Abstract
Background:
Riociguat is a novel soluble guanylate cyclase stimulator, and has been widely used for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (CTEPH). Some studies found that riociguat had better effects on CTEPH and proved to be safe, but the results were not utterly consistent. Therefore, the purpose of this study was to comprehensively evaluate the efficacy and safety of riociguat in the treatment of CTEPH.
Methods:
Randomized controlled trials on riociguat for the treatment of CTEPH were searched through such electronic databases as PubMed, Embase, Cochrane Library, Web of Science, China national knowledge internet, and Wanfang. The outcomes included exercise capacity, pulmonary hemodynamics, and side effects. The fixed-effects or random-effects models were used to analyze the pooled data, and heterogeneity was assessed by the I2 test.
Results:
Four studies involving 520 patients were included in this meta-analysis. Compared with the placebo group, riociguat significantly improved the hemodynamic indexes and increased 6-min walking distance (P < .0001, standardized mean difference (SMD) = −0.24, 95%CI −0.35 to −0.12; P < .00001, SMD = 0.52, 95%CI 0.33 to 0.71), and decreased the Borg dyspnea score (P = .002, SMD = −0.31, 95%CI −0.51 to −0.12). In addition, riociguat could also significantly reduce the living with pulmonary hypertension scores and increase the EQ-5D scores (P = .01, SMD=−0.23, 95%CI −0.42 to −0.05; P < .00001, SMD = 0.47, 95%CI 0.27 to 0.66), but there was no significant difference in the change level of N-terminal pro-hormone B-type natriuretic peptide in patients with riociguat (P = .20, SMD = −0.24, 95%CI −0.61 to −0.13). The common adverse events of riociguat were dyspepsia and peripheral edema, and no other serious adverse reactions were observed.
Conclusions:
We confirmed that riociguat had better therapeutic effects in improving the hemodynamic parameters and exercise capacity in patients with CTEPH without inducing serious adverse events. This will provide a reasonable medication regimen for the treatment of CTEPH.
Keywords: chronic thromboembolic pulmonary hypertension, efficacy, pulmonary arterial hypertension, riociguat, safety
1. Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) belongs to the fourth category of pulmonary arterial hypertension (PAH), and is characterized by pulmonary arterial thrombi or occlusion and microvascular arteriopathy, causing a sustained increase in pulmonary vascular resistance (PVR) and pulmonary arterial pressure, eventually leading to right heart failure.[1,2] According to the world symposium on PAH in 2018, the diagnosis standards of CTEPH include
-
i)
Anticoagulation therapeutic for more than 3 months,
-
ii)
Mean pulmonary arterial pressure ≥25mmHg and pulmonary capillary wedge pressure (PCWP) ≤15 mmHg measured by right heart catheterization
-
iii)
Mismatched perfusion defects on pulmonary perfusion/ventilation scanning or typical signs of CTEPH on multislice spiral computed tomography (MSCT) pulmonary angiography or magnetic resonance imaging.[3]
At present, it is commonly believed that CTEPH is a long-term complication of pulmonary embolism. One study found that the incidence of CTEPH was higher within 3 years of being diagnosed with acute pulmonary embolism, and the risk of CTEPH was associated with lower-limb varicose veins, increased systolic pulmonary artery pressure and intermediate-risk pulmonary embolism.[4] Later, a systematic review and meta-analysis indicated that CTEPH was a common complication with acute pulmonary embolism, and idiopathic pulmonary embolism and right heart dysfunction were the main risky factors.[5]
Currently, the therapeutic strategies of CTEPH include pulmonary endarterectomy (PEA), drugs therapy and interventional therapy. CTEPH is the only pulmonary hypertension that can be cured with PEA. However, some patients were ineligible for PEA due to distal lesions or some severe complications. Balloon pulmonary angioplasty (BPA) is an emerging option that may be curative for patients with CTEPH in the future.[6] Recent study found that BPA could significantly improve pulmonary hemodynamics and exercise capacity of patients with inoperable CTEPH, and better safety was guaranteed.[7] Furthermore, a systematic review assessed the efficacy and safety of BPA for CTEPH, and the results showed that BPA could improve hemodynamics and increase survival rate.[8] On the other hand, such targeted therapies as soluble guanylate cyclase stimulators (riociguat), PDE-5 inhibitors (sildenafil), endothelin receptor antagonists (bosentan and macitentan) and prostacyclin analogues (iloprost, beraprost, and treprostinil) had shown better effects in patients with CTEPH.[9] Additionally, combination therapies and bridging therapy were also used for patients with CTEPH, but no consensus on the therapeutic regimen was achieved.
Riociguat is an orally administered soluble guanylate cyclase stimulator that targets the nitric oxide receptors, and has been approved for the treatment of CTEPH in October 2013.[10] Riociguat has a dual mechanism of action, and mainly acts on the nitric oxide (NO)-soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate signaling pathway, increasing the sensitivity of sGC to NO and directly stimulating sGC in a NO-independent manner, which inhibits the proliferation of pulmonary arterial smooth cells and promotes vasodilation.[11] In an uncontrolled phase II study, the results indicated that riociguat (1.0–2.5 mg t.i.d) was well tolerated, and significantly increased median 6-MWD and reduced pulmonary vascular resistance in patients with CTEPH and PAH.[12] Later, Marra et al[13] found that long-term therapy with riociguat effectively reduced right heart size and bolstered right ventricular function in patients with CTEPH and PAH.
Previous clinical trials had confirmed that riociguat can be used as first-line treatment for CTEPH, and could boost hemodynamics and 6-MWD when combined with treprostinil.[14] However, such serious side effects as hypotension and bleeding, and the high cost of nearly $90,000 per year bring huge burden on patients with CTEPH or PAH. In order to determine the role of riociguat in the treatment of CTEPH and PAH, several clinical trials in different countries had been conducted, but it is difficult to draw reasonable conclusions with insufficient data. Therefore, the purpose of this meta-analysis was to assess the effects and safety of riociguat in the treatment of CTEPH by collecting existing data.
2. Material and methods
2.1. Literature search
A comprehensive literature search was performed in the electronic databases including PubMed, Embase, and the Cochrane library, Web of science, China National Knowledge Infrastructure and Wanfang database from their inceptions to 31 December 2019. The search terms as following: “riociguat” and “chronic thromboembolic pulmonary hypertension” or “CTEPH”, without any restrictions of publication date and languages.
2.2. Inclusion and exclusion criteria
In this study, we only included articles specifically evaluating the effects and safety of riociguat for the treatment of CTEPH. In addition, articles from all databases would be included if they met the following criteria:
-
i)
The studies designed as randomized controlled trials
-
ii)
All patients were aged from 18 to 80 years old and diagnosed with CTEPH by multislice spiral computed tomography pulmonary angiography or magnetic resonance imaging;
-
iii)
Riociguat for the treatment of CTEPH had a minimum treatment duration of 8 weeks and at least one interest outcome should be reported;
-
iv)
The data from all published articles reporting mean and standard deviation. All studies were excluded when they did not meet the criteria described above.
2.3. Data extraction
The baseline characteristics and interested data of all included RCTs were independently extracted by 2 authors (MFY and JS). The primary outcomes included 6-MWD and hemodynamic variables. The second outcomes included N-terminal pro-hormone B-type natriuretic peptide (NT-proBNP), Borg dyspnea scores, EuroQol group five-dimension self-report questionnaire (EQ-5D) scores, living with pulmonary hypertension (LPH) scores, and adverse events.
2.4. Quality assessment
The risk of bias for the chosen studies was evaluated using the Cochrane risk of bias tool, which consisted of the following aspects: random sequence generations (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome (attrition bias), selective reporting (reporting bias) and other bias. Moreover, the quality of all included studies was scored by the Jadad scale. Any disagreements were solved by discussion to reach a consensus. For this type of study, ethical approval and informed consent are not required.
2.5. Statistical analysis
In this meta-analysis, the outcomes were analyzed using RevMan software 5.3 (Cochrane Collaboration, Copenhagen, Denmark). For continuous data, the difference of each outcome was measured by the statistical method of inverse variance, and the results were expressed as standardized mean difference (SMD) with 95% confidence intervals (CIs). I-squared (I2) test was used to analyze the heterogeneity between different studies. Significant heterogeneity was considered when P < .05 or I2 > 50%, and the random effects model was used to analyzed the pooled outcomes. Conversely, the fixed-effect model was applied when no significant heterogeneity was observed (P > .05 or I2 < 50%). The subgroup analysis and sensitivity analysis were also used to reduce heterogeneity. For dichotomous data, the results were expressed as odds ratio (OR) and 95%CIs. P-value less than .05 was regarded as statistically significant.
3. Results
3.1. Description of all included studies
A total of 531 studies were retrieved after searching the electronic databases. The process of literature selection is outlined in Figure 1. A total of 93 records were excluded for duplications, and 355 records were removed after scrutinizing the irrespective reviews, observational trials, meta-analysis, and clinical guidelines. Then 83 articles were eligible for full-text assessment, and 79 articles were excluded because of protocol, non-RCTs, or lack of available data. Finally, 4 studies met the inclusion criteria and were selected.[15–18] The main characteristics of each study were summarized in Table 1. Among the included studies, four studies were multicenter, double-blind, placebo-controlled trials. A total of 483 patients were randomized to receive riociguat (2.5 mg 3 times per day) and 214 to receive placebo. Mean age of all patients ranged from 38 years to 60 years old. The durations of all included trials were more than or equal to 16 weeks. All included studies had a Jadad score, only one of the studies scored less than 4, and the other studies scored more than or equal to 4. In addition, the results of the risk of bias were shown in Figure 2. The main risks of bias were performance bias and reporting bias.
Figure 1.
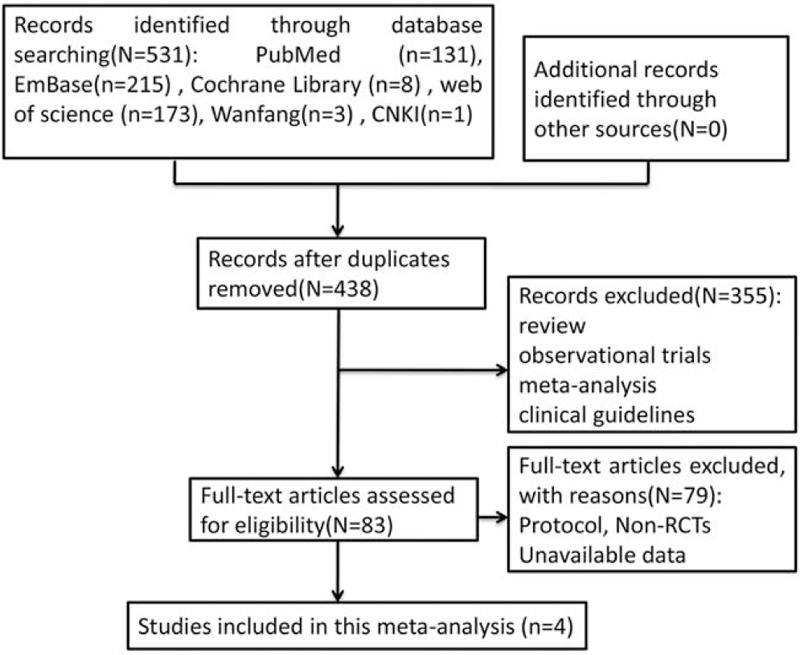
Flow diagram of selected studies in this meta-analysis.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study | Study design | Blind | Patients numberRiociguat /placebo | Age (years)Riociguat /placebo | Dose | Duration(weeks) | Outcomes | Jadadscore |
| Ghofrani et al[15] | RCTs | double-blind | 173/88 | 59 ± 14/59 ± 13 | 2.5 mg, t.i.d | 16 | 6-MWD, Hemodynamics, PVRNT-proBNP, BDS, LPHSEQ-5D,Safety | 5 |
| Marra et al [16] | RCTs | double-blind | 112/20 | 60 ± 13/-- | 1- 2.5 mg, t.i.d | 52 | 6-MWD, NT-proBNP, safety | 4 |
| Simonneau et al [17] | RCTs | double-blind | 155/82 | 59 ± 14/59 ± 12 | 2.5 mg, t.i.d | 52 | 6-MWD, NT-proBNP, BDS, LPHSEQ-5D,Safety | 4 |
| Wang et al [18] | RCTs | double-blind | 21/11 | 47 ± 10/52 ± 13 | 2.5 mg, t.i.d | 16 | 6-MWD, PVR, NT-proBNP, BDSLPHS, EQ-5D,Safety | 3 |
Figure 2.
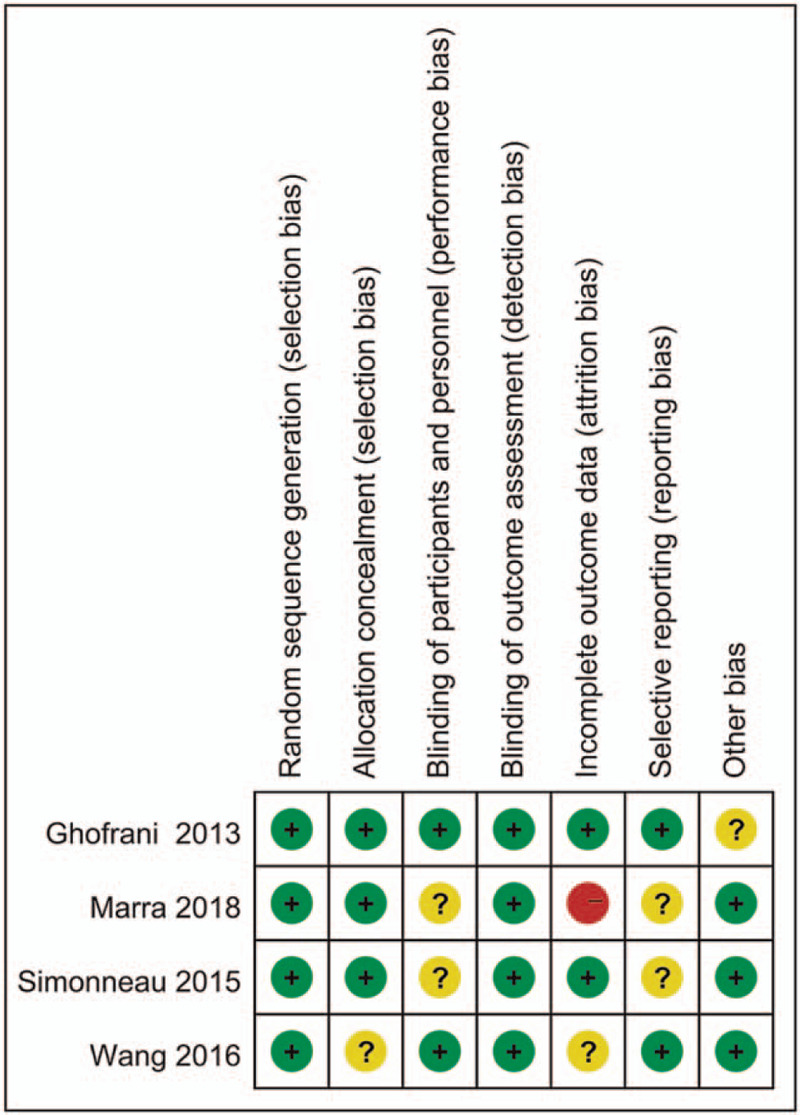
Summarized the risk of bias. Red (−), high risk of bias; yellow (?), unclear risk of bias; green (+), low risk of bias.
3.2. Hemodynamic variables
Hemodynamic parameters include mean arterial pressure (MAP), PVR, PAP, PCWP, right arterial pressure (RAP), and cardiac output. The objective indicators of the state of the pulmonary circulatory system could predict the treating process and outcome of patients with CTEPH.[19,20] In this study, 2 RCTs were included to assess the change of PVR level, and one RCT was used to assess the change of MAP, PAP, PCWP, RAP, and cardiac output. There was no significant heterogeneity between the 2 groups (P = .73, I2 = 0%), and fixed-effects model was used to compare the differences between hemodynamic parameters. Compared with placebo group, riociguat could obviously reduce the levels of PVR, MAP, and PAP (P < .00001, SMD = −0.98, 95%CI −1.25 to −0.71; P < .0001, SMD = −0.73, 95%CI −1.08 to −0.37; P < .00001, SMD=−0.67, 95%CI −0.95 to −0.40), and significantly increase cardiac output (P < .00001, SMD = 0.76, 95%CI 0.48 to 1.03). However, there was no significant differences in the levels of PCWP and RAP when patients were treated with riociguat or placebo (P = .46, SMD = 0.10, 95%CI −0.17 to 0.37; P = .56, SMD = −0.08, 95%CI −0.34 to 0.19) (Fig. 3).
Figure 3.
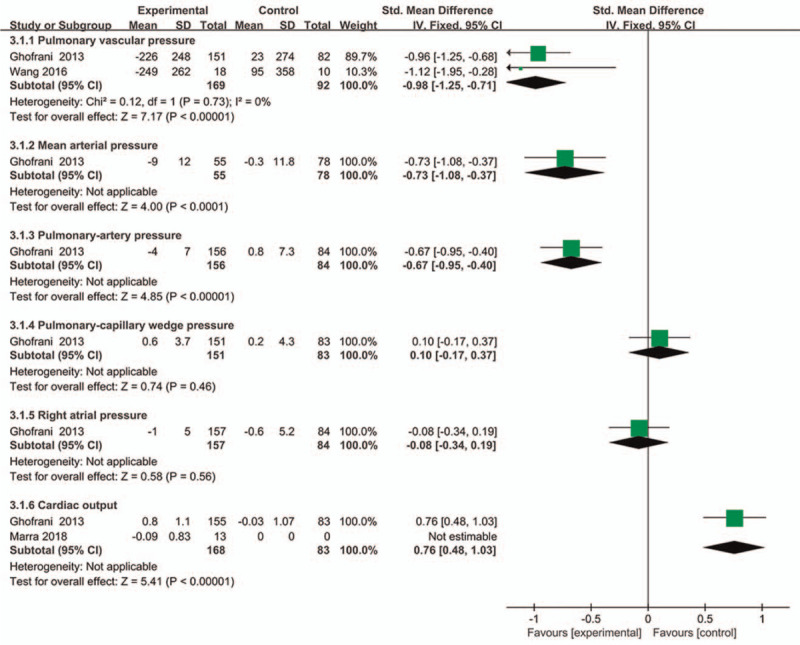
Comparison of the change of hemodynamic parameters between riociguat and placebo groups.
3.3. 6-MWD
Four studies involving 520 patients measured the change of 6-MWD. As shown in Figure 4A, fixed-effects model was applied because no significant heterogeneity was observed (P = .31, I2 = 17%). In contrast to placebo control, the change of 6-MWD was more obvious in patients with riociguat (P < .00001, SMD = 0.52, 95%CI 0.33 to 0.71). This finding indicated that riociguat could significantly increase 6-MWD in patients with CTEPH. In addition, we conducted subgroup analysis to assess this outcome based on the duration of treatment, and the result was consistent (Supplemental Digital Content, Fig. 1).
Figure 4.
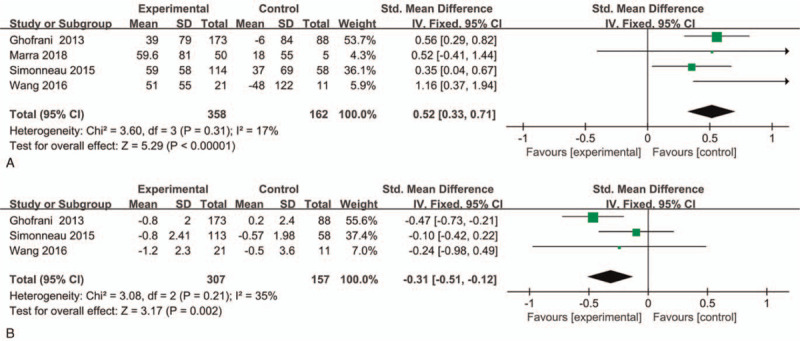
Effect of riociguat on the change of 6-MWD and Borg dyspnea scores in patients with CTEPH. (A) 6- MWD; (B) Borg dyspnea scores.
3.4. Borg dyspnea scores
In the process of measuring the exercise capacity of CTEPH patients, dyspnea could seriously affect the life quality of CTEPH patients, and the degree of dyspnea could be measured by Borg dyspnea scale. We selected three studies to assess this indicator, and fixed effects model was used due to the low heterogeneity (P = .21, I2 = 35%). Riociguat could markedly reduce the score of Borg dyspnea when compared with placebo group (P = .002, SMD = −0.31, 95%CI −0.51 to −0.12) (Fig. 4B). In addition, we conducted subgroup analysis to analyze the data based on the duration of treatment, and the results were consistent (Supplemental Digital Content, Fig. 2).
3.5. NT-proBNP
For this indicator, four studies with 439 patients evaluated the change level of NT-proBNP, and the results were showed in Fig. 5. Random effect model was used because of moderate heterogeneity between the two groups (P = .08, I2 = 59%). Compared with the controlled-placebo group, there was no significant difference in the change level of NT-proBNP in patients with riociguat (P = .20, SMD = −0.24, 95%CI −0.61 to −0.13).
Figure 5.
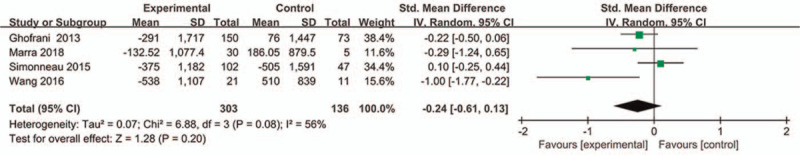
Effect of riociguat on the change level of NT-proBNP.
3.6. The change of LPH score and EQ-5D score
Currently, LPH scale and EQ-5D scale are used to assess health-related quality of life in the prognosis of patients with CTEPH. In this study, 3 RCTs with 493 patients evaluated the change of LPH score after treating with riociguat or placebo. Fixed effect model was used because no significant heterogeneity was observed (P = 0.44, I2 = 0%). Compared with placebo group, riociguat could significantly reduce the score of LPH in patients with CTEPH (P = .01, SMD = −0.23, 95%CI −0.42 to −0.05) (Fig. 6A). In addition, three studies involving 462 patients were used to assess the change of EQ-5D score. Fixed effect model was applied due to low heterogeneity (P = 0.31, I2 = 16%). Riociguat could significantly increase the score of EQ-5D when compared with placebo group (P < .00001, SMD = 0.47, 95%CI 0.27 to 0.66) (Fig. 6B). In addition, subgroup analysis was conducted to analyze the data based on the duration of treatment, and the results were consistent with the comprehensive analysis (Supplemental Digital Content, Fig. 3 and 4)).
Figure 6.
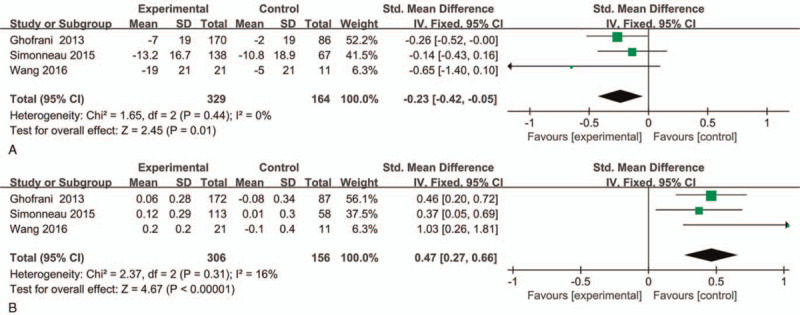
Effect of riociguat on the change of LPH score and EQ-5D score. (A) LPH score; (B) EQ-5D score.
3.7. Safety
During the medication of riociguat, a series of side effects, such as dizziness, dyspepsia, peripheral edema, nasopharyngitis, nausea, vomiting, dyspnea, and upper respiratory tract infection, were observed. In this study, we assessed the safety of riociguat in the treatment of CTEPH. The results were shown in Table 2. There were no significant difference in the incidence of headache, dizziness, nasopharyngitis, nausea, vomiting, diarrhea, hypotension, upper respiratory tract infection, constipation, dyspnea, and cough between riociguat group and placebo group (P = .87, OR = 1.25, 95%CI 0.08 to 19.18; P = .27, OR = 1.61, 95%CI 0.69 to 3.74; P = .28, OR = 1.32, 95%CI 0.79 to 2.20; P = .55, OR = 1.30, 95%CI 0.55 to 3.08; P = .08; P = .53, OR = 1.22, 95%CI 0.65 to 2.30; P = .58, OR = 1.44, 95%CI 0.40 to 5.16; P = .73, OR = 1.13, 95%CI 0.58 to 2.20; P = .11; P = .38, OR = 0.58, 95%CI 0.17 to 1.97; P = .42, OR = 0.79, 95%CI 0.45 to 1.39). In addition, riociguat medication led to higher incidence of dyspepsia and peripheral edema (P = .03, OR = 2.55, 95%CI 1.11 to 5.88; P = .05, OR = 0.63, 95%CI 0.40 to 1.00).
Table 2.
Summary of the common adverse events during the treatment of CTEPH.
| Types | Heterogeneity(I2, P) | OR(95%CI)rioviguat vs placebo | P values |
| Headache | 70%, .07 | 1.25 (0.08,19.18) | .87 |
| Dizziness | 53%, .12 | 1.61 (0.69,3.74) | .27 |
| Dyspepsia | 0%, .93 | 2.55 (1.11,5.88) | .03 |
| Peripheral edema | 0%, .67 | 0.63 (0.40,1.00) | .05 |
| Nasopharyngitis | 0%, .39 | 1.32 (0.79,2.20) | .28 |
| Nausea | 0%, .50 | 1.30 (0.55,3.08) | .55 |
| Vomiting | – | – | .08 |
| Diarrhea | 43%, .19 | 1.22 (0.65, 2.30) | .53 |
| Hypotension | 58%, .12 | 1.44 (0.40, 5.16) | .58 |
| Upper respiratory | 0%, .70 | 1.13 (0.58, 2.20) | .73 |
| Tract infection | |||
| Constipation | – | – | .11 |
| Dyspnea | 73%, .05 | 0.58 (0.17,1.97) | .38 |
| Cough | 10%, .33 | 0.79 (0.45,1.39) | .42 |
3.8. Sensitivity analysis
In the process of data analysis, sensitivity analysis was conducted to identify potential heterogeneity. For the incidence of headache with severe heterogeneity, the pooled outcome changed from (OR = 1.03, 95%CI 0.57 to 1.86) with I2 = 76% to (OR = 1.30, 95%CI 0.31 to 5.42) with I2 = 76%. Therefore, the results were similar and credible.
4. Discussion
Riociguat is an sGC stimulator that works by targeting the NO-sGC-cyclic guanosine monophosphate pathway, and has been the only approved drug for the treatment of PAH and persistent/recurrent or inoperable CTEPH after surgical treatment.[21] The half-life of riociguat is about 12 hours in patients, and could be rapidly absorbed after oral administration. The pharmacokinetic factors of riociguat include smoking, ethnicity, age, sex, and complications.[22] Recent study found that the clearance of riociguat was higher in patients with smoking habit, and riociguat could significantly ameliorate hemodynamic parameters; moreover, 6-MWD was associated with the change of hemodynamic parameters, especially PVR.[23] Therefore, reducing the metabolism and clearance rate of riociguat in patients with CTEPH may be more effective in improving hemodynamic parameters and increasing 6-MWD.
This is the first meta-analysis to assess the efficacy and safety of riociguat in the treatment of CTEPH. The results indicated that riociguat could markedly improve the levels of hemodynamic parameters, except for PCWP and RAP. In a previous meta-analysis, the results indicated that targeted therapies could remarkably enhance pulmonary hemodynamics in patients with CTEPH.[24] Other study demonstrated that riociguat obviously decreased mean pulmonary arterial pressure (47.3– 38.9 mmHg) and PVR (9.2– 5.7 wood units), and increased cardiac index.[25] Later, the study evaluated the effects of riociguat on hemodynamic parameters in Asian patients with inoperable CTEPH, and the results indicated that riociguat significantly reduced PAP (41– 38 mmHg) and PVR (787 to 478 dyn s cm−5), and relieved clinical symptoms.[26] These results were consistent with our study.
Recent studies found that exercise capacity was reduced by 57% in patients with CTEPH, and the right ventricle and its interplay with pulmonary circulation were determinants of exercise capacity.[27,28] Moreover, the main clinical symptoms of CTEPH were exercise capacity limitations, exertional dyspnea, and life quality decline.[29] At present, the trial endpoint and main indicator of risk assessment in PAH were the 6-MWD, which were used to measure the exercise capacity.[30] In an uncontrolled and single-arm clinical trial, the results showed that riociguat was well tolerated and improved the 6-MWD, and no new safety events were observed in patients with CTEPH.[31] In our study, we included four studies to evaluate the effects of riociguat on 6-MWD, and three studies to evaluate the effects of riociguat on dyspnea. The results confirmed that riociguat significantly increased the 6-MWD and decreased the incidence of dyspnea (P < .05). As a consequence, we concluded that riociguat was effective in improving the exercise capacity.
NT-proBNP is correlated with changes in right ventricular function, and has been considered as a biomarker in the prognosis of various cardiovascular diseases. In patients with PAH, the plasma level of NT-proBNP was higher, and this was associated with the prognosis and mortality.[32] In addition, NT-proBNP is also an accurate biomarker of congenital PAH.[33] Suntharalingam et al[34] assessed the role of NT-proBNP and 6-MWD in patients with CETPH, they found that a higher level of NT-proBNP and worse 6-MWD had been become the indicators in predicting mortality, and the capability of NT-proBNP to predict PAH was associated with age. In this meta-analysis, we found that riociguat could not significantly reduce the level of NT-proBNP.
For the effects of riociguat on quality of life, we evaluated the LPH and EQ-5D scores, and the results indicated that riociguat significantly decreased the LPH scores and increased EQ-5D scores. With regard to side effects, the incidence of dyspepsia and peripheral edema were higher in patients receiving riociguat. Previous study showed that headache and hypotension frequently occurred in patients when treated with riociguat.[35] Furthermore, we found that there were no significant differences in the incidence of headache, dizziness, nasopharyngitis, nausea, vomiting, diarrhea, hypotension, upper respiratory tract infection, constipation, dyspnea, and cough between riociguat and placebo groups. These were not consistent with the previous study, and might be caused by the limited sample size of our selected studies. Bleeding is also a serious adverse reaction that happened in CTEPH patients received riociguat, but all included studies had no assessment on this outcome.
Meanwhile, our study has some limitations. First, there were only 4 studies available in this meta-analysis, and the sample size of RCTs conducted in all 4 studies was small, and some of the studies had short follow-up or even no mention of follow-up. Second, the quality of included studies was moderate, and one of the 4 studies was of low quality, which may affect the accuracy of the conclusions. Third, significant heterogeneity was observed, and sensitivity analysis was used to exclude the studies of potential published bias, but no relevant factors were observed and the results were consistent. Therefore, more high-quality studies with sufficient data are necessary to further evaluate the efficacy and safety of riociguat in the treatment of patients with CTEPH in the future.
In conclusion, the results of this meta-analysis suggested that riociguat could efficiently improve the hemodynamic parameters and increase the exercise capacity of patients with CTEPH. Furthermore, riociguat could significantly relieve dyspnea, decrease the LPH scores and increase EQ-5D scores, contributing to life quality improvement. In addition, no serious adverse events were observed in patients receiving riociguat, and the drug could increase the incidence of dyspepsia and peripheral edema. Based on these results, it is safe to conclude that the efficacy and safety of riociguat in the treatment of CTEPH had been substantiated, and this will provide a reasonable medication regimen for the treatment of CTEPH.
Author contributions
Conceptualization: Mingxing Li.
Data curation: Jin Song.
Methodology: Shenglong Gu.
Software: Miaofa Ying.
Supervision: Rui Zhao, Mingxing Li.
Validation: Mingxing Li.
Visualization: Mingxing Li.
Writing – original draft: Miaofa Ying.
Writing – review & editing: Mingxing Li.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 6-MWD = 6-min walking distance, BPA = balloon pulmonary angioplasty, CIs = confidence intervals, CTEPH = chronic thromboembolic pulmonary hypertension, EQ-5D = EuroQol group 5-dimension self-report questionnaire, LPH = living with pulmonary hypertension, MAP = mean arterial pressure, NT-proBNP = N-terminal pro-hormone B-type natriuretic peptide, PAH = pulmonary arterial hypertension, PCWP = pulmonary capillary wedge pressure, PEA = pulmonary endarterectomy, RAP = right arterial pressure, SMD = standardized mean difference.
How to cite this article: Ying M, Song J, Gu S, Zhao R, Li M. Efficacy and safety of riociguat in the treatment of chronic thromboembolic pulmonary arterial hypertension: a meta-analysis. Medicine. 2021;100:22(e26211).
This work was supported by the Natural Science Foundation of Zhejiang Province (Grant no. LYY19H310010, LYY21H310002, and LYY20H310002).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
6-MWD = 6-minute walk distance, BDS = Borg dyspnoea score, LPHS = Living with pulmonary hypertension score, NT-proBNP = N-terminal B-type natriuretic peptide (pg/ml), PVR = pulmonary vascular resistance (dyn·s·cm−5), RCTs = randomized controlled trials, t.i.d = 3 times daily.
References
- [1].Sahay S. Evaluation and classification of pulmonary arterial hypertension. J Thorac Dis 2019;11:S1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lang I. Chronic thromboembolic pulmonary hypertension: a distinct disease entity. Eur Respir Rev 2015;24:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Elwing JM, Vaidya A, Auger WR. Chronic thromboembolic pulmonary hypertension: an update. Clin Chest Med 2018;39:605–20. [DOI] [PubMed] [Google Scholar]
- [4].Yang S, Yang Y, Zhai Z, et al. Incidence and risk factors of chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. J Thorac Dis 2015;7:1927–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang M, Wang N, Zhai Z, et al. Incidence and risk factors of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a systematic review and meta-analysis of cohort studies. J Thorac Dis 2018;10:4751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lang I, Meyer BC, Ogo T, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017;26:160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van Thor MCJ, Lely RJ, Braams NJ, et al. Safety and efficacy of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension in the Netherlands. Neth Heart J 2020;28:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tanabe N, Kawakami T, Satoh T, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: A systematic review. Respir Invest 2018;56:332–41. [DOI] [PubMed] [Google Scholar]
- [9].Zhang Y, Yu X, Jin Q, et al. Advances in targeted therapy for chronic thromboembolic pulmonary hypertension. Heart Fail Rev 2019;24:949–65. [DOI] [PubMed] [Google Scholar]
- [10].Lian T, Jiang X, Jing Z. Riociguat: a soluble guanylate cyclase stimulator for the treatment of pulmonary hypertension. Drug Des Dev Ther 2017;11:1195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mielniczuk LM, Swiston JR, Mehta S. Riociguat: a novel therapeutic option for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Can J Cardiol 2014;30:1233–40. [DOI] [PubMed] [Google Scholar]
- [12].Ghofrani HA, Hoeper MM, Halank M, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J 2010;36:792–9. [DOI] [PubMed] [Google Scholar]
- [13].Marra AM, Egenlauf B, Ehlken N, et al. Change of right heart size and function by long-term therapy with riociguat in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Int J Cardiol 2015;195:19–26. [DOI] [PubMed] [Google Scholar]
- [14].Bishop BM. Riociguat for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Am J Health-Syst Ph 2014;71:1839–44. [DOI] [PubMed] [Google Scholar]
- [15].Ghofrani H, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. New Eng J Med 2013;369:319–29. [DOI] [PubMed] [Google Scholar]
- [16].Marra AM, Halank M, Benjamin N, et al. Right ventricular size and function under riociguat in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (the RIVER study). Resp Res 2018;19:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Simonneau GD, Armini AM, Ghofrani H, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J 2015;45:1293–302. [DOI] [PubMed] [Google Scholar]
- [18].Wang C, Jing Z, Huang Y, et al. Riociguat for the treatment of pulmonary hypertension: Chinese subgroup analyses and comparison. Heart Asia 2016;8:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bonderman D, Skoro-Sajer N, Jakowitsch J, et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 2007;115:2153–8. [DOI] [PubMed] [Google Scholar]
- [20].Condliffe R, Kiely DG, Gibbs JSR, et al. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Respir J 2008;33:332–8. [DOI] [PubMed] [Google Scholar]
- [21].Ranka S, Mohananey D, Agarwal N, et al. Chronic thromboembolic pulmonary hypertension-management strategies and outcomes. J Cardiothor Vasc An 2019;12: S1053-0770(19)31187-5. [DOI] [PubMed] [Google Scholar]
- [22].Frey R, Becker C, Saleh S, et al. Clinical pharmacokinetic and pharmacodynamic profile of riociguat. Clin Pharmacokinet 2018;57:647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Saleh S, Becker C, Frey R, et al. Population pharmacokinetics and the pharmacokinetic/pharmacodynamic relationship of riociguat in patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. Pulm Circ 2016;6:S86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang J, Li JM, Huang ZS, et al. A meta-analysis of randomized controlled trials in targeted treatments of chronic thromboembolic pulmonary hypertension. Clin Respir J 2019;13:467–79. [DOI] [PubMed] [Google Scholar]
- [25].Sulica R, Sangli S, Chakravarti A, et al. Clinical and hemodynamic benefit of macitentan and riociguat upfront combination in patients with pulmonary arterial hypertension. Pulm circ 2019;9:766720768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tsai CH, Wu CK, Kuo PH, et al. Riociguat improves pulmonary hemodynamics in patients with inoperable chronic thromboembolic pulmonary hypertension. Acta Cardiol Sin 2020;36:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Claeys M, Claessen G, La Gerche A, et al. Impaired cardiac reserve and abnormal vascular load limit exercise capacity in chronic thromboembolic disease. JACC 2019;12:1444–56. [DOI] [PubMed] [Google Scholar]
- [28].La Gerche A, Rakhit DJ, Claessen G. Exercise and the right ventricle: a potential Achilles’ heel. Cardiovasc Res 2017;113:1499–508. [DOI] [PubMed] [Google Scholar]
- [29].Ulrich S, Saxer S, Hasler ED, et al. Effect of domiciliary oxygen therapy on exercise capacity and quality of life in patients with pulmonary arterial or chronic thromboembolic pulmonary hypertension: a randomised, placebo-controlled trial. Eur Respir J 2019;54:1900276. [DOI] [PubMed] [Google Scholar]
- [30].Saxer S, Lichtblau M, Berlier C, et al. Physical activity in incident patients with pulmonary arterial and chronic thromboembolic hypertension. Lung 2019;197:617–25. [DOI] [PubMed] [Google Scholar]
- [31].Mclaughlin VV, Jansa P, Nielsen-Kudsk JE, et al. Riociguat in patients with chronic thromboembolic pulmonary hypertension: results from an early access study. BMC Pulm Med 2017;17:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chin KM, Rubin LJ, Channick R, et al. Association of N-Terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation 2019;139:2440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rodriguez-Gonzalez M, Benavente-Fernandez I, Castellano-Martinez A, et al. NT-proBNP plasma levels as biomarkers for pulmonary hypertension in healthy infants with respiratory syncytial virus infection. Biomark Med 2019;13:605–18. [DOI] [PubMed] [Google Scholar]
- [34].Suntharalingam J, Goldsmith K, Toshner M, et al. Role of NT-proBNP and 6MWD in chronic thromboembolic pulmonary hypertension. Resp Med 2007;101:2254–62. [DOI] [PubMed] [Google Scholar]
- [35].Simonneau GP, D’Armini AMP, Ghofrani HP, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016;4:372–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


