Abstract
Introduction:
The efficacy of soy diet for nonalcoholic fatty liver disease remains controversial. We conduct a systematic review and meta-analysis to explore the influence of soy diet vs placebo on the treatment of non-alcoholic fatty liver disease.
Methods:
We search PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases through October 2020 for randomized controlled trials assessing the efficacy of soy diet vs placebo for nonalcoholic fatty liver disease. This meta-analysis is performed using the random-effect model.
Results:
Five randomized controlled trials are included in the meta-analysis. Overall, compared with control group for nonalcoholic fatty liver disease, soy diet is associated with significantly reduced HOMA-IR (standard mean difference [SMD] = −0.42; 95% confidence interval [CI] = −0.76 to −0.08; P = .01), increased insulin (SMD = −0.64; 95% CI = −0.98 to −0.30; P = .0002) and decreased malondialdehyde (SMD = −0.43; 95% CI = −0.74 to −0.13; P = .005), but demonstrated no substantial impact on body mass index (SMD = 0.17; 95% CI = −0.20 to 0.53; P = .37), alanine aminotransferase (SMD = −0.01; 95% CI = −0.61 to 0.60; P = .98), aspartate-aminotransferase (SMD = 0.01; 95% CI = −0.47 to 0.49; P = .97), total cholesterol (SMD = 0.05; 95% CI = −0.25 to 0.35; P = .73) or low density lipoprotein (SMD = 0; 95% CI = −0.30 to 0.30; P = .99).
Conclusions:
Soy diet may benefit to alleviate insulin resistance for nonalcoholic fatty liver disease.
Keywords: meta-analysis, nonalcoholic fatty liver disease, randomized controlled trials, soy diet
1. Introduction
Nonalcoholic fatty liver disease is known as the most common chronic liver disease with high lipid accumulation in the hepatocytes, usually greater than 5% of the liver weight.[1] Type 2 diabetes mellitus commonly occur in these patients and serves as a leading cause of chronic liver disease.[2,3] Progressive degeneration of liver tissue can result in hepatic fibrosis and cirrhosis.[4] Nonalcoholic fatty liver disease are commonly linked to insulin resistance, hypertension, elevated oxidative stress, and increased plasma fibrinogen.[5–7] Liver fat accumulation may also lead to triglyceride accumulation (steatosis), nonalcoholic steatohepatitis, cirrhosis, and even hepatocellular carcinoma.[8]
Current evidence revealed the significance of dietary modification particularly a restricted-calorie diet as the cornerstone treatment of non-alcoholic fatty liver disease.[5,7] Many studies have explored the efficacy of functional foods, as a complementary therapy. For instance, soybean-derived products such as soy milk is rich of isoflavones (e.g., genistein, daidzein, glycitein), bioactive peptides, unsaturated fatty acids and fiber. Several studies revealed the improvement in glycemic measures following supplementation with either soy-isoflavones or -protein in experimental models of fatty liver.[9–11]
However, the benefit of soy diet for nonalcoholic fatty liver disease has not been well established. Recently, several studies on the topic have been published, and the results have been conflicting.[12–15] With accumulating evidence, we therefore perform the meta-analysis of randomized controlled trials (RCTs) to explore the efficacy of soy diet for nonalcoholic fatty liver disease.
2. Materials and methods
Ethical approval and patient consent are not required because this is a systematic review and meta-analysis of previously published studies. The systematic review and meta-analysis are conducted and reported in adherence to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).[16]
2.1. Search strategy and study selection
Two investigators have independently searched the following databases (inception to October 2020): PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases. The electronic search strategy is conducted using the following keywords liver disease or steatohepatitis, and soy. We also check the reference lists of the screened full-text studies to identify other potentially eligible trials.
The inclusive selection criteria are as follows:
-
1.
patients are diagnosed with non-alcoholic fatty liver disease;
-
2.
intervention treatments are soy diet vs control intervention;
-
3.
study design is RCT.
2.2. Data extraction and outcome measures
We have extracted the following information: author, number of patients, age, female, body mass index, weight, and detail methods in each group etc. Data have been extracted independently by 2 investigators, and discrepancies are resolved by consensus. We also contact the corresponding author to obtain the data when necessary.
The primary outcomes are homeostatic model assessment insulin resistance (HOMA IR) and insulin. Secondary outcomes include malondialdehyde (MDA), body mass index (BMI), alanine aminotransferase (ALT), aspartate-aminotransferase (AST), total cholesterol, and low density lipoprotein (LDL).
2.3. Quality assessment in individual studies
Methodological quality of the included studies is independently evaluated using the modified Jadad scale.[17] There are 3 items for Jadad scale: randomization (0–2 points), blinding (0–2 points), dropouts, and withdrawals (0–1 points). The score of Jadad scale varies from 0 to 5 points. An article with Jadad score ≤2 is considered to be of low quality. If the Jadad score ≥3, the study is thought to be of high quality.[18]
2.4. Statistical analysis
We estimate the standard mean difference (SMD) with 95% confidence interval (CI) for all continuous outcomes. The random-effects model is used regardless of heterogeneity. Heterogeneity is reported using the I2 statistic, and I2 > 50% indicates significant heterogeneity.[19] Whenever significant heterogeneity is present, we search for potential sources of heterogeneity via omitting 1 study in turn for the meta-analysis or performing subgroup analysis. All statistical analyses are performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
3. Results
3.1. Literature search, study characteristics, and quality assessment
A detailed flowchart of the search and selection results is shown in Figure 1. One hundred fifty two potentially relevant articles are identified initially. Finally, 5 RCTs that meet our inclusion criteria are included in the meta-analysis.[10,12–15]
Figure 1.
Flow diagram of study searching and selection process.
The baseline characteristics of the 5 eligible RCTs in the meta-analysis are summarized in Table 1. The 5 studies are published between 2014 and 2019, and the total sample size is 260. Among the 5 studies included here, 2 studies report HOMA IR and insulin,[12,15] 3 studies report MDA,[10,12,15] 4 studies report BMI,[10,13–15] and 3 studies report ALT, AST, total cholesterol, and LDL.[10,13,15] Jadad scores of the 5 included studies vary from 3 to 5, and all 5 studies are considered to be high-quality ones according to quality assessment.
Table 1.
Characteristics of included studies.
| Soy group | Control group | |||||||||||||
| No. | Author | Number | Age (yr) | Female (n) | Body mass index (kg/m2) | Weight (kg) | Methods | Number | Age (yr) | Female (n) | Body mass index (kg/m2) | Weight (kg) | Methods | Jada scores |
| 1 | Maleki 2019 | 31 | 46.16 ± 10.53 | 21 | 30.85 ± 3.53 | 83.03 ± 9.98 | 240 mL/day soy milk plus 500-deficit calorie diet for 8 weeks | 31 | 45.16 ± 9.86 | 22 | 31.29 ± 3.77 | 84.97 ± 14.02 | 500-deficit calorie diet | 4 |
| 2 | Eslami 2019 | 32 | 46.25 ± 10.51 | 22 | – | – | 240 mL of soy milk daily plus low-calorie diet for 8 weeks | 32 | 45.16 ± 9.86 | 23 | – | – | low-calorie diet | 4 |
| 3 | Deibert 2019 | 11 | 57.0 (8.0), median (IQR) | 3 | 32.2 (3.4), | 101.1 (30.5) | soy-yogurt-honey preparation | 11 | 54.0 (14.0), median (IQR) | 6 | 32.4 (3.7) | 99.5 (11.1) | guided lifestyle change | 3 |
| 4 | Amanat 2018 | 41 | 44.22 ± 11.8 | - | 29.09 ± 4.67 | 81.6 ± 14.73 | 250 mg genistein for 8 weeks | 41 | 42.94 ± 9.55 | - | 27.83 ± 4.51 | 74.92 ± 11.51 | placebo | 5 |
| 5 | Kani 2014 | 15 | 48.5 ± 3.7 | 8 | 31.3 ± 3.8 | 91.2 ± 9.2 | low calorie, low carbohydrate soy containing diet for 8 weeks | 15 | 49.3 ± 3.5 | 8 | 28 ± 3.2 | 80.1 ± 8.9 | low calorie, low carbohydrate | 4 |
3.2. Primary outcomes: HOMA IR and insulin
These outcome data are analyzed with the random-effects model, and compared to control group for nonalcoholic fatty liver disease, soy diet is associated with significantly reduced HOMA-IR (SMD = −0.42; 95% CI = −0.76 to −0.08; P = .01) with no heterogeneity among the studies (I2 = 0%, heterogeneity P = .32; Fig. 2) and increased insulin (SMD = −0.64; 95% CI = −0.98 to −0.30; P = .0002) with no heterogeneity among the studies (I2 = 0%, heterogeneity P = .76; Fig. 3).
Figure 2.

Forest plot for the meta-analysis of HOMA-IR.
Figure 3.
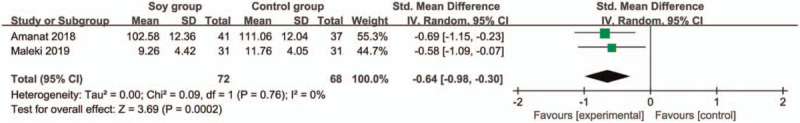
Forest plot for the meta-analysis of insulin.
3.3. Sensitivity analysis
No heterogeneity is observed among the included studies for the primary outcomes, and thus we do not perform sensitivity analysis via omitting 1 study in turn to detect heterogeneity.
3.4. Secondary outcomes
In comparison with control group for non-alcoholic fatty liver disease, soy diet can substantially reduce MDA (SMD = −0.43; 95% CI = −0.74 to -0.13; P = .005; Fig. 4), but showed no obvious impact on BMI (SMD = 0.17; 95% CI = −0.20 to 0.53; P = .37; Fig. 5), ALT (SMD = −0.01; 95% CI = −0.61 to 0.60; P = .98; Fig. 6), AST (SMD = 0.01; 95% CI = −0.47 to 0.49; P = .97; Fig. 7), total cholesterol (SMD = 0.05; 95% CI = −0.25 to 0.35; P = .73; Fig. 8) or LDL (SMD = 0; 95% CI = −0.30 to 0.30; P = .99; Fig. 9).
Figure 4.
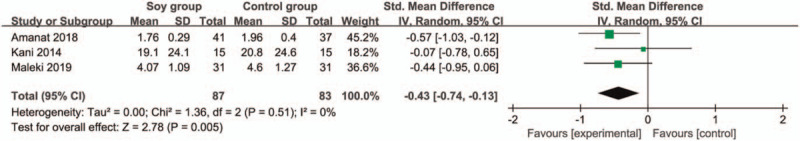
Forest plot for the meta-analysis of MDA. MDA = malondialdehyde.
Figure 5.
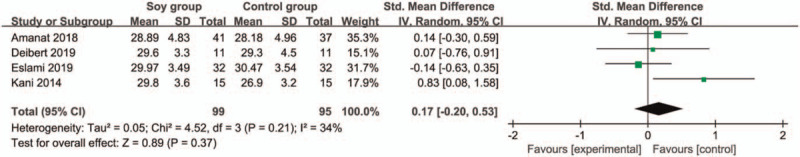
Forest plot for the meta-analysis of BMI. BMI = body mass index.
Figure 6.
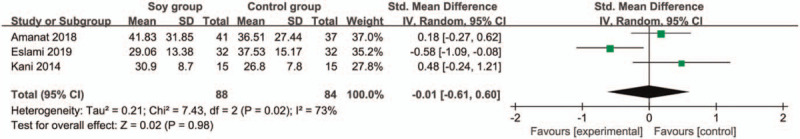
Forest plot for the meta-analysis of ALT. ALT = alanine aminotransferase.
Figure 7.
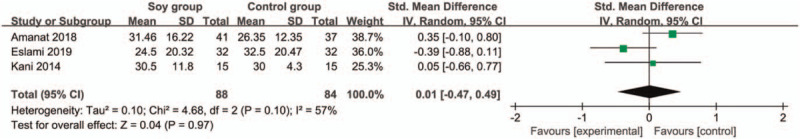
Forest plot for the meta-analysis of AST. AST = aspartate-aminotransferase.
Figure 8.
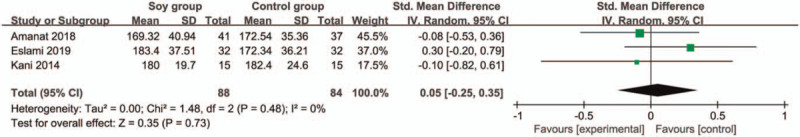
Forest plot for the meta-analysis of total cholesterol.
Figure 9.
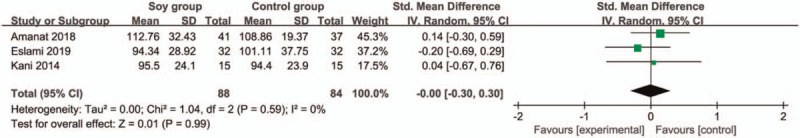
Forest plot for the meta-analysis of LDL. LDL = low density lipoprotein.
4. Discussion
The aggravation of nonalcoholic fatty liver disease may lead to nonalcoholic steatohepatitis which is characterized by hepatocellular damage, inflammation, and liver fibrosis that can progress to cirrhosis.[20–22] Type 2 diabetes mellitus can also increase the risk of developing cirrhosis, hepatocellular carcinoma, and double the death risk from liver cirrhosis.[23] It is crucial to control the metabolic index of diabetes mellitus. However, there is no approved pharmacologic agent for the treatment of nonalcoholic fatty liver disease. Several antidiabetic agents have showed some potential in alleviating insulin resistance for nonalcoholic fatty liver disease, but the results are variable.[24–26]
Our meta-analysis includes 5 RCTs and the results confirmed that soy diet could significantly reduce HOMA IR and increase insulin in patients with nonalcoholic fatty liver disease. Soy supplementation is recommended at the dose of 240 mL soy milk daily.[12,13] Regarding the potential mechanisms of reducing insulin resistance and improving glucose homeostasis in nonalcoholic fatty liver disease, soy isoflavones was reported to decrease the activity of intestinal alpha-glucosidase and protein tyrosine kinases.[27–29] In addition, soy diet may increase the uptake of glucose mediated by glucose transporter type,[30,31] and inhibit expression of lipogenesis transcription factors such as carbohydrate responsive element binding protein, sterol-regulatory element binding protein-1, liver X receptor, and retinoid-X-receptor.[9,32] In addition, the oxidative stress was substantially reduced after soy diet intervention for nonalcoholic fatty liver disease, as evidenced by the reduced MDA.
However, the results of this meta-analysis revealed no obvious impact on BMI, ALT, AST, total cholesterol or LDL after soy diet intervention for nonalcoholic fatty liver disease. The beneficial effect of reducing insulin resistance is not translated to liver function and fat accumulation. Only treatment for 8 weeks is used in the included RCTs, and this treatment duration may be short to assess the beneficial change of liver function and fat accumulation. Additionally, the bioactive peptides found in soy protein act as the inhibitors of angiotensin-converting enzyme, limit the effect of angiotensin II on vasoconstriction, stimulate the activity of Bradykinin vasodilator and consequently decrease blood pressure.[33] The high content of arginine in soy food is a precursor of nitric oxide, a known vasodilator.[34,35] Some types of soy isoflavones such as genistein are shown to have a diuretic activity and subsequently reduce blood pressure.[36]
Our meta-analysis also has some important limitations. Firstly, our analysis is based on 5 RCTs, and all of them have a relatively small sample size (n < 100). Overestimation of the treatment effect was more likely in smaller trials compared with larger samples. Although there is no heterogeneity, different methods and products of soy diet may produce some bias. Finally, treatment duration of 8 weeks may be not sufficient to produce the positive results of liver function.
5. Conclusions
Sitagliptin treatment may provide additional benefits to reduce insulin resistance in nonalcoholic fatty liver disease.
Author contributions
Conceptualization: Pian Xiong.
Data curation: Pian Xiong.
Formal analysis: Pian Xiong.
Supervision: Yong-fen Zhu.
Writing – original draft: Pian Xiong, Yong-fen Zhu.
Writing – review & editing: Pian Xiong.
Footnotes
Abbreviations: CI = confidence interval, RCTs = randomized controlled trials, SMD = standard mean difference.
How to cite this article: Xiong P, Zhu Yf. Soy diet for nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Medicine. 2021;100:22(e25817).
The authors have no funding and conflicts of interests to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
IQR = interquartile range.
References
- [1].Benedict M, Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol 2017;9:715–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].El-Ashmawy HM, Ahmed AM. Serum fetuin-B level is an independent marker for nonalcoholic fatty liver disease in patients with type 2 diabetes. Eur J Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- [3].Smits MM, Tonneijck L, Muskiet MH, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia 2016;59:2588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McCarthy EM, Rinella ME. The role of diet and nutrient composition in nonalcoholic Fatty liver disease. J Academy Nutri Diet 2012;112:401–9. [DOI] [PubMed] [Google Scholar]
- [5].Abd El-Kader SM, El-Den Ashmawy EM. Non-alcoholic fatty liver disease: the diagnosis and management. World J Hepatol 2015;7:846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhatia LS, Curzen NP, Calder PC, et al. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? E Heart J 2012;33:1190–200. [DOI] [PubMed] [Google Scholar]
- [7].Cicero AFG, Colletti A, Bellentani S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): the available clinical evidence. Nutrients 2018;10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bugianesi E, Manzini P, D’Antico S, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology 2004;39:179–87. [DOI] [PubMed] [Google Scholar]
- [9].Ascencio C, Torres N, Isoard-Acosta F, et al. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J Nutr 2004;134:522–9. [DOI] [PubMed] [Google Scholar]
- [10].Kani AH, Alavian SM, Esmaillzadeh A, et al. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non-alcoholic fatty liver disease: a parallel randomized trial. Nutrition 2014;30:814–21. [DOI] [PubMed] [Google Scholar]
- [11].Kim MH, Park JS, Jung JW, et al. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int J Obes 2011;35:1019–30. [DOI] [PubMed] [Google Scholar]
- [12].Maleki Z, Jazayeri S, Eslami O, et al. Effect of soy milk consumption on glycemic status, blood pressure, fibrinogen and malondialdehyde in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Complement Therap Med 2019;44:44–50. [DOI] [PubMed] [Google Scholar]
- [13].Eslami O, Shidfar F, Maleki Z, et al. Effect of soy milk on metabolic status of patients with nonalcoholic fatty liver disease: a randomized clinical trial. J Am Coll Nutri 2019;38:51–8. [DOI] [PubMed] [Google Scholar]
- [14].Deibert P, Lazaro A, Schaffner D, et al. Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis. World J Gastroenterol 2019;25:1116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Amanat S, Eftekhari MH, Fararouei M, et al. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: a randomized, controlled trial. Clin Nutri 2018;37:1210–5. [DOI] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [17].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:01–12. [DOI] [PubMed] [Google Scholar]
- [18].Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982–9. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [20].Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132–8. [DOI] [PubMed] [Google Scholar]
- [21].Park JS, Shim YJ, Kang BH, et al. Hepatocyte-specific clusterin overexpression attenuates diet-induced nonalcoholic steatohepatitis. Biochem Biophys Res Commun 2018;495:1775–81. [DOI] [PubMed] [Google Scholar]
- [22].Jahn D, Dorbath D, Kircher S, et al. Beneficial effects of vitamin D treatment in an obese mouse model of nonalcoholic steatohepatitis. Nutrients 2019;11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. [DOI] [PubMed] [Google Scholar]
- [24].Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone vitamin E, or placebo for nonalcoholic steatohepatitis. NEngl J Med 2010;362:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Therapeut 2009;29:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatob Pancreat Dis Int 2013;12:584–8. [DOI] [PubMed] [Google Scholar]
- [27].Hanhineva K, Törrönen R, Bondia-Pons I, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Molec Sci 2010;11:1365–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jayagopal V, Albertazzi P, Kilpatrick ES, et al. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care 2002;25:1709–14. [DOI] [PubMed] [Google Scholar]
- [29].Liu ZM, Chen YM, Ho SC. Effects of soy intake on glycemic control: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2011;93:1092–101. [DOI] [PubMed] [Google Scholar]
- [30].Arunkumar E, Anuradha CV. Genistein promotes insulin action through adenosine monophosphate-activated protein kinase activation and p70 ribosomal protein S6 kinase 1 inhibition in the skeletal muscle of mice fed a high energy diet. Nutr Res 2012;32:617–25. [DOI] [PubMed] [Google Scholar]
- [31].Ha BG, Nagaoka M, Yonezawa T, et al. Regulatory mechanism for the stimulatory action of genistein on glucose uptake in vitro and in vivo. J Nutrition Biochem 2012;23:501–9. [DOI] [PubMed] [Google Scholar]
- [32].Xiao CW, Wood CM, Weber D, et al. Dietary supplementation with soy isoflavones or replacement with soy proteins prevents hepatic lipid droplet accumulation and alters expression of genes involved in lipid metabolism in rats. Genes Nutri 2014;9:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Miraghajani MS, Najafabadi MM, Surkan PJ, et al. Soy milk consumption and blood pressure among type 2 diabetic patients with nephropathy. J Renal Nutri 2013;23:277–82 e1. [DOI] [PubMed] [Google Scholar]
- [34].De Leo F, Panarese S, Gallerani R, et al. Angiotensin converting enzyme (ACE) inhibitory peptides: production and implementation of functional food. Curr Pharmaceut Des 2009;15:3622–43. [DOI] [PubMed] [Google Scholar]
- [35].Si H, Liu D. Genistein a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutri 2008;138:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Giménez I, Martinez RM, Lou M, et al. Salidiuretic action by genistein in the isolated, perfused rat kidney. Hypertension 1998;31:706–11. [DOI] [PubMed] [Google Scholar]


