Abstract
Accumulating evidence shows that intestinal homeostasis is mediated by cross-talk between the nervous system, enteric neurons and immune cells, together forming specialized neuroimmune units at distinct anatomical locations within the gut. In this review, we will particularly discuss how the intrinsic and extrinsic neuronal circuitry regulates macrophage function and phenotype in the gut during homeostasis and aberrant inflammation, such as observed in inflammatory bowel disease (IBD). Furthermore, we will provide an overview of basic and translational IBD research using these neuronal circuits as a novel therapeutic tool. Finally, we will highlight the different challenges ahead to make bioelectronic neuromodulation a standard treatment for intestinal immune-mediated diseases.
Keywords: enteric nervous system, macrophages, vagus nerve
Bioelectronics as therapy for IBD.
Introduction
Bioelectronic medicine combines the worlds of neuroscience, engineering and computational science to provide a powerful tool for the diagnosis and treatment of a range of neurological and inflammatory diseases using state-of-the-art medical devices. This multidisciplinary field emerged from the revolutionary discovery that the nervous system regulates biological processes underlying health and disease using closed-loop mechanisms called neural reflexes. These include sensing, integration and effector responses (1, 2). Since most organs are neurally innervated, sensory signals could be collected throughout the body by recording nerve activity and subsequently integrated by decoding nerve activity patterns. Conversely, the function of a variety of organs can be modulated by electrically stimulating their innervation, clearly illustrating the potential of bioelectronic medicine with respect to both diagnostic and therapeutic applications. Bioelectronic neuromodulation offers clear advantages compared to standard pharmacological treatments by providing real-time and personalized therapy with a higher anatomical specificity and fewer adverse effects than conventional drug treatments (2, 3).
Since the group of Tracey showed that vagus nerve stimulation (VNS) potently attenuated cytokine production in a model of sepsis (1), the vagus nerve, the main parasympathetic nerve, became an interesting target for neuromodulation. This groundbreaking research led to the introduction of the concept of the cholinergic anti-inflammatory pathway (CAP), a hard-wired connection between the nervous system and the immune system closely interacting to control inflammation (1). This loop consists of sensory neurons detecting inflammation and transmitting neural signals to the central nervous system, which ultimately modulates peripheral inflammation locally via the release of neuronal mediators from efferent nerves (4). In 2005, we and others extended the CAP to the gut revealing anti-inflammatory properties of VNS in different models of intestinal inflammation (5–8), including inflammatory bowel disease (IBD) (9–12).
In the present review, we will discuss the current knowledge and the clinical implications of intestinal neuromodulation with a focus on IBD. In addition, we will describe the different challenges ahead to make bioelectronic medicine a potential treatment for intestinal immune-mediated diseases.
Innervation of the gastrointestinal tract
The gastrointestinal (GI) tract itself is under the intrinsic control of the enteric nervous system (ENS), also referred to as the ‘little brain of the gut’. It is a complex, extensive network of interconnected neurons and glial cells arranged in the sub-mucosal plexus and myenteric plexus, capable of functioning autonomously from the central nervous system (13). The sub-mucosal plexus regulates fluid secretion and nutrient absorption, whereas the myenteric plexus mainly coordinates gut muscle contractility. Both plexi are composed of intrinsic primary afferent neurons (IPANs), interneurons and motor neurons. In particular, IPANs sense mechanical and molecular alterations in the GI tract, information that is relayed to intrinsic motor neurons via other IPANs and interneurons (13). Although the majority of enteric neurons are cholinergic in origin, the ENS communicates via a variety of neurotransmitters and neuropeptides including nitric oxide, adenosine triphosphate, vasoactive intestinal peptide and calcitonin gene-related peptide (CGRP) (4).
The extrinsic neural control of the gut is composed of both sympathetic and parasympathetic nerve fibers, which regulate vital functions such as motility, blood flow and fluid secretion (4). The vagus nerve, representing the principal component of the parasympathetic nervous system, innervates the GI tract from the esophagus to the descending colon. It comprises two major branches, i.e. the left (or anterior) and right (or posterior) vagus nerves. The left branch innervates the GI tract until the duodenum, whereas the right vagus nerve reaches until the colonic splenic flexure (14, 15). To what extent the distal intestine receives vagal input in human is under debate by anatomists (16).
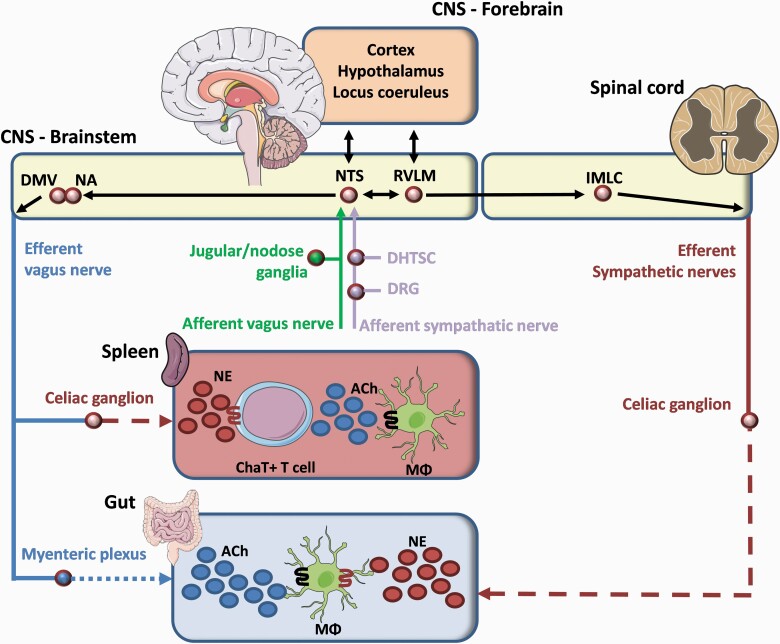
The vagus nerve itself is a mixed nerve composed of 80% afferent and 20% efferent fibers. Vagal afferents relay information about GI function including inflammation centrally to the nucleus tractus solitarius (NTS). In turn, GI function is modulated via efferent vagal fibers originating from two nuclei located in the brain stem, i.e. the nucleus ambiguous (NA) and the dorsal motor nuclei of the vagus nerve (DMV). Of note, gut vagal efferents synapse with neurons located in the ENS (Fig. 1) (17, 18).
Fig. 1.
Brain–gut signaling pathways. Gut afferents from the vagus and sympathetic nerves are transmitted to the NTS interacting with the DMV and NA, the origin of vagal efferents. In the case of overt inflammation, the vagal efferent arm modulates the immune response in the spleen via the celiac ganglion. Particularly, norepinephrine (NE) binds to a subset of ChAT+ T cells leading to the release of ACh interacting with α7nAChR+ Mϕ. During localized intestinal inflammation, the central nervous system (CNS) is alarmed by gut afferents which will lead to the activation of vagal efferents synapsing on myenteric neurons. The latter release ACh modulating intestinal inflammation via the interaction between ACh and α7nAChR+ Mϕ. Notably, intestinal infection promotes the tissue-protective phenotype of Mϕ, an effect mediated by the interaction between NE and Mϕ that express β2 adrenergic receptor. DHTSC, dorsal horn of the thoracolumbar spinal cord; DRG, dorsal root ganglion; IMLC, intermediolateral cell column of spinal cord; RVLM, rostral ventrolateral medulla.
Besides its parasympathetic input, the GI tract is also innervated by the sympathetic nervous system (50% efferent and 50% afferent). Several brain centers such as paraventricular nucleus of the hypothalamus, locus coeruleus, the A5 noradrenergic group and the C1 adrenergic group activate efferent sympathetic pathways (19, 20), which pass through the intermediolateral column of the spinal cord to reach the abdominal pre-vertebral ganglia innervating the GI tract (Fig. 1), i.e. the stomach receives innervation from the celiac ganglion, whereas the small intestine and colon are innervated by the celiac ganglion, superior or inferior mesenteric ganglion or pelvic ganglion. From the pre-vertebral ganglia, post-ganglionic sympathetic noradrenergic fibers arise, which together with arteries enter the gut via the mesenteric serosal surface (Fig. 1). There, they terminate in the ENS and near blood vessels in (sub)mucosa to regulate secretion, motility and vascular tone as well as inflammation (21).
Sympathetic spinal afferents from the GI tract whose cell bodies are located in the dorsal root ganglion synapse in the dorsal horn of the thoracolumbar spinal cord (Fig. 1). These spinal afferents relay information on pain, inflammation and discomfort to the central nervous system.
The sympathetic nervous system also branches to the gut-associated lymphoid tissue (GALT). Interestingly, within the GALT, sympathetic varicosities are found in close approximation to parenchymal immune cells including T cells, macrophages and plasma cells (22). Notably, these sympathetic varicosities release norepinephrine (NE) ‘en passage’ allowing diffusion up to 1 μm before interacting with immune cells (23).
Neuroimmune cross-talk in the gut and beyond
Cholinergic modulation in the intestine
The GI tract is continuously exposed to the lumen harboring commensals, pathogens and dietary food antigens. The gut is therefore equipped with a highly diverse population of innate and adaptive immune cells strategically positioned in the gut wall to protect our body against invading pathogens, while also remaining tolerant towards trillions of commensal bacteria (24, 25). Particularly, macrophages (Mϕ) are historically known for their role in host defense and pathogen clearance, but are now increasingly recognized as a heterogeneous population of tissue-supportive cells, exemplified by their distinct gene expression profile (26). Their function is mainly driven by intrinsic features such as origin, and by environmental factors produced in the niche in which they reside (Fig. 2).
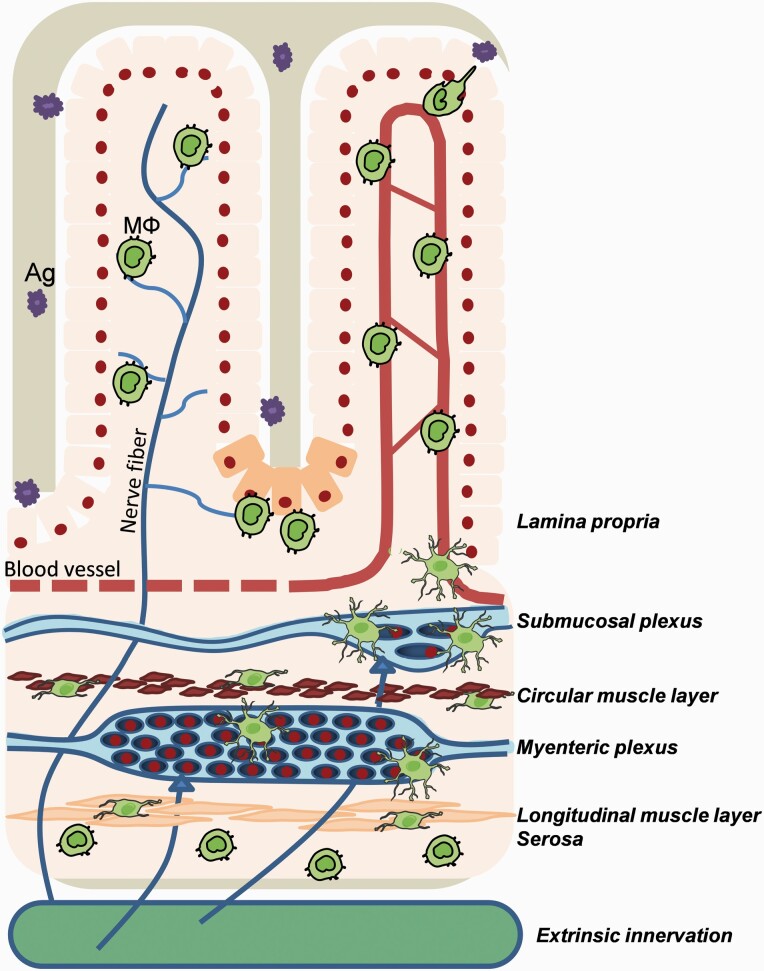
Fig. 2.
Anatomical distribution of intestinal Mϕ. Gut Mϕ reside within different layers of the GI tract. Mϕ (green) are mostly located randomly in the villi of the lamina propria, but are also located in close association with Paneth cells (orange), epithelium, blood vessels (red) and nerve fibers (blue). More distal from the lumen, sub-mucosal Mϕ are found nearby sub-mucosal neurons and blood vessels (red). Within the muscularis externa, stellate Mϕ can be located in the myenteric plexus, and bipolar Mϕ are interspaced between circular and the longitudinal muscle fibers. Finally, a sub-population of intestinal Mϕ resides in the serosa.
In the lamina propria, CX3CR1hi Mϕ are the first defenders against organisms invading from the intestinal lumen, a task which they accomplish by constantly surveilling the environment, phagocytosing harmful antigens and promoting epithelial cell renewal. In addition, lamina propria Mϕ prevent excessive inflammation in response to harmless commensals and promote tolerance by favoring the expansion of antigen-specific CD4+CD25+ regulatory T cells (Tregs), mainly via the production of IL-10, retinoic acid and transforming growth factor β (TGF-β) (Fig. 3a) (27, 28). Thus, lamina propria Mϕ are considered key players in the establishment and maintenance of mucosal homeostasis (27, 29). On the other hand, in the muscularis externa, Mϕ are found in close association with the ENS to maintain neuronal health (30) and promote peristalsis using signaling via bone morphogenetic protein 2 (BMP2) and its receptor (BMP2R) (Fig. 3b) (31). Interestingly, these different populations of intestinal Mϕ express receptors for neuropeptides and neurotransmitters including neurokinin receptors, glycine receptors, α7 and β2 nicotinic acetylcholine receptors (nAChR), β2 adrenergic receptor (β2-AR) and P2 purine receptors (26, 32–35). Moreover, enteric neurons were found to produce CSF1, crucial for the maintenance of the Mϕ compartment (31), indicating that neuronally derived mediators modulate Mϕ function in the gut (Fig. 3b).
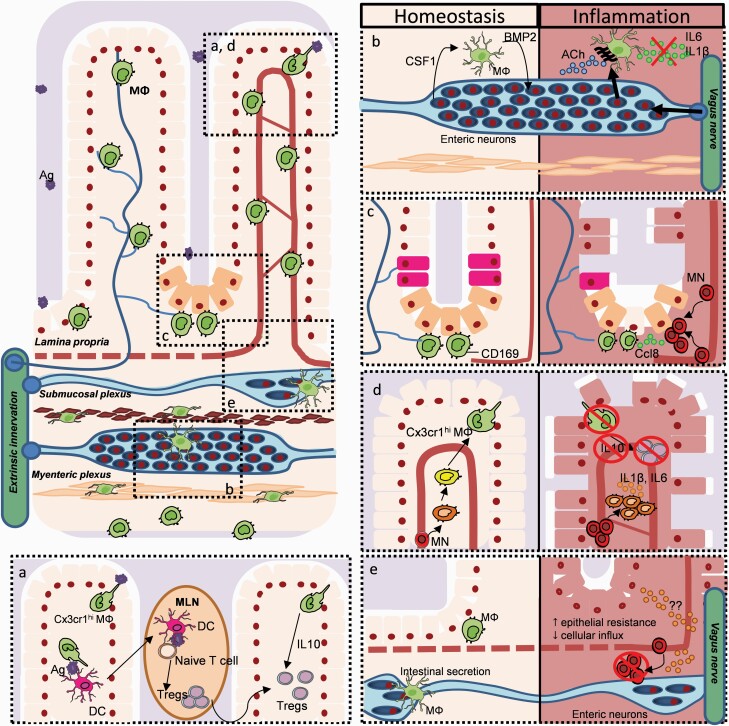
Fig. 3.
Neuroimmune interactions in the gut during health and disease. Areas designated (a–e) in the main part of the figure (upper left) are expanded and adapted in the rest of the figure. For example, in the lower left, (a) lamina propria (LP) CX3CR1hi Mϕ (green) promote oral tolerance via sampling the lumen and transferring trapped antigens (purple) to dendritic cells (DC; pink). The latter cells migrate to mesenteric lymph nodes (MLNs) to induce naive T cells to become FoxP3+ Tregs, which then migrate back to the LP. LP CX3CR1hi Mϕ maintain the Treg population by their IL-10 production. (b) Within the muscular externa, Mϕ are maintained by CSF1 produced by enteric neurons. Reciprocally, Mϕ produce BMP2 regulating peristalsis. During DSS-induced colitis, VNS dampens Mϕ activation via ACh binding to α7nACh+ Mϕ. (c) CD169+ Mϕ are found close to the intestinal crypt where they promote epithelial proliferation (pink) and Paneth cell (light orange) differentiation. During DSS-induced colitis, CD169+ Mϕ release CCL8, thus promoting the influx of blood leukocytes. (d) The LP Mϕ pool is constantly replenished by incoming Ly6Chi monocytes (red) that differentiate into mature CX3CR1hi Mϕ. During intestinal inflammation, the differentiation of Ly6Chi monocytes into CX3CR1hi Mϕ is arrested. As such, immature CX3CR1int Mϕ (orange) are retained in the LP, where they fail to release IL-10, thus reducing the Treg pool. (e) Within the sub-mucosa, Mϕ support neuronal and vascular health. During DSS-induced colitis, VNS reduces the influx of pro-inflammatory leukocytes, but also increases epithelial resistance (N. Stakenborg and G. E. Boeckxstaens, unpublished results).
The concept that the nervous system modulates Mϕ was first introduced in 2000 by the group of Tracey (1). In a model of sepsis, they showed that electrical VNS reduced TNF-α release from splenic α7nAChR+ Mϕ resulting in increased survival (36, 37). This splenic CAP is supposed to be triggered by a direct connection between the efferent vagus nerve and the sympathetic splenic nerve at the level of the celiac ganglion, causing the release of NE within the splenic white pulp (Fig. 1). NE promoted ACh secretion by β2-AR+ ChAT+ T cells interacting with α7nAChR located on adjacent Mϕ (38, 39). Notably, the exact neuronal circuitry of this splenic CAP is still under debate, since some authors argue that the anti-inflammatory properties of VNS is exerted via vago-sympathetic or vago-vagal pathways [for excellent reviews on this topic, we refer to references (40–42)].
In the gut, we and others discovered that the enteric neurons and Mϕ share anatomical niches and interact functionally in certain regions of the intestine to maintain homeostasis, but also modulate inflammation (24, 30, 43, 44). Inflammatory mediators released during a localized inflammation are sensed by gut vagal afferents via binding of cytokines and chemokines such as IL-1 and mast cell mediators (45). For instance, intestinal inflammation in response to Campylobacter jejuni infection or intestinal manipulation increased the expression of c-Fos, a marker of neuronal activity, in the NTS (43, 46) and subsequently the DMV (43). Using antegrade and retrograde tracing experiments, we previously showed that the activated neurons in the DMV are connected to the site of inflammation, supporting the concept of a hard-wired neuronal circuitry able to modify intestinal inflammation (43).
The therapeutic properties of this neuronal circuitry in the gut were clearly demonstrated in a model of postoperative ileus, a condition characterized by an impaired GI motility due to a subtle inflammation of the muscularis externa in response to surgical handling. The key orchestrators of this inflammatory process are the muscularis Mϕ (mMϕ). Interestingly, electrical stimulation of the efferent cervical and abdominal vagus nerve attenuated inflammation in the muscularis externa by activation of cholinergic enteric neurons in close approximation to α7nAChR+ mMϕ (Fig. 3b) (6, 44, 47).
This inhibitory effect on mMϕ is, however, most likely mediated by enteric neurons, as anterograde tracer studies showed that vagal efferents only communicate with cholinergic enteric neurons but not directly with mMϕ (43). In line with this, stimulation of cholinergic enteric neurons using specific 5-hydroxytryptamine receptor 4 agonists dampened the inflammatory response and significantly improved clinical recovery in a model of surgery-induced inflammation, an observation that was confirmed in patients undergoing colonic resection (44, 48, 49).
Whether electrical stimulation of the vagus nerve also has a similar anti-inflammatory effect in the lamina propria remains to be further elucidated. Of interest though, abdominal vagotomy was reported to elevate the basal expression of NF-κB and the production of pro-inflammatory cytokines in the lamina propria (50, 51). In addition, oral tolerance towards commensals and dietary antigens is impaired in vagotomized mice, an observation that is closely related with a reduced induction and expansion of Tregs in the lamina propria (52). Since Hadis et al. revealed that lamina propria CX3CR1+ Mϕ are the key producers of IL-10, crucial to maintain intestinal homeostasis (27), one can argue that vagal input is involved in driving IL-10 production in these immune cells. This hypothesis is indirectly supported by the fact that cholinergic fibers have been observed in close approximation to intestinal monocytes and Mϕ at the level of the sub-mucosal plexus and lamina propria (6, 43).
Interestingly, Teratani et al. recently discovered that the hepatic vagal afferents are also able to indirectly sense the gut microenvironment and relay information to the NTS, and ultimately to the efferent vagus nerve and enteric neurons (53). In particular, surgical or chemical disruption of hepatic vagal afferents significantly decreased the number of colonic Tregs, the T-cell sub-population crucial to maintain homeostasis in the gut lamina propria, because of reduced levels of aldehyde dehydrogenase (ALDH; gene encoding the retinoic acid-synthesizing enzymes RALDH1 and RALDH) and retinoic acid in intestinal antigen-presenting cells including Mϕ. Reciprocally, activation of muscarinic acetylcholine receptors (mAChR) on colonic antigen-presenting cells increased ALDH expression. Notably, disruption of the left hepatic branch also resulted in increased susceptibility to colitis most likely caused by the reduction of the colonic pool of Tregs, thus demonstrating the existence of a novel vago-vagal liver–brain–gut reflex.
Interestingly, capsaicin-sensitive afferent nerves also possess anti-inflammatory properties in the intestine. Indeed, previous studies have showed that electrical and physiological stimulation of afferents causes the release of neurotransmitters such as tachykinins and CGRP (54), which reduced mucosal damage in the gut (55–58). On the other hand, ChAT+ Th17 cells, the cell population previously identified as the effector cells of the splenic CAP, were also discovered in the GALT and lamina propria of the gut. These ChAT+ Th17 cells promoted epithelial AMP production via β2-AR stimulation on dendritic cells in the healthy intestine, potentially protecting the gut from mucosal damage during inflammation (59). Indeed, during the resolution of dextran sulfate sodium (DSS)-induced colitis, ChAT+ Th17 cells supported mucosal healing (60). Hence, both neural and non-neural targets could be used to modulate immune responses during mucosal inflammation.
Sympathetic modulation of the intestine
Besides the parasympathetic nervous system, the GI tract is also densely innervated by the sympathetic nervous system. Here, its main functions are the regulation of blood flow, secretion and motility. The sympathetic fibers mostly innervate the sub-mucosal plexus and myenteric plexus as well as the intestinal mucosa (61). In contrast to the vagus nerve, the sympathetic nervous system was observed to be in direct communication with immune cells especially in the GALT (23, 62). Interestingly, intestinal Mϕ express various (nor)adrenergic receptors, supporting the hypothesis that sympathetic fibers may control the intestinal immune response (38, 63, 64).
Notably, adrenergic receptors were shown to have different thresholds of activation depending on the catecholamine concentration: high concentrations activate β-adrenoreceptors having anti-inflammatory effects, whereas low concentrations preferentially activate α-adrenoreceptors leading to pro-inflammatory mediator release (65). This difference may explain the contrasting data obtained in animal and clinical studies investigating the pro- and anti-inflammatory effects of the sympathetic nervous system during local inflammation. Indeed, chemical sympathectomy using 6-hydroxydopamine treatment was shown to decrease the severity of acute DSS-induced and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis (66, 67), whereas it worsened the disease course in chronic DSS-induced colitis (68).
These controversial results suggest that sympathetic nerve fibers may have a dual role: they promote inflammation during acute disease, but confer resolution during the chronic phase of inflammation (69). Yet again, the method of sympathectomy may also affect results, since surgical sympathectomy, in contrast to chemical sympathectomy, exacerbated colitis severity during acute disease (70). Nevertheless, intermittent electrical stimulation of sympathetic fibers at the level of the superior mesenteric nerve improved disease severity in a DSS colitis model (70). Furthermore, a recent retrospective study showed that β-blockers exacerbated inflammation in IBD patients, increasing relapse risk (71). In line with this, ex vivo catecholaminergic treatment of GALT led to lower rates of Salmonella translocation (72), potentially mitigating infection. Further evidence for sympathetic neuroimmune interaction in the gut is provided by Gabanyi et al. (26). They showed that an enteric infection with Salmonella typhimurium augmented the tissue-protective phenotype of mMϕ, an effect possibly mediated by interaction between extrinsic sympathetic fibers and mMϕ expressing β2-AR (26).
Together, these data suggest that sympathetic anti-inflammatory pathways are activated during gut inflammation, an effect that is most likely mediated via β-AR.
As the sub-mucosal region is highly vascularized and sympathetically innervated, NE could also act as a chemoattractant to recruit circulating immune cells into the lamina propria during inflammation by guiding them to the sympathetic terminals (73). Indeed, Asano et al. have discovered a lamina propria CD169+ Mϕ population that is enriched near the crypt base close to GALT and contributes to disease severity during mucosal inflammation by attracting circulating monocytes through the release of chemoattractant CCL8 (Fig. 3c) (74). As such, neuromodulation of the mucosal sympathetic fibers may potentially halt the influx of inflammatory monocytes during gut inflammation.
Neuromodulation of IBD using bioelectronics
Epidemiology and current treatment strategies
IBD is a debilitating chronic inflammatory disease of the gut with Crohn’s disease (CD) and ulcerative colitis (UC) as its two main clinical presentations (75). Whereas UC is restricted to the mucosal layers of the large intestine, CD is characterized by transmural inflammation involving the entire gut. Although the exact etiology of IBD remains to be elucidated, it is evident that genetic predisposition, environmental factors (e.g. stress, diet, etc.), immunity and the intestinal microbiome are implicated in disease development and its progression (76). In particular, IBD patients are known to have an altered immune response to their microbiome. Moreover, it has become apparent that gut commensals promote immune development and homeostasis in the lamina propria. As such, a disturbed gut microbiota is strongly associated with acute and chronic infections (8). Interestingly, metagenomic studies have found that IBD patients have a decreased intestinal microbiota diversity and stability (77).
In addition, aberrant Mϕ immune responses towards commensals generally lead to a loss of tolerance, which is believed to underlie the inflammation observed in IBD (78). Pre-clinical evidence shows that in DSS colitis models, Ly6Chi monocytes entering the lamina propria rapidly differentiate into pro-inflammatory effector cells (75, 79–81), a finding that was confirmed in inflamed lamina propria tissue of IBD patients (82–85). Of interest, under inflammatory conditions, the differentiation of these incoming Ly6Chi CX3CR1lo monocytes is halted, thus preventing them from fully differentiating into mature CX3CR1hi Mϕ (79, 82). As such, immature CX3CR1int Mϕ are retained in the lamina propria where they fail to release IL-10. Instead, they produce pro-inflammatory mediators and display increased respiratory burst activity, thereby recruiting additional innate and adaptive immune cells including neutrophils, Th1 and Th17 cells (Fig. 3d) (76, 79, 82, 84, 86).
It has been proposed that these recruited inflammatory cells play a pathological role in DSS-induced colitis. Indeed, genetic depletion of CCR2 ameliorates inflammation in acute DSS-induced colitis (87, 88). However, the mechanisms underlying the halted differentiation of recruited Ly6Chi monocytes to mature CX3CR1hi Mϕ in the context of intestinal inflammation remains to be further elucidated (Fig. 3d). Yet, a reduction of essential mediators promoting differentiation including retinoic acid, IL-10 and TGF-β as well as the increased pro-inflammatory milieu in the lamina propria may inhibit proper Mϕ differentiation in the gut.
The phase of intestinal inflammation is followed by a resolution phase. This repeated cycle of inflammation and resolution eventually leads to complications such as intestinal stenosis and fibrosis, bleeding and fistula formation (89). Given the above findings that monocytes and Mϕ appear to be the main orchestrators of the inflammatory cascade during IBD flares, there is renewed interest in targeting the monocyte/macrophage lineage for prophylactic or therapeutic purposes in IBD. Especially as inhibition or even prevention of activation of Mϕ may prove effective in treating/preventing IBD flares.
Current lines of IBD treatments consist of anti-inflammatory, immunosuppressive and biological therapeutics [for excellent review, we refer to references (29, 90)]. Major limitations of these therapies are their side-effects including systemic immunosuppression, loss of effectiveness over time in a certain percentage of patients and development of refractory disease because of chronic treatment (76). In the latter case, surgical resection of the involved gut is inevitable. Unfortunately, this surgical intervention does not prevent recurrence of the disease in a significant proportion of IBD patients despite the patients continuing their biological or pharmacological treatment (76). Therefore, new treatments such as bioelectronic therapy that are more targeted and give fewer side-effects are necessary.
Rationale of targeting the autonomic nervous system in IBD
IBD patients readily present with a blunted vagal tone and a sympathetic overbalance (91, 92). Several studies have demonstrated its predictive value in disease progression of chronic inflammatory disorders including rheumatoid arthritis and IBD (9, 11, 93). Indeed, Pellissier et al. showed that impaired vagal activity is associated with a pro-inflammatory profile in IBD patients (i.e. high levels of serum TNF-α and salivary cortisol levels) (93, 94).
These findings support the hypothesis that autonomic dysfunction could interfere with the anti-inflammatory properties of the CAP, causing increased levels of pro-inflammatory cytokines (95), a finding that was confirmed in pre-clinical studies. In particular, reduced vagal input induced by abdominal vagotomy aggravated the severity of DSS-induced colitis and arrested the expansion of Tregs in the lamina propria following DSS administration (51, 52, 96–98). Interestingly, vagotomized mice deficient in Mϕ, i.e. M-CSFop/op mice, did not develop colitis, underlying a key role for Mϕ in the CAP (96). Therefore, restoring the vago-sympathetic balance through VNS may be crucial to reduce the recurrence of IBD via modulation of gut Mϕ.
Pharmacological modulation of anti-inflammatory pathways in IBD: a tool to unravel the underlying mechanisms of CAP in IBD
The concept of exploiting the autonomic nervous system, especially the CAP, has become appealing and may be a promising approach to treat IBD. Given the potent anti-inflammatory properties of the cholinergic network, one might assume that increased cholinergic activity in the sub-mucosal plexus could impact on mucosal immune homeostasis (69). This can be achieved through acetylcholinesterase (AChE) inhibitors. AChE is an enzyme that rapidly hydrolyzes ACh to terminate synaptic transmission in both central and peripheral pathways.
Inactivation of AChE maximizes the half-life of ACh, increasing the stimulation of muscarinic and nicotinic ACh receptors. For instance, pre-treatment with physostigmine, an AChE inhibitor able to cross the blood–brain barrier (BBB), attenuated the severity of dinitrobenzene sulfonic acid (DNBS)-induced colitis to a greater extent than neostigmine, an AChE inhibitor unable to cross the BBB, suggesting central cholinergic pathways exert a greater protective effect than peripheral pathways (99). Also in DSS-induced colitis, the AChE inhibitors pyridostigmine and rivastigmine ameliorated the disease course evidenced by decreased macroscopic damage, reduced myeloperoxidase (MPO) infiltration and inflammatory mediators (100, 101).
Interestingly, more mechanistic studies using galantamine, yet another AChE inhibitor able to cross the BBB, revealed anti-inflammatory effects in the intestine via interaction with central mAChR (102), provoking increased efferent vagal activity (103). Accordingly, administration of galantamine alleviated mucosal inflammation in DNBS-induced and DSS-induced colitis as observed by reduced major histocompatibility complex class II (MHCII) levels and reduced pro-inflammatory mediators (i.e. IL1β, IL6 and TNF-α) released by splenic CD11c+ cells (104). Similarly, galantamine reduced TNBS-induced ulcers, neutrophil adhesion, MPO infiltration and pro-inflammatory mediators, while increasing anti-apoptotic signaling pathways and IL-10 production (105).
Central activation of the CAP was also effective in alleviating DNBS-induced colitis using the M1 mAChR agonist, McN-A343. Indeed, intra-cerebroventricular injection of this compound decreased colonic inflammation as shown by a reduced pro-inflammatory Th1/Th17 colonic and splenic cytokine release, an effect mediated by modulation of interaction between splenic dendritic cells and CD4+CD25– T cells via α7nAChR and NF-κB signaling pathways. Of note, this anti-inflammatory effect was abrogated in vagotomized and splenectomized mice, indicating that gut inflammation is controlled via a vagus nerve-to-spleen neural connection (50, 104)
Another approach to pharmacologically mimic the anti-inflammatory properties of the CAP is the use of α7nAChR agonists. This strategy is based on the knowledge that the splenic and intestinal CAP is mediated by α7nAChR located on Mϕ. However, the true involvement of this receptor remains ambiguous in colitis and by extension the lamina propria. For example, administration of nicotine, a non-selective agonist for nAChR, consistently improves colitis severity and inflammation in DSS-induced colitis (96, 106, 107). Other studies, however, showed that male α7nAChR−/− mice had a more severe disease course of DSS colitis than their littermate controls (104, 108, 109). In line with this, administration of (partial) α7nAChR agonists (i.e. choline, PHA-543613, GTS21) alleviated the disease activity index (DAI) score and colonic inflammation in DSS-induced colitis (106, 108), whereas other α7nAChR agonists, i.e. AR-R17779 and GSK1345038A, worsened disease activity (110). Thus, to what extent α7nAChR agonists are potential targets to treat IBD require further investigation.
Bioelectronic medicine in IBD
Pre-clinical studies
Animal studies provided initial evidence that chronic neuromodulation has therapeutic properties in TNBS-induced colitis. Using a chronically implanted electrode, the vagus nerve was stimulated electrically 3 h per day for 5 days [Meregnani et al.: 5 Hz, 1 mA, 500 μs, 10 s ON, 90 s OFF; continuous cycle (111); Sun et al.: 0.25 mA, 20 Hz, 500 ms, 30 s ON, 5 min OFF continuously (112)]. This bioelectronic treatment alleviated the disease severity of TNBS colitis, such as body weight loss, bleeding diarrhea and DAI, an effect mediated by the inhibition of NF-κB and mitogen-activated protein kinase (MAPK) nuclear translocation (111–113). Moreover, VNS treatment led to a marked reduction in colonic damage following decreased inflammatory infiltration and improved ulcer healing in comparison to sham-stimulated rats, an effect mostly likely related to decreased IL-6 and TNF-α release.
Moreover, Jin et al. found that VNS decreased the DAI and pro-inflammatory mediators such as TNF-α, IL-1β, IL-6 and MPO in rats with TNBS-induced colitis via the autonomic pathways (113, 114). Interestingly, another study showed that chronic abdominal VNS possessed anti-inflammatory properties similar to chronic cervical VNS in TNBS-induced colitis. In particular, the authors demonstrated that abdominal VNS-treated rats had improved stool quality, decreased pro-inflammatory mediators in the blood and reduced resident inflammatory cell populations within the gut (115).
In an oxazolone colitis model, Meroni et al. recently demonstrated that even a single application of cervical VNS (5 Hz, 1 mA, 1 ms for 5 min) improved intestinal inflammation (i.e. TNF-α, IL-6 and CXCL1) and survival (Fig. 3e) (8). Similarly, we showed that therapeutic treatment with VNS (5 Hz, 1 mA, 1 ms for 5 min) reduced TNF-α and CXCL1 expression in immature intestinal Mϕ and contributed to improved DAI scores (N. Stakenborg and G. E. Boeckxstaens, unpublished results). Interestingly, indirect activation of the vagus nerve via low power therapeutic ultrasound, in which ultrasound oscillation and pressure are capable of inducing biological effects through heating, radiation forces and other mechanotransducive effects, was also shown to alleviate DSS-induced colitis severity (116). Taken together, these experimental data support the concept that bioelectronic medicine could be used as a novel approach to treat IBD in humans.
Human studies
Small open-label pilot studies have also assessed the potential of VNS in patients with ileocolonic CD (11, 12, 93). The group of Bonaz implanted VNS devices in nine patients with moderately active disease (Cyberonics, model 302) who were treated for 1 year (10 Hz, 0.75–1.25 mA and 250–500 μs; duty cycle: 30 s ON/5 min OFF) (93). The device itself was safe and well tolerated. The most common adverse events included voice alteration, cough, dyspnea, nausea and headache, which were well controlled by reducing stimulation intensity. Two patients with more severe disease withdrew from the study after 3 months because of a worsening of the disease, suggesting that VNS is better indicated for mildly to moderately active CD. Notably, among the remaining seven patients, five were still in endoscopic and clinical remission after 12 months and their vagal tone was restored (93).
These results are in line with the preliminary results of D’Haens et al. (117), showing clinical, biomarker and endoscopic improvement in 8 of 16 CD disease patients following 4 months of VNS therapy. Kibleur et al. also evaluated the effects of VNS on inflammation and brain activity in nine CD patients. After 12 months of chronic VNS, CD DAI, fecal calprotectin, anxiety state and vagal tone were improved, which correlated with a decreased power in the alpha frequency band of the electroencephalogram (118). Electrical stimulation of the sacral nerve was also shown to improve mucosal integrity and DAI scores over an 18-month period in a single patient with proctitis (i.e. a condition in which the rectal mucosa is inflamed) (119), a finding which was confirmed in a rat model of TNBS colitis (120–123).
Despite the promising results of these studies, larger randomized double-blinded control studies and a long-lasting follow-up of the patients are required to confirm the current results.
Challenges in performing bioelectronic medicine in humans
Despite the experimental and clinical use of VNS is challenging, several challenges remain before successful translation of bioelectronic neuromodulation to the clinic.
Achieving the optimum stimulation parameters of VNS is challenging, mainly as it remains to be fully elucidated which vagal fibers are involved in the intestinal CAP (124). Especially as the stimulation threshold varies significantly according to the type of nerve fiber targeted (i.e. afferent, efferent, etc.), knowledge about the exact mechanisms underlying the CAP is of great importance in view of the optimal stimulation parameters to be used. Especially as many variations can be applied because of the large combinations with all the possible parameters (frequency, pulse width, current, pulse shape, duty cycle, etc.), so the optimum stimulation parameters in the clinic remain an educated guess based on animal studies, human ex vivo studies and small pilot studies.
Notably, caution is warranted when translating pre-clinical data to humans, since we observed morphological differences between the vagus nerve of small animals (i.e. mice) and humans. Therefore, larger animals (i.e. sheep, pigs, etc.) should be used to optimize stimulation parameters to be used in clinical trials (124). Moreover, the addition of recording electrodes to human VNS devices would enable future clinical trials to study which nerve fibers type are activated with different parameter combinations.
Choosing the best stimulation location for GI disorders is another parameter under debate. Most studies so far have chronically stimulated the left cervical vagus nerve (Cyberonics, model 302) to treat IBD. Yet, this position of the stimulation electrode activates vagal fibers to the larynx, lungs and heart, leading to adverse effects such as cough, hoarseness, voice alteration, paresthesias and rarely bradycardia. Stimulation of the abdominal vagus nerve circumvents the activation of these nerve fibers. In particular, it avoids stimulation of the larynx and minimizes the risk of interfering with cardiopulmonary function in humans (44, 47, 125).
In recent years, the development of non-invasive VNS techniques, i.e. approaches that do not require surgical implantation of the electrode and neurostimulator, has become of interest. These applications can improve the safety and tolerance of VNS, since implanted devices remain a potential source of infection. Moreover, the implanted stimulation electrode may become less effective over time because of fibrosis around the electrode or device failure (e.g. empty battery, leak breakage etc.).
Non-invasive VNS techniques include transcutaneous activation of the vagus nerve via the auricular concha innervated by vagal afferents (126). Some studies have demonstrated that transcutaneous VNS promotes a higher vagal tone (127–130) and reduces inflammatory mediator release in human whole blood (130–132). However, to date, no clinical results have been published about the use of transcutaneous VNS devices in IBD. Currently, 2 transcutaneous VNS devices are commercially available: (i) NEMOS (Cerbomed) is an external device providing transcutaneous stimulation of the auricular branch of the VN and (ii) Gammacore (electroCore LLC,) is another external model consisting of two stainless steel discs delivering transcutaneous low-voltage electrical signal to the cervical vagus nerve (120 s, 25 Hz).
Bioelectronic medicine also faces some ethical issues that undermine researchers and clinicians in conducting well-designed clinical trials. For instance, chronically relapsing and remitting patients cannot be withdrawn for standard care to treat them with VNS, since it would put them at risk. As a result, the true clinical benefit of VNS in inflammatory disorders will be difficult to determine. In addition, sham-stimulated patients cannot be included in a clinical trial as it is unethical to surgically implant a VNS device without switching the stimulator on. As such, clinical studies using implantable VNS devices consistently lack non-stimulated controls and the placebo effect of VNS cannot be accurately measured in such IBD clinical trials.
Conclusion
There has been a rapid expansion of our knowledge regarding the existence and function of neuroimmune units in the intestine and their cross-talk with the brain. Yet, many questions remain unanswered. In particular, discovering the cell types and mediators involved in the neuroimmune signaling in the intestinal mucosa has proven to be difficult due to its complexity and diversity. Future studies should focus on elucidating these mechanisms to better comprehend how they support homeostasis, but also what their contribution is to the intestinal pathology of IBD. Indeed, a clear understanding of all neuroimmune interactions in the gut may enable researchers to discover better targets and further optimize stimulation parameters and location for bioelectronic neuromodulation, as until now, peripheral nerve stimulation has shown only variable success in IBD.
Funding
This work was supported by the European Research Council (ERC) Advanced Grant (ERC-340101-Cholstim) to G.E.B. G.E.B. is also supported by Flanders Fund for Innovation by Science and Technology (IWT-TBM; 110699), and Research Foundation—Flanders (FWO) grants (G.0566.12N and G.0890.18N). N.S. (12V3619N) is supported by a postdoctoral research fellowship of FWO.
Acknowledgement
N.S. and G.E.B. wrote, corrected and approved the manuscript.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Borovikova, L. V., Ivanova, S., Zhang, M.et al. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458. [DOI] [PubMed] [Google Scholar]
- 2. Güemes Gonzalez, A., Etienne-Cummings, R. and Georgiou, P. 2020. Closed-loop bioelectronic medicine for diabetes management. Bioelectron. Med. 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olofsson, P. S. and Tracey, K. J. 2017. Bioelectronic medicine: technology targeting molecular mechanisms for therapy. J. Intern. Med. 282:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stakenborg, N., Viola, M. F. and Boeckxstaens, G. E. 2020. Intestinal neuro-immune interactions: focus on macrophages, mast cells and innate lymphoid cells. Curr. Opin. Neurobiol. 62:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Jonge, W. J., van der Zanden, E. P., The, F. O.et al. 2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6:844. [DOI] [PubMed] [Google Scholar]
- 6. Matteoli, G., Gomez-Pinilla, P. J., Nemethova, A.et al. 2014. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63:938. [DOI] [PubMed] [Google Scholar]
- 7. Bosmans, G., Appeltans, I., Stakenborg, N.et al. 2019. Vagus nerve stimulation dampens intestinal inflammation in a murine model of experimental food allergy. Allergy 74:1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meroni, E., Stakenborg, N., Gomez-Pinilla, P. J.et al. 2018. Functional characterization of oxazolone-induced colitis and survival improvement by vagus nerve stimulation. PLoS ONE 13:e0197487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koopman, F. A., Chavan, S. S., Miljko, S.et al. 2016. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 113:8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine, Y. A., Koopman, F. A., Faltys, M.et al. 2014. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS ONE 9:e104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonaz, B., Sinniger, V., Hoffmann, D.et al. 2016. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol. Motil. 28:948. [DOI] [PubMed] [Google Scholar]
- 12. Clarençon, D., Pellissier, S., Sinniger, V.et al. 2014. Long term effects of low frequency (10 hz) vagus nerve stimulation on EEG and heart rate variability in Crohn’s disease: a case report. Brain Stimul. 7:914. [DOI] [PubMed] [Google Scholar]
- 13. Yoo, B. B. and Mazmanian, S. K. 2017. The enteric network: interactions between the immune and nervous systems of the gut. Immunity 46:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson, R. G. 1949. Anatomy of the vagus nerves in the region of the lower esophagus and the stomach. Anat. Rec. 103:1. [DOI] [PubMed] [Google Scholar]
- 15. McCrea, E. D. 1924. The abdominal distribution of the vagus. J. Anat. 59(Pt 1):18. [PMC free article] [PubMed] [Google Scholar]
- 16. Delmas, J. and Laux, G. 1933. Anatomie médico-chirugicale du système nerveux végétatif. Masson et cie, Paris, France. [Google Scholar]
- 17. Berthoud, H. R., Jedrzejewska, A. and Powley, T. L. 1990. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J. Comp. Neurol. 301:65. [DOI] [PubMed] [Google Scholar]
- 18. Berthoud, H. R., Carlson, N. R. and Powley, T. L. 1991. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 260(1 Pt 2):R200. [DOI] [PubMed] [Google Scholar]
- 19. Abe, C., Inoue, T., Inglis, M. A.et al. 2017. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 20:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bassi, G. S., Dias, D. P. M., Franchin, M.et al. 2017. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav. Immun. 64:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furness, J. B. 1970. The origin and distribution of adrenergic nerve fibres in the Guinea-pig colon. Histochemie 21:295. [DOI] [PubMed] [Google Scholar]
- 22. Elenkov, I. J., Wilder, R. L., Chrousos, G. P.et al. 2000. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52:595. [PubMed] [Google Scholar]
- 23. Felten, D. L., Felten, S. Y., Carlson, S. L.et al. 1985. Noradrenergic and peptidergic innervation of lymphoid tissue. J. Immunol. 135(suppl. 2):755s. [PubMed] [Google Scholar]
- 24. Godinho-Silva, C., Cardoso, F. and Veiga-Fernandes, H. 2019. Neuro-immune cell units: a new paradigm in physiology. Annu. Rev. Immunol. 37:19. [DOI] [PubMed] [Google Scholar]
- 25. De Schepper, S., Stakenborg, N., Matteoli, G.et al. 2018. Muscularis macrophages: key players in intestinal homeostasis and disease. Cell. Immunol. 330:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gabanyi, I., Muller, P. A., Feighery, L.et al. 2016. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadis, U., Wahl, B., Schulz, O.et al. 2011. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34:237. [DOI] [PubMed] [Google Scholar]
- 28. Zigmond, E., Bernshtein, B., Friedlander, G.et al. 2014. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40:720. [DOI] [PubMed] [Google Scholar]
- 29. Na, Y. R., Stakenborg, M., Seok, S. H.et al. 2019. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 16:531. [DOI] [PubMed] [Google Scholar]
- 30. De Schepper, S., Verheijden, S., Aguilera-Lizarraga, J.et al. 2018. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175:400. [DOI] [PubMed] [Google Scholar]
- 31. Muller, P. A., Koscsó, B., Rajani, G. M.et al. 2014. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marques-da-Silva, C., Burnstock, G., Ojcius, D. M.et al. 2011. Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology 216:1.. [DOI] [PubMed] [Google Scholar]
- 33. Froh, M., Thurman, R. G. and Wheeler, M. D. 2002. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G856. [DOI] [PubMed] [Google Scholar]
- 34. Ho, T. W., Connor, K. M., Zhang, Y.et al. 2014. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 83:958. [DOI] [PubMed] [Google Scholar]
- 35. Nemethova, A., Michel, K., Gomez-Pinilla, P. J.et al. 2013. Nicotine attenuates activation of tissue resident macrophages in the mouse stomach through the β2 nicotinic acetylcholine receptor. PLoS ONE 8:e79264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borovikova, L. V., Ivanova, S., Nardi, D.et al. 2000. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton. Neurosci. 85:141. [DOI] [PubMed] [Google Scholar]
- 37. Wang, H., Yu, M., Ochani, M.et al. 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384. [DOI] [PubMed] [Google Scholar]
- 38. Rosas-Ballina, M., Ochani, M., Parrish, W. R.et al. 2008. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105:11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosas-Ballina, M., Olofsson, P. S., Ochani, M.et al. 2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martelli, D., McKinley, M. J. and McAllen, R. M. 2014. The cholinergic anti-inflammatory pathway: a critical review. Auton. Neurosci. 182:65. [DOI] [PubMed] [Google Scholar]
- 41. Martelli, D., Farmer, D. G. and Yao, S. T. 2016. The splanchnic anti-inflammatory pathway: could it be the efferent arm of the inflammatory reflex? Exp. Physiol. 101:1245. [DOI] [PubMed] [Google Scholar]
- 42. Bonaz, B., Sinniger, V. and Pellissier, S. 2017. The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Front. Immunol. 8:1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cailotto, C., Costes, L. M., van der Vliet, J.et al. 2012. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol. Motil. 24:191. [DOI] [PubMed] [Google Scholar]
- 44. Stakenborg, N., Labeeuw, E., Gomez-Pinilla, P. J.et al. 2019. Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut 68:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hansen, M. K., O’Connor, K. A., Goehler, L. E.et al. 2001. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280:R929. [DOI] [PubMed] [Google Scholar]
- 46. Goehler, L. E., Gaykema, R. P., Opitz, N.et al. 2005. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain. Behav. Immun. 19:334. [DOI] [PubMed] [Google Scholar]
- 47. Stakenborg, N., Wolthuis, A. M., Gomez-Pinilla, P. J.et al.. 2017. Abdominal vagus nerve stimulation as a new therapeutic approach to prevent postoperative ileus. Neurogastroenterol Motil. 29:e13075. [DOI] [PubMed] [Google Scholar]
- 48. Kimura, H., Imura, Y. K., Tomiyasu, H.et al. 2019. Neural anti-inflammatory action mediated by two types of acetylcholine receptors in the small intestine. Sci. Rep. 9:5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park, S. J., Choi, E. J., Yoon, Y. H.et al. 2013. The effects of prucalopride on postoperative ileus in guinea pigs. Yonsei Med. J. 54:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Munyaka, P., Rabbi, M. F., Pavlov, V. A.et al. 2014. Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+CD25− T cells in experimental colitis. PLoS ONE 9:e109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O’Mahony, C., van der Kleij, H., Bienenstock, J.et al. 2009. Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297:R1118. [DOI] [PubMed] [Google Scholar]
- 52. Di Giovangiulio, M., Bosmans, G., Meroni, E.et al. 2016. Vagotomy affects the development of oral tolerance and increases susceptibility to develop colitis independently of the alpha-7 nicotinic receptor. Mol. Med. 22:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Teratani, T., Mikami, Y., Nakamoto, N.et al. 2020. The liver–brain–gut neural arc maintains the Treg cell niche in the gut. Nature 585:591. [DOI] [PubMed] [Google Scholar]
- 54. Holzer, P. 1988. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24:739. [DOI] [PubMed] [Google Scholar]
- 55. Lambrecht, N., Burchert, M., Respondek, M.et al. 1993. Role of calcitonin gene-related peptide and nitric oxide in the gastroprotective effect of capsaicin in the rat. Gastroenterology 104:1371. [DOI] [PubMed] [Google Scholar]
- 56. Mazelin, L., Theodorou, V., More, J.et al. 1998. Protective role of vagal afferents in experimentally-induced colitis in rats. J. Auton. Nerv. Syst. 73:38. [DOI] [PubMed] [Google Scholar]
- 57. Eysselein, V. E., Reinshagen, M., Patel, A.et al. 1992. Calcitonin gene-related peptide in inflammatory bowel disease and experimentally induced colitis. Ann. NY Acad. Sci. 657:319. [DOI] [PubMed] [Google Scholar]
- 58. Reinshagen, M., Patel, A., Sottili, M.et al. 1994. Protective function of extrinsic sensory neurons in acute rabbit experimental colitis. Gastroenterology 106:1208. [DOI] [PubMed] [Google Scholar]
- 59. Dhawan, S., De Palma, G., Willemze, R. A.et al. 2016. Acetylcholine-producing T cells in the intestine regulate antimicrobial peptide expression and microbial diversity. Am. J. Physiol. Gastrointest. Liver Physiol. 311:G920. [DOI] [PubMed] [Google Scholar]
- 60. Willemze, R. A., Brinkman, D. J., Welting, O.et al. 2019. Acetylcholine-producing T cells augment innate immune-driven colitis but are redundant in T cell-driven colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 317:G557. [DOI] [PubMed] [Google Scholar]
- 61. Straub, R. H., Wiest, R., Strauch, U. G.et al. 2006. The role of the sympathetic nervous system in intestinal inflammation. Gut 55:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bellinger, D. L., Lorton, D., Felten, S. Y.et al. 1992. Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int. J. Immunopharmacol. 14:329. [DOI] [PubMed] [Google Scholar]
- 63. Abrass, C. K., O’Connor, S. W., Scarpace, P. J.et al. 1985. Characterization of the beta-adrenergic receptor of the rat peritoneal macrophage. J. Immunol. 135:1338. [PubMed] [Google Scholar]
- 64. Sanders, V. M., Baker, R. A., Ramer-Quinn, D. S.et al. 1997. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J. Immunol. 158:4200. [PubMed] [Google Scholar]
- 65. Straub, R. H. 2004. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol. Sci. 25:640. [DOI] [PubMed] [Google Scholar]
- 66. Jacobson, K., McHugh, K. and Collins, S. M. 1997. The mechanism of altered neural function in a rat model of acute colitis. Gastroenterology 112:156. [DOI] [PubMed] [Google Scholar]
- 67. Motagally, M. A., Neshat, S. and Lomax, A. E. 2009. Inhibition of sympathetic N-type voltage-gated Ca2+ current underlies the reduction in norepinephrine release during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296:G1077. [DOI] [PubMed] [Google Scholar]
- 68. Straub, R. H., Grum, F., Strauch, U.et al. 2008. Anti-inflammatory role of sympathetic nerves in chronic intestinal inflammation. Gut 57:911. [DOI] [PubMed] [Google Scholar]
- 69. Di Giovangiulio, M., Verheijden, S., Bosmans, G.et al. 2015. The neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front. Immunol. 6:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Willemze, R. A., Welting, O., van Hamersveld, H. P.et al. 2018. Neuronal control of experimental colitis occurs via sympathetic intestinal innervation. Neurogastroenterol. Motil. 30:e13163. [DOI] [PubMed] [Google Scholar]
- 71. Willemze, R. A., Bakker, T., Pippias, M.et al. 2018. β-Blocker use is associated with a higher relapse risk of inflammatory bowel disease: a Dutch retrospective case–control study. Eur. J. Gastroenterol. Hepatol. 30:161. [DOI] [PubMed] [Google Scholar]
- 72. Brown, D. R. and Price, L. D. 2008. Catecholamines and sympathomimetic drugs decrease early Salmonella typhimurium uptake into porcine Peyer’s patches. FEMS Immunol. Med. Microbiol. 52:29. [DOI] [PubMed] [Google Scholar]
- 73. Sullivan, D. P., Bui, T., Muller, W. A.et al. 2018. In vivo imaging reveals unique neutrophil transendothelial migration patterns in inflamed intestines. Mucosal Immunol. 11:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Asano, K., Takahashi, N., Ushiki, M.et al. 2015. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat. Commun. 6:7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meroni, E., Stakenborg, N., Viola, M. F.et al. 2019. Intestinal macrophages and their interaction with the enteric nervous system in health and inflammatory bowel disease. Acta Physiol. (Oxf.) 225:e13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Payne, S. C., Furness, J. B. and Stebbing, M. J. 2019. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 16:89. [DOI] [PubMed] [Google Scholar]
- 77. Nishino, K., Nishida, A., Inoue, R.et al. 2018. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 53:95. [DOI] [PubMed] [Google Scholar]
- 78. de Souza, H. S. and Fiocchi, C. 2016. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13:13. [DOI] [PubMed] [Google Scholar]
- 79. Zigmond, E., Varol, C., Farache, J.et al. 2012. Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37:1076. [DOI] [PubMed] [Google Scholar]
- 80. Grainger, J. R., Wohlfert, E. A., Fuss, I. J.et al. 2013. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat. Med. 19:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tamoutounour, S., Guilliams, M., Montanana Sanchis, F.et al. 2013. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39:925. [DOI] [PubMed] [Google Scholar]
- 82. Bain, C. C., Scott, C. L., Uronen-Hansson, H.et al. 2013. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thiesen, S., Janciauskiene, S., Uronen-Hansson, H.et al. 2014. CD14(hi)HLA-DR(dim) macrophages, with a resemblance to classical blood monocytes, dominate inflamed mucosa in Crohn’s disease. J. Leukoc. Biol. 95:531. [DOI] [PubMed] [Google Scholar]
- 84. Tamoutounour, S., Henri, S., Lelouard, H.et al. 2012. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 42:3150. [DOI] [PubMed] [Google Scholar]
- 85. Rugtveit, J., Nilsen, E. M., Bakka, A.et al. 1997. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology 112:1493. [DOI] [PubMed] [Google Scholar]
- 86. Bain, C. C., Bravo-Blas, A., Scott, C. L.et al. 2014. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 15:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Platt, A. M., Bain, C. C., Bordon, Y.et al. 2010. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J. Immunol. 184:6843. [DOI] [PubMed] [Google Scholar]
- 88. Becker, F., Kurmaeva, E., Gavins, F. N.et al. 2016. A critical role for monocytes/macrophages during intestinal inflammation-associated lymphangiogenesis. Inflamm. Bowel Dis. 22:1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Neurath, M. F. 2014. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14:329. [DOI] [PubMed] [Google Scholar]
- 90. Verstockt, B., Ferrante, M., Vermeire, S.et al. 2018. New treatment options for inflammatory bowel diseases. J. Gastroenterol. 53:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lindgren, S., Lilja, B., Rosén, I.et al. 1991. Disturbed autonomic nerve function in patients with Crohn’s disease. Scand. J. Gastroenterol. 26:361. [DOI] [PubMed] [Google Scholar]
- 92. Shanks, N., Harbuz, M. S., Jessop, D. S.et al. 1998. Inflammatory disease as chronic stress. Ann. NY Acad. Sci. 840:599. [DOI] [PubMed] [Google Scholar]
- 93. Sinniger, V., Pellissier, S., Fauvelle, F.et al. 2020. A 12-month pilot study outcomes of vagus nerve stimulation in Crohn’s disease. Neurogastroenterol. Motil. 32:e13911. [DOI] [PubMed] [Google Scholar]
- 94. Pellissier, S., Dantzer, C., Mondillon, L.et al. 2014. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS ONE 9:e105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Huston, J. M. and Tracey, K. J. 2011. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J. Intern. Med. 269:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ghia, J. E., Blennerhassett, P., Kumar-Ondiveeran, H.et al. 2006. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131:1122. [DOI] [PubMed] [Google Scholar]
- 97. Ghia, J. E., Blennerhassett, P. and Collins, S. M. 2007. Vagus nerve integrity and experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G560. [DOI] [PubMed] [Google Scholar]
- 98. Ghia, J. E., Blennerhassett, P., El-Sharkawy, R. T.et al. 2007. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G711. [DOI] [PubMed] [Google Scholar]
- 99. Miceli, P. C. and Jacobson, K. 2003. Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Auton. Neurosci. 105:16. [DOI] [PubMed] [Google Scholar]
- 100. Singh, S. P., Chand, H. S., Banerjee, S.et al. 2020. Acetylcholinesterase inhibitor pyridostigmine bromide attenuates gut pathology and bacterial dysbiosis in a murine model of ulcerative colitis. Dig. Dis. Sci. 65:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shifrin, H., Nadler-Milbauer, M., Shoham, S.et al. 2013. Rivastigmine alleviates experimentally induced colitis in mice and rats by acting at central and peripheral sites to modulate immune responses. PLoS ONE 8:e57668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pavlov, V. A., Parrish, W. R., Rosas-Ballina, M.et al. 2009. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 23:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Waldburger, J. M., Boyle, D. L., Edgar, M.et al. 2008. Spinal p38 MAP kinase regulates peripheral cholinergic outflow. Arthritis Rheum. 58:2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ji, H., Rabbi, M. F., Labis, B.et al. 2014. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 7:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wazea, S. A., Wadie, W., Bahgat, A. K.et al. 2018. Galantamine anti-colitic effect: role of alpha-7 nicotinic acetylcholine receptor in modulating Jak/STAT3, NF-κB/HMGB1/RAGE and p-AKT/Bcl-2 pathways. Sci. Rep. 8:5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hayashi, S., Hamada, T., Zaidi, S. F.et al. 2014. Nicotine suppresses acute colitis and colonic tumorigenesis associated with chronic colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 307:G968–G978. [DOI] [PubMed] [Google Scholar]
- 107. Qin, Z., Wan, J. J., Sun, Y.et al. 2017. Nicotine protects against DSS colitis through regulating microRNA-124 and STAT3. J. Mol. Med. (Berl.) 95:221. [DOI] [PubMed] [Google Scholar]
- 108. AlSharari, S. D., Bagdas, D., Akbarali, H. I.et al. 2017. Sex differences and drug dose influence the role of the α7 nicotinic acetylcholine receptor in the mouse dextran sodium sulfate-induced colitis model. Nicotine Tob. Res. 19:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ghia, J. E., Blennerhassett, P., Deng, Y.et al. 2009. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology 136:2280. [DOI] [PubMed] [Google Scholar]
- 110. Snoek, S. A., Verstege, M. I., van der Zanden, E. P.et al. 2010. Selective alpha7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. Br. J. Pharmacol. 160:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Meregnani, J., Clarençon, D., Vivier, M.et al. 2011. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton. Neurosci. 160:82. [DOI] [PubMed] [Google Scholar]
- 112. Sun, P., Zhou, K., Wang, S.et al. 2013. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS ONE 8:e69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jin, H., Guo, J., Liu, J.et al. 2017. Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 313:G192. [DOI] [PubMed] [Google Scholar]
- 114. Jin, H., Guo, J., Liu, J.et al. 2019. Autonomically mediated anti-inflammatory effects of electrical stimulation at acupoints in a rodent model of colonic inflammation. Neurogastroenterol. Motil. 31:e13615. [DOI] [PubMed] [Google Scholar]
- 115. Payne, S. C., Furness, J. B., Burns, O.et al. 2019. Anti-inflammatory effects of abdominal vagus nerve stimulation on experimental intestinal inflammation. Front. Neurosci. 13:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nunes, N. S., Chandran, P., Sundby, M.et al. 2019. Therapeutic ultrasound attenuates DSS-induced colitis through the cholinergic anti-inflammatory pathway. EBioMedicine 45:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. D’Haens, G., Cabrijan, Z., Eberhardson, M.et al. A clinical trial of the effects of vagus nerve stimulation in biological-refractory Crohn’s disease. United European Gastroenterol J. 2016;2:(suppl. 1):S397–S398. [Google Scholar]
- 118. Kibleur, A., Pellissier, S., Sinniger, V.et al. 2018. Electroencephalographic correlates of low-frequency vagus nerve stimulation therapy for Crohn’s disease. Clin. Neurophysiol. 129:1041. [DOI] [PubMed] [Google Scholar]
- 119. Brégeon, J., Neunlist, M., Bossard, C.et al. 2015. Improvement of refractory ulcerative proctitis with sacral nerve stimulation. J. Clin. Gastroenterol. 49:853. [DOI] [PubMed] [Google Scholar]
- 120. Tu, L., Gharibani, P., Yin, J.et al. 2020. Sacral nerve stimulation ameliorates colonic barrier functions in a rodent model of colitis. Neurogastroenterol. Motil. 32:e13916. [DOI] [PubMed] [Google Scholar]
- 121. Guo, J., Jin, H., Shi, Z.et al. 2019. Sacral nerve stimulation improves colonic inflammation mediated by autonomic-inflammatory cytokine mechanism in rats. Neurogastroenterol. Motil. 31:e13676. [DOI] [PubMed] [Google Scholar]
- 122. Tu, L., Gharibani, P., Zhang, N.et al. 2020. Anti-inflammatory effects of sacral nerve stimulation: a novel spinal afferent and vagal efferent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 318:G624. [DOI] [PubMed] [Google Scholar]
- 123. Pasricha, T. S., Zhang, H., Zhang, N.et al. 2020. Sacral nerve stimulation prompts vagally-mediated amelioration of rodent colitis. Physiol. Rep. 8:e14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Stakenborg, N., Gomez-Pinilla, P. J., Verlinden, T. J. M.et al. 2020. Comparison between the cervical and abdominal vagus nerves in mice, pigs, and humans. Neurogastroenterol. Motil. 32:e13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wolthuis, A. M., Stakenborg, N., D’Hoore, A.et al. 2016. The pig as preclinical model for laparoscopic vagus nerve stimulation. Int. J. Colorectal Dis. 31:211. [DOI] [PubMed] [Google Scholar]
- 126. Peuker, E. T. and Filler, T. J. 2002. The nerve supply of the human auricle. Clin. Anat. 15:35. [DOI] [PubMed] [Google Scholar]
- 127. Ylikoski, J., Lehtimäki, J., Pirvola, U.et al. 2017. Non-invasive vagus nerve stimulation reduces sympathetic preponderance in patients with tinnitus. Acta Otolaryngol. 137:426. [DOI] [PubMed] [Google Scholar]
- 128. Frøkjaer, J. B., Bergmann, S., Brock, C.et al. 2016. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol. Motil. 28:592. [DOI] [PubMed] [Google Scholar]
- 129. Farmer, A. D., Albusoda, A., Amarasinghe, G.et al. 2020. Transcutaneous vagus nerve stimulation prevents the development of, and reverses, established oesophageal pain hypersensitivity. Aliment. Pharmacol. Ther. 52:988. [DOI] [PubMed] [Google Scholar]
- 130. Brock, C., Brock, B., Aziz, Q.et al. 2017. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neurogastroenterol Motil. 29:e12999. [DOI] [PubMed] [Google Scholar]
- 131. Lerman, I., Hauger, R., Sorkin, L.et al. 2016. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation 19:283. [DOI] [PubMed] [Google Scholar]
- 132. Addorisio, M. E., Imperato, G. H., de Vos, A. F.et al. 2019. Investigational treatment of rheumatoid arthritis with a vibrotactile device applied to the external ear. Bioelectron. Med. 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]