Abstract
Background:
Providing informal care for a relative or friend with medical or mental needs can extol a physical burden on the caregiver, including impaired aspects of sleep quality such as suboptimal sleep duration, lengthened sleep latency, frequent awakenings, daytime sleepiness, and poor self-rated sleep quality. Diminished sleep quality can worsen the health in the caregiver, including dysregulation of hypothalamic-pituitary-adrenal axis (HPA) activity. Few studies have attempted to describe sleep in young adults who provide regular informal care. This study examines subjective and objective indicators of sleep quality and diurnal cortisol rhythms among young adult caregivers relative to non-caregiving peers. We expect that caregivers will exhibit poorer objective and subjective sleep quality, and greater dysregulation in diurnal cortisol indices, than demographically similar non-caregivers; and that caregivers with poorer sleep will exhibit pronounced cortisol dysregulation.
Methods:
Participants self-reported sleep quality over the prior month via the Pittsburgh Sleep Quality Index and objective sleep quality was observed via wrist actigraph for three consecutive days. Diurnal salivary cortisol was also measured across the three days of actigraph monitoring.
Results:
Informal caregivers exhibited more self-reported sleep disturbance and greater sleep latency than non-caregivers, as well as more objectively measured sleep fragmentation. Caregivers with shorter sleep duration were observed to have flatter diurnal cortisol slopes than caregivers with relatively longer sleep duration.
Conclusions:
Young adult caregivers appear to be at risk for impairment in sleep quality, which in turn might impact health through HPA axis dysregulation. Longitudinal research is needed to identify these relationships across time.
Keywords: Caregiving, Sleep, Young Adults, Actigraphy, Cortisol
Introduction
The burden of informal caregiving, or providing daily help to close friends or family members managing medical or mental illness or disability, can have an impact on caregivers’ overall health and sleep. In fact, insomnia is common among informal caregivers, with rates between 40–76% [1–6], which exceeds prevalence rates in the general population [7]. Caregivers show several impaired sleep characteristics, including suboptimal sleep duration, lengthened sleep latency, frequent awakenings, daytime sleepiness, and poor self-rated sleep quality [1, 3, 5, 8]. Importantly, the moderate to severe symptoms of insomnia experienced by a large proportion of caregivers do not remit naturally [6].
Lack of adequate sleep may result in negative physical and emotional outcomes, worsening the caregiving quality and feelings of fatigue [9–11]. Studies exploring the impact of sleep problems on physical and emotional outcomes in patients with chronic illness (e.g., dementia or cancer) are extensive. Yet few studies have attempted to describe sleep in young adult caregivers [12]. Moreover, young adults might be at heightened risk for dysregulated sleep, including short sleep duration, as sleep timing (both behaviorally and circadian phasing) shift to later hours across young adulthood [13–14]. Therefore it is plausible that sleep quality in young adults may be disproportionately impacted by the burdens of informal caregiving.
Young adults (18 to 29 years old) comprise between 12% to 18% of the total number of adult caregivers and is a population that is grossly understudied [12]. They experience higher levels of caregiving stress and burden than older caregivers, which in turn have a negative impact on their pursuit of educational and career goals [15]. Caregiving in young adulthood likely exerts a negative physiological toll, however, which specific aspect of sleep quality is responsive to caregiving and the impact on dynamics in stress hormone activity are largely unknown.
When an individual perceives events, such as those related to caregiving, as stressful, the hypothalamic-pituitary-adrenal (HPA) axis works to coordinate a cascade of patterned autonomic and neuroendocrine responses in an effort to regain physiologic homeostasis [16]. Among these responses is the secretion of cortisol by the adrenal cortex, which aids in the up- and down-regulation of adaptive stress responses [17]. Under prolonged periods of stress, the HPA axis can reflect over- or under-activation [18–20]. Such dysregulation, often characterized by flattened diurnal slopes, blunted cortisol awakening response (CAR), or elevated daily output, can be detrimental to health over time [21].
The level of chronic stress experienced by long-term caregiving has been shown to impact cortisol patterns, suggesting physiological changes may also be a result of the stress of caregiving [22–23]. Specific patterns of cortisol dysregulation have been noted among caregivers, including blunted CAR [24–25] and flattened diurnal cortisol slope [22]. In addition, cortisol dysregulation may be proportional to the level of caregiving burden [26]. Thus, dysregulation in cortisol may be a particular physiological consequence of caregiver-specific stress. Additionally, HPA axis activity has well-established relationships with various dimensions of sleep quality including nighttime awakenings [27] and poor overall sleep quality [16, 28], and might underscore disruptions in sleep quality [29–30].
Dimensions of sleep quality including sleep duration and fragmentation are associated with coping behaviors and better sleep quality might constitute a critical coping resource. For instance, in a racially diverse sample of adolescence, poor sleep quality was associated with patterns of disengagement coping [31], which might be a poor match to caregiving demands and general life stressors. Thus, the combination of caregiving demands and poor sleep quality might impair individual ability to effectively cope with caregiving demands and together constitutes compounded risk for HPA axis dysregulation.
The current study is designed to contribute to our understanding of the unique stress and coping experiences of this population from their non-caregiving peers. The purpose is to assess the overall sleep quality of young adult informal caregivers relative to non-caregivers. A focus is on examination of one-month subjective sleep quality and objectively measured wrist actigraph sleep monitoring. This study was driven by two primary sets of hypotheses. First, we hypothesized that informal caregivers would have poorer objective and subjective sleep quality, and greater dysregulation in diurnal cortisol indices, than demographically similar non-caregiving young adults. Second, we expected to observe a caregiver status by sleep quality interaction such that caregivers with poorer sleep will exhibit pronounced cortisol dysregulation.
Methods
Participants
Data were collected from young adults attending a large public university in the United States recruited from student subject pools and via fliers posted throughout the university on physical and electronic bulletin boards. All participants were thoroughly screened to determine their current caregiver status. This involved an initial question to establish that informal caregivers “provide significant daily caregiving to a relative, close friend, or household member who needs help because of a physical or mental illness, disability, frailty associated with aging, substance misuse or other condition.” Those responding positively underwent a brief screening interview to discuss their specific caregiving role, level of caregiving effort, and specific caregiving tasks. Only those participants who reported being the primary caregiver (part-time or full-time) and provided daily or near daily caregiving were included.
A total of 76 participants were enrolled and completed study procedures. However, three participants were excluded from data analysis because responses to questionnaire items suggested their caregiving was related to regular parenting or was better characterized as diffuse (inconsistent or in a secondary caregiver role) caregiving responsibilities. Therefore, the final sample included thirty-five caregivers and thirty-eight demographically similar non-caregivers.
Procedures
In a laboratory setting, participants completed questionnaire measures and height and weight were measured. Participants were then fitted with a Motionlogger wrist actigraph (Ambulatory Monitoring, Inc., Ardsley, NY) to wear on their non-dominant wrist outside the laboratory for 72 weekday hours [32] as a noninvasive and ecologically-valid method for obtaining objectively-assessed sleep quality parameters. Devices were programmed to record activity for three consecutive nights of sleep using one-minute epoch lengths. Concurrent with days of at-home sleep monitoring, participants collected saliva upon awakening (morning), 30 minutes after waking, 8 hours post-awakening (afternoon), and at bedtime for three days using Salivette collection tubes (Sarstedt, Inc.) for assessment of diurnal cortisol. They were instructed not to eat, drink, or brush teeth for at least 20 minutes before sampling. Participants refrigerated samples until returning them to the laboratory with the actigraph device. Salivettes were stored in a −20°C freezer until analysis.
All study procedures were approved by the Institutional Review Board of the primary author’s institution, and written informed consent was obtained from all participants.
Measures
Pittsburgh Sleep Quality Index (PSQI) [33] was used as a measure of global subjective sleep quality over the past thirty days. The PSQI provides assessment of various sleep quality parameters. Using standard PSQI scoring procedure [33], we focus on four dimensions: sleep duration, sleep disturbance, sleep latency, and sleep-related daily dysfunction. For each parameter, scores ranged from 0 (better) to 3 (worse).
Caregiver Burden was measured with the Level of Care Index (LCI) [34]. The LCI assesses overall perceived caregiving burden experienced by the caregiver and distinguishes burden level as mild, moderate, or high. A burden index is computed for each caregiver based on hours of weekly caregiving and intensity of care (e.g., assistance with activities of daily living). Burden scores can range from 2 to 8. Cut scores are used to classify index scores as mild (total score of 2 to 4), moderate (total score of 5), or high (total score of 6 to 8) levels of caregiving burden and are used for descriptive characterization. However, scores were treated as continuous variable scores in statistical testing.
Demographics and behavioral factors.
Participants self-reported demographic variables. Relevant behavioral factors were also measured and included the Hospital Anxiety and Depression Scale (HADS) [35]. During the at-home monitoring period, participants completed the Consensus Sleep Diary [36], indicating sleep behaviors (e.g., time to bed).
Wrist actigraphy.
Sleep quality parameters were calculated using the Action-W program and included: sleep duration (total nighttime sleep minutes), sleep efficiency (ratio of nighttime sleep duration to total sleep period), sleep latency (minutes to first sleep epoch), and sleep fragmentation (ratio of number of nighttime awakenings to nighttime sleep duration * 100). The index of sleep fragmentation considers movements of varying intensity.
Diurnal cortisol.
Concentrations of salivary free cortisol were measured in duplicate using a commercially available immunoassay (Salimetrics, Inc). Assay sensitivity was measured to be <.007 ug/dL. The lower detection limit is .33 nmol/L, and inter-assay and intra-assay coefficients of variance are <15%. Three indices were calculated to characterize the distinctive circadian pattern of cortisol secretion: cortisol awakening response (CAR), diurnal slope, and area under the curve (AUCg). Participants recorded adherence to instructions for saliva sample collection across the collection period.
Data Analysis
Actigraph data were downloaded and analyzed with the Action-W (version 2.0) [37] software program using Zero Crossing Mode of activity, which utilizes peer-reviewed sleep cycle algorithms. Data were manually trimmed to the time period of interest by members of the study team in strict adherence to protocols developed in our laboratory and in consultation with participant sleep logs. Time in bed was determined by participant diary, light sensor data, and visual inspection of histogram data.
To account for skewness, raw cortisol values were log transformed prior to analyses. CAR was assessed by changes from awakening (averaged across days) to the second sample (30 minutes post-awakening; averaged across days). Diurnal slope was calculated as the decrease from the first morning sample to evening sample. To assess volume, AUCg was computed and averaged across days using the trapezoidal method based on hours after wakening [38]. The 30-minute post-awakening measure was excluded from AUCg calculation [39].
Descriptive statistics and zero-order correlations were computed for key study variables. Relevant biometric with known relevance to salivary cortisol (e.g., body mass index) and demographic variables were examined to identify possible covariates. Variables that differed significantly by caregiver status were included in subsequent statistical models. Covariate by predictor interactions were also examined.
Independent samples t-tests were conducted to detect between group differences in sleep quality. Multiple linear regression analysis was used to determine the relationship of caregiver status and indices of objective and subjective sleep quality, when controlling for relevant covariates. Analyses were conducted using SPSS v26. Moderator analyses were conducted in accordance with procedures outlined by Aiken and West [40] to test the hypothesized caregiver by sleep quality interactions using the PROCESS macro [41] in SPSS.
Results
Descriptives and Preliminary Analyses
Participant characteristics are reported in Table 1. On average, participants were nearly 21 years of age and the majority were female. Black participants were more likely to be caregivers than were White participants, and although only approaching statistical significance, caregivers had a higher body mass index (BMI) than non-caregivers. Thus, ethnic minority status and BMI were included in model testing as statistical covariates.
Table 1.
Participant Characteristics
| Caregivers (n=35) | Non-Caregivers (n=38) | p-value | |
|---|---|---|---|
| Age, M (SD) (years) | 21.17 (5.93) | 20.47 (4.10) | .564 |
| Female (%) | 71.4% | 71.1% | .972 |
| Race/Ethnicity (%) | |||
| White | 14.3% | 36.8% | .027 |
| Black | 17.1% | 2.6% | .043 |
| Hispanic | 37.1% | 21.1% | .136 |
| Asian | 28.6% | 31.6% | .783 |
| American Indian | 2.9% | 0.0% | .324 |
| Other | 2.9% | 7.9% | .343 |
| Caregiver Burden | |||
| Low | 34.3% | - | - |
| Moderate | 11.4% | - | - |
| High | 54.3% | - | - |
| HADS - Depressive | 6.27 (3.90) | 3.57 (3.17) | .002 |
| HADS - Anxiety | 9.40 (4.69) | 7.34 (3.31) | .036 |
| 30-Day Sleep Medication | 20% | 18.4% | .883 |
| Body Mass Index, M | 25.07 (6.00) | 22.99 (3.76) | .085 |
Note: M (SD) = mean (standard deviation); HADS = Hospital Anxiety and Depression Scale
As reported in Table 1, caregivers had significantly more depressive symptoms than non-caregivers, although both caregivers and non-caregivers’ average scores were within what is typically considered as “normal range” on the HADS depressive symptom scale. On average, caregivers reported anxiety symptoms in the “borderline abnormal” range, and this was significantly higher than the average HADS anxiety symptoms score for non-caregivers (which was in the “normal” range).
Aspects of caregiving activities are reported in Table 2. The majority of caregiving was for a parent, grandparent or sibling, involved 10–29 hours of care per week, and was to care for a physically ill or disabled loved one. Caregiver burden scores ranged from 2 to 8 on the LCI (M = 5.35, SD = 1.69). Notably, the majority of caregivers reported a “high” level of caregiver burden. The LCI reflects a combination of high numbers of weekly caregiving hours and high interference in daily living.
Table 2.
Caregiving Characteristics
| Care Receiver | Reasons for Care Provision | ||
| Parent | 33.2% | Physical Illness | 41.8% |
| Grandparent | 23.7% | Physical Disability | 18.9% |
| Sibling | 21.1% | Age-related Frailty | 17.7% |
| Other Relative | 16.2% | Mental Illness | 16.4% |
| Close Friend | 3.2% | Substance Abuse | 1.3% |
| Offspring | 2.6% | Other | 3.9% |
| Partner/Spouse | 2.6% | ||
| Hours of Weekly Care Provision | Resides with Caregiver | ||
| 0 to 9 | 21.1% | Full-time | 68.4% |
| 10 to 19 | 23.7% | Part-time | 5.3% |
| 20 to 29 | 34.2% | ||
| 30 to 39 | 7.9% | ||
| 40+ | 13.1% | ||
| Caregiving Tasks | |||
| Housework | 36.7% | Dressing | 17.7% |
| Technology Assistance | 32.9% | Eating/Feeding | 13.9% |
| Medication Management | 26.6% | Grooming | 13.9% |
| Cooking | 25.3% | Transportation | 11.4% |
| Laundry | 24.1% | Bathing | 6.3% |
| Household Shopping | 22.8% | Toileting | 5.1% |
| Walking/Mobility Assistance | 19.0% |
Note: Caregiving tasks are not mutually exclusive categories.
Subjective Sleep Quality
Mean values of subjective sleep parameters were examined for caregivers and non-caregivers (see Table 3). Relative to non-caregivers, caregivers reported significantly more sleep disturbance and greater sleep latency in the prior 30-day period. There were no significant group differences on other subjective sleep parameters.
Table 3.
Subjective and Objective Sleep Quality
| Caregivers M(SD) | Non-Caregivers M(SD) | t(df) | 95% CI | p-value | Cohen’s d | |
|---|---|---|---|---|---|---|
| Subjective Sleep Parameters | ||||||
| Sleep Duration | .93(.80) | .59(.88) | 1.70(74) | −.06, .71 | .092 | .40 |
| Sleep Disturbance | 1.92(.55) | 1.64(.58) | 2.09(73) | .01, .54 | .040 | .50 |
| Sleep Latency | 1.68(.82) | 1.31(.69) | 2.12(74) | .02, .71 | .038 | .49 |
| Sleep-Related Daily Dysfunction | 2.14(.86) | 1.80(.86) | 1.73(74) | −.05, .73 | .089 | .01 |
|
Actigraphic Sleep Parameters | ||||||
| Sleep Duration (minutes) | 317.07(81.17) | 353.33(111.23) | −1.41(74) | −78.94, 13.53 | .134 | .37 |
| Sleep Efficiency | 93.80(3.94) | 94.66(3.15) | −.82(74) | −2.41, 1.00 | .329 | .24 |
| Sleep Latency | 10.16(10.03) | 13.12(11.81) | −1.29(74) | −8.61, 1.91 | .279 | .27 |
| Sleep Fragmentation | 6.07(3.71) | 3.22(2.24) | 1.79(74) | −.32, 5.77 | .033 | .93 |
Results did not differ when controlling for ethnic minority status or BMI. However, a significant interaction of caregiver status and ethnic minority status was observed for sleep duration, such that caregiver status was associated with longer sleep duration for White identifying caregivers (β = −.81, p <.001), but not for ethnic minority caregivers (β = .04, p = .744).
Actigraphic Sleep Quality
Mean values of actigraphic sleep parameters for caregivers and non-caregivers are reported in Table 3. Relative to non-caregivers, caregivers demonstrated significantly more sleep fragmentation during the observation period. There were no significant group differences on other sleep indices.
Results did not differ when controlling for ethnic minority status or BMI. However, a significant interaction of caregiver and ethnic minority status was observed for sleep fragmentation, such that caregiver status was associated with less fragmented sleep for White identifying caregivers (β = −.54, p <.00) but not for ethnic minorities (β = −.14, p =.332).
Salivary Diurnal Cortisol
Regression analyses examining caregiver status as a predictor of diurnal cortisol indices, controlling for BMI and ethnic minority status, did not yield any significant direct relationships. However, in hypothesis testing [F(3,59) = 3.06,p=.035, R2 = .14], caregiver status interacted with actigraph measured sleep duration (B=−.01, SE = .001, p<.01) in the prediction of daily cortisol slope. Examination of simple slopes revealed that for caregivers (B=.13, SE = .001,p = .019), but not for non-caregivers (B = .01, SE = .001,p =.722), shorter sleep duration was associated with flatter cortisol slope. This relationship is illustrated in Figure 1.
Figure 1.
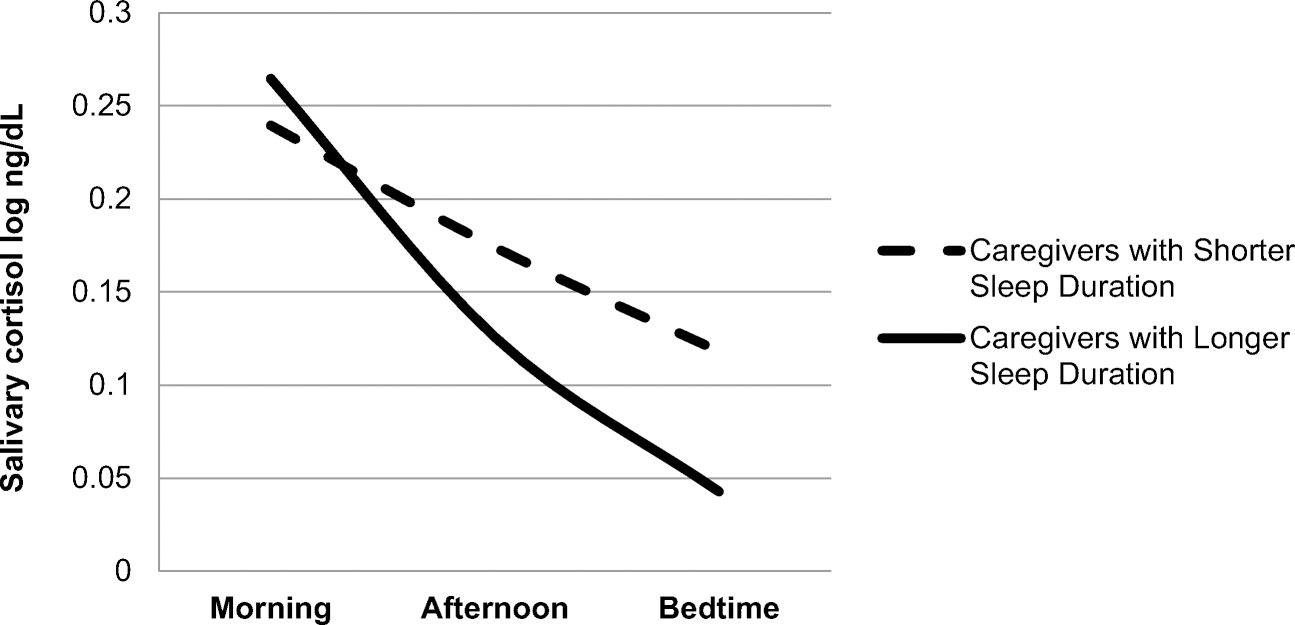
Caregiver Diurnal Cortisol Patterns by Sleep Duration
Figure Note. For illustrative purposes, a median split was used to define shorter and longer sleep duration.
Post-Hoc Analysis of Caregiver Burden
In a set of post-hoc analyses, caregiver burden was regressed on indices of salivary diurnal cortisol to inform that possibility that the burden of caregiving is linearly related to stress processes. Multiple regression testing included BMI and ethnic minority status as potential covariates and tested for sleep by caregiver burden interaction effects.
Caregiver burden (β = −.69, p = .044) and objectively measured sleep duration (total sleep minutes) (β = .25, p = .030) were significantly associated with diurnal cortisol slope. Moreover, a significant caregiver burden by objectively measured sleep duration (total sleep minutes) interaction (p = .032) reveals that the relationship of caregiving burden to flatter cortisol slopes are most pronounced for caregivers experiencing relatively fewer overall minutes of sleep.
Caregiver burden was not associated with other cortisol indices and no other caregiver by sleep parameter interactions were significant.
Discussion
The demands of caregiving can negatively impact caregivers’ functioning across many life domains, particularly among young adult caregivers who are simultaneously engaged in the developmental tasks associated with the transition from adolescence to early adulthood [42]. The current study provides preliminary evidence for the negative impact of informal caregiving on sleep quality in this group. Results suggest that caregiving is associated with several dimensions of diminished sleep quality including self-reported sleep disturbance and sleep latency, as well as objectively measured sleep fragmentation.
Consistent with a primary hypothesis, caregiver status interacted with sleep quality in the case of objective sleep duration to predict diurnal patterns of salivary cortisol. Typically during sleep, blood levels of cortisol fall [43], but chronic disturbances in sleep have been associated with over-activation of the HPA pathway [43]. In this study, caregivers getting less overall sleep exhibited a flatter cortisol slope. That appears (see Figure 1) to be marked by higher evening cortisol levels. This finding provides only preliminary insight into the impact of sleep problems on cortisol dysregulation in the context of caregiving stress; the nature of these relationships remains inconclusive. More careful examination of overall sleep duration in caregivers may be warranted. A study of dementia caregivers also pointed to sleep duration as being associated with dynamic cortisol patterns [44]. Future research should seek to identify modifiable psychological, behavioral, or biological factors that perpetuate or interrupt this pattern.
Our findings are, in part, consistent with literature suggesting decrements in sleep quality potentiate HPA axis reactivity to stressful demands [45]. Our pattern of results support the notion that poor sleep quality might deplete coping resources rendering caregivers more vulnerable to poor stress regulation. Our finding that ethnic minority caregivers may be both more likely to be caregivers and more likely to experience poorer sleep when caregiving, compared to their White counterparts, might be pointing to an important health disparity. Buckhalt and colleagues [46] suggest that an accumulation of stressors is most impactful on sleep outcomes. Ethnic minority caregivers likely experience heightened daily stressors independent of caregiving, further depleting coping resources [47]. An important research direction will be to identify modifiable, explanatory factors underlying such disparities. It is important to note that caregivers were more likely to identify as ethnic minority than White in the current the sample. Moreover, ethnic minority caregivers report an average of more than four hours of caregiving per week compared to White caregivers. It may be that familial or cultural expectations around informal caregiver vary by ethnic group as might reliance on the informal caregiving for higher level needs. Future studies should also seek to identify critical resilience factors that foster better sleep that might vary by ethnicity.
A strength of this study is the focus on dimensions and not simply overall sleep quality. This provides insight into more nuanced aspects of sleep. At the same time, it should be noted that although results provided some support for the hypotheses, results did not hold across sleep quality dimensions. Other study limitations should be noted. This study examined relationships at one moment in time. Future studies should include multiple assessments over time to identify dynamic patterns of relationships and more insight into temporal patterns. Also, although the current study carefully screened to identify caregivers, participants represented caregiving in different contexts. It might be that caregiving within certain contexts (e.g., for a parent with cancer) could vary greatly from others (e.g., caregiving for a depressed spouse). We also note that our recruitment method yielded limited variation in education level; however, racial diversity of the sample was a strength. Finally, we note that actigraphy was measured over three days, which might not reflect fluctuations that would be detected if the monitoring period was of longer duration. It is also possible that actigraph monitoring over a longer duration would better ensure stability in our estimations [48]. We do note that all monitoring occurred on weekdays and on self-reported “typical” days.
This study demonstrated the importance of attending to both subjective and objectively measured sleep. This may be particularly important in caregivers, as they have been known to poorly estimate their sleep quality. One study reported that caregivers of cancer patients tended to overestimate their total sleep time [12], while another study reported that caregivers underestimate their sleep quality [49]. Objective sleep measurement provides important information about sleep behaviors that may not be captured by self-report measures administered during the laboratory visit. Additionally, the impact of chronic general stress in young adults should be considered in future work. Building from our finding on sleep fragmentation, future work should more carefully examine nighttime or “on-call” caregiving duties, as well as the possible impact of night time studying demands or nighttime use of mobile devices.
Taken together, the physical and emotional demands associated with caregiving contribute to and may exacerbate sleep problems, and dysregulation in stress processes among young informal caregivers. Studies of caregivers should, however, not assume that caregiving is universally burdensome. The inclusion of a non-caregiving comparator group provides evidence of caregiving burden in this sample. Moreover, our post-hoc analyses mirrored our findings that caregivers experiencing high levels of caregiver burden and shortened sleep duration are at heightened risk for dysregulation in stress hormones.
The need to assist caregivers in improving sleep quality is paramount. Sleep impairment is associated with elevated risk for a host of physical and psychiatric disorders [50–52], which are also associated with chronic stress. Despite the well-documented insomnia and related distress experienced by caregivers, and caregiving-specific risk factors for insomnia, there are no empirically supported treatments to improve sleep among this vulnerable group. There is, however, emerging evidence for the benefits of Cognitive Behavioral Therapy for Insomnia (CBT-I) [53–54] tailored to the unique setting of cancer caregiving. Future studies are therefore needed to evaluate the efficacy and effectiveness of CBT-I in addressing the unique experience of sleep disturbance in caregivers as well as to further identify dimensions of problem sleep affected by caregivers.
Acknowledgement:
Funding was provided by the National Cancer Institute (SC1 CA187494).
Footnotes
Conflict of Interest Statement: All authors declare they have no conflicts of interest.
Ethical Statement: As stated in the manuscript: All study procedures were approved by the Institutional Review Board of the primary author’s institution and informed consent was obtained from all individual participants included in the study.
References
- 1.Carney S, Koetters T, Cho M, et al. Differences in sleep disturbance parameters between oncology outpatients and their family caregivers. J Clin Oncol. 2011;29:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christakis NA, Allison PD. Mortality after the hospitalization of a spouse. New Engl J Med. 2012;354:719–730. [DOI] [PubMed] [Google Scholar]
- 3.Dhruva A, Lee K, Paul SM, et al. Sleep-wake circadian activity rhythms and fatigue in family caregivers of oncology patients. Cancer Nurs. 2012;35:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Happe S, Berger K, FAQT Study Investigators. The association between caregiver burden and sleep disturbances in partners of patients with Parkinson’s disease. Age Ageing. 2002;31:349–354. [DOI] [PubMed] [Google Scholar]
- 5.Lee KC, Yiin JJ, Lin PC, Lu SH. Sleep disturbances and related factors among family caregivers of patients with advanced cancer. Psycho-Oncol, 2015;24:1632–1638. [DOI] [PubMed] [Google Scholar]
- 6.Morris BA, Thorndike FP, Ritterband LM, Glozier N, Dunn J, Chambers SK. Sleep disturbance in cancer patients and caregivers who contact telephone-based help services. Supportive Care Cancer. 2015;23:1113–1120. [DOI] [PubMed] [Google Scholar]
- 7.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation of chronic insomnia in adults. J Sleep Med. 2008;15:487–504. [PMC free article] [PubMed] [Google Scholar]
- 8.Pawl JD, Lee SY, Clark PC, Sherwood PR. Sleep loss and its effects on health of family caregivers of Individuals with primary malignant brain tumors. Res Nurs Health. 2013;36:386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter PA, Chang BL. Sleep and depression in cancer caregivers. Cancer Nurs. 2000;23:410–415. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz KA. A study of the relationship of caregiving appraisal to depressive symptomatology and home care utilization. J Commun. Health Nurs. 1999;16:95–108. [DOI] [PubMed] [Google Scholar]
- 11.Yates ME, Tenstedt S, Chang BH. Contributors to and mediators of psychological well-being for informal caregivers. J Gerontol Series B Psychol Sci Soc Sci. 1999;54:P12–P22. [DOI] [PubMed] [Google Scholar]
- 12.Carter PA. Bereaved caregivers’ descriptions of sleep: impact on daily life and the bereavement process. Oncol Nurs Forum. 2003;32:741. [DOI] [PubMed] [Google Scholar]
- 13.Crowley S, Van Reen E, LeBourgeois MK, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS ONE. 2014;9:e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagenauer MH, Perryman JI, Lee TM, et al. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31(4):276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine C, Gibson Hunt G, Halper D, Hart AY, Lautz J, Gould DA. Young adult caregivers: a first look at an unstudied population. Amer J Pub Health Assn. 2005;95:2071–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83e98. [DOI] [PubMed] [Google Scholar]
- 18.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA.1992;267:1244e52. [PubMed] [Google Scholar]
- 19.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1e35. [DOI] [PubMed] [Google Scholar]
- 20.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865e71. [DOI] [PubMed] [Google Scholar]
- 21.Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;83:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen AP, Curran EA, Duggan A, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev. 2017;73:123–164. [DOI] [PubMed] [Google Scholar]
- 23.Holland JM, Thompson LW, Cucciare MA, et al. Cortisol outcomes among Caucasian and Latina/Hispanic women caring for a family member with dementia: a preliminary examination of psychosocial predictors and effects of a psychoeducational intervention. J of Intl Soc Investigation Stress. 2011;27: 334–346. [Google Scholar]
- 24.de Vugt ME, Jolles J, van Osch L, et al. Cognitive functioning in spousal caregivers of dementia patients: findings from the prospective MAASBED study. Age Ageing. 2006;35:160–166. [DOI] [PubMed] [Google Scholar]
- 25.Fonareva I, Amen AM, Zajdel DP, Ellingson RM, Oken BS. Assessing sleep architecture in dementia caregivers at home using an ambulatory polysomnographic system. J Geriatr Psychiatry Neurol. 2011;24:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortensen J, Dich N, Clark AJ, et al. Informal caregiving and diurnal patterns of salivary cortisol: results from the Whitehall II cohort study. Pychoneuroendocrinology. 2019;100:41–47. [DOI] [PubMed] [Google Scholar]
- 27.Buckley TM, Schatzberg AF. On the interactions of the hypothalamicpituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106e14. [DOI] [PubMed] [Google Scholar]
- 28.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197e210. [DOI] [PubMed] [Google Scholar]
- 29.Hoyt MA, Bower JE, Irwin MR, Weierich MR, Stanton AL. Sleep quality and depressive symptoms after prostate cancer: The mechanistic role of cortisol. Beh Neurosci. 2016;130:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang WP, Lin CC. Relationships of salivary cortisol and melatonin rhythms to sleep quality, emotion, and fatigue levels in patients with newly diagnosed lung cancer. Euro J Oncol Nurs. 2017;29:79–84. [DOI] [PubMed] [Google Scholar]
- 31.Matthews KA, Hall MH, Cousins J, Lee L. Getting a good night’s sleep in adolescence: do strategies for coping matter? Behav Sleep Med. 2016;14:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 34.National Alliance for Caregiving. e-Connected family caregiver: bringing caregiving into the 21st century. Bethesda, MD: United Healthcare; 2011. [Google Scholar]
- 35.Zigmond AS, Snaith RP. Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 36.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The Consensus Sleep Diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambulatory Monitoring, Inc. Action-W User’s Guide. Ardsley, NY: Ambulatory Monitoring, Inc.; 1999. [Google Scholar]
- 38.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time dependent change. Psychoneuroendocrinology. 2003;28:916–931. [DOI] [PubMed] [Google Scholar]
- 39.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biological Psychol. 2009;80:265–278. [DOI] [PubMed] [Google Scholar]
- 40.Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park: Sage Publications; 1991. [Google Scholar]
- 41.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach, 2nd ed. New York: Guilford Press; 2018. [Google Scholar]
- 42.Arnett JJ. Emerging adulthood: a theory of development from the late teens through the twenties. Amer Psychol. 2000;55:469–480. [PubMed] [Google Scholar]
- 43.Besedovsky L, Lange T, Born J. Sleep and immune function. Eur J Physiol. 2012;463:121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leggett AN, Liu Y, Klein LC, Zarit SH. Sleep duration and the cortisol awakening response in dementia caregivers utilizing adult day services. Health Psychol. 2016;35:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Dalfsen JH, Markus CR. The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: a systematic review. Sleep Med Rev. 2018;39:187–194. [DOI] [PubMed] [Google Scholar]
- 46.Buckhalt JA, El-Sheikh M, Keller P. Children’s sleep and cognitive function: race and socioeconomic status as moderators of effects. Child Dev. 2007;78:213–231. [DOI] [PubMed] [Google Scholar]
- 47.Gallo LC. The Reserve Capacity Model as a framework for understanding psychosocial factors in health disparities. Appl Psychol: Health Well-Being. 2009;1:62–72. [Google Scholar]
- 48.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA Study. Sleep. 2007;30:793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibbins J, McCoubrie R, Kendrick AH, Senior-Smith G, Davies AN, Hanks GW. Sleep-wake disturbances in patients with advanced cancer and their family carers. J Pain Symptom Manage. 2009;38:860–870. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz S, Anderson WM, Cole SR, Cornoni-Huntley J, Hays JC, Blazer D. Insomnia and heart disease: a review of epidemiologic studies. J Psychosom Res. 1999;47(4):313e33. [DOI] [PubMed] [Google Scholar]
- 51.Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14:324e32. [DOI] [PubMed] [Google Scholar]
- 52.Krystal AD. Sleep and psychiatric disorders: future directions. Psychiatr Clin North Am. 2006;29:1115e30. [DOI] [PubMed] [Google Scholar]
- 53.Shaffer KM, Applebaum AJ, DuHamel KN, Garland S, Gerhman P, Mao JJ. Cancer survivors’ beliefs about the causes of their insomnia: associations of causal attributions with survivor characteristics. Beh Sleep Med. 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaffer KM, Carter P, Garland SN, Applebaum AJ. Cognitive behavioral therapy for insomnia for cancer caregivers. In: Applebaum A, editor. Cancer caregivers. New York: Oxford University Press; 2019. [Google Scholar]


