Abstract
Background
Long-term maintenance of functional activity of thyroid cells is an essential requirement for basic in vitro studies on the physiology and pathology of the thyroid. An important prerequisite of thyrocytes’ functional activity in vivo and in vitro is their follicle organization.
Aim
This study aimed at developing a method of cultivation of functionally active rat thyroid follicles in Matrigel under three-dimensional conditions.
Methods
Undamaged rat thyroid follicles were isolated by enzymatic digestion with collagenase/dispase, then embedded into Matrigel, and cultivated for 2 weeks. Thyroglobulin, thyroxine and zonula occludens-1 (ZO-1) localization were revealed by immunofluorescence analysis. Iodide organification was tested by protein-bound 125I (PBI) measurement.
Results
Integrity of the follicles was preserved during the whole period of cultivation and was confirmed by 3D reconstruction of ZO-1 localization. Thyroglobulin was detected in the thyrocyte cytoplasm, as well as in the intrafollicular lumen. Thyroxine was observed predominantly at the apical side of thyrocytes. Also, generated cultures were characterized by a high level of iodide organification: PB125I represented 39% of the total radioactivity in the Matrigel drop embedding the follicles; at the same time, methimazole almost totally inhibited this process (0.2% of total radioactivity).
Conclusion
The method of rat thyrocyte cultivation in Matrigel, as described here allows to maintain the structural integrity and the functional activity of thyroid follicles in vitro and could be used for wide ranges of basic and applied researches in thyroidology.
Keywords: three-dimensional culture of thyroid follicles, thyroglobulin, 125I organification, thyroxine
Introduction
Thyroid cell cultures are widely used to study the basic mechanisms of the gland function, as well as to investigate the processes of carcinogenesis, sensitivity to drugs, toxicological studies, and others. The functional activity of thyroid follicles is closely related to their structural organization (1). Polarization of thyrocytes plays a key role in the production of the thyroid hormones. Therefore, until now, numerous in vitro cellular models were developed, which allow to maintain the essential properties of thyroid gland including cell polarization, intercellular contacts, the presence of intrafollicular lumens, etc. (2). It was previously shown that under conditions of monolayer culture, thyrocytes often lose their functional activity (3), although several functions were sometimes preserved (4, 5). The application of Transwell® (Costar®) cell culture inserts for thyrocyte monolayer culture enables to preserve a polarized state of these cells (6). So, the majority of models to study functionally active thyrocytes are based on three-dimensional cultures. To this end, four main approaches were used for thyrocyte culture: (a) isolation of the whole follicles of functionally active thyrocytes from human/mammalian thyroid tissue with their subsequent cultivation, (b) reconstruction of follicle-like structure by the cells originating from the thyroid gland in three-dimensional conditions, (c) generation of thyroid follicles from stem cells in vitro and (d) application of primary organ culture. Each of these approaches has its own advantages and limitations. The benefits of the first approach (a) include the preservation of the original thyroid histoarchitectonics, namely homotypic intercellular contacts, intrafollicular lumen structure, maintenance of polarization, and high functional activity of the initial cells (7, 8, 9). However, in this case, it is necessary to choose suitable culture conditions that could support long-term functional activity of follicular cells, including the optimal concentration of TSH, iodide salts, and growth factors. In addition, considering the activity of peroxidases (10), it is necessary to provide a balance of factors that control the formation of superoxide radicals. An important requirement for maintaining the polarity (apical localization of microvilli, presence of basal lamina, etc.) and functional activity of thyroid follicles in vitro is the adhesion of thyrocyte to the extracellular matrix (11). Of importance, a shortcoming of this approach is the restriction on the availability of large laboratory animals and postoperative healthy human material. A number of successful studies have been performed on cultures of follicles derived from the pig thyroid, but pigs, like some other large animals, are not always available, due to restrictions related to bioethical standards (12). Moreover the signalling pathway evoked by TSH apparently differs between pigs and humans or rats (13). Mouse, the most common laboratory animal, has a very small thyroid gland and, therefore, is not entirely suitable for this method. Some researches were carried out on primary cultures of rat thyroid follicles. Mainly, suspension cultures of rat follicles were used (6, 14). But in the absence of contact with the extracellular matrix, the phenomenon of follicle reversion takes place (11). Therefore, the presence of an extracellular lattice seems preferable.
Mentioned approach (a) could be also applied to study the fate of the luminal plasma membrane of thyroid follicles in connection to thyroglobulin endocytosis. For this study, the isolated follicles were opened to permit several agents to access the apical membrane to model the process of internalization and endocytosis (15).
Another approach (b) involves the reconstruction of the follicular organization in three-dimensional conditions by cells derived from the thyroid gland. Small cell aggregates from porcine thyrocyte suspension were restructured in reversed follicles in suspension culture. Normal thyroid polarity could be restored after embedding these follicles into collagen gel (16) or reconstituted basement membrane gel (17). It should be noted that regardless of the method of the primary thyroid culture initiation, either by using a suspension of isolated follicles or by a generation of follicles from suspended thyrocytes, the critical factor for maintaining proper polarization of follicles is the contact between the basement membrane of thyrocytes and extracellular matrix components (11, 16, 17). In a recent study, the Fisher rat thyroid (FRT) cell line was shown to reproduce the normal architecture of follicles (18) but the expression of some key thyrocyte proteins necessary for their functional activity was appeared to be lost: the transcription factor Nkx2.1 (also called TTF-1) the thyrotropin receptor, thyroglobulin and the gap junction proteins (connexin 32 and 43) (19).
A protocol for generating thyroid follicles from mesenchymal stem cells (c) has been previously presented as well. These cells reproduced the original follicular structure and contained luminal thyroglobulin. The generation of such cultures is quite difficult and takes about 3 weeks (20). At present time, the technique of obtaining thyroid organoids in three-dimensional conditions seems promising. According to a recent study, the thyrocytes in such cultures are able to synthesize thyroglobulin and to take up radioactive iodide (21).
Organ cultures of the thyroid gland (d) are rarely used. These cultures retain the original follicular structure, heterotypic interactions and functional activity of thyrocytes, but their main limitation is their short survival in culture (22, 23). To overcome this problem, it was proposed to modify the culture conditions. Namely, pieces of mouse thyroid were cultured at 50% oxygen in the gas phase, and 5.6 M of D glucose in the growth medium. This modification of the culture conditions allowed to keep the mini organs structures without disturbances for several weeks (24). Also, there is a method for the maintenance of functional thyroid follicles to determine the thyroid hormone effect on a target tissue. Actually, it has been shown that thyroid follicles transplanted via the portal vein into the liver of rats retained their histoarchitecture and functional activity up to 18 months and caused significant histochemical alterations of liver acini (25).
Thus, the development of cell cultures for the study of physiology and pathology of the thyroid gland remains an important issue in experimental thyroidology. It is important to note that in addition to the structural organization of the thyroid parenchyma, an important role in the functioning of this gland is played by the stroma, primarily the network of blood vessels that tightly entwine the follicles. Moreover, the angiofollicular unit – a follicle with adjacent capillaries – is considered as a structurally functional unit of the thyroid gland (26). Because of that the development of organotypic cultures including thyroid follicles and blood vessel analogs could be a perspective direction of primary thyroid culture modification. Local differences in blood flow between different angiofollicular units most probably explain their different states of activity on which we didn’t focus in the present study.
We propose here a functional model of rat thyroid follicles cultured in Matrigel. It has several important advantages: suspension of purified follicles is obtained by a simple procedure of double filtration using meshes with different pore size; structurally integral and functionally active thyroid follicles could be used for the experiment after 1 day of culture adaptation; cultured follicles retain their functional activity during at least 2 weeks, it is possible to carry out morphological/immunofluorescence analysis of cultured follicles.
In the present work, we have implemented and characterized a method of cultivation of rat thyroid follicles in growth factors reduced Matrigel. Optimal conditions of enzymatic disaggregation of the thyroid tissue and subsequent purification of the follicle suspension were selected. Also, the composition of the growth medium and the conditions of cultivation in Matrigel were optimized, which allowed to cultivate the follicles for at least 2 weeks. Morphological analysis revealed preservation of the follicular organization of thyrocytes. Their functional activity was confirmed by the presence of thyroglobulin, thyroxine (T4) as well as their ability to organify iodide.
Materials and methods
Generation of rat thyroid follicle culture
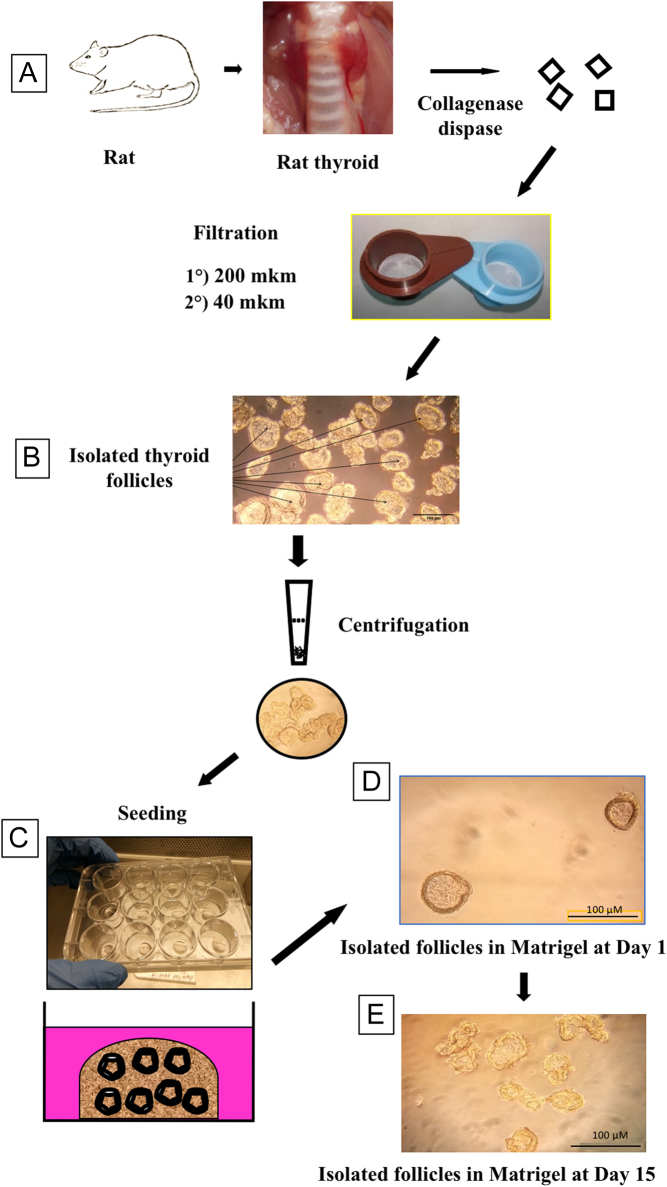
The various steps of the present procedure of rat thyroid follicles culture are summarized in Fig. 1. Two male Wistar rats (4–6 weeks, 140–160 g) were sacrificed by intraperitoneal injection of Nembutal. The lobes of thyroid glands were excised and placed into sterile tubes with Dubelcco's phosphate buffered saline (DPBS, Gibco # 1400133, Thermo Fisher Scientific) containing: Ca2+ 0.9 mM, Mg2+ 0.49 mM, penicillin 500 units/mL, streptomycin 500 µg/mL (Gibco #15070063). All subsequent procedures were carried out in sterile conditions. In a laminar hood, the thyroid lobes were transferred (by pipette) into petri dishes with basal eagle medium (BME), supplemented with penicillin 250 units/mL, streptomycin 250 µg/mL, amphotericin B (Gibco # 152900) 1 µg/mL, fetal bovine serum 5% FBS (Gibco # 10270106) and were freed where it was possible from the fibrous capsule. The thyroid tissue was cut manually into small fragments (1.0–1.5 mm) which were transferred into a 15 mL tube and rinsed twice with 10 mL DPBS. After DPBS aspiration, the first portion (4–5 mL) of prewarmed enzyme mixture (collagenase 0.8 mg/mL (Sigma Cat# C9891)), dispase 3 mg/mL (Sigma Cat# D4693), trypsin inhibitor 0.4 mg/mL (Sigma Cat# T6522) in DPBS was added to the tissue. The tube was placed horizontally and incubated for 20 min at 37°C with soft shaking every 5 min. The enzyme mixture with a lot of debris was removed, and the pieces of thyroid tissue were incubated in 4–5 mL of fresh enzymes for another 10–15 min at 37°C with shaking every 4–5 min. Isolated follicles were observed after placing the tube under the microscope. When the quantity of undamaged follicles was high enough, they were collected. A fresh portion of enzyme mixture (4–5 mL) was added to the remaining pieces and incubated in the same conditions until complete dissociation of the tissue pieces. Thereafter the collected follicles were passed through a polyethylene terephthalate (PET) filter of 200 µm pore diameter (PluriSelect 43-50200-03). BME medium supplemented with 5% FBS, penicillin 50 units/mL, streptomycin 50 µg/mL, amphotericin B 1 µg/mL was used to rinse the filter. Then the follicles were collected on the filter of 40 µm pore diameter (PluriSelect 43-50040-51) and washed with the same medium. The follicles retained on the mesh with pores 40 µm were washed away from the filter into a petri dish by flushing some of the same medium. The quantity of follicles was examined under the microscope (Fig. 1, panel B), and the follicles were then put into a tube. To reach the complete dissociation of thyroid tissue, the procedure was repeated three to five times, yielding the follicle suspension used for culture.
Figure 1.
Panel A: preparation steps of primary rat thyroid follicle culture embedded into Matrigel. Rat thyroid tissue was digested with collagenase/dispase mixture; isolated follicles were purified and collected by double filtration through nylon mesh with pore size 200 µm to remove big connective tissue aggregates and 40 µm to eliminate single cells and damaged follicles. Then follicles were recovered from the 40 µm filter and embedded into a growth factor-reduced Matrigel and cultured for 2 weeks in defined medium. Panel B: suspension of intact rat thyroid follicles after double filtration through nylon mesh. Panel C: 12-well plate with drops of polymerized Matrigel, containing embedded thyroid follicles before the addition of growth media. Panel D: rat thyroid follicles embedded into Matrigel after 1 day of cultivation, bar scale:100 µm. Panel E: thyroid follicles in Matrigel after 14 days of cultivation, bar scale: 100 µm.
Cover glasses of 15 mm diameter were preliminarily treated with 50 mL of 1N HCl for 1 h at room temperature, rinsed three times with 100 mL of milliQ H2O, rinsed two times with 96% ethanol, air-dried, autoclaved and dried at 80°C. Before culture initiation, the cover glasses were put into a 12-well plate and the center of each glass was covered by 25 µL of growth factor reduced Matrigel (Corning 354230) to form a round dot of about 0.7–1 cm2. The plate was placed into a 5% CO2 incubator for 18 min at 37°C to polymerize the Matrigel.
Follicle suspension was centrifuged for 3.5 min at 300 g. After centrifugation, the supernatant was discarded and the tube with follicles was placed on ice. Five hundred microliters of Matrigel was added to the follicles and they were resuspended by gentle aspiration through 1.25 mL tip (Nest 304016). Thirty-five microliters of follicle suspension in Matrigel was added into each well onto the dot of already solidified Matrigel and incubated in CO2 incubator at 37°C for 20 min (Fig. 1, panels C and D).
The growth medium was as follows: F12 Coon’s modified medium (Gibco Cat.# 04191295M) enriched with 5% FBS; growth medium supplement (GMS): L-glutamine (Gibco Cat.#25030024) 2 mM, penicillin 50 U/mL, streptomycin 50 µg/mL, amphotericin B 1 µg/mL, transferrin 5 µg/mL (Sigma Cat.# T8150), insulin 10 ng/mL (Sigma Cat.# I0516), potassium iodide 10 nM (Merck Cat.#.105044), glutathione 5 mg/L (Sigma Cat.# G4251), L-ascorbic acid 5 mg/L (Merck Cat.#.A4403), bovine TSH 1 mU/mL (Sigma Cat.# T8931) (27). Two microliters of growth medium was added into each well and changed every other day.
Several compositions of culture medium and growth medium supplement were tested to choose the optimal for prolonged cultivation of thyroid follicles in growth factor depleted Matrigel: (i) RPMI + GMS, (ii) F12 Coon’s modified + GMS, (iii) RPMI + 0.1% FBS +GMS, (iv) F12 Coon’s modified + 0.1% FBS + GMS, (v) RPMI + 1% FBS, (vi) F12 Coon’s modified + 5% FBS + GMS.
After 14 days of cultivation, the samples on cover glasses were fixed for 3.5 h at room temperature by 4% buffered formaldehyde (Fig. 1 panel E) and finally were stored at 4°C in PBS.
Immunofluorescence analysis
Agarose block preparation
Hundred milliliters of PBS containing 4% agarose (Sigma Cat.# A9539) solution was melted at 100°C and shaked on a magnetic stirrer. Five milliliters of agarose was put into each mold (Polysciences Cat# 18646A-1) and incubated in a water bath for 15–20 min at 65°C. Cover glasses with fixed follicles in Matrigel were placed onto a microscope slide and air-dried for 10–12 min. Then, the samples were put into the molds with agarose and incubated for an additional 15–20 min at 65°C. The molds were removed from the water bath and incubated at room temperature for 5 min. Then, 5 mL of boiled agarose was added to each mold. The samples were incubated for 2 h at room temperature and kept in a refrigerator at 4°C.
Preparation of agarose slices
Excess agarose was cut from agarose block and the sample was glued to the holder of the vibratome (Leica VT1000S) with acrylic glue. Vibratome cuvette was filled with PBS. The cutting was performed at speed 5 and frequency 6, with a slice thickness of 100 µm. Slices were collected by a tassel and put in 24 well-plates containing PBS.
Immunofluorescence analysis
Immunofluorescence analysis on agarose slices was carried out in a 24-well plate. Nonspecific binding blockade and antigen availability were provided by slice treatment with blocking solution (3% BSA, 5% horse serum, 0.15% Triton X-100 in PBS) for 40 min at room temperature. Antibodies were diluted in a solution composed of 3% BSA, 1% horse serum, 0.1% Triton X-100, in PBS. Incubation with primary antibody was performed overnight at 4°C at slow rotation, in a 250–400 µL/well. The following antibody dilutions were applied: mouse anti-thyroglobulin antibody 1:250 (Abcam # ab187378), rabbit anti-thyroglobulin antibody 1:3000 (Dako #A0251), rabbit anti-ZO-1 antibody 1:500 (Invitrogen Cat# 40-2200), goat anti-T4 antibody 1:500 (Biorbyt #orb11479) followed by three times washing with PBS (2 mL/well for 10 min under gentle agitation). Secondary antibodies anti-mouse FITC488 1:300 (Abcam Cat# ab150113), anti-mouse Alexa Fluor 594 1:300 (Invitrogen Cat# A11032), anti-mouse Сy5 1:500 (Invitrogen Cat#A10524), anti-rabbit Alexa Fluor 555 1:500 (Invitrogen Cat# A27039), Cy3 conjugated anti-goat 1:500 (Jackson #705-165-147) were incubated for 1 h 40 min at room temperature under slow agitation. DNA was counterstained with DAPI (Sigma Cat# D9564) for 20 min RT. Slices were embedded into glycergel (Agilent Dako Cat# C0563) supplemented with 25 mg/mL DABCO (Sigma Cat# D27802) and covered by cover glasses. DABCO was used as an anti-fading agent since follicle preparations are rather thick and could be damaged by photobleaching during confocal imaging. Images were taken using a confocal microscope Zeiss 510 Meta.
Iodide uptake assay
The scheme of the procedure is presented in Fig. 2. The culture of rat thyroid follicles was initiated as described above. After 24 h of incubation, the growth medium was changed for fresh medium but the KI concentration was increased to 1 µM. The growth medium was changed every other day. On day 10, the medium was supplemented with 0.5 µCi/mL of 125I (Perkin Elmer Cat# NEZ033001MC), added to all wells. Methimazole (MMI) 1 mM, a thyroperoxidase inhibitor, was added to control wells to measure the background of the method. On day 14, 125I uptake was stopped by discarding and rinsing the wells with 2 mL of 2 mM MMI in PBS. Follicles were recovered by digestion of the Matrigel (see enzyme mixture for follicle isolation from tissue) supplemented with 2 mM MMI in PBS at 37°C for 15 min. After gentle pipetting by blue tip, the suspension of follicles was transferred into a tube for I125 uptake measurement. The well was washed with 1 mL PBS containing 2 mM MMI added to the same tube and the radioactivity was measured in a gamma counter (Perkin Elmer Wizard1470 Automatic Gamma Counter) as total 125I in the Matrigel drop containing the follicles. For estimation of protein-bound iodine (PBI), after digestion of the Matrigel with enzyme mixture (collagenase 0.8 mg/mL, dispase 3 mg/mL, and trypsin inhibitor 0.4 mg/mL in DPBS), 2 mL of 20% TCA (trichloroacetic acid) was added to each tube to precipitate the proteins. Samples were centrifuged for 10 min at 450 g and the pellet was resuspended in 4 mL of 10% TCA. Protein precipitation and washing were repeated twice. Organified 125I was estimated by counting the radioactivity in the washed pellet in a gamma counter. Data were expressed as the ratio of bound 125I to total 125I in the Matrigel drop × 100.
Figure 2.
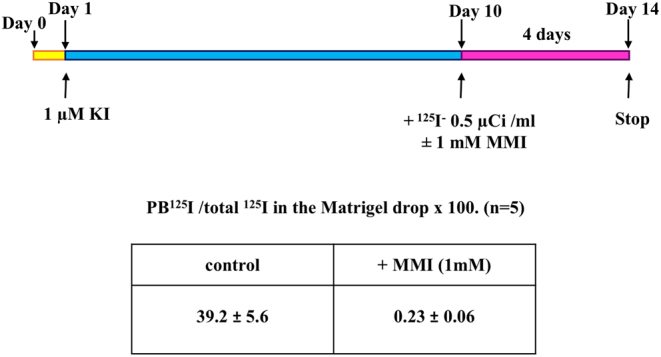
Scheme for experimental detection of 125I organification measured by protein-bound iodine test (PBI) in rat thyroid follicles cultured for 2 weeks in Matrigel. PBI values are expressed as the ratio of PB 125I to total 125I in the Matrigel drop × 100; mean ± s.e.m. of five different preparations.
Ethic statement
Rats were anesthetized with Nembutal in compliance with and after approval of the Ethical and Animal Welfare Committee of the Université libre de Bruxelles (protocol number #709N).
Results and discussion
The maintenance of functionally active thyroid cells in vitro is required for a variety of biomedical investigations. The follicle-like organization of cultured thyrocytes is a necessary condition for the preservation of the functional activity of these cells. Figure 1 represents the scheme of rat thyroid processing to isolate undamaged follicles and ready to be embedded in Matrigel. Mild enzyme treatment with several collections of whole follicles and regular visual control of the obtained material ensured the harvest of suspension of undamaged follicles. Also, supplementing a collagenase/dispase mixture with trypsin inhibitor decreased the nonspecific protease activity and contributed to the preservation of the integrity of a follicular structure. Double filtration of the follicle suspension provided the elimination of crude connective tissue aggregates and small or damaged follicles. It should be noted that in some wells, the fibroblasts from thyroid stroma were present as well and they could produce connective tissue like fibers inside Matrigel. These fibroblasts did not alter the structure of the cultured thyroid follicles.
It is also necessary to monitor the efficiency of recovering the follicles from the 40 μm filter. The best way to wash follicles from the filter was to touch the turned filter with a pipette and apply a gentle stream of liquid. Insufficient washing of the filter led to the loss of a significant quantity of the follicles. Therefore, this step should be performed in a petri dish, which allows to assess the number of follicles by using a microscope.
To minimize the effect of growth factors on the culture of thyroid follicles, growth factor depleted Matrigel was applied. An important step for follicle structure preservation consists of precoating the cover glasses with Matrigel. It completely prevented the spreading of thyroid follicles by abolishing their contact with cover glass.
Several compositions of culture medium and growth medium supplement were compared to detect the most suitable conditions for prolonged cultivation of thyroid follicles in Matrigel. F12 Coon’s modified medium + 5% FBS + GMS was chosen as the optimal combination, based on the morphological assay of thyroid follicles after 2 weeks of cultivation.
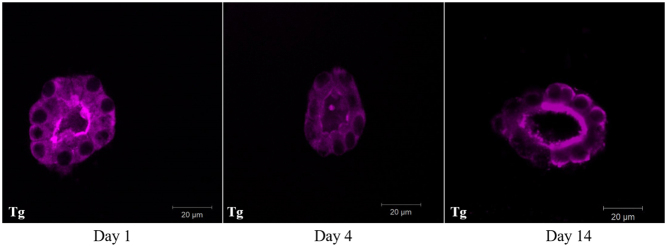
To examine the morphological features of cultured rat thyroid follicles, the immunofluorescence analysis was used. First of all, the presence of thyroglobulin in the follicular cells was revealed. It should be noted that the use of antibodies to thyroglobulin of various origins allowed to detect the thyroglobulin in both cytoplasmic and the intrafollicular lumen. Namely, mouse monoclonal anti-thyroglobulin antibody Abcam ab187378 (Fig. 3) recognized thyroglobulin mainly in the cytoplasm of thyrocytes, while rabbit polyclonal anti-thyroglobulin antibody Dako A0251 showed predominantly intrafollicular localization of thyroglobulin on the serial slices of the same sample. It could be hypothesized that these antibodies react with different epitopes of thyroglobulin. Such epitopes could be more or less accessible in thyroglobulin molecules depending on its actual status, for example, partial proteolysis. Indeed, ‘the cytoplasmic thyroglobulin’ that was observed during all the period of cultivation (Fig. 4) is most probably contained within vesicles, either 'en route' to the follicular lumen (exocytic vesicle containing newly synthesized thyroglobulin) or on the contrary within endocytic vesicles just removed from the follicular lumen. We have no way to distinguish between these possibilities and most probably they both coexist particularly in the presence of 1 mU/mL TSH. In the case of endocytic vesicles, newly added enzymes from peripheral lysosomes may have uncovered new epitopes that are not accessible within the colloid. Indeed thyroglobulin hydrolysis is a complex process sensitive to thyrotropin and mediated at least by three different cathepsins B, D and L. These reactions can uncover some new epitopes. Furthermore, T4 generation was confirmed by a bright positive immunofluorescence reaction predominantly located at the apical membrane of follicular cells (Fig. 5). However, we can only guess whether T4 is free or bound to thyroglobulin. The colocalization of both T4 and Tg may suggest that T4 is still bound within Tg at the apical domain but the lack of colocalization within the colloid may indicate the opposite. Thus, we cannot definitively rule out that the bright immunofluorescence is a reflection of multiple intravesicular T4 molecules released from thyroglobulin but still contained within the same vesicles while after going through the vesicle or lysosomal membrane, free T4 within the cytoplasm would be more diffused and not anymore bright at the basal pole.
Figure 3.
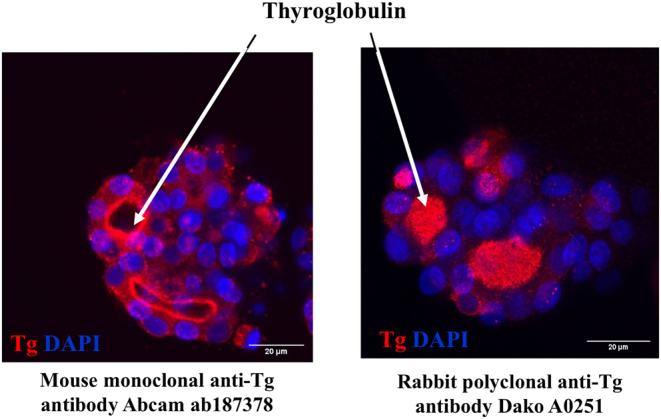
Immunofluorescence detection of thyroglobulin content in thyroid follicles embedded in Matrigel after 2 weeks of cultivation. Mouse monoclonal anti-Tg antibody ab187378 revealed mainly cytoplasmic thyroglobulin (red) whereas rabbit polyclonal anti-Tg antibody Dako A0251 detected thyroglobulin predominantly in intrafollicular lumen (red). Nuclei counterstained with DAPI (blue), bar scale: 20 µm.
Figure 4.
Immunofluorescence analysis of thyroglobulin content (purple) in rat thyroid follicles after 1, 4, and 14 days of cultivation in Matrigel, bar scale: 20 μm.
Figure 5.
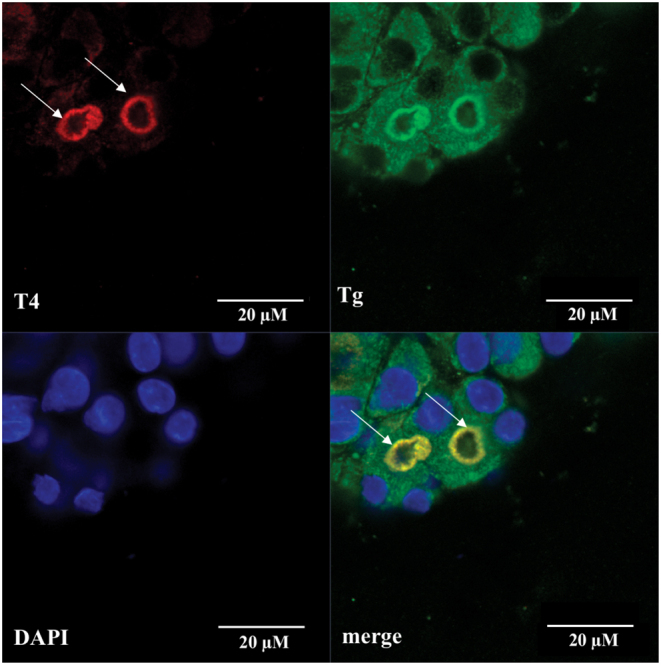
Immunofluorescence detection of T4 (red) and thyroglobulin (green) content in rat thyroid follicles after 14 days of cultivation in Matrigel. Arrows point out bright intraluminal reaction at apical side of thyrocytes, also there is a weak cytoplasmic reaction for T4 in rat thyroid follicles. Nuclei counterstained with DAPI (blue), bar scale 20 µm.
Apical localization of ZO-1-positive tight junction protein was observed in cultured rat thyroid follicles by immunofluorescence analysis (Fig. 6). Moreover, the application of confocal microscopy and 3D reconstruction allowed to confirm the integrity of cultured follicles (Fig. 7). Three-dimensional reconstruction revealed the honeycomb pattern of the ZO-1 distribution at thyrocyte lumen-facing apical membrane as occurring in vivo (28).
Figure 6.
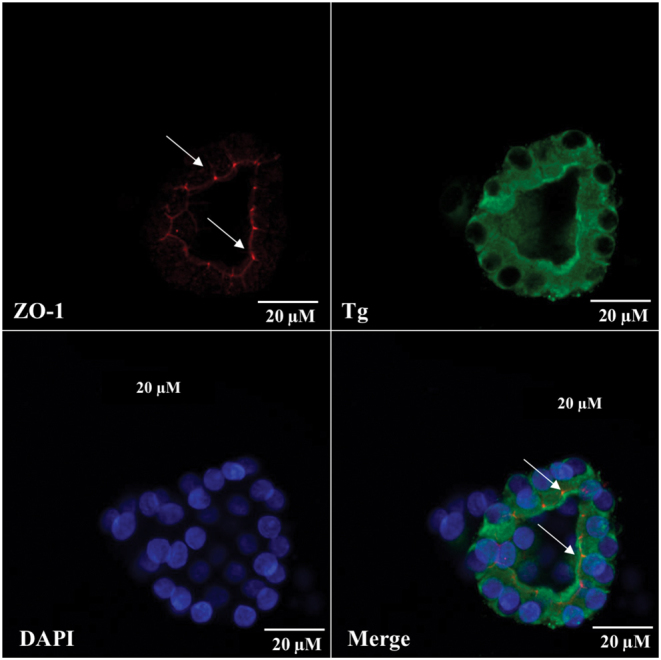
Immunofluorescence analysis of ZO-1 (red) localization and thyroglobulin (green) content in rat thyroid follicles after 14 days of cultivation in Matrigel. Arrows point out the bright apical reaction of ZO-1 in cultured thyrocytes. Nuclei counterstained with DAPI (blue), bar scale: 20 µm.
Figure 7.
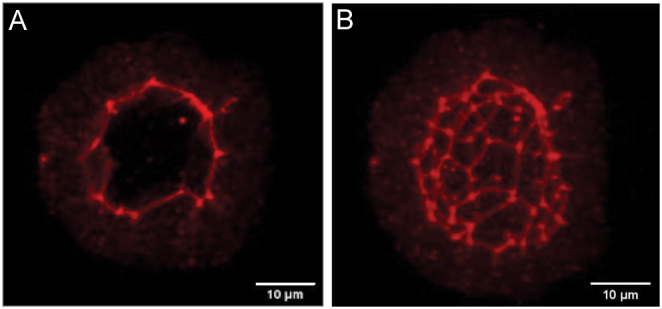
Immunofluorescence detection of ZO-1 (A) and 3D reconstruction of follicle structure based on consecutive confocal optical slices (B) confirm the integrity of follicles cultured in Matrigel. Bar scale:10 µm.
Also, the size of follicles after 1 and 14 days of cultivation was valued. It was determined that after 1 day of cultivation, the mean follicle diameter was 45.88 ± 13.25 µm. The minimum diameter was about 30 µm and the maximum was 72 µm. The presence of some quantity of small follicles (less than 40 µm) could be explained by the following reason: the groups of small follicles were retained on 40 µm filter and then, they were disaggregated at the subsequent step, namely, during pipetting of follicles in Matrigel before they were put into 12-well plate. Also, sometimes, we observed such groups of small follicles in our cultures. The diameter of rat thyroid follicles after 14 days of cultivation was 47.57 ± 12.74. There was no statistically significant difference between the follicle size after 1 and 14 days of cultivation (t-test, GraphPadPrism 6.01).
As the organification of iodide is a major step in thyroid physiology, the uptake of 125Iwas tested in rat follicle cultured for 14 days in Matrigel following the protocol designed in Fig. 2. KI concentration up to 1µM was added on day 1 as a carrier and to stabilize the uptake of 125I. 125I−, 0.5 µCi/mL ± MMI was added on day 10 during 4 days. Follicles were isolated from Matrigel by enzyme digestion. The rest of the digested Matrigel served as protein carriers for subsequent re-precipitation of proteins, in order to reduce the loss of PBI. The uptake of 125I by the follicles in presence of MMI was insignificant: the counted 125I uptake varies in the range of 0.05–0.4% (mean 0.23 ± 0.06%) of total radioactivity counted in the whole Matrigel drop. In contrast, in the absence of MMI, rat thyroid follicles cultured for 2 weeks in Matrigel actively transported and organified iodide. Radioactive iodide counted in the TCA precipitate represented 24.4–54.9% (mean 39.2 ± 5.6%) of the total radioactive iodide in the Matrigel drop. These data serve as convincing evidence that under the proposed experimental conditions, the follicular cells of the rat thyroid gland retain their functional activity.
In conclusion, we have developed a technique of primary rat thyroid follicle culture keeping its initial follicular organization and high functional activity of thyrocytes during 2 weeks of cultivation. The proposed model will be useful for both research of the basic mechanisms underlying the functioning of the thyroid gland, and evaluation of the effect of different factors on the whole behavior and functional activity of thyroid cells.
Declaration of interest
The authors declare that this research was conducted in the absence of any financial or other potential conflict of interest.
Funding
This study was supported by the 'Fonds Dr Jean-Pierre Naet' (#J1813300) managed by the 'Fondation Roi Baudouin', Belgium.
References
- 1.Rousset B, Dupuy C, Miot F, Dumont JE.Chapter 2. Thyroid hormone synthesis and secretion. In Endotext, Eds Feingold KRB, Anawalt A, Boyceet al. South Dartmouth, MA, USA: MDText.com, Inc, 2015. (available at: https://www.ncbi.nlm.nih.gov/books/NBK285550) [Google Scholar]
- 2.Samimi H, Atlasi R, Parichehreh-Dizaji S, Khazaei S, Rahnama MA, Seifirad S, Haghpanah V.A systematic review on thyroid organoid models: time-trend and its achievements. American Journal of Physiology: Endocrinology and Metabolism 2021. 320 E581–E590. ( 10.1152/ajpendo.00479.2020) [DOI] [PubMed] [Google Scholar]
- 3.Bocanera LB, Aphalo P, Pisarev MA, Gärtner R, Silberschmidt D, Juvenal GJ, Beraldi G, Krawiec L.Presence of a soluble inhibitor of thyroid iodination in primary cultures of thyroid cells. European Journal of Endocrinology 1999. 141 55–60. ( 10.1530/eje.0.1410055) [DOI] [PubMed] [Google Scholar]
- 4.Roger PP, Christophe D, Dumont JE, Pirson I.The dog thyroid primary culture system: a model of the regulation of function, growth and differentiation expression by cAMP and other well-defined signaling cascades. European Journal of Endocrinology 1997. 137 579–5. ( 10.1530/eje.0.1370579) [DOI] [PubMed] [Google Scholar]
- 5.Di Lauro R, Damante G, De Felice M, Arnone MI, Sato K, Lonigro R, Zannini M.Molecular events in the differentiation of the thyroid gland. Journal of Endocrinological Investigation 1995. 18 117–11. ( 10.1007/BF03349716) [DOI] [PubMed] [Google Scholar]
- 6.Pocard T, Le Bivic A, Galli T, Zurzolo C.Distinct v-SNAREs regulate direct and indirect apical delivery in polarized epithelial cells. Journal of Cell Science 2007. 120 3309–3320. ( 10.1242/jcs.007948) [DOI] [PubMed] [Google Scholar]
- 7.Spinel-Gomez C, Colin I, van den Hove MF, Denef JF.Correlated morphological and functional study of isolated rat thyroid follicles in suspension culture. Molecular and Cellular Endocrinology 1990. 71 141–1. ( 10.1016/0303-7207(9090251-3) [DOI] [PubMed] [Google Scholar]
- 8.Khoruzhenko A.New approaches for thyrocyte cultivation in vitro with retention of their follicular organization. Experimental Oncology 2002. 24 99–104. [Google Scholar]
- 9.Sato K, Yamazaki K, Shizume K, Kanaji Y, Obara T, Ohsumi K, Demura H, Yamaguchi S, Shibuya M.Stimulation by thyroid-stimulating hormone and Grave’s immunoglobulin G of vascular endothelial growth factor mRNA expression in human thyroid follicles in vitro and mRNA expression in the rat thyroid in vivo. Journal of Clinical Investigation 1995. 96 1295–1302. ( 10.1172/JCI118164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghaddhab C, Kyrilli A, Driessens N, Van Den Eeckhaute E, Hancisse O, De Deken X, Dumont JE, Detours V, Miot F, Corvilain B.Factors contributing to the resistance of the thyrocyte to hydrogen peroxide. Molecular and Cellular Endocrinology 2019. 481 62–70. ( 10.1016/j.mce.2018.11.010) [DOI] [PubMed] [Google Scholar]
- 11.Garbi C, Nitsch L, Wollman SH.Embedding in a collagen gel stabilizes the polarity of epithelial cells in thyroid follicles in suspension culture. Experimental Cell Research 1984. 151 458–4. ( 10.1016/0014-4827(8490395-1) [DOI] [PubMed] [Google Scholar]
- 12.Fröhlich E, Wahl R, Reutter K.Basal lamina formation by porcine thyroid cells grown in collagen- and laminin-deficient medium. Histochemical Journal 1995. 27 602–60. ( 10.1007/BF02388459) [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Massart C, Chico-Galdo V, Jin L, De Maertelaer V, Decoster C, Dumont JE, Van Sande J.Species specific thyroid signal transduction: conserved physiology, divergent mechanisms. Molecular and Cellular Endocrinology 2010. 319 56–62. ( 10.1016/j.mce.2010.01.024) [DOI] [PubMed] [Google Scholar]
- 14.Simons PJ, Delemarre FG, Drexhage HA.Antigen-presenting dendritic cells as regulators of the growth of thyrocytes: a role of interleukin-1β and interleukin-6. Endocrinology 1998. 139 3148–3156. ( 10.1210/endo.139.7.6110) [DOI] [PubMed] [Google Scholar]
- 15.Herzog V, Miller F.Membrane retrieval in epithelial cells of isolated thyroid follicles. European Journal of Cell Biology 1979. 19 203–2. [PubMed] [Google Scholar]
- 16.Chambard M, Verrier B, Gabrion J, Mauchamp J.Polarity reversal of inside-out thyroid follicles cultured within collagen gel: reexpression of specific functions. Biology of the Cell 1984. 51 315–3. ( 10.1111/j.1768-322x.1984.tb00310.x) [DOI] [PubMed] [Google Scholar]
- 17.Espanet H, Alquier C, Mauchamp J.Polarity reversal of inside-out thyroid follicles cultured on the surface of a reconstituted basement membrane matrix. Experimental Cell Research 1992. 200 473–4. ( 10.1016/0014-4827(9290198-h) [DOI] [PubMed] [Google Scholar]
- 18.Koumarianou P, Goméz-López G, Santisteban P.Pax8 controls thyroid follicular polarity through cadherin-16. Journal of Cell Science 2017. 130 219–231. ( 10.1242/jcs.184291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonoli H, Flachon V, Audebet C, Callé A, Jarry-Guichard T, Statuto M, Rousset B, Munari-Silem Y.Formation of three-dimensional thyroid follicle-like structures by polarized FRT cells made communication competent by transfection and stable expression of the connexin-32 gene. Endocrinology 2000. 141 1403–1413. ( 10.1210/endo.141.4.7400) [DOI] [PubMed] [Google Scholar]
- 20.Antonica F, Figini Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, Refetoff S, Peremans K, Manto M, Kyba M.Generation of functional thyroid from embryonic stem cells. Nature 2012. 491 66–71. ( 10.1038/nature11525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito Y, Onishi N, Takami H, Seishima R, Inoue H, Hirata Y, Kameyama K, Tsuchihashi K, Sugihara E, Uchino S.Development of a functional thyroid model based on an organoid culture system. Biochemical and Biophysical Research Communications 2018. 497 783–789. ( 10.1016/j.bbrc.2018.02.154) [DOI] [PubMed] [Google Scholar]
- 22.Young BA, Baker TG.The ultrastructure of rat thyroid glands under experimental conditions in organ culture. Journal of Anatomy 1982. 135 407–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Asakawa H, Hanafusa T, Oda Y, Katsura H, Miyagawa J, Mashita K, Kono N, Tarui S.Human recombinant interleukin 1 inhibits TSH-stimulated morphological changes in thyroid follicles cultured as semi-organs. Acta Endocrinologica 1991. 125 80–8. ( 10.1530/acta.0.1250080) [DOI] [PubMed] [Google Scholar]
- 24.Bauer MF, Herzog V.Mini organ culture of thyroid tissue: a new technique for maintaining the structural and functional integrity of thyroid tissue in vitro. Laboratory Investigation 1988. 59 281–2. [PubMed] [Google Scholar]
- 25.Dombrowski F, Klotz L, Hacker HJ, Li Y, Klingmüller D, Brix K, Herzog V, Bannasch P.Hyperproliferative hepatocellular alterations after intraportal transplantation of thyroid follicles. American Journal of Pathology 2000. 156 99–113. ( 10.1016/S0002-9440(1064710-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colin IM, Denef JF, Lengelé B, Many MC, Gérard AC.Recent insights into the cell biology of thyroid angiofollicular units. Endocrine Reviews 2013. 34 209–238. ( 10.1210/er.2012-1015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bechtner G, Fröschl H, Sachse A, Schopohl D, Gärtner R.Induction of apoptosis in porcine thyroid follicles by transforming growth factor beta1 and epidermal growth factor. Biochimie 1999. 81 315–3. ( 10.1016/s0300-9084(9980076-5) [DOI] [PubMed] [Google Scholar]
- 28.Rebuffat SA, Kammoun-Krichen M, Charfeddine I, Ayadi H, Bougacha-Elleuch N, Peraldi-Roux S.IL-1β and TSH disturb thyroid epithelium integrity in autoimmune thyroid diseases. Immunobiology 2013. 218 285–2. ( 10.1016/j.imbio.2012.05.016) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a