Abstract
Background:
Recent cardiovascular outcome trials (CVOTs) changed the therapeutic strategy of guidelines for type 2 diabetes. We compared the characteristics of patients from real-world hospital settings with those of participants in recent pragmatic randomized trials.
Methods:
This electronic medical record (EMR)-based retrospective observational study investigated the data of patients with diabetes from inpatient and outpatient settings in West China Hospital of Sichuan University from January 1, 2011, to June 30, 2019. We identified patients meeting the inclusion criteria of a pragmatic randomized trial (EMPA-REG OUTCOME) based on EMRs and compared their baseline characteristics with those of the trial participants. The cutoff for the clinical significance of each characteristic was set as its minimal clinically important difference based on expert consultation.
Results:
We included 48,257 inpatients and 36,857 outpatients with diabetes and found that 8389 (17.4%) inpatients and 2646 (7.2%) outpatients met the inclusion criteria for the EMPA-REG OUTCOME trial. Compared with the trial population, the real-world inpatients meeting the eligibility criteria of the EMPA-REG OUTCOME had similar age, blood pressure, and lipid profiles but comprised of fewer males, metformin users, anti-hypertensive drug users, and aspirin users, and had a lower body mass index. The group of outpatients meeting the eligibility criteria had fewer males, similar age, fewer metformin users, fewer insulin users, fewer anti-hypertensive drug users, and fewer aspirin users compared with the trial population.
Conclusions:
The trial population in EMPA-REG OUTCOME represents only a small portion of patients with diabetes from the inpatient and outpatient departments of a Chinese tertiary medical center. Evidence localization in different clinical settings and validation are essential to enabling extrapolation of the results from CVOTs in patients with diabetes to Chinese clinical practice.
Keywords: Cardiovascular outcome trials, Empagliflozin, Indirectness of evidence
Introduction
Randomized controlled trials (RCTs) are recognized as the gold standard for evaluating the safety and efficacy of medicinal products and supporting clinical decision-making.[1,2] However, to ensure study precision and quality, and patient safety, RCTs typically have restrictive eligibility criteria and, therefore, include highly specific populations, which leads to poor representativeness of real-world populations and limited generalizability.[3] Considering these shortcomings, in addition to that RCTs usually adopt surrogate outcomes, pragmatic RCT designs with fewer recruitment restrictions are increasingly used to improve population representation and applicability to real-world practice.[4–7] This type of trial design can provide robust evidence for guideline development and clinical decision-making, especially in the study and management of non-communicable chronic diseases.[8,9]
Type 2 diabetes is among the most common non-communicable chronic diseases and is a major risk factor for cardiovascular disease.[10–14] Since 2008, to ensure the cardiovascular safety of anti-diabetic agents for the treatment of type 2 diabetes, the Food and Drug Administration has required that new drugs show evidence not only of glucose-lowering efficacy but also of cardiovascular safety, as demonstrated in a cardiovascular outcome trial (CVOT).[14,15] CVOTs, most of which are pragmatic RCTs, play a key role in the development of clinical practice guidelines for diabetes.[9,16–19] However, recent studies have reported that CVOTs do not adequately represent real-world patient populations.[20,21]
The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) study[22] was a landmark randomized, double-blind, placebo-controlled CVOT that investigated the cardiovascular outcomes and long-term safety of empagliflozin, a sodium-glucose co-transporter 2 (SGLT-2) inhibitor, in patients with type 2 diabetes.[22–24] This study has been widely cited and has contributed significantly to recent guidelines on diabetes.[17–19] However, it remains unclear whether the study population of EMPA-REG represents real-world Chinese patients with diabetes and adequately informs clinical decision-making in Chinese hospitals.
In the present study, we aimed to explore the representativeness of the EMPA-REG OUTCOME trial with respect to Chinese real-world patients with diabetes by describing and comparing the baseline characteristics of the EMPA-REG OUTCOME trial population with those of patients in a hospital setting who would have been eligible for inclusion in EMPA-REG OUTCOME.[25]
Methods
Ethics
This study was approved by the ethical committee of West China Hospital (WCH) of Sichuan University (No. 2018-379).
Data source and study population
WCH is one of the largest tertiary hospitals in China.[26] We performed a retrospective cross-sectional analysis of data obtained from the electronic medical records (EMRs) of all patients with diabetes treated at WCH from January 1, 2011 to June 30, 2019 as a part of an ongoing EMR-based diabetes network, namely West China Electronic medical record Collaboration Of DiabEtes (WECODe).
We included inpatients if they (1) attended the inpatient department with a discharge diagnosis according to International Classification of Diseases 10th Revision (ICD-10), including codes E10–E14, fasting glucose >7.0 mmol/L, 2-hour blood glucose after 75 g glucose challenge >11.1 mmol/L, random glucose >11.1 mmol/L, or glycated hemoglobin A1c (HbA1c) >6.5%; (2) were ≥18 years old; and (3) were Chinese. We included outpatients if they attended the outpatient department and had a diagnosis of “diabetes” in the free text or ICD-10 codes including E10–E14 in the EMR. We excluded inpatients or outpatients with missing key laboratory test data including HbA1c, serum creatinine, and lipid profiles.
Next, we identified patients who would have met the inclusion criteria of the EMPA-REG OUTCOME trial who had (1) a first HbA1c value of 7.0% to 10.0%, (2) a first estimated glomerular filtration rate (eGFR) value of ≥30 mL/min per 1.73 m2, and (3) high cardiovascular risk. We defined each cardiovascular risk factor using the closest matching diagnosis ICD-10 codes available for inpatients, and diagnosis, disease description, and pharmaceutic therapy records for outpatients [Supplementary Tables 1 and 2].
Data collection
We collected the following data from the inpatient EMR system: anonymous identification number, age, sex, weight, height, systolic blood pressure, diastolic blood pressure, prescription records, laboratory tests, and discharge diagnosis with ICD-10 codes. We used data from the first hospitalization of patients who were admitted more than once. We also included laboratory test data from 1 month before admission to the outpatient clinic or emergency room as the baseline data for the inpatients. eGFR was calculated using the CKD-EPI creatinine equation (2009).[27] We described the baseline characteristics based on the first available data in the records.
We collected the following data from the outpatient EMR system: the anonymous identification number, age, sex, prescription records, laboratory tests, and diagnosis with ICD-10 codes and/or free text. We described the baseline characteristics using the first record within the 3 years after the first outpatient visit.
Data extraction from EMPA-REG OUTCOME
We extracted the following baseline characteristics of patients in the empagliflozin group in the EMPA-REG trial from the published appendix data:[22] mean and standard deviation (SD) for age, weight, body mass index (BMI), HbA1c, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, total cholesterol, eGFR, systolic blood pressure, and diastolic blood pressure; the frequency and percentage of users of metformin, insulin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB), beta-blockers, statins, and acetylsalicylic acid; the frequency and percentage of male and categories of eGFR and urine albumin-to-creatinine ratio.
Statistical analysis
Continuous variables were described as means and SDs and compared using the mean difference (MD), which is the mean in the EMPA-REG OUTCOME trial population minus that for each study population. Categorical variables were shown as frequencies and percentages and were compared by the difference in the proportion (PD), which is the percentage in the EMPA-REG OUTCOME trial population minus that for each study population.
We used the minimally clinically important difference (MCID) to describe the smallest magnitude of change that might affect clinical decision-making in real-world practice.[28–31] We consulted seven senior clinical diabetologists from different provinces in China to determine the MCID of each baseline characteristic [Supplementary Table 3]. We used ±10% as the MCID for categorical variables. The clinically significant difference for each variable was identified if its MD or PD was out the range of its MCID. For those continuous or categorical variables with clinical significance, we used Student's t test or the Chi-square test, respectively, to further evaluate the statistically significant difference with a significance level of α = 0.001. All analyses were conducted using R-Studio (R Pack Version 3.6.1; R Studio, Boston, MA, USA).
Results
Among a total of 131,695 inpatients and 201,798 outpatients with diabetes registered in the EMR system of WCH from January 1, 2011, to June 30, 2019, we included 48,257 inpatients and 36,857 outpatients, among which 8389 (17.4%, 8389/48,257) and 2646 (7.2%, 2646/36,857), respectively, met the inclusion criteria for the EMPA-REG OUTCOME trial. The details of the study population selection process are summarized in Figure 1.
Figure 1.
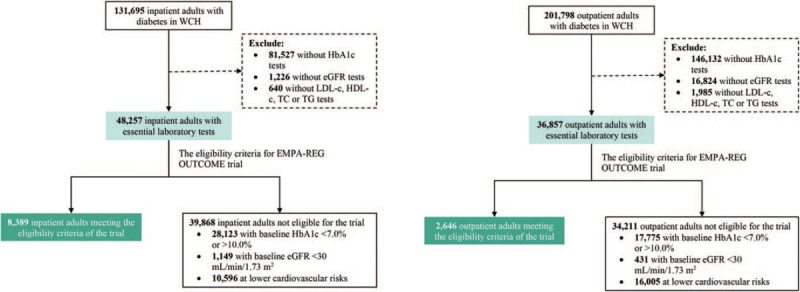
Selection of inpatient and outpatient adults with diabetes. eGFR: Estimated glomerular filtration rate; HbA1c: Glycated hemoglobin A1c; HDL-c: High-density lipoprotein cholesterol; LDL-c: Low-density lipoprotein cholesterol; TC: Total cholesterol; TG: Triglycerides.
Comparison of baseline characteristics between the EMPA-REG OUTCOME population and all included patients
Compared with the EMPA-REG OUTCOME trial population, the inpatients with diabetes included fewer metformin users (73.8% vs. 21.6%, PD: 52.2%), fewer anti-hypertensive agent users (94.9% vs. 54.4%, PD: 40.5%), fewer ACEI/ARB users (81.0% vs. 29.0%, PD: 52.0%), fewer beta-blocker users (65.2% vs. 21.5%, PD: 43.7%), fewer statins users (77.4% vs. 41.3%, PD: 36.1%), fewer acetylsalicylic acid users (82.7% vs. 30.3%, PD: 52.4%), and fewer males (71.2% vs. 61.0%, PD: 10.2%), but more patients with better kidney function (eGFR ≥ 90 subgroup: 22.4% vs. 45.3%, PD: −22.9%), and had clinically lower BMI (30.6 kg/m2vs. 24.5 kg/m2, MD: 6.1 kg/m2, MCID: ±3.0 kg/m2), and clinically similar blood pressure, average age, and lipid profiles (MD < MCID). Outpatients with diabetes included fewer metformin users (73.8% vs. 42.4%, PD: 31.4%), fewer insulin users (48.0% vs. 26.6%, PD: 21.4%), fewer anti-hypertensive agent users (94.9% vs. 24.4%, PD: 70.5%), fewer ACEI/ARB users (81.0% vs. 18.0%, PD: 63.0%), fewer beta-blocker users (65.2% vs. 6.6%, PD: 58.6%), fewer statins users (77.4% vs. 26.8%, PD: 50.6%), fewer acetylsalicylic acid users (82.7% vs. 10.7%, PD: 72.0%), and fewer males (71.2% vs. 58.0%, PD: 13.2%), but more patients with better kidney function (eGFR ≥90 subgroup: 22.4% vs. 59.4%, PD: −37.0%), but had clinically similar average age and lipid profiles (MD < MCID) [Table 1].
Table 1.
Comparison of baseline characteristics between the EMPA-REG OUTCOME population and identified patients in each group.
| Empagliflozin trial population | Inpatients | Eligible inpatients | Outpatients | Eligible outpatients | ||||||
| Characteristics | MCID | N = 4687 | N = 48,257 | Δ∗ | N = 8389 | Δ | N = 36,857 | Δ | N = 2646 | Δ |
| Age (years) | ±10.0 | 63.1 ± 8.6 | 62.9 ± 13.7 | 0.2 | 67.3 ± 11.6 | −4.2 | 55.7 ± 12.7 | 7.4 | 62.6 ± 11.1 | 0.5 |
| Male, n (%) | ±10.0% | 3336 (71.2) | 29,416 (61.0) | 10.2%† | 5414 (64.5) | 6.7% | 21,366 (58.0) | 13.2%† | 1580 (59.7) | 11.5%† |
| Weight (kg) | 86.2 ± 18.9 | 65.0 ± 12.9 | 21.2 | 65.6 ± 12.1 | 20.6 | |||||
| BMI (kg/m2) | ±3.0 | 30.6 ± 5.3 | 24.5 ± 4.1 | 6.1 | 24.7 ± 3.9 | 5.9 | ||||
| Cardiovascular risk factor, n (%) | 4657 (99.4) | 20,239 (41.9) | 8389 (100) | 4971 (13.5) | 2646 (100) | |||||
| LDL-c (mmol/L) | ±1.0 | 2.2 ± 0.9 | 2.4 ± 1.0 | −0.2 | 2.3 ± 0.9 | −0.1 | 2.8 ± 1.0 | −0.6 | 2.6 ± 1.1 | −0.4 |
| HDL-c (mmol/L) | ±0.5 | 1.2 ± 0.3 | 1.1 ± 0.4 | 0.1 | 1.1 ± 0.4 | 0.1 | 1.3 ± 0.4 | −0.1 | 1.3 ± 0.4 | −0.1 |
| TG (mmol/L) | ±2.0 | 1.9 ± 1.5 | 2.0 ± 2.2 | −0.1 | 1.8 ± 1.5 | 0.1 | 2.2 ± 2.2 | −0.3 | 2.0 ± 1.8 | −0.1 |
| TC (mmol/L) | ±1.0 | 4.2 ± 1.1 | 4.3 ± 1.5 | −0.1 | 4.2 ± 1.2 | −0.1 | 4.9 ± 1.3 | −0.7 | 4.7 ± 1.4 | −0.5 |
| HbA1c (%) | ±1.0 | 8.1 ± 0.9 | 7.6 ± 1.9 | 0.5 | 8.1 ± 0.9 | 0.0 | 8.4 ± 2.1 | −0.3 | 8.1 ± 0.9 | 0.0 |
| SBP (mmHg) | ±10.0 | 135.3 ± 16.9 | 135.2 ± 21.5 | 0.1 | 139.2 ± 22.0 | −3.9 | ||||
| DBP (mmHg) | ±5.0 | 76.6 ± 9.7 | 79.5 ± 12.9 | −2.9 | 79.6 ± 13.0 | −3.0 | ||||
| Kidney function | ||||||||||
| eGFR (mL/min per 1.73 m2) | ±10.0 | 74.2 ± 21.6 | 81.4 ± 28.4 | −7.2 | 79.4 ± 20.9 | −5.2 | 90.2 ± 22.4 | −16.0 | 82.4 ± 19.7 | −8.2 |
| eGFR, n (%) | ||||||||||
| <60 mL/min per 1.73 m2 | ±10.0% | 1212 (25.9) | 9812 (20.3) | 5.6% | 1674 (20.0) | 5.9% | 3697 (10.0) | 15.9%† | 400 (15.1) | 10.8%† |
| 60 to <90 mL/min per 1.73 m2 | 2423 (51.7) | 16,584 (34.4) | 17.3%† | 3765 (44.9) | 6.8% | 11,253 (30.5) | 21.2%† | 1141 (43.1) | 8.6% | |
| ≥90 mL/min per 1.73 m2 | 1050 (22.4) | 21,861 (45.3) | −22.9%† | 2950 (35.2) | −12.8%† | 21,907 (59.4) | −37.0%† | 1105 (41.8) | −19.4%† | |
| UACR, n (%) | ||||||||||
| <30 mg/g | ±10.0% | 2789 (59.5) | 4945 (54.7) | 4.8% | 1158 (52.6) | 6.9% | 7026 (62.5) | −3.0% | 544 (54.9) | 4.6% |
| 30–300 mg/g | 1338 (28.5) | 2292 (25.4) | 3.1% | 643 (29.2) | −0.7% | 2888 (25.7) | 2.8% | 300 (30.3) | −1.8% | |
| >300 mg/g | 509 (10.9) | 1801 (19.9) | −9.0% | 400 (18.2) | −7.3% | 1334 (11.9) | −1.0% | 147 (14.8) | −3.9% | |
| Treatment, n (%) | ||||||||||
| Metformin | ±10.0% | 3459 (73.8) | 10,415 (21.6) | 52.2%† | 2809 (33.5) | 40.3%† | 15,618 (42.4) | 31.4%† | 1351 (51.1) | 22.7%† |
| Insulin | ±10.0% | 2252 (48.0) | 22,337 (46.3) | 1.7% | 4561 (54.4) | −6.4% | 9795 (26.6) | 21.4%† | 812 (30.7) | 17.3%† |
| Anti-hypertensive therapy | ±10.0% | 4446 (94.9) | 26,273 (54.4) | 40.5%† | 5956 (71.0) | 23.9%† | 8992 (24.4) | 70.5%† | 1660 (62.7) | 32.2%† |
| ACEI/ARB | ±10.0% | 3798 (81.0) | 13,973 (29.0) | 52.0%† | 3847 (45.9) | 35.1%† | 6647 (18.0) | 63.0%† | 1303 (49.2) | 31.8%† |
| Beta-blocker | ±10.0% | 3056 (65.2) | 10,384 (21.5) | 43.7%† | 2563 (30.6) | 34.6%† | 2440 (6.6) | 58.6%† | 711 (26.9) | 38.3%† |
| Statins | ±10.0% | 3630 (77.4) | 19,909 (41.3) | 36.1%† | 6386 (76.1) | 1.3% | 9882 (26.8) | 50.6%† | 2107 (79.6) | −2.2% |
| Acetylsalicylic acid | ±10.0% | 3876 (82.7) | 14,637 (30.3) | 52.4%† | 5138 (61.2) | 21.5%† | 3935 (10.7) | 72.0%† | 1524 (57.6) | 25.1%† |
ACEI: Angiotensin-converting enzyme inhibitors; ARB: Angiotensin receptor blockers; BMI: Body mass index; DBP: Diastolic blood pressure; eGFR: Estimated glomerular filtration rate; HbA1c: Glycated hemoglobin A1c; HDL-c: High-density lipoprotein cholesterol; LDL-c: Low-density lipoprotein cholesterol; MCID: Minimally clinically important difference; MD: Mean difference; PD: Difference in the proportion; SBP: Systolic blood pressure; TC: Total cholesterol; TG: Triglycerides; UACR: Urine albumin-to-creatinine ratio.∗Δ for continuous variables is the MD, equal to the mean in the EMPA-REG OUTCOME trial population minus that in each study population. Δ for categorical variables is the PD, equal to the percentage in the EMPA-REG OUTCOME trial population minus that in each study population. †P-value for these categorical variables with Δ out of range, −10% to 10%, is <0.001.
Comparison of baseline characteristics between the EMPA-REG OUTCOME population and eligible patients
As shown in Figure 2, compared with the EMPA-REG OUTCOME trial population, the real-world eligible inpatients included fewer metformin users (73.8% vs. 33.5%, PD: 40.3%), fewer anti-hypertensive agent users (94.9% vs. 71.0%, PD: 23.9%), fewer ACEI/ARB users (81.0% vs. 45.9%, PD: 35.1%), fewer beta-blocker users (65.2% vs. 30.6%, PD: 34.6%), fewer acetylsalicylic acid users (82.7% vs. 61.2%, PD: 21.5%), and more patients with better kidney function (eGFR ≥90 subgroup: 22.4% vs. 35.2%, PD: −12.8%), and had clinically lower BMI (30.6 kg/m2vs. 24.7 kg/m2, MD: 5.9 kg/m2, MCID: ±3.0 kg/m2), but clinically similar blood pressure, average age, and lipid profiles (MD < MCID). The real-world eligible outpatients included fewer metformin users (73.8% vs. 51.1%, PD: 22.7%), fewer insulin users (48.0% vs. 30.7%, PD: 17.3%), fewer anti-hypertensive agent users (94.9% vs. 62.7%, PD: 32.2%), fewer ACEI/ARB users (81.0% vs. 49.2%, PD: 31.8%), fewer beta-blocker users (65.2% vs. 26.9%, PD: 38.3%), fewer acetylsalicylic acid users (82.7% vs. 57.6%, PD: 25.1%), fewer males (71.2% vs. 59.7%, PD: 11.5%), and more patients with better kidney function (eGFR ≥ 90 subgroup: 22.4% vs. 41.8%, PD: −19.4%), but presented clinically similar average age and lipid profiles (MD < MCID) [Table 1].
Figure 2.
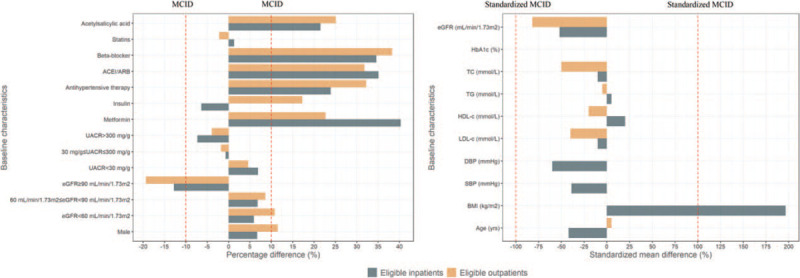
Comparison of baseline characteristics between EMPA-REG OUTCOME trial population and eligible inpatient and outpatient adults with diabetes. MCID: Minimally clinically important difference; HbA1c: Glycated hemoglobin A1c; eGFR: Estimated glomerular filtration rate; LDL-c: Low-density lipoprotein cholesterol; HDL-c: High-density lipoprotein cholesterol; TG: Triglycerides; TC: Total cholesterol; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; UACR: Urine albumin-to-creatinine ratio; ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin receptor blocker. The percentage difference is equal to the percentage in the EMPA-REG OUTCOME trial population minus that in each identified population. The mean difference is equal to the mean in the EMPA-REG OUTCOME trial population minus that in each identified population. Standardized MCID = MCID/MCID×100%. Standardized mean difference = mean difference/its MCID × 100%.
Discussion
This retrospective cross-sectional real-world study demonstrated that only a small proportion of patients with diabetes in the Chinese tertiary medical center would have met the eligibility criteria of the EMPA-REG OUTCOME trial. Our analyses also indicate that, compared with the trial population, patients meeting the trial criteria from the inpatient and outpatient departments had similar age, lipid profiles, and statins use, but better kidney function; fewer patients were male, and fewer were users of metformin, anti-hypertensive agents, and acetylsalicylic acid. This is the first study to demonstrate population differences between a pragmatic RCT on diabetes and the real-world patient population in the mainland of China.
Using data from the EMRs of a large tertiary hospital, our study described the baseline characteristics of patient populations in the outpatient and inpatient departments, which represented two major clinical scenarios in real-world practice. However, neither population was aligned with the EMPA-REG OUTCOME trial population. One reason for the differences observed is that the EMPA-REG OUTCOME trial focused solely on patients with both type 2 diabetes and cardiovascular disease; according to our findings, such patients represent a minority in both the outpatient and inpatient settings in Chinese hospitals. Nevertheless, the population difference remained significant when we examined the characteristics of patients meeting the inclusion criteria of the EMPA-REG OUTCOME. Differences can further be explained by ethnic differences between Chinese patients and patients in the global EMPA-REG OUTCOME study. For example, mean BMI is lower among Chinese patients with diabetes compared with that in Caucasian patients with type 2 diabetes.[32] Some differences might also be attributable to patient recruitment practices for pragmatic RCTs. Comorbidities of hypertension and chronic kidney disease were markedly less frequent among outpatients and inpatients with diabetes in our study compared with those in the trial population; this was in contrast with findings in previous population-based studies in various ethnicities.[26,33–36] Of note, lower use of antiplatelet therapy may be indicative of clinical inertia with respect to initiating such therapies.
Clinical practice guidelines for diabetes are a critical reference for clinicians, and the recommendations in these guidelines rely heavily on evidence from pragmatic RCTs such as EMPA-REG OUTCOME.[17–19] The significant diversity observed between Chinese real-world patients and trial participants in the present analysis indicates that the findings may not be generalizable to patients in China, regardless of the source of the difference. Our study highlights the need for obtaining evidence from pragmatic RCTs of diabetes treatments in China and validating this evidence in real-world populations using existing patient cohorts before clinical use.[25] Our findings also highlight the need for caution while introducing clinical practice guidelines from Western countries.
Our findings are in line with those from previous studies in Catalonia, the US, and the UK, which reported that only 8.2%, 4.1%, and 15.7% of patients with diabetes, respectively, met eligibility criteria for EMPA-REG OUTCOME.[21,37,38] Previous studies[21,39] found that over 80% and 90% of new users of SGLT-2 inhibitors, respectively, would not have been eligible for the EMPA-REG OUTCOME trial. These findings suggest that most clinical decision-making is not based on the direct interpretation of this pragmatic RCT. Our study also found that eligible patients with diabetes from inpatient and outpatient departments had a lower risk of cardiovascular events compared with the trial population. The difference in baseline characteristics between real-world and trial populations was also identified in the UK study,[21] in which the population features also differed from those in the present study population. These differences could be region- or ethnic-specific, supporting the necessity for evidence localization in different clinical settings.
Our study had several strengths. First, to our knowledge, this is the first study to explore the applicability of SGLT-2 inhibitor CVOTs to a real-world population in the mainland of China. Second, our database provided a large sample size with relatively comprehensive medical information and reliable data. Third, we developed MCIDs to explore how the baseline characteristics of the EMR population were clinically different from those of the trial population, which augmented the reliability and power of our study.
Our study also had some limitations. First, this study was single-centered, limiting the generalizability of the results. However, our findings show that the results of the EMPA-REG OUTCOME may not apply to all real-world populations. Second, this retrospective study based on EMR data could not completely replicate the eligibility criteria of the EMPA-REG OUTCOME, with its prospective design and real-time clinical estimates. This limitation may have contributed to a slight overestimation of the proportion of eligible patients. Moreover, in the EMRs of outpatients, most diagnoses were not recoded as ICD-10 codes but were entered as free text, reducing the accuracy of identification of outpatients with a high cardiovascular risk equivalent to that in EMPA-REG OUTCOME. Given that each diagnosis was recorded with various characters and inconsistent formats, our study may have missed some patients with a high cardiovascular risk. However, this limitation was deemed unlikely to have affected the overall results.
Conclusion
Our study shows that only a small proportion of inpatients and outpatients with diabetes at a Chinese tertiary medical center would have been eligible to enter the EMPA-REG OUTCOME trial and that the baseline characteristics of the patients at the tertiary medical center differed somewhat from those of the trial population. Our findings highlight the need for evidence localization and validation within Chinese clinical practice while interpreting data from CVOTs of diabetes medications and the clinical practice guidelines that refer to these data.
Statement
The abstract of this manuscript was presented in the oral presentation section at the 2020 International Congress of Diabetes and Metabolism, held by the Korean Diabetes Association, on 18–19 September 2020.
Funding
The study was supported by grants from the Sichuan Science and Technology Program (No. 2019YFH0150) and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Nos. ZYGD18022 and 2020HXF011). She-Yu Li also received grants from the National Natural Science Foundation of China (No. 21534008) and the Chief Scientist Office Project (No. CGA/19/10).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zhou YL, Zhang YG, Zhang R, Zhou YL, Li N, Wang MY, Tian HM, Li SY. Population diversity of cardiovascular outcome trials and real-world patients with diabetes in a Chinese tertiary hospital. Chin Med J 2021;134:1317–1323. doi: 10.1097/CM9.0000000000001407
Yi-Ling Zhou and Yong-Gang Zhang contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence—What is it and what can it tell us? N Engl J Med 2016; 375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 2.Franklin JM, Pawar A, Martin D, Glynn RJ, Levenson M, Temple R, et al. Nonrandomized real-world evidence to support regulatory decision making: process for a randomized trial replication project. Clin Pharmacol Ther 2020; 107:817–826. doi: 10.1002/cpt.1633. [DOI] [PubMed] [Google Scholar]
- 3.Akhras KS, Alsheikh-Ali AA, Kabbani S. Use of real-world evidence for healthcare decision-making in the Middle East: practical considerations and future directions. Expert Rev Pharmacoecon Outcomes Res 2019; 19:245–250. doi: 10.1080/14737167.2019.1568243. [DOI] [PubMed] [Google Scholar]
- 4.Chalkidou K, Tunis S, Whicher D, Fowler R, Zwarenstein M. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials 2012; 9:436–446. doi: 10.1177/1740774512450097. [DOI] [PubMed] [Google Scholar]
- 5.Mentz RJ, Hernandez AF, Berdan LG, Rorick T, O’Brien EC, Ibarra JC, et al. Good clinical practice guidance and pragmatic clinical trials: Balancing the best of both worlds. Circulation 2016; 133:872–880. doi: 10.1161/CIRCULATIONAHA.115.019902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roland M, Torgerson DJ. What are pragmatic trials? BMJ 1998; 316:285.doi: 10.1136/bmj.316.7127.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware JH, Hamel MB. Pragmatic trials — Guides to better patient care? N Engl J Med 2011; 364:1685–1687. doi: 10.1056/NEJMp1103502. [DOI] [PubMed] [Google Scholar]
- 8.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marx N, Rydén L, Brosius F, Ceriello A, Cheung M, Cosentino F, et al. Proceedings of the Guideline Workshop 2019 — Strategies for the optimization of guideline processes in diabetes, cardiovascular diseases and kidney diseases. Diabetes Res Clin Pract 2020; 162:108092.doi: 10.1016/j.diabres.2020.108092. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010; 304:1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 11.Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies [published correction appears in Lancet. 2010 Sep 18;376:958. Hillage, H L [corrected to Hillege, H L]]. Lancet 2010; 375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes [published correction appears in N Engl J Med. 2015 Aug 6;373: 586]. N Engl J Med 2015; 373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 14. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes | FDA [Internet]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-jardiance-reduce-cardiovascular-death-adults-type-2-diabetes. [Accessed Jul 22, 2020]. [Google Scholar]
- 15.Cefalu WT, Kaul S, Gerstein HC, Holman RR, Zinman B, Skyler JS, et al. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care editors’ expert forum. Diabetes Care 2018; 41:14–31. doi: 10.2337/dci17-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnell O, Standl E, Cos X, Heerspink HJL, Itzhak B, Lalic N, et al. Report from the 5th cardiovascular outcome trial (CVOT) summit. Cardiovasc Diabetol 2020; 19:47.doi: 10.1186/s12933-020-01022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association Of Clinical Endocrinologists and American College Of Endocrinology on the comprehensive type 2 diabetes management algorithm — 2020 Executive Summary. Endocr Pract 2020; 26:107–139. doi: 10.4158/CS-2019-0472. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Addendum. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care 2020; 43:S98–S110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 19.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 20.Birkeland KI, Bodegard J, Norhammar A, Kuiper JG, Georgiado E, Beekman-Hendriks WL, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab 2019; 21:968–974. doi: 10.1111/dom.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGovern A, Feher M, Munro N, de Lusignan S. Sodium-glucose co-transporter 2 (SGLT2) inhibitor: Comparing trial data and real-world use. Diabetes Ther 2017; 8:365–376. doi: 10.1007/s13300-017-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 23.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005; 54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Hamo CE, Filippatos G, Pocock SJ, Bernstein RA, Brueckmann M, et al. The potential role and rationale for treatment of heart failure with sodium-glucose co-transporter 2 inhibitors. Eur J Heart Fail 2017; 19:1390–1400. doi: 10.1002/ejhf.933. [DOI] [PubMed] [Google Scholar]
- 25.Shen Y, Zhou J, Hu G. Practical use of electronic health records among patients with diabetes in scientific research. Chin Med J 2020; 133:1224–1230. doi: 10.1097/CM9.0000000000000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Yu C, Li Y, Li Q, Zhang R, Hou Q, et al. Study design and baseline characteristics of inpatients with diabetes mellitus in a tertiary hospital in China: A database study based on electronic medical records. J Evid Based Med 2018; 11:152–157. doi: 10.1111/jebm.12291. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. Erratum in: Ann Intern Med 2011;155:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Clinical Significance Consensus Meeting Group. Methods to explain the clinical significance of health status measures. Mayo Clin Proc 2002; 77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 29.Maltenfort MG. The minimally important clinical difference. Clin Spine Surg 2016; 29:383.doi: 10.1097/BSD.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 30.Curtis JR, Yang S, Chen L, Pope JE, Keystone EC, Haraoui B, et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2015; 67:1345–1353. doi: 10.1002/acr.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston BC, Ebrahim S, Carrasco-Labra A, Furukawa TA, Patrick DL, Crawford MW, et al. Minimally important difference estimates and methods: a protocol. BMJ Open 2015; 5:e007953.doi:10.1136/bmjopen-2015-007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 33.Plantinga LC, Crews DC, Coresh J, Miller ER, 3rd, Saran R, Yee J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol 2010; 5:673–682. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, He J, Ji XL, Zhao YM, Lou HY, Song XX, et al. Association of chronic kidney disease with coronary heart disease and stroke risks in patients with type 2 diabetes mellitus: An observational cross-sectional study in Hangzhou, China. Chin Med J 2017; 130:57–63. doi: 10.4103/0366-6999.196564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Wang MY, Wang H, Liu HW, Lu R, Duan TQ, et al. Status of glycosylated hemoglobin and prediction of glycemic control among patients with insulin-treated type 2 diabetes in North China: a multicenter observational study. Chin Med J 2020; 133:17–24. doi: 10.1097/CM9.0000000000000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu JK, Charles LE, Fekedulegn D, Allison P, Ma CC, Violanti JM, et al. Temporal trends in prevalence of cardiovascular disease (CVD) and CVD risk factors among U.S. older workers: NHIS 2004-2018 [published online ahead of print, 2020 Oct 10]. Ann Epidemiol 2020; S1047-2797:30389–30396. doi: 10.1016/j.annepidem.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canivell S, Mata-Cases M, Vlacho B, Gratacòs M, Real J, Mauricio D, et al. How many patients with type 2 diabetes meet the inclusion criteria of the cardiovascular outcome trials with SGLT2 inhibitors? Estimations from a population database in a Mediterranean area. J Diabetes Res 2019; 2019:2018374.doi: 10.1155/2019/2018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wittbrodt ET, Eudicone JM, Bell KF, Enhoffer DM, Latham K, Green JB. Eligibility varies among the 4 sodium-glucose cotransporter-2 inhibitor cardiovascular outcomes trials: Implications for the general type 2 diabetes US population. Am J Manag Care 2018; 24:S138–S145. [PubMed] [Google Scholar]
- 39.Shao SC, Lin Y-H, Chang KC, Chan YY, Hung MJ, Kao Yang YH, et al. Sodium glucose co-transporter 2 inhibitors and cardiovascular event protections: How applicable are clinical trials and observational studies to real-world patients? BMJ Open Diabetes Res Care 2019; 7:e000742.doi: 10.1136/bmjdrc-2019-000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


