Abstract
It is widely accepted that SES is a fundamental cause of health inequality. There is evidence, however, that race is also a fundamental cause of disparities in health. Based on this idea, the weathering hypothesis developed by Geronimus and her colleagues views the elevated rates of illness and disability seen among Black Americans as a physiological response to the structural barriers, daily slights, and other threats to identity that comprise the Black experience. The current study tests the weathering hypothesis using chronic inflammation as an indicator of biological weathering. Specifically, we examine the extent to which persistent exposure to racial discrimination predicts elevated inflammation and, in turn, diagnosed chronic illness, after taking into account SES and several control variables. This mediation model was tested using zero-inflated Poisson path modeling with five waves of data collected from 391 African American women participating in the Family and Community Health Study (FACHS). A 13-item index was used to assess exposure to racial discrimination across eight years. ELISA blood assays of seven cytokines central to the inflammatory response were used to construct an inflammatory index. Respondents reported their diagnosed chronic diseases. Consonant with the weathering hypothesis, persistent exposure to discrimination predicted inflammation which, in turn, predicted number of chronic diseases. This indirect effect was statistically significant. SES predicted having a chronic disease and the various controls showed no effect. The findings support the idea that race, like SES, is a fundamental cause of health inequalities.
Keywords: Racial discrimination, Inflammation, African American women, Social determinants of illness
Introduction
In the U.S., individuals of color, especially Black Americans, suffer a greater prevalence and earlier onset of chronic illness and disability than other ethnic groups [1]. For instance, Blacks are over twice as likely to develop diabetes [2] or to die of sudden cardiac arrest [3] compared to white Americans. They also demonstrate higher rates and earlier onset of elevated blood pressure, arthritis, and cancer, and they are more likely to suffer from comorbidity of these conditions [4, 5]. The most prominent race differences in health emerge in middle age, and the mortality rates for middle-aged Black women have actually worsened since 1990 [6].
In large measure, medicine and public health use a risk factor approach to account for the poorer health of Black Americans. This perspective considers the earlier onset of chronic illness and disability experienced by Blacks to be rooted in the various health-risk behaviors associated with being poor [7, 8] such as an unhealthy diet, lack of exercise, and smoking. While these factors have been shown to be important, they leave most of the elevated rates of illness seen among African Americans unexplained.
An alternative to the risk factor perspective is the fundamental cause hypothesis developed by Link and Phelan (9, 10) to explain the persistently reported association between socioeconomic status (SES) and health. They argue that this enduring association is a consequence of the fact that SES embodies an array of resources such as money, knowledge, prestige, power, and social connections that are beneficial for good health. Using this perspective, Blacks display poorer health than whites because they are disproportionately located in the lower social strata of society. Consistent with this view, controlling for SES substantially reduces the relationship between race and health (11). Importantly, however, significant racial differences in morbidity and mortality continue to be evident after taking socioeconomic characteristics into account. As noted by Phelan and Link (11), this finding suggests that, in addition to SES, race operates as a fundamental cause of inequalities in health. That is, having to endure racial stereotypes, discrimination, and institutionalized racism has an effect on health above and beyond socioeconomic circumstances.
The assertion that race is a fundamental cause of health inequities is consonant with the “weathering hypothesis” developed by Geronimus and her colleagues [12, 13]. They posit that the health inequality suffered by Blacks is, in large measure, a consequence of the cumulative impact of living in a society where they suffer social, economic, and political exclusion. Although health risk behaviors are seen as having some influence, Geronimus and associates assert that this explanation ignores the extent to which health inequalities in the U.S. are fostered by a racial divide where Black Americans occupy a marginalized, stigmatized, subordinate status in relation to Whites. The weathering hypothesis [12] views the elevated rates of illness and disability seen among Black Americans as a physiological response to the structural barriers and daily slights, stereotypes, and other threats to identity that comprise the Black experience.
Geronimus and colleagues [14] tested this idea using allostatic load as an indicator of weathering. Consistent with the weathering argument, Black Americans showed higher levels of allostatic load than whites and this difference continued to be significant after adjusting for poverty. McDade and associates [15] found comparable results using inflammation as a biomarker. Race was related to C-reactive protein even after taking into account educational attainment, household wealth, various health behaviors, and usage of medications. Similarly, Nowakowski and Sumerau [16] reported that being Black was associated with higher levels of C-reactive protein after adjusting for gender, education, income, and marriage. Such findings provide support for the weathering hypothesis and the idea that racism, like SES, is a fundamental cause of health inequities.
Even stronger support for these contentions, however, would be provided by analyses that went beyond comparing Blacks and whites to actually examining the link between experiences of racial bias and health deterioration. That is the focus of the present study. Like McDade et al. (15) and Nowakshi and Sumerau (16), we use chronic inflammation as a biomarker of health. Inflammation has been shown to be a robust predictor of age-related chronic diseases such as heart disease, diabetes, arthritis, and cancer [17–19]. And, there is evidence that Blacks tend to have higher levels of inflammation than Whites [15, 16, 20]. This suggests that inflammation may play a role in explaining the poor health of African Americans. To test this idea, the present study uses a sample of roughly 400 middle age African American women participating in the Family and Community Health Study (FACHS) to interrogate the association between elevated peripheral inflammation and onset of chronic illness. Such a sample is particularly appropriate given evidence that women, especially Black women, score higher than men on biomarkers of poor health (14, 16). We begin by investigating the extent to which persistent exposure to various forms of racial discrimination predicts elevated inflammation. We then examine the degree to which exposure to racial discrimination has an indirect effect on chronic illness through its impact on inflammation. All of these analyses take into account the effect of SES and control for BMI, health risk behaviors, and childhood adversity.
Inflammation and Chronic Illness
The various chronic illnesses of old age tend to be highly correlated so that individuals who suffer early onset of one illness tend to develop others as well. This correlation of illnesses appears to be a function of the fact that they share a common set of biological risk factors [21, 22]. Perhaps chief among these risk factors is prolonged, elevated inflammation. A profusion of studies has accrued showing that chronically elevated inflammation directly contributes to the pathophysiology of chronic illnesses such as cardiovascular disorders, type II diabetes, osteoporosis, rheumatoid arthritis, Alzheimer’s disease, and certain cancers [17–19]. A proinflammatory state is characterized by high circulating levels of various proinflammatory cytokines such as the interleukins (IL1, IL2, IL6, IL17), tumor necrosis factor (TNFα), and macrophage inflammatory protein (MIP). Although increases in inflammation is a necessary defense against infections and injury, when it is sustained and protracted it becomes damaging to health.
In recent years, much progress has been made in identifying the biological mechanisms whereby inflammation promotes various chronic diseases [17, 19]. Inflammatory cytokines, for example, lead to atherosclerosis (and hence CVD), cause insulin resistance (and hence diabetes), and influence the processing and accumulation of amyloid beta (and hence Alzheimer’s disease). Further, chronic inflammation is a primary contributor to progression of chronic kidney disease and to cancer initiation, promotion, and metastasis. It is also the case, however, that onset of chronic illness can elevate levels of inflammation. While there is evidence for such effects, the primary causal flow seems to be from chronic inflammation to chronic illness [17].
Discrimination and Inflammation
In recent years, behavioral scientists have documented the importance of social adversity as a predictor of inflammation [23, 24]. Loneliness, bereavement, caregiver burnout, and economic hardship are among the range of adverse conditions that have been linked to elevated inflammation. Consonant with such findings, animal models show that low social rank and repeated social defeat are associated with increased inflammation [25]. Explanations for the connection between adversity and inflammation tend to emphasize the way the immune system has evolved to address threatening conditions [17, 24]. Cole and colleagues [24, 26], for example, note that the immune system contains a pro-inflammatory program designed to combat tissue damage, bacteria, and other extracellular pathogens. They argue that adversity (threat or danger) leads to increased expression of the inflammatory program as the organism prepares for possible attack and injury. The sympathetic nervous system (SNS) is viewed as a major component of the pathway leading from threat and rejection to inflammation as it stimulates β-adrenergic-responsive transcription factors that facilitate transcription of genes that encode for proinflammatory cytokines [24, 25].
Presumably this pattern of gene expression evolved to help adapt molecular physiology to the types of sporadic and transient threats that characterized our ancestral environments [24]. In contemporary society, however, purely symbolic or anticipated threats undermine health by fostering chronic activation of the inflammatory program [24]. Arguments such as those proffered by Cole and his colleagues suggest that social environments that pose a persistent threat of hostility, denigration, and disrespect promote chronically high levels of inflammation. This is, of course, everyday life for members of ethnic minorities living in a racially charged society. In the U.S., this is particularly the case for Black Americans [27]. Most whites do not recognize the multitude of ways that they unknowingly typecast, patronize, or exclude stigmatized minorities. As a consequence, persons of color often enter situations with uncertainty and vigilance regarding how they will be perceived and treated [12, 28]. In other words, the psychological orientation that has been linked to inflammation (viz. vigilance and preparedness for threat) is likely a common experience among Black Americans as they deal with the cultural and structural challenges of a racialized society. To the extent that this is true, one would expect a robust association between Blacks’ reports of racial discrimination and their level of inflammation.
The few studies that have examined the link between discrimination and inflammation have produced inconsistent findings. Although some of these investigations find support for this association [29, 30], many do not [31–34]. There is reason to believe, however, that these discrepancies are a consequence of methodological limitations that have plagued this research [35]. Discrimination is often assessed with a single item [31] or at a single point in time, or with a scale that fails to distinguish whether a discriminatory event was in response to the person’s race or to other social characteristics such as weight or gender [36].
There have also been problems in the way that past research has measured inflammation. In recent years a profusion of studies has investigated the potential link between social adversity and elevated inflammation. Albeit, whether the adversity be racial discrimination or some other stressful condition, inflammation has almost always been assessed using a single marker, usually C-reactive protein (CRP) or Interleukin-6 (IL-6). Because each cytokine considered individually has a range of potentially confounding influences (e.g., IL-6 is significantly elevated by exercise, CRP is significantly associated with BMI) the result has been very modest and often inconsistent associations between social variables and inflammation, including racial discrimination. The inflammatory system, however, is extensive and complex; one inflammatory cytokine often stimulates and amplifies the production of others, setting up a cascade of reactions [37]. This argues for using an index containing several proinflammatory cytokines to assess inflammation.
All of this suggests that the contradictory findings that have been reported regarding a potential link between discrimination and elevated inflammation may be, to a large degree, a consequence of unreliable measurement of both experiences of discrimination and pro-inflammatory response. In support of this contention, two recent studies that used more comprehensive measures of both discrimination and inflammation found a robust effect [29, 35]. Both of these studies, however, focused on emerging or young adults. Thus it is unclear whether this association continues to be evident in late middle age when onset of chronic illness begins to occur. Further, no study has examined the extent to which elevated levels of inflammation might mediate the association between racial discrimination and onset of chronic illness. Accordingly, although these studies indicate that discrimination fosters inflammation, they do not go on to establish that this inflammation, in turn, compromises an individual’s health and leads to illness.
The Current Study
Using longitudinal data from a sample of 391 middle-aged African American women, the present study investigates the associations between racial discrimination, inflammation, and chronic illness after taking into account the contribution of SES. Based on the SES as fundamental cause argument [9, 10], we expect that SES will be negatively associated with reports of chronic disease. The theory suggests that SES is related to illness through a wide variety of avenues and hence we expect to find an association between SES and chronic disease even after controlling for health risk behaviors. The primary concern of our study, however, is the extent to which the physiological consequences of racial mistreatment predict chronic illness above and beyond SES. Specifically, based on the race as fundamental cause argument and the idea of weathering, we predict that persistent exposure to racial discrimination will be related to inflammation, that inflammation will be related to increased chronic disease, and that discrimination will affect level of chronic illness indirectly through inflammation.
We use measures of discrimination and inflammation that overcome many of the problems inherent in many past studies. First, we use a self-report measure of discrimination that asks the respondent to report how often they have experienced each of a wide variety of racist events. These data were obtained from the first four waves of data collection covering roughly an eight year period. This allowed us to construct an index that assesses persistent exposure to various types of racial discrimination prior to assessment of inflammation at wave 5. Second, using blood collected at wave 5, we construct a measure of inflammation based upon seven cytokines central to the inflammatory response. The seven cytokines were highly correlated and loaded on a single factor. Third, we used a count index of co-morbidity of chronic disease that asks respondents to report whether they have ever been told by a doctor that they have each of six chronic conditions: cardiovascular problems, diabetes, arthritis, liver disease, glaucoma, thyroid problems, or asthma. Finally, our analyses control for age, exposure to childhood adversity, BMI, and lifestyle variables such as smoking, exercise, and diet. These controls are necessary as some studies have found one or more these variables to be associated with either inflammation or chronic illness.
Methods
Participants and Procedures
We tested our hypothesized model using data collected at Waves 1 through 5 from the primary caregivers (PCs) in the Family and Community Health Study (FACHS). FACHS is a longitudinal study of several hundred African American families that was initiated in 1997. At study inception, all of the families had a 5th grader and a primary caregiver. A stratified random sampling procedure was used to generate families representing a range of socioeconomic statuses and neighborhood settings. Details regarding recruitment are described elsewhere [38, 39]. The protocol and all study procedures were approved by the Institutional Review Board at the University of Georgia (Title: FACHS IV; Protocol # Study00000172). At each wave, questionnaires were administered via computer in the respondent’s home and took on average about 2 hours to complete.
At Wave 1, about half of the sample resided in Georgia (n = 422) and the other half in Iowa (n = 467). Most of the PCs (93%) were women. The 1st, 2nd, 3rd, 4th, and 5th Waves of data were collected in 1997, 1999, 2002, 2005, and 2008, respectively. Mean PC age at wave 5 was 48.47 years (SD = 9.23), 17.82% had less than a 12th grade education, and 24.8% were married. The majority (68.5%) lived in large urban areas, 12.2% lived in the suburbs, and 19.3 lived in rural areas. Average annual household income was between $30,000 and $35,000. Based on the small number of male PCs, the present study only focuses on women.
Within two weeks of the wave 5 psychosocial interview, a certified phlebotomist visited the home and collected four tubes of blood (30 ml) from each consenting participant. Given the logistics of scheduling home visits by phlebotomists, only members of the sample still residing in Georgia or Iowa at wave 5 were identified as eligible for the blood draw. Blood was obtained from 433 (72%) of the roughly 600 women who met this criteria. Comparisons of these PCs with those who did not provide blood did not reveal any significant differences with regard to either demographic characteristics or the independent variables [e.g., household income: t = 1.042, ns; financial pressure: t = 1.231, ns, or chronological age t = 1.133, ns] at the initial wave of the FACHS study. The only exception was diet. PCs who gave blood had a slightly poorer diet than those who did not (about 3/20ths of a standard deviation, p < .05).
Measurements
Inflammation.
Our measure of inflammation utilized seven cytokines central to the inflammatory response: interleukin 1-beta (IL-1β), interleukin 2 (IL-2), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 17 (IL-17), tumor necrosis factor (TNFα), and macrophage inflammatory protein 1-b (MIP-1b). To assess multiple cytokines simultaneously, we used ELISA blood assays obtained with the Bio-Plex Pro Human Cytokine Immunoassay. Useable assays were available for 408 of the respondents, 391 of whom had participated in all 5 waves of data collection. Alloquots of blood for each participant were randomly assigned to eight “plates.” To correct for potential method variance reflecting “plate” rather than variables of interest, the cytokines used in the study were corrected for plate-to-plate variation by linear regression that included the eight plates as categorical covariates. For each of the cytokines, we used the residuals after removal of plate effects in subsequent analyses. The seven cytokines were highly correlated and loaded on a single factor with loadings ranging from .84 to .97. Given this clustering, we formed a composite inflammatory index by summing the seven z-scored cytokine values [40]. Coefficient alpha for the index was .971.
Discrimination.
At waves 1 – 4 of data collection, respondents completed 13 items from the Schedule of Racist Events [41]. This instrument has strong psychometric properties and has been used extensively in studies of African Americans [29, 35, 43, 44]. The items assess the frequency (1 = never, 4 = several times) with which various discriminatory events have been experienced. The items focus on events such as being the victim of disrespectful treatment by sales clerks or co-workers, false accusations by authority figures, racial slurs, being hassled by the police, exclusion from social activities, and not being expected to do well because of being African American. At wave 1, respondents were asked to report how often they had experienced each of these events in the past, whereas at waves 2–4 they were asked to report how often they had experienced each of these events during the previous year. Coefficient alpha for the scale was above .75 at every wave. A composite measure of discrimination was obtained by averaging scores across waves 1–4.
Index of Chronic Disease.
At wave 5, respondents reported whether a doctor had ever told them (no=0, yes=1) that they were suffering from each of six chronic diseases (cardiovascular problems, diabetes, arthritis, liver disease, glaucoma, thyroid problems, or asthma) that have been associated with elevated inflammation. Such indices are a common approach to assessing multmorbidity [45]. While this approach involves self-reports, it avoids the biases and recall problems associated with most self-report health measures as individuals are unlikely to either forget or incorrectly recall being told by a doctor that they have a major chronic disease. An individual’s chronic disease score consisted of the number of illness reported (0–6).
Socioeconomic status.
Respondents reported on their highest level of education at wave 5. Categorical response options were 0 (less than high school), 1 (some high school), 2 (high school completion or GED), 3 (some college), 4 (bachelor’s degree or technical training), and 5 (postbaccalaureate degree). At wave 5, respondents also reported on their household income in the past year from all family members and all sources. Scales were standardized and then averaged to form a measure of SES.
Controls.
Our analyses control for various health risk behaviors, BMI, and exposure to childhood adversity, as one or more studies has reported associations between these variables and assessments of either inflammation or chronic illness.
Childhood Adversity.
Childhood and early adolescent adversity was assessed at Wave 1 using retrospect reports of five types of adversity [47]. Respondents reported (no=0; yes=1) whether they had experienced various stressful events when they were growing up. The stressors included living without both biological parents, parental divorce or separation, parental death, family violence, and parental conflict. These items were intended to be easily recalled and salient for children. Scores were summed across items to form an index of childhood stress exposure. Sixty-nine percent of respondents reported they had experienced at least one of these stressful events while growing up. Spearman-Brown coefficient for this scale was 0.70 (mean=1.587,SD=1.307).
BMI.
At wave 5, respondents were asked to report their height and weight, and this information was used to calculate a Body Mass Index (BMI) score. BMI score was calculated according to the following formula provided by the Center for Disease Control (47): weight/height in inches2 x .703 [48].
Health Risk Behaviors.
We assessed three health risk behaviors: regular exercise, healthy diet, and smoking behavior.
Exercise.
At Waves 4 and 5, respondents’ involvement in exercise over the previous week was assessed using an item: “How often do you exercise” with responses ranging from 1 (never) to 4 (regularly). We created a measure of exercise by averaging the scores across waves.
Diet.
At Waves 4 and 5, a 3-item scale developed for FACHS was used to assess healthy diet. Respondents were asked to report on their regular eating behavior including, “how often do you watch what you eat (i.e., nutrition - such as eating fruits and vegetable regularly)?”, “how often do you eat fatty foods like potato chips or ice cream”?, and “how often do you eat fast food”? with responses ranging from 1 (never) to 4 (regularly). We reverse coded the last two items and then averaged the scores. Finally, we created a measure of healthy diet by averaging the scores across waves.
Smoking.
At Wave 5, the enzyme linked immunoassay (ELISA) assessment of serum cotinine levels was accomplished using a cotinine direct assay from Abnova (Taipei City, Taiwan) according to the manufacturer’s directions. Cotinine has an in vivo half-life of approximately 20 hours, and is typically detectable for several days (up to one week) after the use of tobacco. For African Ameicans, scores above 10 indicate some use of tobacco and scores above 100 indicate a heavy smoker. Following recommended procedures, the variable was log transformed to correct for skew.
Statistical Analysis
Full information maximum likelihood (FIML) was utilized for those few instances of missing data. We began by running a correlation matrix for the study variables. Next, we assessed the extent to which discrimination at each wave of data collection (W1 – W4) was related to each of the eight inflammatory cytokines in our inflammatory index. This allowed us to rule out the possibility that any significant correlation between our overall measure of discrimination and our composite inflammatory index was being driven by discrimination assessed at a particular wave, or was driven primarily by strong associations between discrimination and just one or two inflammatory cytokines rather than displaying a consistent effect across these biomarkers.
Next, we examined the correlation of each of the thirteen items in the discrimination index (averaged across waves) with the inflammatory and chronic disease measures. Finally, we correlated each of the inflammatory cytokines with each of the six chronic illnesses to examine the possibility that inflammation was associated with only a few of the chronic illnesses rather than being positively related to each of the chronic diseases.
We used structural equation modeling to test our study hypotheses regarding the effect of SES, discrimination, and inflammation on chronic disease. Given that chronic disease is a count variable with an excessive number of zeroes (53%), we used the zero-inflated Poisson (ZIP) model [48] available in MPlus 8.0 [49]. This model allows simultaneous estimation of a binary model (having a chronic disease) and a count model (number of chronic diseases). We present incident rate-ratios and odds ratios from our zero-inflated Poisson model if the outcome is a count or a binary variable, respectively. Although model fit indices are not available for the ZIP model, the fit indices for the continuous model are presented. To assess model fit [50], we used Pearson’s χ2, Steiger’s root mean square error of approximation (RMSEA), and the comparative fit index. Finally, the 95% confidence interval (CI) was estimated using bias-corrected and accelerated bootstrapping with 1,000 resamples to assess the significance of indirect effects. The bootstrap method involves resampling the data. That is, bootstrap samples of the same size as the original sample are randomly drawn from the original sample with replacement, and then the empirical sampling distribution is constructed using statistics that are calculated from the bootstrap samples. Thus, this method can capture the asymmetric nature of the indirect effects sampling distribution [51].
Results
Means, standard deviations, and frequencies for the study variables are presented in Table 1. Although not evident in this table, the respondents reported relatively high exposure to many of the items in the discrimination index. For example, 63% indicated that at least twice a year they had encountered people who did not expect them to do well because of their race, 33% indicated that at least twice a year someone had discouraged them from trying to achieve an important goal because of their race, and 62% % indicated that at least twice a year a close friends had been treated unfairly because of their race. None of the respondents indicated at any of the waves that they had not experienced any of the discriminatory events during the preceding year.
Table 1.
Descriptive statistics for study variables (N = 391)
| Variables | % or Mean (SD) |
|---|---|
| Chronic disease (W5), Mean (SD) | .739 (1.004) |
| Pro-inflammatory (W5), Mean (SD) | −.014 (.891) |
| Racial discrimination (W1–4), Mean (SD) | 1.872 (.562) |
| SES (W5), Mean (SD) | .009 (.828) |
| Education, % | |
| <12th Grade | 17.9% |
| High school graduate or GED | 37.3% |
| Some college or associate degree | 34.0% |
| Above bachelor’s degree | 10.7% |
| Household income, Mean (SD) | 37570.876 (33959.879) |
| Healthy diet (W4–5), Mean (SD) | 2.586 (.590) |
| Exercise (W4–5), Mean (SD) | 2.289 (.851) |
| Cotinine (W5), Mean (SD) | 36.069 (51.909) |
| Childhood vulnerability | 1.870 (1.489) |
| Age (W5), Mean (SD) | 48.782 (8.083) |
| BMI (W5), Mean (SD) | 34.704 (8.813) |
Similarly, roughly half of the sample reported having been told that they had one or more chronic illness. For example, 33% reported that they had been told they had arthritis, 10% indicated that they had been told that they had heart problems, and 15% reported they had been told they had diabetes. The number of individuals reporting no illness was 207 (52.8%), while 120 (30.7%) reported one illness, 33 (8.4%) reported two illnesses, and 33 (8.4%) reported 3 or more illnesses.
Table 2 presents the correlation matrix for the study variables. As expected, the table shows that chronic illness is significantly correlated with inflammation (r = .170) and racial discrimination (r = .120), and that racial discrimination is associated with inflammation (r = .114). This is the necessary pattern of correlations if inflammation mediates the effect of discrimination on chronic illness. Consonant with the SES as fundamental cause argument, SES is negatively related to chronic disease (r = −.133). And, consistent with the contention that the effect of racism is not fully explained by SES, Table 2 shows that SES has a positive, rather than negative, correlation with discrimination (r = .319).
Table 2.
Correlation matrix for the study variables (N = 391)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Chronic disease (W5) | - | ||||||||
| 2. Pro-inflammatory (W5) | .170** | - | |||||||
| 3. Racial discrimination (W1–4) | .120* | .114* | - | ||||||
| 4. SES (W5) | −.133** | −.020 | .319** | - | |||||
| 5. Healthy diet (W4–5) | .178** | .079 | .195** | .093† | - | ||||
| 6. Exercise (W4–5) | −.056 | .041 | .181** | .121* | .264** | - | |||
| 7. Cotinine (W5) | .110* | .035 | .003 | −.292** | .027 | −.052 | - | ||
| 8. Childhood adversity | −.026 | −.031 | .130* | .007 | −.015 | −.088† | .008 | - | |
| 9. Age (W5) | .425** | .033 | .155** | −.027 | .338** | .030 | .027 | −.116* | - |
| 10. BMI (W5) | .144** | .001 | −.004 | −.071 | −.023 | −.067 | −.131** | .014 | −.088† |
p ≤ .10
p ≤ .05
p ≤ .01 (two-tailed tests).
Turning to the various controls included in our analysis, cotinine (smoking) and age are positively related to chronic illness, and discrimination is related to healthy diet, exercise, childhood adversity, and age. The latter correlation is interesting as it suggests that racial discrimination increases with age, perhaps because ageism enhances racial discrimination.
Next, various preliminary analyses were conducted prior to performing structural equation modeling to test our mediation hypothesis. First, there was the possibility that the association between discrimination and inflammation reported in Table 2 was being driven by discrimination assessed at a particular wave rather than there being a constant or persistent effect of this variable on inflammation across waves. Another possibility was that the association between discrimination and inflammation was a consequence of discrimination being strongly associated with just one or two inflammatory cytokines rather than displaying a consistent effect across inflammatory cytokines. Analysis showed, however, that discrimination scores at each wave and the Discrimination Index summed across waves were correlated with each of the various inflammatory cytokines and with the Inflammatory Index. (see online supplement Table S1). Also, discrimination, regardless of wave, was significantly correlated with the Chronic Disease Index (see online supplement Table S2). Further, each of the 13 types of discrimination in the Discrimination Index was correlated with the Inflammatory Index as well as with the Chronic Disease Index (see online supplement Table S3). Finally, we were concerned with whether the correlation reported in Table 2 between the Inflammatory index and the Chronic Disease Index might be driven by just a subset of cytokines or a subset of illnesses. Albeit, analyses showed that each of the seven cytokines was correlated with each of the chronic diseases, with the exception of thyroid problems. And, each of the cytokines showed a significant correlation with the Chronic Disease Index (see online Supplement Table S2).
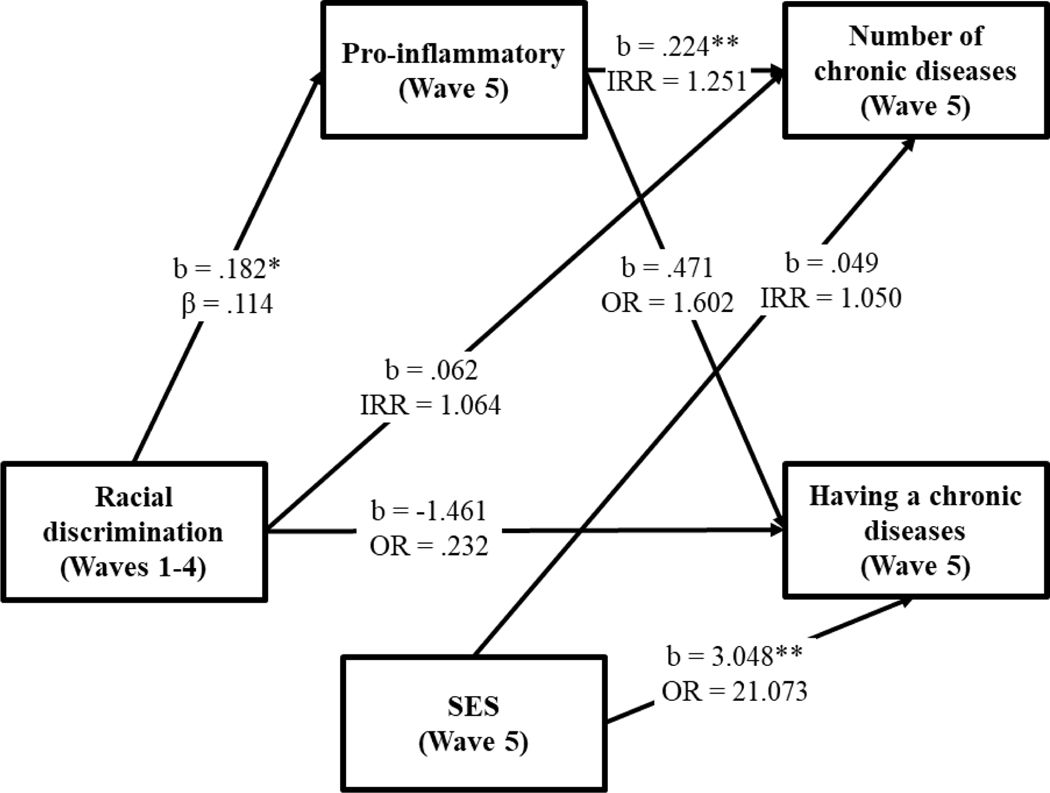
Having established the consistency of the pattern of associations between racial discrimination, inflammation, and chronic disease, structural equation modeling was used to test the indirect effect of discrimination on chronic disease through inflammation, as well as the main effect of SES on chronic disease. Given that chronic disease is a count variable with an excessive number of zeroes (over 50% of the cases reported no chronic disease), we used the zero-inflated Poisson (ZIP) model [48] available in MPlus 8.0 [49]. This allowed simultaneous estimation of a binary model (having a chronic disease) and a count model (number of chronic diseases). Incident rate-ratios (IRRs) are presented for the count variable and odds ratios (ORs) are presented for the binary variable. The model controls for BMI, diet, exercise, childhood adversity, and age. Of these controls, only age was significantly related to either having a chronic disease or number of chronic diseases. The paths for the control variables are not depicted in the SEM model in order to display a less cluttered figure.
The results are shown in Figure 1. First, the path (OR = 21.073, p < .01) from SES to the binary variable (having a chronic illness) is significant whereas the path from SES to the count variable (number of illnesses) is not. Also, SES not related to discrimination or inflammation. Second, inflammation is related to number of diseases (IRR = 1.25, p <.01) but not to the binary measure of chronic illness. These findings suggest that inflammation influences the number of chronic diseases that one develops while SES influences whether a person contracts a chronic disease of some sort. Third, there is a significant path from racial discrimination to inflammation (β = .114, p < .05)), but racial discrimination is not related to either the binary or count measures of chronic illness. This pattern of findings suggests that inflammation may mediate the effect of discrimination on number of chronic diseases. Consonant with this idea, the bootstrapping method with 1000 replications revealed the indirect effect of racial discrimination on number of chronic diseases through the Inflammatory Index to be significant. It was possible, however, that the model is misspecified with the correct causal ordering being from discrimination to chronic diseases to inflammation. As shown in the online supplement (Figure S1), this model is not supported as there is no significant path from racial discrimination to chronic disease while there is a significant path from racial discrimination to inflammation.
Figure 1.
Zero-inflated Poisson path modeling predicting the effect of racial discrimination on the frequency and occurrence of chronic disease through pro-inflammation.
Note: Fit stats taken from a continuous: χ2 = 3.871, df = 7, p = .795, CFI = .000, RMSEA = .000. b = unstandardized coefficients; β = standardized coefficients; IRR = incident rate-ratios; OR =odds ratio. Healthy diet, exercise, cotinine, childhood vulnerability, and age are controlled. *p < .05. **p < .01. ***p < .001.
Discussion
The contention that SES is a fundamental cause of illness [9, 10] is a popular idea in medical sociology that has received substantial empirical support. This perspective posits that SES is not merely a placeholder for other variables. Rather, SES embodies an array of resources such as money, knowledge, prestige, power, and social connections that are all beneficial for good health. Consequently, SES would be expected to maintain a relationship with health even when various controls are taken into consideration. Consistent with this assertion, the present study found that SES was negatively related to the probability of having a chronic illness, and this association held while controlling for health risks such as diet, exercise, BMI, and smoking. This finding suggests that the African American women in our sample suffer from a chronic illness, at least in part, because of their low socioeconomic status.
Past research has demonstrated, however, that SES is only part of the story as significant racial differences in morbidity and mortality continue to be evident after taking socioeconomic characteristics into account (11). As noted by Phelan and Link (11), this finding suggests that, in addition to SES, race operates as a fundamental cause of inequalities in health. That is, having to endure racial stereotypes, discrimination, and institutionalized racism has an effect on health above and beyond socioeconomic circumstances. Years earlier Geronimus and her colleages [12, 13] advocated a similar idea with their weathering hypothesis. They posited that the health inequality suffered by Blacks is, in large measure, a consequence of the cumulative physiological wear and tear of living in a society where they suffer social, economic, and political exclusion. This idea has been supported by studies showing that, net a variety of controls, Blacks show higher levels of allostatic load [14] and inflammation than whites [15, 16].
The present study extended this research in three respects. First, rather just comparing Blacks to whites, we assessed variation in exposure to racial mistreatment within a Black sample over an eight-year period, providing a more stringent test of the role of discrimination. Second, we used a more comprehensive measure of inflammation than has been employed in past research. Finally, we tested the extent to which inflammation, our measure of weathering, mediates the impact of discrimination on chronic disease. Our analyses supported this hypothesis. Among our sample of Black women, persistence of exposure to discriminatory acts was related to inflammation across several inflammatory cytokines, and this elevated inflammation was, in turn, associated with an increased number of doctor diagnosed chronic diseases. Further, analysis indicated that, in large measure, heightened inflammation (physiological weathering) accounted for (mediated) much of the association between racial discrimination and number of chronic diseases reported.
A strength of our analysis was the use of zero-inflated Poisson path modeling. This allowed us to distinguish between having a chronic disease (a binary outcome) and number of diseases (a count variable). This distinction was important given that 50% of our sample reported no illness. Distinguishing between the two outcomes did produce, however, a rather puzzling finding: inflammation was related to number of diseases but not whether one simply had a disease, whereas SES predicted whether one had a disease but not number of diseases. While we are not sure why this pattern emerged, we offer the following speculation. Inflammation has been linked to the onset of virtually every chronic disease – CHD, diabetes, cancer, arthritis, etc. [17, 19]. Indeed, it is a risk factor for all the diseases listed in our index of chronic diseases. Thus it might be viewed as increasing an individual’s risk not just of developing a disease but for developing multiple diseases. While this seems plausible, the effect of SES is more difficult to explain. Albeit, it may be that the wide variety of avenues linking SES to health do not increase the probability of any particular disease; rather they simply elevate the probability that a person develops at least one chronic illness, while the number of chronic ailments is determined by other factors, such as inflammation. It will be important to see if future research can replicate these findings.
Surprisingly, health risk behaviors had no significant association with either inflammation, SES, or chronic disease in our SEM. We speculate that the marginal impact of these lifestyle variables might be attributable, at least in part, to the well-known challenges of using short, self-report measures to assess them. Also, there was minimal variation for some of the variables. For example, few of the women in our sample were heavy smokers, and most engaged in just moderate exercise. Consequently, our finding of no effect should not be interpreted as suggesting that diet and exercise are not relevant for long-term health outcomes or that changes in these health behaviors would not be beneficial. They do suggest, however, that these factors are not likely to be major explanations for chronic disease among African American women.
While our study included several strengths, it also suffered from various limitations. For example, a more powerful test of our mediation model requires multiple assessments of inflammation over time so that one might investigate the extent to which chronic discrimination fosters change in inflammation, with such change predicting subsequent increases in chronic illness. Unfortunately, inflammation assays were only available at wave 5 in the present study.
The sample used in the study also presents certain limitations. First, the participants all resided in either the Southeast or Midwest. While there is little reason to believe that the associations found between discrimination, inflammation, and chronic illness are specific to these regions, it is important that our findings be replicated with a more nationally representative sample. Another limitation related to the study sample is that it consisted only of middle-age, African American women. On the one hand this was appropriate based on the heavy burdens placed upon this group. Given the high unemployment and incarceration rates experienced by Black males [52, 53], economic survival of the family must often be shouldered by Black women who must engage in sustained high-effort coping [10]. As a result, they are at high risk for biological wear and tear or weathering [10]. Still, while it is important to investigate discrimination and inflammation among Black women, our results need to be replicated with other stigmatized groups such as Black males, Latinos, Native Americans, and members of the LGBTQ community.
Turning to policy considerations, there are few if any effective pharmacological treatments for chronically elevated inflammation. Hence modern medicine advocates personal lifestyle changes as a remedy for reducing inflammation. Unfortunately, however, cost, neighborhood availability, transportation issues, and many other factors serve as barriers to changing dietary and exercise habits [54–56]. Perhaps more importantly, however, such life style changes, even when they are successful, do not address the stress and challenges of living in a racially charged society [8]. Findings from the present study suggest that the elevated inflammation of African Americans is rooted, at least in part, in racial mistreatment. To the extent that this is the case, political and cultural changes are required in order to address the high levels of chronic disease experienced by the African American community. Social policies must be implemented that eliminate cultural messages and practices that stigmatize and discredit Black Americans. Unfortunately, there is little sign that this is taking place in contemporary society. Indeed, increases in white nationalism, police targeting of Blacks, and political rhetoric denigrating Black congresspersons and the citizens living in their districts all undoubtedly contribute to a sense of fear and anxiety among the African American community. Findings from the current study suggest that these perceptions likely increase inflammation and, in turn, illness.
On a more positive note, however, it may be that coping resources such as family support, a strong friendship network, and religious involvement can buffer the effects of racial discrimination on inflammation. African Americans often display strong family ties and meaningful involvement in a religious organization. Recently, Simons et al. [57] reported that both religious involvement and having close, black friends served to counter levels of inflammation in a sample of young African Americans. And, Beach et al. [58] recently reported that romantic partner support buffers the effect of stress on inflammation in young African Americans. More research is needed on the way that supportive relationships and empowering activities might help individuals cope with the stress of living in a society where they are often stereotyped and stigmatized. Albeit, concomitant with this focus on personal coping resources, it is important that studies examine the way that changes in social policy and cultural dialogue, whether at the level of the community or the broader society, might influence inflammatory levels and chronic illness among Black Americans. An approach that stresses personal lifestyle while ignoring these structural and cultural factors is likely to have only a limited impact upon the health disparities suffered by the African American community.
Supplementary Material
Acknowledgments
Funding. This work was supported by the National Institute on Aging (R01 AG055393), the National Heart, Lung, Blood Institute (R01 HL118045), the National Institute on Child Health and Human Development (R01 HD080749), the National Institute on Drug Abuse (R21 DA034457), and the National Institute of Mental Health (R01 MH62699, R01 MH62666). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
All procedures performed in this study involving human participants were in accordance with the Institutional Review Board of the University of Georgia.
Conflict of Interest The authors declare no conflicts of interest
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Ronald L. Simons, Department of Sociology, University of Georgia, 324 Baldwin Hall, Athens, GA, USA 30602
Man-Kit Lei, Department of Sociology, University of Georgia, 217B Baldwin Hall, Athens, GA, USA 30605.
Eric Klopack, Department of Sociology, University of Georgia, 104 Baldwin Hall, Athens, GA, USA 30602.
Yue Zhang, Department of Sociology, University of Georgia, 104 Baldwin Hall, Athens, GA, USA 30602.
Frederick X. Gibbons, Department of Psychological Sciences, University of Connecticut, 406 Babbidge Road, Storrs, CT, USA 06269
Steven R. H. Beach, Department of Psychology, University of Georgia, 157 IBR Psychology Building, Athens GA, USA 30602
References
- 1.Williams DR. Miles to go before we sleep: Racial inequities in health. J Health Soc Behav. 2012;53:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard G, Safford MM, Moy CS, Howad VJ, Kleindorder DO, et al. J Am Geriatr Soc. 2017;65:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carethon MR, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular health in African Americans: A scientific statement from the American Heart Association. Curculation. 2017;doi: 10.1161/CIR.000000000000534 [DOI] [PubMed] [Google Scholar]
- 4.Lim E, Gandhi K, David J, Chen JJ. Prevalence of chronic conditions and multimorbidities in a geographiclly defined geriatric population with diverse races and enthicities. J Aging Health. 2018;30:421–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorpe RJ, Gesahazion RG, Parker L, Wilder T, Rooks RN, Bowie JV, Bell CN, Szanton SL, LaVeist TA. Accelerated health declines among African Americans in the USA. J Urban Health. 2016;93:808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US black women experience stress-related accelerated biological aging? Hum Nat. 2010:21:19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockerham WC, Hamby BW, Oates GR. The social determinants of chronic illness. Am J Prev Med. 2017;doi: 10.1016/j.amepre.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodai MD, Lawenda S, Rehbein M, Vif P, Greger M. Lifestyle medicine: A brief review of its dramatic impact on health and survival. Perm J. 2018;22:17–025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Link BG, and Phelan JC Social conditions as fundamental causes of disease. J Health and Soc Behavior. 1995;35:80–84. [PubMed] [Google Scholar]
- 10.Pelan JC, Link BG, Tehranifar TP. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51:S28–40. [DOI] [PubMed] [Google Scholar]
- 11.Phelan JC, Link BG Is racism a fundamental cause of inequalities in health? 2015;41:311–30. [Google Scholar]
- 12.Geronimus AT, James S, Destin M, Graham L, Hatzenbuehler M, Murphy M, Pearson J, Omari A, Thompson JP. Jedi Public Health: Co-Creating an Identity-Safe Culture to Promote Health Equity. SSM Popul Health. 2016;2:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geronimus AT, Thompson JP. To denigrate, ignore, or disrupt: racial inequality in health and the impact of policy-induced breakdown of urban African American communities of support. Du Bois Review, 2004;1(2):247–279. [Google Scholar]
- 14.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States. 2006. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrucci L, Fabbri E. Inflammaging: Chronic inflammation in ageing, cardiovascular disease, and fraity. National Review of Cardiology. 2018;15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(1)1: S4–9. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y-Z, Wang Y-X, Jiang C-L. Inflammation: The common pathway of stress-related diseases. Frontiers in Human Neuroscience, 2017;doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowakowski ACH, Sumerau JE. Swell foundations: Funadmental causes and chronic inflammation. Sociological Spectrum. 2015;35:161–178. [Google Scholar]
- 19.Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethnicity & Disease. 2011;21(2):142–149. [PMC free article] [PubMed] [Google Scholar]
- 20.Hayflick L. Biological aging is no longer an unsolved problem. Annuals of the New York Academy of Sciences. 2007;1100:1–13. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy BK, Berger S, Brunet A, et al. Geroscience: Linking Aging to Chronic Disease. Cell. 2014;159:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browning CR, Cagney KA, Iveniuk J. Neighborhood stressors and cardiovascular health: Crime and C-reactive protein in Dallas, USA. Soc Sci Med. 2012;75(7):1271–1279. [DOI] [PubMed] [Google Scholar]
- 23.Cole SW. Human social genomics. PLoS Genet. 2014;10(8):e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell ND, Sloan EK, Bailey MT, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Science U.S.A. 2013;110(41): 16574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slavich GM, Cole SW. The emerging field of human social genomics. Clin Psychol Sci, 2013;1:331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll G. Mundane extreme environmental stress and African American families: A case for recognizing different realities. J Comp Fam Stud. 1998;29:271–284. [Google Scholar]
- 27.Emerson KTU, Murphy MC. Identity threat at work: How social identity threat and situational cues contribute to racial and ethnic disparities in the workplace. Cultural Diversity and Ethnic Minority Psychology. 2014;20:508–520. [DOI] [PubMed] [Google Scholar]
- 28.Brody GH, Yu T, Miller GE, Chen C. Discrimination, racial identity, and cytokine levels among African-American adolescents. J Adolesc Health, 2015:56:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behavior and Immunity. 2010;24(3):438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albert MZ, Ravenell J, Glynn RJ, Khera A, Halevy N, de Lemos JA. Cardiovascular risk indicators and perceived discrimination in the Dallas Heart Study. Am Heart J. 2008;156:1103–1109. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, Berkman LF. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the Cardia cohort of 4 US communities. Soc Sci Med, 2012;75:922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kershaw KN, Lewis TT, Diez Roux AV, Jenny NS, Liu K, Penedo FJ, Carnethon MR. Self-reported experiences of discrimination and inflammation among men and women: The multi-ethnic study of atherosclerosis. Health Psychol. 2016;35:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moody DLB, Brown C, Matthews KA, Bromberger JT. Everyday discrimination prospectively predicts inflammation across 7 years in racially diverse midlife women: Study of women’s health across the nation. J Soc Issues, 2014;70:298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simons RL, Lei MK, Beach SRH, Barr AB, Simons LG, Gibbons FX, Philibert RA. Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Dev Psychol. 2018;54:1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. J Health Psychol. 1997;2:335–351. [DOI] [PubMed] [Google Scholar]
- 36.Abbas AK, Lichtman AH, Pillai S. Cellular and molecular Immunology. 8th Ed. Philadelphia: Elsevier; 2015. [Google Scholar]
- 37.Gibbons FX, Gerrard M, Cleveland MJ, Wills TA, Brody G. Perceived discrimination and substance use in African American parents and their children: A panel study. J Pers Soc Psychol. 2004;86(4):517–529. [DOI] [PubMed] [Google Scholar]
- 38.Simons RL, Lei MK, Beach SRH, Brody GH, Philibert RA, Gibbons FX. Social environment, genes, and aggression: Evidence supporting the differential susceptibility perspective. Am Sociol Rev, 2011;76(6):883–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc Natl Acad Sci. 2014;31:11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landrine H, Klonoff EA. The schedule of racist events: A measure of racial discrimination and a study of its negative physical and mental health consequences. J Black Psychol. 1996;22:144–168. [Google Scholar]
- 41.Brody GH, Chen Y-F, Murry VM, et al. Perceived Discrimination and the Adjustment of African American Youths: A Five-Year Longitudinal Analysis with Contextual Moderation Effects. Child Development. 2006;77(5):1170. [DOI] [PubMed] [Google Scholar]
- 42.Burt HC, Simons RL, Gibbons FX. Racial discrimination, ethnic-racial socialization, and crime. Am Sociol Rev. 2012;77:648–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons RL, Murry V, McLoyd V, Lin KH, Cutrona C, Conger RD. Discrimination, crime, ethnic identity, and parenting as correlates of depressive symptoms among African American children: A multilevel analysis. Development and Psychopathology. 2002;14(2):371–393. [DOI] [PubMed] [Google Scholar]
- 44.Diederichs CP, Wellmann J, Bartels DB, Ellert U, Hoffman W, Berger K. How to weight chronic disease in multimorbidity indices? Development of a new method on the basis of individual data from five population-based studies. J Clin Epidemiol. 2012;65: 679–685. [DOI] [PubMed] [Google Scholar]
- 45.Conger RD, Elder GH, Lorenz FO, Simons RL, Whitbeck LB. Famililes in troubled times: adapting to change in rural America. Hawthorne, NY: Aldine de Gruyter;1994. [Google Scholar]
- 46.Von Korff M, Alonso J, Ormel J, et al. Childhood psychosocial stressors and adult onset arthritis: Broad spectrum risk factors and allostatic load. Pain. 2009;143(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. BMI—Body Mass Index: BMI for Adults. 2004. Department of Health and Human Services Web site. http://www.cdc.gov/nccdphp/dnpa/bmi/bmi-adult-formula.htm [Google Scholar]
- 48.Loeys T. Moerkerke B, DeSmet O. Buysse A. The analysis of zero-inflated count data: Beyond zero inflated Poisson regression. Br J Math Stat Psychol, 2012;1:163–80. [DOI] [PubMed] [Google Scholar]
- 49.Muthen LK, Muthen BO. Mplus version 7.04: Base program and combination add-on. Los Angeles, CA: Muthen & Muthen; 2018. [Google Scholar]
- 50.Browne MW, Cudeck R. Alternative ways of assessing model fit. Soc Methods & Res.1992;21:230–258. [Google Scholar]
- 51.Lee S, Lei MK, & Brody GH (2015). Constructing confidence intervals for effect size measures of an indirect effect. Multivariate behavioral research, 50, 600–613. [DOI] [PubMed] [Google Scholar]
- 52.Alexander M. (2010). The New Jim Crow. New York: The New Press. [Google Scholar]
- 53.Western B, & Wildeman C. (2009). The black family and mass incarceration. Annals of the American Academy of Political and Social Sciences, 621, 221–242. [Google Scholar]
- 54.Hilmer A, Hilmers D, Dave J. Neighborhood disparities in access to healthy foods and their effects on social justice. Am J Public Health, 2012;102:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sequin R, Connor L, Nelson M, LaCroix A, Eldridge G. Understanding barriers and facilitators to healthy eating and active in rural communities. J Nutr Metab. 2014; 10.1155/2014/146502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: A review of food deserts literature. 2010;16:876–884. [DOI] [PubMed] [Google Scholar]
- 57.Simons RL, Lei KM, Carter S, Beach SRH, Gibbons FX, Gerrard M, Philibert RA. Inflammation mediates the effect of discrimination, religiosity, and friendship network on expression of the TP53 cancer suppression gene. SSM- Population Health. 2019;7:1–6. doi: 10.1016/j.ssmph.2019.100389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beach SRH, Lei M-K, Simons RL, Cutrona CE, Philibert RA. Perceived relationship support moderates the association of contextual stress with inflammation among African Americans. J Family Psychology, 2019; 33:338–348. doi: 10.1037/fam0000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.