133+ cells, including signaling pathways, non-coding RNA and the cellular microenvironment. Beyond the molecular mechanisms of CSCs, the expression of CD133+ in tumors contributes to the accuracy of prognosis in HCC patients. Although increasing studies suggest that CD133-targeted‐based therapies could be a novel promising option for the treatment of HCC (Figure 2), they currently remain far removed from clinical applications for their controversial specificity.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Disclosure statement
All authors declare that they have no conflict of interests.
References
- 1.Bruix J, Gores GJ, Mazzaferro V.. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koren E, Fuchs Y. The bad seed: cancer stem cells in tumor development and resistance. Drug Resist Updat. 2016;28:1–12. doi: 10.1016/j.drup.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123(5):1911–1918. doi: 10.1172/JCI66024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 5.Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer. 2017;16(1):4. doi: 10.1186/s12943-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang NZ, Wang SS, Li MY, Hu BG, Liu LP, Yang SL, Yang SC, Gong ZQ, Lai PBS, Chen GG. Cancer stem cells in hepatocellular carcinoma: an overview and promising therapeutic strategies. Ther Adv Med Oncol. 2018;10:1758835918816287. doi: 10.1177/1758835918816287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7(1):18. doi: 10.1186/s40169-018-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351(4):820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 9.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Yin SY, Li JJ, Hu C, Chen XH, Yao M, Yan MX, Jiang GP, Ge C, Xie HY, Wan DF, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120(7):1444–1450. DOI: 10.1002/ijc.22476 [DOI] [PubMed] [Google Scholar]
- 11.Corbeil D, Röper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2(2):82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- 12.Corbeil D, Karbanová J, Fargeas CA, Jászai J. Prominin-1 (CD133): molecular and cellular features across species. Adv Exp Med Biol. 2013;777:3–24. doi: 10.1007/978-1-4614-5894-4_1. [DOI] [PubMed] [Google Scholar]
- 13.Jang JW, Song Y, Kim SH, Kim J-S, Kim KM, Choi EK, Kim J, Seo HR. CD133 confers cancer stem-like cell properties by stabilizing EGFR-AKT signaling in hepatocellular carcinoma. Cancer Lett. 2017;389:1–10. doi: 10.1016/j.canlet.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Cho CY, Lee D, Song DG, Kim HJ, Jun JW, Kim JE, Park D, Lee H, Um H, et al. CD133-induced TM4SF5 expression promotes sphere growth via recruitment and blocking of protein tyrosine phosphatase receptor type F (PTPRF). Cancer Lett. 2018;438:219–231. doi: 10.1016/j.canlet.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Hyun SK, Kim HB, Kang CD, Kim SH. Potential role of CD133 expression in the susceptibility of human liver cancer stem-like cells to TRAIL. Oncol Res. 2016;24(6):495–509. doi: 10.3727/096504016x14685034103950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohga K, Tatsumi T, Takehara T, Tsunematsu H, Shimizu S, Yamamoto M, Sasakawa A, Miyagi T, Hayashi N, et al. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J Hepatol. 2010;52(6):872–879. DOI: 10.1016/j.jhep.2009.12.030 [DOI] [PubMed] [Google Scholar]
- 17.Kang M, Kim S, Ko J. Roles of CD133 in microvesicle formation and oncoprotein trafficking in colon cancer. FASEB J. 2019;33(3):4248–4260. doi: 10.1096/fj.201802018R. [DOI] [PubMed] [Google Scholar]
- 18.Karbanová J, Laco J, Marzesco AM, Janich P, Voborníková M, Mokrý J, Fargeas CA, Huttner WB, Corbeil D, et al. Human prominin-1 (CD133) is detected in both neoplastic and non-neoplastic salivary gland diseases and released into saliva in a ubiquitinated form. PloS One. 2014;9(6):e98927. DOI: 10.1371/journal.pone.0098927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HY, Luo ZL, Dong LW, Tan YX, Yang JM, Feng GH, Wu MC, Li Z, Wang HY. CD133/prominin-1-mediated autophagy and glucose uptake beneficial for hepatoma cell survival. PloS One. 2013;8(2):e56878. DOI: 10.1371/journal.pone.0056878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, Ng IO, Man K, To KF, Lai PB, et al. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55(3):807–820. DOI: 10.1002/hep.24739 [DOI] [PubMed] [Google Scholar]
- 21.Jin YL, Mao J, Wang HX, Hou ZH, Ma W, Zhang J, Wang B, Huang YH, Zang SZ, Tang JW, et al. Enhanced tumorigenesis and lymphatic metastasis of CD133+ hepatocarcinoma ascites syngeneic cell lines mediated by JNK signaling pathway in vitro and in vivo. Biomed Pharmacother. 2013;67(4):337–345. DOI: 10.1016/j.biopha.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 22.Na DC, Lee JE, Yoo JE, Oh BK, Choi GH, Park YN. Invasion and EMT-associated genes are up-regulated in B viral hepatocellular carcinoma with high expression of CD133-human and cell culture study. Exp Mol Pathol. 2011;90(1):66–73. doi: 10.1016/j.yexmp.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Xu YX, Lai Y, Weng HQ, Tan LP, Li YS, Chen GC, Luo XX, Ye YB, et al. MiR-124 sensitizes cisplatin-induced cytotoxicity against CD133(+) hepatocellular carcinoma cells by targeting SIRT1/ROS/JNK pathway. Aging. 2019;11(9):2551–2564. DOI: 10.18632/aging.101876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27(12):1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 25.Hemati H, Kaur J, Sobti RC, Trehanpati N. Inhibition of NOTCH signaling pathway chemosensitizes HCC CD133(+) cells to vincristine and 5-fluorouracil through upregulation of BBC3. Biochem Biophys Res Commun. 2020;525(4):941–947. doi: 10.1016/j.bbrc.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Kahraman DC, Kahraman T, Cetin-Atalay R. Targeting PI3K/Akt/mTOR pathway identifies differential expression and functional role of IL8 in liver cancer stem cell enrichment. Mol Cancer Ther. 2019;18(11):2146–2157. doi: 10.1158/1535-7163.Mct-19-0004. [DOI] [PubMed] [Google Scholar]
- 27.Piao LS, Hur W, Kim TK, Hong SW, Kim SW, Choi JE, Sung PS, Song MJ, Lee BC, Hwang D, et al. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315(2):129–137. DOI: 10.1016/j.canlet.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 28.Lee YK, Hur W, Lee SW, Hong SW, Kim SW, Choi JE, Yoon SK. Knockdown of 14-3-3ζ enhances radiosensitivity and radio-induced apoptosis in CD133(+) liver cancer stem cells. Exp Mol Med. 2014;46(2):e77. doi: 10.1038/emm.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong SW, Hur W, Choi JE, Kim JH, Hwang D, Yoon SK. Role of ADAM17 in invasion and migration of CD133-expressing liver cancer stem cells after irradiation. 2016;Oncotarget. 7(17):23482–23497. doi: 10.18632/oncotarget.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang HL, Wang MD, Zhou X, Qin CJ, Fu GB, Tang L, Wu H, Huang S, Zhao LH, Zeng M, et al. Blocking preferential glucose uptake sensitizes liver tumor-initiating cells to glucose restriction and sorafenib treatment. Cancer Lett. 2017;388:1–11. doi: 10.1016/j.canlet.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 32.El Hout M, Cosialls E, Mehrpour M, Hamaï A. Crosstalk between autophagy and metabolic regulation of cancer stem cells. Mol Cancer. 2020;19(1):27. doi: 10.1186/s12943-019-1126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song YJ, Zhang SS, Guo XL, Sun K, Han ZP, Li R, Zhao QD, Deng WJ, Xie XQ, Zhang JW, et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013;339(1):70–81. DOI: 10.1016/j.canlet.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 34.Salnikov AV, Kusumawidjaja G, Rausch V, Bruns H, Gross W, Khamidjanov A, Ryschich E, Gebhard MM, Moldenhauer G, Büchler MW, et al. Cancer stem cell marker expression in hepatocellular carcinoma and liver metastases is not sufficient as single prognostic parameter. Cancer Lett. 2009;275(2):185–193. DOI: 10.1016/j.canlet.2008.10.015 [DOI] [PubMed] [Google Scholar]
- 35.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6(7):1146–1153. doi: 10.1158/1541-7786.Mcr-08-0035. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Pomyen Y, Hernandez MO, Li C, Livak F, Tang W, Dang H, Greten TF, Davis JL, Zhao Y, et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology. 2018;68(1):127–140. DOI: 10.1002/hep.29778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu JY, Zhu PP, Lu TK, Du Y, Wang YY, He LY, Ye BQ, Liu BY, Yang LL, Wang J, et al. The long non-coding RNA LncHDAC2 drives the self-renewal of liver cancer stem cells via activation of Hedgehog signaling. J Hepatol. 2019;70(5):918–929. DOI: 10.1016/j.jhep.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 38.Wang YY, He L, Du Y, Zhu PP, Huang GL, Luo JJ, Yan XL, Ye BQ, Li C, Xia PY, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16(4):413–425. DOI: 10.1016/j.stem.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 39.Zhu Z, Hao XF, Yan MX, Yao M, Ge C, Gu JR, Li JJ. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126(9):2067–2078. doi: 10.1002/ijc.24868. [DOI] [PubMed] [Google Scholar]
- 40.Hou Y, Zou QF, Ge RL, Shen F, Wang YZ. The critical role of CD133(+)CD44(+/high) tumor cells in hematogenous metastasis of liver cancers. Cell Res. 2012;22(1):259–272. doi: 10.1038/cr.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JH, Hur W, Hong SW, Kim JH, Kim SM, Lee EB, Yoon SK. ELK3 promotes the migration and invasion of liver cancer stem cells by targeting HIF-1α. Oncol Rep. 2017;37(2):813–822. doi: 10.3892/or.2016.5293. [DOI] [PubMed] [Google Scholar]
- 42.Gao XM, Sheng YY, Yang J, Wang CQ, Zhang R, Zhu Y, Zhang Z, Zhang KL, Yan SC, Sun HT, et al. Osteopontin alters DNA methylation through up-regulating DNMT1 and sensitizes CD133+/CD44+ cancer stem cells to 5 azacytidine in hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37(1):179. DOI: 10.1186/s13046-018-0832-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Yu DK, Zhang H, He HW, Zhang CX, Zhao WL, Shao RG. CD133(+)EpCAM(+) phenotype possesses more characteristics of tumor initiating cells in hepatocellular carcinoma Huh7 cells. Int J Biol Sci. 2012;8(7):992–1004. doi: 10.7150/ijbs.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Firtina Karagonlar Z, Koç D, Şahin E, Avci ST, Yilmaz M, Atabey N, Erdal E. Effect of adipocyte-secreted factors on EpCAM+/CD133+ hepatic stem cell population. Biochem Biophys Res Commun. 2016;474(3):482–490. doi: 10.1016/j.bbrc.2016.04.137. [DOI] [PubMed] [Google Scholar]
- 45.Delman M, Avcı ST, Akçok İ, Kanbur T, Erdal E, Çağır A. Antiproliferative activity of (R)-4ʹ-methylklavuzon on hepatocellular carcinoma cells and EpCAM(+)/CD133(+) cancer stem cells via SIRT1 and Exportin-1 (CRM1) inhibition. Eur J Med Chem. 2019;180:224–237. doi: 10.1016/j.ejmech.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Karagonlar ZF, Akbari S, Karabicici M, Sahin E, Avci ST, Ersoy N, Ates KE, Balli T, Karacicek B, Kaplan KN, et al. A novel function for KLF4 in modulating the de-differentiation of EpCAM(-)/CD133(-) nonStem cells into EpCAM(+)/CD133(+) liver cancer stem cells in HCC cell line HuH7. Cells. 2020;9(5):1198. DOI: 10.3390/cells9051198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang RH, Li YW, Tsung A, Huang H, Du Q, Yang MQ, Deng MH, Xiong S, Wang XJ, Zhang LY, et al. iNOS promotes CD24(+)CD133(+) liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc Natl Acad Sci U S A. 2018;115(43):E10127–e36. DOI: 10.1073/pnas.1722100115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chai S, Ng KY, Tong M, Lau EY, Lee TK, Chan KW, Yuan YF, Cheung TT, Wang XQ, Wong N, et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology. 2016;64(6):2062–2076. DOI: 10.1002/hep.28821 [DOI] [PubMed] [Google Scholar]
- 49.Hou J, Zhao NP, Zhu PX, Chang J, Du Y, Shen W. Irradiated mesenchymal stem cells support stemness maintenance of hepatocellular carcinoma stem cells through Wnt/β-catenin signaling pathway. Cell Biosci. 2020;10:93. doi: 10.1186/s13578-020-00449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinkenbaugh AL, Baldwin AS. The NF-κB pathway and cancer stem cells. Cells. 2016;5(2):16. doi: 10.3390/cells5020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma DQ, Zhang YH, Ding DP, Li J, Chen LL, Tian YY, Ao KJ. Effect of Bmi-1-mediated NF-κB signaling pathway on the stem-like properties of CD133+ human liver cancer cells. Cancer Biomark. 2018;22(3):575–585. doi: 10.3233/cbm-181329. [DOI] [PubMed] [Google Scholar]
- 52.Lai FB, Liu WT, Jing YY, Yu GF, Han ZP, Yang X, Zeng JX, Zhang HJ, Shi RY, Li XX, et al. Lipopolysaccharide supports maintaining the stemness of CD133(+) hepatoma cells through activation of the NF-κB/HIF-1α pathway. Cancer Lett. 2016;378(2):131–141. DOI: 10.1016/j.canlet.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 53.Won C, Kim BH, Yi EH, Choi KJ, Kim EK, Jeong JM, Lee JH, Jang JJ, Yoon JH, Jeong WI, et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015;62(4):1160–1173. DOI: 10.1002/hep.27968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang YW, Wu G, Fu XY, Xu SL, Wang TL, Zhang Q, Yang Y. Aquaporin 3 maintains the stemness of CD133+ hepatocellular carcinoma cells by activating STAT3. Cell Death Dis. 2019;10(6):465. doi: 10.1038/s41419-019-1712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maehara O, Ohnishi S, Asano A, Suda G, Natsuizaka M, Nakagawa K, Kobayashi M, Sakamoto N, Takeda H. Metformin regulates the expression of CD133 through the AMPK-CEBPβ pathway in hepatocellular carcinoma cell lines. Neoplasia. 2019;21(6):545–556. DOI: 10.1016/j.neo.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semba T, Sammons R, Wang XP, Xie XM, Dalby KN, Ueno NT. JNK signaling in stem cell self-renewal and differentiation. Int J Mol Sci. 2020;21(7):2613. doi: 10.3390/ijms21072613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong M, Fung TM, Luk ST, Ng KY, Lee TK, Lin CH, Yam JW, Chan KW, Ng F, Zheng BJ, et al. ANXA3/JNK signaling promotes self-renewal and tumor growth, and its blockade provides a therapeutic target for hepatocellular carcinoma. Stem Cell Rep. 2015;5(1):45–59. DOI: 10.1016/j.stemcr.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rawal P, Siddiqui H, Hassan M, Choudhary MC, Tripathi DM, Nain V, Trehanpati N, Kaur S. Endothelial cell-derived TGF-β promotes epithelial-mesenchymal transition via CD133 in HBx-infected hepatoma cells. Front Oncol. 2019;9:308. doi: 10.3389/fonc.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu FC, Kong X, Lv L, Gao J. TGF-β1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015;359(2):288–298. doi: 10.1016/j.canlet.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 60.Chen GL, Ye T, Chen HL, Zhao ZY, Tang WQ, Wang LS, Xia JL. Xanthine dehydrogenase downregulation promotes TGFβ signaling and cancer stem cell-related gene expression in hepatocellular carcinoma. Oncogenesis. 2017;6(9):e382. doi: 10.1038/oncsis.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.You H, Ding W, Rountree CB. Epigenetic regulation of cancer stem cell marker CD133 by transforming growth factor-beta. Hepatology. 2010;51(5):1635–1644. doi: 10.1002/hep.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen CL, Tsukamoto H, Liu JC, Kashiwabara C, Feldman D, Sher L, Dooley S, French SW, Mishra L, Petrovic L, et al. Reciprocal regulation by TLR4 and TGF-β in tumor-initiating stem-like cells. J Clin Invest. 2013;123(7):2832–2849. DOI: 10.1172/jci65859 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Ran RZ, Chen J, Cui LJ, Lin XL, Fan MM, Cong ZZ, Zhang H, Tan WF, Zhang GQ, Zhang YJ. miR-194 inhibits liver cancer stem cell expansion by regulating RAC1 pathway. Exp Cell Res. 2019;378(1):66–75. doi: 10.1016/j.yexcr.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Bai HY, Liao YJ, Cai MY, Ma NF, Zhang Q, Chen JW, Zhang JX, Wang FW, Wang CY, Chen WH, et al. Eukaryotic initiation factor 5A2 contributes to the maintenance of CD133(+) hepatocellular carcinoma cells via the c-Myc/microRNA-29b axis. Stem Cells. 2018;36(2):180–191. DOI: 10.1002/stem.2734 [DOI] [PubMed] [Google Scholar]
- 65.Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, Ng I, Man K, Wong N, To KF, et al. miR-130b promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell. 2010;7(6):694–707. DOI: 10.1016/j.stem.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 66.Liu FC, Kong X, Lv L, Gao J. MiR-155 targets TP53INP1 to regulate liver cancer stem cell acquisition and self-renewal. FEBS Lett. 2015;589(4):500–506. doi: 10.1016/j.febslet.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Ma S, Cheng JP, Wang HY, Ding NL, Zhou F, Ji RN, Zhu L, Zhu CW, Pan YZ. A novel regulatory loop miR-101/ANXA2/EGR1 mediates malignant characteristics of liver cancer stem cells. Carcinogenesis. 2021;42(1):93–104. doi: 10.1093/carcin/bgaa055. [DOI] [PubMed] [Google Scholar]
- 68.Cao XC, Liu LH, Cao XZ, Cui YH, Zou C, Chen A, Qiu YB, Quan MF, Ren KQ, Chen XD, et al. The DNMT1/miR-34a/FOXM1 axis contributes to stemness of liver cancer cells. J Oncol. 2020;2020:8978930. doi: 10.1155/2020/8978930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu XM, Zhu XY, Qin FJ, Zhang Y, Lin JZ, Ding YC, Yang ZH, Shang YM, Wang L, Zhang QX, et al. Linc00210 drives Wnt/β-catenin signaling activation and liver tumor progression through CTNNBIP1-dependent manner. Mol Cancer. 2018;17(1):73. DOI: 10.1186/s12943-018-0783-3 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Yang C, Cai WC, Dong ZT, Guo JW, Zhao YJ, Sui CJ, Yang JM. lncARSR promotes liver cancer stem cells expansion via STAT3 pathway. Gene. 2019;687:73–81. doi: 10.1016/j.gene.2018.10.087. [DOI] [PubMed] [Google Scholar]
- 71.Huang GQ, Jiang H, Lin Y, Xia WZ, Luo YW, Wu YP, Cai WL, Zhou XK, Jiang XH. LncGPR107 drives the self-renewal of liver tumor initiating cells and liver tumorigenesis through GPR107-dependent manner. J Exp Clin Cancer Res. 2018;37(1):121. DOI: 10.1186/s13046-018-0794-3 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Guo JC, Yang YJ, Zheng JF, Zhang JQ, Guo M, Yang X, Jiang XL, Xiang L, Li Y, Ping H, et al. Silencing of long noncoding RNA HOXA11-AS inhibits the Wnt signaling pathway via the upregulation of HOXA11 and thereby inhibits the proliferation, invasion, and self-renewal of hepatocellular carcinoma stem cells. Exp Mol Med. 2019;51(11):1–20. DOI: 10.1038/s12276-019-0328-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang HL, Bamodu OA, Ong JR, Lee WH, Yeh CT, Tsai JT. Targeting the epigenetic non-coding RNA MALAT1/Wnt signaling axis as a therapeutic approach to suppress stemness and metastasis in hepatocellular carcinoma. Cells. 2020;9(4):1020. doi: 10.3390/cells9041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng Z, Li XF, Ding J. Characteristics of liver cancer stem cells and clinical correlations. Cancer Lett. 2016;379(2):230–238. doi: 10.1016/j.canlet.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 75.Liu K, Hao MJ, Ouyang Y, Zheng JS, Chen DX. CD133(+) cancer stem cells promoted by VEGF accelerate the recurrence of hepatocellular carcinoma. Sci Rep. 2017;7:41499. doi: 10.1038/srep41499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L, Zhang LX, Li H, Ge C, Zhao FY, Tian H, Chen TY, Jiang GP, Xie HY, Cui Y, et al. CXCL3 contributes to CD133(+) CSCs maintenance and forms a positive feedback regulation loop with CD133 in HCC via Erk1/2 phosphorylation. Sci Rep. 2016;6:27426. doi: 10.1038/srep27426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitra A, Yan J, Xia XQ, Zhou SH, Chen J, Mishra L, Li SL. IL6-mediated inflammatory loop reprograms normal to epithelial-mesenchymal transition(+) metastatic cancer stem cells in preneoplastic liver of transforming growth factor beta-deficient β2-spectrin(±) mice. Hepatology. 2017;65(4):1222–1236. doi: 10.1002/hep.28951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei YY, Shi DF, Liang ZW, Liu YM, Li YN, Xing Y, Liu WT, Ai ZL, Jiang JH, Chen XN, et al. IL-17A secreted from lymphatic endothelial cells promotes tumorigenesis by upregulation of PD-L1 in hepatoma stem cells. J Hepatol. 2019;71(6):1206–1215. DOI: 10.1016/j.jhep.2019.08.034 [DOI] [PubMed] [Google Scholar]
- 79.Chen YL, Lin PY, Ming YZ, Huang WC, Chen RF, Chen PM, Chu PY. The effects of the location of cancer stem cell marker CD133 on the prognosis of hepatocellular carcinoma patients. BMC Cancer. 2017;17(1):474. doi: 10.1186/s12885-017-3460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sasaki A, Kamiyama T, Yokoo H, Nakanishi K, Kubota K, Haga H, Matsushita M, Ozaki M, Matsuno Y, Todo S. Cytoplasmic expression of CD133 is an important risk factor for overall survival in hepatocellular carcinoma. Oncol Rep. 2010;24(2):537–546. doi: 10.3892/or_00000890. [DOI] [PubMed] [Google Scholar]
- 81.Song W, Li H, Tao K, Li R, Song Z, Zhao Q, Zhang F, Dou K. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62(8):1212–1218. doi: 10.1111/j.1742-1241.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 82.Yu HJ, Zhu XQ, Lin HC, Pan HY, Zhao FY, Zhu MX, Sun L, Chai WJ, Yao M, Yan MX. A new risk model comprising genes highly correlated with CD133 identifies different tumor-immune microenvironment subtypes impacting prognosis in hepatocellular carcinoma. Aging. 2020;12(12):12234–12250. doi: 10.18632/aging.103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan AW, Tong JH, Chan SL, Lai PB, To KF. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology. 2014;64(7):935–950. doi: 10.1111/his.12342. [DOI] [PubMed] [Google Scholar]
- 84.Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, Zhang BH, Wu WZ, Shi YH, Wu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59(7):953–962. DOI: 10.1136/gut.2008.176271 [DOI] [PubMed] [Google Scholar]
- 85.Dai XM, Yang SL, Zheng XM, Chen GG, Chen J, Zhang T. CD133 expression and α-fetoprotein levels define novel prognostic subtypes of HBV-associated hepatocellular carcinoma: a long-term follow-up analysis. Oncol Lett. 2018;15(3):2985–2991. doi: 10.3892/ol.2017.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen D, Li Z, Cheng Q, Wang Y, Qian L, Gao J, Zhu JY. Genetic alterations and expression of PTEN and its relationship with cancer stem cell markers to investigate pathogenesis and to evaluate prognosis in hepatocellular carcinoma. J Clin Pathol. 2019;72(9):588–596. doi: 10.1136/jclinpath-2019-205769. [DOI] [PubMed] [Google Scholar]
- 87.Su RJ, Nan HC, Guo H, Ruan ZP, Jiang LL, Song YY, Nan KJ. Associations of components of PTEN/AKT/mTOR pathway with cancer stem cell markers and prognostic value of these biomarkers in hepatocellular carcinoma. Hepatol Res. 2016;46(13):1380–1391. doi: 10.1111/hepr.12687. [DOI] [PubMed] [Google Scholar]
- 88.Hagiwara S, Kudo M, Nagai T, Inoue T, Ueshima K, Nishida N, Watanabe T, Sakurai T. Activation of JNK and high expression level of CD133 predict a poor response to sorafenib in hepatocellular carcinoma. Br J Cancer. 2012;106(12):1997–2003. doi: 10.1038/bjc.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim BH, Park JW, Kim JS, Lee SK, Hong EK. Stem cell markers predict the response to sorafenib in patients with hepatocellular carcinoma. Gut Liver. 2019;13(3):342–348. doi: 10.5009/gnl18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zen C, Zen Y, Mitry RR, Corbeil D, Karbanová J, O’Grady J, Karani J, Kane P, Heaton N, Portmann BC, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl. 2011;17(8):943–954. DOI: 10.1002/lt.22314 [DOI] [PubMed] [Google Scholar]
- 91.Vilchez V, Turcios L, Zaytseva Y, Stewart R, Lee EY, Maynard E, Shah MB, Daily MF, Tzeng CWD, Davenport D, et al. Cancer stem cell marker expression alone and in combination with microvascular invasion predicts poor prognosis in patients undergoing transplantation for hepatocellular carcinoma. Am J Surg. 2016;212(2):238–245. DOI: 10.1016/j.amjsurg.2015.12.019 [DOI] [PubMed] [Google Scholar]
- 92.Chen HY, Luo ZL, Sun W, Zhang CZ, Sun HL, Zhao NJ, Ding J, Wu MC, Li Z, Wang HY. Low glucose promotes CD133mAb-elicited cell death via inhibition of autophagy in hepatocarcinoma cells. Cancer Lett. 2013;336(1):204–212. doi: 10.1016/j.canlet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 93.Marcucci F, Caserta CA, Romeo E, Rumio C. Antibody-drug conjugates (ADC) against cancer stem-like cells (CSC)—is there still room for optimism? Front Oncol. 2019;9:167. doi: 10.3389/fonc.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, Zabinski RF, Sutherland MK, Gerber HP, Orden KLV, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008;99(1):100–109. DOI: 10.1038/sj.bjc.6604437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma HT, Liu JP, Ali MM, Mahmood MA, Labanieh L, Lu MR, Iqbal SM, Zhang Q, Zhao WA, Wan YA. Nucleic acid aptamers in cancer research, diagnosis and therapy. Chem Soc Rev. 2015;44(5):1240–1256. doi: 10.1039/c4cs00357h. [DOI] [PubMed] [Google Scholar]
- 96.Jiang JX, Chen HW, Yu C, Zhang YY, Chen MY, Tian S, Sun CY. The promotion of salinomycin delivery to hepatocellular carcinoma cells through EGFR and CD133 aptamers conjugation by PLGA nanoparticles. Nanomedicine. 2015;10(12):1863–1879. doi: 10.2217/nnm.15.43. [DOI] [PubMed] [Google Scholar]
- 97.Zhou G, Da Won Bae S, Nguyen R, Huo XQ, Han SL, Zhang ZQ, Hebbard L, Duan W, Eslam M, Liddle C, et al.. An aptamer-based drug delivery agent (CD133-apt-Dox) selectively and effectively kills liver cancer stem-like cells. Cancer Lett. 2021;501:124–132. doi: 10.1016/j.canlet.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 98.Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat Biomed Eng. 2018;2(6):377–391. doi: 10.1038/s41551-018-0235-9. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Chen MX, Wu ZQ, Tong C, Dai HR, Guo YL, Liu Y, Huang JH, Lv HY, Luo C, et al.. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology. 2018;7(7):e1440169. DOI: 10.1080/2162402x.2018.1440169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dai HR, Tong C, Shi DW, Chen MX, Guo YL, Chen DY, Han X, Wang H, Wang Y, Shen PP. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: a single-arm, open-label, phase II trial. Oncoimmunology. 2020;9(1):1846926. doi: 10.1080/2162402x.2020.1846926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bach P, Abel T, Hoffmann C, Gal Z, Braun G, Voelker I, Ball CR, Johnston IC, Lauer UM, Herold-Mende C, et al. Specific elimination of CD133+ tumor cells with targeted oncolytic measles virus. Cancer Res. 2013;73(2):865–874. DOI: 10.1158/0008-5472.Can-12-2221 [DOI] [PubMed] [Google Scholar]
- 102.Terai K, Bi DS, Liu Z, Kimura KG, Sanaat Z, Dolatkhah R, Soleimani M, Jones C, Bright A, Esfandyari T, et al. A novel oncolytic herpes capable of cell-specific transcriptional targeting of CD133± cancer cells induces significant tumor regression. Stem Cells. 2018;36(8):1154–1169. DOI: 10.1002/stem.2835 [DOI] [PubMed] [Google Scholar]
- 103.Song Y, Jang J, Shin TH, Bae SM, Kim JS, Kim KM, Myung SJ, Choi Ek SHR, Seo HR. Sulfasalazine attenuates evading anticancer response of CD133-positive hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2017;36(1):38. doi: 10.1186/s13046-017-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Song Y, Kim IK, Choi I, Kim SH, Seo HR. Oxytetracycline have the therapeutic efficiency in CD133(+) HCC population through suppression CD133 expression by decreasing of protein stability of CD133. Sci Rep. 2018;8(1):16100. doi: 10.1038/s41598-018-34301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ye JF, Sun D, Yu Y, Yu JH. Osthole resensitizes CD133(+) hepatocellular carcinoma cells to cisplatin treatment via PTEN/AKT pathway. Aging. 2020;12(14):14406–14417. doi: 10.18632/aging.103484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang KZ, Zhang QB, Zhang QB, Sun HC, Ao JY, Chai ZT, Zhu XD, Lu L, Zhang YY, Bu Y, et al. Arsenic trioxide induces differentiation of CD133+ hepatocellular carcinoma cells and prolongs posthepatectomy survival by targeting GLI1 expression in a mouse model. J Hematol Oncol. 2014;7:28. doi: 10.1186/1756-8722-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song Y, Kim S, Lee H, No JH, Ryu HC, Kim J, Lim JW, Kim M, Choi I, Seo HR. Chromenopyrimidinone controls stemness and malignancy by suppressing CD133 expression in hepatocellular carcinoma. Cancers. 2020;12(5):1193. doi: 10.3390/cancers12051193. [DOI] [PMC free article] [PubMed] [Google Scholar]


