Abstract
Objectives
Circulating concentrations of endogenous steroids have systemic implications on health in elderly. However, population-based age- and ethnicity-specific data are scarce. The aim was to report sex-specific plasma concentrations of endogenous sex and adrenal steroids in elderly Swedish Caucasians, to examine the impact of BMI and to present concentrations in apparently healthy subjects.
Methods
A population-based observational study of 70-year olds, including 684 community-dwelling men and women enrolled in the PIVUS study, Sweden. Median plasma concentrations were determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS) for pregnenolone, 17-hydroxypregnenolone, 17-hydroxy-progesterone, 11-deoxycortisol, DHEA, androstenedione, testosterone, estrone and estradiol.
Results
Plasma concentrations were significantly higher in men (n = 452) than in women (n = 232) for estradiol: median 61.3 pmol/L (95% CI, 11.4, 142.7) vs 18.4 (4.0, 127.3), for estrone: 92.8 (33.3, 206) vs 71.6 (17.8, 209) pmol/L, and for testosterone 13.8 (5.7, 28.0) vs 0.7 (0.2, 2.0) nmol/L. Higher concentrations of estrone and estradiol were observed in obese than non-obese women. Compared to non-obese men, obese men had lower concentrations of testosterone and its precursors: 17-hydroxypregnenolone, 17-hydroxyprogesterone, androstenedione and DHEA. The subgroup of apparently healthy individuals had median values > 20% lower for estrone and estradiol in women but slightly higher for testosterone in both sexes.
Conclusions
Concentrations of estradiol, estrone and testosterone were higher in 70-year-old men than in women. BMI associated positively to estradiol and estrone in women and negatively to testosterone in men. Apparently healthy women had lower median concentrations of estradiol and estrone and men had higher median testosterone compared to all individuals.
Keywords: endogenous, sex steroids, elderly, population-based, liquid chromatography-tandem mass spectrometry
Introduction
Sex hormones have traditionally been considered most important during early and reproductive periods of life, as they play an important role in regulating growth, development of the gonads, and reproduction (1). However, recent epidemiological studies suggest that sex hormones may have an impact on various body functions and diseases in older individuals, for example, bone mineral density (2, 3), diabetes (4) and development of cardiovascular disease (5, 6, 7) as well as Alzheimer’s disease and impaired cognitive function (8). The levels of sex hormones may also be related to BMI), and diabetes status (4), prevalent cardiovascular disease (CVD) (9), and vascular aging in elderly women and men (10). Higher testosterone concentrations in older men have been associated with reduced all-cause mortality and higher dihydrotestosterone (DHT) with reduced ischemic heart disease mortality (11). Estrone is the most prevalent estrogen in menopause and has less intra- and inter-variation than estradiol (7, 12), which declines with the onset of menopause. Estradiol levels are very low in postmenopausal women (7) but play an important role for physiological functions. Although data on endogenous sex steroid concentrations have been presented for children (13, 14, 15, 16, 17), adolescents (18) and adults (13, 14, 16, 17, 19, 20), sex-specific and age-specific population-based concentration are scarce, especially for elderly. In addition, there are very few studies aimed on measurement of bioactive sex hormones and their precursors in elderly apparently healthy individuals (21) and individuals with pathology (22, 23).
Accurate quantification of the low concentrations of sex steroids present in elderly is important. Immunoassays (IAs) are often used to quantify sex hormones (19), however, due to their low specificity and assay-specific bias (19, 20, 24), IAs have been shown to be inaccurate to quantify the low steroid concentrations seen in elderly (25, 26, 27). We recently developed highly sensitive and specific liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods to identify and quantify a large number of steroids in small sample volumes (13, 14, 16, 17, 28).
The aim of this study was to evaluate distributions of concentrations of endogenous steroids in a population-based sample of 70-year-old Swedish Caucasian men and women, evaluate association of the concentrations with BMI, and to assess distribution of concentrations in apparently healthy 70-year-old men and women.
Subjects and methods
Study population
The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) is a population-based study in Uppsala community, Sweden (www.medsci.uu.se/pivus/pivus.htm). Eligible for the study were all Caucasian individuals aged 70 years and living in the community at enrolment between April 2001 and June 2004. The participants were randomly chosen from the national register of residence and received an invitation by letter within 2 months of their 70th birthday. Of the invited 2025 subjects, a total of 1016 individuals participated in the PIVUS (participation rate 50%). A comparison with non-respondents has been described (29). All the study participants underwent a physical examination; information on history of coronary heart disease (CHD) and menopausal hormone therapy (MHT) was available, as previously described (29).
Study design
CVD was defined as the self-reported data on the presence or history of heart failure, hospital-treated myocardial infarction or stroke, coronary artery bypass graft (CABG) surgery or percutaneous transluminal coronary angioplasty (PTCA) but excluding subjects with hypertension alone. Respiratory disease was defined as the self-reported data on the presence or history of asthma, chronic bronchitis, COPD or emphysema. For this study, diabetes was defined as fasting blood sugar > 6.2 mmol/L or if there is history of diabetes. Lipid lowering treatment was defined as the current treatment with statins or other lipid-lowering medication. Common cardiovascular disease-related drugs were defined as any regular pharmaceutical treatment. All participants were investigated in the morning after an over-night fast with blood drawn between 08:00 and 10:00 h. No medication or smoking was allowed after midnight. BMI was defined as body weight in kilograms divided by the squared body height in meters and was based on measurements performed at the enrolment. Smoking status was defined based on the self-report and categorized into current, former and never-smokers (30). Plasma samples were stored at −70°C until analysis and transported between the participating centere on dry ice. Each participant provided an informed consent form, and the study was approved by the human ethics committee of Uppsala University, Sweden.
Reagents and standards
Testosterone, estrone, estradiol, pregnenolone, 17-hydroxypregnenolone, 17-hydroxyprogesterone, 11-deoxycortisol, hydroxylamine, formic acid, trifluoroacetic acid, dansyl chloride, and sodium carbonate were purchased from MilliporeSigma. Androstenedione and DHEA were purchased from Steraloids (Newport, RI). The internal standards were deuterium-labeled analogs of the steroids d3-testosterone, d4-pregnenolone, d2-11deoxycortisol, d8-17-hydroxyprogesterone, and d3-17-hydroxypregnenolone (purchased from Cambridge Isotope Laboratories, Andover, MA) and d4-estrone and d3-estradiol (purchased from CDN Isotopes, Toronto, ON, Canada). Methanol, acetonitrile, and methyl-tert-butyl ether were all high-performance LC grade (purchased from VWR, West Chester, PA). All other chemicals were of the highest purity commercially available.
Methods
Nine endogenous steroids were quantified in plasma samples using high-sensitivity LC-MS/MS assays at ARUP Laboratories (Salt Lake City, UT) (13, 14, 16, 17). In short, estrogens were analyzed as dansyl derivatives (13) and ketosteroids were analyzed as oxime derivatives (14, 17). The HPLC system consisted of series 1200 HPLC pumps (Agilent), six-port switching valve, a vacuum degasser, and an autosampler HTC PAL (LEAP Technologies, Carrboro, NC). An API 5500 (AB SCIEX, Foster City, CA) tandem mass spectrometer was used in the positive ion mode with TurboIonspray ion source. The analytic procedures are described in details elsewhere (13, 14, 17, 28, 31). The quadrupoles Q1 and Q3 were tuned to unit resolution, and the mass spectrometer conditions were optimized for maximum signal intensity and specificity. Two mass transitions were monitored for each steroid and its IS. Concentrations of each steroid were determined using the primary mass transitions; specificity of the analysis for each steroid was evaluated by comparing concentrations determined using the primary and the secondary mass transitions of each steroid and its IS (32). Quantitative data analysis was performed using Analyst 1.5.2 software (AB SCIEX). The assays had a within-run coefficient of variation (CV) less than 10% and a between-run CV less than 12% (13, 14, 17, 28). Calibration curves were generated for every batch of samples, using six calibration standards; three quality control samples were included with every batch of samples. The analytical sensitivity of the assays for the nine endogenous steroids is presented in Table 1.
Table 1.
Limit of quantification of the LC-MS/MS assays.
| Analyte | Limit of quantitation, pmol/L |
|---|---|
| Estrone | 4 |
| Estradiol | 4 |
| 11-deoxycortisol | 145 |
| Testosterone | 35 |
| DHEA | 170 |
| Androstenedione | 35 |
| 17-hydroxypregnenolone | 750 |
| Pregnenolone | 150 |
| 17-hydroxyprogesterone | 150 |
SHBG levels were measured using an immunofluorometric assay (DELFIA-Wallac, Inc., Turku, Finland) (32, 33). Inter-assay coefficient of variations were 8.3, 7.9, and 10.9%, and intra-assay coefficient of variations 7.3, 7.1, and 8.7%, respectively, in the low, medium, and high pools. The analytical sensitivity of the assays was 0.5 nmol/L.
Statistical analyses
Subjects on current corticosteroid treatment and on previous or current hormone therapy were excluded. Demographic data are presented as median and range, or percentage. Steroid concentrations are presented as median and 2.5 and 97.5 percentiles (i.e. central 95%) by sex and by sex and BMI category (normal weight (BMI ≤ 25 kg/m2), overweight (25 < BMI ≤ 30 kg/m2) or obese (BMI > 30 kg/m2)). Individuals without prevalent cardiovascular disease and diabetes, not taking lipid-lowering medications, and non-smokers were considered as apparently health subjects. Normality of distributions for the continuous variables was evaluated using the skewness and kurtosis tests for Normality. Comparison of concentrations for binary variables (e.g. sex) was performed using the non-parametric Wilcoxon rank-sum test as concentrations of all steroids were non-normally distributed. For three level categorical variable (i.e. BMI), sex comparisons were made applying the non-parametric Kruskal–Wallis test. To adjust for multiple testing, the conservative Bonferroni correction of the P-value for statistical significance was applied and set to 0.05/10 (i.e. P < 0.005) when evaluating among-groups differences in the steroid concentrations. Concentrations of the measured steroids in apparently healthy subjects are presented excluding statistical outliers, based on the conventional percentile method to exclude extreme values according to Tukey (34). STATA 12 (StataCorp, College Station, TX, USA) was used for most data management and analysis, and Medcalc statistical software (v.14.8.1, Frank Schoonjans, Mariakerke, Belgium) was used to assess distribution of concentrations in apparently healthy individuals.
Results
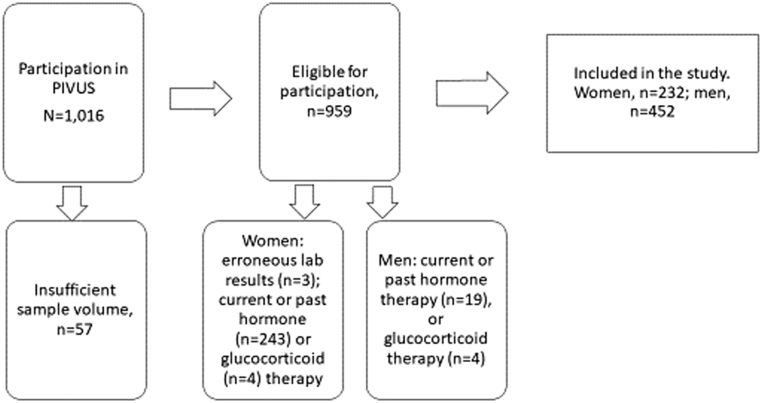
The selection of participants in this study from the PIVUS sample of 1016 men and women is shown in Fig. 1. After exclusions the total number included was 684 (232 women, 34%). The characteristics of the study population are presented in Table 2. The steroid concentrations and the continuous demographic variables (except height) were non-normally distributed. The prevalence of CVD was statistically significantly higher in men (20%) than in women (8%), as was alcohol intake (median 6.6 vs 2.7 g per day). There was a higher proportion of overweight men and a higher proportion of obese women, whereas the exercise habits were similar in both sexes.
Figure 1.
Selection of the study participants (exclusion criteria shown in the lower three boxes).
Table 2.
Characteristics of the study population including 232 women and 452 men after removing observations with no available sex steroid data (n = 57), observations with known erroneous laboratory test results (n = 3, all women), persons on hormone replacement therapy (n women = 243, n men = 19) and persons on glucocorticoid therapy (n women = 6, n men = 4). Statistical significant difference between men and women as evaluated by chi-squared test (categorical variables) and two-sample Wilcoxon rank-sum (Mann–Whitney) test (continuous variables).
| Women | Men | |||
|---|---|---|---|---|
| n | Median (range) or percentage | n | Median (range) or percentage | |
| Weight (kg) | 232 | 72 (42, 126)* | 452 | 82 (57, 138) |
| Height (cm) | 232 | 162 (149, 184)* | 452 | 176 (155, 198) |
| Waist circumference (cm) | 228 | 88 (62, 134)* | 447 | 94 (64, 134) |
| BMI (kg/m2) | 232 | 26.9 (17.7, 49.8) | 452 | 26.8 (19.2, 43.4) |
| BMI ≤ 25.0 kg/m2 | 79 | 34*† | 143 | 32 |
| BMI > 25.0–≤30 kg/m2 | 89 | 38*† | 230 | 51 |
| BMI > 30 kg/m2 | 64 | 28*† | 79 | 17 |
| Diabetes | 23 | 10 | 57 | 13 |
| Cardiovascular disease | 18 | 8* | 90 | 20 |
| Respiratory disease | 16 | 7 | 28 | 6 |
| Hypertension | 180 | 78* | 314 | 69 |
| Lipid lowering therapy | 39 | 17 | 81 | 18 |
| Cardiovascular disease therapy | 162 | 70 | 284 | 63 |
| Alcohol intake (g/day) | 192 | 2.7 (0, 32.1)* | 384 | 6.6 (0, 61.3) |
| Current smoker | 31 | 13 | 42 | 9 |
| Exercise habits, per week | ||||
| Very light, <2 times | 21 | 9 | 54 | 13 |
| Light, twice per week | 152 | 68 | 262 | 62 |
| Moderate (sweat), 1–2times | 42 | 19 | 81 | 19 |
| Heavy exercise (sweat) > 2 per week | 8 | 4 | 25 | 6 |
†BMI categories were evaluated in total, not pairwise. *indicates statistical significance after adjusting P values for multiplicity using Bonferroni correction (0.05/10); P = 0.005.
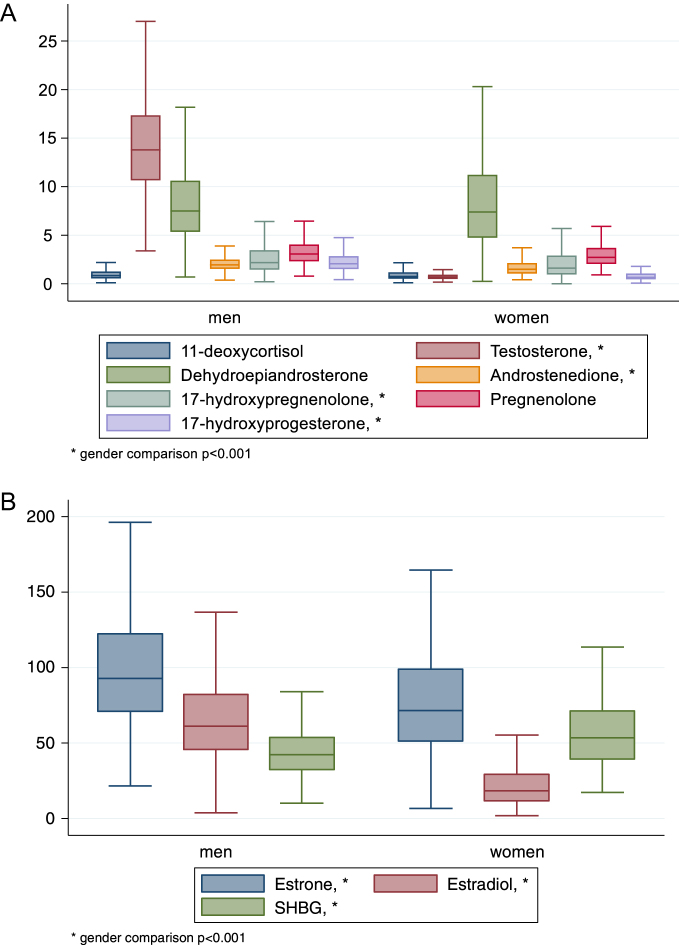
Out of the measured endogenous steroids, concentrations in men were significantly higher than women for estrone, estradiol, testosterone, androstenedione, 17-hydroxypregnenolone, 17-hydroxy-progesterone and sex hormone binding globuline (Table 3). The distribution of the steroid concentrations by sex are presented in Fig. 2 as median and 50th percentile. Steroid concentrations in a subgroup of apparently healthy individuals (56 women and 135 men) are shown in Table 3. The median values for estrone and estradiol were >20% lower in healthy women than the median in all women, whereas median concentration of testosterone was 5–6.5% higher in healthy women and men, as compared to the entire study population (Table 3).
Table 3.
Concentration of endogenous steroids in 70-year-old women (n = 232) and men (n = 452) (after removing observations with no available sex steroid data (n = 57), observations with known erroneous laboratory test results (n = 3, all women), persons on hormone replacement therapy (n women = 243, n men = 19) and persons on glucocorticoid therapy (n women = 6, n men = 4) presented as median and the 2.5 and 97.5 percentile values (i.e. the central 95% range). Gender comparisons were made applying the non-parametric Wilcoxon rank-sum test. For apparently healthy subjects, concentrations of steroids in 70-year-old women and men are shown after removing also persons with diabetes and/or cardiovascular disease (n women = 40, n men = 127), persons with asthma, COPD or chronic bronchitis (n women = 12, n men = 21), persons on lipid lowering medication (n women = 23, n men = 19), persons on any common cardiovascular disease-related drugs (n women = 91, n men = 133), current smokers (n women = 10, n men = 17), and outliers identified according to Tukey (34) using the non-parametric percentile method (CLSI C28-A3).
| Variable | Women | Men | Comparing All women and all men | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All subjects | Healthy subjects | All subjects | Healthy subjects | ||||||
| n | Median (central 95%) | n | Median (central 95%) | n | Median (central 95%) | n | Median (central 95%) | P-value* | |
| Estrone (pmol/L) | 232 | 71.6 (17.8, 209) | 50 | 63,1 (23.3, 124) | 452 | 92.8 (33.3, 206) | 131 | 89.5 (30.7, 169) | <0.001* |
| Estradiol (pmol/L) | 232 | 18.4 (4.0, 127) | 48 | 14.8 (6.20, 33.0) | 452 | 61.3 (11.4, 143) | 130 | 56.0 (11.4, 121) | <0.001* |
| 11-deoxycortisol (nmol/L) | 232 | 0.749 (0.179, 3.63) | 55 | 0.726 (0.202, 2.13) | 450 | 0.840 (0.259, 2.85) | 128 | 0.842 (0.259, 1.87) | 0.095 |
| Testosterone (nmol/L) | 232 | 0.694 (0.243, 2.01) | 55 | 0.729 (0.222, 1.42) | 450 | 13.8 (5.69, 28.0) | 131 | 14.7 (6.94, 27.0) | <0.001* |
| DHEA (nmol/L) | 232 | 7.39 (1.35, 22.7) | 51 | 6.97 (1.97, 16.5) | 450 | 7.50 (2.01, 23.6) | 130 | 7.55 (2.01, 17.0) | 0.77 |
| Androstenedione (nmol/L) | 232 | 1.49 (0.590, 3.61) | 56 | 1.45 (0.590, 3.12) | 450 | 1.94 (0.833, 4.16) | 134 | 1.93 (0.968, 3.40) | <0.001* |
| 17-hydroxypregnenolone (nmol/L) | 232 | 1.61 (0.211, 9.33) | 5 | 1.51 (0.289, 5.24) | 450 | 2.18 (0.602, 8.88) | 127 | 2.41 (0.602, 5.63) | <0.001* |
| Pregnenolone (nmol/L) | 232 | 2.72 (1.12, 5.75) | 55 | 2.69 (1.24, 4.87) | 450 | 3.07 (1.29, 6.45) | 133 | 3.03 (1.37, 6.35) | 0.002* |
| 17-hydroxyprogesterone (nmol/L) | 232 | 0.665 (0.242, 2.85) | 54 | 0.688 (0.294, 1.58) | 450 | 2.06 (0.761, 4.64) | 132 | 2.12 (1.06, 4.58) | <0.001* |
| Sex hormone-binding globulin (nmol/L) | 222 | 53.5 (20.2, 123) | 54 | 50.3 (27.4, 96.3) | 438 | 42.2 (18.4, 91.5) | 126 | 44.2 (20.6, 76.8) | <0.001* |
*indicates statistical significance after adjusting P values for multiplicity using Bonferroni correction (0.05/10); P = 0.005.
Figure 2.
Distributions of endogenous steroids in 70-year-old men and women, presented as median and 25th and 75th percentile. (A) Median concentration of testosterone, androstenedione, pregnenolone, 17-hydroxypregnenolone, 17-hydroxyprogesterone, 11-deoxycortisol, and DHEA, in nmol/L with the outline of the boxes representing the interquartile range and the errorbars extending to the adjacent values for 452 men and 232 women. (B) Median concentrations of estrone, estradiol (in pmol/L), and SHBG (in nmol/L) with the outline of the boxes representing the interquartile range and the errorbars extending to the adjacent values for 452 men and 232 women.
Distribution of steroid concentrations by BMI category are presented in Tables 4 (women) and Table 5 (men); individuals on hormone replacement and glucocorticoid therapy or on lipid-lowering medication were excluded. In both sexes, BMI was negatively associated with SHBG concentrations. In women, higher BMI category had higher concentrations of estrone and estradiol (Table 3); in men, the higher BMI category had higher concentrations of estrone and lower concentrations of testosterone as well as lower concentrations of the precursors of sex steroids; DHEA, androstenedione, 17-hydroxypregnenolone, and 17-hydroxyprogesterone (Table 4).
Table 4.
Concentrations of steroids in 70-year-old women (n = 193) (after excluding observations with no available sex steroid data (n = 57), observations with known erroneous test results (n = 3, all women), on hormone replacement therapy (n = 243)), on glucocorticoid therapy (n = 6) and/or lipid lowering medication (n = 39), by BMI categories normal weight (BMI < 25 kg/m2), overweight (BMI 25–30 kg/m2) and obese (BMI > 30 kg/m2), presented as median and the 2.5 and 97.5 percentile values (i.e. the central 95%). BMI category comparisons were made applying the non-parametric Kruskal–Wallis test.
| Variable | Normal weight | Over weight | Obese | P-valuea | |||
|---|---|---|---|---|---|---|---|
| n | Median (2.5th, 97.5th percentiles) | n | Median (2.5th, 97.5th percentiles) | n | Median (2.5th, 97.5th percentiles) | ||
| Estrone (pmol/L) | 74 | 64.9 (18.1, 235) | 68 | 70.4 (14.4, 177) | 51 | 86.1 (30.0, 246) | <0.005* |
| Estradiol (pmol/L) | 74 | 16.4 (3.67, 131) | 68 | 18.4 (4.63, 151) | 51 | 24.7 (9.58, 120) | <0.005* |
| 11-deoxycortisol (nmol/L) | 74 | 0.723 (0.201, 3.57) | 68 | 0.770 (0.141, 3.68) | 51 | 0.835 (0.173, 2.10) | 0.53 |
| Testosterone (nmol/L) | 74 | 0.729 (0.242, 1.70) | 68 | 0.694 (0.232, 2.61) | 51 | 0.729 (0.382, 1.98) | 0.84 |
| Dehydroepi-androsterone (nmol/L) | 74 | 8.42 (2.71, 21.7) | 68 | 7.37 (0.746, 30.2) | 51 | 7.11 (2.95, 16.3) | 0.28 |
| Androstenedione (nmol/L) | 74 | 1.49 (0.590, 3.47) | 68 | 1.40 (0.555, 3.61) | 51 | 1.49 (0.486, 3.12) | 0.80 |
| 17-hydroxypregnenolone (nmol/L) | 74 | 1.99 (0.21, 8.61) | 68 | 1.67 (0.205, 11.2) | 51 | 1.48 (0.33, 5.15) | 0.17 |
| Pregnenolone (nmol/L) | 74 | 3.14 (1.45, 5.56) | 68 | 2.58 (1.21, 7.43) | 51 | 2.65 (1.36, 4.04) | 0.016 |
| 17-hydroxyprogesterone (nmol/L) | 74 | 0.773 (0.261, 2.36) | 68 | 0.715 (0.203, 3.27) | 51 | 0.757 (0.212, 1.757) | 0.94 |
| Sex hormone-binding globulin (nmol/L) | 72 | 68.8 (27.4, 127) | 66 | 55.7 (31.5, 103) | 47 | 39.7 (18.8, 79.7) | <0.005* |
aKruskal–Wallis test was used to test differences between BMI categories; *indicates statistical significance after adjusting the P-values for multiplicity using Bonferroni correction (0.05/10); P-value for significance 0.005.
Table 5.
Concentrations of steroids in 70-year-old men (n = 371) after excluding persons on glucocorticoid therapy (n = 4), hormone treatment (n = 19) and/or lipid lowering medication (n = 81), by BMI categories normal weight, (BMI < 25 kg/m2) overweight (BMI 25–30 kg/m2) and obese (BMI > 30 kg/m2), presented as median and the 2.5 and 97.5 percentile values (i.e. the central 95%). BMI category comparisons were made applying the non-parametric Kruskal–Wallis test.
| Variable | Normal weight | Over weight | Obese | P-valuea | |||
|---|---|---|---|---|---|---|---|
| n | Median (2.5th, 97.5th percentiles) | n | Median (2.5th, 97.5th percentiles) | n | Median (2.5th, 97.5th percentiles) | ||
| Estrone (pmol/L) | 122 | 94.3 (31.4, 184) | 191 | 89.9 (28.5, 192) | 58 | 112 (44.4, 261) | <0.005* |
| Estradiol (pmol/L) | 122 | 62.0 (21.5, 141) | 191 | 57.9 (15.8, 147) | 58 | 75.4 (11.2, 131) | 0.03 |
| 11-deoxycortisol (nmol/L) | 121 | 0.979 (0.317, 2.51) | 191 | 0.749 (0.259, 2.94) | 57 | 0.95 (0.253,3.43) | <0.005* |
| Testosterone (nmol/L) | 121 | 16.9 (9.37, 29.4) | 191 | 13.1 (6.91, 28.0) | 57 | 11.3 (4.20, 18.7) | <0.005* |
| Dehydroepi-androsterone (nmol/L) | 121 | 9.02 (2.05, 25.3) | 191 | 7.08 (2.46, 21.0) | 57 | 7.60 (1.90, 22.6) | <0.005* |
| Androstenedione (nmol/L) | 121 | 2.12 (1.15, 3.61) | 191 | 1.87 (0.832, 4.03) | 57 | 1.84 (0.892, 5.76) | <0.005* |
| 17-hydroxypregnenolone (nmol/L) | 121 | 2.98 (0.810, 8.52) | 191 | 2.02 (0.696, 8.70) | 57 | 2.08 (0.686, 10.4) | <0.005* |
| Pregnenolone (nmol/L) | 121 | 3.35 (1.51, 7.33) | 191 | 3.00 (1.29, 6.45) | 57 | 3.03 (1.05, 6.38) | 0.02 |
| 17-hydroxyprogesterone (nmol/L) | 121 | 2.55 (1.24, 4.64) | 191 | 1.93 (0.788, 5.18) | 57 | 1.97 (0.757, 10.5) | <0.005* |
| Sex hormone-binding globulin (nmol/L) | 118 | 52.9 (22.4, 99.7) | 184 | 39.1 (19.1, 91,5) | 58 | 37.8 (17.9, 59.1) | <0.005* |
aKruskal–Wallis test was used to test differences between BMI categories; *indicates statistical significance after adjusting the P-values for multiplicity using Bonferroni correction (0.05/10); P-value for significance 0.005.
Discussion
Measurement of low endogenous concentrations of sex steroids, characteristic of elderly, requires use of sensitive and specific methods (27). During the last decade, LC-MS/MS has become the method of choice for quantitative analysis of endogenous steroids in biological samples (24). This study provides information on concentrations of multiple endogenous steroids in a population-based sample of elderly of a certain age and ethnicity in Uppsala, Sweden.
In general, lower concentrations of DHEA, androstenedione and testosterone at advanced age are expected as an effect of reduced adrenal and gonadal function with aging (10, 19).
Table 3 summarizes data of this study on distribution of the measured steroid concentrations in all men and women and in the subgroups of apparently healthy men and women, respectively, the distributions are presented as central 95thpercentile, which is often used for establishing age-appropriate reference values for healthy individuals (35). We applied a rather strict definition of healthy and excluded individuals with prevalent CVD, on lipid-lowering medication, on sex hormone therapy, on corticosteroids, diabetics and current smokers, as some of these conditions have been reported to be associated with concentrations of plasma estrone, estradiol, testosterone (36, 37, 38), SHBG concentrations (37, 38), DHEA and androstenedione (38).
As there is evidence of sex, age, geographical, and ethnic variation in sex hormone levels (33), reports on specific populations are necessary to contrast and evaluate clinical relevance, thus, varying data is to be expected across studies. Distributions of the steroid concentrations reported in this study differed between women and men, with generally higher concentrations of estrone, estradiol, testosterone, androstenedione, 17-hydroxy-pregnenolone, pregnenolone and 17-hydroxy-progesterone in men. Compared to a recent large community-based study reporting the distribution of sex steroids in women over 70 years of age, using LC-MS/MS methods (39), levels of estrone were considerably higher and median concentrations of testosterone were lower in the sub-group of 70–74-year-old women than observed in our study. Further, they reported that the concentrations of estradiol were below the limit of quantitation of the assay in 66% of the participants indicating lack of sensitivity for the quantification method or very low concentrations in the study population. When comparing a population-based sample of 20 to 80+ year-old men and women in the United States to our data, the central 95% concentrations for testosterone observed in our study were similar to their central 80% concentrations in all men and women, respectively (40), which is somewhat surprising considering the different demographic characteristics of the study populations. Furthermore, they reported higher testosterone concentrations in fasting than non-fasting individuals, which has been confirmed in a more recent study on Australian men (41) but did not report data by fasting/non-fasting status. Thus, lack of detailed information on testosterone levels by age, ethnicity and fasting status prevents further analysis of the higher variation seen in their data and detailed comparison with our data. A study of elderly men in Australia (4) reported a mean value of testosterone comparable to the median value reported in the present study but considerably higher mean estradiol concentration than the median value reported here. Similar median levels of testosterone were also reported in >10,000 men of 35–99 year of age (median 70.9 year) but higher median E2 levels (42) compared to our study.
Increase in BMI had comparable effect on concentrations of the measured steroids in men and women, while less pronounced in women. The lower concentrations of total testosterone in overweight/obese men reported here are in concordance with two Australian studies including 76 ± 3 year-old men (4) and 10,900 men 35–99 year of age (median 70.9 year) (42). On the contrary, we observed significantly higher concentrations of estradiol and estrone in women with higher BMI, which was in agreement with earlier findings (3, 39). However, an association between overweight and obese women and higher testosterone levels has been reported previously (39) but was not seen in our data. Difference in the observed distributions of concentration may be related to the study population, inclusion and exclusion criteria applied during the recruitment, BMI of the participants and the methods’ specificity. In our study, women with normal BMI showed higher concentrations of the precursors dihydroepiandrosterone, 17-hydroxypregnenolone and pregnenolone compared to the obese groups. A similar pattern was present in men regarding testosterone concentrations with higher concentrations among men with normal BMI than in the overweight and obese, most likely explained by consumption of the precursors for biosynthesis of estrogens in the fat tissue.
It has not been established in what way sex steroids are associated with cardiovascular disease. However, lower levels of androgens and higher levels of estrogens have been associated with a more unhealthy artery wall and prevalent cardiovascular disease (9) suggested to be caused by an endogenous response to the arteriosclerotic inflammatory process rather than a causal relationship between endogenous steroid concentrations and prevalent CVD. On the contrary, no association between blood pressure and sex steroids was reported in adults from the HERITAGE Family Study, Canada (43).
This study included only 70-year-old Caucasian subjects in Sweden, and, therefore, caution should be raised about the representativeness of the data to other geographic, ethnic and age groups. The definition of prevalent CVD was conservative as it was based on self-reported data. On the other hand, hypertension data was measured and the definition used for CVD (hospitalization due to myocardial infarction or stroke or doctor’s diagnosis of heart failure) can be expected to be well-remembered by the subjects, thus, reducing the risk of the recall bias.
Conclusions
This population-based study provides LC-MS/MS-based concentrations of endogenous adrenal and sex steroids in 70-year-old Caucasian men and women. Concentrations were often lower in women than men. Higher BMI category in women was associated with higher concentrations of estrone and estradiol and lower SHBG whereas in men, higher BMI was associated with lower concentrations of testosterone as well as lower precursors (except pregnenolone) and SHBG but higher estrone levels. In both sexes, apparently healthy individuals had comparable median concentrations compared to all men and women, respectively, but with narrower central 95% distributions indicating reduction of variation in data when including only apparently healthy subjects.
Declaration of interest
All authors have approved the submission to the European Journal of Endocrinology and the manuscript is not considered for publication elsewhere. The authors have nothing to disclose.
Funding
Swedish Research Council (621-2011-4423, 2015-4870 J B), ARUP Institute for Clinical and Experimental Pathology (Salt Lake City, USA), Selanders foundation Uppsala and ALF funding from Uppsala University Hospital.
Author contribution statement
T N, J B2 and L L conceived the study; J P conceived, designed, and performed the data analysis, and wrote the manuscript; T N and M M K supported the design of the data analysis and the scope of the manuscript; M M K developed and validated the LC-MS/MS methods; M M K, J B1 and J B2 performed testing of the study samples; and all authors contributed to providing and interpreting the data and drafting the manuscript.
References
- 1.Dorgan JF, Fears TR, McMahon RP, Aronson Friedman L, Patterson BH, Greenhut SF.Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry. Steroids 2002. 67 151–158. ( 10.1016/s0039-128x(01)00147-7) [DOI] [PubMed] [Google Scholar]
- 2.Paller CJ, Shiels MS, Rohrmann S, Basaria S, Rifai N, Nelson W, Platz EA, Dobs A.Relationship of sex steroid hormones with bone mineral density (BMD) in a nationally representative sample of men. Clinical Endocrinology 2009. 70 26–34. ( 10.1111/j.1365-2265.2008.03300.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger B, Pressman A, Sklarin P, Bauer DC, Cauley JA, Cummings SR.Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. Journal of Clinical Endocrinology and Metabolism 1998. 83 2239–2243. ( 10.1210/jcem.83.7.4708) [DOI] [PubMed] [Google Scholar]
- 4.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Norman PE, Flicker L.Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. Journal of Clinical Endocrinology and Metabolism 2012. 97 4030–4039. ( 10.1210/jc.2012-2265) [DOI] [PubMed] [Google Scholar]
- 5.Tivesten A, Hulthe J, Wallenfeldt K, Wikstrand J, Ohlsson C, Fagerberg B.Circulating estradiol is an independent predictor of progression of carotid artery intima-media thickness in middle-aged men. Journal of Clinical Endocrinology and Metabolism 2006. 91 4433–4437. ( 10.1210/jc.2006-0932) [DOI] [PubMed] [Google Scholar]
- 6.Tivesten A, Mellstrom D, Jutberger H, Fagerberg B, Lernfelt B, Orwoll E, Karlsson MK, Ljunggren O, Ohlsson C.Low serum testosterone and high serum estradiol associate with lower extremity peripheral arterial disease in elderly men. The MrOS Study in Sweden. Journal of the American College of Cardiology 2007. 50 1070–1076. ( 10.1016/j.jacc.2007.04.088) [DOI] [PubMed] [Google Scholar]
- 7.Bernini GP, Moretti A, Sgro M, Argenio GF, Barlascini CO, Cristofani R, Salvetti A.Influence of endogenous androgens on carotid wall in postmenopausal women. Menopause 2001. 8 43–50. ( 10.1097/00042192-200101000-00008) [DOI] [PubMed] [Google Scholar]
- 8.Lv W, Du N, Liu Y, Fan X, Wang Y, Jia X, Hou X, Wang B.Low testosterone level and risk of Alzheimer’s disease in the elderly men: a systematic review and meta-analysis. Molecular Neurobiology 2016. 53 2679–2684. ( 10.1007/s12035-015-9315-y) [DOI] [PubMed] [Google Scholar]
- 9.Naessen T, Sjogren U, Bergquist J, Larsson M, Lind L, Kushnir MM.Endogenous steroids measured by high-specificity liquid chromatography-tandem mass spectrometry and prevalent cardiovascular disease in 70-year-old men and women. Journal of Clinical Endocrinology and Metabolism 2010. 95 1889–1897. ( 10.1210/jc.2009-1722) [DOI] [PubMed] [Google Scholar]
- 10.Naessen T, Bergquist J, Lind L, Kushnir MM.Higher endogenous estrogen levels in 70-year-old women and men: an endogenous response to counteract developing atherosclerosis? Menopause 2012. 19 1322–1328. ( 10.1097/gme.0b013e31825ea8c1) [DOI] [PubMed] [Google Scholar]
- 11.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Almeida OP, Golledge J, Norman PE, Flicker L.In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. Journal of Clinical Endocrinology and Metabolism 2014. 99 E9–E18. ( 10.1210/jc.2013-3272) [DOI] [PubMed] [Google Scholar]
- 12.Cauley JA, Gutai JP, Kuller LH, Powell JG.Reliability and interrelations among serum sex hormones in postmenopausal women. American Journal of Epidemiology 1991. 133 50–57. ( 10.1093/oxfordjournals.aje.a115801) [DOI] [PubMed] [Google Scholar]
- 13.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, Bunker AM, Meikle AW.High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. American Journal of Clinical Pathology 2008. 129 530–539. ( 10.1309/LC03BHQ5XJPJYEKG) [DOI] [PubMed] [Google Scholar]
- 14.Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Owen WE, Bunker AM, Meikle AW.Development and performance evaluation of a tandem mass spectrometry assay for 4 adrenal steroids. Clinical Chemistry 2006. 52 1559–1567. ( 10.1373/clinchem.2006.068445) [DOI] [PubMed] [Google Scholar]
- 15.Kulle AE, Riepe FG, Melchior D, Hiort O, Holterhus PM.A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age- and sex-specific reference data. Journal of Clinical Endocrinology and Metabolism 2010. 95 2399–2409. ( 10.1210/jc.2009-1670) [DOI] [PubMed] [Google Scholar]
- 16.Kushnir MM, Rockwood AL, Yue B, Meikle AW.High sensitivity measurement of estrone and estradiol in serum and plasma using LC-MS/MS. Methods in Molecular Biology 2010. 603 219–228. ( 10.1007/978-1-60761-459-3_20) [DOI] [PubMed] [Google Scholar]
- 17.Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Bunker AM, Fitzgerald RL, Meikle AW.Performance characteristics of a novel tandem mass spectrometry assay for serum testosterone. Clinical Chemistry 2006. 52 120–128. ( 10.1373/clinchem.2005.052167) [DOI] [PubMed] [Google Scholar]
- 18.Fanelli F, Gambineri A, Belluomo I, Repaci A, Di Lallo VD, Di Dalmazi G, Mezzullo M, Prontera O, Cuomo G, Zanotti L.et al. Androgen profiling by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in healthy normal-weight ovulatory and anovulatory late adolescent and young women. Journal of Clinical Endocrinology and Metabolism 2013. 98 3058–3067. ( 10.1210/jc.2013-1381) [DOI] [PubMed] [Google Scholar]
- 19.Fanelli F, Belluomo I, Di Lallo VD, Cuomo G, De Iasio R, Baccini M, Casadio E, Casetta B, Vicennati V, Gambineri Aet al. Serum steroid profiling by isotopic dilution-liquid chromatography-mass spectrometry: comparison with current immunoassays and reference intervals in healthy adults. Steroids 2011. 76 244–253. ( 10.1016/j.steroids.2010.11.005) [DOI] [PubMed] [Google Scholar]
- 20.Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, Handelsman DJ.Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. Journal of Clinical Endocrinology and Metabolism 2005. 90 5928–5936. ( 10.1210/jc.2005-0962) [DOI] [PubMed] [Google Scholar]
- 21.Mezzullo M, Pelusi C, Fazzini A, Repaci A, Di Dalmazi G, Gambineri A, Pagotto U, Fanelli F.Female and male serum reference intervals for challenging sex and precursor steroids by liquid chromatography – tandem mass spectrometry. Journal of Steroid Biochemistry and Molecular Biology 2020. 197 105538. ( 10.1016/j.jsbmb.2019.105538) [DOI] [PubMed] [Google Scholar]
- 22.Mathews L, Subramanya V, Zhao D, Ouyang P, Vaidya D, Guallar E, Yeboah J, Herrington D, Hays AG, Budoff MJet al. Endogenous sex hormones and endothelial function in postmenopausal women and men: the multi-ethnic study of atherosclerosis. Journal of Women’s Health 2019. 28 900–909. ( 10.1089/jwh.2018.7441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oluleye OW, Kronmal RA, Folsom AR, Vaidya DM, Ouyang P, Duprez DA, Dobs AS, Yarmohammadi H, Konety SH.Association between statin use and sex hormone in the multi-ethnic study of atherosclerosis cohort. Journal of Clinical Endocrinology and Metabolism 2019. 104 4600–4606. ( 10.1210/jc.2019-00530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushnir MM, Rockwood AL, Roberts WL, Yue B, Bergquist J, Meikle AW.Liquid chromatography tandem mass spectrometry for analysis of steroids in clinical laboratories. Clinical Biochemistry 2011. 44 77–88. ( 10.1016/j.clinbiochem.2010.07.008) [DOI] [PubMed] [Google Scholar]
- 25.Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P.Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clinical Chemistry 2003. 49 1381–1395. ( 10.1373/49.8.1381) [DOI] [PubMed] [Google Scholar]
- 26.Herold DA, Fitzgerald RL.Immunoassays for testosterone in women: better than a guess? Clinical Chemistry 2003. 49 1250–1251. ( 10.1373/49.8.1250) [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS.Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. Journal of Clinical Endocrinology and Metabolism 2004. 89 534–543. ( 10.1210/jc.2003-031287) [DOI] [PubMed] [Google Scholar]
- 28.Kushnir MM, Naessen T, Kirilovas D, Chaika A, Nosenko J, Mogilevkina I, Rockwood AL, Carlstrom K, Bergquist J.Steroid profiles in ovarian follicular fluid from regularly menstruating women and women after ovarian stimulation. Clinical Chemistry 2009. 55 519–526. ( 10.1373/clinchem.2008.110262) [DOI] [PubMed] [Google Scholar]
- 29.Lind L, Fors N, Hall J, Marttala K, Stenborg A.A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arteriosclerosis, Thrombosis, and Vascular Biology 2005. 25 2368–2375. ( 10.1161/01.ATV.0000184769.22061.da) [DOI] [PubMed] [Google Scholar]
- 30.Lee DH, Lind L, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind PM.Does mortality risk of cigarette smoking depend on serum concentrations of persistent organic pollutants? Prospective investigation of the vasculature in Uppsala seniors (PIVUS) study. PLoS ONE 2014. 9 e95937. ( 10.1371/journal.pone.0095937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, Bunker AM, Meikle AW.Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clinical Chemistry 2010. 56 1138–1147. ( 10.1373/clinchem.2010.143222) [DOI] [PubMed] [Google Scholar]
- 32.Kushnir MM, Rockwood AL, Nelson GJ, Yue B, Urry FM.Assessing analytical specificity in quantitative analysis using tandem mass spectrometry. Clinical Biochemistry 2005. 38 319–327. ( 10.1016/j.clinbiochem.2004.12.003) [DOI] [PubMed] [Google Scholar]
- 33.Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar Aet al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. Journal of Clinical Endocrinology and Metabolism 2011. 96 2430–2439. ( 10.1210/jc.2010-3012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tukey JW.Exploratory Data Analysis. Reading, MA, USA: Addison-Wesley Publishing Co., 1977, xvi, pp 688. [Google Scholar]
- 35.Ridefelt P, Hagstrom E, Svensson MK, Akerfeldt T, Larsson A.Age- and sex-specific reference values for non-HDL cholesterol and remnant cholesterol derived from the Nordic Reference Interval Project (NORIP). Scandinavian Journal of Clinical and Laboratory Investigation 2019. 79 39–42. ( 10.1080/00365513.2018.1550809) [DOI] [PubMed] [Google Scholar]
- 36.de Keyser CE, de Lima FV, de Jong FH, Hofman A, de Rijke YB, Uitterlinden AG, Visser LE, Stricker BH.Use of statins is associated with lower serum total and non-sex hormone-binding globulin-bound testosterone levels in male participants of the Rotterdam Study. European Journal of Endocrinology 2015. 173 155–165. ( 10.1530/EJE-14-1061) [DOI] [PubMed] [Google Scholar]
- 37.Kim C, Halter JB.Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Current Cardiology Reports 2014. 16 467. ( 10.1007/s11886-014-0467-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB.The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. Journal of Clinical Endocrinology and Metabolism 1994. 79 1310–1316. ( 10.1210/jcem.79.5.7962322) [DOI] [PubMed] [Google Scholar]
- 39.Davis SR, Bell RJ, Robinson PJ, Handelsman DJ, Gilbert T, Phung J, Desai R, Lockery JE, Woods RL, Wolfe RSet al. Testosterone and estrone increase From the age of 70 years: findings from the sex hormones in older women study. Journal of Clinical Endocrinology and Metabolism 2019. 104 6291–6300. ( 10.1210/jc.2019-00743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vesper HW, Wang Y, Vidal M, Botelho JC, Caudill SP.Serum total testosterone concentrations in the US household population from the NHANES 2011–2012 study population. Clinical Chemistry 2015. 61 1495–1504. ( 10.1373/clinchem.2015.245969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sartorius G, Spasevska S, Idan A, Turner L, Forbes E, Zamojska A, Allan CA, Ly LP, Conway AJ, McLachlan RIet al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clinical Endocrinology 2012. 77 755–763. ( 10.1111/j.1365-2265.2012.04432.x) [DOI] [PubMed] [Google Scholar]
- 42.Handelsman DJ, Yeap B, Flicker L, Martin S, Wittert GA, Ly LP.Age-specific population centiles for androgen status in men. European Journal of Endocrinology 2015. 173 809–817. ( 10.1530/EJE-15-0380) [DOI] [PubMed] [Google Scholar]
- 43.He Z, Rankinen T, Leon AS, Skinner J, Tchernof A, Bouchard C.Plasma steroids are not associated with resting and exercise blood pressure. International Journal of Sports Medicine 2018. 39 967–971. ( 10.1055/a-0660-0121) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a