Abstract
Background
Acute ST elevation myocardial infarction (STEMI) is a medical emergency and is most commonly due to atherosclerotic plaque rupture and occlusion of coronary vessels. This case demonstrates that eosinophilic granulomatosis with polyangiitis (EGPA) myocarditis can mimic acute STEMI.
Case summary
A 44-year-old woman presented with acute chest pain, shortness of breath, and collapse with ST elevation on electrocardiography. Coronary angiogram showed unobstructed coronaries and chest film revealed left-sided consolidation. Together with a thorough history, serum eosinophilia, cardiac magnetic resonance (CMR), and computated tomography imaging, the patient was diagnosed with acute EGPA myocarditis. She responded tremendously to steroid and cyclophosphamide immunosuppression and subsequent CMR imaging demonstrated complete resolution of myocarditis.
Discussion
CMR played a crucial role in the diagnosis and follow-up of this rare presentation. In patients who present as a STEMI but show unobstructed coronary vessels, EGPA may be a possible diagnosis.
Keywords: ST elevation myocardial infarction, Eosinophilic granulomatosis with polyangiitis (Churg-Strauss), Cardiac magnetic resonance imaging, Myocarditis, Case report
Learning points
This case represents a rare mimic of an acute ST elevation myocardial infarction that cardiologists should be aware of.
Cardiac magnetic resonance is an invaluable tool in assessment of patients with eosinophilic granulomatosis with polyangiitis myocarditis and response to cyclophosphamide and steroids can be dramatic.
Expedited cyclophosphamide and steroid administration may help to minimize subsequent myocardial fibrosis and thereby optimize prognosis.
Introduction
Primary percutaneous intervention is the preferred revascularization strategy for ST-segment elevation myocardial infarction (STEMI).1 However, a diagnostic conundrum is faced when a culprit lesion is not identified on angiography. In these ‘false activation’ patients, alternative diagnoses need to be sought. We present a case of eosinophilic granulomatosis with polyangiitis (EGPA) myocarditis, mimicking the clinical features of an acute STEMI. The patient had a dramatic response to immunosuppressive therapy, with complete resolution of the myocarditis. Cardiac magnetic resonance imaging (CMR) has enhanced the diagnosis and assessment of myocarditis, and played a crucial non-invasive role in this case.
Timeline
| 3 years prior | New asthma diagnosis (age 41) |
| 6 months prior | Patient experiences nasal congestion and discharge |
| Day of presentation | Acute chest pain with anterior ST elevation on ECG, coronary angiogram showed no culprit lesion, chest x-ray showed extensive left-sided lung consolidation |
| 1 day post | Persistent chest pain, computed tomography pulmonary angiogram showed consolidation in left upper and middle zones and small pulmonary emboli |
| 2 days post | Bronchoalveolar lavage was negative. Cardiac magnetic resonance imaging (CMR) showed panmyocarditis |
| 3 days post | High-dose intravenous steroids and cyclophosphamide are initiated, and patient has a rapid improvement in symptoms. A further five cycles of cyclophosphamide with oral steroids are administered over the next 14 weeks |
| 4 months post | Interval CMR imaging shows complete resolution of the myocarditis |
| 12 months post | Patient is in remission on oral azathioprine, has been weaned off steroids, and shows no signs of ongoing myocarditis |
Case presentation
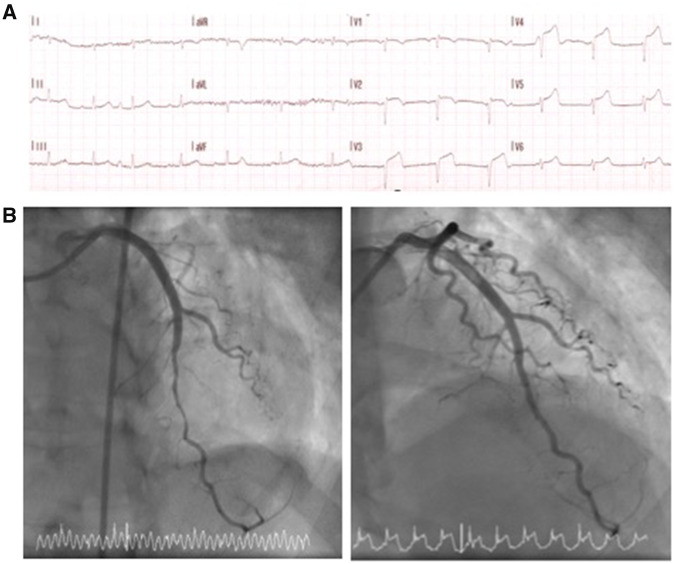
A 44-year-old Caucasian woman presented with sudden onset central crushing chest pain, shortness of breath, and collapse. An ECG showed anterior ST elevation across leads V2–V5 (Figure 1A) prompting management via the primary percutaneous angioplasty pathway. Past medical history was unremarkable except for recently diagnosed asthma, treated with inhaled corticosteroid and β-agonist. The patient did not have any risk factors for ischaemic heart disease. Cardiovascular examination was unremarkable and respiratory examination revealed bi-basal crepitations, more prominent on the left. Urgent coronary angiography showed unobstructed coronary arteries, with initial coronary artery spasm that resolved with intracoronary nitrate (Figure 1B). Troponin-T was 772 ng/L (normal range <14 ng/L) and a chest radiograph showed extensive left-sided consolidation (Figure 2A). A working diagnosis of myopericarditis secondary to pneumonia was made and the patient received intravenous co-amoxiclav with supportive treatment and monitoring.
Figure 1.
(A) ECG showing anterior ST elevation. (B) Angiogram of the left anterior descending artery showing diffuse coronoary vasospasm before (left image) and after (right image) intracoronary nitrate administration.
Figure 2.
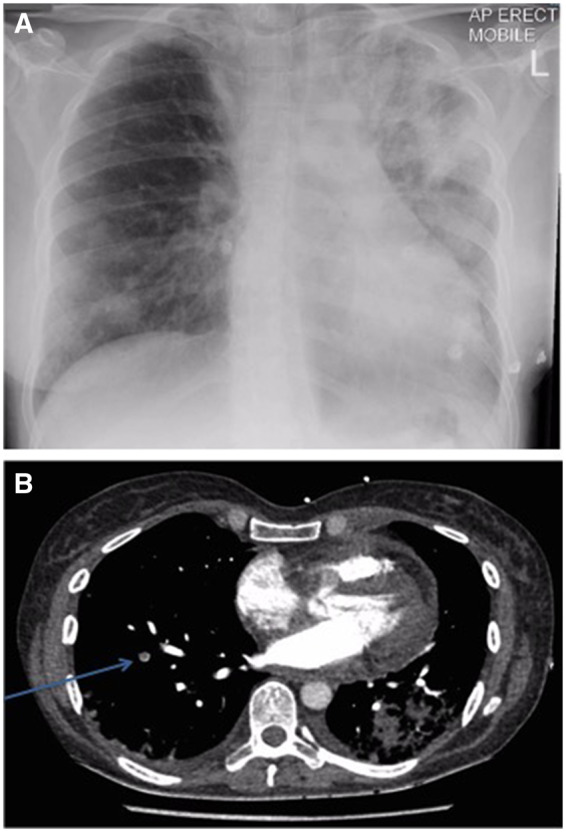
(A) Chest x-ray showing extensive left upper and middle zone sided consolidation. (B) Computed tomography pulmonary angiogram showing left-sided consolidation and small right-sided filling defect (arrow).
Viral swabs and urinary antigens for atypical pneumonia were negative. Serological viral and autoimmune screens for myocarditis were negative and the patient did not improve despite antibiotic treatment. Peripheral blood counts revealed a white cell count of 17.34 × 109/L (normal range 3–10.5 × 109/L) consisting of an eosinophilia of 7.23 × 109 (normal range <0.5 × 109/L). The patient denied any recent travel history, had a stable weight and no bowel symptoms. A computed tomography (CT) pulmonary angiogram was undertaken which confirmed the consolidation seen on the chest x-ray, and showed multiple small filling defects suggestive of pulmonary emboli. A bronchoalveolar lavage showed abundant polymorphonuclear leucocytes, lymphocytes, macrophages, and bronchial epithelial and squamous cells, in keeping with acute inflammation, though no evidence of organisms or eosinophilic damage. Serum troponin-T remained elevated at 1131 ng/L.
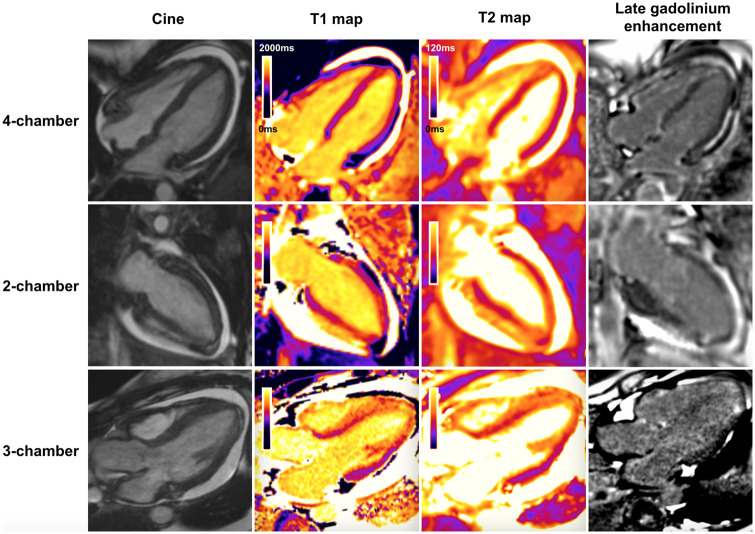
A CMR was undertaken showing extensive myocardial oedema on multi-parametric native myocardial mapping, patchy subendocardial late gadolinium enhancement (LGE) particularly in the apical segments and right ventricle, and a circumferential pericardial effusion (Figure 3). These features were in keeping with a myopericarditis, but not indicating a specific underlying aetiology.
Figure 3.
Panel showing cardiac magnetic resonance long axis cine images, native myocardial T1 and T2 maps, and late gadolinium enhancement images prior to treatment. There is a circumferential pericardial effusion. The native myocardial T1 and T2 maps show high values, signified by regions of orange colour, in the apical and anteroseptal segments (compared with normal myocardium that is coloured purple). There is patchy subendocardial late gadolinium enhancement seen in the apex and in the right ventricle.
Taken together with the eosinophilia and late onset asthma, which was poorly responsive to standard inhaled treatment, but responsive to oral steroids, and CT sinus imaging revealing sinusitis and polyposis, the patient met the American College of Rheumatology diagnostic criteria for EGPA [asthma, eosinophilia (>10% of peripheral white cell count), pulmonary infiltrates, and paranasal sinus abnormalities].2
The patient was treated with pulsed intravenous methylprednisolone (500 mg) once daily for 5 days and cyclophosphamide (15 mg/kg) and rapidly showed improvement clinically. She received a further five cycles of cyclophosphamide with tapering oral prednisolone and was initiated on azathioprine as a maintenance agent. The patient had a good response symptomatically, biochemically (normalized troponin-T), electrocardiographically, and radiologically.
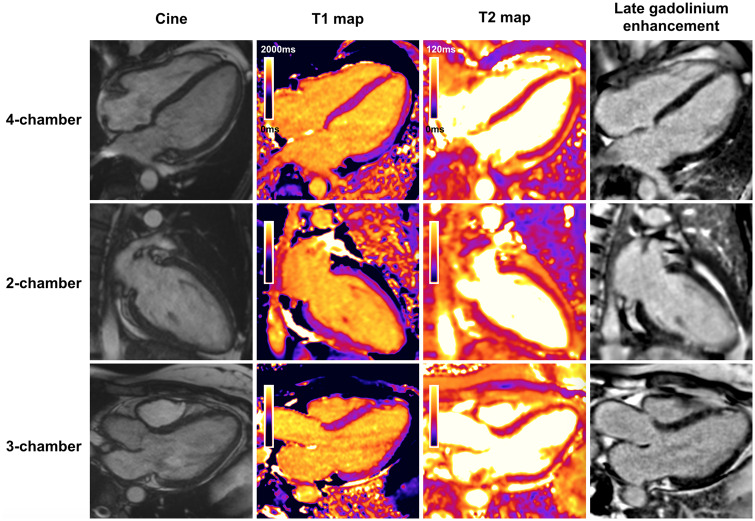
Interval CMR imaging showed complete resolution of myocardial oedema (Figure 4) indicating a dramatic response to therapy. Some LGE remained in the apical segments which is likely representative of focal areas of fibrosis, but may also represent a limited area of infarct due to initial coronary vasospasm. Coronary vasculitis also remains a differential, but the response to intracoronary nitrate on angiogram would not be expected with vasculitis (Figure 1B). These findings suggest that fast administration of cyclophosphamide with high-dose steroids may reduce the burden of post-myocarditis fibrosis in EGPA. The patient was maintained on oral azathioprine and oral steroids were weaned off. She remains in clinical and radiological remission.
Figure 4.
Panel showing cardiac magnetic resonance long axis cine images, native myocardial T1 and T2 maps, and late gadolinium enhancement images following treatment. The pericardial effusion has resolved, and global biventricular systolic function was preserved. The T2 maps show normal values throughout the myocardium, signifying resolution of myocardial oedema. The previously high regions of T1 have largely resolved, with the remaining small discrete areas of high T1 near the apex corresponding to the residual foci of subendocardial late gadolinium enhancement. Overall, this suggests some residual focal apical subendocardial scar in the absence of ongoing inflammation.
Discussion
The underlying cause for EGPA is unclear; it appears to involve both environmental factors and genetic pre-determinants. HLA-DRB1 and HLA-DRB4 are associated with EGPA substantiating some genetic component to the disease. Furthermore, mutations in genes encoding interleukin-10 may play a role in EGPA pathogenesis.3 It is unclear whether drugs, infections, or allergens represent the environmental trigger for EGPA.
Histologically, EGPA is characterized by ‘Eosinophil-rich and necrotizing granulomatous inflammation often involving the respiratory tract, and necrotising vasculitis predominantly affecting small to medium vessels, and associated with asthma and eosinophilia’.4 Disease progression is divided into an allergic phase, eosinophilic stage, and a vasculitic stage. Cardiac manifestations typically occur in the eosinophilic stage of the disease and are associated with a poor prognosis. There are numerous cardiac manifestations; myopericarditis, pericardial effusions, coronary artery vasculitis, arrhythmia (most commonly heart block), and intramural thrombus formation. Myocardial injury in EGPA is a direct effect of eosinophil-mediated necrosis and induction of apoptosis rather than myocardial vasculitis. Cardiac involvement is more clinically overt in ANCA-negative EGPA, as was the case with our patient. It is also associated with high peripheral eosinophil counts (>8.1 × 109/L or >20% of total white cell count, normal range <0.5 × 109/L).5–7
Thrombus formation may due to the disease predisposition to affect localized ventricular segments or due to independent procoagulant effect of the hypereosinophilic state. Usually this displays as a propensity for venous thrombosis,8 and as we saw in this case, the patient had evidence of multiple small pulmonary emboli.
EGPA is a disease with variable clinical course, and cardiac involvement is a prognostic factor as arrhythmia secondary to fibrosis is main cause of mortality in these patients.9 STEMI presentation in EGPA may also be due to coronary vasculitis or severe sustained coronary vasospasm.10
Consensus for the management of EGPA myocarditis is poor owing to the lack of randomized controlled trials in this patient group. Therapies typically include high-dose steroids and IV cyclophsophamide11 as induction agents and azathioprine or methotrexate as maintenance agents.
CMR is the clinical standard of practice for the evaluation of all forms of myocarditis and heart muscle disease, with contemporary multi-parametric techniques providing excellent diagnostic accuracy for the diagnosis of acute myocarditis when compared against endomyocardial biopsy (EMB).12 EGPA has numerous manifestations that can be found on CMR, including myocardial oedema and LGE. The most common distribution of LGE is subendocardial, but non-ischaemic myocarditic patterns are also seen.7,13 Furthermore, CMR is more sensitive at detecting mural thrombi that may otherwise be missed by transthoracic echocardiogram.
In this case, prompt diagnosis with the aid of CMR facilitated treatment with cyclophosphamide and remission of myocarditis. Patients with established EGPA but without cardiac symptomatology also benefit from the use of CMR to detect clinically silent myocardial involvement.13 Treatment is aimed at suppressing the systemic inflammatory process, the cardiac response to which can be monitored by interval follow-up CMR study.7
Whilst EMB remains the reference standard for diagnosing EGPA myocarditis, CMR has several advantages aside from the avoidance of the potential risks inherent with cardiac biopsy. Myocardial biopsy can potentially sample tissue not involved with the acute disease process, whereas CMR allows evaluation of the whole myocardium. A non-invasive approach is also more desirable for the follow-up assessment of response to treatment. Finally, internationally guidelines restrict the role of EMB in myocarditis to those patients who already have demonstrable left ventricular dysfunction.14 As such, CMR is a safe non-invasive assessment that can be applied across the spectrum of myocarditis irrespective of aetiology. When the CMR data are considered alongside the clinical findings both on the index study and following treatment, EGPA can reliably be diagnosed as a unifying diagnosis without EMB.
Lead author biography

Jaspal Singh Gill graduated from University College London with an intercalated BSc in Pharmacology in 2016 and completed Foundation Medical Training in London hospitals. Currently, he is training in Internal Medicine and has a keen interest in cardiology, a field that he would like to pursue in his later career.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M; ESC Committee for Practice Guidelines (CPG). Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2008;29:2909–2945. [DOI] [PubMed] [Google Scholar]
- 2. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lightfoot RW, McShane DJ, Mills JA, Stevens MB, Wallace SL, Zvaifler NJ.. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 2010;33:1094–1100. [DOI] [PubMed] [Google Scholar]
- 3. Wieczorek S, Hellmich B, Arning L, Moosig F, Lamprecht P, Gross WL, Epplen JT.. Functionally relevant variations of the interleukin-10 gene associated with antineutrophil cytoplasmic antibody-negative Churg-Strauss syndrome, but not with Wegener’s granulomatosis. Arthritis Rheum 2008;58:1839–1848. [DOI] [PubMed] [Google Scholar]
- 4. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA.. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Qiao L, Gao D.. A case report and literature review of Churg–Strauss syndrome presenting with myocarditis. Medicine 2016;95:e5080.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corradi D, Vaglio A, Maestri R, Legname V, Leonardi G, Bartoloni G, Buzio C.. Eosinophilic myocarditis in a patient with idiopathic hypereosinophilic syndrome: Insights into mechanisms of myocardial cell death. Hum Pathol 2004;35:1160–1163. [DOI] [PubMed] [Google Scholar]
- 7. Mavrogeni S, Karabela G, Gialafos E, Stavropoulos E, Spiliotis G, Katsifis G, Kolovou G.. Cardiac involvement in ANCA (+) and ANCA (-) Churg-Strauss syndrome evaluated by cardiovascular magnetic resonance. Inflamm Allergy Drug Targets 2013;12:322–327. [DOI] [PubMed] [Google Scholar]
- 8. Maino A, Rossio R, Cugno M, V. Marzano A, Tedeschi A.. Hypereosinophilic syndrome, Churg-Strauss syndrome and parasitic diseases: possible links between eosinophilia and thrombosis. Curr Vasc Pharmacol 2012;10:670–675. [DOI] [PubMed] [Google Scholar]
- 9. Neumann T, Manger B, Schmid M, Kroegel C, Hansch A, Kaiser WA, Reinhardt D, Wolf G, Hein G, Mall G, Schett G, Zwerina J.. Cardiac Involvement in Churg-Strauss Syndrome. Medicine 2009;88:236–243. [DOI] [PubMed] [Google Scholar]
- 10. Chai JT, McGrath S, Lopez B, Dworakowski R.. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) masquerading as acute ST-elevation myocardial infarction with complete resolution after immunosuppressive therapy: a case report. Eur Heart J Case Rep 2018;2:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Groot K, Harper L, Jayne DRW, Flores Suarez LF, Gregorini G, Gross WL, Luqmani R, Pusey CD, Rasmussen N, Sinico RA, Tesar V, Vanhille P, Westman K, Savage CO; EUVAS (European Vasculitis Study Group). Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670–680. [DOI] [PubMed] [Google Scholar]
- 12. Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, de Waha S, Rommel K-P, Lurz JA, Klingel K, Kandolf R, Schuler G, Thiele H, Gutberlet M.. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-trial. J Am Coll Cardiol 2016;67:1800–1811. [DOI] [PubMed] [Google Scholar]
- 13. Yune S, Choi D, Lee B, Lee J, Jeon E, Kim S, Choe YH.. Detecting cardiac involvement with magnetic resonance in patients with active eosinophilic granulomatosis with polyangiitis. Int J Cardiovasc Imaging 2016;32(Suppl 1):155–162. [DOI] [PubMed] [Google Scholar]
- 14. Cooper L, Baughman K, Feldman A, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R; American Heart Association; American College of Cardiology; European Society of Cardiology; Heart Failure Society of America; Heart Failure Association of the European Society of Cardiology. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 2007;50:1914–1931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.