Abstract
Background
Non-bacterial thrombotic endocarditis (NBTE) is a rare form of endocarditis notably described in patients with advanced malignancy and auto-immune diseases. It is characterized by the formation of sterile, fibrin-containing vegetations on cardiac endothelium, in the absence of positive blood cultures. It is predominantly located on the mitral- and aortic valve (AV). Vegetations in NBTE are prone to embolize. Trousseau syndrome (TS) is defined as unexplained thrombotic events that precede the diagnosis of malignancy.
Case summary
A 49-year-old pre-menopausal woman with a history of visual disturbances, recurrent deep vein thrombosis (DVT) with concurrent pulmonary emboli (PE), and uterine myomas with dysfunctional uterine bleeding was resuscitated for ventricular fibrillation. While echocardiography revealed vegetations on the AV, blood cultures remained negative. Additional work-up for the aetiology of sterile vegetations revealed a low-grade ovarian carcinoma. Cardiac analysis showed evidence of myocardial infarction in the absence of coronary atherosclerosis as a cause for ventricular fibrillation.
Discussion
Unexplained thrombotic events (venous, arterial, or both) warrant further investigation, e.g., with regard to TS. NBTE is a potential source of thromboembolism in TS and a rare ante-mortem finding, which prompts additional investigation of the underlying cause. In our patient, a triad of (suspected) (i) arterial/systemic embolization (i.e. visual disturbances, splenic infarction, coronary embolism), (ii) peripheral thrombophlebitis/hypercoagulability (i.e. DVT and PE), and (iii) malignancy (i.e. gynaecological abnormalities) raised suspicion of NBTE in the setting of TS. Early diagnosis and treatment of NBTE is of importance due to the high incidence of embolization, with possible fatal outcome.
Keywords: Case report, Trousseau syndrome, Non-bacterial thrombotic endocarditis, Ovarian carcinoma, Ventricular fibrillation, Myocardial infarction, Anticoagulation, Literature review
Learning points
In case of unexplained recurrent venous and arterial thrombo-embolic events, underlying pathology should be considered (Trousseau Syndrome).
Echocardiography should be considered after arterial thromboembolism, notably in the setting of concurrent venous thromboembolism and/or suspected malignancy, to exclude non-bacterial thrombotic endocarditis.
Ventricular fibrillation might result from myocardial ischaemia and/or infarction secondary to coronary emboli in the setting of non-bacterial thrombotic endocarditis.
Low molecular weight heparines are preferred above (direct) oral anticoagulants in the setting of non-bacterial thrombotic endocarditis.
Whether prophylactic antibiotic therapy is indicated in the presence of non-bacterial thrombotic endocarditis to prevent infective endocarditis remains unknown.
Primary specialties involved other than cardiology
Internal medicine, gynaecology, oncology
Introduction
The association of both arterial and venous thromboembolism (VTE) with malignancy has been mentioned as early as 1823 by Jean Baptiste Bouillard and was systematically studied by Armand Trousseau in 1865.1 Trousseau’s name has been famously linked to this syndrome not only because he first described it but also suffered from it himself and subsequently succumbed to gastric cancer. Although many definitions of Trousseau syndrome exist, it was recently proposed to restrict its use to unexplained thrombotic events that precede the diagnosis of an occult visceral malignancy or that appear concomitantly with the tumour.2
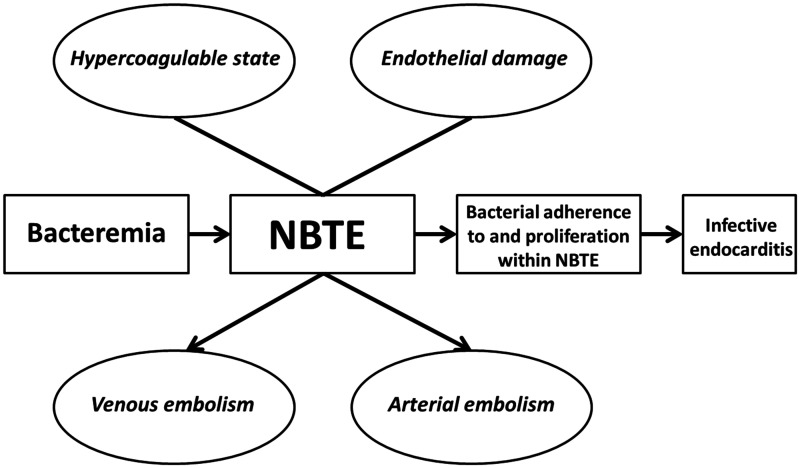
Non-bacterial thrombotic endocarditis (NBTE) is a potential source of thromboembolism in Trousseau syndrome. NBTE is a rare form of endocarditis, characterized by the formation of sterile thrombi, consisting of a mixture of platelets, fibrin, inflammatory cells, and immune complexes on valvular endothelium in the setting of endothelial injury and/or a hypercoagulable state.3 NBTE is usually discovered post-mortem. It has been proposed that NBTE should be suspected in the presence of a triad of (i) a predisposing disease to be associated with NBTE, (ii) a cardiac murmur, and (iii) evidence of (multiple) systemic emboli.4 Others proposed a triad of (i) suspected (mucinous) malignancy, (ii) arterial emboli, and (iii) peripheral thrombophlebitis, to be strongly suggestive of NBTE.5 The diagnosis is additionally supported by a laboratory diagnosis of disseminated intravascular coagulation (DIC) and the obligatory absence of positive blood cultures.6
We describe a case of ventricular fibrillation secondary to (a previous) myocardial infarction, considered to be one of the thrombo-embolic manifestations of Trousseau syndrome, which eventually led to the discovery of NBTE and previously undiagnosed ovarian adenocarcinoma. The current case underscores that screening for underlying pathology should be considered, when clinical signs of coagulopathy such as venous or arterial thromboembolism, and notably both, are observed in the presence of a new cardiac murmur or in patients without cardiovascular risk factors since early diagnosis of e.g. a malignancy might impact patient morbidity and mortality.
Timeline
| Weeks prior to index event) | Symptoms | Imaging | Diagnosis | Action |
|---|---|---|---|---|
| −112 | Visual disturbances | None | Migraine | None |
| −28 | Leg pain and dyspnoea | Echo-duplex | Deep vein thrombosis and clinical pulmonary emboli (PE) |
Rivaroxaban 20 mg Regular check-ups |
| −18 | Vaginal bleeding, dizziness, diplopia | Transvaginal sonography (TVS)/abdominal echography | Anaemia secondary to use of novel anticoagulant and uterine myoma |
Regular check-up Continue Rivaroxaban 20 mg |
| −6 | Gynaecological check-up | TVS/abdominal echography | Possible ovarian abnormality |
Rivaroxaban reduced to 10 mg Magnetic resonance imaging (MRI) uterus ordered |
| −2 | Leg pain and dyspnoea | CT-angiography (CT-A) | Recurrent PE | Rivaroxaban increased to 20 mg |
| −1 | MRI abdomen | Suspected ovarian malignancy | CT thorax/abdomen | |
| Index event | In hospital cardiac arrest during computed tomography (CT)-scan | Electrocardiogram | Ventricular fibrillation |
Transthoracic echocardiography, transoesophageal echocardiography, CT-A + calcium score and MRI-heart Switch to Tinzaparin |
| +4 | Start chemotherapy | CT thorax | Regression of pulmonary emboli | Continuation of Tinzaparin |
| +6 | Haematoma on injection site | None | Haematomas due to Tinzaparin injections | Switch from tinzaparin to fenprocoumon |
| +8 | Leg pain and dyspnoea | Echo-duplex | Deep vein thrombosis | Switch from fenprocoumon to Tinzaparin |
Case presentation
A 49-year-old woman known with migraine and a recent history of recurrent deep venous thrombosis (DVT) and subsegmental pulmonary emboli (PE) lost consciousness during a CT-scan which was performed to further evaluate a suspected gynaecological malignancy. Upon connection to the monitor, ventricular fibrillation was shown, and resuscitation was started. She was intubated and after four shocks she had return of spontaneous circulation. Electrocardiogram (ECG) showed sinus rhythm, a normal heart axis but no signs of myocardial ischaemia or infarction. Quick-look transthoracic echocardiography (TTE) revealed signs of right ventricle-pressure overload but no regional wall motion abnormalities. Having a low a priori risk for atherosclerotic cardiovascular disease, acute coronary angiography or intervention was considered unnecessary at that moment. She was transferred to the intensive care unit (ICU) for further analysis and treatment.
In retrospect (see Timeline section), her complaints had started 7 months earlier when she had visited our emergency department (ED) with leg pain and dyspnoea. Deep vein thrombosis was diagnosed by Doppler ultrasound and due to desaturation up to 88% oxygen saturation during slight exertion PE was considered likely. No other abnormalities in the patient history, physical examination, and routine laboratory testing were found. Anticoagulant therapy with rivaroxaban 20 mg once daily was started and she was quickly discharged. Three months later, she presented to our ED with vaginal bleeding, dizziness, and diplopia. Abdominal and vaginal palpation revealed an enlarged mobile lump just below the umbilicus, suspect for myomatous uterus. Laboratory analysis showed severe anaemia (Hb 3.4 mmol/L), and transvaginal sonography (TVS) revealed uterine myomas. She received a blood transfusion, an oral progesterone antagonist (Orgametril), and oral GnRH agonist (Lucrin). At subsequent visits, she reported improved exercise tolerance but episodes of recurrent blood loss. After 5 months, it was decided to lower the dose of rivaroxaban to 10 mg daily, since her symptoms of DVT and PE had improved, but she continued to complain of vaginal blood loss. Meanwhile, a repetitive TVS revealed another ovarian abnormality, besides the uterine myomas. An outpatient MR of her pelvis was scheduled. Four weeks later, she was readmitted with recurrent DVT and PE. She was discharged the same day with increased rivaroxaban dosage (20 mg). Two weeks after this event, she underwent the MR pelvis, which showed a 20 cm large mixed cystic and solid lesion suspect for ovarian malignancy next to adenomyosis uteri. Another abdominal/thoracic CT was planned for 2 days later. During this CT-scan, she was resuscitated for ventricular fibrillation.
Upon admittance to the ICU, laboratory results revealed a normal haemoglobin level (8.9 × 109 mmol/L), elevated leucocyte count (43 × 109), normal thrombocyte count (193 × 109), elevated D-dimer (16.6 mg/L), activated partial thromboplastin time (aPTT; 46.0 s) and partial thromboplastin time (15.1 s), and Howell–Jolly bodies in the blood smear suggestive of splenic afunctionality. Cardiac enzymes in the acute setting were low (CK-MB 1.2 μg/L), but repetitive measurements were not taken. CT-thorax confirmed the presence of subsegmental pulmonary emboli but excluded major thrombi that could have caused acute deoxygenation resulting in ventricular fibrillation. CT-abdomen confirmed a solid multilocular lesion suspect for ovarian carcinoma. Further laboratory analysis showed elevated tumour markers: Carcinoembryonic Antigen (CEA) 9.9 ng/mL (normal <5) and Ca-125 954 U/mL (normal <35). She was extubated after 2 days in the ICU and transferred with maximum Glasgow Coma Scale Eye-Motor-Verbal (EMV)-scores to the cardiology ward.
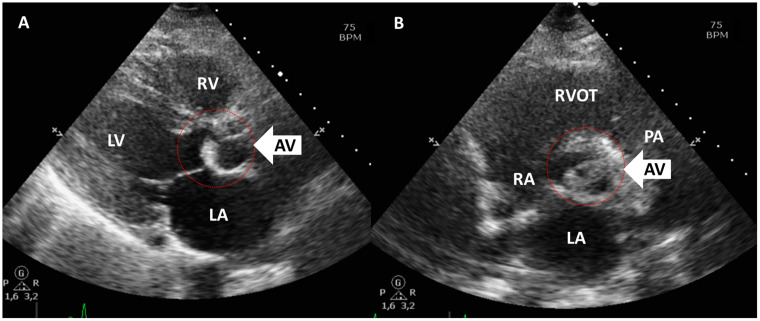
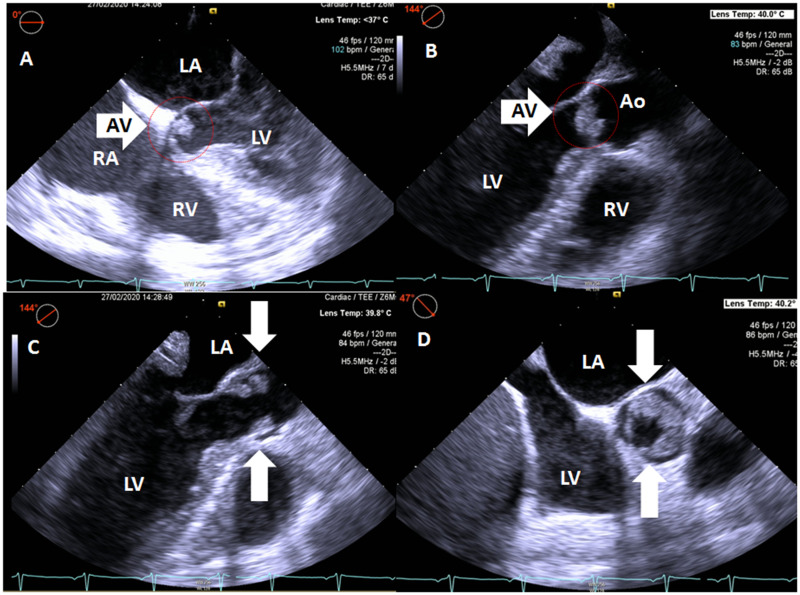
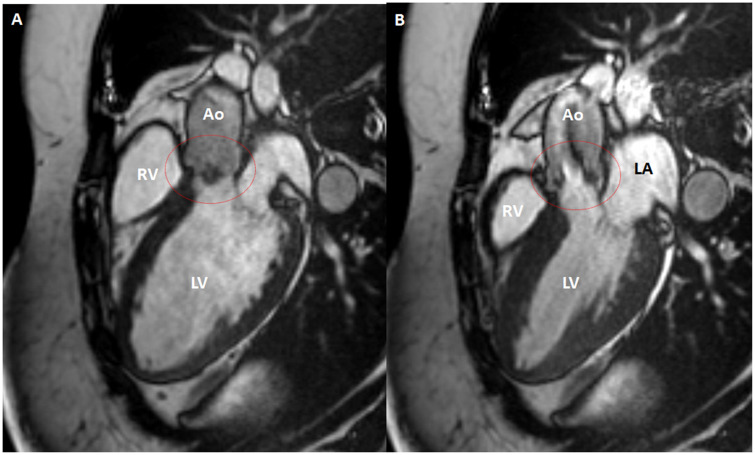
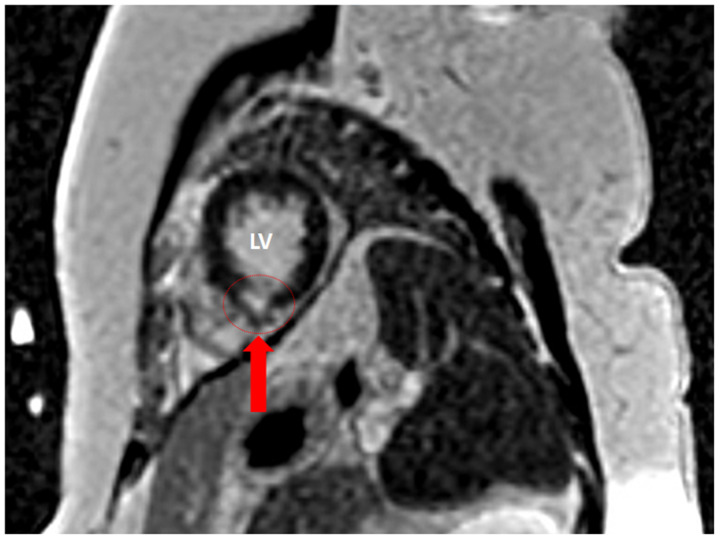
To substantiate the aetiology of the arrhythmia a second, complete TTE was performed 3 days after admittance which showed a normal left and right ventricular function without regional wall motion abnormalities or pressure overload. The aortic valve revealed severely thickened cusps (Figure 1, Videos 1 and 2). Cardiac CT revealed a calcium score of zero, normal coronary anatomy, and vessel patency. Transoesophageal echocardiography showed large oscillating structures in long axis (Figure 2A–C, Video 3) and severe thickening of the tips of the left and non-coronary cusps in short-axis view (Figure 2D, Video S1). Cardiac MRI performed after 1 week confirmed the presence of abnormally thickened aortic valvular cusps (Figure 3) but also showed focal subendocardial late-enhancement and oedema in the apical inferoseptal region (Figure 4). Due to the absence of clinical, laboratory electrocardiographic, and echocardiographic signs of acute ischaemia at presentation, a previous myocardial infarction with scar formation was considered the most likely cause of the arrhythmia. Blood cultures remained negative and laboratory investigations revealed no signs of auto-immune disease.
Figure 1.
Non-bacterial thrombotic endocarditis as visualized with transthoracic echocardiography parasternal long axis (A) and short axis (B) views of the aortic valve. Note thickened aortic valve cusps (arrows). LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract.
Figure 2.
Non-bacterial thrombotic endocarditis as visualized with transoesophageal echocardiography in five-chamber view (A), three-chamber long-axis view with the aortic valve in closed (B) and opened (C) position, and short-axis view (D) of the aortic valve. Note thickened aortic valve cusps (arrows). Ao, aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 3.
Non-bacterial thrombotic endocarditis of the aortic valve (within red circle) as visualized by MRI in three-chamber long axis during late diastole (A) and early systole (B). Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
Figure 4.
Inferoseptal late gadolinium enhancement on MRI indicating regional scar and/or myocardial fibrosis. LV, left ventricle.
In the setting of a metastatic adenocarcinoma, NBTE was suspected. Taken together, ventricular fibrillation secondary to a previous myocardial infarction after coronary embolization from NBTE in the setting of metastatic ovarian cancer was diagnosed.
Upon the diagnosis of NBTE, rivaroxaban was replaced by low molecular weight heparin (LMWH: tinzaparin 175 International Units (IU)/kg subcutaneously once daily). However, in the outpatient setting LMWH was changed to a vitamin K antagonist when the patient complained of haematomas at the injection site of the LMWH (see Timeline section). When signs of DVT recurred, she was put on LMWH once again. Due to the remaining aortic vegetations in the setting of NBTE and consequent increased risk of recurrent embolic cardiac events and the fact that the arrhythmia was considered to be related to myocardial scar, a subcutaneous implantable cardioverter-defibrillator was implanted for secondary prevention. She was started on platinum-based neoadjuvant chemotherapy followed by a complete debulking operation including hysterectomy, salpingectomy, omentectomy, stripping of the bladder peritoneum, removal of the ovaries, and multiple random peritoneal biopsies. Pathological examination showed endometrioid type adenocarcinoma restricted to the right ovary (Figo stage I). Transthoracic echocardiography’s performed 4 weeks and 9 months after discharge showed a similar picture to the TTE performed upon admittance. At present, she is continuing adjuvant chemotherapy and is doing relatively well, she has had no signs of recurrent thromboembolism or heart failure. Device follow-ups were unremarkable.
Discussion
We describe a rare case of survived ventricular fibrillation considered to be secondary to (a previous) arterial thromboembolism in the setting of Trousseau syndrome, which led to the discovery of NBTE and ovarian malignancy.
Up to one in four patients presenting with unexplained thrombotic events has underlying malignancy, i.e., presents with Trousseau syndrome.1 The incidence rate of VTE during malignancy ranges from 0.2 to 20%, depending on the type and stage of the malignancy. On post-mortem examinations, however, 50% of cancer patients have evidence of VTE. There has been discussion whether screening for malignancy in patients with unprovoked VTE will be beneficial. One study concluded that early detection of occult cancers may be associated with improved treatment possibilities, although it remains uncertain whether this improves prognosis.7 However, a subsequent study8 has shown that the prevalence of occult cancer is low among patients with a first unprovoked VTE and that routine screening with advanced imaging does not provide a clinically significant benefit. The epidemiology of arterial thrombosis in cancer patients has received much less attention but was recently estimated to range from 0.25 to 2.6%.1 The development of arterial thromboembolism in cancer patients was associated with a 3- to 5-fold increased risk of death. Conversely, the risk of cancer in patients with arterial embolism was 2.5% after 6 months and 17.9% after 20 years of follow-up.9 The development of both venous and arterial thromboembolism should raise the suspicion of underlying pathology even more, but descriptive data on the incidence rate of malignancy in this situation are lacking.
Valvular vegetations in the absence of bacterial infection were described as early as 1888 by Ziegler.3 Early in the 20th century Libman and Sacks10 classified the various forms of endocarditis into five major classes: acute bacterial, subacute bacterial (or lenta), syphilitic, rheumatic (or typical verrucous), and indeterminate. The latter was subclassified into terminal/cachectic endocarditis and atypical verrucous endocarditis. The former type was also known as marantic endocarditis, deriving from the Greek ‘marantikos’, which means wasting away, referring to the ‘wasted’ state of many of the patients with this condition at the end of a chronic and debilitating disease such as malignancy or severe infection. The latter form (atypical verrucous endocarditis) is presently known as Libman–Sacks endocarditis, which is most often observed in the context of auto-immune diseases,6 notably systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS). The term NBTE was first introduced by Gross and Friedberg in 1936 to replace the terms marantic and terminal/cachectic endocarditis. NBTE is often accompanied by (signs of) DIC.6 With advancing insights and technology, the term NBTE has been used less specific, to include every non-infected thrombotic valvular vegetation.
NBTE is a rare disorder found in 1.2% of adult autopsies, with a range of 0.3–9.3% depending on the subset of studied patients and the meticulosity of the pathologist.6,11,12 By prospective echocardiographic screening of cancer patients with solid tumours, the incidence of NBTE can reach 19%.13 See Table 1 for an overview of characteristics of patients diagnosed with NBTE in published case series. Generally, there is no sex predilection, and it predominantly affects those between the 4th and 8th decades of life.15,16 NBTE has been associated with conditions ranging from chronic and acute inflammatory diseases, such as (metastatic) malignancies, auto-immune diseases, trauma, stress, sepsis, and burns, to an allergy to porcine proteins after a porcine bioprosthesis, and as a result of an indwelling catheter.6 In essence, NBTE is thought to be the result of a combination of a hypercoagulable state and endothelial damage, caused by several underlying aetiologies (Figure 5).6,18 Several autopsy studies6,14,15 have shown that malignancy and infection about equally contribute to the incidence of NBTE. Malignancies most often associated with NBTE seem to differ with time and geographical location, but notably include mucin-producing adenocarcinomas, since they produce a certain proteolytic enzyme (cancer procoagulant).19 Frequently associated malignancies include pancreas, gastric, lung, colon, gall tract, and bladder, haematological and ovarian cancer (Table 1). NBTE has been observed in 4% of all end-stage cancer patients and in up to 19% of patients with disseminated adenocarcinoma.3 The fact that our patient developed NBTE in the absence of metastatic malignancy is remarkable. Although some case reports of patients with NBTE and less advanced gynaecological malignancies have been reported previously, NBTE mostly occurs in advanced malignancies. It has been suggested that thromboembolism is in fact a surrogate marker of aggressive tumour biology. In particular, the presence of advanced or metastatic cancers implies greater tumour bulk, more necrosis, higher growth rate, and excessive release of proteolytic enzymes and cytokines.20 In patients with ovarian cancer, levels of interleukin-6 and tumour necrosis factor-α in serum and ascites were shown to be elevated. It has been hypothesized that the interaction between monocytes/macrophages and malignant cells may result in endothelial damage thus inducing a thrombogenic surface, and in hypercoagulability by activating platelets and clotting factors, which could further result in thrombosis and growth on endothelial surfaces, such as cardiac valves (Figure 5). Tissue factor, which is expressed by many malignant tissues and mononuclear cells in response to inflammatory cytokines has also been reported to be essential for the initiation of NBTE and DIC in malignancies.21 Compared to infective endocarditis, NBTE has a higher frequency of embolization, ranging from 14% to 90% (mean 42%) in various reports, predominantly into the cerebral vasculature, followed by the spleen, kidneys, coronary arteries, and extremities.3,6,22 When signs of cerebrovascular embolization are present in patients with cancer, such as could be argued for our patient since she reported diplopia, the prevalence of NBTE may be as high as 32%. NBTE is usually diagnosed when signs of its complications, such as embolization and/or valve dysfunction, or signs of the underlying disease, either malignancy, auto-immune, or infectious disease occur.3
Table 1.
Characteristics of patients diagnosed with non-bacterial thrombotic endocarditis over the last decades in various case series
| Authors | Lopez et al.6 | Steiner14 | Eiken et al.12 | Llenas-Garcia et al.15 | Bussani et al.16 | Zmaili et al.17 | |
|---|---|---|---|---|---|---|---|
| Study type | Autopsies review | Autopsy Case series | Surgical case series | Autopsy case series | Clinical autopsies | Echocardiography cases | |
| Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | ||
| Year | 1987 | 1993 | 2001 | 2007 | 2019 | 2020 | |
| Inclusion | 1954–84 | 1971–93 | 1985–2000 | 1974–2004 | 1983–2006 | 1999–2019 | |
| N | 1071 | 171 | 30 | 22 | 405 | 42 | |
| Prevalance of NBTE | 1.3 (0.3–9.3) | 0.93 | 1.08 | 3.7 | |||
| Mean age | 49 | 63 | 83 | 54 | |||
| Age modus | 1 | 70–79 (27%) | 60–69 | ||||
| 2 | 60–69 (21%) | 70–79 | |||||
| 3 | >80 (19%) | 50–59 | |||||
| 4 | 50–59 (13%) | 80–90 | |||||
| Sex | No difference | No difference | Female (60%) > male | No difference | Female (53%) > male | Female (67%) > male | |
| Aetiology | 1 | Malignancy (52%) | Malignancy (59%) | Immuno-mediated (60%) | Infection (55%) | Malignancy (59%) | Malignancy (41%) |
| 2 | – | APS (26%) | Malignancy (32%) | APS (36%) | |||
| RHD (20%) | Immuno-mediated (5%) | SLE (33%) | |||||
| SLE (7%) | |||||||
| 3 | RA (7%) | ||||||
| Malignancy | 1 | Lung (41%) | Ovaries | Pancreatic | Gastric | Lung (47%) | |
| 2 | Pancreas (15%) | Biliary | Renal | Pancreatic | Breast (24%) | ||
| 3 | Stomach (14%) | Pancreas | Colonic | Pancreatic (18%) | |||
| 4 | Colon/rectal (11%) | Lung | Urogenital (6%) | ||||
| 5 | Gall bladder (7%) | Stomach | Unknown primary (6%) | ||||
| 6 | Leukaemia (5%) | ||||||
| 7 | Ovarian (5%) | ||||||
| Metastatic cancer | 86% | ||||||
| Infection | 1 | Sepsis (50%) | |||||
| 2 | Tuberculosis (25%) | ||||||
| 3 | Pneumonia (17%) | ||||||
| Localization | 1 | Mitral (43%) | Mitral (64%) | Mitral (63%) | Mitral (37%) | Mitral (43%) | Mitral (62%) |
| 2 | Aortic (36%) | Aortic (24%) | Aortic (27%) | Aortic (23%) | Aorta (36%) | Aortic (24%) | |
| 3 | AV/MV (13.1%) | AV/MV (7%) | AV/MV (27%) | AV/MV (13%) | |||
| 4 | Tricuspid (3.6%) | Tricuspid (5%) | Tricuspid (4%) | ||||
| 5 | Trivalvular (1.3%) | Tric/aort (5%) | Pulmonic (1%) | ||||
| Valve morphology | Normal (82%) | Normal (55%) | Normal (65%) | ||||
| Systemic emboli | 42% | 41% | 33% | 41% | 38% | 33% | |
| Localization of emboli | 1 | Splenic | Cerebral (80%) | Spleen (23%) | Cardiac | Stroke | |
| 2 | Cerebral | Kidney (18%) | Limb | ||||
| 3 | Renal | Cerebral (18%) | |||||
| Concurrent PE | 50% | 43% | |||||
| Death due to NTBE | 9.1% | ||||||
| Prevalence of DIC | 14.2% | 0% |
APS, antiphosphlipid syndrome; NBTE, non-bacterial thrombotic endocarditis; RA, rheumatic arthritis; RHD, rheumatic heart disease.
Figure 5.
Pathogenesis and possible clinical consequences of non-bacterial thrombotic endocarditis. After Lopez et al.6 and Liesenborghs et al.18
The diagnosis of NBTE is often established post-mortem. A recent study of 22 autopsy cases of NBTE15 reported that in none of these cases NBTE had been suspected in the ante-mortem situation. Vegetations range from microscopic to large and are characterized by the endothelial deposition of an amorphous mixture of fibrin, platelets, inflammatory monocytes, and immune complexes.3 Characteristically, vegetations manifest on the coaptation edges of previously injured aortic/mitral valves,6,15,22 but may also involve healthy and other valves, chordae tendineae, papillary muscles, or the mural myocardium.3 The differential diagnosis of NBTE consists of infectious endocarditis, cardiac masses, such as giant Lambl’s excrescences, papillary fibroelastoma, myxoma and carcinoid heart disease, thrombus, and certain degenerative heart diseases, such as rheumatic or Barlow’s heart disease (Table 2). Echocardiographic features of NBTE and resembling structures are depicted in Figure 6. For a definite diagnosis of NBTE, several blood cultures should be taken to rule out microbiological infection. Special care should be taken to rule out infective blood culture-negative endocarditis, including (i) bacterial endocarditis with blood cultures sterilized by previous antibacterial treatment; (ii) endocarditis related to fastidious micro-organisms in which prolonged incubation is necessary; (iii) true blood culture-negative endocarditis, due to intra-cellular bacteria that cannot be routinely cultured in blood with currently available blood culture systems.
Table 2.
The differential diagnosis of valvular abnormalities resembling non-bacterial thrombotic endocarditis
| Clinical characteristics | NBTEa | Papillary fibroelastomab | Lambl's excrescenceb | IE | Toxic valvulopathy c | Rheumatic HDd | Myxoma |
|---|---|---|---|---|---|---|---|
| Incidence | Rare | 85% of valvular tumours | Rare | Common | Rare | Common | 30% of cardiac tumours |
| 8% of cardiac tumours | Rarely on valves | ||||||
| Age of the patient | Elderly (50–80 years) | Elderly (60–80 years) | Elderly (60–70 years) | Elderly (50–70 years) | Mostly elderly | All ages | Middle aged (30–60 years) |
| Sex predilection | M = F | M > V (2:1) | F > M (2:1) | ||||
| Predisposing conditions/agents | Hypercoagulability | Hypercoagulability | Unknown | Bacteraemia | Endocrine tumour | Previous infection | Possible familial |
| Endothelial damage | Endothelial damage | Hypercoagulability? | Endothelial damage | Serotonin-like drugs# | S. pyogenes | Mostly unknown | |
| Immune complexes | Possible oncogenic (KRAS) | Endothelial damage? | Endothelial damage | Auto-immune reaction | |||
| Hypoxia | |||||||
| Fever | No | No | No | Yes | No | No | No |
| Cardiac murmur | Rare | Possible | No | Often | Rare | Often | Possible |
| Laboratory markers of infection | Possible | No | No | Yes | Possible | No | No |
| Blood cultures | Negative | Negative | Negative | Positive | Negative | Negative | Negative |
| Echocardiographic features | |||||||
| Shape | Verrucous, friable | Round, oval, often stalked | Thin and long | Verrucous, irregular | Nodular | Nodular, verrucous | Round, oval |
| Rounded | Frond-like, sea-anemone | Filifom strands | Irregularly shaped | Cluster of grapes | |||
| Broad based | Stippling along edges | Irregular or smooth | |||||
| Mobility | Moderately mobile | Often mobile | Hypermobile | Often mobile | Immobile | Immobile | Mobile |
| Penduncated | No | Often (50%) | Yes | No | No | No | Often |
| Homogeneity | Homogenous | Homogenous | Homogenous | Homogenous | Homogenous | Homogenous | Non-homogenous |
| Size/length | Mostly < 3–4 mm | 1–2 cm | Thin (<1 mm) | Variable | <1 mm | <1 mm | 4–8 cm |
| Maximum 1 cm | Range 0.2–4.6 | Long (upto 1–2 cm) | Upto several cm | Range 2–12 | |||
| Preferred side of the heart | Left | Left | Left | Left | Right | Left | Left |
| Preferred cardiac valve | Mitral>aortic | Aortic>mitral | Aortic>pulmonic | – | Tricuspid>pulmonic | Mitral>aortic | — |
| Location to the valve | Mostly upstream | Mostly downstream | Mostly upstream | Mostly upstream | Mostly downstream | — | Downstream |
| Location on the valve | Anywhere on valve | Anywhere on valve | Along closure lines | Anywhere | Entire leaflet | Starting at the tip | — |
| Valvular involvement | Thickening, fibrosis | Minimal at base | None | Thickening | Diffuse thickening | Thickening, calcification | Minimal at base |
| Abces, perforation | Immobility | Domed appearance | |||||
| Valvular fusion | |||||||
| Valvular regurgitation | Possible | Rare | No | Often | Very often | Often | Possible |
| Histological composition | Platelets, fibrin | Avascular | Avascular | Micro-organisms | Fibroblasts | Granulomatous lesions | Vascularized |
| Granulation tissue | Fibro-elastic tissue | Fibro-elastic tissue | Platelets, fibrin | Smooth muscle cells | Macrophages | Myxoid matrix | |
| Neovascularization | Endothelial layer | Endothelial layer | Neutrophils | Collagen, calcified | |||
| Embolic events | Frequent (>30%) | Frequent (33%) | Considered possible | Often (∼10%) | No | No | Frequent (>30%) |
| Association with stroke | Yes | Yes | Yes | Yes | No | No | Yes |
Although arguably different in aetiology, marantic (also known as terminal or cachectic endocarditis; and notably due to carcinomatosis), and Libman–Sacks (also known as atypical verrucous endocarditis; notably due to SLE and APS) are considered indistinguishable by echocardiography and histology.
Various authors consider papillary fibroelastoma's to be giant Lambl's excrescences.
Including carcinoid Syndrome (also known as Hedinger syndrome), in which case an endocrine tumour releases high levels of seretonin/tryptophan resulting in endothelial damage/inflammation, and carcinoid-like syndrome or diet-drug valvulopathy, in which case high serotonin levels are caused by diet and/or drugs, e.g.: ergotamin, methylsergide, pergolide, fenfluramine-phentermine, methylenedioxymethamphatamine (MDMA). NB: Carcinoid(-like) syndrome mainly involves the right heart due to inactivation of serotonin-related metabolites in the lungs (although the left side of the heart might be involved in case of right–left shunting or pulmonary metastases).
Rheumatic heart disease, also known as typical verrucous endocarditis.
F, female; IE, infective endocarditis; M, male.
Figure 6.
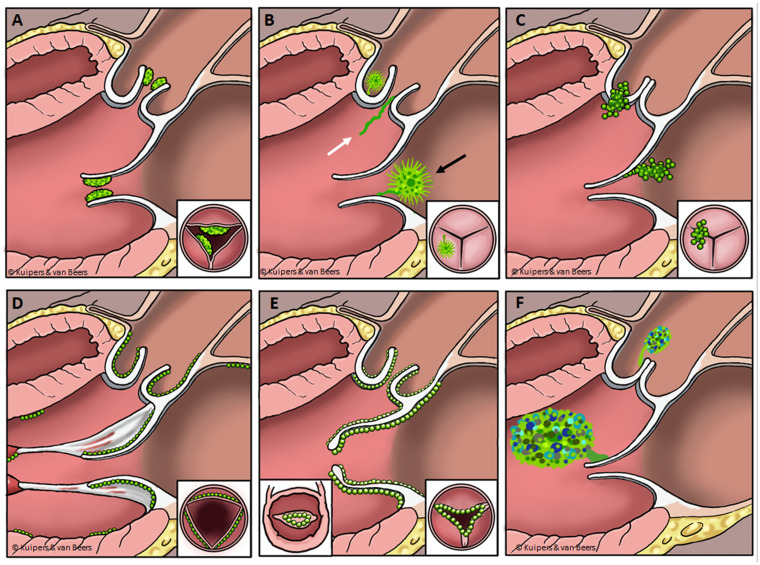
The differential diagnosis of non-bacterial thrombotic endocarditis (NBTE). From left upper to right lower corner. (A) NBTE: Small, broad based, friable vegetations, on the upstream side of the valve that give the valve a thickened appearance. (B) Papillary fibroelastoma (black arrow): Sea-anemone or frond-like, often stalked and mobile structure located on both the up- and downstream side of cardiac valves, and Lambl’s excrescences (white arrow): Thin filiform strands, mostly visualized upstream. (C) Infective endocarditis: Irregular, often mobile, structures mostly on the upstream side of a valve, often causing observable valvular damage. (D) Toxic valvulopathy: Small, nodular, immobile structures on the downstream side of the valve, predominantly located on the right side of the heart, but can be left-sided. (E) Rheumatic heart disease: Small, verrucous, immobile structures on the heart valve, characteristically resulting in valvular thickening, fusion and doming. (F) Myxoma: Relatively large, non-homogenous, rounded, often mobile structures, seen very rarely on the downstream side of cardiac valves.
Typically, abnormal cardiovascular manifestations are absent at the initial presentation.15 Heart failure, heart murmurs, and atrial fibrillation are noted in 23, 27, and 27% of patients post-mortem diagnosed with NBTE, respectively.15 Chest pain, ECG changes, or laboratory measurements suggesting myocardial ischaemia may be present, indicating myocardial infarction resulting from coronary artery emboli, but cardiovascular events are often asymptomatic and only diagnosed with additional imaging or even after death.6 On the other hand, the effects of coronary occlusion may be more severe in patients experiencing embolic myocardial infarction from NBTE, due to the likely absence of collateral vessels. Supporting this hypothesis, myocardial infarction, ventricular fibrillation, and sudden cardiac death secondary to NBTE have been described in several hospital and forensic autopsies. Although no clear data exist on the incidence of coronary embolism in NBTE, rates of cardiac embolism up to 68% have been reported22 and a much higher rate of myocardial infarction and scar (51%) was shown in NBTE compared to control autopsies (39%), while the extent of coronary atherosclerosis was less in the NBTE group, suggesting that the origin of the myocardial ischaemic lesion was embolism from NBTE.11
Treatment involves systemic anticoagulation, surgical intervention, and the diagnosis and treatment of underlying disease.3 The 2015 European Society of Cardiology guideline for endocarditis recommends anticoagulation with unfractioned, low molecular weight heparin (LMWH) or warfarin, but mentions there is little evidence to support this strategy. Vitamin K antagonists—as supported by our case—are known to be less effective, notably in the case of underlying malignancy.23 Direct-acting oral coagulants (DOACs) have not been evaluated in the setting of NBTE. A meta-analysis of LMWH, vitamin K antagonist, and DOACs in cancer-associated thrombosis however, showed DOACs to be superior with regard to recurrence of DVT, at the expense of an 14% increased risk of major bleeding with DOAC compared to LMWH.24 Conversely, in auto-immune mediated (i.e. Libman–Sacks) NBTE, vitamin K antagonists are the anticoagulant of choice, and DOACs are generally not recommended.25 With regard to long-term management, surgery, including valve repair and vegetation excision, has been described for 18% of patients with and 28% of patients without malignancy in a single centre after 20 years of experience with NBTE. Reasons for surgical intervention were severe valvular regurgitation, the large size of the vegetation (>2 cm), and recurrent embolic strokes.17 None of these was present in our patient.
Retrospective studies show a high rate of recurrent stroke, cognitive disability, and a poor prognosis of NBTE due to the association with advanced malignancy and other ‘wasting’ diseases. NBTE associated with malignancy showed a 40% survival rate at 6 months, while NBTE not associated with malignancy revealed a 40% survival rate at 6 years.17 The fact that our patient had low-grade carcinoma, however, makes our case difficult to compare. Life-long anticoagulation therapy is recommended to lower the risk of recurrent thromboembolism. Regular echocardiographic follow-up has been advocated since both progression and resolution of valvular vegetations have been observed. Interestingly, although NBTE has been described as requisite (see Figure 5) for infective endocarditis,18 the use of antibiotic prophylaxis has been studied nor recommended in the setting of NBTE thus far.
In conclusion, as far as we are aware of, this is the first case of survived ventricular fibrillation considered to be secondary to a previous coronary embolization in the setting of Trousseau syndrome, which led to the discovery of NBTE in the setting of previously undiagnosed ovarian malignancy. We argue that echocardiography should be performed, when a triad of suspected malignancy, peripheral arterial embolism, and VTE is present, since a diagnosis of NBTE may convene these into an underlying aetiology of hypercoagulability in the setting of (metastatic) malignancy or auto-immune disease. Early diagnosis of such an underlying aetiology may have an impact on patient morbidity.
Lead author biography

Dr Remko S. Kuipers was educated as a medical doctor and pharmacologist at the University of Groningen in the Netherlands. He earned his MD and PhD from the same university. He specialized in Cardiology at the OLVG in Amsterdam, the Netherlands. His interests are preventive cardiology, imaging, and devices. At present he works as a cardiologist in Amsterdam.
Supplementary material
Supplementary material is available at European Heart Journal—Case Reports online.
Supplementary Material
Acknowledgements
The authors would like to thank C.A. de Groot, E.E.A. de Groot, T.L. Braber, W.J. Kikkert, F. Nijland, R. Bholasingh, and Z.Y. Yong for their contribution to diagnostics, imaging, and treatment of the patient.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: None declared.
References
- 1.De Stefano V.Arterial thrombosis and cancer: the neglected side of the coin of Trousseau syndrome. Haematologica 2018;103:1419–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varki A.Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood 2007;110:1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Frishman WH.. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev 2016;24:244–247. [DOI] [PubMed] [Google Scholar]
- 4.Mckay DG, Wahle GH.. Disseminated thrombosis in colon cancer. Cancer 1955;8:970–978. [DOI] [PubMed] [Google Scholar]
- 5.Rohner RF, Prior JT, Sipple JH.. Mucinous malignancies, venous thrombosis and terminal endocarditis with emboli. A syndrome. Cancer 1966;19:1805–1812. [DOI] [PubMed] [Google Scholar]
- 6.Lopez JA, Ross RS, Fishbein MC, Siegel RJ.. Nonbacterial thrombotic endocarditis: a review. Am Heart J 1987;113:773–784. [DOI] [PubMed] [Google Scholar]
- 7.Piccioli A, Lensing AW, Prins MH, Falanga A, Scannapieco GL, Ieran M. et al. ; for the SOMIT Investigators Group. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost 2004;2:884–889. [DOI] [PubMed] [Google Scholar]
- 8.Carrier M, Lazo-Langner A, Shivakumar S, Tagalakis V, Zarychanski R, Solymoss S. et al. ; SOME Investigators. Screening for occult cancer in unprovoked venous thromboembolism. N Engl J Med 2015;373:697–704. [DOI] [PubMed] [Google Scholar]
- 9.Sundbøll J, Veres K, Horváth-Puhó E, Adelborg K, Sørensen HT.. Risk and prognosis of cancer after lower limb arterial thrombosis. Circulation 2018;138:669–677. [DOI] [PubMed] [Google Scholar]
- 10.Libman E.B. A hitherto undescribed form of valvular and mural endocarditis. Arch Intern Med 1924;33:701. [Google Scholar]
- 11.Kuramoto K, Matsushita S, Yamanouchi H.. Nonbacterial thrombotic endocarditis as a cause of cerebral and myocardial infarction. Jpn Circ J 1984;48:1000–1006. [DOI] [PubMed] [Google Scholar]
- 12.Eiken PW, Edwards WD, Tazelaar HD, McBane RD, Zehr KJ.. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985-2000. Mayo Clin Proc 2001;76:1204–1212. [DOI] [PubMed] [Google Scholar]
- 13.Edoute Y, Haim N, Rinkevich D, Brenner B, Reisner SA.. Cardiac valvular vegetations in cancer patients: a prospective echocardiographic study of 200 patients. Am J Med 1997;102:252–258. [DOI] [PubMed] [Google Scholar]
- 14.Steiner I.Nonbacterial thrombotic endocarditis – a study of 171 case reports. Cesk Patol 1993;29:58–60. [PubMed] [Google Scholar]
- 15.Llenas-García J, Guerra-Vales JM, Montes-Moreno S, López-Ríos F, Castelbón-Fernández FJ, Chimeno-García J.. Nonbacterial thrombotic endocarditis: clinicopathologic study of a necropsy series. Rev Esp Cardiol 2007;60:493–500. [PubMed] [Google Scholar]
- 16.Bussani R, DE-Giorgio F, Pesel G, Zandonà L, Sinagra G, Grassi S. et al. Overview and comparison of infectious endocarditis and non-infectious endocarditis: a review of 814 autoptic cases. In Vivo 2019;33:1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zmaili MA, Alzubi JM, Kocyigit D, Bansal A, Samra GS, Grimm R. et al. Contemporary 20-year Cleveland clinic experience of nonbacterial thrombotic endocarditis: etiology, echocardiographic imaging, management, and outcomes. Am J Med 2021;134:361–369. [DOI] [PubMed] [Google Scholar]
- 18.Liesenborghs L, Meyers S, Vanassche T, Verhamme P.. Coagulation: at the heart of infective endocarditis. J Thromb Haemost 2020;18:995–1008. [DOI] [PubMed] [Google Scholar]
- 19.Gordon SG, Mielicki WP.. Cancer procoagulant: a factor X activator, tumor marker and growth factor from malignant tissue. Blood Coagul Fibrinolysis 1997;8:73–86. [PubMed] [Google Scholar]
- 20.Heath OM, van Beekhuizen HJ, Nama V, Kolomainen D, Nobbenhuis MAE, Ind TE. et al. Venous thromboembolism at time of diagnosis of ovarian cancer: survival differs in symptomatic and asymptomatic cases. Thromb Res 2016;137:30–35. [DOI] [PubMed] [Google Scholar]
- 21.Moradi MM, Carson LF, Weinberg B, Haney AF, Twiggs LB, Ramakrishnan S.. Serum and ascitic fluid levels of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in patients with ovarian epithelial cancer. Cancer 1993;72:2433–2440. [DOI] [PubMed] [Google Scholar]
- 22.Bryan CS.Nonbacterial thrombotic endocarditis with malignant tumors. Am J Med 1969;46:787–793. [DOI] [PubMed] [Google Scholar]
- 23.el-Shami K, Griffiths E, Streiff M.. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518–523. [DOI] [PubMed] [Google Scholar]
- 24.Sobieraj DM, Baker WL, Smith E, Sasiela K, Trexler SE, Kim O. et al. Anticoagulation for the treatment of cancer-associated thrombosis: a systematic review and network meta-analysis of randomized trials. Clin Appl Thromb Hemost 2018;24:182S–187S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N. et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019;78:1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.